Abstract
Sleep abnormalities are coexpressed with human communication disorders. Recent data from the birdsong system, the best model for human speech, indicate that sleep has a critical role in vocal learning. To understand the neural mechanisms that underlie behavioral changes during sleep, we recorded sleep activity in the song control area HVC longitudinally during song development in zebra finches. We focused on the sensorimotor phase of song learning, when the finch shapes his song behavior toward a learned tutor song model. Direct comparison of sleep activity in adults and juveniles revealed that the juvenile HVC has a lower spike rate and longer silent periods than the adult. Within individual finches, sleep silent periods decreased and spike rate increased with age. We next systematically compared neural sleep activity and song behavior. We now report that spike rate during sleep was significantly correlated with overnight changes in song behavior. Collectively, these data indicate that sleep activity in the vocal control area HVC increases with age and may affect song behavior.
INTRODUCTION
Although the majority of children develop the ability to speak by the age of 4 yr with no formal instruction, many cases exist where speech is delayed or never acquired. Mounting evidence indicates that children with developmental disorders, such as autism, also have atypical sleep architecture (Harvey and Kennedy 2002). Appreciable movement toward treatment and cure of these disorders will require an understanding of the fundamental biological principles of vocal learning and the role of sleep in acquiring this complex motor skill (Wise 2003).
Potential animal models for the investigation of speech and language development are rare. The most attractive vocal learning model has been the songbird (Doupe and Kuhl 1999). Songbirds, as with humans, learn their species-typical vocalizations in two phases: a sensory phase of explicit memorization during which the memory of a tutor’s song is formed and a sensorimotor phase of implicit skill learning during which the comparison of the tutor song memory to auditory feedback shapes the bird’s own song (Bolhuis & Gahr 2006; Konishi 1965). The zebra finch songbird has been particularly valuable for developmental studies because it has a defined period of vocal plasticity after which song behavior is relatively “crystallized” or insensitive to all but the most extreme environmental perturbations (Leonardo and Konishi 1998; Nordeen and Nordeen 1992; Zevin et al. 2004). The relative lack of seasonal variability in this species simplifies adult–juvenile comparisons.
Previous data indicate that motor patterns may be ’“replayed” or rehearsed during sleep in the neural song system of the zebra finch, which underlies song behavior (Dave and Margoliash 2000). Further, during developmental learning of song in this species, dramatic and apparently destabilizing changes occur in learned vocalizations during sleep (Deregnaucourt et al. 2005). These authors analyzed the development of song vocalization-by-vocalization and compared earlier songs to the final, mature song. Early in the sensorimotor phase of song learning, they found that vocalizations appeared to become less mature after night sleep, such that the song followed a pattern of apparent maturation during each day, followed by a significant loss of maturation after a night of sleep (termed postsleep deterioration). The same study also showed that the amount of song that was ultimately learned (as measured by similarity to a tutor song model) was directly proportional to the amount of postsleep deterioration in the sensorimotor phase. After the period of sensorimotor plasticity, the song behavior stopped showing the postsleep deterioration that was measured earlier in development. Collectively, these previous studies (Dave and Margoliash 2000; Deregnaucourt et al. 2005) indicate that the zebra finch presents an excellent model for examining the role of sleep in the development and learning of a complex motor skill.
To understand the brain mechanisms during sleep that underlie plasticity in vocal behavior, we examined population spiking activity during sleep in the song nucleus HVC (Reiner et al. 2004) during sensorimotor development. HVC both receives auditory input (Katz and Gurney 1981; Margoliash 1983; McCasland and Konishi 1981) and controls song behavior (Nottebohm et al. 1976; Vu et al. 1994). We compared HVC sleep activity in juveniles (that produced plastic song) to sleep activity in adults (that produced mature, crystallized song). Further, we recorded from the brains of zebra finches longitudinally throughout the period of song learning and crystallization, enabling us to systematically compare brain sleep activity and song behavior.
METHODS
Subjects
In all, 36 juvenile (61–90 days; in the late sensorimotor stage of song development) and 18 adult (>200 days) male zebra finches (Taeniopygia guttata) were surgically implanted with chronic population recording electrodes. Of the juveniles, 11 had high-quality neural recordings [premotor root mean square (RMS) signal-to-noise ratio (SNR) >2]. Of the adults, five had high-quality neural recordings. One implanted juvenile’s recording lasted until 142 days of age (Red-87). This bird did not make the >200 day cutoff for our adult group analysis, but his raw population data are shown in Fig. 1D as an example of a young adult. All song recordings were made in the absence of a female (undirected). Undirected song is thought to reflect song practice (Jarvis et al. 1998) and is thus most relevant to learning studies. All juvenile and 2 of the adult finches were reared in our facility on a 12 h:12 h light cycle. The rearing environment contained either a single breeder pair or five breeder pairs [in 18 × 16 × 16- or 36 × 24 × 48-in. (length × width × height) cages, respectively]. The cages contained all offspring of the resident breeders <45 days of age. All birds were removed from the rearing cage at 45 days of age and the males were placed in acoustic isolation chambers either alone or with same-clutch male siblings. Three adult birds were obtained from Magnolia Bird Farm (Anaheim, CA). None of the finches used in these experiments was ever exposed to auditory playback. The finches were allowed to hear their own vocalizations. All procedures were approved by the University of Minnesota Institutional Animal Care and Use Committee.
FIG. 1.

Sleep activity in the song nucleus HVC differs between juveniles and adults. Raw population recordings of 5 randomly selected 10-s sleep epochs from the HVCs of 3 juveniles (A–C), a young adult (D), and 2 adults age >200 days (E and F). Scale bar: 100 μV, 1 s.
Chronic physiological recording
Basic methods were previously described (Crandall et al. 2007). For population recordings, finches were implanted with a set of recording electrodes: one or two 50-μm nichrome–formvar electrodes in HVC (not plated, 1.1–1.8 MΩ, AM Systems, Carlsborg, WA), a 50-μm nichrome–formvar electrode adjacent to HVC for use as a reference electrode, and 75-μm silver ground and electroencephalogram (EEG) electrodes. The headstage and recording environment were previously described (Nick and Konishi 2001; Schmidt and Konishi 1998).
All data were acquired with custom-written (Datafleet, Minneapolis, MN) LabView software (National Instruments, Austin, TX) at a sampling frequency of 44.1 kHz. During recording, a lightweight operational amplifier was attached to the bird and connected to a mercury commutator by a flexible cable. HVC neural activity was amplified and filtered 300 –10,000 Hz. Song was monitored with a microphone (Earthworks, Milford, NH), high-pass in-line filtered at 100 Hz (Shure, Chicago, IL), and recorded. Localization of electrodes to HVC was confirmed with premotor activity in all cases and cresyl-violet histology in six of 16 finches.
Population activity on one or two chronic electrodes was collected for 10 s out of every 60 s, from 1 h after lights out to 1 h before lights on. EEG data were collected in 12 of 16 birds.
HVC population sleep activity analysis
Throughout the manuscript we will use “sleep activity” to refer to HVC population activity recorded during sleep. All data were analyzed with custom-written Matlab functions. For each 10-s recording epoch, the power of the EEG 1– 4 Hz was calculated and saved for further analysis. Trials with large, relatively fast deflections of the EEG, associated with movement, were discarded. For population activity, spike thresholds for each night of data were computed as the mean + (3 × SD) for 50 randomly selected trials from that night. As a quality control for the computed threshold, a trained user inspected the threshold relative to five random 10-s recording traces to subjectively confirm that the threshold was just above the noise band for the recording.
Segregation of data collected between 8 pm (1 h after lights out) and 6 am (1 h before lights on) based on slow-wave EEG did not significantly affect several measurements of population spiking activity. For each 10-s epoch, we calculated the power of the EEG between 1 and 4 Hz. We discarded all epochs that were less than the mean + SD, leaving approximately 16% epochs that were highest-power EEG (1– 4 Hz). We compared these highest-power EEG epochs to all epochs and found no significant difference in three separate measurements of sleep activity [n = 132 nights; spike rate: highest EEG = 53.29 ± 2.52 spikes/s, all = 57.38 ± 2.70 spikes/s, P = 0.27; median interspike interval (ISI): highest EEG = 11.62 ± 0.96 ms, all = 12.07 ± 0.93 ms, P = 0.74; maximum ISI: highest EEG = 430.50 ± 36.40 ms, all = 368.32 ± 23.37 ms, P = 0.15, two-tailed Student’s t-test]. Thus we analyzed all data between 8 pm and 6 am without regard for EEG.
Single-unit data (Crandall et al. 2007) suggest that single extracellular spikes last <0.75 ms. Thus continuous points above threshold were considered a single spike for 0.75 ms after the first threshold crossing. This provides a conservative estimate of population activity. Spike rate (in spikes/s) was computed as the number of spikes in a 10-s epoch divided by 10 s. ISIs were computed as the time between every spike (except the last in the epoch) and the next. We used a robust measure of the longest ISIs: 95th percentile (results also held with maximum ISI; TA Nick, unpublished observations). The 95th percentile ISI was computed as the median of the 95th percentile ISI across all 10-s recording epochs for a given night. Median ISI was calculated as the median of the median ISI across all 10-s recording epochs.
Behavioral (song) analysis
Initial song analysis consisted of the sorting of sound data and exclusion of movement noise. Sound data were further sorted according to temporal properties. Preliminary songs were defined as sounds lasting ≥500 ms with time gaps of no more than 20 ms. Preliminary songs were then converted to wave files. The Sound Analysis Pro (Tchernichovski et al. 2000) program was used to extract syllable features. Feature calculation was tuned to zebra finch sounds (default; see the Sound Analysis Pro manual for specific details: http://ofer.sci.ccny.cuny.edu/html/sound__analysis.html).
The clustering algorithm KlustaKwik (by K Harris) was used to cluster syllables based on the following features: syllable duration, mean pitch, mean entropy, mean frequency modulation (FM), and mean goodness of pitch. KlustaKwik was used instead of the built-in clustering functions of Sound Analysis Pro because we sought to cluster a large number of syllables (>60,000 in three cases) in a single clustering run. We were unable to cluster or export such large numbers of syllables from Sound Analysis Pro. A subset of Matlab functions taken from the MClust suite (by AD Redish) was used to feed these parameters to KlustaKwik and to assess the quality of clusters using the L-ratio (“good” quality <0.05) and isolation distance (“good” quality >16) metrics (Schmitzer-Torbert et al. 2005).
After clustering and cluster quality checking, syllable clusters were screened to exclude calls and very simple call-like syllables that were unlikely to show quantifiable developmental change. Calls that are of relatively short duration and consist of harmonic stacks (which have low FM) are unlearned and thus should not change during vocal learning. Only syllables with a mean duration >30 ms and a mean FM >30 were analyzed for entropy variance across development (for details on the FM feature, see Tchernichovski et al. 2000). Running median entropy variances (Fig. 6) were calculated using a 100-syllable sliding window.
FIG. 6.

Overnight behavioral change paralleled sleep activity. Data shown are from bird Blue-81, electrode 1. For display purposes only, A–C show the last 6 song motifs from each evening and the first 6 from each morning. Motifs appeared to become less mature and stereotyped overnight after days 74 (A) and 78 (C). Note how the morning syllables look less defined relative to those from the night before in A and C. In contrast, the song motif appeared to stabilize overnight before day 76 (B). D: sleep spike rate (black) was higher on the night before day 76, when the overnight ratio of entropy variance (red) of a motif syllable (indicated by arrowheads in A–C) increased (the vocalization was more stable overnight). E: overnight ratio of entropy variance and sleep spike rate data shown in D were strongly and significantly correlated.
Statistical analysis
The distribution of ISIs was not normal and, importantly, appeared to change over development (Supplementary video).1 With this in mind, we obtained the median spike rate, median ISI, and the 95th percentile ISI across all 10-s recording epochs from each night. These values were normally distributed across all of our development/age/electrode data, so we used the parametric two-tailed Student’s t-test, to compare juvenile and adult data. Each bar graph shows means ± SEs. All comparisons used a two-tailed Student’s t-test unless otherwise noted. For within-juvenile comparisons of spike rate across ≥5 days, we used the Wilcoxon signed-rank test because of the relatively low numbers of data available and the variation across juveniles (at least partially resulting from the range in juvenile ages).
Sleep activity and behavior were compared in juvenile singing finches that were recorded during development. If <100 copies of a given noncall, a “good” clustered syllable were produced either the evening before or the morning after a night of sleep, that syllable was discarded. If no syllable made the cut, that night was excluded from further sleep-behavior comparisons. Four singing juvenile (<90 days) zebra finches produced ≥100 copies of at least one noncall, a clustered syllable after noon on the prior day and ≥100 copies before noon on the next day (Ns = 6 electrodes, 41 nights of recording, and 12 clustered syllables). To be included, clusters had to be judged “good” by our criteria (see above). None of our adults produced 100 copies of any song syllable after noon. To allow direct comparisons of adult song to juvenile undirected song (see above), no females were presented to elicit directed song.
Entropy variance is a syllable feature calculated by Sound Analysis Pro that shows considerable change during development (Deregnaucourt et al. 2005). This feature reports variability in the spectral entropy of the sound during the syllable. Depending on the syllable, entropy variance may increase, decrease, or not change with development. The overnight ratio of entropy variance was measured as the ratio of the mean entropy variance of the first 100 syllable copies produced before noon the day after measured sleep to the last 100 syllable copies produced after noon the day before.
RESULTS
HVC sleep activity differs between juveniles and adults
Comparison of raw HVC population recordings during sleep (termed “sleep activity”) from juveniles and adults revealed clear differences (Fig. 1). By eye, the most obvious difference was the presence of long silent periods in the juvenile. Long silent periods were also apparent in thresholded data (>250 ms highlighted in red in Fig. 2). To quantify these data, we measured the spike rate, the median of the 95th percentile ISI per 10-s recording epoch, and the median of the median ISI per epoch. We found that spike rate was lower in juveniles (Fig. 3A; P < 0.04). Consistent with this finding, the 95th percentile ISI was significantly greater in juveniles (Fig. 3B; P < 0.05), confirming that they have longer silent periods. The median ISI tended to be greater in juveniles, but this difference was not significant (Fig. 3C; P = 0.41).
FIG. 2.
Long silent periods and decreased spike rate characterize sleep activity in the juvenile (A; bird Blue-81, age 72 days) as compared with the adult (B; Black-161, age >200 days). 100 randomly selected 500-ms thresholded population recordings are shown for each. Black dots indicate spikes, whereas red bars indicate periods of silence that were longer than 250 ms in duration.
FIG. 3.
Analysis of group data revealed that the spike rate per 10-s epoch was lower in juveniles compared with that of adults (A; P < 0.04, t-test) and that the 95th percentile interspike interval (ISI; an indicator of the longest silent periods) was longer (B; P < 0.05). Median ISI per 10-s epoch was not significantly different between juveniles and adults [C; P = 0.41; for A–C, juveniles n = 11 finches (age: median = 60; range = 48 –90), 18 electrodes; adults n = 5 finches, 9 electrodes]. Each electrode contributed one datum to this analysis. For each electrode in a juvenile, only the youngest age available was used.
The preceding data indicate that sleep spiking activity is greater in adults than in juveniles. In contrast, measurement of combined subthreshold and spiking activity in the same data set using the RMS of HVC voltage during sleep revealed no difference between adults and juveniles (P = 0.66, t-test). The RMS tended to be greater in juveniles (9.37 ± 1.09 μV, n = 18) than that in adults (8.51 ± 1.64 μV, n = 9). The trend in these data is consistent with that of a previous study (Nick and Konishi 2005b), which reported that the RMS voltage in HVC decreases with development during both sleep and waking.
To better understand the differences in sleep activity between juveniles and adults, we examined the properties of sleep bursts (Table 1). Analysis of bursts is inherently subject to bias because of the arbitrary criteria used to define a burst. Because of this, we chose three different burst criteria, all based on the maximum allowable ISI within a burst (10, 20, and 50 ms). We found that burst rate (not spike rate within a burst) was significantly higher in adults compared with juveniles when the burst criterion was 10- or 20-ms maximum ISI (Table 1; P < 0.003, t-test). The interburst interval tended to be higher in juveniles when the burst criterion was 20- or 50-ms maximum ISI (P < 0.05). Collectively, these data indicate that there are significant differences in HVC population bursting activity between juveniles and adults.
TABLE 1.
Properties of HVC sleep bursts
| Maximum ISI Within a Burst, ms | Juvenile (n = 18 electrodes) | Adult (n = 9 electrodes) | P |
|---|---|---|---|
| Burst duration, ms | |||
| 10 | 4.17 ± 0.10 | 4.15 ± 0.11 | 0.9179 |
| 20 | 5.56 ± 0.20 | 5.44 ± 0.19 | 0.7028 |
| 50 | 7.75 ± 0.58 | 6.70 ± 0.52 | 0.2538 |
| Spike rate within burst, spikes/s | |||
| 10 | 747.32 ± 18.74 | 765.25 ± 26.85 | 0.5870 |
| 20 | 462.17 ± 20.66 | 440.19 ± 28.53 | 0.5417 |
| 50 | 228.44 ± 16.41 | 186.74 ± 16.13 | 0.1208 |
| Burst rate, bursts/s | |||
| 10 | 9.31 ± 15.51 | 18.82 ± 2.59 | 0.0026* |
| 20 | 7.04 ± 1.04 | 13.19 ± 1.05 | 0.0010* |
| 50 | 3.38 ± 0.43 | 4.49 ± 0.56 | 0.1422 |
| Interburst interval, ms | |||
| 10 | 48.71 ± 5.51 | 32.25 ± 3.98 | 0.0596 |
| 20 | 57.22 ± 5.86 | 38.03 ± 4.37 | 0.0409 |
| 50 | 68.81 ± 7.08 | 44.21 ± 5.65 | 0.0321 |
Significant with Bonferroni-corrected α = 0.05, P < 0.017, t-test. Juvenile age: median = 60; range = 48 –90. Each electrode contributed one datum to this analysis. For each electrode in a juvenile, only the youngest age available was used.
Within single juveniles, sleep activity increases with development
To further analyze the development of sleep activity, we compared HVC population spiking activity in the same juvenile finches. Figure 4 shows raw HVC population data during sleep from the same electrodes in the same animals recorded 8 (Fig. 4A) and 37 (Fig. 4B) days apart.
FIG. 4.
Sleep activity increased with development when recorded with the same electrodes in the same HVC. Comparison of raw population recordings of 5 randomly selected 10-s sleep epochs from the HVC of 2 juveniles. A: HVC population activity recorded from Bird Red-2 at 73 days and 81 days. B: HVC population activity recorded from Bird Blue-70 at 71 and 108 days. Scale bar: 100 μV, 1 s.
Group data indicated that the spike rate increased dramatically during development in juvenile finches. We compared two nights of HVC activity recorded from the same electrode that occurred ≥5 days apart in the same animal. Using these criteria, we were able to compare data from five juveniles and seven electrodes. Consistent with adult–juvenile comparisons, we found that the 95th percentile ISI decreased with age (young: median = 161.96 ms; range = 40.86 –212.54 ms; old: median = 80.89 ms; range = 25.96 –154.15 ms; P < 0.008, Wilcoxon signed rank) and that spike rate increased with age (young: median = 26.99 spikes/s; range = 25.18 –73.32 spikes/s; old: median = 60.57 spikes/s; range = 38.51–92.81 spikes/s; P < 0.008, Wilcoxon signed rank). These data indicate that sleep activity in HVC increases with development.
HVC sleep activity correlates with song behavior
We found that sleep activity appeared to vary with developmental changes in behavior (Fig. 5). For a single developing finch, the median entropy variance of a clustered syllable (black dots; 100-syllable sliding window) is shown for each day and the sleep spike rate per 10-s epoch (gray) is shown for each night. Note that as the sleep spike rate increased, the entropy variance of the song syllable also increased.
FIG. 5.
Neural sleep activity and a measure of song behavior, entropy variance, correlate across many days of development. In this case, spike rate and entropy variance both increased with age. All data shown are from a single animal recorded over multiple days (Blue-70). Neural sleep activity (gray) was measured from 8 pm to 6 am each night. Median spike rates for 10 s of every minute are shown. Running median entropy variances of a single clustered syllable type (black) are plotted in temporal order. Sleep data were not available at the end of day 82. For unknown reasons, the finch stopped singing on day 84 and resumed on day 85.
During nights over which the behavior was relatively stable, the sleep activity was high. For example, the song motif shown in Fig. 6B appeared more mature or defined on the morning of day 76 than the night before. During the night before day 76, the spike rate during sleep was unusually high (note the peak in the black line in Fig. 6D). In contrast, the motifs produced on the mornings of days 75 and 79 appeared less defined than the night before (Fig. 6, A and C) and the sleep spike rate was correspondingly low (Fig. 6D).
We next assessed the day-by-day relationship between sleep activity and behavior across several finches that produced enough high-quality song data for rigorous analysis of behavioral and neural data (n = 4 finches). We compared HVC population spiking activity during each night of recording, which increased with age (Fig. 7A), with the overnight change in song behavior. We identified syllables across days by coclustering the entire developmental song database of each finch, using five parameters that describe vocalizations. We analyzed a sixth parameter, entropy variance, which was previously shown to vary dramatically across development (Deregnaucourt et al. 2005).
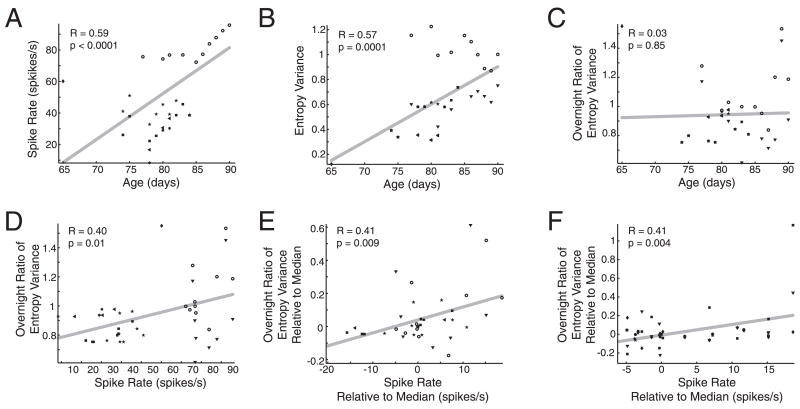
FIG. 7.
HVC sleep activity was significantly correlated with overnight changes in vocal behavior. A–E: group data are shown for 7 syllables (n = 4 juvenile finches) that had a developmental trend in entropy variance (either increasing or decreasing; Deregnaucourt et al. 2005). A: spike rate in HVC during sleep increased with development. B: entropy variance increased with age for most syllables (solid symbols, each symbol represents a different syllable), but decreased for one syllable (hollow circles). C: overnight change in entropy variance was not correlated with age. D: overnight change in entropy variance was significantly correlated with spike rate. E: controlling for developmental age-related changes by subtracting the median, the overnight change in entropy variance was still significantly correlated with spike rate. F: entropy variances of syllables that showed no developmental trend also had nightly changes that correlated with spike rate.
Consistent with a prior study (Deregnaucourt et al. 2005), the entropy variance of six of 12 syllables increased with development, whereas the entropy variance of one of 12 decreased (Fig. 7B; five syllables did not change with regard to entropy variance; see following text). The blurriness or definition of a syllable can be quantified using the entropy variance parameter, which describes the change in noise across the syllable. Syllables that appear less defined have lower entropy variance. The overnight ratio of entropy variance (ONEV) for each syllable was computed as the mean entropy variance of 100 clustered syllable copies produced before noon the day after divided by that of 100 syllable copies produced after noon the day before. An ONEV of 1 would indicate that the syllable was completely stable overnight, an ONEV <1 would indicate that the entropy variance decreased overnight (“deteriorated” as defined by Deregnaucourt et al. 2005), and an ONEV >1 would indicate that the entropy variance increased overnight. Sleep spike rate and the ONEV were strongly and significantly correlated over the several nights of recording for the finch shown in Fig. 6, D and E. Across recordings, we found that ONEV was not linearly correlated with age (Fig. 7C), but was significantly correlated with HVC population spike rate during sleep on the corresponding night (Fig. 7D). To test whether the ONEV–sleep activity relationship was affected by the differences in firing rate between recording sites, we normalized differences between animals by subtracting the median for spike rate and entropy variance for each site from all data points from that site. In the normalized data, sleep spike rate and ONEV were significantly correlated (Fig. 7E).
We were surprised to find that the ONEV of a syllable that decreased entropy variance with age (Fig. 7B, hollow circles) was positively correlated with spike rate, similar to the syllables that increased entropy variance with age (Fig. 7E). These data suggest that HVC population spiking activity during sleep stabilizes or increases entropy variance, regardless of whether such stabilization or increase works against the maturation of the syllable. To further investigate this question, we examined the ONEV and corresponding nightly spike rates of five syllables that did not change their entropy variance during the period of development that we examined. We found that these syllables that did not show net developmental changes in entropy variance nevertheless had overnight changes in entropy variance that were positively correlated with spike rate during sleep (Fig. 7F).
DISCUSSION
We found that HVC spiking activity during sleep increases with development. Within the song control area HVC, the spike rate increased and the duration of long silent periods decreased with development. These data are consistent with a recent study that indicated that HVC activity is developmentally modulated (Crandall et al. 2007). In addition, they confirm and extend studies in humans and other animals that indicate that gross brain activity during sleep is developmentally regulated (as measured by EEG; Frank and Heller 1997; Gramsbergen 1976; Marshall et al. 2002).
The song system allowed direct comparison of neural sleep activity and a specific behavior. This is possible because the neural song system is dedicated to the production and plasticity of learned vocal behavior. Through systematic comparison of chronic sleep recordings and behavior, we found that sleep activity is significantly correlated with overnight changes or stability in song behaviors. Our data strongly suggest that HVC activity during sleep has a role in vocal learning. We found a significant correlation, but not causation. Future experiments will test causality by selectively perturbing sleep activity and analyzing behavior.
Comparisons between RMS and population spiking
We found that population spiking activity in HVC increased with development, whereas a measure of overall activity (the RMS of the voltage) remained stable with development with a trend toward decreasing with development, consistent with a previous study (Nick and Konishi 2005b). However, in the current study, the RMS voltages in the HVC of juveniles and adults were not significantly different. There were several differences between these two studies, including the electrodes [rhodium-plated (prior study) vs. not plated], the EEG criteria for inclusion, genetics of the finches, and rearing environment. However, the most salient difference between these studies may have been that RMS activity was measured during silent periods in a background of auditory playback stimuli in the first study (Nick and Konishi 2005b), whereas the current study used no auditory playback stimuli at any time. Our preliminary studies indicate that auditory stimuli affect ongoing HVC activity during sleep in adults (Nick 2001). Future experiments will examine differential developmental effects of auditory stimuli on HVC activity during sleep, which may illuminate mechanisms underlying the auditory shaping of vocal behavior.
Why the RMS decreases or remains stable and the spiking activity increases during development remains an intriguing question. RMS measures all activity, whereas thresholded spiking activity reports only the largest voltage deflections. Although the largest voltage deflections (that will also be counted as population spikes) will dominate the RMS, other subthreshold activity will influence it. This subthreshold activity may include synaptic activity, calcium transients, and smaller spikes that when summed become suprathreshold. The higher spike rate indicates that the adult HVC has more suprathreshold activity than the juvenile. Thus the juvenile may have less synchronous population spiking activity and/or a larger amount of activity that does not drive action potentials compared with the adult. If so, this would be consistent with current understanding of the developing nervous system. Asynchronous activity was previously reported in developing cortical and hindbrain neurons (Corlew et al. 2004; Gust et al. 2003). A large amount of synaptic activity in the juvenile compared with that in the adult is consistent with the overgrowth of synapses that occurs during synapse and circuit formation (Cohen-Cory 2002). In addition, activity below the action potential threshold plays critical roles in early stages of neural development, such as differentiation and axonogenesis (Spitzer 2006), which would occur in the newly generated neurons that appear in HVC during the sensorimotor phase (Bottjer et al. 1986; Kirn and DeVoogd 1989).
Stability of longitudinal recordings
Our neural population recordings are very stable, which enabled this longitudinal study. A common criticism of longitudinal electrophysiological investigations is that they are not stable. We saw increases in juvenile HVC activity with 12 of 16 electrodes that recorded sleep population activity for ≥2 days. If the electrodes moved randomly, then the probability of HVC activity increasing may be estimated at 0.333 (with the probability of decreasing or staying the same equal to 0.666). With these values, the binomial probability distribution indicates that the probability that 12 of 16 electrodes would increase their activity is small (P = 0.0007). In other studies, we found that the overall pattern of HVC activity during singing is relatively stable across many days (Crandall et al. 2007). In addition, the auditory responses during sleep on two different electrodes in the same HVC are similar in magnitude (see supplementary RMS data; Nick and Konishi 2005a) and the spiking activity patterns on two different electrodes in the same HVC are very similar during singing (Nick, unpublished observations). Both of these findings indicate that our recordings monitor HVC activity at the neural population level. One may still speculate that the electrodes in juveniles typically move or degrade in such a way as to increase the measured spiking activity as the animal matures, but a more parsimonious explanation is that population spiking activity increases with development.
Cellular mechanisms
The current study shows that population spiking activity during sleep increases with development (for the first time in any system) and links the reported changes in sleep activity to behavior. Because the recordings were of many neurons, we could not tell whether the changes in sleep activity that we observed arose primarily from changes in neuronal density or from selective changes in the activity of all or a subset of HVC neurons. Because HVC contains at least three major subtypes of neuron based on projection target, future experiments will determine what specific HVC neurons show developmental modulation of sleep activity. This knowledge will illuminate the mechanisms through which sleep affects song behavior.
The relationship between HVC sleep activity and song behavior
Tchernichovski and colleagues (Deregnaucourt et al. 2005) previously found that song behavior appears to deteriorate during sleep in the early sensorimotor phase. These data are consistent with our findings because we found that juveniles have less HVC sleep activity and that sleep activity appears to stabilize most song syllables (by stabilizing entropy variance). During nights of apparent behavioral deterioration (i.e., vocal behavior after sleep appears less mature than that before sleep), HVC activity is relatively low, which is typical of juveniles. During nights over which the song is unusually stable (i.e., vocal behavior after sleep is similar to that before sleep), HVC activity is relatively high.
Our data presented in Fig. 7C (Age vs. Overnight Ratio of Entropy Variance) may appear to contradict the findings of Deregnaucourt and colleagues (2005). However, the previous study focused on the early sensorimotor phase when more dramatic changes occur in learned song. During the period when these larger vocal changes occur (~5– 60 days), it seems reasonable that there would be corresponding larger overnight changes in entropy variance that would decrease with age. If so, future studies will reveal a correlation between overnight ratio of entropy variance and age in these younger finches. We should also note that the previous study trained finches with the tutor song using an operant conditioning paradigm starting at day 43, whereas we used live tutors until day 45. These two types of rearing conditions may differentially affect the amount of learning relative to age and the underlying neural mechanisms.
The authors of two previous papers speculated on the mechanisms underlying the role of sleep in the destabilization or “degradation” of song behavior. Deregnaucourt and colleagues (2005) suggested that the occurrence of sleep bursting activity (putative “replay”) in the absence of auditory feedback would enable drifts in song structure. Margoliash (2005) suggested that random or structured modifications of bursting activity during sleep enable vocal plasticity. In contrast to these hypotheses, our data indicate that the lack of sleep bursting activity enables plasticity (a greater overnight change in entropy variance). However, it should be noted that the rare syllable that we recorded that showed a decrease in entropy variance with development proved to be the exception to this rule; this syllable was most plastic over nights with the greatest sleep activity. Thus there may not be a simple relationship between sleep bursting activity and song maturation, but rather a direct relationship between sleep activity and plasticity of a specific aspect of song behavior (entropy variance; Fig. 8).
FIG. 8.

Simple schematic illustrating the observed effects of HVC activity during sleep on the entropy variance of a song syllable. With low HVC activity (top), the entropy variance of the same syllable the next morning was low relative to the night before. With high HVC activity (bottom), the entropy variance the next morning was the same as or greater than the night before. x-axis: 0 –50 ms; y-axis: 500 –10,000 Hz. Images produced by Sound Analysis Pro (Tchernichovski et al. 2000).
We report that sleep activity, in general, stabilizes song behavior. These data are consistent with two hypotheses of the function of sleep: replay (Wilson and McNaughton 1994) and synaptic homeostasis (Tononi and Cirelli 2006). With replay, HVC neural patterns that produced song would be rehearsed during sleep, which would stabilize and/or improve behavior. Consistent with this hypothesis, in most but not all cases (see preceding paragraph), we see a stabilization of song behavior with increased sleep bursting activity. The synaptic homeostasis hypothesis (Tononi and Cirelli 2006) would predict that HVC slow-wave sleep activity serves to increase the SNR in HVC neural activity by ubiquitously downscaling all synaptic strengths. Increasing the SNR in HVC may increase SNR in the behavior. If so, the spectral structure of the song would rise further out of the noise with increased bursting activity in HVC during sleep. This would cause the variance in noise across a given syllable (entropy variance) to parallel sleep activity (Fig. 8), as we observed. If strictly applied, the synaptic homeostasis hypothesis would predict that less sleep activity would result in less change in behavior because synapses would not be down-scaled. In contrast, we observed that low levels of HVC sleep activity led to a decrease in the entropy variance of most syllables. This may reflect the fact that HVC alone does not control song behavior, but works in partnership with several efferent nuclei (Schmidt et al. 2004; Wild 2004).
HVC spiking activity during sleep may serve to maintain and shape its synaptic contacts with its targets. During slow-wave sleep, asynchrony between the bursting of target neurons and HVC synaptic activity may lead to synaptic decrement through activity-dependent plasticity (i.e., synapses from HVC that are silent or only weakly active during ongoing bursting of target neurons may be weakened). The requirement for spiking/bursting activity in the target neurons can explain why changes occur during sleep and not during quiet waking (Deregnaucourt et al. 2005). Because HVC drives song behavior (Nottebohm et al. 1976; Vu et al. 1994), decrement of HVC synaptic inputs to its targets may degrade song behavior. Simultaneous recording of HVC and its synaptic targets (Area X and the robust nucleus of the arcopallium) will test whether song system sleep activity is less correlated during vocal learning.
Supplementary Material
Acknowledgments
We thank N. Aoki, T. Balmer, V. Carels, A. Craig, D. Cygnar, J. Despinoy, M. Negia, and L. Onikoro for technical assistance; and M. Konishi for reviewing a preliminary draft of the manuscript.
GRANTS
This work was supported by the John Merck Scholars Program, National Institute on Deafness and Other Communication Disorders Grants R01-DC-007384 and K02-DC-008521, Minnesota Medical Foundation, Grant-in-Aid of Research, Artistry, and Scholarship from the University of Minnesota Graduate School to T. A. Nick, and University of Minnesota Undergraduate Research Opportunities Program grants to A. K. Kinnischtzke and M. Adam.
Footnotes
The online version of this article contains supplemental data.
References
- Bolhuis JJ, Gahr M. Neural mechanisms of birdsong memory. Nat Rev Neurosci. 2006;7:347–357. doi: 10.1038/nrn1904. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Miesner E, Arnold AP. Changes in neuronal number, density and size account for increases in volume of song-control nuclei during song development in zebra finches. Neurosci Lett. 1986;67:263–268. doi: 10.1016/0304-3940(86)90319-8. [DOI] [PubMed] [Google Scholar]
- Cohen-Cory S. The developing synapse: construction and modulation of synaptic structures and circuits. Science. 2002;298:770–776. doi: 10.1126/science.1075510. [DOI] [PubMed] [Google Scholar]
- Corlew R, Bosma MM, Moody WJ. Spontaneous, synchronous electrical activity in neonatal mouse cortical neurones. J Physiol. 2004;560:377–390. doi: 10.1113/jphysiol.2004.071621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall SR, Aoki N, Nick TA. Developmental modulation of the temporal relationship between brain and behavior. J Neurophysiol. 2007;97:806 – 816. doi: 10.1152/jn.00907.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave AS, Margoliash D. Song replay during sleep and computational rules for sensorimotor vocal learning. Science. 2000;290:812–816. doi: 10.1126/science.290.5492.812. [DOI] [PubMed] [Google Scholar]
- Deregnaucourt S, Mitra PP, Feher O, Pytte CL, Tchernichovski O. How sleep affects the developmental learning of bird song. Nature. 2005;433:710–716. doi: 10.1038/nature03275. [DOI] [PubMed] [Google Scholar]
- Doupe AJ, Kuhl PK. Birdsong and human speech: common themes and mechanisms. Annu Rev Neurosci. 1999;22:567–631. doi: 10.1146/annurev.neuro.22.1.567. [DOI] [PubMed] [Google Scholar]
- Frank MG, Heller HC. Development of REM and slow wave sleep in the rat. Am J Physiol Regul Integr Comp Physiol. 1997;272:R1792–R1799. doi: 10.1152/ajpregu.1997.272.6.R1792. [DOI] [PubMed] [Google Scholar]
- Gramsbergen A. The development of the EEG in the rat. Dev Psychobiol. 1976;9:501–515. doi: 10.1002/dev.420090604. [DOI] [PubMed] [Google Scholar]
- Gust J, Wright JJ, Pratt EB, Bosma MM. Development of synchronized activity of cranial motor neurons in the segmented embryonic mouse hindbrain. J Physiol. 2003;550:123–133. doi: 10.1113/jphysiol.2002.038737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey MT, Kennedy CH. Polysomnographic phenotypes in developmental disabilities. Int J Dev Neurosci. 2002;20:443–448. doi: 10.1016/s0736-5748(02)00008-4. [DOI] [PubMed] [Google Scholar]
- Jarvis ED, Scharff C, Grossman M, Ramos JA, Nottebohm F. For whom the bird sings: context-dependent gene expression. Neuron. 1998;21:775–788. doi: 10.1016/s0896-6273(00)80594-2. [DOI] [PubMed] [Google Scholar]
- Katz LC, Gurney ME. Auditory responses in the zebra finch’s motor system for song. Brain Res. 1981;221:192–197. doi: 10.1016/0006-8993(81)91073-8. [DOI] [PubMed] [Google Scholar]
- Kirn JR, DeVoogd TJ. Genesis and death of vocal control neurons during sexual differentiation in the zebra finch. J Neurosci. 1989;9:3176–3187. doi: 10.1523/JNEUROSCI.09-09-03176.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi M. The role of auditory feedback in the control of vocalization in the white-crowned sparrow. Z Tierpsychol. 1965;22:770–783. [PubMed] [Google Scholar]
- Leonardo A, Konishi M. Decrystallization of adult birdsong by perturbation of auditory feedback. Nature. 1998;399:466 – 470. doi: 10.1038/20933. [DOI] [PubMed] [Google Scholar]
- Margoliash D. Acoustic parameters underlying the responses of song-specific neurons in the white-crowned sparrow. J Neurosci. 1983;3:1039 –1057. doi: 10.1523/JNEUROSCI.03-05-01039.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margoliash D. Song learning and sleep. Nat Neurosci. 2005;8:546–548. doi: 10.1038/nn0505-546. [DOI] [PubMed] [Google Scholar]
- Marshall PJ, Bar-Haim Y, Fox NA. Development of the EEG from 5 months to 4 years of age. Clin Neurophys. 2002;113:1199–1208. doi: 10.1016/s1388-2457(02)00163-3. [DOI] [PubMed] [Google Scholar]
- McCasland JS, Konishi M. Interaction between auditory and motor activities in an avian song control nucleus. Proc Natl Acad Sci USA. 1981;78:7815–7819. doi: 10.1073/pnas.78.12.7815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nick TA. Song playback phase-locks ongoing activity during sleep in the birdsong nucleus HVC. Soc Neurosci Abstr. 2001;31:724.18. [Google Scholar]
- Nick TA, Konishi M. Dynamic control of auditory activity during sleep: correlation between song response and EEG. Proc Natl Acad Sci USA. 2001;98:14012–14016. doi: 10.1073/pnas.251525298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nick TA, Konishi M. Neural auditory selectivity develops in parallel with song. J Neurobiol. 2005a;62:469 – 481. doi: 10.1002/neu.20115. [DOI] [PubMed] [Google Scholar]
- Nick TA, Konishi M. Neural song preference during vocal learning in the zebra finch depends on age and state. J Neurobiol. 2005b;62:231–242. doi: 10.1002/neu.20087. [DOI] [PubMed] [Google Scholar]
- Nordeen KW, Nordeen EJ. Auditory-feedback is necessary for the maintenance of stereotyped song in adult zebra finches. Behav Neural Biol. 1992;57:58 – 66. doi: 10.1016/0163-1047(92)90757-u. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Stokes TM, Leonard CM. Central control of song in the canary, Serinus canarius. J Comp Neurol. 1976;165:457– 486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- Reiner A, Perkel DJ, Mello CV, Jarvis ED. Songbirds and the revised avian brain nomenclature. Ann NY Acad Sci. 2004;1016:77–108. doi: 10.1196/annals.1298.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt MF, Ashmore RC, Vu ET. Bilateral control and interhemispheric coordination in the avian song motor system. Ann NY Acad Sci. 2004;1016:171–186. doi: 10.1196/annals.1298.014. [DOI] [PubMed] [Google Scholar]
- Schmidt MF, Konishi M. Gating of auditory responses in the vocal control system of awake songbirds. Nat Neurosci. 1998;1:513–518. doi: 10.1038/2232. [DOI] [PubMed] [Google Scholar]
- Schmitzer-Torbert N, Jackson J, Henze D, Harris K, Redish AD. Quantitative measures of cluster quality for use in extracellular recordings. Neuroscience. 2005;131:1–11. doi: 10.1016/j.neuroscience.2004.09.066. [DOI] [PubMed] [Google Scholar]
- Spitzer NC. Electrical activity in early neuronal development. Nature. 2006;444:707–712. doi: 10.1038/nature05300. [DOI] [PubMed] [Google Scholar]
- Tchernichovski O, Nottebohm F, Ho CE, Pesaran B, Mitra PP. A procedure for an automated measurement of song similarity. Anim Behav. 2000;59:1167–1176. doi: 10.1006/anbe.1999.1416. [DOI] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10:49 – 62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Vu ET, Mazurek ME, Kuo Y-C. Identification of a forebrain motor programming network for the learned song of zebra finches. J Neurosci. 1994;14:6924 – 6934. doi: 10.1523/JNEUROSCI.14-11-06924.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild JM. Functional neuroanatomy of the sensorimotor control of singing. Ann NY Acad Sci. 2004;1016:438 – 462. doi: 10.1196/annals.1298.016. [DOI] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676 – 679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- Wise RJS. Language systems in normal and aphasic human subjects: functional imaging studies and inferences from animals studies. Br Med Bull. 2003;65:95–119. doi: 10.1093/bmb/65.1.95. [DOI] [PubMed] [Google Scholar]
- Zevin JD, Seidenberg MS, Bottjer SW. Limits on reacquisition of song in adult zebra finches exposed to white noise. J Neurosci. 2004;24:5849 –5862. doi: 10.1523/JNEUROSCI.1891-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.