Abstract
Objective
C-type natriuretic peptide (CNP) has recently been suggested to represent an endothelium-derived hyperpolarizing factor (EDHF) in the mammalian resistance vasculature, important in the regulation of local blood flow and systemic blood pressure. Additionally, this peptide has been shown to protect against ischaemia-reperfusion injury and inhibits leukocyte and platelet activation. Herein, we use a novel, selective natriuretic peptide receptor-C (NPR-C) antagonist (M372049) to highlight the pivotal contribution of CNP/NPR-C signalling in the EDHF-dependent regulation of vascular tone and investigate the mechanism(s) underlying the release and biological activity of CNP and EDHF.
Methods
In vitro pharmacological investigation was conducted in rat (Sprague-Dawley) aorta and mesenteric resistance arteries. Relaxant responses to CNP, atrial natriuretic peptide (ANP), the nitric oxide donor spermine-NONOate (SPER-NO) and the endothelium-dependent vasodilator, acetylcholine (ACh) were examined in the absence and presence of M372049 or inhibitor cocktails shown previously to block endothelium-dependent dilatation in the resistance vasculature. RT-PCR was employed to characterize the expression of NPR subtypes in the vessels studied.
Results
M372049 produced concentration-dependent inhibition of the vasorelaxant activity of CNP in rat isolated mesenteric resistance arteries but not aorta; in contrast, M372049 did not affect relaxations to ANP or SPER-NO in either vessel. M372049 or ouabain alone produced small, significant inhibition of EDHF-dependent relaxations in mesenteric arteries and in combination acted synergistically to abolish such responses. A combination of M372049 with established inhibitors of EDHF-dependent relaxation revealed that multiple, distinct pathways coordinate the bioactivity of EDHF in the resistance vasculature, and that CNP/NPR-C signalling represents a major component.
Conclusions
These data substantiate CNP/NPR-C signalling as a fundamental pathway underlying EDHF-dependent regulation of vascular tone in the rat mesenteric resistance vasculature. An increased understanding of the physiological roles of CNP/NPR-C signalling in the vasculature (now facilitated by the identification of a selective NPR-C antagonist) should aid determination of the (patho)physiological importance of EDHF and might provide the rationale for the design of novel therapeutics.
Keywords: Blood flow, natriuretic peptide, endothelium-derived hyperpolarizing factor, microcirculation, vasodilation
INTRODUCTION
C-type natriuretic peptide (CNP) affords a key paracrine element to the biological activity of the natriuretic peptide family of vasoactive mediators that are important in the maintenance of blood volume and blood pressure [1,2,3]. In contrast to the cardiac-derived, endocrine activity of atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP), CNP is expressed abundantly in vascular endothelial cells and thought to act in a local fashion to regulate vascular tone and blood flow [4,5,6,7]. The importance of endothelium-derived CNP to cardiovascular homeostasis has been highlighted recently by work identifying this peptide as an endothelium-derived hyperpolarizing factor (EDHF) in the coronary and mesenteric resistance vasculature [8,9]. These investigations have also elucidated, in part, the signal transduction pathway(s) underlying the vasorelaxant activity of CNP and identified a novel system involving activation of a G-protein-coupled inwardly rectifying K+ channel (GIRK) [8]. Moreover, it has been shown that endothelium-dependent vasorelaxants, such as ACh, release CNP acutely from the vascular endothelium [8,9]. Subsequently, it has been demonstrated that CNP can also procure protection against myocardial ischaemia-reperfusion (I/R) injury [9] and inhibits leukocyte recruitment and platelet aggregation [10]. In sum, these observations suggest that the remit of CNP (and perhaps EDHF) includes regulation of circulating elements (i.e. platelets, leukocytes) in addition to effects on vascular smooth muscle tone; as such, it is likely that CNP maintains an important anti-atherogenic influence of the blood vessel wall akin to endothelium-derived NO. Consequently, interventions that can mimic the effects of CNP may offer therapeutic potential in combating cardiovascular disorders.
The biological activity of CNP is thought to be mediated exclusively via activation of natriuretic peptide receptor (NPR)-B, for which CNP represents the sole endogenous ligand [11]. However, CNP (akin to other natriuretic peptides) also binds to NPR-C, which is thought to clear natriuretic peptides from the circulation by binding, internalization and subjecting them to lysosomal degradation [12]. In addition to this ‘clearance’ function, NPR-C also possesses an intracellular G-protein binding domain and can couple to Gi/o G-proteins to inhibit adenylate cyclase activity and activate phospholipase-Cβ3 [13]. This positive signalling role is exemplified by the CNP-dependent inhibition of neuronal catecholamine release [14]. Previous studies have provided indirect evidence that NPR-C may be important in the vasodilator activity of EDHF in the mesenteric and coronary resistance vasculature [8,9] (in contrast, in conduit arteries CNP elicits a NPR-B, cGMP-dependent reduction in vascular tone [15]). Unfortunately, differentiation between NPR-B, cGMP-dependent and NPR-C, cGMP-independent effects of CNP (and other natriuretic peptides) is difficult due to the lack of selective receptor antagonists. HS-142-1, apolysaccharide derived from Aureobasidium sp., has been used in this regard since it appears to block NPR-A and NPR-B activity [16,17]. However, to provide definitive evidence for a key, positive signalling role for NPR-C in mediating the vasorelaxant (and anti-atherogenic) activity of CNP and EDHF, a selective NPR-C antagonist would be required.
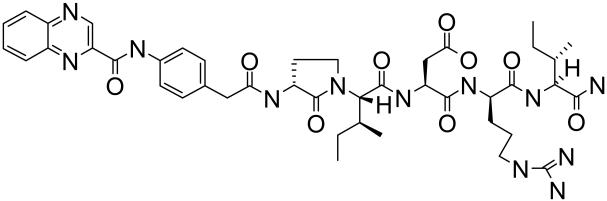
To address this deficit, we used a novel NPR-C selective antagonist, M372049 (Figure 1). This compound is one member of a family of homologous molecules developed to prevent binding of all natriuretic peptides to NPR-C [18]. Herein, using M372049 we demonstrate that NPR-C is responsible for CNP-dependent and EDHF-dependent dilation in the rat mesenteric resistance vasculature. Hence, we have established NPR-C as an important effector pathway in the regulation of vascular tone, identified CNP as a major component of the EDHF response in this vascular bed and highlighted a selective pharmacological tool for the elucidation of the (patho)physiological significance of CNP.
Figure 1.
Chemical structure of M372049.
METHODS
All experiments were conducted according to the Animals (Scientific Procedures) Act 1986, United Kingdom and conform to the Guide for the Care and Use of Laboratory Animals (NIH publication No. 85-23, 1996).
Rat isolated mesenteric artery
Rat isolated mesenteric resistance arteries were used as a model of EDHF bioactivity. Male rats (Sprague-Dawley; 200-250g) were stunned and killed by cervical dislocation. The mesentery was removed and third-order arteries mounted in an automated tension myograph (Danish Myotechnology, Denmark), as previously described [8]. After an equilibration period of 45 min, vessels were normalized according to published protocols and vessel diameter determined [19].
Following normalization, each vessel was contracted repeatedly with the thromboxane A2-mimetic 9,11-dideoxy-11α,9α-epoxymethano-prostaglandin F2α (U46619; 1μM) until the response was reproducible. The vessels were then washed to restore basal tone before contracting to approximately 50% of the maximum U46619-induced response. Once a stable response to U46619 was achieved, cumulative concentration-response curves were constructed to SPER-NO (0.001-10μM), ACh (0.001-10μM), ANP (0.001-1μM) or CNP (0.001-1μM) in the absence or presence of stated interventions. Only one curve to any one agonist was constructed in any single tissue and all experiments were conducted in the presenceof L-NAME (300μM) and indomethacin (5μM). In certain experiments tissues were exposed to various inhibitors (that have been shown previously to block EDHF-dependent relaxation in the resistance vasculature [20]), either alone or in combination, including the small conductance calcium-activated potassium channel (SKCa) inhibitor apamin (100nM [21,22]), the intermediate conductance calcium-activated potassium channel (IKCa) inhibitors TRAM-34 (10μM [23,24]) or charybdotoxin (100nM [22,24]), the inwardly-rectifying potassium channel (KIR) blocker Ba2+ (30μM [22,25]), the Na+/K+-ATPase inhibitor ouabain (1mM [21,22]) and M372049 (100nM).
Membrane potential measurements
In situ membrane potential measurements were recorded in rat isolated small mesenteric arteries to link blockade of functional EDHF responses with inhibition of smooth muscle cell hyperpolarization. Small mesenteric arteries were mounted in a tension myograph, normalized and equilibrated using U46619 as described above. Vessels were incubated with L-NAME (300μM) and indomethacin (5μM) and membrane potential was measured continuously using aluminum silicate microelectrodes (1mm in diameter, World Precision Instruments, USA) that had resistances between 50 and 90MΩ when filled with 2M KCl. Membrane potential (mV) was measured using an oscilloscope (Gould, UK) connected to an amplifier (Intra 767 electrometer, World Precision Instruments, USA) and recorded on a chart recorder (BBC Goertz Metrawatt). Electrode entry into a vascular smooth muscle cell was determined by an abrupt drop in voltage, followed by a sharp return to baseline on exit, with a minimal change (no more than 10%) in resistance [26].
Electrophysiological studies
HEK293 cells stably-expressing a G-protein-gated inwardly rectifying potassium channel (KIR3.1 + KIR 3.2A) and the M4-muscarinic receptor [27] were employed to ascertain if M372049 was a direct KIR channel blocker. Whole-cell membrane currents were recorded at room temperature with an Axopatch 200B amplifier, and digitised with a Digidata 1322A interface (both Axon Instruments) and analysed with pClamp software (version 8.0; Axon Instruments). Cells were perfused using a gravity-fed bath perfusion system. Pipette solution: 107 mM glutamate, 20 mM KCl, 10 mM NaCl, 1 mM MgCl2, 2 mM EGTA, 10 mM Hepes, 2 mM MgATP, 0.01 mM Na2GTP; KOH to pH 7.3. Bath solution:120 mM NaCl, 20 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM Hepes, NaOH to pH 7.4. Cell capacitance was ~15-20 pF.
Under voltage-clamp condition, cells were held at 0 mV, stepped to −80 mV for 20 ms and pulsed for 400 ms from −120 mV to + 60 mV in 12 mV increments. KIR currents induced by carbachol (10μM; measured at 120 mV) were compared in the absence and presence of M372049 (10μM) and Ba2+ (30μM).
Rat isolated thoracic aorta
Rat isolated thoracic aortae were employed as a model of endothelium-derived relaxing factor (EDRF) bioactivity and to provide a comparison between the conduit and resistance vasculature in terms of the importance of CNP/NPR-C signalling. Male rats (Sprague-Dawley; 200-250g) were stunned and killed by cervical dislocation. The thoracic aortae were carefully removed and mounted in organ baths for isometric tension recordings, as described previously [15]. Tissues were then pre-contracted with phenylephrine (PE; EC80). Once a stable response to PE was achieved, cumulative concentration-response curves to SPER-NO (0.001-10μM), ACh (0.001-1μM), ANP (0.001-1μM) or CNP (0.001-1μM) were constructed in the absence or presence of M372049 (1-100nM).
NPR mRNA expression
Reverse transcription-polymerase chain reaction (RT-PCR) was used to assess the expression of mRNA for each NPR in aortic and mesenteric vascular smooth muscle following a protocol identical to that we have described previously [28,29]. Thermal cycling conditions for PCR were as follows: 35 cycles of denaturation at 94°C for 1 min, annealing at 60°C for 1 min, and polymerization at 72°C for 1 min, followed by a final extension at 72°C for 10 min. RT-PCR products were resolved by agarose gel electrophoresis (2% gel) and stained with ethidium bromide.
Primer sequences were as follows:
| PRIMER | SEQUENCE (5′→3′) | PRODUCT LENGTH |
| NPR-A sense | CATCCTGGACAACCTGC | 714bp |
| NPR-A antisense | TAGGTCCGAACCTTGCC | |
| NPR-B sense | CATGGCAGGACAATCGAACC | 720bp |
| NPR-B antisense | TGCCTGCACCTTTGTGATATCG | |
| NPR-C sense | GGCTCAATGAGGAGGATTACGTG | 554bp |
| NPR-C antisense | AATCTTCCCGCAGCTCTCGATG |
Materials
All drugs used were obtained from Sigma except ANP (Calbiochem), Apamin (Calbiochem), CNP (Calbiochem), M372049 (kind gift of Dr. C. Veale, AstraZeneca, USA), TRAM-34 (kind gift of Dr. H. Wulff, California, USA) and U-46619 (Biomol). U-46619 was dissolved in ethanol and then diluted in saline. Indomethacin was dissolved in 5% NaHCO3 and diluted in saline. TRAM-34 was dissolved in DMSO; all other drugs were prepared using saline (0.9% NaCl).
Calculations and statistics
Relaxations are expressed as percentage reversal of induced tone. All data are shown as mean ± SEM. Tests of significance between curves were conducted using Two-way ANOVA for multiple comparisons or a Students t-test for differences between two data groups, where P<0.05 was considered significant. The n values quoted similarly indicate the number of experiments and animals used
RESULTS
Characterisation of CNP-induced relaxation in rat mesenteric resistance arteries
CNP caused concentration-dependent relaxations of rat mesenteric resistance arteries that were unaffected by endothelium removal or blockade of Na+/K+-ATPase using ouabain but were suppressed following blockade of KIR using Ba2+ (Figure 2). The selective NPR-C antagonist, M372049 (0.1-100nM), potently inhibited CNP-induced relaxations in a concentration-dependent manner (Figure 2). M372049 (100nM) completely abolished responses to CNP and this concentration was therefore used in all further experimentation. The effect of M372049 (100nM) appeared to be selective for NPR-C since responses to SPER-NO were unaffected (Figure 2). Whilst ANP had little relaxant activity in control resistance arteries, exposure of these vessels to M372049 resulted in relaxation of arteries at the lower (1 and 10nM) concentrations of ANP (Figure 2).
Figure 2.
Relaxation of rat isolated mesenteric artery by CNP (0.001-1μM; upper panels), ANP (0.001-1μM; lower left panel) and SPER-NO (0.001-10μM; lower right panel) in the absence and presence of M372049 (0.1-100nM), Ba2+ (30μM), ouabain (1mM) or following endothelial denudation. Data is represented as mean±s.e.mean; n≥6; *P<0.05, significantly different to control.
NPR-C antagonism blocks EDHF responses in rat mesenteric resistance arteries
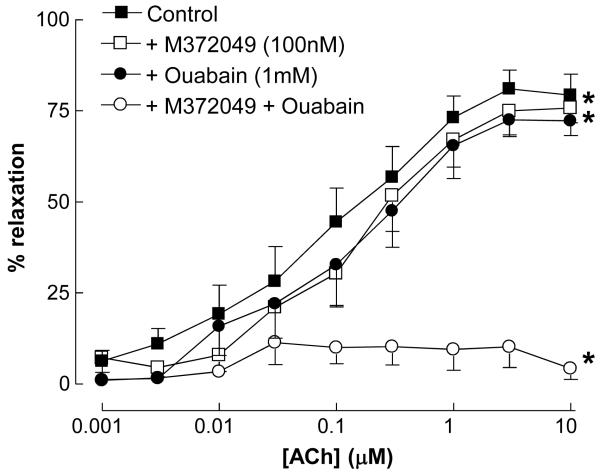
In the presence of L-NAME and indomethacin, ACh induced concentration-dependent relaxations that have previously been attributed to the activity of EDHF [8,30,31]. These responses were reduced marginally, but significantly, by treatment with M372049 (100nM) or ouabain (1mM) alone. However, a combination of M372049 and ouabain abolished responses to ACh (Figure 3).
Figure 3.
Relaxation of rat isolated mesenteric artery to ACh (0.001-10μM) in the absence and presence of M372049 (100nM), ouabain (1mM) or a combination of M372049 plus ouabain. Data is represented as mean±s.e.mean; n≥6; *P<0.05, significantly different to control.
NPR-C antagonism blocks EDHF-mediated smooth muscle hyperpolarisation in rat mesenteric resistance arteries
ACh-mediated vascular smooth muscle hyperpolarisation was examined in rat small mesenteric arteries that were depolarized by 27 ± 1.3 mV (n=6) from a resting membrane potential of −58.4 ± 3.9 mV (n=6) with an approximate EC50 concentration of U46619. In the presence of L-NAME (300 μM) and indomethacin (5μM), a maximum hyperpolarisation of 20.5 ± 0.8 mV (n=3) was obtained with 10μM ACh. ACh-mediated hyperpolarisation and vasorelaxation were virtually abolished in the presence of M372049 (100nM) in combination with ouabain (1mM; Figure 4). Interestingly, although the hyperpolarisation to ACh was significantly inhibited by M372049 (100nM), the relaxant response to ACh was only slightly reduced (Figure 4). Hyperpolarisation and relaxation to the KATP channel opener, levcromakalim (10μM) was sustained in the presence of M372049 (100nM) and ouabain (1mM; data not shown); M372049 (100nM) alone did not affect resting membrane potential (data not shown).
Figure 4.
Relaxation (upper panel) and hyperpolarisation (lower panel) of rat isolated mesenteric artery by ACh (0.01-10μM) in the absence and presence of M372049 (100nM) or M372049 plus ouabain (1mM). Data is represented as mean±s.e.mean; n≥6; *P<0.05, significantly different to control.
NPR-C antagonism does not alter agonist-induced vasorelaxation in rat aorta
M372049 (100nM) had no inhibitory activity on vasorelaxant responses to CNP, ANP, ACh or SPER-NO in isolated rat aortic rings (Figure 5). Moreover, combination of M372049 with ouabain, unlike in resistance arteries, had no effect on ACh-induced relaxations (data not shown).
Figure 5.
Relaxation of rat isolated aorta by CNP (0.001-1μM; upper left panel), ANP (0.001-1μM; upper right panel), ACh (0.001-1μM; lower left panel) and SPER-NO (0.001-10μM; lower right panel) in the absence and presence of M372049 (100nM). Data is represented as mean±s.e.mean; n≥6; *P<0.05, significantly different to control.
Combination of IKCa or SKCa inhibitors with M372049 inhibits EDHF responses in rat mesenteric resistance arteries
Since we had identified M372049 as a selective antagonist of NPR-C/EDHF responses in rat mesenteric resistance arteries, we proceeded to use this pharmacological tool to dissect the mechanisms involved in EDHF release and smooth muscle activity in this vascular bed. Since only a combination of M372049 plus ouabain abolished EDHF responses, this intimated that the EDHF response in these vessels is comprised of CNP and at least one additional mediator (since M372049 abolished, but ouabain did not affect, CNP-induced relaxations). Thus, we explored whether blockade of NPR-C/KIR in combination with inhibition of the individual pathways involved in EDHF release (i.e. SKCa and IKCa) and bioactivity (i.e. KIR and Na+/K+-ATPase) would also result in compete blockade of EDHF and thereby provide insight into the mechanisms governing release of (individual) EDHF(s) in this vascular bed.
Apamin and TRAM-34 had no significant effect on ACh-induced relaxations alone but in combination they abolished EDHF responses (Figure 6). In the presence of M372049 and apamin, no additional blockade of EDHF response was observed beyond that achieved with either drug alone (Figure 6); the same was true of a combination of ouabain plus TRAM-34 (data not shown). However, a combined treatment of tissues with M372049 or Ba2+ plus TRAM-34, or ouabain plus apamin, significantly inhibited EDHF responses (Figure 6).
Figure 6.
Relaxation of rat isolated mesenteric artery by ACh (0.001-10μM) in the absence and presence of TRAM-34 (10μM), apamin (100μM) or both (upper left panel); apamin, M372049 (100nM) or both (upper right panel); apamin, ouabain (1mM) or both (lower left panel); or TRAM-34, M372049/Ba2+ (30μM) or both (lower right panel). Data is represented as mean±s.e.mean; n≥6; *P<0.05, significantly different to control.
RT-PCR analysis of NPR expression in rat mesenteric resistance vessels
All three NPR subtypes (NPR-A, NPR-B and NPR-C) are present (at least at mRNA level) in the rat conduit and resistance vasculature (Figure 7). It should be noted that both endothelial and smooth muscle cells are present in these preparations so a defined localisation/distribution of each NPR between these two cells types cannot be determined. However, we have found that all NPRs are expressed on primary rat and human aortic vascular smooth muscle cells and human umbilical vein endothelial cells in culture (unpublished observations), suggesting both endothelial and smooth muscle cells express a replete quota of NPR subtypes.
Figure 7.
RT-PCR analysis of NPR subtypes present in isolated rat aorta and mesenteric small vessels. Each receptor subtype (i.e. NPR-A, NPR-B & NPR-C) was found in both vessels. Expected product sizes: NPR-A, 698bp; NPR-B 699bp; NPR-C, 533bp. This is representative of 3 separate experiments.
M372049 does not directly inhibit KIR channel activity
One explanation for the inhibitory effect of M372049 against CNP- and EDHF- mediated responses is that this compound may act as a direct inhibitor of KIR channel function. To rule out this possibility we conducted electrophysiological (patch-clamp) studies in a HEK293 cell line expressing a GIRK channel coupled to an M4 receptor [27]. In these cells, addition of carbachol (10μM) caused a archetypal GIRK-dependent current (Control: 188.19±30.58 pA; Carbachol: 2863.04±384.85 pA; P<0.05 versus control) that was abolished in the presence of Ba2+ (30μM; 365.02±27.72 pA; P<0.05 versus carbachol alone) but unaffected in the presence of M372049 (10μM; 3467.96±471.67 pA; P>0.05 versus carbachol alone; all n=8).
DISCUSSION
Endothelium-derived mediators including NO and prostacyclin play a key role in regulating vascular tone (and the reactivity of circulating immune cells and platelets [32,10]) in resistance vessels, and are therefore thought to contribute significantly to the control of local blood flow, peripheral vascular resistance and systemic blood pressure [33,20]. We have recently published evidence that CNP acts as an EDHF in mesenteric and coronary resistance vessels and also regulates the activation of leukocytes and platelets [8,9,10]. Moreover, we provided evidence that NPR-C, as opposed to NPR-B, might be the receptor subtype activated by CNP in this context, thereby intimating a key signalling role to this ‘clearance receptor’ in the mammalian resistance vasculature. Investigation of the biological activity of CNP (and other natriuretic peptides) and NPR-C has been hampered by a lack of selective antagonists, limiting delineation of the physiological importance of CNP/NPR-C signalling. Herein, we demonstrate that blockade of NPR-C using the selective antagonist, M372049, inhibits CNP- and EDHF- dependent responses in rat isolated mesenteric arteries, intimating that NPR-C-dependent signalling acts as an important regulatory pathway governing vessel tone and blood flow; moreover, we show that NPR-C antagonism inhibits the smooth muscle hyperpolarization associated with EDHF-dependent dilation in these arteries and confirm the expression of all three NPR subtypes in resistance vessels. Further, using M372049 and specific inhibitor combinations that block the EDHF-dependent dilation in resistance arteries [20], we further elucidate the mechanism involved in the release and bioactivity of CNP.
The selective NPR-C antagonist, M372049, was first described by Veale et al. (2000) [18] as a result of a comprehensive screening exercise identifying antagonists at NPR-C that might be useful in the treatment of heart failure and other disorders where blockade of the removal of circulating natriuretic peptides (via this ‘clearance’ receptor) would be potentially beneficial. Since this compound has not been characterized from a pharmacological perspective in vascular tissue, we initially conducted a number of studies to ascertain the selectivity and potency of M372049 in both conduit and resistance arteries in vitro. In rat isolated aorta, M372049 did not alter relaxant responses to CNP, ANP, SPER-NO and ACh. The significance of this finding is three-fold. First, it indicates that the natriuretic peptides are not mediating vascular smooth muscle relaxation via NPR-C in this vessel (this is consistent with an NPR-A or NPR-B - mediated, cGMP-dependent relaxation of this tissue, as we have demonstrated previously [15]). Second, it demonstrates that the in vitro ‘clearance’ activity of NPR-C does not appear to significantly alter the steady-state concentrations, and therefore activity, of natriuretic peptides (otherwise an increase in potency would be expected as ‘clearance’ is blocked by M372049). Third, endothelium-derived vasodilators released by the aortic endothelium do not invoke NPR-C to induce vasorelaxation (consistent with NO acting as the sole endothelium-derived vasodilator in this tissue [34]). In marked contrast, a very different pattern of activity of M372049 was observed in rat isolated mesenteric resistance arteries. M372049 produced a potent and concentration-dependent inhibition of CNP-induced relaxations such that at 100nM the antagonist abolished CNP-mediated responses. According to ligand-binding data obtainedin the characterisation of the compound [18], M372049 is a competitive antagonist with a Ki = 21nM. Unfortunately, due to the inability to administer sufficiently high concentrations of CNP to overcome this competitive inhibition in our functional pharmacological investigations, we were unable to obtain a definitive pA2 value. Nonetheless, based on extrapolation of the data sets obtained, an approximate pA2 value of 11.14 (~IC50 = 4nM) was determined. Importantly, patch-clamp studies revealed that M372049 does not inhibit KIR channel activity directly (at a concentration two-orders of magnitude greater than that which completely attenuates functional responses to CNP), intimating this potential mechanism of action is not confounding interpretation.
Interestingly, despite the fact that all natriuretic peptides bind NPR-C [12,35], and that RT-PCR analysis revealed NPR-A, NPR-B and NPR-C mRNA expression in conduit and resistance vessels of the rat, ANP was found to be a very poor relaxant of mesenteric resistance vessels; indeed, at higher concentrations ANP elicited a contraction (as has been reported previously [36]). Relaxant responses observed at lower concentrations of ANP were not blocked by M372049 (indeed, if anything they were enhanced) suggesting that the biological activity of ANP in this resistance vascular bed is dependent on NPR-A activation and, unlike conduit vessels (i.e. aorta), blockade of NPR-C might be affecting the steady-state concentrations of natriuretic peptides. These observations also reveal that binding of ANP and CNP at NPR-C produces a distinct profile of activity; this may be related to the differential binding of these peptides to the receptor [37]. Our data also reveal that CNP mediates idiosyncratic vasorelaxation in conduit and resistance vessels via activation of NPR-B and NPR-C, respectively. Based on pharmacological data obtained with M372049, we postulated that NPR-B expression might be limited to conduit vessels and NPR-C expression to resistance vessels; however, all three NPRs appear to be expressed by both vessel types, intimating that vessel-specific pathways are activated by CNP.
When investigating EDHF-dependent relaxations, M372049 produced a marginal, but significant inhibition. In an analogous fashion, ouabain also caused a small rightward shift in the ACh concentration-response curve. However, in combination M372049 and ouabain completely abolished responses to EDHF. This is in accord with studies in which Ba2+ plus ouabain is required to completely attenuate EDHF responses in a number of vascular beds [20]. Since we observed that ouabain alone does not block responses to exogenous CNP in these vessels, such observations give rise to the following thesis: EDHF responses in the rat mesenteric resistance vasculature are comprised of at least two distinct mediators/pathways; one that involves the release of CNP that activates an NPR-C-linked and Ba2+-sensitive K+ channel (likely a GIRK [KIR3.x], as we have described previously [8]) to induce smooth muscle hyperpolarisation; and a second that is CNP-independent but sensitive to blockade by ouabain (Figure 8).
Figure 8.
Schematic representation of the proposed multiple EDHF pathways present in rat mesenteric resistance arteries. One ‘arm’ of the EDHF response is triggered by SKCa channels, is dependent on the release of CNP, activation of NPR-C and opening of a Ba2+-sensitive GIRK. An additional component is provided by an as yet unidentified mediator that is released following IKCa channel activation and is dependent on stimulation of the ouabain-sensitive Na+/K+-ATPase.
A key role for CNP/NPR-C signalling in EDHF-mediated relaxations was substantiated in studies exploring the hyperpolarisation associated with EDHF release. ACh caused a marked hyperpolarization (20-25mV) of the U46619-depolarized tissues, characteristic of an EDHF response. In accord with the isometric tension recordings, in the presence of M372049 plus ouabain this hyperpolarisation was abolished. Interestingly, M372049 alone had a significant inhibitory effect on EDHF-dependent hyperpolarisation whilst only exerting a marginal effect on smooth muscle tone. This suggests that complete blockade of endothelium-dependent hyperpolarization is required before the corresponding vasorelaxant activity is impaired significantly. Indeed, this has been demonstrated previously in rat arteries in which barium alone substantially suppresses ACh-induced vascular smooth muscle hyperpolarization with only a minor effect on relaxation, whereas ouabain alone has little or no effect on EDHF-dependent hyperpolarisation (a combination of the two abolishes both the hyperpolarisation and relaxation) [22].
We confirmed the multiplicity of EDHF responses in the rat mesenteric resistance vessels by using combinations of inhibitors previously characterised to inhibit EDHF responses in a number of vessels in different species [20]. We found that a combination of IKCa (TRAM-34) and KIR (M372049/Ba2+) or SKCa (apamin) and Na+/K+-ATPase (ouabain) blockade significantly inhibited EDHF responses (as did the ‘conventional’ cocktails described earlier) whereas any other combination of these selective inhibitors was unable to significantly alter EDHF-induced relaxations. These data have led to the hypothesis and schematic proposed in Figure 8. Such a mechanism is certainly applicable to the rat mesenteric vasculature, but might hold true for other vessels exhibiting a significant EDHF response which necessitates the use of Ba2+ and ouabain in combination to abolish EDHF-dependent relaxation (as is the case for human vessels in vivo [38]); in such cases, it is likely that there are two pathways and (at least) two mediators that constitute the EDHF phenomenon. From our observations we conclude that one pathway, involving the release of CNP is triggered by activation of SKCa channels on the endothelium and this results in NPR-C activation and hyperpolarisation [8]. The second pathway involves an as yet unidentified mediator/pathway that is initiated via opening of IKCa on the endothelium and culminates in vasorelaxation via Na+/K+-ATPase activation (i.e. ouabain sensitive). These pathways are redundant in as much as if a single mechanism is blocked only a fraction of the EDHF-dependent dilation is lost and therefore the alternate pathways must act in a compensatory fashion.
The phenomenon of multiple pathways underlying the biological activity of EDHF, inferring the existence of more than one EDHF in a single vascular bed, has been suspected as a result of the heterogeneity of EDHF (in terms of identity and function) across vascular beds and species [20]; however, the observations contained herein provide the first definitive evidence of such an occurrence. The identity of EDHF appears to vary considerably between vascular beds and the inhibitor combinations we have employed in this study have differing potencies against EDHF responses between species and vessel. For instance, in some vessels ouabain alone can almost abolish EDHF-dependent relaxation [39,40,41] whereas in other vessels it is the Ba2+-sensitive component that predominates [38,21]. Moreover, the actual identity of EDHF appears to differ between species and vascular bed and this may be explained by the release of more than one EDHF (as we have shown in the mesenteric vasculature); inhibitors of one ‘arm’ of the EDHF response in any given vessel may not be sufficient alone to block endothelium-dependent relaxation and therefore the candidate being investigated may be excluded erroneously. Moreover, the different sensitivity to inhibitor combinations suggests that in different tissues the balance between the two (or more) ‘arms’ of EDHF-mediated effects varies markedly. This latter thesis also gives rise to the possibility that different stimuli can release different EDHFs. For instance, SKCa channels (including the SK3 subtype that is thought to be expressed on endothelial cells [42,43]) require higher concentrations of calcium (Kd=600-700nM) to open than the IKCa channels (Kd=100-300nM) [44], suggesting that agonists that induce large, rapid increases in [Ca2+]i (e.g. ACh, bradykinin) may activate the SKCa-, CNP-dependent pathway preferentially. Indeed, this thesis is supported by a previous report of differential activation of SKCa and IKCa in rat mesenteric small arteries [45]. This may explain some of the heterogeneity in EDHF responses when different agonists are used to evoke endothelium-dependent relaxation/hyperpolarisation. Moreover, the multiplicity of EDHF responses suggests it may be possible to produce organ- or tissue- specific manipulation of EDHF by targeting the ‘arm’ of the EDHF response that predominates in particular vascular beds.
In sum, we have characterized and employed a selective NPR-C antagonist as a pharmacological tool to inhibit EDHF-dependent relaxations (and hyperpolarisation) in the rat mesenteric resistance vasculature and thereby demonstrated a key role for CNP/NPR-C in the regulation of vascular tone; in so doing, we have identified a unique tool for the study of CNP and EDHF biology. The importance of CNP/NPR-C signalling in EDHF-dependent hyperpolarisation in rat mesenteric resistance vessels revealed by this study, in combination with previous work by ourselves and others, highlights a novel target for potential therapeutic intervention in the treatment of cardiovascular disorders.
ACKNOWLEDGEMENTS
This work was funded by The British Heart Foundation. AJH is the recipient of a Wellcome Trust Senior Research Fellowship.
REFERENCES
- [1].Nakao K, Ogawa Y, Suga S, Imura H. Molecular biology and biochemistry of the natriuretic peptide system. I: Natriuretic peptides. J Hypertens. 1992;10:907–912. [PubMed] [Google Scholar]
- [2].Levin ER, Gardner DG, Samson WK. Natriuretic peptides. N Engl J Med. 1998;339:321–328. doi: 10.1056/NEJM199807303390507. [DOI] [PubMed] [Google Scholar]
- [3].Ahluwalia A, MacAllister RJ, Hobbs AJ. Vascular actions of natriuretic peptides. Cyclic GMP-dependent and -independent mechanisms. Basic Res Cardiol. 2004;99:83–89. doi: 10.1007/s00395-004-0459-6. [DOI] [PubMed] [Google Scholar]
- [4].Chen HH, Burnett JC., Jr C-type natriuretic peptide: the endothelial component of the natriuretic peptide system. J Cardiovasc Pharmacol. 1998;32(Suppl 3):S22–S28. [PubMed] [Google Scholar]
- [5].Stingo AJ, Clavell AL, Heublein DM, Wei CM, Pittelkow MR, Burnett JC., Jr Presence of C-type natriuretic peptide in cultured human endothelial cells and plasma. Am J Physiol. 1992;263:H1318–H1321. doi: 10.1152/ajpheart.1992.263.4.H1318. [DOI] [PubMed] [Google Scholar]
- [6].Suga S, Nakao K, Itoh H, Komatsu Y, Ogawa Y, Hama N, et al. Endothelial production of C-type natriuretic peptide and its marked augmentation by transforming growth factor-beta. Possible existence of “vascular natriuretic peptide system”. J Clin Invest. 1992;90:1145–1149. doi: 10.1172/JCI115933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Igaki T, Itoh H, Suga S, Komatsu Y, Ogawa Y, Doi K, et al. Insulin suppresses endothelial secretion of C-type natriuretic peptide, a novel endothelium-derived relaxing peptide. Diabetes. 1996;45(Suppl 3):S62–S64. doi: 10.2337/diab.45.3.s62. [DOI] [PubMed] [Google Scholar]
- [8].Chauhan SD, Nilsson H, Ahluwalia A, Hobbs AJ. Release of C-type natriuretic peptide accounts for the biological activity of endothelium-derived hyperpolarizing factor. Proc Natl Acad Sci U S A. 2003;100:1426–1431. doi: 10.1073/pnas.0336365100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hobbs A, Foster P, Prescott C, Scotland R, Ahluwalia A. Natriuretic peptide receptor-C regulates coronary blood flow and prevents myocardial ischemia/reperfusion injury: novel cardioprotective role for endothelium-derived C-type natriuretic peptide. Circulation. 2004;110:1231–1235. doi: 10.1161/01.CIR.0000141802.29945.34. [DOI] [PubMed] [Google Scholar]
- [10].Scotland RS, Cohen M, Foster P, Lovell M, Mathur A, Ahluwalia A, et al. C-type natriuretic peptide inhibits leukocyte recruitment and platelet-leukocyte interactions via suppression of P-selectin expression. Proc Natl Acad Sci U S A. 2005;102:14452–14457. doi: 10.1073/pnas.0504961102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Drewett JG, Fendly BM, Garbers DL, Lowe DG. Natriuretic peptide receptor-B (guanylyl cyclase-B) mediates C-type natriuretic peptide relaxation of precontracted rat aorta. J Biol Chem. 1995;270:4668–4674. doi: 10.1074/jbc.270.9.4668. [DOI] [PubMed] [Google Scholar]
- [12].Maack T, Suzuki M, Almeida FA, Nussenzveig D, Scarborough RM, McEnroe GA, et al. Physiological role of silent receptors of atrial natriuretic factor. Science. 1987;238:675–678. doi: 10.1126/science.2823385. [DOI] [PubMed] [Google Scholar]
- [13].Anand-Srivastava MB, Sehl PD, Lowe DG. Cytoplasmic domain of natriuretic peptide receptor-C inhibits adenylyl cyclase. Involvement of a pertussis toxin-sensitive G protein. J Biol Chem. 1996;271:19324–19329. doi: 10.1074/jbc.271.32.19324. [DOI] [PubMed] [Google Scholar]
- [14].Trachte GJ. Depletion of natriuretic peptide C receptors eliminates inhibitory effects of C-type natriuretic peptide on evoked neurotransmitter efflux. J Pharmacol Exp Ther. 2000;294:210–215. [PubMed] [Google Scholar]
- [15].Madhani M, Scotland RS, MacAllister RJ, Hobbs AJ. Vascular natriuretic peptide receptor-linked particulate guanylate cyclases are modulated by nitric oxide-cyclic GMP signalling. Br J Pharmacol. 2003;139:1289–1296. doi: 10.1038/sj.bjp.0705365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Morishita Y, Sano T, Ando K, Saitoh Y, Kase H, Yamada K, et al. Microbial polysaccharide, HS-142-1, competitively and selectively inhibits ANP binding to its guanylyl cyclase-containing receptor. Biochem Biophys Res Commun. 1991;176:949–957. doi: 10.1016/0006-291x(91)90374-g. [DOI] [PubMed] [Google Scholar]
- [17].Sano T, Morishita Y, Yamada K, Matsuda Y. Effects of HS-142-1, a novel non-peptide ANP antagonist, on diuresis and natriuresis induced by acute volume expansion in anesthetized rats. Biochem Biophys Res Commun. 1992;182:824–829. doi: 10.1016/0006-291x(92)91806-2. [DOI] [PubMed] [Google Scholar]
- [18].Veale CA, Alford VC, Aharony D, Banville DL, Bialecki RA, Brown FJ, et al. The discovery of non-basic atrial natriuretic peptide clearance receptor antagonists. Part 1. Bioorg Med Chem Lett. 2000;10:1949–1952. doi: 10.1016/s0960-894x(00)00387-5. [DOI] [PubMed] [Google Scholar]
- [19].Mulvany MJ, Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- [20].Busse R, Edwards G, Feletou M, Fleming I, Vanhoutte PM, Weston AH. EDHF: bringing the concepts together. Trends Pharmacol Sci. 2002;23:374–380. doi: 10.1016/s0165-6147(02)02050-3. [DOI] [PubMed] [Google Scholar]
- [21].Zygmunt PM, Hogestatt ED. Role of potassium channels in endothelium-dependent relaxation resistant to nitroarginine in the rat hepatic artery. Br J Pharmacol. 1996;117:1600–1606. doi: 10.1111/j.1476-5381.1996.tb15327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Edwards G, Dora KA, Gardener MJ, Garland CJ, Weston AH. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature. 1998;396:269–272. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- [23].Wulff H, Miller MJ, Hansel W, Grissmer S, Cahalan MD, Chandy KG. Design of a potent and selective inhibitor of the intermediate-conductance Ca2+-activated K+ channel, IKCa1: a potential immunosuppressant. Proc Natl Acad Sci U S A. 2000;97:8151–8156. doi: 10.1073/pnas.97.14.8151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gluais P, Edwards G, Weston AH, Falck JR, Vanhoutte PM, Feletou M. Role of SK(Ca) and IK(Ca) in endothelium-dependent hyperpolarizations of the guinea-pig isolated carotid artery. Br J Pharmacol. 2005;144:477–485. doi: 10.1038/sj.bjp.0706003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].White R, Hiley CR. A comparison of EDHF-mediated and anandamide-induced relaxations in the rat isolated mesenteric artery. Br J Pharmacol. 1997;122:1573–1584. doi: 10.1038/sj.bjp.0701546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mulvany MJ, Nilsson H, Flatman JA. Role of membrane potential in the response of rat small mesenteric arteries to exogenous noradrenaline stimulation. J Physiol. 1982;332:363–373. doi: 10.1113/jphysiol.1982.sp014418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Leaney JL, Tinker A. The role of members of the pertussis toxin-sensitive family of G proteins in coupling receptors to the activation of the G protein-gated inwardly rectifying potassium channel. Proc Natl Acad Sci U S A. 2000;97:5651–5656. doi: 10.1073/pnas.080572297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].McLean PG, Ahluwalia A, Perretti M. Association between kinin B(1) receptor expression and leukocyte trafficking across mouse mesenteric postcapillary venules. J Exp Med. 2000;192:367–380. doi: 10.1084/jem.192.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Connelly L, Jacobs AT, Palacios-Callender M, Moncada S, Hobbs AJ. Macrophage endothelial nitric-oxide synthase autoregulates cellular activation and pro-inflammatory protein expression. J Biol Chem. 2003;278:26480–26487. doi: 10.1074/jbc.M302238200. [DOI] [PubMed] [Google Scholar]
- [30].Garland JG, McPherson GA. Evidence that nitric oxide does not mediate the hyperpolarization and relaxation to acetylcholine in the rat small mesenteric artery. Br J Pharmacol. 1992;105:429–435. doi: 10.1111/j.1476-5381.1992.tb14270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Doughty JM, Plane F, Langton PD. Charybdotoxin and apamin block EDHF in rat mesenteric artery if selectively applied to the endothelium. Am J Physiol. 1999;276:H1107–H1112. doi: 10.1152/ajpheart.1999.276.3.H1107. [DOI] [PubMed] [Google Scholar]
- [32].Selemidis S, Cocks TM. Endothelium-dependent hyperpolarization as a remote anti-atherogenic mechanism. Trends Pharmacol Sci. 2002;23:213–220. doi: 10.1016/s0165-6147(02)01998-3. [DOI] [PubMed] [Google Scholar]
- [33].Scotland RS, Madhani M, Chauhan S, Moncada S, Andresen J, Nilsson H, et al. Investigation of vascular responses in endothelial nitric oxide synthase/cyclooxygenase-1 double-knockout mice: key role for endothelium-derived hyperpolarizing factor in the regulation of blood pressure in vivo. Circulation. 2005;111:796–803. doi: 10.1161/01.CIR.0000155238.70797.4E. [DOI] [PubMed] [Google Scholar]
- [34].Rees DD, Palmer RM, Schulz R, Hodson HF, Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 1990;101:746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Maack T. Receptors of atrial natriuretic factor. Annu Rev Physiol. 1992;54:11–27. doi: 10.1146/annurev.ph.54.030192.000303. [DOI] [PubMed] [Google Scholar]
- [36].Osol G, Halpern W, Tesfamariam B, Nakayama K, Weinberg D. Synthetic atrial natriuretic factor does not dilate resistance-sized arteries. Hypertension. 1986;8:606–610. doi: 10.1161/01.hyp.8.7.606. [DOI] [PubMed] [Google Scholar]
- [37].He XL, Dukkipati A, Garcia KC. Structural determinants of natriuretic Peptide receptor specificity and degeneracy. J Mol Biol. 2006;361:698–714. doi: 10.1016/j.jmb.2006.06.060. [DOI] [PubMed] [Google Scholar]
- [38].Dwivedi R, Saha S, Chowienczyk PJ, Ritter JM. Block of inward rectifying K+ channels (KIR) inhibits bradykinin-induced vasodilatation in human forearm resistance vasculature. Arterioscler Thromb Vasc Biol. 2005;25:e7–e9. doi: 10.1161/01.ATV.0000152610.40086.31. [DOI] [PubMed] [Google Scholar]
- [39].Dong H, Jiang Y, Cole WC, Triggle CR. Comparison of the pharmacological properties of EDHF-mediated vasorelaxation in guinea-pig cerebral and mesenteric resistance vessels. Br J Pharmacol. 2000;130:1983–1991. doi: 10.1038/sj.bjp.0703474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bussemaker E, Popp R, Binder J, Busse R, Fleming I. Characterization of the endothelium-derived hyperpolarizing factor (EDHF) response in the human interlobar artery. Kidney Int. 2003;63:1749–1755. doi: 10.1046/j.1523-1755.2003.00910.x. [DOI] [PubMed] [Google Scholar]
- [41].Edwards G, Gardener MJ, Feletou M, Brady G, Vanhoutte PM, Weston AH. Further investigation of endothelium-derived hyperpolarizing factor (EDHF) in rat hepatic artery: studies using 1-EBIO and ouabain. Br J Pharmacol. 1999;128:1064–1070. doi: 10.1038/sj.bjp.0702916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Eichler I, Wibawa J, Grgic I, Knorr A, Brakemeier S, Pries AR, et al. Selective blockade of endothelial Ca2+-activated small- and intermediate-conductance K+-channels suppresses EDHF-mediated vasodilation. Br J Pharmacol. 2003;138:594–601. doi: 10.1038/sj.bjp.0705075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Burnham MP, Bychkov R, Feletou M, Richards GR, Vanhoutte PM, Weston AH, et al. Characterization of an apamin-sensitive small-conductance Ca(2+)-activated K(+) channel in porcine coronary artery endothelium: relevance to EDHF. Br J Pharmacol. 2002;135:1133–1143. doi: 10.1038/sj.bjp.0704551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wei AD, Gutman GA, Aldrich R, Chandy KG, Grissmer S, Wulff H. International Union of Pharmacology. LII. Nomenclature and molecular relationships of calcium-activated potassium channels. Pharmacol Rev. 2005;57:463–472. doi: 10.1124/pr.57.4.9. [DOI] [PubMed] [Google Scholar]
- [45].Crane GJ, Gallagher N, Dora KA, Garland CJ. Small- and intermediate-conductance calcium-activated K+ channels provide different facets of endothelium-dependent hyperpolarization in rat mesenteric artery. J Physiol. 2003;553:183–189. doi: 10.1113/jphysiol.2003.051896. [DOI] [PMC free article] [PubMed] [Google Scholar]