Abstract
Cytoskeletal proteins are crucial in maintaining cellular structure and, in certain cell types, also play an essential role in motility and shape change. Nitric oxide (NO) is an important paracrine mediator of vascular and platelet function and is produced in the vasculature by the enzyme NO synthase type 3 (NOS-3). Here, we demonstrate in human platelets that the polymerization state of β-actin crucially regulates the activation state of NOS-3, and hence NO formation, through altering its binding of heat shock protein 90 (Hsp90). We found that NOS-3 binds to the globular, but not the filamentous, form of β-actin, and the affinity of NOS-3 for globular β-actin is, in turn, increased by Hsp90. Formation of this ternary complex among NOS-3, globular β-actin, and Hsp90, in turn, results in an increase in both NOS activity and cyclic guanosine-3′,5′-monophosphate, an index of bioactive NO, as well as an increased rate of Hsp90 degradation, thus limiting the duration for which NOS-3 remains activated. These observations suggest that β-actin plays a critical role in regulating NO formation and signaling in platelets.
Platelets are circulating anucleate blood cells derived from bone marrow megakaryocytes, whose main physiological function is to contribute to hemostasis following injury; under certain pathophysiological conditions, abnormal platelet activation gives rise to vascular thrombosis. Following activation, platelets adhere to the endothelium or subendothelial connective tissue, and this is followed by aggregation and recruitment of further platelets. Prevention of excessive platelet activation is achieved physiologically by a number of mediators, but one of the most important is nitric oxide (NO), produced both by the vascular endothelium and by platelets themselves (1). Indeed, arterial atherothrombotic disease occurs in a number of diseases associated with deficient endothelial and/or platelet NO biosynthesis (2–4), although it remains to be proven in clinical studies that amelioration of NO production in these conditions gives rise to an improvement in clinical outcomes.
NO in platelets is formed through the action of nitric oxide synthase type 3, or NOS-3 (1); this enzyme catalyzes the conversion of the amino acid l-arginine to l-citrulline and NO. Its actions in target cells are largely mediated through the activation of soluble guanylyl cyclase and, hence, increased formation of cyclic guanosine-3′,5′-monophosphate (cGMP). Inhibition of NO production by platelets gives rise to their activation as well as increased adhesion to vascular endothelium (5, 6), which is a precursor to intravascular thrombosis. Thus, platelet NO biosynthesis is crucial for the maintenance of blood fluidity and the prevention of thrombus formation in uninjured healthy vessels physiologically.
The cytoskeleton is vital in maintaining structural integrity in all cell types, and, in certain cells, it also plays an essential role in motility and shape change, for example in platelets in response to a proaggregatory stimulus. NOS-3 has been reported to associate with β-actin, an important cytoskeletal protein, and this association gives rise to significant increases in NOS-3 activity, which are especially marked when NOS-3 associates with the globular as opposed to the filamentous form of actin (7). This suggests that NOS-3 activity can be crucially regulated by the cytoskeleton, and this can explain how in endothelial cells NOS-3 activity can be increased in response to cellular deformation and shear stress (8–10). In platelets, aggregation is accompanied by cytoskeletal rearrangement (11), and this may have an important influence on platelet NO biosynthesis and, hence, thrombogenicity. However, the mechanism by which this association between NOS-3 and β-actin can regulate NOS-3 activity remains unclear.
The activity of NOS-3 is regulated by a number of factors, including intracellular Ca2+ levels, availability of cofactors (dihydronicotinamide adenine dinucleotide phosphate, flavin adenine dinucleotide, flavin mononucleotide, tetrahydrobiopterin), phosphorylation (by a number of protein kinases, including protein kinase A, Akt, and protein kinase C), and interactions with other proteins both within the cell membrane and the cytosol (12). These latter proteins include calmodulin (which binds Ca2+ when intracellular Ca2+ increases, and this in turn increases its affinity for NOS-3), heat shock protein 90 (Hsp90), and caveolin-1 (a protein found specifically in specialized structures within the plasma membrane termed caveolae, where NOS-3 is normally situated) as well as the nitric oxide synthase-interacting protein (NOSIP) and dynamin-2, although the precise physiological role of the latter two proteins is speculative at present (13–15).
We hypothesized that NOS-3 activity is crucially regulated by the polymerization state of β-actin in platelets, being increased to a greater degree by association with the globular as compared with the filamentous form of β-actin and that this may be mediated through a change in intracellular Ca2+ flux, an alteration in NOS-3 phosphorylation, and/or a modification in binding of other proteins. We therefore investigated the role of globular as compared with filamentous β-actin in NOS-3 regulation in platelets as well as the mechanism by which β-actin may influence NOS-3 activity, focusing on Ca2+ flux, NOS-3 phosphorylation state and associations with Hsp90 and caveolin-1.
Results and Discussion
NOS-3 Binds to Globular but Not Filamentous β-Actin in Platelets.
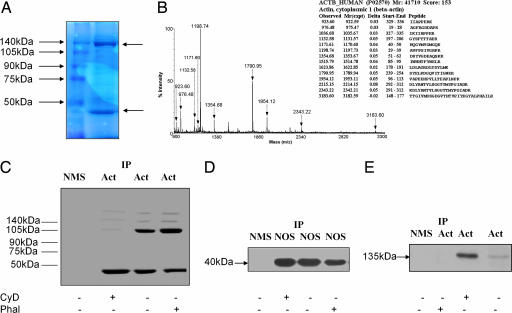
To confirm that NOS-3 binds β-actin, isolated platelets from healthy human subjects were lysed and NOS-3 immunoprecipitated by using a specific anti-NOS-3 antibody. The immunoprecipitate was subjected to SDS/PAGE, and the gel was subsequently stained with Coomassie Brilliant blue. Two predominant bands were seen (Fig. 1A), one at 135 kDa (which is the molecular mass of NOS-3) and the other at 40 kDa (the known molecular mass of globular β-actin). To verify its identity, the 40-kDa band was excised from the gel and subjected to trypsin digestion, followed by measurement of the molecular masses of the resulting peptides by using MALDI-TOF mass spectrometry; the peptide mass fingerprint, coupled with a database search using the Mascot algorithm, allowed successful identification of the protein as β-actin (Fig. 1B). Fourteen peptides were matched within 50 ppm mass accuracy.
Fig. 1.
NOS-3 binds to the globular, but not the filamentous, form of β-actin in platelets. (A) A protein of molecular mass 40 kDa coimmunoprecipitates with NOS-3. Human platelets, isolated from blood of healthy subjects (n = 4), were lysed, and NOS-3 was immunoprecipitated. This immunoprecipitate was subjected to SDS/PAGE on a 10% polyacrylamide gel, which was stained with Coomassie Brilliant blue. The left lane shows molecular mass markers, and the right lane is NOS-3 immunoprecipitate from platelets. Two bands predominate (arrowed), one at 135 kDa (the known molecular mass of NOS-3) and another at 40 kDa. (B) The 40-kDa protein is β-actin. The 40-kDa band was excised from the gel and digested with trypsin, and the digest analyzed by MALDI-TOF. The peptide mass fingerprint is that of β-actin. (C) β-Actin exists in both globular and filamentous forms in platelets. Human platelets, isolated from blood of healthy subjects (n = 6), were treated with vehicle, cytochalasin D 5 μmol/liter or phalloidin 5 μmol/liter, and lysed, and β-actin was immunoprecipitated from cell lysates. This immunoprecipitate was subjected to nondenaturing PAGE, followed by Western blotting for β-actin. Two predominant bands are seen, at 40 kDa (representing globular β-actin) and 105 kDa, as well as weaker bands at higher molecular mass. Treatment with cytochalasin D increases the density of the 40-kDa band and reduces the density of higher-molecular-mass bands. By contrast, treatment with phalloidin decreases the density of the 40-kDa band while increasing the density of higher-molecular-mass bands, especially the 105-kDa band. (D) NOS-3 binds globular, but not filamentous, β-actin. NOS-3 was immunoprecipitated from platelet lysates obtained from healthy subjects (n = 6), run on nondenaturing PAGE, and Western blotted for β-actin. Only a 40-kDa band was seen, with no evidence of higher-molecular-mass forms of β-actin present in the NOS-3 immunoprecipitates. Cytochalasin D increases, and phalloidin decreases, the density of the 40-kDa band. (E) Immunoprecipitation of β-actin pulls down NOS-3. β-Actin was immunoprecipitated from platelet lysates obtained from healthy subjects (n = 6) and was then run on SDS/PAGE and Western blotted for NOS-3. A 135-kDa band is seen, corresponding to the known molecular mass of NOS-3. Cytochalasin D increases, and phalloidin decreases, the density of the 135-kDa band. IP, immunoprecipitation; NMS, normal mouse serum; Act, anti-β-actin antibody; NOS, anti-NOS-3 antibody; CyD, cytochalasin D 5 μmol/liter; Phal, phalloidin 5 μmol/liter.
To show that β-actin exists in both globular and filamentous forms in platelets, lysates were subjected to nondenaturing PAGE and β-actin was immunoprecipitated and then detected by Western blotting using a monoclonal anti-β-actin antibody (Fig. 1C). Two predominant bands were detected, one at 40 kDa (the predicted molecular mass of monomeric β-actin) and another at 105 kDa, although other bands of higher molecular mass were also seen that were only weakly detected; to confirm that these higher-molecular-mass bands corresponded to filamentous forms of β-actin, isolated platelets were treated with either cytochalasin D 5 μmol/liter, an inhibitor of actin polymerization (16), phalloidin 5 μmol/liter, a chemical derived from Amanita phalloides that specifically binds the filamentous form of actin and stabilizes it (16), or vehicle for 20 min, after which β-actin was immunoprecipitated from platelet lysates and detected by Western blotting as before (Fig. 1C). After cytochalasin D, the 40-kDa band density increased (by 27.4 ± 3.2%, n = 6, P < 0.001), and this was accompanied by a clear reduction in the density of the higher molecular mass bands (especially the 105-kDa band). On the other hand, after phalloidin treatment, the 40-kDa band density was significantly reduced (by 44.8 ± 4.6%, n = 6, P < 0.001), with an accompanying increase in the density of the higher-molecular-mass bands (again, the change was most evident in the 105-kDa band). These results confirm that both globular and filamentous forms of β-actin are present in platelets under basal conditions and that their relative abundance can be manipulated pharmacologically by using these agents.
To ascertain whether NOS-3 bound both globular and filamentous β-actin, NOS-3 immunoprecipitates from platelet lysates were subjected to nondenaturing PAGE, and β-actin was detected by Western blotting. Only a 40-kDa band could be detected, suggesting that NOS-3 binds solely to the globular form of β-actin (Fig. 1D). Cytochalasin D treatment resulted in an increase in density of the 40-kDa band, of 22.1 ± 4.2% (n = 6, P < 0.01), consistent with an increase in NOS-3 binding by globular β-actin, whereas phalloidin caused a decrease in its density, of 35.6 ± 6.4% (n = 6, P < 0.001).
We also wished to confirm that the apparent observed association of NOS-3 with β-actin was not simply because of cross-reactivity of the anti-NOS-3 antibody with β-actin. For this purpose, we immunoprecipitated β-actin from platelet lysates by using the anti-β-actin antibody, and subjected these immunoprecipitates to nondenaturing PAGE followed by Western blotting, this time probing the blots with a monoclonal anti-NOS-3 antibody. This showed the presence of a band at 135 kDa (Fig. 1E), confirming that immunoprecipitation of either NOS-3 or of β-actin gave rise to coimmunoprecipitation of the other protein. Treatment of platelets with cytochalasin D increased the degree of NOS-3 coimmunoprecipitation, as determined from its band density, by 74.3 ± 6.7% (P < 0.001), again consistent with an increased association of NOS-3 with globular β-actin, whereas phalloidin decreased the density of this band by 80.4 ± 8.8% (P < 0.001).
Polymerization Status of β-Actin Regulates NOS-3 Activity and NO Bioactivity in Platelets.
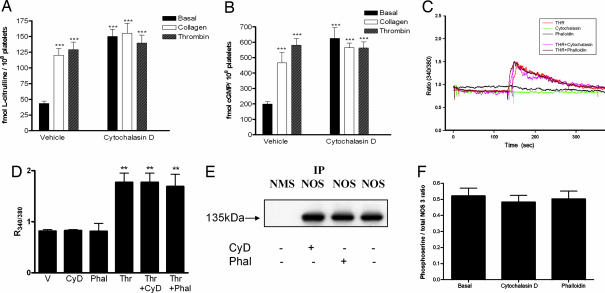
Although we found β-actin to associate with NOS-3, this might be of limited or no importance physiologically, because actin is a frequent contaminant in immunoprecipitates of many cellular proteins. However, the actin/NOS-3 association has been reported to have functional consequences (7). In accordance with this reported finding in pulmonary artery endothelial cells, we hypothesized that increased association in platelets of globular β-actin with NOS-3 would give rise to an increase in NOS-3 activity, and hence NO biosynthesis. To address this question, NOS activity was measured in isolated human platelets from the conversion of l[3H]arginine to l[3H]citrulline, and cGMP (an index of bioactive NO) was determined by RIA, as described (6, 17); these experiments were performed in platelets untreated or treated with cytochalasin D 5 μmol/liter for 20 min and incubated with collagen 0.8 mg/liter, thrombin 1 unit/ml or vehicle, for 2 min. In the absence of cytochalasin D, collagen and thrombin each increased platelet NOS activity (18, 19); in the presence of cytochalasin D, however, basal platelet NOS activity was significantly increased, by 368.2 ± 32.6% (n = 6, P < 0.001), and no further increase in NOS activity could be elicited with collagen or thrombin (Fig. 2A). Similarly, we found that cytochalasin D increased basal intraplatelet cGMP by 304.2 ± 26.8% (n = 6, P < 0.001) and, whereas collagen and thrombin could increase cGMP in the absence of cytochalasin D, they could not increase cGMP any further in the presence of cytochalasin D (Fig. 2B). Coincubation with the NOS antagonist NG-nitro-l-arginine methyl ester (0.1 mmol/liter) inhibited measured NOS activity and cGMP completely (data not shown), confirming that the effects observed were indeed because of alterations in platelet NOS activity and bioactive NO. Although these data do not exclude the possibility that cytochalasin D might exert direct effects on NOS-3 and soluble guanylyl cyclase, because β-actin associates with NOS-3 it is much more likely that modulation of this association by effects of cytochalasin D on the β-actin polymerization state is responsible for its observed effects on NO signaling. Furthermore, binding of cytochalasin D to targets other than actin has not been reported in the literature.
Fig. 2.
Polymerization status of β-actin regulates NOS-3 activity and bioactive NO, with no change in intracellular Ca2+ or serine phosphorylation of NOS-3. (A) NOS activity was determined, from l-[3H]arginine to l-[3H]citrulline conversion, in platelets isolated from healthy human subjects treated with cytochalasin D 5 μmol/liter or vehicle, which were coincubated with collagen 0.8 mg/liter, thrombin 1 unit/ml or corresponding vehicle. In platelets not treated with cytochalasin D, collagen and thrombin increase NOS activity. In platelets treated with cytochalasin D, basal NOS activity increases, and no further increase occurs with collagen or thrombin cotreatment. (n = 6, ± SD, ∗∗∗, P < 0.001 as compared with basal NOS activity in untreated platelets). (B) cGMP was assayed in platelets isolated from healthy human subjects treated with cytochalasin D 5 μmol/liter or vehicle, which were coincubated with collagen 0.8 mg/liter, thrombin 1 unit/ml or corresponding vehicle. In platelets not treated with cytochalasin D, collagen and thrombin increase intraplatelet cGMP. In platelets treated with cytochalasin D, basal cGMP is increased, and no further increase occurs with collagen or thrombin cotreatment. (n = 6, ± SD, ∗∗∗, P < 0.001 as compared with basal cGMP in untreated platelets). (C) A typical experiment showing changes in intraplatelet Ca2+ levels, as determined by fura-2 fluorescence and expressed as the ratio of emission at 510 nm after excitation at 340 and 380 nm (R340/380); tracings show no effect of cytochalasin D 5 μmol/liter or phalloidin 5 μmol/liter, whereas thrombin 1 unit/ml used as a positive control elicits an increase in intracellular Ca2+. The combination of thrombin with either cytochalasin D or phalloidin yields traces not different to that seen with thrombin alone. (D) Accumulated results showing effects of cytochalasin D (CyD), phalloidin (Phal) and thrombin (Thr), either alone or in combination as indicated, as compared with vehicle (V), on intraplatelet Ca2+, as determined from R340/380 (n = 6, ± SD, ∗∗, P < 0.01 as compared with vehicle). (E) Phosphoserine-modified NOS-3 was detected by Western blotting for phosphoserine in NOS-3 immunoprecipitates. IP, immunoprecipitation; NMS, normal mouse serum; NOS, anti-NOS-3 antibody; CyD, cytochalasin D 5 μmol/liter; Phal, phalloidin 5 μmol/liter. (F) Accumulated results showing phosphoserine-modified NOS-3, quantitated as the ratio of phosphoserine to NOS-3 bands as determined by scanning densitometry (n = 6, ±SD).
We next wished to determine whether the activation of NOS-3 by globular β-actin could be explained by an increase in intracellular Ca2+ or by serine phosphorylation of NOS-3. Changes in intracellular Ca2+ were examined by using platelets loaded with the fluorescent dye fura 2-AM (n = 6). No change in intracellular Ca2+ was detected in platelets treated with cytochalasin D or phalloidin; by contrast, intracellular Ca2+ increased in response to thrombin 1 unit/ml, which was used as a positive control, and the thrombin response was not altered by coincubation with either cytochalasin D or phalloidin (Fig. 2 C and D). We next examined the alternative possibility, that the polymerization state of β-actin might regulate the serine phosphorylation status of NOS-3, such that binding of globular β-actin to NOS-3 might promote an increase in its serine phosphorylation and hence in its activity. We therefore determined NOS-3 serine phosphorylation, following immunoprecipitation of NOS-3, by Western blotting using an anti-phosphoserine antibody (n = 6); no change in serine phosphorylation was detected, in response to incubation of platelets with cytochalasin D or phalloidin (Fig. 2 E and F).
Hsp90 Colocalizes with NOS-3 and β-Actin in Platelets.
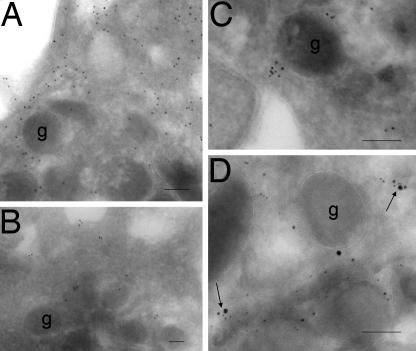
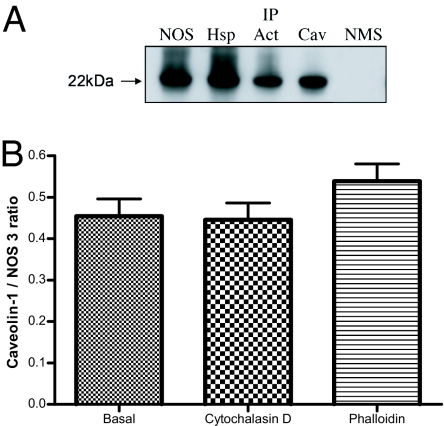
In view of the known association of Hsp90 with NOS-3, and the known stimulatory effect of Hsp90 on NOS-3 activity (20, 21), we examined the distribution of NOS-3, β-actin, and Hsp90 in platelets by immunogold labeling. All three proteins were detected in platelets, with labeling of β-actin being predominant over that of NOS-3 or Hsp90 (Fig. 3 A–C). By the use of triple labeling, we demonstrated a number of regions where all three proteins colocalized (Fig. 3D). Localization of proteins to caveolae was confirmed by Western blotting detection of caveolin-1 (an integral protein of plasmalemmal caveolae) after immunoprecipitation of any of these three proteins (Fig. 4A). Caveolin-1 association with NOS-3 was not altered by treatment of platelets with cytochalasin D or phalloidin (Fig. 4B).
Fig. 3.
Hsp90 colocalizes with NOS-3 and β-actin. β-Actin, Hsp90, and NOS-3 were each detected (A–C, respectively) in fixed platelets from six healthy subjects, by ImmunoGold labeling with electron microscopy. β-Actin shows more extensive labeling than either Hsp90 or NOS-3, and this is distributed throughout the cytoplasm, whereas the labeling for Hsp90 and NOS-3 is clustered. Triple labeling shows multiple areas (arrowed) where β-actin (5-nm gold particles), NOS-3 (10-nm gold particles), and Hsp90 (15-nm gold particles) colocalize (D). g, platelet granule.
Fig. 4.
Hsp90, NOS-3 and β-actin localize to caveolae. (A) Platelets isolated from healthy subjects were lysed and, after immunoprecipitation of NOS-3 (NOS), Hsp90 (Hsp), β-actin (Act) or caveolin-1 (Cav) by using specific antibodies coated onto protein A-Sepharose beads, immunoprecipitates were run on SDS/PAGE by using a 12.5% polyacrylamide gel and Western blotted for caveolin-1. A 22-kDa protein is seen in all immunoprecipitates, which accords with the known molecular mass of caveolin-1. IP, immunoprecipitation; NMS, normal mouse serum used for immunoprecipitation as control. (B) Coimmunoprecipitation of caveolin-1 and NOS-3, quantitated as the ratio of caveolin-1 to NOS-3 bands as determined by scanning densitometry of Western blots of NOS-3 immunoprecpitates probed with anti-caveolin-1 or anti-NOS-3 antibody; cytochalasin D 5 μmol/liter and phalloidin 5 μmol/liter have no effect on caveolin-1 association to NOS-3 (n = 6, ±SD).
To establish whether the degree of colocalization of Hsp90, β-actin, and NOS-3 is altered by an agonist known to increase NOS-3 activity, we measured colocalization by triple ImmunoGold labeling in platelets that were treated for 10 min with collagen 0.8 mg/liter, which activates NOS through an increase in intracellular Ca2+ (18, 19), or corresponding vehicle. Collagen significantly increased the percentage of colocalization, from 8.3 ± 3.5% to 16.0 ± 2.5% (n = 6, P < 0.05). It is likely, however, that these figures represent an underestimate of the true degree of colocalization, because the process of triple labeling reduces the overall degree of labeling because of steric hindrance caused by the large (principally the 15-nm, but also to some degree the 10-nm) gold particles.
Globular, but Not Filamentous, β-Actin Forms a Tight Ternary Complex with NOS-3 and Hsp90.
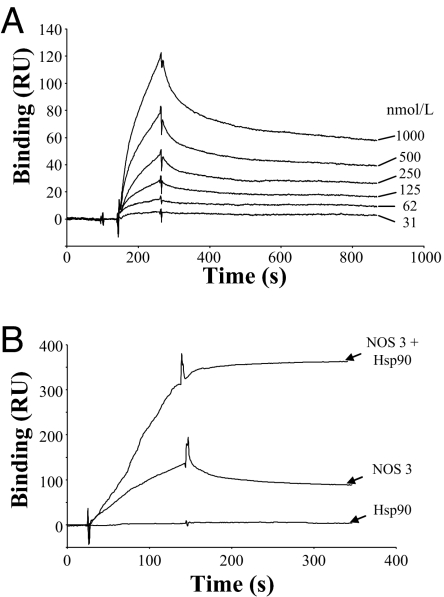
The characteristics of binding of β-actin to NOS-3 were examined in vitro by surface plasmon resonance analysis. Sensor chip CM5 was coated with purified monomeric β-actin, after which recombinant NOS-3 was injected and binding measured from the change in refractive index. We found that NOS-3 binding to monomeric β-actin was concentration-dependent (Fig. 5A), with a KD value of 240 ± 57 nmol/liter (kon = 1.4 × 104 M−1 s−1; koff = 3.3 × 10−3 s−1), and reversible. By contrast, coated polymerized β-actin on the sensor chip gave rise to no observable binding of NOS-3 (data not shown). Additionally, we found no detectable binding of Hsp90 to immobilized β-actin, whether polymerized or nonpolymerized.
Fig. 5.
Globular β-actin forms a ternary complex with NOS-3 and Hsp90. (A) NOS-3 binds monomeric β-actin in a concentration-dependent manner. A CM5 sensor chip was chemically coated with purified monomeric β-actin [11,000 resonance units (RU)] and, after injection of different concentrations of recombinant NOS-3 (31 nmol/liter to 1 μmol/liter), binding was determined by surface plasmon resonance analysis. A representative experiment is shown from a total of three experiments. (B) Hsp90 increases binding of NOS-3 to monomeric β-actin. Binding was determined to monomeric β-actin immobilized on a CM5 sensor chip (13,000 RU), by surface plasmon resonance analysis, after preincubation of NOS-3 with Hsp90 in a 1:1 ratio. Although Hsp90 alone (500 nmol/liter) was found not to bind to monomeric β-actin, its preincubation with NOS-3 was found to increase the binding of NOS-3 by 2.8 ± 1.2-fold, and this binding became irreversible. A representative experiment is shown from a total of three experiments.
We found that preincubation of NOS-3 with Hsp90 (in a 1:1 ratio) increased the degree of binding to immobilized monomeric β-actin, by 2.8 ± 1.2-fold, and this binding became irreversible (Fig. 5B). Taken together, these results suggest the formation of a tightly associated ternary complex onto the sensor chip.
These data indicate that filamentous β-actin does not bind either NOS-3 or Hsp90, whereas, by contrast, globular β-actin binds NOS-3 with an affinity in the submicromolar range, and this binding is increased substantially by Hsp90, resulting in the irreversible formation of a ternary complex. Hsp90 has been reported to cause activation of NOS-3; our results suggest that Hsp90 only binds NOS-3 in the presence of globular β-actin, so that this facilitatory action of Hsp90 on NOS-3 activity can only occur as a result of the formation of the globular β-actin-NOS-3-Hsp90 ternary complex.
Hsp90 Binding Increases Its Degradation in Vivo.
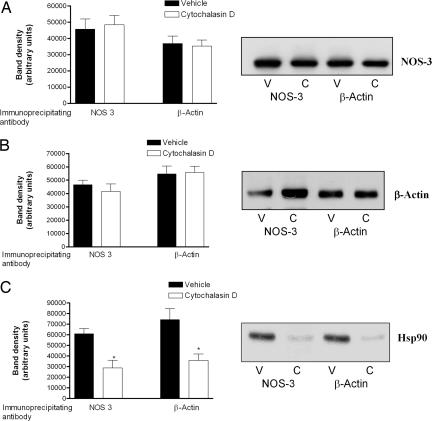
We next determined whether promoting the formation of globular over filamentous β-actin in platelets gives rise to an alteration in either cellular levels or degree of coassociation of β-actin, Hsp90 and NOS-3. Treatment of platelets with cytochalasin D 5 μmol/liter for 20 min did not affect the amount of Hsp90, NOS-3, or β-actin detectable by Western blotting in whole-cell lysates (data not shown), suggesting no change in overall level of these proteins in the cell. Nor did such treatment affect the amount of NOS-3 or β-actin detected after immunoprecipitation of NOS-3 or β-actin (Fig. 6 A and B). By contrast, cytochalasin D decreased the amount of Hsp90 that was detectable by Western blotting, after immunoprecipitation with antibody to either NOS-3 or β-actin (Fig. 6C). This suggests that promoting the formation of β-actin-NOS-3-Hsp90 ternary complex, by using cytochalasin D to increase the amount of globular over filamentous β-actin, leads to a rapid loss of Hsp90 from this complex.
Fig. 6.
Formation of a ternary complex among Hsp90, β-actin, and NOS-3 leads to a decrease in bound Hsp90. Platelets from healthy subjects were treated with cytochalasin D 5 μmol/liter or vehicle, after which they were lysed and, after immunoprecipitation of NOS-3 or β-actin, immunoprecipitates were run on SDS/PAGE and Western blotted for either NOS-3 (A) or β-actin (B). Typical blots are shown (V, vehicle; C, cytochalasin D treatment), and results are presented for band density, as determined by scanning densitometry (n = 6, ±SD). Cytochalasin D does not affect the degree of coimmunoprecipitation of NOS-3 and β-actin. (C) Cytochalasin D treatment decreases Hsp90 levels in NOS-3 and β-actin immunoprecipitates. Platelets from healthy subjects were treated with cytochalasin D or vehicle, after which they were lysed and, after immunoprecipitation of NOS-3 or β-actin, immunoprecipitates were run on SDS/PAGE and Western blotted for Hsp90. A typical blot is shown (V, vehicle; C, cytochalasin D treatment), and results are presented for band density, as determined by scanning densitometry (n = 6, ±SD, ∗, P < 0.01 for cytochalasin D- versus vehicle-treated platelets).
To determine whether cytochalasin D treatment gives rise to an increase in the rate of Hsp90 degradation in the ternary complex itself, we made use of Mycograb, a human recombinant antibody derived from the anti-Hsp90 antibody cDNA of patients recently recovered from invasive candidiasis (22); it consists of the antigen-binding variable domains of antibody heavy and light chains linked together to create a recombinant protein that is expressed in Escherichia coli, and detects the cellular breakdown products of Hsp90 (23). We found, by Western blotting of NOS-3 immunoprecipitates from platelets, that this antibody detected a band at 90 kDa, corresponding to the known molecular mass of intact Hsp90, as well as three other lesser bands, at 47, 40, and 30 kDa, corresponding to Hsp90 degradation products [see supporting information (SI) Fig. 7A]. Treatment of platelets with cytochalasin D 5 μmol/liter for 20 min decreased the intensity of the 90-kDa band, consistent with our data using anti-Hsp90 antibody, and increased the density of the smaller-molecular-mass bands (SI Fig. 7A). In whole-platelet lysates, no change was detectable in the 90-kDa band, but once again, the lower-molecular-mass bands were increased after cytochalasin D treatment (SI Fig. 7B).
Taken together, these experiments suggest that Hsp90 forms a tight complex with NOS-3 and globular β-actin in platelets and that this binding gives rise to an increase in the rate of Hsp90 degradation on the ternary complex in vivo. This may serve to provide a negative-feedback mechanism, thereby limiting the time for which NOS-3 is in the activated state, such that, once Hsp90 is degraded, NOS-3 is released from β-actin.
Our results suggest that, physiologically, NOS-3 activity would be expected to be increased under conditions that favor the existence of β-actin in globular rather than filamentous form. Activation of platelets by any prothrombotic stimulus produces a reproducible sequence of morphologic events, whether in suspension or during spreading on glass: rounding, filopodial projection, attachment, spreading, and, ultimately, contraction (24–29). These morphologic changes depend on the reorganization of the actin cytoskeleton, including severing of existing filaments, which causes the discoid platelet to round and depends on gelsolin (26, 30, 31), and polymerization of actin monomers into new filaments (26, 32–34). These new actin filaments organize into four distinct structures: filopodia, lamellipodia, stress fibers, and a contractile ring (27). Each of these structures performs a different function, and each contains a different complement of actin-binding proteins (27–29). Thus, increased conversion of globular to filamentous β-actin is an integral component of platelet activation, shape change, adhesion and aggregation. Our data suggest that this conversion will concomitantly result in a decrease in platelet NO biosynthesis, which, in turn, will give rise to an increase in platelet activation and subsequent thrombotic events. The modulation of NOS-3 activity by β-actin conformation through effects on Hsp90 binding and degradation as described here represents a hitherto unrecognized mechanism of regulation of platelet function by the actin cytoskeleton.
Materials and Methods
Platelet Preparation.
Subjects recruited were healthy volunteers, on no drug treatment and with no evidence of disease clinically, as judged from history and examination. All subjects gave informed consent, and approval for the study was granted by the St. Thomas' Hospital Research Ethics Committee. By using a 19-gauge butterfly needle (Abbott, Sligo, Ireland), 120 ml of venous blood was obtained from an antecubital vein and collected into trisodium citrate (0.38% final concentration). Platelet-rich plasma (PRP), prepared by centrifuging the whole blood (8 min, 800 × g, 20°C), was applied to a Sepharose CL-2B gel column (Amersham Biosciences, Little Chalfont, U.K.). Gel-filtered platelets (GFP) were eluted with and collected in sodium tyrode buffer [final composition: NaCl 125.0 mmol/liter, glucose 5.6 mmol/liter, Hepes 5.0 mmol/liter, KCl 2.8 mmol/liter, KH2PO4 0.8 mmol/liter, MgCl2 0.4 mmol/liter (pH 7.4)]. The platelet count was measured in the eluate by using a Coulter counter (Gen.S System 2, Beckman Coulter, High Wycombe, U.K.).
Platelet NOS Activity and cGMP Measurement.
NOS activity was assessed by measuring the conversion of l-[3H]arginine to l-[3H]citrulline, and cGMP was determined by RIA, both in GFP in suspension (unstirred) at 37°C, as described (6, 17, 35).
Assessment of Changes in Cytoplasmic Ca2+.
PRP (stirred at 300 rpm) were incubated with fura-2-AM 3 μmol/liter (1 h, 37°C), after which the suspension was allowed to cool to room temperature and acidified to pH 6.5 by the addition of citric acid 6 mmol/liter. Platelets were then pelleted (650 × g, 15 min, room temperature) and resuspended in buffer [final composition: NaCl 140.0 mmol/liter, KCl 5.0 mmol/liter, MgCl2 1.0 mmol/liter, glucose 5.0 mmol/liter, NaH2PO4 0.42 mmol/liter, NaHCO3 12.0 mmol/liter, indomethacin 0.003 mmol/liter (pH 7.35)] with added apyrase (1 unit/ml), to a platelet concentration of 1.5–2 × 108 platelets per ml, in the dark at 37°C. Samples were constantly stirred at 300 rpm by using a magnetic stirrer. After addition of EGTA 0.1 mmol/liter, and equilibration for 2 min, samples were incubated with cytochalasin D 5 μmol/literL, phalloidin 5 μmol/liter, or 1 unit/ml thrombin (as a positive control). Changes in cytoplasmic Ca2+ were examined as a function of time, over 3 min, from the ratio of emission at 510 nm after excitation at 340 nm and 380 nm, in a dual excitation wavelength LS50 luminescence spectrometer (6).
Immunoprecipitation.
NOS-3, Hsp90 and β-actin were immunoprecipitated from GFP, as detailed in SI Materials and Methods.
PAGE and Western Blotting.
PAGE and electroblotting onto a polyvinylidene fluoride membrane were performed as described (36). Western blotting detection of NOS-3, Hsp90 (or its degradation products), β-actin, caveolin-1, or phosphoserine was performed as elaborated in SI Materials and Methods.
Peptide Mass Fingerprinting.
In-gel reduction, alkylation, and digestion with trypsin were performed on the gel slice before analysis by mass spectrometry, as outlined in SI Materials and Methods.
Immunoelectron Microscopy.
Immunoelectron microscopy was carried out as described (37). Single, double, and triple labeling were carried out as detailed in SI Materials and Methods.
Surface Plasmon Resonance Analysis of in Vitro Protein Binding.
Binding experiments and kinetic analysis were performed at 25°C by using a Biacore 3000 biosensor system (Biacore International, Neuchâtel, Switzerland). Full details are given in SI Materials and Methods.
Statistical Analysis.
All data are presented as mean ± standard deviation. Statistical comparisons were made by using ANOVA with or without repeated measures, as appropriate, and with post hoc analyses using Dunnett's test (Prism version 4; GraphPad Software, San Diego, CA). A value of P < 0.05 (two-tailed) was taken as indicating statistical significance.
Supplementary Material
Acknowledgments
We thank Professor James Burnie (NeuTec Pharma plc) for his kind gift of Mycograb antibody. This work was supported by a Wellcome Trust Traveling Research Fellowship and Jiangsu Provincial Science Foundation of China Project Grant BK2005145 (to Y.J.) and by a Guy's and St Thomas' Charitable Foundation Project Grant (to A.F.). E.G. was supported by the Greek Foundation for State Scholarships. A.F. also receives funding from the British Heart Foundation and Diabetes U.K.
Abbreviations
- NO
nitric oxide
- NOS
nitric oxide synthase
- Hsp
heat shock protein
- cGMP
cyclic guanosine-3′,5′-monophosphate
- GFP
gel-filtered platelets
- PRP
platelet-rich plasma.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0611416104/DC1.
References
- 1.Radomski MW, Moncada S. Adv Exp Med Biol. 1993;344:251–264. doi: 10.1007/978-1-4615-2994-1_20. [DOI] [PubMed] [Google Scholar]
- 2.Kinlay S, Ganz P. Am J Cardiol. 1997;80:11I–16I. doi: 10.1016/s0002-9149(97)00793-5. [DOI] [PubMed] [Google Scholar]
- 3.Ross R. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 4.Anderson TJ. Heart Fail Rev. 2003;8:71–86. doi: 10.1023/a:1022199021949. [DOI] [PubMed] [Google Scholar]
- 5.Radomski MW, Palmer RM, Moncada S. Lancet. 1987;7:1057–1058. doi: 10.1016/s0140-6736(87)91481-4. [DOI] [PubMed] [Google Scholar]
- 6.Queen LR, Xu B, Horinouchi K, Fisher I, Ferro A. Circ Res. 2000;87:39–44. doi: 10.1161/01.res.87.1.39. [DOI] [PubMed] [Google Scholar]
- 7.Su Y, Edwards-Bennett S, Bubb MR, Block ER. Am J Physiol. 2003;284:C1542–C1549. doi: 10.1152/ajpcell.00248.2002. [DOI] [PubMed] [Google Scholar]
- 8.Furchgott RF, Vanhoutte PM. FASEB J. 1989;3:2007–2018. [PubMed] [Google Scholar]
- 9.Berk BC, Corson MA, Peterson TE, Tseng H. J Biomech. 1995;28:1439–1450. doi: 10.1016/0021-9290(95)00092-5. [DOI] [PubMed] [Google Scholar]
- 10.Corson MA, James NL, Latta SE, Nerem RM, Berk BC, Harrison DG. Circ Res. 1996;79:984–991. doi: 10.1161/01.res.79.5.984. [DOI] [PubMed] [Google Scholar]
- 11.Chen R, Liang N. Cell Biol Int. 1998;22:429–435. doi: 10.1006/cbir.1998.0271. [DOI] [PubMed] [Google Scholar]
- 12.Fleming I, Busse R. Am J Physiol. 2003;284:R1–R12. doi: 10.1152/ajpregu.00323.2002. [DOI] [PubMed] [Google Scholar]
- 13.Dedio J, Konig P, Wohlfart P, Schroeder C, Kummer W, Muller-Esterl W. FASEB J. 2001;15:79–89. doi: 10.1096/fj.00-0078com. [DOI] [PubMed] [Google Scholar]
- 14.Cao H, Thompson HM, Krueger EW, McNiven MA. J Cell Sci. 2000;113:1993–2002. doi: 10.1242/jcs.113.11.1993. [DOI] [PubMed] [Google Scholar]
- 15.Cao S, Yao J, Shah V. J Biol Chem. 2003;278:5894–5901. doi: 10.1074/jbc.M212546200. [DOI] [PubMed] [Google Scholar]
- 16.Cooper JA. J Cell Biol. 1987;105:1473–1478. doi: 10.1083/jcb.105.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Queen LR, Ji Y, Goubareva I, Ferro A. Diabetologia. 2003;46:1474–1482. doi: 10.1007/s00125-003-1219-0. [DOI] [PubMed] [Google Scholar]
- 18.Malinski T, Radomski MW, Taha Z, Moncada S. Biochem Biophys Res Commun. 1993;194:960–965. doi: 10.1006/bbrc.1993.1914. [DOI] [PubMed] [Google Scholar]
- 19.Zhou Q, Hellermann GR, Solomonson LP. Thromb Res. 1995;77:87–96. doi: 10.1016/0049-3848(95)90868-g. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Cardena G, Fan R, Shah V, Sorrentino R, Cirino G, Papapetropoulos A, Sessa WC. Nature. 1998;392:821–824. doi: 10.1038/33934. [DOI] [PubMed] [Google Scholar]
- 21.Russell KS, Haynes MP, Caulin-Glaser T, Rosneck J, Sessa WC, Bender JR. J Biol Chem. 2000;275:5026–5030. doi: 10.1074/jbc.275.7.5026. [DOI] [PubMed] [Google Scholar]
- 22.Matthews R, Burnie J. Curr Opin Investig Drugs. 2001;2:472–476. [PubMed] [Google Scholar]
- 23.Matthews RC, Rigg G, Hodgetts S, Carter T, Chapman C, Gregory C, Illidge C, Burnie J. Antimicrob Agents Chemother. 2003;47:2208–2216. doi: 10.1128/AAC.47.7.2208-2216.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allen RD, Zacharski LR, Widirtsky ST, Rosenstein R, Zaitlin LM, Burgess DR. J Cell Biol. 1979;83:126–142. doi: 10.1083/jcb.83.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Escolar G, Krumwiede M, White JG. Am J Pathol. 1986;123:86–94. [PMC free article] [PubMed] [Google Scholar]
- 26.Hartwig JH. J Cell Biol. 1992;118:1421–1442. doi: 10.1083/jcb.118.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bearer EL. Cell Motil Cytoskeleton. 1995;30:50–66. doi: 10.1002/cm.970300107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bearer EL, Abraham M. Eur J Cell Biol. 1999;78:117–126. doi: 10.1016/S0171-9335(99)80013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bearer EL, Prakash JM, Manchester RD, Allen PG. Cell Motil Cytoskeleton. 2000;47:351–364. doi: 10.1002/1097-0169(200012)47:4<351::AID-CM8>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Witke W, Sharpe AH, Hartwig JH, Azuma T, Stossel TP, Kwiatkowski DJ. Cell. 1995;81:41–51. doi: 10.1016/0092-8674(95)90369-0. [DOI] [PubMed] [Google Scholar]
- 31.Kwiatkowski D. Curr Opin Cell Biol. 1999;11:103–108. doi: 10.1016/s0955-0674(99)80012-x. [DOI] [PubMed] [Google Scholar]
- 32.Nachmias VT. J Cell Biol. 1980;86:795–802. doi: 10.1083/jcb.86.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fox JE, Phillips DR. Semin Hematol. 1983;20:243–260. [PubMed] [Google Scholar]
- 34.Hartwig JH, Bokoch GM, Carpenter CL, Carpenter CL, Janmey PA, Taylor LA, Toker A, Stossel TP. Cell. 1995;82:643–653. doi: 10.1016/0092-8674(95)90036-5. [DOI] [PubMed] [Google Scholar]
- 35.O'Kane PD, Queen LR, Ji Y, Reebye V, Stratton P, Jackson G, Ferro A. Cardiovasc Res. 2003;59:152–159. doi: 10.1016/s0008-6363(03)00323-7. [DOI] [PubMed] [Google Scholar]
- 36.Xu B, Chibber R, Ruggiero D, Kohner E, Ritter J, Ferro A. FASEB J. 2003;17:1289–1291. doi: 10.1096/fj.02-0490fje. [DOI] [PubMed] [Google Scholar]
- 37.Tokuyasu KT. J Microscopy. 1986;143:139–149. doi: 10.1111/j.1365-2818.1986.tb02772.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.