Abstract
The assembly of the polymerase complex of influenza A virus from the three viral polymerase subunits PB1, PB2, and PA is required for viral RNA synthesis. We show that peptides which specifically bind to the protein-protein interaction domains in the subunits responsible for complex formation interfere with polymerase complex assembly and inhibit viral replication. Specifically, we provide evidence that a 25-amino-acid peptide corresponding to the PA-binding domain of PB1 blocks the polymerase activity of influenza A virus and inhibits viral spread. Targeting polymerase subunit interactions therefore provides a novel strategy to develop antiviral compounds against influenza A virus or other viruses.
Influenza A viruses are human pathogens that are responsible for both annual epidemics and recurring devastating pandemics. The recent emergence of highly pathogenic avian influenza virus strains of the H5N1 subtype and the lethality associated with these viruses reflect the continuing threat of influenza viruses. Despite the existence of vaccines against annually recurring influenza A and B virus strains, as well as antiviral drugs targeting either the viral neuraminidase (9) or M2 proteins (14), the protection against epidemic and pandemic influenza is still incomplete. The viral RNA-dependent RNA polymerase consisting of three subunits, PA, PB1, and PB2 (the P proteins), is a potential target for the development of new anti-influenza drugs. PB1 is the central protein, containing two different domains interacting with the PB2 and the PA subunits (1, 7, 13, 16). We hypothesized that viral RNA synthesis could be blocked by the specific inhibition of viral polymerase complex formation by using small peptides which bind to the protein-protein interaction domains responsible for hetero-oligomerization between the individual subunits. Here, we describe the characterization of a PB1-derived short peptide that fulfils these criteria.
The first 25 aa of PB1 fused to GFP bind to the viral polymerase subunit PA and inhibit polymerase activity.
To design an inhibitory peptide, we selected the region encompassing amino acids (aa) 1 to 25 of the PB1 polymerase subunit of influenza A virus (A/WSN/33), which has been demonstrated previously to bind the PA subunit (16, 17) and is highly conserved among influenza A virus strains (Fig. 1A). To confirm that this region indeed binds PA, we fused this sequence to the N terminus of the green fluorescent protein (GFP), transiently expressed the fusion protein (PB11-25-GFP) together with hemagglutinin (HA)-tagged PA (PA-HA) in HEK293T cells, and immunoprecipitated PA-HA from cell extracts by using anti-HA antibodies. As shown in Fig. 1B, PB11-25-GFP, but not Flag-tagged GFP, was specifically immunoprecipitated with PA-HA (lane 3). In a similar experiment, we also tested whether the PB1 aa 715 to 740, which are embedded in a larger PB2-binding domain (13), were sufficient for binding PB2 when fused to GFP (PB1715-740-GFP). However, PB1715-740-GFP could not be immunoprecipitated with HA-tagged PB2 (Fig. 1B, lane 6), indicating that this minimal region is insufficient for mediating a stable interaction with PB2.
FIG. 1.
The first 25 aa of PB1 fused to GFP are sufficient to bind to the viral polymerase subunit PA. (A) Alignment of the first 25 N-terminal amino acids of PB1 subunits from the 2,485 influenza A virus strains with sequences available in the NCBI data bank. Unique sequences contain one or more amino acid changes compared to the top sequence. (B) GFP fusion proteins containing the PA-binding (PB11-25-GFP) or a putative PB2-binding (PB1715-740-GFP) domain were expressed with either C-terminally HA-tagged PA or PB2 in HEK293T cells. These cells are highly transfectable human embryonic kidney (HEK) cells expressing the simian virus 40 T antigen. Cell extracts were prepared 24 h posttransfection and subjected to immunoprecipitation (IP) using anti-HA antibodies (αHA) bound to protein A-Sepharose. Precipitated material was separated on a sodium dodecyl sulfate-10% polyacrylamide gel and analyzed by Western blotting for the presence of GFP and HA-tagged polymerase subunits by using anti-GFP antibodies (αGFP) or αHA. Control immunoprecipitations were carried out with extracts of cells expressing (+) Flag-GFP and HA-tagged PA or PB2 (lanes 1 and 4) or untagged PA or PB2 and PB11-25-GFP or PB1715-740-GFP (lanes 2 and 5). Flu A, influenza A virus.
We next tested whether the binding of PB11-25-GFP to PA can disturb the polymerase activity of influenza A virus by measuring the polymerase activity in an influenza A virus minireplicon assay (19) in the presence or absence of this fusion protein. Cotransfection with the PB11-25-GFP expression plasmid resulted in an 85% inhibition of reporter gene activity, whereas cotransfection with the plasmid encoding PB1715-740-GFP had no effect (Fig. 2). Demonstrating the specificity of this inhibition, PB11-25-GFP did not interfere with influenza B virus polymerase activity (Fig. 2) in an influenza B virus minireplicon system (15). This result was unsurprising, since the N-terminal region of influenza B virus PB1 differs substantially from that of influenza A virus strains and since it is not possible to functionally interchange the P subunits between influenza A and B viruses (2, 10).
FIG. 2.
The PA-binding domain of PB1 blocks influenza virus polymerase activity. Approximately 105 HEK293T cells were transiently transfected with a mixture containing plasmids expressing either influenza A virus (A/WSN/33) or B virus (B/Yamagata/73) PB1, PB2, and PA (90 ng) and NP (300 ng); polymerase I expression plasmids (50 ng) carrying an influenza virus-like RNA coding for the reporter protein firefly luciferase (influenza A virus) or chloramphenicol acetyltransferase (influenza B virus) to monitor viral polymerase activity; and expression plasmids coding for the indicated GFP fusion proteins (1,800 ng). Both minigenome RNAs were flanked by noncoding sequences of segment 8 of the respective influenza A or B virus. The transfection mixtures also contained a plasmid constitutively expressing renilla luciferase (100 ng), which served to normalize variations in transfection efficiency. The reporter activity was determined 24 h posttransfection and normalized. The activity observed with transfection reaction mixtures containing Flag-GFP was set at 100%. A transfection mixture with PB2 omitted served as a negative control. Levels of the indicated GFP fusion proteins from one representative experiment were determined by Western blot analysis using anti-GFP antibodies (upper panels). FluA, influenza A virus; FluB, influenza B virus; Rel., relative.
Inhibition of viral replication by PB1-derived peptides.
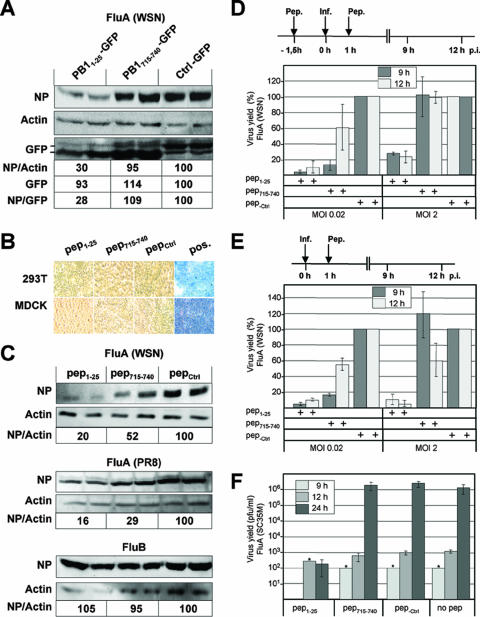
To test whether PB11-25-GFP also blocks viral growth, we first transfected HEK293T cells with plasmids expressing PB11-25-GFP, PB1715-740-GFP, or a control GFP fusion construct (ctrl-GFP) containing a protein-protein interaction sequence (aa 69 to 93) from the unrelated phosphoprotein of Borna disease virus (BDV-P). Approximately 24 h later, when 80 to 90% of the cells expressed the GFP constructs as determined by green light emission, the cultures were infected with influenza A virus (A/WSN/33) at a multiplicity of infection (MOI) of 0.02. Viral replication was measured by quantifying nucleoprotein (NP) levels 12 h postinfection (p.i.) by Western blot analysis. This analysis revealed a decrease in NP expression of 70% in cells transfected with plasmids encoding PB11-25-GFP compared to that in cells transfected with plasmids expressing ctrl-GFP, while no significant change in cells transfected with PB1715-740-GFP-encoding plasmids was observed (Fig. 3A). We next tested whether small peptides alone, corresponding to either aa 1 to 25 (pep1-25) or aa 715 to 740 (pep715-740) of PB1, were sufficient to inhibit viral replication. Peptides were synthesized (4) with an additional C-terminal sequence from the human immunodeficiency virus Tat protein (aa 47 to 59), which has been shown to mediate cell entry (5, 21). HEK293T and MDCK cells incubated with the highest concentrations of peptides used for further experiments showed no signs of toxicity after a trypan blue exclusion test (Fig. 3B). HEK293T cells pretreated with these peptides or a control peptide harboring aa 69 to 93 of BDV-P (pep-ctrl) were infected with influenza A virus (A/WSN/33). Cells pretreated with the PB1-derived PA-binding site (pep1-25) showed an 80% decrease in NP levels 4 h p.i. (Fig. 3C, top panel) and a 70% decrease 8 h p.i. (data not shown) compared to cells pretreated with pep-ctrl. Surprisingly, the pretreatment of the cultures with pep715-740 resulted in a 50% decrease in NP levels (Fig. 3B), suggesting that this peptide maintained residual binding affinity for PB2 despite its inability to coimmunoprecipitate this protein or inhibit viral replication when fused to GFP (Fig. 1B and 3A). Similar results were obtained with influenza A virus strain A/PR8/34 (Fig. 3C, middle panel). The inhibition of protein expression by pep1-25 and pep715-740 was specific for influenza A virus, as these peptides did not block influenza B virus (B/Lee/40) replication (Fig. 3C, lower panel). Furthermore, viral titers in pep1-25-treated MDCK cells were reduced by 95 and 85% compared to those in pep-ctrl-treated cells at 9 and 12 h p.i., respectively, when the peptides were administered before and after infection with influenza A virus (A/WSN/33) at an MOI of 0.02 (Fig. 3D). Significant decreases in viral titers (90%) in pep715-740-treated MDCK cells at 9 h p.i. were also observed; however, at 12 h p.i., the reduction in titers was only 40% (Fig. 3D). At an MOI of 2, viral titers were reduced by ca. 75% only in pep1-25-treated cells at 9 and 12 h p.i. (Fig. 3D). The administration of the peptides after infection revealed a similar picture: whereas pep1-25 efficiently decreased viral titers by approximately 90% at low and high MOIs, pep715-740 showed significant activity only at a low MOI (Fig. 3E). Furthermore, pep1-25 conferred significant inhibition of a mouse-adapted influenza A virus SC35M (H7N7) of avian origin (6), yielding a ca. 4-log difference (Fig. 3F). Similar infection experiments carried out with influenza A/WSN/33 at a low MOI (0.02) also revealed significant inhibition by pep1-25 at 24 h p.i. (data not shown).
FIG. 3.
Peptide-specific inhibition of influenza A virus growth. (A) HEK293T cells were transfected with plasmids expressing the indicated GFP fusion proteins. After 24 h, cells were infected with influenza A virus (FluA) A/WSN/33 (WSN) at an MOI of 0.02. Western blot analysis was carried out 12 h p.i. with total cell extract to determine the levels of the viral NP, actin, and the GFP fusion proteins by using specific antibodies recognizing these proteins. Results from duplicate experiments are shown. The relative ratios of NP and actin signal intensities (NP/actin), the relative GFP signal intensities, and the relative ratios of NP and GFP signal intensities (NP/GFP) are shown. The ratio for cells expressing ctrl-GFP was set at 100. (B) HEK293T (293T) and MDCK cells were incubated with medium containing 10% fetal calf serum and 100 and 300 μM peptides, respectively, consisting of the transmembrane spanning region of TAT (aa 47 to 59) fused to aa 1 to 25 (pep1-25) or 715 to 740 (pep715-740) of PB1 or 25 aa of an unrelated viral sequence (pepCtrl). After 9 h (HEK293T) and 24 h (MDCK), cells were incubated with trypan blue solution (0.4%) for 5 min and washed two times with phosphate-buffered saline. Pos., positive control comprising Triton X-100-damaged cells. (C) HEK293T cells were incubated with medium containing 10% fetal calf serum and 100 μM peptides as indicated. After 1.5 h, cells were washed with phosphate-buffered saline and infected with influenza A virus strain A/WSN/33 for 4 h (upper panel) or A/PR8/34 for 5 h (middle panel) or influenza B virus (B/Lee/40) for 8 h (lower panel) in medium containing 2% fetal calf serum at an MOI of 0.2. Western blot analysis was performed using total cell extract and influenza A and B virus-specific anti-NP and anti-actin antibodies. Results from duplicate experiments are shown. The means of the relative ratios of NP and actin signal intensities are shown, and the ratios observed for cells incubated with pep-ctrl were set at 100. (D) MDCK cells were incubated with 300 μM peptides (Pep., −1.5 h) and infected (Inf., 0 h) with influenza A virus (A/WSN/33) as described in the legend to panel B at an MOI of 0.02 or 2. One hour p.i., cells were washed and further incubated in culture medium containing 2% fetal calf serum and 300 μM peptides (Pep., 1 h) as indicated. The viral titers in the cell culture supernatants were determined by plaque assays. The relative virus yields from pep-ctrl-treated cells observed at 9 and 12 h p.i., corresponding to 2 × 104 and 3 × 105 PFU/ml at an MOI of 0.02 and 5 × 104 and 3 × 105 PFU/ml at an MOI of 2, respectively, were set at 100%. Experiments were performed with triplicate assays. Error bars represent standard deviations of the mean values. +, present. (E) MDCK cells were infected with influenza A virus (A/WSN/33) at an MOI of 0.02 or 2 as described in the legend to panel D but without preincubation with peptides. The viral titers in the cell culture supernatants were determined by plaque assays. The relative virus yields from pep-ctrl-treated cells observed at 9 and 12 h p.i., corresponding to 1 × 103 and 1 × 104 PFU/ml at an MOI of 0.02 and 5 × 104 and 3 × 105 PFU/ml at an MOI of 2, respectively, were set at 100%. (F) MDCK cells were infected with influenza A virus (A/SC35M) at an MOI of 0.02 and treated with peptides as described in the legend to panel E. Stars indicate values from early time points at which titers could be detected in only one of three independent experiments.
In conclusion, we provide a proof of principle that PB1-derived peptides, corresponding to the PA-binding domain, inhibit the growth of influenza A virus by interfering with the viral polymerase activity. Based on these results, we propose that targeting the individual polymerase subunit interaction domains provides exciting possibilities to develop new antivirals that specifically block viral polymerase complex assembly and activity. Since the PA interaction domain of PB1 encompassing aa 1 to 25 is highly conserved (Fig. 1A), such antivirals may block most, if not all, influenza A virus strains. In addition, the possibility of targeting additional interaction sites in the polymerase complex (1, 7, 13, 20) may allow the generation of an antiviral cocktail that could reduce the probability of the appearance of escape mutants. The inhibition of protein complex formation by synthetic peptides that bind to interfaces of viral multiprotein complexes is an emerging tool for interfering with viral replication. Other examples include the peptide-mediated block of the oligomerization of Ebola virus transcription factor VP30 (8), the assembly of DNA polymerase subunits in herpes simplex viruses (11, 18), the activity of human immunodeficiency virus integrase (12), and the intramolecular interactions occurring in viral glycoproteins during fusion (3). In the case of the influenza A virus polymerase complex, the next task will be to screen for molecules other than the peptides used here, ones which are preferably of low toxicity, efficiently penetrate the plasma membrane, and bind with high affinity to their targets.
Acknowledgments
We thank Peter Staeheli and Thorsten Wolff for providing SC35M and B/Lee/40, respectively.
D.M. was partially supported by a grant from the Deutsche Forschungsgemeinschaft (DFG). This work was partially supported by NIH grants to A.G.-S. and a grant from the Müller-Fahnenberg Foundation to M.S.
Footnotes
Published ahead of print on 9 May 2007.
REFERENCES
- 1.Biswas, S. K., and D. P. Nayak. 1996. Influenza virus polymerase basic protein 1 interacts with influenza virus polymerase basic protein 2 at multiple sites. J. Virol. 70:6716-6722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crescenzo-Chaigne, B., N. Naffakh, and S. van der Werf. 1999. Comparative analysis of the ability of the polymerase complexes of influenza viruses type A, B and C to assemble into functional RNPs that allow expression and replication of heterotypic model RNA templates in vivo. Virology 265:342-353. [DOI] [PubMed] [Google Scholar]
- 3.De Clercq, E. 2004. Antivirals and antiviral strategies. Nat Rev. Microbiol. 2:704-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dostmann, W. R., C. Nickl, S. Thiel, I. Tsigelny, R. Frank, and W. J. Tegge. 1999. Delineation of selective cyclic GMP-dependent protein kinase Ialpha substrate and inhibitor peptides based on combinatorial peptide libraries on paper. Pharmacol. Ther. 82:373-387. [DOI] [PubMed] [Google Scholar]
- 5.Fawell, S., J. Seery, Y. Daikh, C. Moore, L. L. Chen, B. Pepinsky, and J. Barsoum. 1994. Tat-mediated delivery of heterologous proteins into cells. Proc. Natl. Acad. Sci. USA 91:664-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gabriel, G., B. Dauber, T. Wolff, O. Planz, H. D. Klenk, and J. Stech. 2005. The viral polymerase mediates adaptation of an avian influenza virus to a mammalian host. Proc. Natl. Acad. Sci. USA 102:18590-18595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonzalez, S., T. Zurcher, and J. Ortin. 1996. Identification of two separate domains in the influenza virus PB1 protein involved in the interaction with the PB2 and PA subunits: a model for the viral RNA polymerase structure. Nucleic Acids Res. 24:4456-4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartlieb, B., J. Modrof, E. Muhlberger, H. D. Klenk, and S. Becker. 2003. Oligomerization of Ebola virus VP30 is essential for viral transcription and can be inhibited by a synthetic peptide. J. Biol. Chem. 278:41830-41836. [DOI] [PubMed] [Google Scholar]
- 9.Hay, A. J., A. J. Wolstenholme, J. J. Skehel, and M. H. Smith. 1985. The molecular basis of the specific anti-influenza action of amantadine. EMBO J. 4:3021-3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jambrina, E., J. Barcena, O. Uez, and A. Portela. 1997. The three subunits of the polymerase and the nucleoprotein of influenza B virus are the minimum set of viral proteins required for expression of a model RNA template. Virology 235:209-217. [DOI] [PubMed] [Google Scholar]
- 11.Loregian, A., and D. M. Coen. 2006. Selective anti-cytomegalovirus compounds discovered by screening for inhibitors of subunit interactions of the viral polymerase. Chem. Biol. 13:191-200. [DOI] [PubMed] [Google Scholar]
- 12.Maroun, R. G., S. Gayet, M. S. Benleulmi, H. Porumb, L. Zargarian, H. Merad, H. Leh, J. F. Mouscadet, F. Troalen, and S. Fermandjian. 2001. Peptide inhibitors of HIV-1 integrase dissociate the enzyme oligomers. Biochemistry 40:13840-13848. [DOI] [PubMed] [Google Scholar]
- 13.Ohtsu, Y., Y. Honda, Y. Sakata, H. Kato, and T. Toyoda. 2002. Fine mapping of the subunit binding sites of influenza virus RNA polymerase. Microbiol. Immunol. 46:167-175. [DOI] [PubMed] [Google Scholar]
- 14.Palese, P., and R. W. Compans. 1976. Inhibition of influenza virus replication in tissue culture by 2-deoxy-2,3-dehydro-N-trifluoroacetylneuraminic acid (FANA): mechanism of action. J Gen. Virol. 33:159-163. [DOI] [PubMed] [Google Scholar]
- 15.Paragas, J., J. Talon, R. E. O'Neill, D. K. Anderson, A. Garcia-Sastre, and P. Palese. 2001. Influenza B and C virus NEP (NS2) proteins possess nuclear export activities. J. Virol. 75:7375-7383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perez, D. R., and R. O. Donis. 1995. A 48-amino-acid region of influenza A virus PB1 protein is sufficient for complex formation with PA. J. Virol. 69:6932-6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perez, D. R., and R. O. Donis. 2001. Functional analysis of PA binding by influenza A virus PB1: effects on polymerase activity and viral infectivity. J. Virol. 75:8127-8136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pilger, B. D., C. Cui, and D. M. Coen. 2004. Identification of a small molecule that inhibits herpes simplex virus DNA polymerase subunit interactions and viral replication. Chem. Biol. 11:647-654. [DOI] [PubMed] [Google Scholar]
- 19.Pleschka, S., R. Jaskunas, O. G. Engelhardt, T. Zurcher, P. Palese, and A. Garcia-Sastre. 1996. A plasmid-based reverse genetics system for influenza A virus. J. Virol. 70:4188-4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poole, E., D. Elton, L. Medcalf, and P. Digard. 2004. Functional domains of the influenza A virus PB2 protein: identification of NP- and PB1-binding sites. Virology 321:120-133. [DOI] [PubMed] [Google Scholar]
- 21.Vives, E., J. P. Richard, C. Rispal, and B. Lebleu. 2003. TAT peptide internalization: seeking the mechanism of entry. Curr. Protein Pept. Sci. 4:125-132. [DOI] [PubMed] [Google Scholar]