Abstract
MscS is a mechanosensitive channel that is ubiquitous among bacteria. Recent progress in the genome projects has revealed that homologs of MscS are also present in eukaryotes, but whether they operate as ion channels is unknown. In this study we cloned MSC1, a homolog of MscS in Chlamydomonas, and examined its function when expressed in Escherichia coli. Full-length MSC1 was not functional when expressed in E. coli cells. However, removal of the N-terminal signal sequence (ΔN-MSC1) reversed this effect. ΔN-MSC1 was found to open in response to membrane stretch and displayed a preference for anions over cations as permeable ions. ΔN-MSC1 exhibited marked hysteretic behavior in response to ascending and descending stimuli. That is, channel gating occurred in response to significant stimuli but remained open until the stimulus was almost completely removed. Indirect immunofluorescence revealed that MSC1 is present as punctate spots in the cytoplasm and chloroplasts. Moreover, knockdown of MSC1 expression resulted in the abnormal localization of chlorophyll. These findings show that MSC1 is an intracellular mechanosensitive channel and is responsible for the organization of chloroplast in Chlamydomonas.
Keywords: MscS, hysteresis, heterogeneous expression, knockdown, green algae
Mechanosensation is typically involved in auditory perception, touch sensation, proprioception, and gravity perception. The mechanoreceptor potential in sensory cells is mediated by mechanosensitive channels, which are activated by stretching or deformation of the membrane. The patch-clamp technique has revealed that mechanosensitive channels are present in almost every cell type, not just sensory cells. In fact, mechanosensitive channels were first recorded with the patch-clamp method in the muscle cell (1). Mechanosensitive channels in nonsensory cells are believed to be responsible for monitoring cell deformation evoked by contact with surrounding materials and by changes in osmolarity.
Bacteria harbor the mechanosensitive channels MscS and MscL, which act to protect against hypoosmotic downshock (2). The presence of MscS homologs in virtually all eubacteria and archaea suggests that MscS has an essential function in prokaryotes, but whether every MscS homolog serves as a mechanosensitive channel is uncertain. For instance, although there are at least six MscS homologs in the Escherichia coli genome, only two of them (MscS and MscK) have been detected by electrophysiological methods (3, 4). Recent genomic projects on eukaryotes have uncovered the presence of MscS homologs in some plants (Arabidopsis and Oryza), protists (Chlamydomonas), and fungi (Schizosaccharomyces). Curiously, MscS homologs have not been found in animals (5, 6). The MscS homologs of Arabidopsis (MSL2 and MSL3) have been observed as one or two foci on the plastid envelope, and their double knockout results in an abnormal size and shape of the plastids (6). This finding suggests that one of the possible functions of the MscS homologs is to control the size and shape of the intracellular organelles.
Whereas previous studies have mostly focused on mechanoreception initiated at the plasma membrane, more recent studies have shown that the mechanosensitive channels are also present in intracellular membranes. A TRP channel (TrpY1) is present in the vacuolar membrane of yeast and functions as a mechanosensitive channel (7, 8). The finding that TrpY1 releases Ca2+ upon hyperosmotic shock suggests that this intracellular mechanosensitive channel responds to environmental stress.
The presence of MscS homologs in eukaryotic genomes and the localization of the Arabidopsis MscS homologs in plastids is of special interest with respect to the symbiotic origin of the chloroplast as well as the function of this channel in the intracellular space. However, whether the eukaryotic MscS homologs work as mechanosensitive channels has not yet been directly demonstrated. In this study, we expressed a Chlamydomonas MscS homolog (MSC1) in E. coli cells and found that this protein displays mechanosensitive channel activity with characteristics different from E. coli MscS. In addition, our results revealed that MSC1 exhibited a punctuate distribution that partially overlapped with chloroplasts.
Results
Molecular Identification of MSC1.
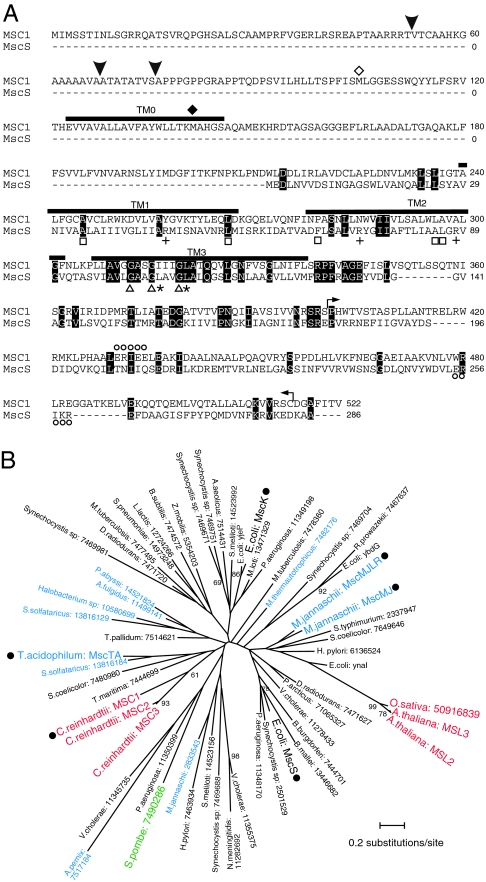
Chlamydomonas cells express at least three genes (MSC1, MSC2, and MSC3) homologous to E. coli MscS. Of these, MSC1 exhibits the highest homology to MscS and has 23.8% identity to the MscS transmembrane helices TM1, TM2, and TM3 (Fig. 1A). MSC1 shows highest homology to E. coli MscS in the TM3 region, which forms the channel pore. Of note, three glycine residues that are known to be important for close packing of TM3 are conserved (9). MSC1 contains similar residues at the positions of the leucines that form the pore constriction (10), the hydrophobic residues that are important for sensing the membrane tension (11), and the charged residues that detect pH (12) (Fig. 1A). However, the three arginine residues that are postulated to be voltage sensors are absent (10).
Fig. 1.
MSC1 sequence and phylogenic relationships. (A) MSC1 aligned with E. coli MscS. Identical residues are highlighted. Thick lines indicate the predicted transmembrane domains. Symbols represent the glycine residues important for TM3 packing (triangles), the leucine residues that form a pore constriction (asterisks), the hydrophobic residues involved in tension sensing (squares), the residues serving as a pH sensor (circles), the residues that are a possible voltage sensor (crosses), and predicted cleavage sites (arrowheads). The start codons (methionine) for ΔN-MSC1 and ΔTM0-MSC1 are indicated by open and filled diamonds, respectively. The sequence bounded by the two arrows was used as antigen. (B) The maximum likelihood (ML) tree of the MscS family proteins inferred by the JTT (Jones, Taylor, and Thornton) model. Colors represent bacteria (black), archaea (blue), fungi (green), and eukarya (red). Bootstrap proportions (BPs) by the 100 repetitions of the ML method are attached to internal branches. Unmarked branches have <60 bootstrap proportions. Filled circles indicate that these products have been identified as mechanosensitive channels by electrophysiological means (2, 4, 35, 36). The accession numbers are provided after the names of the species.
MSC1 has an extended N-terminal segment compared with MscS. A prediction program for the subcellular localization of eukaryotic proteins (TargetP) suggests that MSC1 is likely to be localized to chloroplasts (score = 0.587) or in mitochondria (score = 0.433). The program indicates that the signal peptide is possibly cleaved at residue 52/53 (TargetP) or 75/76 (ChloroP). Another program (Genetyx) suggests that the signal peptide will be cleaved at residue 67/68. A transmembrane helix (designated as TM0; predicted by SOSUI) is predicted to lie downstream of the putative cleavage site.
Phylogenic analysis of MscS family proteins revealed that bacterial, archaeal, and eukaryotic homologs cannot be divided into discrete branches (5) (Fig. 1B). The log-likelihood values and the bootstrap proportion values suggest that the MscS homologs of Chlamydomonas reinhardtii and the two homologs of Arabidopsis thaliana have developed independently.
Electrophysiological Characterization of MSC1.
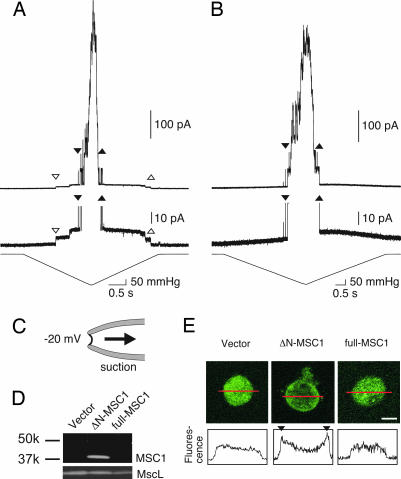
The channel activity of Chlamydomonas MSC1 was examined by patch clamping of mscS- and mscK-null E. coli spheroplasts expressing MSC1. When the full-length MSC1 was expressed and negative pressure was applied to the excised patch membrane through the patch pipette (see Fig. 2C for recording conditions), no channel activities could be detected (10 spheroplasts examined). We speculated that the signal sequence at the N terminus interferes with functional expression of the protein. Thus, we removed the N-terminal 104 residues and used Met-105 as a start codon (ΔN-MSC1) (Fig. 1A). Application of a negative pressure to the membrane of ΔN-MSC1-expressing spheroplasts elicited a unitary conductance of ≈0.4 nS (Fig. 2A). Further increases in the negative pressure opened MscL, which has a unitary conductance of ≈4 nS. The channel with the smaller conductance opened in the majority of cells expressing ΔN-MSC1 (38/50; from six preparations). In total, 16 preparations of the ΔN-MSC1-expressing cells were made in this study, and the 0.4-nS conductance was consistently observed in all preparations. On the other hand, the 0.4-nS conductance was never observed when an empty vector was incorporated (0/20; from three preparations) (Fig. 2B).
Fig. 2.
Channel activity and expression of MSC1 in mscs- and msck-null E. coli. (A and B) Current recordings from an inside-out patch excised from giant spheroplasts expressing ΔN-MSC1 (A) or harboring an empty vector (B). The second trace is an expanded view of the upper trace. The pressure applied to the patch is presented with the expanded trace. The downward and upward triangles indicate the beginning and the end of ΔN-MSC1 (open triangles) and E. coli MscL (filled triangles) activity, respectively. The membrane potential was held at −20 mV where not indicated. Voltage represents the potential of the intracellular (bath) side to that of the periplasmic (pipette) side. (C) Configuration of the patch-clamp experiment. (D) Western blot analysis of membrane fraction prepared from cells harboring empty vector or expressing ΔN-MSC1 or full-length MSC1. Anti-MSC1 (Upper) and anti-MscL (Lower) were used as the primary antibodies. (E) Immunostaining of a giant spheroplast for MSC1 (Upper). Fluorescence intensity profiles (arbitrary units) of the confocal sections along the red line are shown in Lower. Fluorescence peaks were observed at the cell membrane (triangles) in cells expressing ΔN-MSC1. (Scale bar: 2 μm.)
The expression of ΔN-MSC1 at the membrane was examined by Western blotting and indirect immunofluorescence by using an antibody raised against the C terminus of MSC1. The antibody detected a single band with a molecular mass of 37 kDa in membrane fraction from E. coli cells expressing ΔN-MSC1 (Fig. 2D). However, no signal was detected in the cells expressing the full-length MSC1 or harboring empty vector. Immunofluorescent labeling revealed intense staining concentrated at the cellular membrane in spheroplasts expressing ΔN-MSC1 (Fig. 2E). In contrast, the fluorescence intensity was uniform throughout cells expressing full-length MSC1 and in the cells harboring empty vector. Therefore, the presence of the MSC1 epitope at the cell membrane coincides with the activity of 0.4-nS conductance. Based on the observations above, it is likely that the channel current with the smaller conductance is due to ΔN-MSC1 and that ΔN-MSC1 forms a mechanosensitive channel when expressed in E. coli cells.
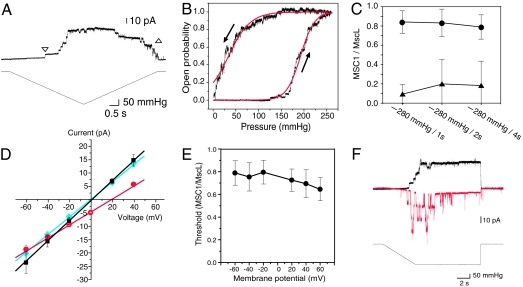
As can be seen in Fig. 2A, ΔN-MSC1 opened at a pressure of ≈130 mmHg but closed at pressures as low as 30 mmHg. On the other hand, MscL opened and closed at a similar pressure (240 mmHg). The closing threshold for ΔN-MSC1 was consistently lower than the opening threshold in all patch preparations. The pressure for closing was 0.18 ± 0.20 times the pressure for opening in ΔN-MSC1, whereas it was 1.07 ± 0.06 times in MscL (mean ± SD; n = 16). The opening and closing kinetics of ΔN-MSC1 in ΔmscLΔmscS E. coli cells were examined in greater detail. Again, the ΔN-MSC1 channels began to open at ≈130 mmHg but closed at a pressure close to 0 mmHg (Fig. 3A). Indeed, the open probability increased at pressures between 150 and 250 mmHg and decreased at pressures between 0 and 100 mmHg (Fig. 3B). Both curves fit well with the Boltzmann equation, which is based on an assumption that the channel gating depends on the membrane tension (3, 13). The half-activation pressure to the ascending pressure (p1/2,open) was 192 mmHg, and that to the descending pressure (p1/2,close) was 34 mmHg. The ratio of p1/2,close/p1/2,open was 0.33 ± 0.18 (n = 10). These observations indicate that MSC1 shows a pronounced hysteretic behavior upon gating. The threshold for opening was significantly larger than that for closing at every ramp rate examined (Fig. 3C).
Fig. 3.
The conductance of ΔN-MSC1 recorded from the inside-out patches from the giant spheroplasts of mscl- and mscs-null E. coli (A, B, D, and F) and mscs- and msck-null E. coli (C and E). (A) Channel activity in response to increasing and decreasing pressure. The beginning and the end of the channel activity are shown by downward and upward triangles, respectively. (B) Change in the open probability in response to increasing and decreasing pressure. Current traces from a single patch were summed and normalized. Dose–response curves were fitted with a two-state Boltzmann-type model (3). (C) Opening and closing threshold of ΔN-MSC1 to increasing (circles) and decreasing (triangles) pressure, respectively. The ratio of ΔN-MSC1 gating threshold relative to MscL threshold is plotted. (D) Current–voltage curve of ΔN-MSC1 in bath and pipette solutions containing 200 mM KCl, 40 mM MgCl2, 10 mM CaCl2, 0.1 mM EDTA, and 5 mM Hepes·KOH (black). Alternatively, the KCl concentration of the bath solution was reduced to 20 mM (red), or the CaCl2 concentration of the bath solution was reduced to 1 mM (blue). The data are fitted with linear regression lines. (E) Threshold for ΔN-MSC1 opening at various voltages. The threshold for ΔN-MSC1 is normalized to that of E. coli MscL (n = 10). (F) Inactivation of MSC1. Shown are current traces at −20 mV (black) and +60 mV (red). Bars represent standard deviations.
The conductance of ΔN-MSC1 was 0.39 ± 0.04 nS (n = 5) when both the bath and pipette solutions contained 200 mM KCl, 40 mM MgCl2, 10 mM CaCl2, 0.1 mM EDTA, and 5 mM Hepes·KOH (pH 7.2). This conductance is approximately one-half that of MscS. Reducing the KCl concentration of the pipette solution to 20 mM shifted the reversal potential to approximately +20 mV (Fig. 3D). Because this value is close to the reversal potential for chloride [+23 mV; see supporting information (SI) Table 1], MSC1 is likely to have higher permeability to Cl− than to K+. ΔN-MSC1 was almost equally permeable to Br− or I− (see SI Fig. 5). Evaluation of these data with the Goldman–Hodgkin–Katz equation indicated that the ratio of the permeability is gK:gCl:gBr:gI = 1:7:9:9. This anion preference is stronger than that of MscS, which has a gCl/gK of 1.2–1.5 (14, 15). Decreasing the bath CaCl2 concentration to 1 mM did not shift the reversal potential, showing that the permeability to Ca2+ was insignificant (Fig. 3D). Therefore, the major ion species that passes through ΔN-MSC1 in vivo is likely to be chloride ion.
The threshold for the activation of MSC1 did not change significantly over voltages ranging from −60 mV to +60 mV (Fig. 3E). In contrast, the inactivation of ΔN-MSC1 was influenced by the voltage. After application of a negative pressure at which approximately one-half of the MSC1 channels were activated (at −20 mV), most opened ΔN-MSC1 channels remained open as long as the negative pressure persisted (Fig. 3F). However, at +60 mV, the MSC1 channels repeatedly opened and closed for the first 4–6 sec and rarely opened thereafter (Fig. 3F).
MSC1 has an extended N-terminal sequence compared with E. coli MscS. Although MSC1 resembles E. coli MscK in this respect (2, 4), this region does not exhibit homology to MscK. Deletion of the N-terminal 140 residues, which encompass TM0 and the signal sequence, abolished MSC1 channel activity (10 spheroplasts examined). Thus, the TM0 region appears to be essential for the functional expression of MSC1.
Localization of MSC1.
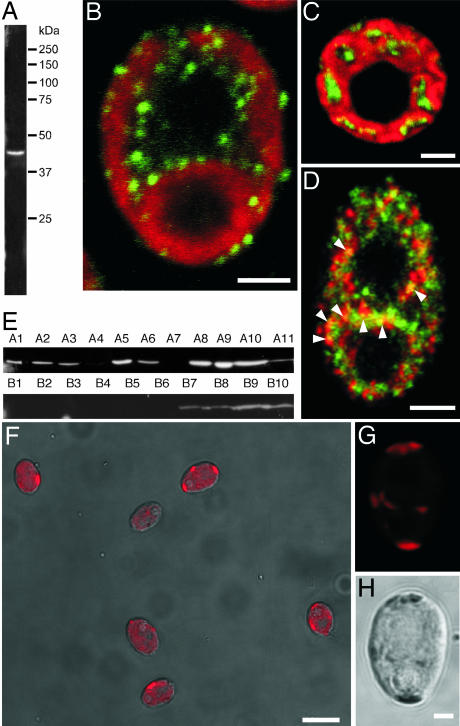
The localization of MSC1 in Chlamydomonas cells was examined by indirect immunofluorescence microscopy using an antibody generated against MSC1. The anti-MSC1 antibody recognized a single band with an apparent molecular mass of 45 kDa in Western blots of whole-cell extract (Fig. 4A). Numerous punctate spots in the cytoplasm and chloroplasts of Chlamydomonas cells were reactive for the anti-MSC1 (Fig. 4 B–D, green). Labeling was present within chloroplast but did not overlap with chlorophyll autofluorescence (Fig. 4C). In contrast, nuclei and pyrenoids were devoid of labeling. This distinct punctate pattern of labeling was not observed when the primary antibody was omitted. As shown in Fig. 4D, a small proportion of MSC1 immunolabeling overlapped with labeling for KDEL, the endoplasmic reticulum retention signal sequence that is known to label punctate spots in the cytoplasm and chloroplast of Chlamydomonas (16). No MSC1 labeling colocalized with the markers for other organelles such as the phosphate body, Golgi apparatus, mitochondria, and lysosome (data not shown).
Fig. 4.
Localization of MSC1 in Chlamydomonas cells. (A) Western blot analysis of total cell lysate for MSC1. (B and C) Representative images of indirect immunofluorescence labeling of MSC1 (green) and chloroplast autofluorescence (red) in longitudinal (B) and transverse (C) confocal microscopic sections of wild-type cells. (D) Immunofluorescent labeling of MSC1 (green) and KDEL (red). MSC1 partially colocalizes with KDEL (arrowheads, yellow). (E) Western blot analysis of total cell lysate from MSC1 RNAi transformants for MSC1. MSC1 gene expression was successfully suppressed in eight transformants. Strains A4, A7, B1, and B2 were chosen for further analysis. (F) Overlap of a chlorophyll autofluorescence image (red) and a phase contrast image from the B2 strain. (G and H) Chloroplast autofluorescence (G) and phase contrast (H) images obtained from the same B2 strain cell, in which MSC1 was knocked down. Chlorophyll fluorescence was restricted to a small region in MSC1 knockdown cell. (Scale bars: B–D and H, 2 μm; F, 10 μm.)
RNAi-mediated MSC1 knockdown suppressed the MSC1 expression to undetectable levels in 8 of 21 colonies examined (Fig. 4E). The growth rate of the MSC1 knockdown cells was similar to that of the wild-type cells in either hetero- or autotrophic culture medium. However, chlorophyll autofluorescence was greatly reduced as a result of MSC1 knockdown, and this autofluorescence was limited to small regions near the cell periphery (Fig. 4 F–H). This restricted localization of chlorophyll autofluorescence was consistently observed in all four of the RNAi strains examined (A4, A7, B1, and B2); however, it was not observed in transformants that did not exhibit reduced MSC1 expression.
Discussion
In the present study, we have cloned MSC1, an MscS homolog of Chlamydomonas, and found that ΔN-MSC1 opens on membrane stretch when expressed in E. coli cells. MSC1 was localized as intracellular punctate spots that are partially present within chloroplast.
The finding that 0.4-nS conductance is present in E. coli cells expressing ΔN-MSC1, but not in those harboring empty vector, strongly supports the hypothesis that the 0.4-nS conductance is attributable to ΔN-MSC1. Moreover, localization of ΔN-MSC1 at the cellular membrane provides additional support for this hypothesis. However, the possibility that the 0.4-nS conductance is due to proteins intrinsic to E. coli cannot be excluded. It has previously been shown that MscM, which was obtained via reconstitution of proteoliposomes from an inner membrane fraction of E. coli, exhibits a conductance of 0.1–0.15 nS in 0.1 M KCl solution (17). This conductance is comparable to 0.4 nS in a 0.2 M KCl solution, as was used here. However, the 0.4-nS conductance is probably not due to MscM because MscM is rarely present in the spheroplast preparations (18). In a previous study by our laboratory, an MscM-like conductance (0.3 nS in 0.2 M KCl) was detected in only 1 of 170 patches from 31 preparations, in which the same strain hosted MscS expression (11). It is also unlikely that OmpC and PhoE porins, outer-membrane channels that show mechanosensitive gating upon reconstitution in liposomes (19), contaminated the patch of the inner membrane. It is important to note that MscL, an inner membrane channel, and ΔN-MSC1 were recorded in the same patch (Fig. 2A).
In contrast to E. coli MscS, MSC1 has an extended N-terminal segment that possibly contains a signal sequence and a transmembrane helix. The observation that the channel activity appeared only when the signal sequence was removed indicates that MSC1 becomes functional after the signal sequence is removed by an endopeptidase acting at the predicted cleavage site. Indeed, Western blot and immunofluorescence data indicate that MSC1 fails to be integrated into membrane when the signal peptide is present. A number of membrane proteins are known to become functional after the removal of the N-terminal segment (see refs. 20 and 21 as examples). In contrast to the signal sequence, TM0 was found to be essential for the functional expression of MSC1.
Immunolabeling experiments revealed that MSC1 exhibits a punctate distribution within the intracellular space. Overlap of a portion of MSC1 label with endoplasmic reticulum retention signal labeling supports the idea that the nucleus-encoded gene MSC1 is translated at the endoplasmic reticulum. However, the major target of MSC1 expression seems to be chloroplasts because the majority of MSC1 labeling was present here. To the best of our knowledge, there is no small spherical membrane in the chloroplast of Chlamydomonas. Nevertheless, it is possible that MSC1 localizes to the distinct area of the thylakoidal membrane. The absence of any overlap between MSC1 labeling and chlorophyll autofluorescence indicates that MSC1 and the membrane proteins for photosynthesis are situated in separate membrane areas within the chloroplast. It is also worth noting that the prediction programs indicated that mitochondria may be a minor target of MSC1, although no mitochondrial MSC1 could be detected.
MSC1 knockdown experiments revealed that MSC1 is involved in the organization of the chloroplast. We suspect that MSC1 is activated by local expansion and infolding of the inner membrane of the chloroplast, which occurs during the formation of the thylakoid membrane (22). It is also possible that MSC1 responds to some environmental stress such as hyperosmotic shock. In Chlamydomonas cells, hyperosmotic shock results in an increase in the volume of the thylakoid lumen and in the level of phosphatidic acid (PA) (23, 24). Because PA has considerable negative spontaneous curvature, it is possible that the increased PA content of the intracellular membrane leads to the opening of MSC1 such as has been shown to occur with MscS, which opens upon addition of conical amphypaths to the membrane (25, 26).
At positive voltages, the voltage-dependent inactivation of MSC1 is similar to that observed in E. coli MscS (27). This finding was unexpected because MSC1 is devoid of arginine residues that were postulated to act as voltage sensors (10, 27). Therefore, the voltage-dependent inactivation of MSC1 is probably not due to charge movement in the transmembrane domain. On the other hand, the activation threshold did not change with the membrane potential. Recent studies on MscS have also demonstrated that the activation threshold does not change with voltage (15, 27). Thus, voltage-independent activation of ΔN-MSC1 resembles that of MscS, although MscS and ΔN-MSC1 may exhibit voltage-sensitive gating under certain experimental conditions.
MSC1 shows significant hysteresis such that, once it is activated, it does not close until the membrane tension is released almost completely. It is unlikely that this hysteresis is attributable to artifacts originating from the high pressure applied to the membrane because MscL in the same patch did not exhibit hysteresis. In theory, any channel should display hysteresis when the gating stimulus changes faster than the opening and closing kinetics. However, this explanation is probably not valid in the case of MSC1 because the thresholds for the channel opening and closing did not change with the rate of the pressure change. In addition, when the pressure was kept constant at the level of half activation, no additional opening of MSC1 was observed (Fig. 3F). An alternative and more attractive explanation for the hysteresis is that the tension dependence of MSC1 is different between the opening and closing kinetics. Hysteresis in the mechanical properties also has been observed in other molecules such as titin (connectin), which is responsible for muscle elasticity (28). Titin molecules unfold in response to strong stretch, whereas refolding occurs at significantly weak stretch. If membrane stretch is the only factor affecting the closing of MSC1, then fine regulation of MSC1 opening may be difficult because, once activated, MSC1 leaks chloride ions until almost entire release of stretch. Consequently, chloride ion would be expected to be closely involved in the swelling of the organelles and intracellular vesicles of Chlamydomonas, but this hypothesis awaits testing. An alternative possibility is that MSC1 closes in response to signal molecules situated downstream of phospholipase D, which is activated by hyperosmotic stress (24). It is also possible that MSC1 closes through the voltage-dependent inactivation.
MSC1 is distinct from MSL2 and MSL3 in Arabidopsis in various aspects. MSC1 is present as a numerous punctae within the chloroplast and cytoplasm, whereas MSL2 and MSL3 are localized as one or two foci on the plastid envelope (6). MSL2 and MSL3 double knockout results in the enlargement of chloroplasts, whereas knockdown of MSC1 disarranges chlorophyll autofluorescence. In addition, phylogenic analysis did not support the idea that MSC1 and MSL2/3 are derived from the same origin. Despite these differences, it is still of interest to determine whether MSL2 and MSL3 serve as mechanosensitive channels.
Opening of MSC1 probably equilibrates the chloride ion concentration with the transmembrane potential until total relaxation of membrane occurs. The hysteresis and strong anion preference of MSC1 are not shared by bacterial MscS. The present study indicates that, at some point of evolution, Chlamydomonas and probably other cell-walled organisms began to make use of an intracellular mechanosensitive channel with a developed function of maintaining proper plastid organization.
Materials and Methods
Strain and Culture Conditions.
C. reinhardtii 137c was used throughout this study. Cells were cultured at 25°C in Tris acetic acid phosphate (TAP) medium in a 12-hour light, 12-hour dark cycle (29). E. coli PB112 (ΔmsclΔmscs) and PB113 (ΔmscsΔmsck) were used for the patch-clamp experiments (4). DH5α and XL1-Blue E. coli strains (Stratagene, La Jolla, CA) were used for cloning.
Gene Cloning.
Searches for genes homologous to E. coli MscS were performed by using the Chlamydomonas genome database version 3.0 of the Department of Energy Joint Genome Institute (http://genome.jgi-psf.org/Chlre3/Chlre3.home.html). Protein ID 10143 was found to be most similar to E. coli MscS and was designated MSC1. MSC1 cDNA was amplified from total RNA extracted from Chlamydomonas cells by RT-PCR using SuperScript 3 reverse transcriptase (Invitrogen, Carlsbad, CA) and KOD plus DNA polymerase (Toyobo, Osaka, Japan). The sequence toward the 5′ end was obtained from the cDNA library constructed by the Kazusa DNA Research Institute. MSC1 was cloned into expression vector pB10b along with the 5′ untranslated region of E. coli MscS.
TargetP (www.cbs.dtu.dk/services/TargetP), Genetyx (Genetyx, Tokyo, Japan), and SOSUI (http://bp.nuap.nagoya-u.ac.jp/sosui) were used to predict the subcellular localization and secondary structure of MscS, respectively.
Electrophysiology.
Giant spheroplasts were prepared from E. coli cells as described elsewhere (30). In short, cells in log-growth phase were treated with cephalexin to block septation. Cell walls of the elongated cells were digested with lysozyme. The resulting spheroplasts were laid onto the sucrose cushion and collected by centrifugation. Channel currents were recorded from inside-out membrane patches from the giant spheroplast with conventional methods. The pipette solution contained 200 mM KCl, 40 mM MgCl2, 10 mM CaCl2, 0.1 mM EDTA, and 5 mM Hepes·KOH (pH 7.2). The bath solution consisted of the pipette solution supplemented with 300 mM sucrose. The pressure for suction was controlled by HSPC-1 (ALA Scientific Instruments, Westbury, NY).
Protein Analysis.
Residues 401–514 of MSC1 (Fig. 1A) were tagged with six histidine residues. Tagged MSC1 was expressed in XL1-Blue (Stratagene) and purified by using a Ni-NTA Superflow column (Qiagen, Hilden, Germany). Rabbits were then immunized with the purified product. The resulting antibodies were affinity-purified with the purified antigen.
Membrane proteins from E. coli were obtained as described previously (30). The cells were grown in LB medium containing 1 mM IPTG for 2 h and disrupted by sonication. The debris was removed by centrifugation, and the supernatant was dissolved in 2% octyl glucoside. Insoluble particles were removed by ultracentrifugation. Whole-cell proteins from Chlamydomonas were prepared from cells in the logarithmic growth phase. The cells were suspended in SDS sample buffer (Wako, Osaka, Japan), boiled for 5 min, and vortexed for 5 min in the presence of glass beads.
Proteins were separated on a SDS/10% PAGE gel and electrotransferred to Immobilon-P membrane (Millipore, Bedford, MA). The membrane was blocked with PBS containing 2% nonfat dry milk and 0.1% Tween 20 for 1 h. Membranes were incubated in the anti-MSC1 suspended in blocking solution for 1 h at room temperature. Membranes were then incubated in secondary antibody (HRP-conjugated anti-rabbit IgG; GE Healthcare, Little Chalfont, U.K.) in blocking solution (1:75,000) for 1 h. The secondary antibody was detected with Immobilon Western Chemiluminescent HRP Substrate kit (Millipore) according to the manufacturer's protocol.
Immunofluorescent Labeling.
Cells were fixed with 1.8% formaldehyde (EM grade; TAAB, Aldermaston, England) for 20 min and washed with PBS containing 0.1 M glycine for 30 min. The cells were allowed to adhere to the slide glass, permeabilized with 0.2% Triton X-100 for 8 min, and blocked with PBS containing 2% nonfat dried milk for 1 h. Glass slides were subsequently incubated with the primary and secondary antibodies diluted in PBS containing 2% nonfat dried milk. MSC1 was detected by the affinity-purified antibody generated by our laboratory. Endoplasmic reticulum was detected with anti-KDEL mouse monoclonal antibody (31) (Calbiochem/EMD Biosciences, Darmstadt, Germany). Golgi and polyphosphate bodies were detected with N2 mouse monoclonal antibody provided by R. D. Allen (University of Hawaii, Honolulu, HI) (32). Mitotracker Red (Invitrogen) and Lysotracker (Invitrogen) were used to visualize mitochondria and lysosomes, respectively. Nuclei and polyphosphate bodies were detected with 0.2 mg/ml DAPI. Cy3-conjugated anti-rabbit IgG (Jackson ImmunoResearch, West Grove, PA) and FITC-conjugated anti-mouse IgG (Jackson ImmunoResearch) were used as secondary antibodies and were used at a 1:400 dilution. Immunolabeled cells were observed under a LSM 510 laser scanning confocal microscope (Zeiss, Oberkochen, Germany). All procedures were carried out at room temperature and, where appropriate, protected from light.
RNAi-Mediated Knockdown of MSC1 Expression.
Plasmids for RNAi experiments were designed according to the method of Sineshchekov et al. (33). Genomic DNA spanning exon 1 to exon 3 of MSC1 was joined with corresponding cDNA in the reverse direction. The sequences of the primers used to amplify these DNA fragments are listed in SI Table 2. The DNA fragments were mixed and amplified by PCR. The PCR product was digested with NdeI and EcoRI and introduced into the pGenD-ble expression vector (34). C. reinhardtii cells were transformed by electroporation and selected on TAP-agar plates containing 10 μg/ml Zeocin (Invitrogen). Only the colonies that appeared on the selective culture plate very early were analyzed so as to reduce the number of false positives. Transformants were evaluated for MSC1 expression by Western blot analysis. Cells were fixed with formaldehyde and resuspended in PBS for observation.
Supplementary Material
Acknowledgments
We acknowledge Dr. T. Hashimoto for help with phylogenic analysis, Dr. K. Takahashi for critically reading the manuscript, and Dr. H. Yanagisawa (University of Tokyo, Tokyo, Japan) for pGenD-ble. This work was supported by Ministry of Education, Culture, Sports, Science, and Technology (Japan) Grants-in-Aid 16GS0308 (to M.S.) and 16370034 (to K.Y.), and a grant from the Japan Science and Technology Agency.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
Data deposition: The sequence reported in this paper has been deposited in the DNA Data Bank of Japan (accession no. AB288852).
This article contains supporting information online at www.pnas.org/cgi/content/full/0609996104/DC1.
References
- 1.Guharay F, Sachs F. J Physiol. 1985;363:119–134. doi: 10.1113/jphysiol.1985.sp015699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levina N, Tötemeyer S, Stokes NR, Louis P, Jones MA, Booth IR. EMBO J. 1999;18:1730–1737. doi: 10.1093/emboj/18.7.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martinac B, Buechner M, Delcour AH, Adler J, Kung C. Proc Natl Acad Sci USA. 1987;84:2297–2301. doi: 10.1073/pnas.84.8.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Y, Moe PC, Chandrasekaran S, Booth IR, Blount P. EMBO J. 2002;21:5323–5330. doi: 10.1093/emboj/cdf537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pivetti CD, Yen MR, Miller S, Busch W, Tseng YH, Booth IR, Saier MH., Jr Microbiol Mol Biol Rev. 2003;67:66–85. doi: 10.1128/MMBR.67.1.66-85.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haswell ES, Meyerowitz EM. Curr Biol. 2006;16:1–11. doi: 10.1016/j.cub.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 7.Palmer CP, Zhou XL, Lin J, Loukin SH, Kung C, Saimi Y. Proc Natl Acad Sci USA. 2001;98:7801–7805. doi: 10.1073/pnas.141036198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou XL, Batiza AF, Loukin SH, Palmer CP, Kung C, Saimi Y. Proc Natl Acad Sci USA. 2003;100:7105–7110. doi: 10.1073/pnas.1230540100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edwards MD, Li Y, Kim S, Miller S, Bartlett W, Black S, Dennison S, Iscla I, Blount P, Bowie JU, Booth IR. Nat Struct Mol Biol. 2005;12:113–119. doi: 10.1038/nsmb895. [DOI] [PubMed] [Google Scholar]
- 10.Bass RB, Strop P, Barclay M, Rees DC. Science. 2002;298:1582–1587. doi: 10.1126/science.1077945. [DOI] [PubMed] [Google Scholar]
- 11.Nomura T, Sokabe M, Yoshimura K. Biophys J. 2006;91:2874–2881. doi: 10.1529/biophysj.106.084541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kloda A, Ghazi A, Martinac B. Biophys J. 2006;90:1992–1998. doi: 10.1529/biophysj.105.075481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamill OP, Martinac B. Physiol Rev. 2006;81:685–740. doi: 10.1152/physrev.2001.81.2.685. [DOI] [PubMed] [Google Scholar]
- 14.Sukharev S. Biophys J. 2002;83:290–298. doi: 10.1016/S0006-3495(02)75169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sotomayor M, Vasquez V, Perozo E, Schulten K. Biophys J. 2007;92:886–902. doi: 10.1529/biophysj.106.095232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levitan A, Trebitsh T, Kiss V, Pereg Y, Dangoor I, Danon A. Proc Natl Acad Sci USA. 2005;102:6225–6230. doi: 10.1073/pnas.0500676102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berrier C, Besnard M, Ajouz B, Coulombe A, Ghazi A. J Membr Biol. 1996;151:175–187. doi: 10.1007/s002329900068. [DOI] [PubMed] [Google Scholar]
- 18.Sukharev SI, Blount P, Martinac B, Kung C. Annu Rev Physiol. 1997;59:633–657. doi: 10.1146/annurev.physiol.59.1.633. [DOI] [PubMed] [Google Scholar]
- 19.Le Dain AC, Häse C, Tommassen J, Martinac B. EMBO J. 1996;15:3524–3528. [PMC free article] [PubMed] [Google Scholar]
- 20.Cao J, Drews M, Lee HM, Conner C, Bahou WF, Zucker S. J Biol Chem. 1998;273:34745–34752. doi: 10.1074/jbc.273.52.34745. [DOI] [PubMed] [Google Scholar]
- 21.Ossovskaya VS, Bunnett NW. Physiol Rev. 2003;84:579–621. doi: 10.1152/physrev.00028.2003. [DOI] [PubMed] [Google Scholar]
- 22.Murakami S, Packer L. Biochim Biophys Acta. 1969;180:420–423. doi: 10.1016/0005-2728(69)90128-5. [DOI] [PubMed] [Google Scholar]
- 23.Cruz JA, Salbilla BA, Kanazawa A, Kramer DM. Plant Physiol. 2001;127:1167–1179. [PMC free article] [PubMed] [Google Scholar]
- 24.Munnik T, Meijer HJG, Riet Bt, Hirt H, Frank W, Bartles D, Musgrave A. Plant J. 2000;22:147–154. doi: 10.1046/j.1365-313x.2000.00725.x. [DOI] [PubMed] [Google Scholar]
- 25.Martinac B, Adler J, Kung C. Nature. 1990;348:261–263. doi: 10.1038/348261a0. [DOI] [PubMed] [Google Scholar]
- 26.Perozo E, Kloda A, Cortes DM, Martinac B. Nat Struct Biol. 2002;9:696–703. doi: 10.1038/nsb827. [DOI] [PubMed] [Google Scholar]
- 27.Akitake B, Anishkin B, Sukharev S. J Gen Physiol. 2005;125:143–154. doi: 10.1085/jgp.200409198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kellermayer MS, Smith SB, Granzier HL, Bustamante C. Science. 1997;276:1112–1116. doi: 10.1126/science.276.5315.1112. [DOI] [PubMed] [Google Scholar]
- 29.Gorman DS, Levine RP. Proc Natl Acad Sci USA. 1965;54:1665–1669. doi: 10.1073/pnas.54.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blount P, Sukharev SI, Moe PC, Martinac B, Kung C. Methods Enzymol. 1999;294:458–482. doi: 10.1016/s0076-6879(99)94027-2. [DOI] [PubMed] [Google Scholar]
- 31.Levitan A, Trebitsh T, Kiss V, Pereg Y, Dangoor I, Danon A. Proc Natl Acad Sci USA. 2005;102:6225–6230. doi: 10.1073/pnas.0500676102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fok AK, Clarke M, Ma L, Allen RD. J Cell Sci. 1993;106:1103–1113. doi: 10.1242/jcs.106.4.1103. [DOI] [PubMed] [Google Scholar]
- 33.Sineshchekov OA, Jung HK, Spudich JL. Proc Natl Acad Sci USA. 2002;99:8689–8694. doi: 10.1073/pnas.122243399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fischer N, Rochaix JD. Mol Genet Genomics. 2001;265:888–894. doi: 10.1007/s004380100485. [DOI] [PubMed] [Google Scholar]
- 35.Kloda A, Martinac B. Biophys J. 2001;80:229–240. doi: 10.1016/S0006-3495(01)76009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kloda A, Martinac B. EMBO J. 2001;8:1888–1896. doi: 10.1093/emboj/20.8.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.