Abstract
Fibronectin (FN) is a prototypic adhesive glycoprotein that is widely expressed in extracellular matrices and body fluids. The fibronectin molecule is dimeric, and composed of a series of repeating polypeptide modules. A recombinant fragment of FN incorporating type III repeats 12-15, and including the alternatively-spliced type three connecting segment (IIICS), was found to bind Ni2+, Cu2+ and Zn2+ divalent cations, whereas a similar fragment lacking the IIICS did not. Mutation of two pairs of histidine residues in separate spliced regions of the IIICS reduced cation binding to near the level of the variant lacking the IIICS, suggesting a zinc finger-like mode of cation coordination. Analysis of native FNs purified from plasma or amniotic fluid revealed significant levels of zinc associated with those isoforms that contain the complete IIICS. Taken together, these data demonstrate that the IIICS region of FN is a novel zinc-binding module.
Keywords: fibronectin, zinc, IIICS region, cation-binding, alternative splicing
Introduction
Fibronectin (FN) is a dimeric glycoprotein that is ubiquitously expressed in extracellular matrices (ECM) and body fluids, and which has a widespread adhesive role during development, haemostasis and tissue repair (Hynes, 1990). FN is a modular protein, composed of an array of independently-folding type I, II and III repeats. These modules form a series of functional domains which are specialised to bind other matrix proteins or eukaryotic cell membrane receptors. In addition, three regions of FN undergo alternative splicing. Of these, the type III connecting segment (IIICS, or V), situated between the 14th and 15th type III repeats, has the most complex splice pattern in man, with three sub-domains (A-C) combining to generate five variants (Kornblihtt et al., 1985). The IIICS lies adjacent to the main proteoglycan-binding domain in FN (HepII), and contains the major binding sites for the integrins α4β1 and α4β7 in sub-domains A and C (Mould et al., 1990, Walsh et al., 1996) together with a minor site for binding the proteoglycan syndecan-4 in sub-domain B (Mostafavi-Pour et al., 2001). Based on analysis of natural, recombinant and peptidic fragments containing the IIICS, this region has been shown to play an important role in the adhesion of leukocytes, melanoma cells and peripheral neurons to FN (Humphries et al., 1987, 1988, Wayner et al., 1989).
Zinc is a trace element essential for enzyme catalysis and stabilisation of protein conformation (Vallee and Auld, 1990, Jacobs, 1992). In the ECM, zinc is required for the activity of matrix metalloproteinases that mediate the glycoprotein removal and remodelling that are required during development and wound healing. These enzymes also contribute to pathological conditions such as cancer, arthritis and osteoporosis (Woessner, 1991). In addition, several ECM proteins have been shown to bind zinc ions, including nidogen (Reinhardt et al., 1993), biglycan (Liu, et al., 1994), laminin (Ancsin and Kisilevsky, 1996), COMP (Rosenberg, et al., 1998), and decorin (Yang, et al., 1999).
In previous studies, fragments of FN spanning type III repeats 12-15 and including the different spliced forms of the IIICS region, have been expressed in recombinant form and characterised for their adhesive activity (Makarem et al., 1994). While attempting to purify a C-terminally His-tagged variant containing the full-length IIICS (termed H/120) from its proteolytic fragments, we observed that fragments lacking the tag were retained by Ni2+-NTA-agarose. This led us to speculate that the recombinant protein itself contained a cation-binding site. We report here that the IIICS region of FN contains a divalent cation-binding site which is coordinated by four histidine residues located in the IIICS-A and IIICS-C sub-domains (Fig. 1). We provide evidence that the physiological cation is zinc.
Figure 1.
Diagram of FN structure showing the recombinant fragments of the HepII/IICS region (H variants) used in this study. The figure depicts the structure of a full-length human FN subunit, with its component type I, II and III repeats, together with the regions subject to alternative splicing. The numbers assigned to each recombinant H variant refer to the number of amino acids remaining in the spliced IIICS. The figure also shows the amino acid sequence of the IIICS-A and IIICS-C regions. The histidine residues mutated in this study are underlined, and the cell adhesion motifs LDV and REDV highlighted.
Results
Recombinant FN fragments containing all or part of the IIICS bind to Ni-NTA-agarose
The initial observation leading to the findings in this report was made while attempting to purify full-length H/120 away from its proteolytic breakdown products using a doubly-tagged construct with a C-terminal His-tag and N-terminal GST tag. While only full-length H/120 would be expected to bind following sequential glutathione-agarose and Ni-NTA-agarose affinity chromatography, it was noted that the eluate yielded almost the same profile on SDS-PAGE as the solution applied to the column (Fig. 2A). Similar profiles were obtained with H/120 that lacked a His tag (data not shown). This finding led us to speculate that H/120 contains a cation-binding site which enabled shorter fragments, as well as the full-length molecule, to bind to the Ni-NTA resin.
Figure 2.
Binding of H/120 fragments to Ni-NTA-agarose. A. 50μg of H/120 without His-tag were mixed with 80μl of Ni-NTA resin for 30 minutes. The unbound material was retained, the resin washed, and bound protein eluted with 100mM imidazole. Starting material (lane 1), unbound (lanes 2,3,4) and bound (lanes 5,6,7) protein was separated by SDS-PAGE and the gel stained with Coomassie Blue. Note that the gel gives a qualitative rather than quantitative analysis of the different fractions. B. Bands from unbound and bound fractions were excised, subjected to trypsin digestion, and analysed by tandem mass spectrometry. Peptides consistently identified in unbound (dark grey) and bound grey) samples are highlighted.
When different preparations of non-tagged H/120 were subjected to Ni-NTA chromatography, the profiles of breakdown products in the bound and unbound fractions demonstrated a consistent pattern (Fig. 2A). Bands specific to the bound and unbound fractions were excised, subjected to trypsin digestion, and analysed by mass spectrometry. Results from replicate experiments identified several peptides from type III repeats 12-14 in the unbound fraction bands. Fewer peptides were identified from the bound fraction bands, but one from the IIICS region was consistently retrieved together with a fragment from type III repeat 15 (Fig. 2B). These results suggested that the IIICS region of H/120 might contain a cation-binding site.
This result was reinforced by a second experiment in which samples of all five recombinant H variants, H/120, H/95, H/89, H/64 and H/0 (without His-tags), were mixed with Ni-NTA-agarose and bound and unbound fractions analysed by SDS-PAGE. As shown in Fig. 3, application of saturating levels of H/120, H/95 and H/89 led to retention by the nickel matrix, but H/0 was not retained. The result for H/64 was ambiguous. These results indicated that the IIICS region contains one or more cation-binding sites that depend on the IIICS-A and IIICS-C regions for co-ordination. In a complementary approach, the binding of radioactive 63Ni2+ to immobilised H variants was analysed using an overlay assay. H/120 bound three-fold higher levels of 63Ni2+ than either H/95 or H/89 (Fig. 4). Ni2+ binding to H/0 was the same as background binding to the control protein ovalbumin. These data suggest that a full-length IIICS region is required for efficient Ni2+ binding.
Figure 3.
Binding of H variants to Ni-NTA-agarose. 50μg of each H variant was mixed with 80μl of Ni-NTA-agarose for 30 minutes. The unbound material was retained, the resin washed, and bound protein eluted with 100mM imidazole. The starting material, bound and unbound fractions were analysed by SDS-PAGE. Numbers refer to recombinant H variant.
Figure 4.
Binding of radioactive Ni2+ to H variants immobilised on nitrocellulose. 500 pmol of each H variant or ovalbumin was bound to nitrocellulose using a slot blotter, and endogenous cations removed by washing with EDTA. The membrane was incubated with 0.5μCi/ml 63NiCl2 for 10 minutes, washed, and exposed to a phosphorimaging plate. Results are expressed as percent binding relative to Ni2+ bound by H/120.
Zn2+ and Cu2+, as well as Ni2+, compete for 63Ni2+ binding to immobilised H/120
To ascertain whether radioactive Ni2+ binding to H/120 was specific, and also to test if other cations could compete for binding, radioactive Ni2+overlay assays were carried out in the presence of a constant 1mM concentration of a range of unlabelled divalent and monovalent cations. As shown in Figs. 5A and 5B, unlabelled Ni2+, Cu2+ and Zn2+ all reduced the binding of labelled Ni2+ in a dose-dependent manner with similar half-maximal inhibition values (approximately 10μM). This result indicates that, as well as Ni2+ , both Cu2+ and Zn2+ are also able to bind to H/120. Other divalent and monovalent cations tested bound more weakly or not at all to immobilised H/120 (Fig. 5A), underlining the specificity of the Ni2+, Cu2+ and Zn2+ interaction.
Figure 5.
Binding of cations to H/120. A. H/120 was immobilised on nitrocellulose and incubated with 63NiCl2 in the presence of 1mM of a series of monovalent and divalent cations as chloride salts. Results are expressed as percent labelled Ni2+ binding to H/120 with no added cation. B. Dose response of Cu2+, Ni2+ and Zn2+ inhibition of Ni2+ binding to H/120. H/120 was immobilised on nitrocellulose and incubated with 63NiCl2 in the presence of varying concentrations of CuCl2(■), NiCl2 (●) and ZnCl2(▲). Results are expressed as percent labelled Ni2+ binding to H/120 with no added cation.
The binding of Zn2+ by H/120 and H/0 was also investigated by adding excess (200μM) ZnCl2 to equimolar amounts of each protein followed by extensive dialysis to remove unbound cation. The protein solutions were then analysed for Zn2+ content by ICP-OES and ICP-MS. As shown in Table 1, after correcting for free Zn2+ remaining in the sample, the stoichiometry of H/120-Zn2+ binding was 1:1, while H/0 exhibited background levels of binding. This result confirms that H/120 can bind Zn2+ as well as Ni2+ and that cation binding is a specific association with the IIICS region.
Table 1.
Direct binding of Zn2+ to H/120 and H/0, and analysis of Zn2+ and Ni2+ content of purified FNs
(a) Equimolar amounts of H/120 or H/0 were incubated with ZnCl2 at various concentrations. After extensive dialysis, the proteins were analysed for Zn2+ content. Results are expressed as atoms of zinc found per molecule of H variant. (b) FN was purified from different sources and analysed for Zn2+ and Ni2+ content by ICP-OES or ICP-MS. Results are expressed as atoms of metal per monomeric FN molecule.
| Analysis by ICP-OES Zn:FN |
Analysis by ICP-MS Zn:FN |
|
|---|---|---|
| (a) H/120 | 1.27 ± 0.21 (n=3) | 1.00 |
| H/0 | 0 (n=3) | ND |
| (b) Amniotic fluid | 1.19 ± 1.08 | ND |
| Plasma | ND | 0 |
ND = not determined
To quantify the H variant-Zn2+ association, the binding of Zn2+ to H/120, H/95, H/89 and H/0 was characterised further using isothermal titration calorimetry (ITC). As shown in Table 2, Zn2+ was found to bind best to H/120, with a valency value of 1 in agreement with the previous data, with a Kd of 18μM. Very low valency values were obtained for H/95 and H/89, suggesting a small degree of non-specific binding to these variants, while binding to H/0 was at background levels. These data confirm the conclusions from overlay experiments; namely, that a full-length IIICS region is required for efficient cation binding.
Table 2.
Analysis of Zn2+ binding to H variants by ITC
| Sample | Valency | KD |
|---|---|---|
| H/120 | 1.11 ± 0.09 | 17.95 ± 3.12μM |
| H/95 | 0.005 ± 0.01 | - |
| H/89 | 0.005 ± 0.03 | - |
| H/0 | 0 | - |
Histidine residues within the IIICS region are essential for cation binding
The IIICS-A and IIICS-C regions of FN each contain two histidine residues which reside in a motif similar to that found in zinc metalloproteinases, H-X3-H (Fig. 1). To test the contribution of these residues to Zn2+ binding, either pair of histidines, or all four residues, were mutated to alanine and then the ability of the mutant proteins [termed H/120(12), H/120(78) and H/120(1278)] to bind divalent cations was investigated. In the overlay assay using radioactive Ni2+, binding to H/120(12) and H/120(78) was reduced to the level exhibited by H/95 or H/89. Binding to H/120(1278) was further reduced, although not eliminated (Fig. 6). These data suggest that all four histidine residues are required for full co-ordination of the cation and that the partial binding observed with different H variants or with mutants containing only one pair of histidine residues represents partial saturation. The fact that H/120(1278) shows levels of Zn2+ binding above that of H/0, suggests that residues other than the mutated histidines may also contribute to the co-ordination of the metal ion.
Figure 6.
Binding of 63Ni2+ to wild type and mutated H/120. 500 pmol of wild-type and His-mutated H/120 were immobilised on nitrocellulose and incubated with 63NiCl2. Results are expressed as percent labelled Ni2+ bound compared to wild type H/120.
FN purified from amniotic fluid contains bound zinc
As one way to test the physiological relevance of H/120- Zn2+ binding, purified full-length FNs were assayed for their divalent cation content. FN was purified from plasma or amniotic fluid. Previous analyses have demonstrated that amniotic fluid contains a higher proportion of molecules expressing the complete IIICS region, whereas plasma FN is mostly composed of molecules containing a partially spliced IIICS (Herschberger and Culp, 1990). Table 1 shows the results obtained from analysis of the FNs for Zn2+ by either ICP-OES or ICP-MS. Significant amounts of Zn2+ were detectable in the amniotic fluid sample, but none was detectable in plasma FN or in the dialysis buffer. No Ni2+ was found in any of the samples. The stoichiometry of cation binding to FN was variable, perhaps because of loss during purification, but on average amniotic fluid samples contained 1 zinc ion for every 3 monomeric FN molecules. Taken together, these findings demonstrate that native FN is associated with at least one zinc ion.
Zinc does not play a functional role in receptor binding the IIICS region
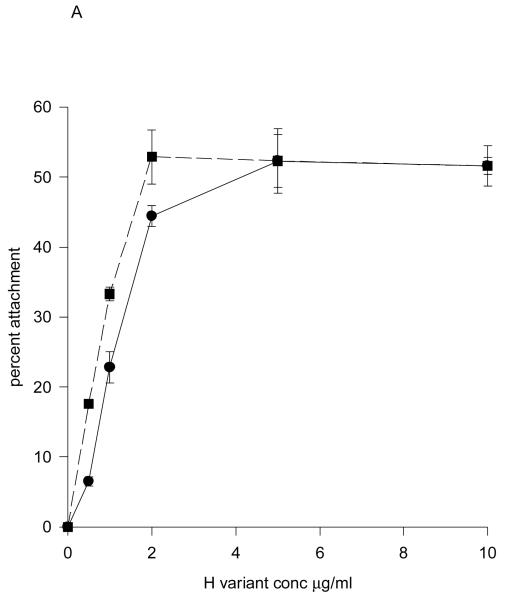
It is well documented that integrin activity is regulated by divalent cations (Mould et al., 1995), and since the histidine residues identified above are located adjacent to both the LDV (in IIICS-A) and REDV (in IIICS-C) α4β1 integrin-binding sites, this might imply a role in integrin-ligand binding. To test this possibility, the ability of H/120 or H/120(1278) to promote attachment and spreading was carried out using MOLT-4 and B16-F10 cells, respectively. As shown in Figs. 7A and B, neither adhesive function was affected by removal of the cation co-ordination site. Similar results were obtained in more sensitive solid-phase assays using purified α4β1 integrins in place of cells (data not shown).
Figure 7.
Cell adhesion to wild-type and mutated H/120. Attachment of Molt-4 cells (A) and spreading of B16-F10 cells (B) on H/120 (■) and H/120(1278) (●). Background adhesion to BSA alone was subtracted. Results are expressed as percent cells attached or spread.
Discussion
By using metal ion affinity chromatography and direct binding of divalent cations in an overlay assay, we demonstrate that the IIICS region of FN is able to bind copper, nickel and zinc ions. Direct analysis of purified human FN demonstrated association with zinc in vivo, while site-directed mutagenesis of recombinant fragments spanning the IIICS region suggests that one metal ion is co-ordinated by four histidine residues present in independently spliced segments of this domain. Further quantification of this association by ITC confirmed the 1:1 stoichiometry.
There are few published reports concerning metal ion binding by FN. Both whole FN (Smilenov et al., 1992) and its proteolytic fragments (Gmeiner et al., 1995) have been subjected to metal chelate chromatography. In the latter study, a 40kDa collagen-binding fragment and a 110kDa central cell-binding domain fragment both bound to a zinc-charged column, while a C-terminal 35kDa heparin-binding fragment, equivalent to the H/0 fragment used in this study, showed very poor retention by the same affinity matrix. These studies were carried out using plasma FN, which contains a low proportion of molecules with the full 120 amino acid residues of the IIICS (Herschberger and Culp, 1990), but it is possible that regions other than the IIICS of FN have the ability to bind zinc. In this regard, it has been reported that a specific sequence in the collagen-binding domain (AAHEEIC) has high affinity for zinc (Lambert-Vidmar et al., 1991). The present study is, however, the first to report zinc binding by the IIICS region of FN.
Evidence that the association with zinc occurs in the native FN molecule was gained from the analysis of human FN purified from two different sources - plasma and amniotic fluid. No zinc was found to be associated with plasma FN, but an average of 1 zinc atom per 3 monomers of amniotic fluid FN was detected. Plasma FN is known to contain very few molecules containing the full IIICS (Herschberger and Culp, 1990), while amniotic fluid FN has a higher proportion of full-length IIICS (Ruoslahti et al., 1981, Linnala et al., 1994). Thus, the stoichiometry of associated zinc correlates with IIICS content. A previous study reported two atoms of zinc associated with plasma FN when analysed by atomic absorption spectroscopy (Sinosich et al., 1983); however differences in sample preparation, which did not include heparin-agarose chromatography, and analysis method could account for the discrepancy. It is also possible that some zinc could be lost during isolation but since both plasma and amniotic fluid FNs were compared using the same purification methods, the correlation of zinc and IIICS content remains valid.
The IIICS region of FN contains a total of eight histidine residues, four of which reside in motifs reminiscent of the zinc-binding motifs in matrix metalloproteinases (HNPLH and HLYPH). Mutation of these four histidine residues to alanine in recombinant H/120 diminished cation binding, as shown by reduced binding of nickel in the overlay assay, while mutation of either pair severely reduced this interaction. ICP-MS, ICP-OES and ITC analysis confirmed that H/120 was able to bind only one atom of zinc and therefore we conclude that all four of these histidine residues are required for full cation binding.
Cell adhesion and solid phase assays performed with recombinant wild-type and mutant H/120 (1278) suggested that the cation does not play a functional role in receptor binding, at least in the context of the abbreviated recombinant fragment; however this does not rule out the possibility that the zinc ion is required for the structural integrity of the IIICS region of FN. In addition, Zn2+ binding to H/120(1278) was not completely abrogated and the possibility that other residues in this H variant are still available to bind enough metal to mask any differences cannot be ruled out. However, one could speculate that, due to the proximity of the two α4β1 recognition sites to the two pairs of histidine residues, cation co-ordination might regulate the presentation of the integrin-binding motifs to activated integrin. This possibility is not precluded by our data as the LDV and REDV motifs are likely to be constitutively exposed in this fragment of FN, particularly when adsorbed to plastic. There is evidence that the same might not be true in the full-length FN molecule (Ugarova et al., 1996).
Zinc co-ordination could be required not only for intra-molecular structural interactions but also for inter-molecular stability. Recently, a number of matrix proteins have been shown to bind zinc, and this association is required to mediate interactions with other matrix molecules [for example, laminin 1 with entactin (Ancsin and Kisilevsky, 1996), COMP with collagens I and II (Rosenberg et al., 1998), and decorin with fibrinogen (Dugan et al., 2003)]. Furthermore, the latter study indicated that decorin interacted with FN in a zinc-dependent manner. Further studies are underway to determine if Zn2+ affects the structure, stability or interaction of the fibronectin fragments. Interactions between FN molecules are required for fibrillogenesis. It is known that fibril formation requires both the interaction of FN with cells (via integrins) and the stretching of individual molecules to expose cryptic inter- and intra-molecular binding sites (Geiger et al., 2001). Both the N-terminal region of FN and a dimeric structure is essential for the formation of an insoluble FN matrix (Schwarzbauer, 1991). Interestingly, FN dimers containing an excised IIICS fail to be secreted from the cell (Schwarzbauer, 1989), suggesting that the IIICS is needed for fibril formation. The possibility that dimerisation might involve the zinc-binding site within the IIICS will be the subject of further investigation. In addition, although the Kd of the H/120: zinc association was fairly low at 18μM this is well within the physiological range for this element (12-25μM) and therefore supports a potential role for a FN: Zn interaction in vivo.
While the structure of much of the FN molecule is known (Williams et al., 1994, Leahy et al., 1996, Pickford et al., 1997, Sharma et al., 1999), the IIICS remains unsolved and impossible to predict due to a large number of proline residues. The cation-chelating motif present in the IIICS, His-X3-His, has been engineered into proteins to facilitate applications such as metal-chelate chromatography (Arnold and Haymore, 1991, Lu et al., 1996); however, high affinity chelation only occurs in the context of an α-helix (Arnold and Haymore, 1991). If this type of secondary structure were present in the IIICS, it would be noteworthy since the rest of the FN structure contains no helical elements.
Experimental procedures
Production of HepII/IIICS recombinant FN fragments
Human FN cDNA clones encoding the five different variants of the HepII/IIICS region of FN were obtained by RT-PCR from primary human skin fibroblast mRNA, amplified by PCR, and subcloned into a pGex expression vector as previously described (Makarem et al., 1994). The constructs were transformed into E. coli BLR (De3) plysS (Novagen) for expression. Fifty ml of overnight cultures of each clone were grown for 16 hours in LB containing 50μg/ml ampicillin, 30μg/ml chloramphenicol and then diluted 1:10 in the same medium. The cells were grown for 1 hour and protein expression induced by the addition of 0.1M IPTG (Sigma). Growth was continued for a further 4 hours before harvesting the cells by centrifugation. The cell pellet was resuspended in 20ml Tris-buffered saline (150mM NaCl, 10mM Tris/HCl, pH7.4; TBS) containing 0.1% (w/v) Triton X-100, 1mM phenylmethylsulfonyl fluoride, 20μg/ml leupeptin and frozen overnight. Bacteria were lysed by thawing and RNase and DNase (500μg of each; Roche) were added to the lysate and incubated at 4°C until the solution was no longer viscous. Cell debris was removed by centrifugation at 40000g for 10min and the cleared supernatant added to 5ml of pre-swollen glutathione-agarose (Sigma) equilibrated in TBS. This mixture was rotated for 30min at room temperature to allow the induced GST-FN fragment fusion protein to bind, and then unbound proteins were removed by centrifugation. The gel with the bound fusion protein was washed three times in TBS, twice in low salt TBS (50mM NaCl, 10mM Tris/HCl, pH 7.4) and resuspended in 5ml of low salt TBS. 25U human thrombin (Sigma) were added and the mixture rotated for 2 hours at room temperature to cleave the recombinant FN away from the GST-matrix. The supernatant, containing FN fragment only, was retained following centrifugation. H variant proteins were further purified by heparin-agarose affinity chromatography (Sigma) as previously described (Makarem et al., 1994). The recombinant fragments were named H/120, H/95, H/89, H/64 and H/0 according to the number of amino acid residues in the IIICS and are described in detail in Fig.1.
Mutagenesis
Three mutant clones of H/120 were produced by site-directed mutagenesis. The first two histidine residues in IIICS-A (in the sequence HNPLH) were mutated to alanine; second, the last two histidine residues in IIICS-C (in the sequence HLYPH) were similarly changed; and third, all four histidine residues were mutated (residues are shown underlined in Fig. 1). The clones were named H/120(12), H/120(78) and H/120(1278) respectively. The mutant proteins were expressed and purified as described above for the native protein.
In-gel trypsin digestion for identification of peptides by tandem MS
Silver-stained SDS-PAGE gels were washed with distilled water the bands of interest excised and cut into pieces. These were washed with 20mM NH4HCO3 followed by acetonitrile, before reduction with 10mM dithiothreitol (DTT) for 60 minutes at 56° and alkylation with 55mM iodoacetamide for 30 minutes at room temperature. After washing, the gel pieces were dried in a vacuum centrifuge and then rehydrated in 12.5ng/μl trypsin (Sigma) for 45 mins. The trypsin was replaced with 20mM NH4HCO3 and incubated for a further 16 hours at 37°C. Supernatants were concentrated and tryptic peptides analysed by positive ion ES-MS/MS using a Q-ToF micro mass spectrometer (Waters). Data acquired was searched against SwissProt and Trembl databases using Proteinlynx global server 1.1 software (Waters).
Binding of H variants to nickel-NTA-agarose
Samples of purified H variants (50μg), diluted 1: 10 in 300mM NaCl, 50mM sodium phosphate, pH 8.0, were mixed with 80μl aliquots of washed Ni-NTA-agarose (Qiagen). The suspensions were rotated at room temperature for 30min and the gel separated by centrifugation. The unbound material in the supernatants was retained and the resin washed extensively with dilution buffer. Bound protein was eluted by the addition of 100μl 100mM imidazole in the same buffer, the supernatant again retained, and all samples analysed by SDS-PAGE.
Overlay assay
The binding of radioactive 63Ni to H variant proteins was investigated using the method described by Michishita et al. (1993). Briefly, equimolar amounts of purified recombinant proteins were blotted onto nitrocellulose using a Minifold II slot blot system (Schleicher and Schuell). The membrane was washed twice for 10 minutes at room temperature in overlay buffer (60mM KCl, 10mM imidazole, pH7.5) containing 10mM EDTA to remove any protein-bound cations, and then four times in overlay buffer alone to remove EDTA. The membrane was incubated for 10 minutes at room temperature in overlay buffer containing 0.5μCi/ml 63NiCl2 (Amersham). The nitrocellulose was washed twice with 50% ethanol and exposed to a BAS-III Fuji phosphorimaging plate for 20 hours at room temperature. Results were analysed using a Fuji FUJIX BAS2000 phosphorimager and associated software.
Isothermal Titration Calorimetry
Isothermal titration calorimetry (ITC) was performed on a VP-ITC calorimeter (Microcal, Northampton MA). In each experiment a total of 30 4-μl aliquots of 100μM ZnCl2 were injected into the cell containing 10mM fibronectin fragment at 30°C. All solutions were dialysed against TBS before commencing titrations. Experimental data were analysed using Microcal Origin software provided by the ITC manufacturer.
Purification of FN
Human FN was purified from two different sources, plasma (obtained from Manchester Blood Transfusion Service) and amniotic fluid (from mid-term amniocentesis samples kindly provided by the Department of Cytogenetics, St Mary’s Hospital, University of Manchester) by a method adapted from Miekka et al. (1982). ε-aminocaproic acid was added to each starting material before centrifugation at 10000g for 15 minutes to remove any debris. The supernatants were applied to 15ml pre-columns of Sepharose CL-4B (Pharmacia) equilibrated with 50mM ε-aminocaproic acid, 50mM Tris/HCl, pH 7.5 and the flow-through collected. Meanwhile a 20ml gelatin-agarose column (Sigma) was first washed with 4M urea, 0.5M NaCl, 10mM Tris/HCl, pH 7.5 and then equilibrated as above. The pre-column flow-through was applied to the gelatin-agarose column and the bound material washed first with 50mM ε-aminocaproic acid, 1M NaCl, 50mM Tris/HCl, pH 7.5, and then with the same buffer without added salt. The FN was eluted with 4M urea, 10mM Tris/HCl, pH 7.5, precipitated with 50% ammonium sulphate, resuspended in TBS, and dialysed against TBS. FN purified from fibroblasts was resuspended in 0.2M 3-(cyclohexylamino)-1-propane sulphonic acid (CAPS) buffer pH11 followed by dialysis against CAPS/saline (10mM CAPS/150mM NaCl) pH11. Plasma FN samples were further purified by heparin-agarose (Sigma) affinity chromatography to remove traces of a serum gelatinase that has been reported to bind divalent cations (Smilenov et al.., 1992). Analysis by reducing SDS-PAGE showed bands at the expected Mr of 200-250kDa (not shown).
Analysis of the zinc content of FN
Samples of purified FN were analysed for the presence of Zn2+ and Ni2+ by either inductively coupled plasma optical emission spectroscopy (ICP-OES) by the Department of Chemistry, University of Manchester, or inductively coupled plasma mass spectroscopy (ICP-MS) by the Department of Earth Sciences, University of Manchester. In experiments where binding of exogenous zinc by the recombinant fragments was investigated by these methods, ZnCl2 was added to the proteins at various concentrations, followed by extensive dialysis to remove unbound cation before analysis.
Attachment and spreading assays
Attachment assays were carried out using MOLT-4, a human lymphoblastic leukaemia cell line obtained from the European Cell and Culture Collection. Cells were cultured in RPMI 1640 supplemented with 10% (v/v) fetal bovine serum and 2mM glutamine (Invitrogen). Assays were performed as previously described (Mould et al., 1994). Briefly, 96-well microtitre plate wells (Costar) were coated with 100μl aliquots of recombinant proteins diluted in PBS and non-specific adhesion sites blocked with 100μl of 10mg/ml heat-denatured BSA. MOLT-4 cells were resuspended at 106/ml in DMEM with 25mM HEPES (4-(2-Hydroxyethyl)piperazine-1-ethanesulphonic acid, Invitrogen), 1mM MnCl2, and 100μl aliquots added to the wells. The plate was incubated for 20min at 37°C in humidified atmosphere of 6% (v/v) CO2. Unbound cells were removed by gentle tapping and the remainder fixed by the addition of 100μl 5% (w/v) glutaraldehyde. After 30min, the fixative was aspirated, and the attached cells were washed and stained with 100μl 0.1% (w/v) crystal violet in 200mM MES (methylethanesulphonic acid, Sigma), pH6 for 1 hour. Excess dye was removed by washing, and bound dye was solubilised with 100μl 10% (v/v) acetic acid. The absorbance at 570nm of each well was measured using a Multiscan ELISA reader (Dynatech). For cell spreading assays, the method of Humphries et al. (1986) was used. The microtitre plates were coated and blocked as for attachment. B16-F10 mouse melanoma cells (provided by I.J. Fidler, University of Texas) were detached with 0.05% (w/v) trypsin, 0.02% (w/v) EDTA, resuspended at 2×105/ml in DMEM containing 25mM HEPES, and 100μl aliquots added to each well. The plates were incubated as above for 60min and the cells fixed with 3% (w/v) glutaraldehyde (Sigma). The degree of spreading was monitored using phase-contrast microscopy
Acknowledgements
We would like to express our thanks to Maurice Hart and Paul Lythgoe for ICP-OES and ICP-MS analysis, respectively. This work was supported by grants 045225 and 074941 from the Wellcome Trust (to MJH).
References
- Ancsin JB, Kisilevsky R. Laminin interactions important for basement membrane assembly are promoted by zinc and implicate laminin zinc finger-like sequences. J. Biol. Chem. 1996;271:6845–6851. doi: 10.1074/jbc.271.12.6845. [DOI] [PubMed] [Google Scholar]
- Arnold FH, Haymore BL. Engineered metal-binding proteins: purification to protein folding. Science. 1991;252:1796–1797. doi: 10.1126/science.1648261. [DOI] [PubMed] [Google Scholar]
- Borsi L, Balza E, Leprini A, Ponassi M, Zardi L. Procedure for the purification o the fibronectin proteolytic fragments containing the ED-B oncofetal domain. Analytical Biochem. 1991;192:372–379. doi: 10.1016/0003-2697(91)90551-4. [DOI] [PubMed] [Google Scholar]
- Geiger B, Bershadsky A, Pankov R, Yamada K. Transmembrane extracellular matrix-cytoskeleton crosstallk. Nature Reviews Mol. Cell Biol. 2001;2:793–799. doi: 10.1038/35099066. [DOI] [PubMed] [Google Scholar]
- Gmeiner B, Leibl H, Zerlauth G, Seelos C. Affinity binding of distinct functional fibronectin domains to immobilised metal chelates. Arch. Biochem. Biophys. 1995;321:40–42. doi: 10.1006/abbi.1995.1365. [DOI] [PubMed] [Google Scholar]
- Herschberger RP, Culp LA. Cell-type-specific expression of alternatively spliced human fibronectin IIICS mRNAs. Mol. Cell. Biol. 1990;10:662–671. doi: 10.1128/mcb.10.2.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries MJ, Akiyama SK, Komoriya A, Olden K, Yamada KM. Identification of an alternatively spliced site in human plasma fibronectin that mediates cell-type specific adhesion. J.Cell. Biol. 1986;103:2637–2647. doi: 10.1083/jcb.103.6.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries MJ, Komoriya A, Akiyama SK, Olden K, Yamada KM. Identification of two distinct regions of the type III connecting segment of human plasma fibronectin that promote cell type-specific adhesion. J. Biol. Chem. 1987;262:6886–6892. [PubMed] [Google Scholar]
- Humphries MJ, Akiyama SK, Komoriya A, Olden K, Yanada KM. Neurite extension of chicken peripheral nervous system neurons on fibronectin: relative importance of specific adhesion sites in the central cell-binding domain and the alternatively-spliced type III connecting segment. J. Cell Biol. 1988;106:1289–97. doi: 10.1083/jcb.106.4.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Fibronectins. Springer-Verlag; New York: 1990. [Google Scholar]
- Jacobs GH. Determination of the base recognition positions of zinc fingers from sequence analysis. EMBO J. 1992;11:4507–17. doi: 10.1002/j.1460-2075.1992.tb05552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblitt AR, Umezawa K, Vibe-Pedersen K, Baralle FE. Primary structure of human fibronectin. Differential splicing may generate at least 10 polypeptides from a single gene. EMBO J. 1985;3:1755–1759. doi: 10.1002/j.1460-2075.1985.tb03847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert-Vidmar S, Lottspeich F, Emod I, Imhoff J-M, Keil-Dlouha V. Collagen-binding domain of human plasma fibronectin contains a latent type-IV collagenase. Eur. J. Biochem. 1991;201:79–84. doi: 10.1111/j.1432-1033.1991.tb16258.x. [DOI] [PubMed] [Google Scholar]
- Leahy DJ, Aukil I, Erickson HP. 2.0 A crystal structure of a four-domain segment of human fibronectin encompassing the RGD loop and synergy region. Cell. 1996;84:155–164. doi: 10.1016/s0092-8674(00)81002-8. [DOI] [PubMed] [Google Scholar]
- Linnala A, von Koskull H, Virtanen I. Isoforms of cellular fibronectin and tenascin in amniotic fluid. FEBS Lett. 1994;337:167–170. doi: 10.1016/0014-5793(94)80266-1. [DOI] [PubMed] [Google Scholar]
- Liu J, Laue TM, Choi HU, Tang L-H, Rosenberg L. The self-association of biglycan from bovine articular cartilage. J. Biol. Chem. 1994;269:28366–28373. [PubMed] [Google Scholar]
- Lu Z, DiBlasio EA, Grant KL, Warne NW, LaVallie ER, Collins-Racie LA, Follettie MT, Williamson MJ, McCoy JM. Histidine Patch Thioredoxins. Mutant forms of thioredoxin with metal chelating affinity that provide for convenient purifications of thioredixin fusion proteins. J. Biol. Chem. 1996;271:5059–5065. [PubMed] [Google Scholar]
- Makarem R, Newham P, Askari JA, Green LJ, Clements J, Edwards M, Humphries MJ, Mould AP. Competitive binding o vascular cell adhesion molecule-1 and HepII/IIICS domain of fibronectin to the integrin α4β1. J. Biol. Chem. 1994;269:4005–4011. [PubMed] [Google Scholar]
- Michishita M, Videm V, Arnaout MA. A novel divalent cation-binding site in the A domain of the α2 integrin CR3 (CD11b/CD18) is essential for ligand binding. Cell. 1993;72:857–867. doi: 10.1016/0092-8674(93)90575-b. [DOI] [PubMed] [Google Scholar]
- Miekka SI, Ingham KC, Menache D. Rapid methods for isolation of human plasma fibronectin. Thromb. Res. 1982;27:1–14. doi: 10.1016/0049-3848(82)90272-9. [DOI] [PubMed] [Google Scholar]
- Mould AP, Askari JA, Craig SE, Garratt AN, Clements J, Humphries MJ. Integrin α4β1-mediated melanoma cell adhesion and migration on vascular cell adhesion molecule-1 (VCAM-1) and the alternatively spliced IIICS region of fibronectin. J. Biol. Chem. 1994;269:27224–27230. [PubMed] [Google Scholar]
- Mould AP, Akiyama SK, Humphries MJ. Regulation of integrin α5β1-fibronectin interactions by divalent cations. J. Biol. Chem. 1995;270:26270–26277. doi: 10.1074/jbc.270.44.26270. [DOI] [PubMed] [Google Scholar]
- Pickford AR, Potts JR, Bright JR, Phan I, Campbell ID. Solution structure of a type 2 module from fibronectin: implications for the structure and function of the gelatin-binding domain. Structure. 1997;5:359–370. doi: 10.1016/s0969-2126(97)00193-7. [DOI] [PubMed] [Google Scholar]
- Reinhardt D, Mann K, Nischt R, Fox J.w., Chu ML, Kreig T, Timpl R. Mapping of nidogen binding sites for collagen type IV, heparan sulphate proteoglycan and zinc. J. Biol. Chem. 1993;268:10881–10887. [PubMed] [Google Scholar]
- Rosenberg K, Olsson H, Mörgelin M, Heingård D. Cartilage oligomeric matrix protein shows high affinity zinc-dependent interaction with triple helical collagen. J. Biol. Chem. 1998;273:20397–20403. doi: 10.1074/jbc.273.32.20397. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E, Engvall E, Hayman EG, Spiro RG. Comparative studies on amniotic fluid and plasma fibronectins. Biochem. J. 1981;193:295–299. doi: 10.1042/bj1930295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Askari JA, Humphries MJ, Jones EY, Stuart DI. Crystal structure of a heparin- and integrin-binding segment of human fibronectin. EMBO J. 1999;18:1468–1479. doi: 10.1093/emboj/18.6.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzbauer JE. Identification of the fibronectin sequences required for assembly of a fibrillar matrix. J. Cell Biol. 1991;113:1463–1473. doi: 10.1083/jcb.113.6.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzbauer JE, Spencer CS, Wilson CL. Selective secretion of alternatively spliced fibronectin variants. J. Cell Biol. 1989;109:3445–3453. doi: 10.1083/jcb.109.6.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinosich MJ, Davey MW, Teisner B, Grudzinskas JG. Comparative studies of pregnancy associated plasma protein-A and α2-macroglobulin using metal chelate chromatography. Biochem. International. 1983;201:79–84. [PubMed] [Google Scholar]
- Smilenov L, Forsberg E, Zeligman I, Sparrman M, Johanson S. Separation of fibronectin from a plasma gelatinase using immobilised metal affinity chromatography. FEBS Letts. 1992;302:227–230. doi: 10.1016/0014-5793(92)80447-o. [DOI] [PubMed] [Google Scholar]
- Ugarova TP, Ljubimov AV, Deng L, Plow EF. Proteolysis regulates exposure of the IIICS-1 adhesive sequence in plasma fibronectin. Biochemistry. 1996;35:10913–10921. doi: 10.1021/bi960717s. [DOI] [PubMed] [Google Scholar]
- Vallee BL, Auld DS. Zinc coordination and structure of zinc enzymes and other proteins. Biochemistry. 1990;29:5647–5659. doi: 10.1021/bi00476a001. [DOI] [PubMed] [Google Scholar]
- Walsh GM, Symon FA, Lazarovils AL, Wardlaw AJ. Integrin alpha4beta7 mediates human eosinophil interaction with MAdCAM-1, VCAM-1 and fibronectin. Immunology. 1996;89:112–119. doi: 10.1046/j.1365-2567.1996.d01-713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner EA, Garcia-Pardo A, Humphries MJ, McDonald JA, Carter WG. Identification and characterization of the T lymphocyte adhesion receptor for an alternative cell attachment domain (CS-1) in plasma fibronectin. J. Cell Biol. 1989;109:1321–1330. doi: 10.1083/jcb.109.3.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MJ, Phan I, Harvey TS, Rostango A, Gold L, Campbell ID. Solution structure of a pair of fibronectin type I modules with fibrin-binding activity. J. Mol. Biol. 1994;119:923–933. doi: 10.1006/jmbi.1994.1083. [DOI] [PubMed] [Google Scholar]
- Woessner JF. Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991;5:2145–54. 1991. [PubMed] [Google Scholar]
- Yang VW-C, LaBrenz SR, Rosenberg LC, McQuillan D, Höök M. Decorin is a Zn2+ metalloprotein. J. Biol. Chem. 1999;274:12454–12460. doi: 10.1074/jbc.274.18.12454. [DOI] [PubMed] [Google Scholar]