Abstract
Recent reports of slow tonic myosin heavy chain (MHCst) in human masticatory and laryngeal muscles suggest that MHCst may have a wider distribution in humans than previously thought. Because of the novelty of this finding, we sought to confirm the presence of MHCst in human masticatory and laryngeal muscles by reacting tissue from these muscles and controls from extraocular, intrafusal, cardiac, appendicular and developmental muscle with antibodies (Abs) ALD-58 and S46 considered highly specific for MHCst. At Ab dilutions producing minimal reaction to muscle fibers positive for MHCI, only extraocular, intrafusal and fetal tongue tissue reacted with Ab S46 had strong immunoreaction in an appreciable number of muscle fibers. In immunoblots Ab S46, but not Ab ALD-58, labeled adult extraocular muscles; no other muscles were labeled with either Ab. We conclude that, in humans, Ab S46 has greater specificity for MHCst than does Ab ALD-58. We suggest that reports of MHCst in human masticatory and laryngeal muscles reflect false-positive identification of MHCst due to cross-reactivity of Ab ALD-58 with another MHC isoform.
Keywords: Slow tonic, myosin heavy chain, muscle, antibody, mastication
INTRODUCTION
Slow tonic muscle fibers are distinguished by a number of anatomical, molecular and physiological features including multiple innervation, the expression of a unique slow tonic myosin heavy chain (MHCst) and the lack of muscle potentials and/or capacity for tonic contraction2,5,6,13,19.20. In birds and mammals, immunohistochemical studies with isoform-specific antibodies (Abs) demonstrate MHCst in muscle spindles (MS)18, the chick anterior latissimus dorsii muscle (ALD)32 and mammal extraocular muscles (EOM)14,25. In both ALD and EOM, muscle fibers with a slow tonic contractile profile are present5,27 and presumptive slow tonic fibers are distinguished by the presence of punctate motor endplates (MEPs), multiple MEPs along muscle fiber length and/or innervation from multiple motoneurons13,24,29. Recently, MHCst has been reported in many muscle fibers in human anterior digastric (AD), mylohyoid (MH) and thyroarytenoid vocalis (TAV)11,23,33, but several factors leave uncertainty as to the presence and/or extent of MHCst in these muscles. First, evidence for MHCst in these muscles is primarily based on reaction with Ab ALD-58 which has a high affinity for MHCst but also reacts with MHCI and MHCcardiacα11,30. Second, other studies specifically seeking MHCst have not found evidence for an appreciable population of slow tonic fibers in the human TAV4,10. Third, with the exception of the multiple MEPs on some TAV fibers26,28, none of these muscles are known to exhibit innervation features typical of slow tonic muscle fibers. Immunoreactivity is a subjective measure, the interpretation of which can be influenced by the intensity of local staining or background. To confirm the presence of MHCst in cranial muscles, we reacted human laryngeal, lingual and masticatory muscles in the presence of positive (EOM and MS) and negative (extrafusal biceps brachii and medial gastrocnemius) controls with Abs S46 and ALD-58, Abs thought to be highly specific for MHCst. At Ab concentrations producing minimal reaction to muscle fibers positive for MHCI and MHCcardiacα, only tissue sections of EOM, MS and fetal tongue had strong immunoreaction in an appreciable number of muscle fibers. We suggest that reports of MHCst in human anterior digastric, mylohyoid, and thyroarytenoid vocalis reflect false-positive identification of MHCst due to cross-reactivity of anti-MHCst Ab with another MHC isoform.
MATERIALS AND METHODS
Subjects and Tissue Preparation
Tissue from the following muscles was obtained within 10 hours post-mortem from an 86yo female (H1) and/or a 63yo female (H2) (Table 1): anterior digastric (AD), biceps brachii (BB), genioglossus (GG), geniohyoid (GH), hyoglossus, (HG), medial rectus (MR), medial gastrocnemius (MG), mylohyoid (MH), styloglossus (SG), superior longitudinalis (SL), superior oblique (SO), superior rectus (SR), thyroarytenoid vocalis (TAV), thyroarytenoid muscularis (TAM), transversus (T) and verticalis (V) (Emory University School of Medicine Body Donor Program). Additional tissue for control studies was obtained within 12 hours post-mortem from the anterior latissimus dorsus (ALD) of a chick, the ventricle and atrium of an adult rhesus macaque (Yerkes Regional Primate Center) and two human fetal tongues (FT1 and FT2; National Disease Research Interchange). Tissue samples were quick-frozen in isopentane cooled by liquid nitrogen, and stored at -80°C. For each muscle sample, serial 12 μm thick cross-sections were cut on a cryostat at -23°C and mounted onto gelatin-subbed slides. To insure uniformity in the assessment of immunoreactivity, all samples were reacted with a positive control (EOM or EOM and MS) and a negative control (BB or MG extrafusal muscle fibers) on the same slide.
Table 1.
Human Tissue Samples for Immunohistochemistry
| Muscle | Subject |
|---|---|
| Extraocular Muscles: | |
| Medial Rectus | H1* |
| Superior Oblique | H1 |
| Superior Rectus | H1*, H2 |
| Laryngeal Muscles: | |
| Thyroarytenoid muscularis | H1, H2 |
| Thyroarytenoid vocalis | H1*, H2 |
| Lingual Muscles: | |
| Fetal Tongue | FT1, FT2 |
| Genioglossus | H1, H2 |
| Hyoglossus | H1, H2 |
| Styloglossus | H1, H2 |
| Superior Longitudinalis | H1, H2 |
| Transversus | H1, H2 |
| Verticalis | H1, H2 |
| Muscles of Mastication: | |
| Anterior Digastric | H1*, H2 |
| Mylohyoid | H2 |
| Post-cranial Muscles: | |
| Biceps Brachii | H1*, H2 |
| Medial Gastrocnemius | H1 |
| Suprahyoid Muscles: | |
| Geniohyoid | H1 |
tissue also used for immunoblots.
Immunohistochemical Methods
Serial sections of each muscle were reacted with the following Abs: Ab ALD-58 (Developmental Studies Hybridoma Bank, DSHB) which in humans is considered specific for MHCst23 or reactive with MHCst, MHCcardiacα and MHCI11,30; Ab A4.84 (DSHB) and/or Ab BA-D5 (ATCC) which in humans are specific for MHCI(cardiacβ)17,18,21; Ab S46 (gift of Dr. Frank Stockdale, also DSHB) which is considered specific for avian SM-1 and SM-2 MHC isoforms22,32 and reacts with MHCst-positive intrafusal fibers in the rat MHCst16; and Ab MY-32 (Sigma) which is specific for MHCII isoforms in humans12. Fetal tissue was additionally reacted with Ab F1.625 (DSHB) which in mammals is specific for MHCembryonic7 and Ab NCL-MHCn (Vector) which in humans is specific for MHCneonatal9. Ab MY-32 was reacted at dilution of 1:400, Ab BA-D5 was reacted at dilution of 1:30, and Abs ALD-58 and S46 were reacted at dilutions of 1:5 (strong dilution) and 1:25 (weak dilution) to determine the optimal solution for label of MHCst but not MHCI. All other Abs were reacted at dilution of 1:5. In some reactions, the anti-laminin Ab D18 (DSHB, dilution 1:25) was added to assist in muscle fiber and muscle spindle identification. Tissue was reacted following the protocol of Eason et al8. Briefly, tissue sections were incubated in a blocking solution composed of 2% normal goat serum, 0.03% Triton-X, and 0.1M Tris-HCl (T-NGS) at room temperature for one hour, followed by incubation overnight with primary antibody in blocking solution in a humid chamber at -4 degrees. Tissue was then washed in Tris-HCl buffer and incubated with secondary antibody (peroxidase-conjugated goat IGG fraction to mouse immunoglobulins, Capel; dilution 1:100) for 1 hour at room temperature. A standard DAB reaction was used to visualize label (0.5 mg DAB/mL, 0.1 M PBS, 0.03% H2O2). Slides were then washed with H20 for 1 minute, dehydrated and coverslipped in permount.
Tissue sections were viewed on an Olympus BX51 microscope at 100x, 200x and 400x magnification. Images were collected with Neurolucida software (Microbrightfield, Burlington, VT) and a MicroFire Digital Microscope Camera (Optronics, Goleta, CA) and stored onto computer (Dell Optiplex GX270, 1280 × 1024 pixel resolution). To facilitate analysis, muscle fibers positive for Ab A4.84 were classified as phenotype “slow”, muscle fibers negative for Ab A4.84 and positive for Ab MY-32 were classified as phenotype “fast” and muscle fibers strongly reactive for both Ab S46 and Ab ALD-58 in EOM and muscle spindles (MS, in biceps brachii) were classified as phenotype ‘slow tonic’ (Figure 1). For each subject, reaction of fibers with each anti-MHCst Ab was scored as “strong,” “moderate”, or “absent.” The threshold intensity to identify a fiber as “strong” was determined from the strong label of slow tonic fibers in EOM and MS at 1:5 dilution and as “absent” was determined from the negative reaction of fast fibers at 1:25 dilution in extrafusal BB and MG control tissue. This was necessary because Ab ALD-58, particularly at higher concentration, produced variegated staining intensities that did not appear to correlate with reports by other investigators, and specific reference to known positive and negative control muscles was expected to reveal any non-specific or cross reactivity.
Figure 1.
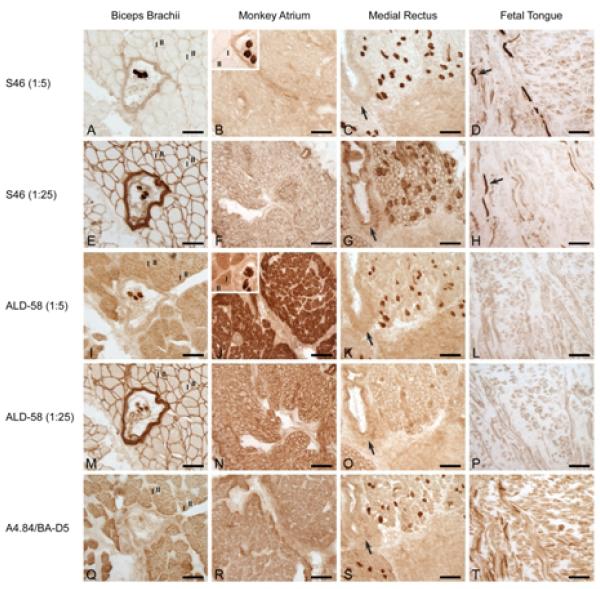
Serial sections from the adult human biceps brachii, monkey atrium, adult human medial rectus and human fetal tongue illustrating immunoreaction with Ab S46 at 1:5 dilution (A-D), Ab S46 at 1:25 dilution (E-H), Ab ALD-58 at 1:5 dilution (I-L), Ab ALD-58 at 1:25 dilution (M-P) and Abs A4.84 or BA-D5 (Q-T). At both dilutions, Ab S46 stongly labels intrafusal (muscle spindle) fibers in biceps brachii (A,E) and a subset of muscle fibers in the medial rectus (C,G) and fetal tongue (D,H, arrows), but does not label extrafusal fibers in biceps brachii or muscle fibers in monkey atrium. Inset in B shows tissue from biceps brachii reacted on same slide and photographed under the same optical conditions as monkey atrium in B; note the strong label of intrafusal but not extrafusal or atrial fibers. At strong dilution Ab ALD-58 strongly labels intrafusal fibers in a muscle spindle in biceps brachii (I), a subset of fibers in the medial rectus (K) and fibers in the monkey atrium (J). At strong dilution Ab ALD-58 moderately labels ‘slow’ fibers in biceps brachi (I, also inset in J). Inset in J shows tissue from biceps brachii reacted on the same slide and photographed under the same optical conditions as monkey atrium in J; note the strong label of both intrafusal and atrial fibers and the moderate label of extrafusal slow (“I”) fibers. At weak dilution, Ab ALD-58 does not label slow fibers, and either does not label, moderately labels or strongly labels slow tonic fibers in muscle spindle (M) and medial rectus (O). “I” denotes fibers positive for MHCI (labeled with Ab A4.84 or Ab BA-D5). “II” denotes fibers negative for MHCI and positive for MHCII (labeled with Ab MY-32, data not shown). Arrows in C, G, K. O, and S indicate same region in sections. Arrows in D and H indicate slow tonic fibers in human fetal tongue. For E, F, G, M, N and O, reaction solution also included the anti-laminin Ab D-18 to enable visualization of muscle fiber perimeters. Calibration bar = 100μm.
Electrophoretic Immunoblotting
Tissue for imunoblots was obtained from chick ALD and from the same tissue blocks used for immunohistochemical study of AD, BB, MR, SR and TAV in HT1. Approximately 15-50 milligrams of muscle tissue was placed in 2 ml of non-denaturing lysis buffer (50 mM Tris, 150 mM NaCl, 5 mM EDTA, 1% Triton X-100, with 0.02% NaN3 and 0.1% protease inhibitors (Sigma) and homogenized for 30 seconds with a Tissuemiser at 15,000 rpm in an ice bath and cleared by centrifugation at 6,000 xg for 5 minutes. Protein content was assayed by bicinchronic acid assay (BCA, Pierce) according to the manufacturer’s instructions. Protein was separated by SDS-page through a 7.5% acrylamide resolving gel at 25 mA for 90 minutes. To insure detection of MHCst, even if expressed at moderate levels, all samples were overloaded (9.75-11.40 μgrams/lane) with the exception of chick ald (0.2 μgrams/lane). Migration of MHC was determined by running a 204 kDa molecular weight standard (Bio-Rad Kaleidoscope Prestained Standards) on each gel. Gels were transferred to a nitrocellulose membrane using a wet transfer system. Membranes were blocked for 60 minutes in 5% non-fat dry milk in TTBS prior to incubation with primary Abs ALD-58, S46 or A4.84 (1:500 dilution). Well rinsed membranes were conjugated with secondary HRP-conjugated goat anti-mouse IgG (1:2,000 dilution), detected by enhanced chemiluminescence (ECL, Amersham), and visualized by exposure to radiographic film.
RESULTS
Tests of Ab S46 and Ab ALD-58 Working Dilutions for Immunohistochemistry
Because Ab ALD-58 is known to cross-react with MHCI11,30, we compared reaction of MR, SO, SR and MS (positive controls) and BB and MG (negative controls) to strong (1:5) and weak (1:25) dilutions of Ab S46 and Ab ALD-58 to determine optimal dilutions for label of MHCst but not MHCI. At strong and weak dilutions, Ab S46 did not label slow fibers in BB and MG but strongly labeled slow tonic fibers in MR, SO, SR and MS (Figure 1). At strong dilution, Ab ALD-58 moderately to strongly labeled slow fibers in BB and MG and strongly labeled slow tonic fibers in MR, SO, SR and MS (Figure 1). At weak dilution Ab ALD-58 did not label slow fibers, and either did not label, moderately labeled or strongly labeled slow tonic fibers in EOM and MS. Thus only Ab S46 provided consistently specific and strong label of MHCst in these muscles.
Tests of Ab S46 and Ab ALD-58 Cross-Reactivity with MHCcardiacα, MHCembryonic, MHCneonatal and MHCeom
To control for possible false-positive identification of other MHC isoforms, we tested immunoreactivity of Ab S46 and Ab ALD-58 at strong and weak dilutions in human EOM (positive control for MHCeom14), in human fetal tongue (positive control for MHCemb and MHCneo verified by immunoreactivity for Abs F1.625 and NCL-MHCn, data not shown) and in monkey atrium and ventricle (positive control for MHCcardiacα and MHCI). These tests demonstrated strong reactivity of Ab ALD-58 for atrium and ventricle (Figure 1) and no or minimal reactivity with other isoforms known to be expressed in these muscles (i.e., MHCemb, MHCeom, MHCneo). In contrast, Ab S46 had no or minimal reactivity in all muscles with the exception of fetal human tongue in which a subset of slow fibers was strongly labeled (Figure 1).
Immunoreaction of Ab S46 to AD, GG, GH, HG, MH, SG, SL, T, TAM, TAV and V
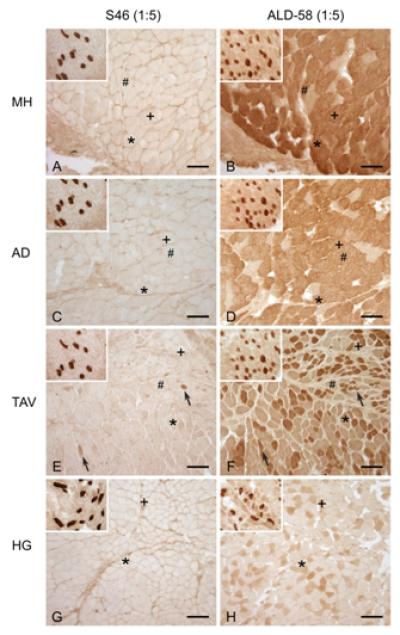
We tested for the presence of MHCst in adult human cranial muscles with Ab S46 because of the high specificity of this Ab for MHCst demonstrated in control experiments. No fibers were labeled in any of these muscles with the exception of a few isolated fibers in AD, GG, MH, T, V, TAV (1-20 fibers/muscle) and in SL and TAM (20-70 fibers/muscle) (Figure 2; reaction with Ab ALD-58 shown for comparison).
Figure 2.
Serial sections of the adult human mylohyoid (A,B), adult human anterior digastric (C,D), adult human thyroarytenoid vocalis (E,F) and adult human hyoglossus (G,H) reacted with 1:5 dilutions of Ab S46 (A,C,E,G) and Ab ALD-58 (B,D,F,H). Inset in each figure shows medial rectus tissue reacted on the same slide and photographed under the same optical conditions (as a positive control for MHCst). Ab S46 strongly labels a subset of fibers in the medial rectus but labels few or no fibers in the other muscles (isolated labeled fibers marked with arrows in Figure E). Ab ALD-58 strongly labels a subset of fibers in the medial rectus and moderately to strongly labels slow muscle fibers in all other muscles. Symbols “#”, “+” and “*” identify the same fibers in sections. Calibration bar = 100μm.
Immunoblots of Chick ALD and Human AD, BB, MR, SR and TAV with Ab S46 and Ab ALD-58
Ab S46 labeled human MR, SR and chick ALD but did not label other muscles tested. Ab ALD-58 labeled chick ALD, but did not label other muscles tested (Figure 3).
Figure 3.
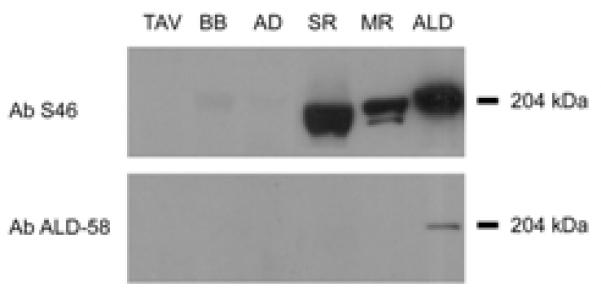
Immunoblots of human thyroarytenoid vocalis (TAV), biceps brachi (BB), anterior digastric (AD), superior rectus (SR), medial rectus (MR) and chick anterior latissimus dorsi muscle (ALD) reacted with Ab S46 (top panel) and with Ab ALD-58 (bottom panel). Equivalent amounts of whole muscle protein were loaded for each human muscle tested. Note the strong reaction of Ab S46 to ALD and to the extraocular muscles SR and MR, the absence of Ab S46 reaction to TAV, BB and AD and the weak reaction of Ab ALD-58 to ALD only.
DISCUSSION
Summary of Data
The primary finding of this study is that muscle fibers expressing MHCst are present in human extraocular muscles, muscle spindles and fetal tongue but are absent in appreciable numbers in all other tested muscles, including AD, MH and TA. This observation differs substantially from previous work indicating robust expression of MHCst in AD, MH and TA based on immunolabeling with Ab ALD-58. Based on extensive observation of these muscles, in comparison with both positive and negative control tissue, we also conclude that Ab ALD-58 reacts with human MHCI and/or MHCcardiacα and should not be considered a specific indicator of MHCst in human muscle. To our knowledge, this study is the first to demonstrate the presence of MHCst in the human fetal tongue and the absence of MHCst in the AD and MH.
Comparison to Other Studies
EOM and MS
Immunohistochemical studies using different Abs have demonstrated MHCst in human EOM and MS2,3,4,10,14,18,25. In the present study both Ab S46 and Ab ALD-58 (at high concentration only) strongly labeled fibers in human EOM and MS which we thus classify as MHCst-positive. Greater immunohistochemical specificity of Ab S46 for MHCst was demonstrated by the strong label of slow tonic fibers at both strong and weak Ab dilutions and the absence of label in negative control muscles; in contrast, consistently strong label of EOM and MS fibers with Ab ALD-58 was only achieved at high antibody concentrations that also produced moderate to strong label of atrial tissue and MHCI fibers in limb muscles. Specificity of Ab S46 for MHCst was further demonstrated in immunoblots: Ab S46 labeled chick ALD more strongly than Ab ALD-58 and only Ab S46 labeled human EOM (Figure 3). To our knowledge, this is the first positive immunoblot of human EOM MHCst.
AD, MH, TAM and TAV
Primarily on the strength of immunohistochemical and immunoblot studies with Ab ALD-58, MHCst has recently been reported to be present in a large number of fibers in the human AD, MH and TAV (from 15.5%-44% in regions of the AD and MH23,33). We therefore compared reaction of Ab S46 and Ab ALD-58 to AD, MH, TAM, TAV, positive control tissue (EOM, MS) and negative control tissue (BB, MG). Ab S46 did not label negative control tissue and did not label more than occasional fibers in AD, MH, TAM and TAV (see4 for similar finding in TA). In contrast, at concentrations likely similar to those used in prior studies (undiluted supernatant in23,33), Ab ALD-58 moderately to strongly labeled atrial tissue and slow fibers in all muscles including AD, MH, TAM and TAV. When Ab ALD-58 was reacted at dilution that did not label slow fibers in negative control muscles, no fibers were labeled in AD, MH, TAM and TAV. In immunoblots Ab S46 strongly labeled EOM but did not label equivalent amounts of AD, BB and TAV protein. From these data we conclude that AD, MH, and TAV do not express MHCst in more than a few fibers. We attribute prior immunoblot identification of MHCst in AD, MH and TAV to the large amount of protein (10-40μg/lane) run in these studies11,23,33 and the cross-reactivity of Ab ALD-58 with MHCI and/or MHCcardiacα, either or both of which are present in these muscles11,15,23,33.
GG, GH, HG, SL, SG, T, V
Immunoreaction of GH and of muscles in the adult tongue with Ab S46 did not label any fibers with the exception of occasional, isolated fibers in GG, SL, T and V (reaction for HG shown in Figure 2). To our knowledge this is the first investigation of MHCst in adult human tongue muscles or geniohyoid. Thus, despite the possible importance of maintaining tongue posture and shape in many oromotor behaviors, we do not find evidence for appreciable MHCst in adult human tongue muscles. Previous studies have not found evidence for an EOM-like multiple innervation of adult human tongue muscles1,31. In contrast, Ab S46 positively labeled slow muscle fibers in the human fetal tongue. To our knowledge MEP innervation of individual fibers has not been described in human fetal tongue, and thus whether EOM-like multiple MEP innervation is present, compatible with transient EOM-like innervation of these fibers, is not known.
ACKNOWLEDGEMENTS
Grant Support: DC005017 from the National Institute on Deafness and Other Communication Disorders to AJS.
This work was supported by grant DC005017 from the National Institute on Deafness and Other Communication Disorders to AJS. The authors would like to thank Dr. Arthur W. English and Dr. Frank Stockdale for the generous donation of Abs used in this study. Human tissue was kindly provided by the Emory University School of Medicine Body Donor Program and the National Disease Research Interchange. Primate tissue was kindly provided by the Yerkes Regional Primate Center (Research Center Base Grant RR-00165, National Center for Research Resources, NIH).
ABBREVIATIONS
- Ab
Antibody
- AD
Anterior Digastric Muscle
- ALD
Anterior Latissimus Dorsii Muscle
- BB
Biceps Brachii Muscle
- EOM
Extraocular Muscles
- GG
Genioglossus Muscle
- GH
Geniohyoid Muscle
- HG
Hyoglossus Muscle
- MEP
Motor Endplate
- MH
Mylohyoid Muscle
- MHC
Myosin Heavy Chain
- MHCemb
Myosin Heavy Chain Embryonic
- MHCeom
Myosin Heavy Chain Extraocular
- MHCneo
Myosin Heavy Chain Neonatal
- MHCst
Myosin Heavy Chain Slow Tonic
- MR
Medial Rectus Muscle
- SG
Styloglossus Muscle
- SL
Superior Longitudinalis Muscle
- SO
Superior Oblique Muscle
- SR
Superior Rectus Muscle
- T
Transversus Muscle
- TAM
Thyroarytenoid Muscularis Muscle
- TAV
Thyroarytenoid Vocalis Muscle
- V
Verticalis Muscle
REFERENCES
- 1.Arenal A, Alvarez A, Villa Vigil MA, Rodriguez González A, Pérez Casas S, Suárez Garnacho JA, et al. Morfoestrucura de las sinapsis mioneurales en al musculature lingual. Avances en Odontoestiomatologia. 1989;5:191–196. [PubMed] [Google Scholar]
- 2.Bormioli SP, Torresan P, Sartore S, Moschini GB, Schiaffino S. Immunohistochemical identification of slow-tonic fibers in human extrinsic eye muscles. Invest Ophthalmol Vis Sci. 1979;18:303–306. [PubMed] [Google Scholar]
- 3.Bormioli SP, Sartore S, Vitadello M, Schiaffino S. “Slow” myosins in vertebrate skeletal muscle. J Cell Biology. 1980;85:672–681. doi: 10.1083/jcb.85.3.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandon CA, Rosen C, Georgelis G, Horton MJ, Mooney MP, Sciote JJ. Staining of human thyroarytenoid muscle with myosin antibodies reveals some unique extrafusal fibers, but no muscle spindles. J Voice. 2003;17:245–54. doi: 10.1016/s0892-1997(03)00013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Canfield SP. The mechanical properties and heat production of chicken latissimus dorsi muscles during tetanic contractions. J Physiol. 1971;219:281–302. doi: 10.1113/jphysiol.1971.sp009662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiarandini DJ. Activation of two types of fibres in rat extraocular muscles. J Physiol. 1976;259:199–212. doi: 10.1113/jphysiol.1976.sp011461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho M, Hughes SM, Karsch-Mizrachi I, Travis M, Leinwand LA, Blau HM. Fast myosin heavy chains expressed in secondary mammalian muscle fibers at the time of their inception. J Cell Sci. 1994;107:2361–71. doi: 10.1242/jcs.107.9.2361. [DOI] [PubMed] [Google Scholar]
- 8.Eason JM, Schwartz G, Shirley KA, English AW. Investigation of sexual dimorphism in the rabbit masseter muscle showing different effects of androgen deprivation in adult and young adult animals. Arch Oral Biol. 2000;45:683–690. doi: 10.1016/s0003-9969(00)00030-3. [DOI] [PubMed] [Google Scholar]
- 9.Ecob-Prince M, Hill M, Brown W. Immunocytochemical demonstration of myosin heavy chain expression in human muscle. J Neurol Sci. 1989;91:71–8. doi: 10.1016/0022-510x(89)90076-2. [DOI] [PubMed] [Google Scholar]
- 10.Fujii Y, Abe J, Nunomura S, Moriuchi T, Hizawa K. Immunohistochemical study of fiber types in human extraocular muscles. Acta Pathol Jpn. 1990;40:808–14. doi: 10.1111/j.1440-1827.1990.tb02493.x. [DOI] [PubMed] [Google Scholar]
- 11.Han Y, Wang J, Fischman DA, Biller HF, Sanders I. Slow tonic muscle fibers in the thyroarytenoid muscles of human vocal folds; a possible specialization for speech. Anat Rec. 1999;256:146–157. doi: 10.1002/(SICI)1097-0185(19991001)256:2<146::AID-AR5>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 12.Horton MJ, Brandon CA, Morris TJ, Braun TW, Yaw KM, Sciote JJ. Abundant expression of myosin heavy-chain IIB RNA in a subset of human masseter muscle fibres. Arch Oral Biol. 2001;46:1039. doi: 10.1016/s0003-9969(01)00066-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacoby J, Chiarandini DJ, Stefani E. Electrical properties and innervation of fibers in the orbital layer of rat extraocular muscles. J Neurophys. 1989;61:116–125. doi: 10.1152/jn.1989.61.1.116. [DOI] [PubMed] [Google Scholar]
- 14.Kjellgren D, Thornell L-E, Andersen J, Pedrosa-Domello F. Myosin heavy chain isoforms in human extraocular muscles. Invest Ophthalmol Vis Sci. 2003;44:1419–1425. doi: 10.1167/iovs.02-0638. [DOI] [PubMed] [Google Scholar]
- 15.Korfage JAM, Brugman P, Van Eijden TMGJ. Intermuscular and intramuscular differences in myosin heavy chain composition of the human masticatory muscles. J Neurol Sci. 2000;178:95–106. doi: 10.1016/s0022-510x(00)00372-5. [DOI] [PubMed] [Google Scholar]
- 16.Kucera J, Walro JM. Origin of intrafusal fibers from a subset of primary myotubes I the rat. Anat Embryol. 1995;192:149–158. doi: 10.1007/BF00186003. [DOI] [PubMed] [Google Scholar]
- 17.Li ZB, Lehar M, Nakagawa H, Hoh JF, Flint PW. Differential expression of myosin heavy chain isoforms between abductor and adductor muscles in the human larynx. Otolaryngol Head Neck Surg. 2004;130:217–22. doi: 10.1016/j.otohns.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Liu JX, Eriksson PO, Thornell LE, Pedrosa-Domellof Myosin heavy chain composition of muscle spindles in human biceps brachii. J Histochem Cytochem. 2002;50:171–83. doi: 10.1177/002215540205000205. [DOI] [PubMed] [Google Scholar]
- 19.Luff AR, Proske U. Properties of motor units of the frog iliofibularis muscle. Am J Physiol. 1979;236:C35–40. doi: 10.1152/ajpcell.1979.236.1.C35. [DOI] [PubMed] [Google Scholar]
- 20.Lutz GJ, Lieber RL. Myosin isoforms in anuran skeletal muscle: their influence on contractile properties and in vivo muscle function. Microsc Res Tech. 2000;50:443–57. doi: 10.1002/1097-0029(20000915)50:6<443::AID-JEMT3>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 21.Mercadier JJ, Schwartz K, Schiaffino S, Wisnewsky C, Ausoni S, Heimburger M, et al. Myosin heavy chain gene expression changes in the diaphragm of patients with chronic lung hyperinflation. Am J Physiol. 1998;274:L527–34. doi: 10.1152/ajplung.1998.274.4.L527. [DOI] [PubMed] [Google Scholar]
- 22.Miller JB, Crow MT, Stockdale FE. Slow and fast myosin heavy chain content defines three types of myotubes in early muscle cell cultures. J Cell Biol. 1985;101:1643–50. doi: 10.1083/jcb.101.5.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mu L, Su H, Wang J, Han Y, Sanders I. Adult human mylohyoid muscle fibers express slow-tonic, alpha-cardiac, and developmental myosin heavy-chain isoforms. Anat Rec A Discov Mol Cell Evol Biol. 2004;279:749–60. doi: 10.1002/ar.a.20065. [DOI] [PubMed] [Google Scholar]
- 24.Oda K. Motor innervation and acetylcholine receptor distribution of human extraocular muscle fibres. J Neurol Sci. 1986;74:125–33. doi: 10.1016/0022-510x(86)90099-7. [DOI] [PubMed] [Google Scholar]
- 25.Pedrosa-Domellöf F, Holmgren Y, Lucas CA, Hoh JF, Thornell LE. Human extraocular muscles: unique pattern of myosin heavy chain expression during myotube formation. Invest Ophthalmol Vis Sci. 2000;41:1608–1616. [PubMed] [Google Scholar]
- 26.Perie S, St Guily JL, Callard P, Sebille AJ. Innervation of adult human laryngeal muscle fibers. Neurol Sci. 1997;149:81–6. doi: 10.1016/s0022-510x(97)05395-1. [DOI] [PubMed] [Google Scholar]
- 27.Porter JD, Baker RS, Ragusa RJ, Brueckner JK. Extraocular muscles: basic and clinical aspects of structure and function. Surv Ophthalmol. 1995;39:451–484. doi: 10.1016/s0039-6257(05)80055-4. [DOI] [PubMed] [Google Scholar]
- 28.Rossi G, Cortesina G. Morphological study of the laryngeal muscles in man. Acta Otolaryngol. 59:575–92. doi: 10.3109/00016486509124588. 965. [DOI] [PubMed] [Google Scholar]
- 29.Rouaud T, Toutant JP. Histochemical properties and innervation pattern of fast and slow-tonic fibre types of the anterior latissimus dori muscle of the chick. Histochemical Journal. 1982;14:415–428. doi: 10.1007/BF01011854. [DOI] [PubMed] [Google Scholar]
- 30.Shafiq SA, Shimizu T, Fischman DA. Heterogeneity of type 1 skeletal muscle fibers revealed by monoclonal antibody to slow myosin. Muscle Nerve. 1984;7:380–7. doi: 10.1002/mus.880070507. [DOI] [PubMed] [Google Scholar]
- 31.Slaughter K, Li H, Sokoloff AJ. Neuromuscular organization of the superior longitudinalis muscle in the human tongue I: motor endplate morphology and muscle fiber architecture. Cells Tissues Organs. 2005;181:51–64. doi: 10.1159/000089968. [DOI] [PubMed] [Google Scholar]
- 32.Stewart AFR, Kennedy JM, Bandman E, Radovan Z. Myosin isoform repressed in hypertrophied ALD muscle of the chicken reappears during regeneration following cold injury. Developmental Biology. 1989;135:367–375. doi: 10.1016/0012-1606(89)90186-3. [DOI] [PubMed] [Google Scholar]
- 33.Wang J, Han Y, Su H, Mu L. Expression of unique and developmental myosin heavy chain isoforms in adult human digastric muscle. J Histochem Cytochem. 2004;52:851–859. doi: 10.1369/jhc.3A6136.2004. [DOI] [PubMed] [Google Scholar]