Abstract
Relaxometry and solution thermodynamic measurements show that Gd-H(2,2)-1,2-HOPO is a good candidate as a contrast agent for Magnetic Resonance Imaging (MRI-CA). Acidic, octadentate H(2,2)-1,2-HOPO forms a very stable Gd(III) complex [pGd 21.2 (2)]. The coordination sphere at the Gd(III) center is completed by one water molecule that is not replaced by common physiological anions. In addition, this ligand is highly selective for Gd(III) binding in the presence of Zn(II) or Ca(II).
The symmetric charge distribution of the 1,2-HOPO chelates is associated with favorably long electronic relaxation time T1,2e comparable to those of GdDOTA. This, in addition to the fast water exchange rate typical of HOPO chelates improves relaxivity to r1p = 8.2 mM−1 s−1 (0.47 T). This remarkably high value is unprecedented for small molecule, q = 1 MRI-CA.
Introduction
Recently, the Eu(III) luminescence sensitizing properties of 1,2-HOPO chelates were discovered.2 Ternary complexes of tetradentate 1,2-HOPO ligands support very efficient Eu(III) sensitization with quantum yields as high as ΦEu 21.5 %. However, octadentate ligands such as H(2,2)-1,2-HOPO (1) (Chart 1), which were investigated for improved aqueous stability3 gave a quantum yield for [Eu(1)]− of ΦEu 3.6 %, almost one order of magnitude lower. This decrease was ascribed to the presence of one water molecule in the first coordination sphere and a change of symmetry at the Eu(III) center. While both properties are unfavorable for highly luminescent Eu(III) chelates, they increase relaxivity of Gd(III) complexes, which can be used as MRI-CA. Particularly, the electronic relaxation time is one of the limiting factors for most of the commercially available MRI-CA. The favorable electronic relaxation times of 1,2-HOPO chelates versus 3,2-HOPO have been recognized previously for hexadentate TREN-1,2-HOPO (2).4 However, the relatively low selectivity of 2 for Gd(III) over Zn(II) may represent a problem utilizing this compound as MRI-CA.5 For this reason relaxometric and solution thermodynamic properties of [Gd(1)]− have been investigated.
Chart 1.
Potential MRI-CA based on 1,2-HOPO chelates.
Results
The 1/T1 NMRD profile of a 0.42 mM aqueous solution of [Gd(1)]− was recorded at pH 7.4 at 25 and 37 C (Figure 1). Relaxivities of [Gd(1)]− are very high compared to commercial MRI-CA.6 The low field relaxivity r1p 15 mM−1s−1 (25C) is twice the corresponding value for [Gd(DTPA)(H2O)]2− (r1p 7.8 mM−1s−1) and ca. 25 % higher than for [Gd(DOTA)(H2O)]− with (r1p 12 mM−1s−1). Furthermore, relaxivities at relevant field strengths r1p 8.2 mM−1s−1 (20 MHz) and r1p 7.9 mM−1s−1 (60 MHz) are approximately double to those of both [Gd(DOTA)(H2O)]− and [Gd(DTPA)(H2O)]2−. These relaxivity values, very high considering [Gd(1)]− as a small, monohydrated (q = 1) complex, can be rationalized by fitting the NMRD profiles to equations for inner- and outer spheres paramagnetic relaxation (Table 1).7 The predominant contribution arises from a longer rotational correlation time, τR, about twice that of [Gd(DOTA)(H2O)]−. This implies a rather rigid structure since the increase in size of [Gd(1)]− is efficiently transferred into a corresponding increase of τR. Compared to other MRI-CA and Gd(III) chelates studied,8 Δ2 is small and τV long, which indicates a very favorable longitudinal electronic correlation time T1e of ca. 70 ns at 60 MHz. This is also the case for [Gd(DOTA)(H2O)]− (T1e = 10 ns at 60 MHz), which, however, is limited by a long water residence time τM 472 ps.8
Figure 1.
1/T1 NMRD profile of a solution (pH 7.4) of [Gd(1)]− at 25 and 37 C.
Table 1.
Parameters from refining NMRD profiles of [Gd(1)]− at 25 and 37 C. The third column lists those for [Gd(DOTA)(H2O)] as comparison.9
| Parameters | [Gd(1)]− (298 K) |
[Gd(1)]− (310 K) |
[Gd(DOTA)(H2O)] (298 K) |
|---|---|---|---|
| r1p (mM−1s−1) | 8.2 | 6.3 | 3.9 |
| 20 MHz | |||
| Δ2 (s−2; × 1019) | 0.9 (1) | 1.0 (2) | 1.60 |
| τV (ps) | 51 (2) | 47 (3) | 11 |
| τR (ps) | 157 (6) | 107 (2) | 77 |
| τM (ns) | 10* | 8* | 473 |
| q | 1* | 1* | 1 |
| r (Å) | 3.0* | 3.0* | 3.13 |
| a (Å) | 4* | 4* | - |
| D (cm2s−1; ×105) | 2.24* | 3.0 (1) | 2.2 |
these parameters were fixed during refinement.
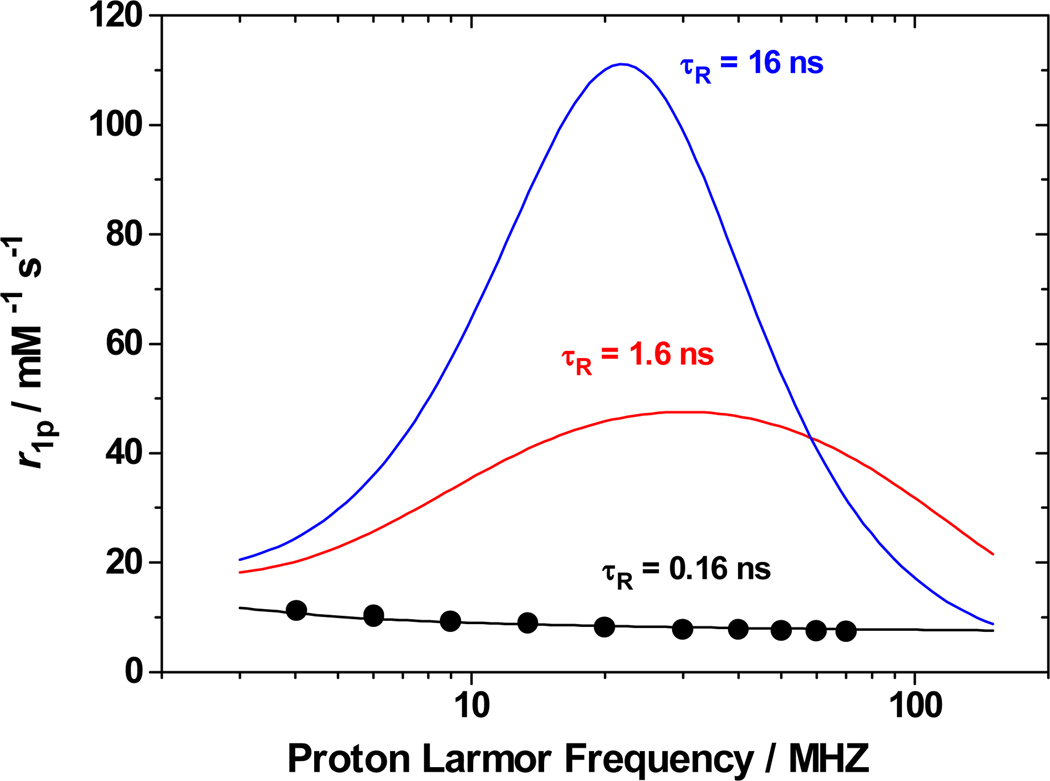
The relation between the parameters Δ2, τV, τR, τM, and relaxivity r1p at high field has been detailed recently.10 Further improvement of the relaxivity of [Gd(1)]− can be achieved by increasing the rotational correlation time τR. T1e can be estimated from Δ2 and τV to be significantly longer than for other HOPO chelates reported.11 Thus T1e and τM represent optimal values for a significant improvement of r1p to over 100 mM−1s−1 by slowing rotational tumbling (Figure 2).
Figure 2.
Simulation of relaxivity r1p for [Gd(1)]− calculated as a function of τR using parameters of Table 1 (298 K).
Attaching such a small molecule contrast agent to a large and rigid molecule can significantly improve τR. For this reason one may take advantage of the association of a MRI-CA to human serum albumin (HSA).12 However, no interaction of [Gd(1)]− with HSA was observed in serum samples. Attachment of a HSA binding moiety to [Gd(1)]− is in progress and will be reported.
The most important parameters for evaluating the toxicity of [Gd(1)]−are complex stability and selectivity of 1 for Gd(III) binding versus Zn(II) and Ca(II).13 The protonation constants 3 illustrate the acidity of 1 compared to 3,2-HOPO chelates.14 The four acidic protonation steps are associated with deprotonation of the 1,2-HOPO moieties while the tertiary nitrogen atoms represent the two basic protonation sites.
Gd(III) binding of 1 versus the benchmark ligand DTPA (pGd 19.11) was monitored by spectrophotometry. Batch titration techniques were employed because of slow protonation kinetics of the complex preventing variable pH titrations. Conditional stability constants were determined at pH 6.0, 7.4, and 9.0 (Table 2). From this data two protonation constants of the Gd(III) complex and logβ values could be calculated. The two basic protonation sites of [Gd(1)]− are located at the scaffold amines because relaxivities of GdL−, GdLH, and GdLH2 + are nearly identical (Figures 3 and S5). The high solubility of [Gd(1)]− (> 0.5 mM) is not affected by protonation at pKa 9.7 (5). However, the protonation of the second scaffold amine with pKa 6.9 (3) reduces the solubility to 0.1 mM. Under physiological conditions the monoprotonated, neutral complex GdLH is dominant (75 %) with one proton being shared by the two amine functions in the scaffold.
Table 2.
Metal Binding Constants of 1.*
| logβGdL | 21.5 (5) | pGd9.0 | 21.7 (1) |
| logβGdLH | 31.2 (2) | pGd7.4 | 21.2 (2) |
| logβGdLH2 | 38.1 (3) | pGd6.0 | 20.0 (2) |
| pZn | 14.5 (1) | pCa | 8.3 (2) |
pM = − log[Mfree] in a solution with cLtot 10 µM and cMtot 1 µM at pH 7.4 or as specified by superscript.
Figure 3.
Calculated speciation of a 0.1 mM solution of [Gd(1)]−.
Zn(II) and Ca(II) binding of 1 is weak (Table 2) as determined by pM values obtained from competition batch titration versus DTPA. The addition of a fourth 1,2-HOPO moiety to the ligand does not improve the stability of the Zn(II) complex resulting in a high selectivity of 1 for lanthanides versus five or six-coordinate transition metals. The difference between pGd and pZn is 6.7 (3). This is well beyond the ability of the benchmark compound DTPA (4.2) and comparable to more basic 3,2-HOPO chelates.15
Calcium binding of 1 is stronger than that of DTPA by almost one order. However, the resulting pCa 8.3 (2) indicates that a 13 log unit excess of Ca(II) is required to remove Gd(III) from [Gd(1)]− although the formation of a mixed complex with Ca(II) cannot be excluded.
[Gd(H1)] and [Eu(H1)] show no affinity for carbonate, biphosphate, and fluoride as established by luminescence spectroscopy (Figure 4) and relaxometry at 60 MHz (Supp. Info), respectively, upon addition of 1100 to 1600 equivalents of anion. The lack of changes in luminescence intensity or relaxation rate observed indicates affinities KA for all three anions well below 10 M−1. Thus, binding constants of [Eu(H1)] are far lower than the already uncritical affinities of Gd-DOTA, Gd-DTPA, and Gd-DTPA-BMA for biphosphate ranging from 100 to 160 M−1.11
Figure 4.
Obtained emission spectra between 600 and 625 nm (J = 2 band) of [Eu(1)]− (black; 17 µM) and after addition of 1100 equivalents of phosphate (blue), 1100 eq. carbonate (green) or 1600 eq. fluoride (blue) at pH 7.4 after 24 hr. equilibration.
Summary and Outlook
[Gd(1)]− reveals excellent properties for utilization as MRI-CA. The very high relaxivity is achieved by optimizing all relevant parameters for this small molecule. Solution thermodynamic assessment predicts very low toxicity based on the high stability of the Gd(III) complex and the excellent selectivity of 1 for Gd(III) over Zn(II) and Ca(II). In addition the low affinity for phosphate, carbonate and protein interactions are valuable for good performance as MRI-CA in vivo and clean excretion. Current efforts aim on exploring strategies to reduce rotational tumbling.
Supplementary Material
Acknowledgement
This work (UCB) was partially supported by NIH grant HL69832 and NATO travel grant PST.CLG.980380. CJJ thanks the German Research Foundation (DFG) for a postdoctoral fellowship. Support from MIUR (COFIN 2005) is also gratefully acknowledged (M.B., S.A.). The authors thank Professor Chang (UCB) for use of a 60 MHz relaxometer.
Footnotes
Supporting Information Available. Additional stability data, figures and tables. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- *.Paper no. 21 in the series “High Relaxivity Gadolinium MRI Agents”. For the previous paper in the series see: Werner EJ, Avedano S, Botta M, Hay BP, Moore EG, Aime S, Raymond KN. J. Am. Chem. Soc. 2007;129:1870–1871. doi: 10.1021/ja068026z.
- 2.Moore EG, Xu J, Jocher CJ, Werner EJ, Raymond KN. J. Am. Chem. Soc. 2006;128:10648–10649. doi: 10.1021/ja062597+. [DOI] [PubMed] [Google Scholar]
- 3.Moore EG, Jocher CJ, Werner EJ, Xu J, Raymond KN. Inorg.Chem. 2007;46 doi: 10.1021/ic700364t. accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu J, Churchill DG, Botta M, Raymond KN. Inorg. Chem. 2004;43:5492–5494. doi: 10.1021/ic049028s. [DOI] [PubMed] [Google Scholar]
- 5.Jocher CJ, Raymond KN. unpublished result. [Google Scholar]
- 6.Laurent S, Vander Elst L, Muller RN. Contrast Med. Mol. Imaging. 2006;1:128–137. doi: 10.1002/cmmi.100. [DOI] [PubMed] [Google Scholar]
- 7.Aime S, Botta M, Terreno E. Gd(III)-based Contrast Agents for MRI. In: van Eldik R, Bertini I, editors. Advances in Inorganic Chemistry. Vol. 57. S. Diego: Elsevier; 2005. pp. 173–237. [Google Scholar]
- 8.Caravan P, Ellison JJ, McMurray TJ, Lauffer RB. Chem. Rev. 1999;99:2293–2352. doi: 10.1021/cr980440x. [DOI] [PubMed] [Google Scholar]
- 9.Powell DH, Ni Dhubhghaill OM, Pubanz D, Helm L, Lebedev YS, Schlaepfer W, Merbach AE. J. Am. Chem. Soc. 1996;118:9333–9346. [Google Scholar]
- 10.Caravan PC. Chem. Soc. Rev. 2006;35:512–523. doi: 10.1039/b510982p. [DOI] [PubMed] [Google Scholar]
- 11.Raymond KN, Pierre VC. Bioconjugate Chem. 2005;16:3–8. doi: 10.1021/bc049817y. [DOI] [PubMed] [Google Scholar]
- 12.Merbach AE, Tóth E. The Chemistry of Contrast Agents. Chichester: Wiley; 2001. [Google Scholar]
- 13.Cacheris WP, Quay SC, Rocklage SM. Magn Reson. Imaging. 1990;8:467–481. doi: 10.1016/0730-725x(90)90055-7. [DOI] [PubMed] [Google Scholar]
- 14.Doble DMJ, Melchior M, O´Sullivan B, Siering C, Xu J, Pierre VC, Raymond KN. Inorg. Chem. 2003;42:4930–4937. doi: 10.1021/ic026240s. [DOI] [PubMed] [Google Scholar]
- 15.Pierre VC, Botta M, Aime S, Raymond KN. Inorg. Chem. 2006;45:8355–8364. doi: 10.1021/ic061262q. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.