Summary
Objectives
To assess the risk of transmission of viral haemorrhagic fevers in northern Ghana.
Design
A two-year cross-sectional entomological study was carried out in four communities in the northern part of Ghana. Standard WHO methods were used to collect adult and larvae of Aedes mosquitoes to estimate man-vector contact rates and larval indices.
Results
A total of 2804 households were surveyed to estimate larval indices and man-vector contacts of potential vectors of viral haemorrhagic fevers such as Yellow fever and Dengue. Over 56% households in each study site were positive for Aedes larvae. Relatively higher Breteaux index (BI) and Container index (CI) were estimated in Damongo (BI: 180 and CI: 44.8) and Jirapa (BI: 149.7 and CI: 41.5) compared to Tumu (BI: 76.1 and CI: 19.5) and Bolgatanga (BI: 72.4 and CI: 20.6). Man-biting rates of 9.8 and 18.5 bites /man/hour were estimated for Damongo and Jirapa respectively whilst Bolgatanga recorded 10 B/M/H. Generally, man-vector contact rates in all the study sites were higher during the dry season than the wet season. Larval indices showed seasonal variations and the dry season was identified as the high-risk period for transmission of viral haemorrhagic fevers and possible disease outbreaks. No flavivirus was detected in the 2034 Aedes mosquitoes from the study sites by RT-PCR.
Conclusions
Aedes mosquito larval densities and adult biting rates, in all the study areas were sufficient to promote outbreaks of viral haemorrhagic fevers.
Keywords: Viral haemorrhagic fevers, Aedes, transmission, Ghana
Introduction
Epidemics of viral haemorrhagic fevers including Yellow fever (YF), Dengue haemorrhagic fever (DHF) and Dengue (DEN) transmitted mainly by mosquitoes pose significant public health problems in the tropics1, 2. Yellow fever is endemic in Ghana and major outbreaks that occurred between 1969–1970 in Damongo and Pong-Tamale in the Northern Region, Bolgatanga and Navrongo in the Upper East Region, and Nandom and Jirapa in the Upper West Region involved 319 cases with 79 deaths3. Yellow fever outbreaks are known to recur every ten to twelve years in the southern sector of Ghana.4 In a recent study of a suspected yellow fever epidemic in northern Ghana, only two of 96 blood samples of patients suspected to have haemorrhagic fever were yellow fever positive; the identity of the rest could not be established using conventional methods (Osei-Kwasi, personal Communication, 2004.). This suggests that other viruses or perhaps yellow fever virus presenting different epitopes may also have been involved. Aedes aegpyti, Ae. luteocephalus and Ae. Africanus, vectors of both YF and DEN, have been implicated in some of the YF outbreaks in Ghana3,4,5.
Dengue has been reported in 18 of the 46 countries in the WHO African Region (AFRO), including seven in West Africa and the average mortality rate is about 5%6. Although DEN, has not been reported in Ghana, it has been detected in Côte d'Ivoire and Burkina Faso, which share common borders with Ghana6. Thus, with increasing migration of people across borders of countries belonging to the Economic Community of West Africa States (ECOWAS) and the absence of organized mosquito control in Ghana it is possible that the status of viral haemorrhagic fever transmission in the country may be changing.
However, there is no comprehensive data on the risk of transmission of viral haemorrhagic fevers in Ghana. The objective of the study was to assess the risk of transmission of viral haemorrhagic fevers in the study areas to be able to develop a surveillance system for the disease in the country. This paper reports on the entomological assessment of risk of transmission of viral haemorrhagic fevers (YF and DEN) in some communities in the northern regions of the country.
Patients and Methods
Study areas
The study was carried out in four towns: Damongo (Northern region), Jirapa and Tumu (Upper West region) and Bolgatanga (Upper East region) of Ghana (Figure 1) during the wet (June–October) and dry (November–May) seasons of 1999 and 2000. The towns were selected on the basis of previous histories of outbreaks of yellow fever and suspected viral haemorrhagic fevers. These areas are characterized by prolonged dry season and a short rainy season covering about five months with annual rainfall under 900mm. Bolgatanga is more cosmopolitan and serves as the Upper East regional capital. Damongo is about 30km from Mole, the biggest game reserve in the country. The game reserve is about 300km2 and harbours different species of animals including chimpanzees, baboons and elephants. Damango and Jirapa are served by Catholic mission hospitals while Tumu and Bolgatanga have Ghana Government hospitals. The vegetation in all these area is typical sahel savannah with short trees including baobab and shea butter trees and long grass.
Figure 1.
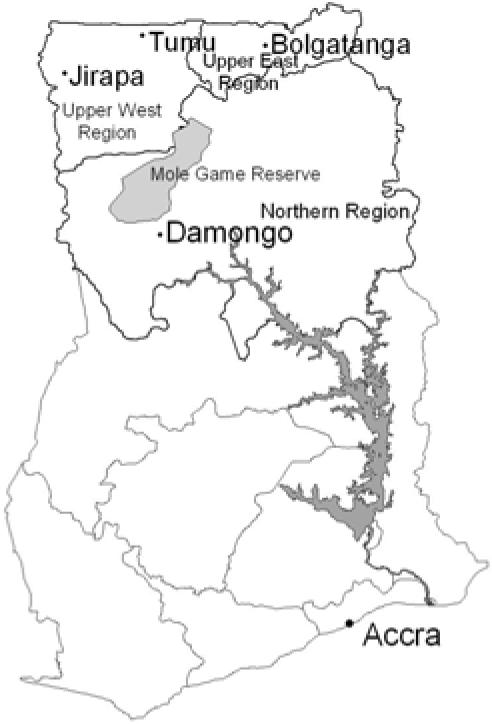
Map of Ghana showing the study sites
Majority of the inhabitants in the study areas are subsistent farmers who grow cashew, millet, and groundnut and keep livestock. Most of the people live in multi-family compounds of dispersed settlements. The houses are built largely of mud with either thatched, mud or iron roofing. During the prolonged dry season when farming activities are reduced, brewing of ‘pito’ a local drink brewed from millet becomes the main occupation.
Household sampling and mosquito surveys
A survey frame of households in each study area was obtained and a simple random sampling was used without replacement to select fifty households for the study. Each household was examined for water storage containers for the presence of pre-adult stages of Aedes mosquitoes for larval indices estimation.
Man-vector contact studies and mosquito identification
Adult Aedes mosquitoes were collected off three persons who exposed their legs for a period of three hours, usually between 4–7pm. All mosquitoes which landed on the men and thus presumed to be biting were collected off them and each other using aspirators and transferred into paper cups; killed with chloroform and identified to species, counted and immediately stored in liquid nitrogen. For each season two landing catches per evening per site for three consecutive days were carried out. Larvae and adult Aedes mosquitoes were identified using morphological characters7. Mosquito collectors were vaccinated against yellow fever and the study was approved by the Ethical Committee of the Ministry of Health.
RT-PCR Detection of Flavivirus in mosquitoes
Mosquitoes were processed in pools of 20 for viral RNA extraction. Reverse transcriptase-polymerase chain reaction (RT-PCR) was performed as previously described 8. The 10-µl PCR reaction contained 2 µl of 5x1st strand buffer, 1 µl of 0.1M dithiothreitol (DTT), 0.5 µl of 10mM dNTPs, 3 of RNA template, 1.25 µl of reverse primer (cFD2) (10 µM concentration), 2 µl of DEPC pre-treated water with 0.25 µl of Avian Myeloblastosis virus (AMV) reverse transcriptase added in each reaction. Forty cycles of amplification were carried out (94°C for 2 min, 95°C for 1 min 50°C for 1 min, 72°C for 1 min and ended with an extension step of 72°C for 7 min) using a thermal cycler [Techne Ltd., UK]. Yellow fever and Dengue 1 infected mosquitoes (female Ae. aegyti after 12 days post-infection) were used as positive controls while uninfected male Ae. aegypti mosquitoes were used as negative controls. The amplified products were electrophoresed in a 2% agarose gel stained with ethidium bromide and visualized under UV light.
Data analysis
The entomological indices were estimated as follows:-
For the larval indices: House index (HI) was expressed as the percentage of houses infested with Aedes larvae; Container index (CI) as the percentage of containers infested; and Breteaux index (BI) as the number of positive containers per 100 inspected houses. The man-vector contact (man biting rate) was estimated as the number of biting adult female Aedes mosquitoes per man-hour. Potential risk areas were estimated based on WHO criteria,9 which states that where the BI, HI and CI exceeded 50, 35 and 20 respectively, the risk of Ae. aegypti-transmitted VHF was considered high. Where the BI was between 5 and 50, the density of Ae. aegypti is considered sufficient to promote an outbreak of VHF disease. Where the BI was less than 5, the HI less than 4, and the CI was also less than 3, it was considered unlikely for urban transmission of VHF to occur. In addition, man-vector count exceeding two female bites per man-hour was also indicative of a significant risk of transmission of the disease9.
Results
Household surveys for the estimation of larval indices
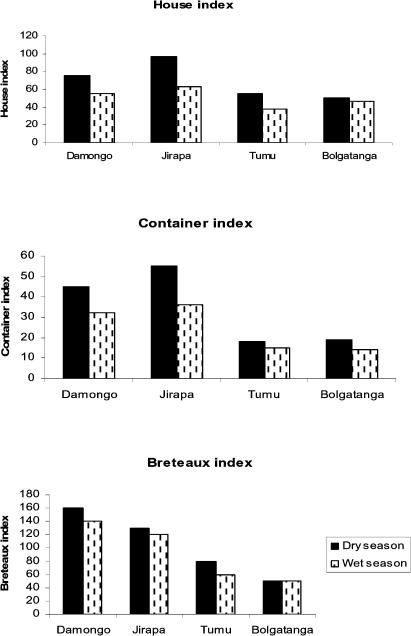
A total of 2804 households 799 at Damongo, 639 at Jirapa 621 at Tumu and 745 at Bolgatanga) were surveyed during the study period. Out of these, more than 56% households at each study site were positive for Aedes larvae, and Damongo and Jirapa recorded the highest larval indices (Table 1).
Table 1.
Aedes mosquito larval indices (House, Container and Breteax) estimated for some sites in the Northern Regions of Ghana
| Indices | Study area Damongo | Jirapa | Tumu | Bolgatanga |
| House Index | 87.7 | 88.3 | 55.9 | 57.6 |
| Container Index | 44.8 | 41.5 | 19.5 | 20.6 |
| Breteaux Index | 180.9 | 149.7 | 76.1 | 72.4 |
House indices ranged from average of 55.9 in Tumu to as high as 88.3 in Jirapa, the CI from 19.5 in Tumu to 44.8 in Damongo and BI from 72.4 in Bolgatanga to 180.9 in Damongo. Ovitrap index of 300 was recorded in Jirapa.
Seasonal variations in the larval indices observed were generally higher during the dry season than the wet season (Figure 2). Damongo and Jirapa recorded the highest estimates of BI and CI indices respectively in the dry season with Bolgatanga recording the least values.
Figure 2.
Seasonal variations in larval indices (House, Container and Breteaux)
Man vector-contact studies and detection of Flavivirus in mosquitoes
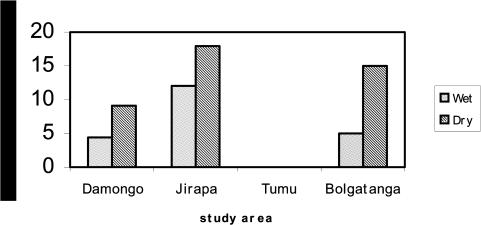
A total of 2034 adult Aedes mosquitoes comprising 1050 and 984 were collected during the dry and wet season respectively in all the study areas. Aedes aegypti was identified as the major mosquito species biting, forming 94% of the total collection with Ae..africanus forming 6%. No Aedes mosquito was collected in Tumu.
Generally, man-vector contact rates in all the study sites except Tumu were higher during the dry season than the wet season. Jirapa recorded the highest average man-vector contact rate in both seasons (18.3 and 12.1 bites per man-hour in the dry and rainy seasons respectively), followed by Bolgatanga and Damongo (Figure 3).
Figure 3.
Man-vector contact rates of Aedes mosquitoes estimated during the wet and dry seasons in some sites in the Northern Regions of Ghana
Out of the total of 2034 Aedes mosquitoes analysed by RT-PCR for flaviviruses (DEN1 and yellow fever), none were positive for any strain of the flavivirus
Discussion
The present study assessed the risk of transmission of viral haemorrhagic fevers (YF and DEN) in some communities in the northern parts of Ghana. All the sites studied were identified as being potential risk areas for transmission of yellow fever, DEN/DHF and other arboviruses. This is evident in the high larval indices and man-vector contact or biting rates recorded in the study areas. For example, Breteaux index estimated in Jirapa was about ten fold higher than what was estimated during the 1980 outbreaks 3,4. This suggests that the risk of transmission of viral haemorrhagic fevers to individuals living in this area has increased ten times over the years. Although no flavivirus was detected in the mosquitoes caught from the study areas, Ae. aegypti and Ae. Africanus were identified as the potential vector species in all the study areas. Several species of Aedes including, Ae. aegypti, Ae. africanus and Ae. Luteocephalus, have also been found to be potential vectors of yellow fever in Ghana5,10. Aedes africanus is known primarily to transmit arboviruses between monkeys but can also transmit to humans when opportunities created by ecological changes, such as deforestation exists. Studies have shown that estimates of Ae. aegypti distribution and density are affected by the life-limiting factors of latitude, altitude, temperature, rainfall, humidity, season, habitat and dispersal11,12,13,14. The main contributory factor to the high larval indices recorded in these study areas was the unavailability of pipe-borne water especially in Damongo and Jirapa. Households therefore stored water in pots and barrels for long periods especially during the dry seasons which allowed the breeding of Aedes. This was apparent in the high larval indices recorded in these areas as compared to Tumu and Bolgatanga where pipeborne water is readily available. Seasonal variation in population density and distribution is common for Ae. aegypti since it is sensitive to changes in temperature and available moisture. Essentially, low mosquito populations are evident in dry and cool seasons and they increase when temperatures increase and the wet season commences15. However, in the contrary, the present study showed that Aedes populations tended to increase during the dry season than the wet season. In Tumu, the pipes are opened every third day and most water containers are cleaned regularly. This practice therefore prevents larvae from reaching adult stage and this may explain the apparent absence of adult Aedes there during the survey. There was a general reduction in the larval indices and man-vector contact rates in the wet season. The observed seasonal changes in transmission indices corroborate previous findings that outbreaks of yellow fever in the northern region occurred mainly at the end of the rainy seasons and peaked during the dry seasons4. These reductions were due to the availability of water from the water impoundments and small dams present in the study sites. It was also observed that storage of water for brewing ‘pito’ (a local brewed drink) in some of the study sites especially in Jirapa and Damongo encourages breeding of Aedes mosquitoes in domestic containers.
Frequent emptying and scouring of water storage containers to remove Aedes eggs; covering or screening of water storage containers to prevent access to mosquitoes are some of the measures that can be taken. In this regard, the provision of regular water supply in the form of wells, or piped water where possible, would reduce the need to store water for long periods. The movement of animals especially baboons and other monkeys from game reserves to human habitations in Damongo need to be monitored, since the existence of Ae. africanus in the area can promote sylvatic (animal-man) transmission of viral haemorrhagic fevers. Vector densities were found to be sufficient to promote outbreak of viral haemorrhagic fevers; thus non-immunized susceptible individuals in the study areas are at risk since viruses could either be introduced by travels or could originate from a local sylvatic cycle (e.g. monkeys). Unlike YF, there is presently no vaccine against DEN/DHF and until such time that safe, affordable and effective vaccine is available, vector control is the only option. Dengue has been reported in Cote d'Ivoire and Burkina Faso6 and although this study did not find any flavi-viruses in the mosquitoes collected, there is the need to develop a nationwide surveillance system to monitor the vector densities and viral infection so as to prevent future outbreaks of viral haemorrhagic fevers including Dengue.
Acknowledgement
We wish to acknowledge the cooperation of the chiefs, opinion leaders and people of Damongo, Jirapa, Tumu and Bolgatanga. Gratitude also goes to all the Regional and District Directors of the Ministry of Health for permitting us to do this project in their regions and districts. The local field workers are also thanked for their devotion to work that ensured the successful completion of the project. We give thanks to Saturo Takeo of the Faculty of Medicine, Graduate School of Medicine and International Health, Tokyo University, Japan for his advice. We are most grateful to Japan International Cooperation Agency (JICA) and Noguchi Memorial Institute for Medical Research (NMIMR) for sponsoring the project as part of the Infectious Diseases project.
References
- 1.Halstead SB. Dengue haemorrhagic fever-a public health problem and a field for research. Bull WHO. 1980;58:1–21. [PMC free article] [PubMed] [Google Scholar]
- 2.Monath TP. Pathobiology of the flaviviruses. In: Schlesinger S, Schlesinger MJ, editors. The Togaviridae and Flaviviridae. New York: Plenum Press; 1986. pp. 375–442. [Google Scholar]
- 3.Agadzi VK, Boatin Boakye A, Appawu MA, Mingle A A, Addy P A. Yellow fever in Ghana, 1977–80. Bull WHO. 1984;62(4):577–583. [PMC free article] [PubMed] [Google Scholar]
- 4.Addy PAK, Minami K, Agadzi VK. Recent yellow fever epidemics in Ghana (1969–1983) East African Med J. 1986;63(6):422–433. [PubMed] [Google Scholar]
- 5.Burton GJ, Noamesi GK, McRae TM. A survey for the vector of yellow in the Damongo area, northern region, Ghana. Ghana Med J. 1964:9–15. [Google Scholar]
- 6.WHO, author. WHO CTD/FIL (DEN)/IC/96.1. Geneva: 1995. Report of the Consultation on: Key Issues in dengue vector control towards the operationalization of a global strategy. [Google Scholar]
- 7.White GB. Family Culicidae. In: Crosskey RW, editor. Catalogue of the Diptera of the Afro-tropical Region. London: British Museum (Natural History); 1980. pp. 114–148. [Google Scholar]
- 8.Tanaka M. Rapid identification of flavivirus using polymerase chain reaction. Journal of Virological Methods. 1993;41:311–322. doi: 10.1016/0166-0934(93)90020-r. [DOI] [PubMed] [Google Scholar]
- 9.WHO, author. Technical guide for a system of yellow fever surveillance. Weekly Epidemiological Records. 1971;46(49):493–500. [Google Scholar]
- 10.Boorman JPT, Porterfield JS. A small outbreak of yellow fever in Gold Coast. Trans Roy Soc Trop Med Hyg. 1957;51:227. doi: 10.1016/0035-9203(57)90079-2. [DOI] [PubMed] [Google Scholar]
- 11.Surtees G. The distribution, density and seasonal prevalence of Aedes aegypti in West Africa. Bull WHO. 1967;36:539–540. [PMC free article] [PubMed] [Google Scholar]
- 12.Chinery WA. A survey of mosquito breeding in Accra, Ghana during a two year period (Sept. 1964 – Aug. 1966) of larval mosquito control. III. The breeding of Aedes (Stegomyia) aegypti, Linnaeus, in Accra. Ghana Med J. 1970;9:197–200. [Google Scholar]
- 13.Service MW. Survey of the relative prevalence of potential yellow fever vectors in north-west Nigeria. Bull WHO. 1974;50:487–494. [PMC free article] [PubMed] [Google Scholar]
- 14.Russell RC. Seasonal abundance of mosquitoes in a native forest of the Murray Valley of Victoria, 1979–1985. J of the Australian Entomol Soc. 1986;25:235–240. [Google Scholar]
- 15.Schultz GW. Seasonal abundance of dengue vectors in Manila, Republic of the Philippines. Southeast Asian Journal of Tropical Medicine and Public Health. 1993;24:369–375. [PubMed] [Google Scholar]