Abstract
We report a case of a 62-year-old woman with renal cell carcinoma (RCC) presenting with a hypercalcemia-induced coma. A laboratory evaluation indicated nonparathyroid-mediated hypercalcemia with an initial serum calcium level of 18.6 mg/dL. Our patient’s parathyroid hormone (PTH)-related peptide level was undetectable. Initial imaging was negative, but PET scan identified a mass in the upper pole of the left kidney. Our patient underwent partial nephrectomy, and the mass was identified as RCC on final pathology. After surgery, her hypercalcemia resolved and PTH returned to normal limits. This case report describes a patient with RCC with the unusual presentation of hypercalcemic coma. We review the differential diagnosis of malignant hypercalcemia and the evaluation of hypercalcemia occurring with RCC. This case illustrates the need to carefully review and interpret all available data, especially when conventional testing in the work-up of hypercalcemia is unrevealing.
KEY WORDS: hypercalcemia, renal cell carcinoma, interleukin-6, parathyroid hormone, PTHrP
Introduction
Malignant hypercalcemia can occur in patients with cancer as part of a paraneoplastic syndrome. Traditionally, humoral hypercalcemia of malignancy is referred to the overproduction of parathyroid hormone-related peptide (PTHrP). However, other cytokines have been discovered to cause hypercalcemia such as interleukin-6 (IL-6), prostaglandins, and tumor necrosis factor alpha (TNF-α). This report describes an unusual case of paraneoplastic hypercalcemic coma occurring with renal cell carcinoma (RCC) and a discussion of the differential diagnosis and laboratory evaluation of this condition.
Presentation of Case
A 62-year-old white woman was admitted to an outside medical facility with profound onset of hypercalcemia causing marked mental status changes. The patient had been in good health until the day of her admission when she was found unconscious at her home. She was transported to the emergency department where she was found to have a serum calcium level of 18.6 mg/dL.
Her medical history includes chronic bronchiectasis, atrial fibrillation, duodenal ulcer, depression, urolithiasis, polymyalgia rheumatica, and maxillary sinusitis with history of sinus abscess. Her home medications included weekly rotating antibiotics for chronic sinusitis: ciprofloxacin 500 mg bid, followed by cefuroxime axetil 500 mg bid, followed by trimethoprim/sulfamethoxazole 160/800 bid, and then followed by doxycycline 100 mg bid; acetaminophen prn; amitriptyline 50 mg qhs; bumetanide 1 mg daily prn; potassium chloride 60 meq bid; spironolactone 25 mg bid; Premarin® 1.25 mg daily; digoxin 0.25 mg bid; trazodone 150 mg prn insomnia; pantoprazole 40 mg daily; docusate 100 mg TID prn constipation; gabapentin 300 mg qhs; metoprolol extended release 100 mg qhs; and prednisolone 15 mg daily.
She was treated with approximately 8 L of normal saline; once daily intravenous (IV) furosemide; six doses of subcutaneous calcitonin at 100 U every 6 hours; 20 mg of solumedrol twice daily; and 90 mg of IV pamidronate. The following morning her serum calcium was 12.9 mg/dL. By the fifth hospital day, her serum calcium was normal at 8.7 mg/dL, and her mental status normalized. Her evaluation revealed a suppressed parathyroid hormone (PTH) level of 2.7 pg/mL, undetectable PTHrP, and low 1,25-dihydroxyvitamin D (1,25(OH)2D). Her hypercalcemia was thought to be because of an underlying malignancy, and she was discharged with plans for outpatient imaging.
One month later, she presented again in an unconscious state with a serum calcium level of 11.0 mg/dL. She was treated again with IV fluids and pamidronate. She underwent a comprehensive search for malignancy, which was negative. CT scans of the head, neck, chest, abdomen, and pelvis did not identify suspicious masses. A total body bone scan and skeletal survey x-ray did not identify osteolytic lesions. An upper endoscopy and colonoscopy did not visualize malignant lesions. Mammogram and thyroid uptake scans were normal. The patient was discharged with plans for evaluation at an outside facility.
Within 2 months of her initial presentation, the patient was referred to our academic medical center for further evaluation of her hypercalcemia. She denied any history of fevers, night sweats, hematuria, flank pain, or parathyroid disease but did report mild constipation, a history of nephrolithiasis, and 20-lb unintentional weight loss within the previous 3 months.
Physical examination revealed a blood pressure of 124/64 mmHg, pulse rate of 84/minute, respiratory rate 18/minute, and temperature at 97.5°F. Head, ear, nose, and throat examination was normal without thyroid enlargement, carotid bruits, or cervical lymphadenopathy. Pulmonary and cardiovascular exams were normal. No costovertebral angle tenderness or flank masses were found on abdominal exam. Her neurological exam was normal except difficulty with concentration. Her skin had no bruises or rash.
Repeat laboratory tests included normal blood counts, electrolytes, transaminases, and alkaline phosphatase. Calcium was elevated to 12.1 mg/dL. Albumin was 3.8 g/dL. Intact PTH was 8 pg/mL (normal 10–65 pg/mL), PTHrP was undetectable, midsegment PTHrP was undetectable, 1,25(OH)2D was suppressed at 6.7 pg/mL (normal 25–66 pg/mL), and 25-hydroxy vitamin D was 52 ng/mL. Twenty-four-hour urine calcium was elevated at 400 mg (normal 100–300 mg), and phosphorus was 310 mg (normal 400–1300 mg). Calcitonin, thyrotropin, and serum protein electrophoresis were normal. The initial CT images performed at the outside facility were not available for review.
We suspected a paraneoplastic syndrome due to an occult malignancy because of her weight loss, hypercalcemia, and suppressed PTH value. Our patient underwent a PET scan to investigate for a potential malignancy, revealing reactive inflammation in subcentimeter mediastinal lymph nodes and a 2.7 × 2.3-cm exophytic mass at the superior pole of the left kidney, which was not previously reported in prior radiologic imaging. A repeat abdominal CT scan was performed and confirmed the left-sided kidney mass (Fig. 1).
Figure 1.
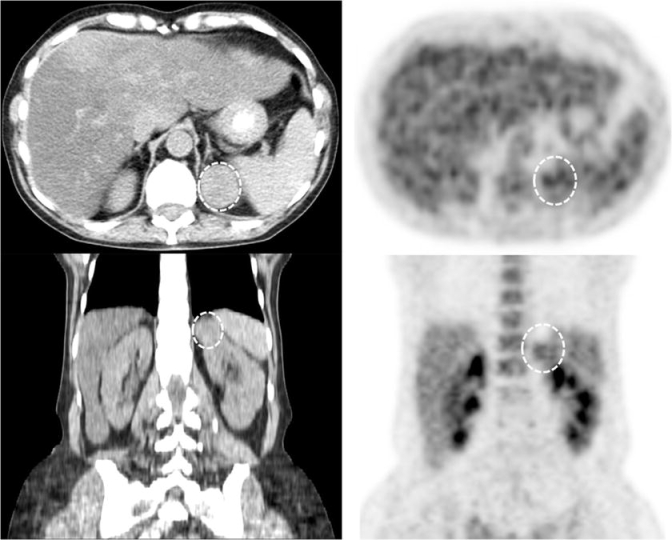
Abdominal CT and PET scan in our patient with hypercalcemia. Top left = CT axial, top right = PET axial, bottom left = CT coronal, bottom right = coronal PET, 1 = left kidney, 2 = right kidney, 3 = spleen, 4 = liver, 5 = abdominal aorta, 6 = stomach with oral contrast, 7 = thoracic vertebral body, circle = tumor. CT images demonstrate a 3.0 × 2.7-cm exophytic tumor emanating from the superior pole of the left kidney. On PET scan, fluorodeoxyglucose activity within the 2.7 × 2.3-cm tumor is equal to that of normal renal parenchyma.
She underwent a partial left nephrectomy 10 weeks after her initial presentation. Surgical pathology demonstrated a 3.9-cm RCC, clear cell type with Fuhrman’s nuclear grade 2 (Fig. 2), with staging of T1aN0M0. After surgery, she became hypocalcemic and required IV infusion of calcium. She was discharged home 3 days after surgery. Twenty-six days after surgery, she was seen in our outpatient endocrinology clinic with a serum calcium level of 10.7 mg/dL. Two months after her surgery, our patient’s serum calcium level was measured at 10.4 mg/dL with an intact PTH level of 45 pg/mL (Fig. 3). Follow-up CT scan of the abdomen revealed no recurrence of the RCC.
Figure 2.
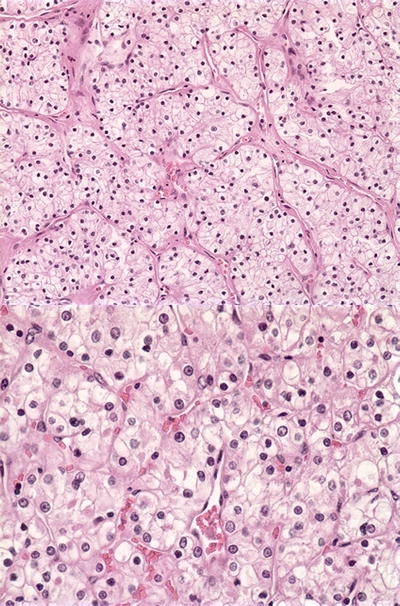
Pathology from the kidney mass. Tissue from partial nephrectomy revealed renal cell carcinoma, clear cell type, Fuhrman grade 2, magnified at ×200 (above) and ×400 (below).
Figure 3.
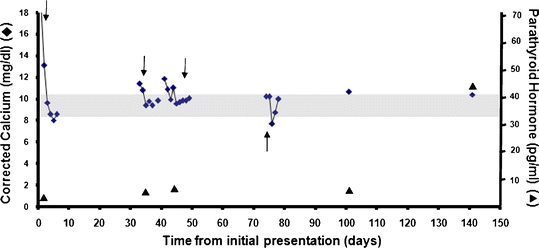
Calcium and PTH levels in our patient with renal cell carcinoma. The downward arrows represent days when a bisphosphonate was administered intravenously. The upward arrows represents the day surgery was performed.
We analyzed presurgery serum samples for TNFα and IL-6 levels by ELISA (Alpco Diagnostics, Salem, NH, USA); however, both of these cytokines were undetectable. Immunohistochemistry for IL-6 (Santa Cruz Biotechnology, Inc., Santa Cruz, Calif, USA) and PTH (BioGenex, San Ramon, Calif, USA) was performed on two separate areas of the tumor with adjacent normal kidney. Normal renal parenchyma and parathyroid adenoma served as positive controls for IL-6 and PTH, respectively. Negative controls were performed by replacing the primary antibody with buffer. The RCC was negative for both IL-6 and PTH immunohistochemistry. TNFα and PTHrP stains were not available.
Discussion
Renal cell carcinoma accounts for 2% of cancers worldwide.1 The incidence of RCC has risen over the past several decades, due largely to incidental detection by imaging modalities such as CT scan, ultrasound, and MRI.2 Nearly 50% of cases are identified incidentally by imaging,3 and accuracy of diagnosis with CT scan is greater than 95%.4 Given this high level of accuracy and the size of her tumor, we must attribute our patient’s initial negative CT scan to human error. When we finally obtained the original images, the tumor was, in fact, present. Detection of unknown primary tumors ranges from 33% to 57% with PET-CT,5–7 but there is a paucity of data on the use of PET-CT in paraneoplastic syndromes.
The most common clinical presentations of RCC are hematuria (50% to 60% of patients), abdominal pain (40%), and a palpable abdominal mass (30%); this classic triad of symptoms is present in less than 10% of cases.8 Paraneoplastic manifestations of RCC, including hypercalcemia, polycythemia, hepatic dysfunction, amyloidosis, fever, and weight loss, are present in up to 20% of patients.3,8,9 Unusual presentations of RCC include pancreatitis,10 life-threatening polyneuropathy,11 gastrointestinal bleeding,12 and polymyalgia rheumatica,13 but hypercalcemia-induced coma has not been reported.
In retrospective studies to determine the etiology of hypercalcemia in all patients, 28–36% of cases are attributed to malignancy.14,15 Of patients with known malignancy who develop hypercalcemia, the most common malignancies are lung and kidney.15,16 Hypercalcemia is the most common paraneoplastic complication of RCC, and approximately 17% of all patients with RCC develop hypercalcemia during the course of their disease.17–19 Albright20 first reported hypercalcemia occurring in a patient with RCC in 1941. He postulated that the hypercalcemia was because of a humoral factor secreted by the tumor. Later reports confirmed that a protein was secreted from RCC, which could increase urinary cyclic adenosine monophosphate levels similar to PTH.21,22 In the late 1980s, the substance was identified and named PTHrP.23
Parathyroid hormone-related peptide is present in several isoforms. The N-terminal sequence is identical in 8 of the first 13 amino acids compared to PTH and binds to the PTH/PTHrP receptor.24 Parathyroid hormone-related peptide undergoes posttranscriptional modification, dividing itself into the N-terminal fragment, midsection, and C-terminal fragment.25 The C-terminal fragment is cleared by the kidney and is present in higher serum concentrations, particularly in kidney failure. There is some evidence that the C-terminal fragment inhibits RCC growth, whereas the N-peptide stimulates growth.24,26 In addition, the C-peptide plays an inhibitory role in calcium metabolism.26
It is reported that PTHrP levels are elevated in up to 47% of patients with malignancy and hypercalcemia.27 Parathyroid hormone-related peptide is elevated in up to 15% of all patients with RCC,19 but it is also expressed in normal tissue.28 This peptide binds to the common PTH/PTHrP receptor to cause increased resorption of bone and increased renal calcium absorption.29–31
Other humoral factors that have been associated with hypercalcemia in RCC include IL-6, IL-1, TNFα, and transforming growth factor alpha and beta.32,33 Many studies have attempted to determine the exact role of IL-6 in hypercalcemia of RCC. Interleukin-6 has been shown to activate osteoclastic bone resorption, and it acts synergistically when coexpressed with PTHrP. Some studies suggest that IL-6 stimulates tumor growth.34,35 It is not clear whether this cytokine causes hypercalcemia either directly or indirectly by increasing the effect of PTHrP on bone resorption.36–38 At this time, IL-6-mediated hypercalcemia appears to be multifactorial.
Increased prostaglandins have been reported as a cause for hypercalcemia in patients with RCC by stimulating bone resorption.39,40 In these cases, nonsteroidal inflammatory drugs may be effective in reducing calcium by inhibiting prostaglandin production by the tumor.41,42 Our patient was on chronic steroids for treatment of polymyalgia rheumatica, therefore prostaglandin-mediated hypercalcemia is unlikely in this case.
Medications are also a common cause of hypercalcemia. After review of our patient’s medications, we found that Premarin has rarely been associated with hypo- and hypercalcemia. However, our patient had been on this medication for many years with normal calcium levels, and none of her other medications are commonly associated with hypercalcemia. Other causes of hypercalcemia occurring in RCC, which were ruled out in our patient, include lytic bone lesions43 and ectopic PTH secretion.44 The presence of hypercalcemia in metastatic RCC portends a poor prognosis,19 but this has not been proven in localized disease. The most reliable prognostic indicator is the tumor-node-metastasis staging guidelines.45
Treatment of hypercalcemia associated with RCC includes restoration of volume status, saline diuresis, and IV bisphosphonates such as zoledronate or pamidronate.46 Bisphosphonates are the cornerstone treatment in malignant hypercalcemia as they inhibit osteoclastic bone resorption, alleviate bone pain, and may prevent growth of bony metastatic lesions.47 Oral bisphosphonates have poor absorption and are not used first line for treatment of acute hypercalcemia.48 Approximately 50% of patients who undergo nephrectomy and/or tumor debulking will revert to normocalcemia.43
Conclusions
Hypercalcemia is a frequent occurrence in patients with RCC, but it is an unusual presenting symptom. The initial evaluation should focus on whether the hypercalcemia is parathyroid-dependent or medication-related. Basic laboratory investigations for hypercalcemia include PTH, PTHrP, and 1,25(OH)2D. Bone-resorbing cytokines may be present in those patients in which the cause of the hypercalcemia is unclear. In our case, we could not determine the specific cytokine that caused the hypercalcemia, as IL-6 and TNF were below the limit of detection by commercially available ELISA kits. If laboratory tests are unrevealing, bone scan, CT scan, or PET-CT should be considered to evaluate for lytic bone lesions or underlying malignancy. Evaluation for carcinoma should be persistently pursued in a patient who has symptoms suggestive of paraneoplastic hypercalcemia. Finally, IV fluids and bisphosphonates are often helpful to control the hypercalcemia. Nephrectomy will improve hypercalcemia in many patients with RCC.
Acknowledgments
The authors acknowledge Dr. John Anderson and Dr. Kenneth Ogan in the preparation of this case report. Research funding was provided by the Atlanta Research and Education Foundation, Atlanta Veterans Administratrion Medical Center, and the UV Foundation.
Conflict of interest statement Dr. Tangpricha has received grant funding from the UV Foundation, Atlanta Research and Education Foundation, National Institutes of Health, and Proctor & Gamble. He has received speaker honoraria from Merck and Abbott. The remaining authors declare no conflicts of interest in the preparation of this manuscript.
Footnotes
Supported by the Atlanta Research and Education Foundation, Atlanta Veterans Administration Medical Center, and UV Foundation
References
- 1.McLaughlin J, Lipworth L, Tarone R. Epidemiologic aspects of renal cell carcinoma. Semin Oncol. 2006;33(5):527–33. [DOI] [PubMed]
- 2.Chow W, Devesa S, Warren J, et al. Rising incidence of renal cell cancer in the United States. JAMA. 1999;281(17):1628–31. [DOI] [PubMed]
- 3.Cohen H, McGovern F. Renal-cell carcinoma. N Engl J Med. 2005;353(23)2477–90. [DOI] [PubMed]
- 4.Zagoria R, Dyer R, Wolfman N, Hinn G, Chen Y. Radiology in the diagnosis and staging of renal cell carcinoma. Crit Rev Diagn Imaging. 1990;31(1):81–115. [PubMed]
- 5.Gutzeit A, Antoch G, Kuhl H, et al. Unknown primary tumors: detection with dual-modality PET/CT—initial experience. Radiology. 2005;234(1):227–34. [DOI] [PubMed]
- 6.Nanni C, Rubello D, Castellucci P, et al. Role of 18F-FDG PET-CT imaging for the detection of an unknown primary tumor: preliminary results in 21 patients. Eur J Nucl Med Mol Imaging. 2005;32(5):589–92. [DOI] [PubMed]
- 7.Ambrosini V, Nanni C, Rubello D, et al. (18)F-FDG PET/CT in the assessment of carcinoma of unknown primary origin. Radiol Med (Torino). 2006;111(8):1146–55. [DOI] [PubMed]
- 8.Motzer R, Bander N, Nanus D. Renal-cell carcinoma. N Engl J Med. 1996;335(12):865. [DOI] [PubMed]
- 9.Gold P, Fefer A, Thompson J. Paraneoplastic manifestations of renal cell carcinoma. Semin Urol Oncol. 1996;14(4):216–22. [PubMed]
- 10.Nabi G, Chowdhary A. Renal cell carcinoma presenting as acute pancreatitis. Urol Int. 2002;68(3):202–3. [DOI] [PubMed]
- 11.Roy M, May E, Jabbari B. Life-threatening polyneuropathy heralding renal cell carcinoma. Mil Med. 2002:167(12):986–9. [PubMed]
- 12.Klasuner J, Rozin R, Lelcuck S, Ilie B. Renal cell carcinoma presenting as acute pancreatitis and GI bleeding. J Surg Oncol. 1983;22(4):265–8. [DOI] [PubMed]
- 13.Sidhom O, Basalaevm, Sigal L. Renal cell carcinoma presenting as polymyalgia rheumatica. Resolution after nephrectomy. Arch Intern Med. 1993;153(17):2043–5. [PubMed]
- 14.Newman E, Bouvet M, Borgehi S, Herold D, Deftos L. Causes of hypercalcemia in a population of military veterans in the United States. Endocr Pract. 2006;12(5):535–41. [DOI] [PubMed]
- 15.Lee C, Yang C, Lam K, et al. Hypercalcemia in the emergency department. Am J Med Sci. 2006;331(3):119–23. [DOI] [PubMed]
- 16.Vassilopoulou-Sellin R, Newman B, Taylor S, Guinee V. Incidence of hypercalcemia in patients with malignancy referred to a comprehensive cancer center. Cancer. 1993;71(4):1309–12. [DOI] [PubMed]
- 17.Chasan S, Pothel L, Huben R. Management and prognostic significance of hypercalcemia in renal cell carcinoma. Urology. 1989;33(3):167–70. [DOI] [PubMed]
- 18.Fahn H, Lee Y, Chen M, Huang J, Chen K, Chang L. The incidence and prognostic significance of humoral hypercalcemia in renal cell carcinoma. J Urol. 1991;145(2):248–50. [DOI] [PubMed]
- 19.Papworth K, Grankvist K, Ljungberg B, Rasmuson T. Parathyroid hormone-related protein and serum calcium in patient with renal cell carcinoma. Tumour Biol. 2005;2(4):201–6. [DOI] [PubMed]
- 20.Albright F. Case records of the Massachusetts General Hospital. N Engl J Med. 1941;225:789–91.
- 21.Powell D, McPartlin J, Skrabanek P. Humoral hypercalcemia in a patient with renal-cell carcinoma. Eur J Clin Invest. 1978;8(6):425–6. [DOI] [PubMed]
- 22.Gottleib S, Rude R, Sharp C, Singer F. Humoral hypercalcemia of malignancy: a syndrome in search of a hormone. Am J Med. 1982;73(5):751–5. [DOI] [PubMed]
- 23.Suva L, Winslow G, Wettenhall R, et al. A parathyroid hormone-related protein implicated in malignant hypercalcemia: cloning and expression. Science. 1987;237(4817):893–6. [DOI] [PubMed]
- 24.Iwamura M, Wu W, Maramoto M, et al. Parathyroid hormone-related protein is an independent prognostic factor for renal cell carcinoma. Cancer. 2000;86(6):1028–34. [DOI] [PubMed]
- 25.Soifer N, Insogna K, Brutis W, et al. Parathyroid hormone-related protein. Evidence for secretion of a novel mid-region fragment by three different cell types. J Biol Chem. 1992;267(25):18236–43. [PubMed]
- 26.D’Amour P. Circulating PTH molecular forms: what we know and what we don’t. Kidney Int Suppl. 2006;(102):S29–33. [DOI] [PubMed]
- 27.Kao P, Klee G, Taylor R, Heath H. Parathyroid hormone-related peptide in plasma of patients with hypercalcemia and malignant lesions. Mayo Clin Proc. 1990;65(11):1399–407. [DOI] [PubMed]
- 28.Frankin H, Epstein M, Strewler G. The physiology of parathyroid hormone-related protein. N Engl J Med. 2000;342:177–85. [DOI] [PubMed]
- 29.Stewart A, Vignery A, Silvergate A. Quantitative bone histomorphometry in humoral hypercalcemia of malignancy: uncoupling of bone cell activity. J Clin Endocrinol Metab. 1982;55:219–27. [DOI] [PubMed]
- 30.Horwitz M, Tedesco M, Sereika S, Hollis B, Garcia-Ocaña A, Stewart A. Direct comparison of sustained infusion of human parathyroid hormone-related protein-(1-36) [hPTHrP-(1-36)] versus hPTH-(1-34) on serum calcium, plasma 1,25-dihydroxyvitamin D concentrations, and fractional calcium excretion in healthy human volunteers. J Clin Endocrinol Metab. 2003;88:1603–9. [DOI] [PubMed]
- 31.Onuma E, Azuma Y, Saito H, et al. Increased renal calcium reabsorption by parathyroid hormone-related protein is a causative factor in the development of humoral hypercalcemia of malignancy refractory to osteoclastic bone resorption inhibitors. Clin Cancer Res. 2005;11(11):4198–203. [DOI] [PubMed]
- 32.Takahashi S, Hakuta M, Aiba K, et al. Elevation of circulating plasma cytokines in cancer patients with high plasma parathyroid hormone-related protein levels. Endocr Relat Cancer. 2003;10(3):403–7. [DOI] [PubMed]
- 33.Palapattu G, Kristo B, Rajfer J. Paraneoplastic syndromes in urologic malignancy: the many faces of renal cell carcinoma. Rev Urol. 2002;4(4):163–70. [PMC free article] [PubMed]
- 34.Paule B. Interleukin-6 and bone metastasis of renal cancer: molecular bases and therapeutic implications. Prog Urol. 2001;11(2):368–75. [PubMed]
- 35.Weissglas M, Schamhart D, Papapoulos S, Theuns H, Kurth K. The role of interleukin-6 in the induction of hypercalcemia in renal cell carcinoma transplanted into nude mice. Endocrinology. 1997;138(5):1879–85. [DOI] [PubMed]
- 36.Greenwald RA, Stein B, Miller F. Rapid skeletal turnover and hypercalcemia associated with markedly elevated interleukin-6 levels in a young black man. Bone. 1998;22(3):285–8. [DOI] [PubMed]
- 37.Ueno M, Ban S, Nakanoma T, et al. Hypercalcemia in a patient with renal cell carcinoma producing parathyroid hormone-related protein and interleukin-6. Int J Urol. 2000;7(6):239–42. [DOI] [PubMed]
- 38.Motellon J, Jimenez F, de Miguel F, et al. Relationship of plasma bone cytokines with hypercalcemia in cancer patients. Clin Chim Acta. 2000;302(1–2):59–68. [DOI] [PubMed]
- 39.Robertson R, Baylink D, Marini B, et al. Elevated prostaglandins and suppressed parathyroid hormone associated with hypercalcemia and renal cell carcinoma. J Clin Endocrinol Metab. 1975;41(1):164–7. [DOI] [PubMed]
- 40.Klein D, Raisz L. Prostaglandins: stimulation of bone resorption in tissue culture. Endocrinology. 1970;86:1436–40. [DOI] [PubMed]
- 41.Brereton H, Halushka P, Alexander R, et al. Indomethacin-responsive hypercalcemia in a patient with renal-cell adenocarcinoma. N Engl J Med. 1974;291(2):83–5. [DOI] [PubMed]
- 42.Cummings K, Robertson R. Prostaglandin: increased production by renal cell carcinoma. J Urol. 1977;118(5):720–3. [DOI] [PubMed]
- 43.Walther M, Alexander R, Weiss G, et al. Cytoreductive surgery prior to interleukin-2-based therapy in patients with metastatic renal cell carcinoma. Urology. 1993;42(3):250–7. [DOI] [PubMed]
- 44.Goldberg R, Pilcher D, Yates J. The aggressive surgical management of hypercalcemia due to ectopic parathormone production. Cancer. 1980;45(10):2652–4. [DOI] [PubMed]
- 45.Kirkail Z, Lekili M. Renal cell carcinoma: new prognostic factors? Curr Opin Urol. 2003;13(6):433–8. [DOI] [PubMed]
- 46.Stewart AF. Clinical practice. Hypercalcemia associated with cancer. N Engl J Med. 2005;352(4):373–9. [DOI] [PubMed]
- 47.Endo I, Inoue D. Treatment of malignancy-associated hypercalcemia. Clin Calcium. 2006;16(4)133–7. [PubMed]
- 48.Fogelman I, Smith L, Mazess R, et al. Absorption of oral bisphosphonate in normal subjects. Clin Endocrinol. 1986;24(1):57–62. [DOI] [PubMed]


