Abstract
Tyrosine hydroxylase (TH), the rate-limiting enzyme in dopamine synthesis, can be regulated by phosphorylation at multiple serine residues, including serine-40. In the present study, we report a novel interaction between a key member of the novel PKC family, protein kinase Cδ (PKCδ), and TH, in which the kinase modulates dopamine synthesis by negatively regulating TH activity via protein phosphatase 2A (PP2A). We observed that PKCδ is highly expressed in nigral dopaminergic neurons and colocalizes with TH. Interestingly, suppression of PKCδ activity with the kinase inhibitor rottlerin, PKCδ-small interfering RNA, or with PKCδ dominant-negative mutant effectively increased a number of key biochemical events in the dopamine pathway, including TH-ser40 phosphorylation, TH enzymatic activity, and dopamine synthesis in neuronal cell culture models. Additionally, we found that PKCδ not only physically associates with the PP2A catalytic subunit (PP2Ac) but also phosphorylates the phosphatase to increase its activity. Notably, inhibition of PKCδ reduced the dephosphorylation activity of PP2A and thereby increased TH-ser40 phosphorylation, TH activity, and dopamine synthesis. To further validate our findings, we used the PKCδ knock-out (PKCδ −/−) mouse model. Consistent with other results, we found greater TH-ser40 phosphorylation and reduced PP2A activity in the substantia nigra of PKCδ −/− mice than in wild-type mice. Importantly, this was accompanied by an increased dopamine level in the striatum of PKCδ−/− mice. Collectively, these results suggest that PKCδ phosphorylates PP2Ac to enhance its activity and thereby reduces TH-ser40 phosphorylation and TH activity and ultimately dopamine synthesis.
Keywords: PKCδ, TH phosphorylation, dopamine synthesis, Parkinson's disease, PP2A, RNAi
Introduction
Tyrosine hydroxylase (TH) is a rate-limiting enzyme in the biosynthesis of catecholamines and catalyzes the first step of a biochemical synthetic pathway in which l-tyrosine is converted to l-3,4-dihydroxyphenylalanine (l-DOPA). Phosphorylation and dephosphorylation of TH represent important posttranslational regulatory mechanisms of the enzymatic activity that mainly determine the amount of catecholamine synthesis. A number of phosphorylation sites have been identified in TH, and phosphorylation of TH greatly influences the enzyme activity (Lee et al., 1989). Phosphorylation of TH at N-terminal serine (Ser) amino sites at Ser8, Ser19, Ser31, and Ser40 leads to activation of TH. A number of protein kinases have been shown to phosphorylate these serine residues to varying degrees. For example, THser19 is phosphorylated by calcium calmodulin-dependent protein kinase II (CaMKII), TH-ser40 by protein kinase A (PKA), protein kinase G, mitogen and stress-activated protein kinase, protein kinase C (PKC), and TH-ser31 by extracellular signal-regulated kinase 1 (ERK1)/ERK2 kinases and indirectly by PKC (Haycock, 1990). Among these serine phosphorylation sites, TH-ser40 is a major residue that positively regulates the TH activity in vivo (Campbell et al., 1986; Wu et al., 1992). The phosphorylation state of TH can also be regulated by dephosphorylation reactions mediated by phosphatases. Haavik et al. (1989) demonstrated that phosphatase 2A (PP2A) is the major serine/threonine phosphatase that dephosphorylates TH, resulting in reduced TH activity.
The PKC family consists of >12 isoforms and is subdivided into three major subfamilies, which include conventional PKC (α, βI, βII, γ), novel PKC (δ, ε, μ, η, θ), and atypical PKC (τ, λ, ζ) (Gschwendt, 1999; Dempsey et al., 2000; Maher, 2001; Kanthasamy et al., 2003). PKCδ, a key member of the novel PKC family, plays a role in a variety of cell functions, including cell differentiation, proliferation, and secretion. Our recent studies demonstrate that PKCδ is an oxidative stress-sensitive kinase, and activation of this kinase via caspase-3-dependent proteolysis induces apoptotic cell death in cell culture models of Parkinson's disease (Kanthasamy et al., 2003; Kaul et al., 2003; Yang et al., 2004; Latchoumycandane et al., 2005). General PKCs can phosphorylate TH-ser40 and TH-ser31 (Albert et al., 1984; McTigue et al., 1985; Tachikawa et al., 1987; Cahill et al., 1989; Haycock et al., 1992; Bunn and Saunders, 1995; Bobrovskaya et al., 1998); however, direct phosphorylation of TH by PKA and not by PKC results in activation of the enzymatic activity (Funakoshi et al., 1991). The role of PKC isoforms in the regulation of TH activity is not well studied. After the characterization of a key proapoptotic role of PKCδ in dopaminergic neuronal cell death during neurotoxic insults (Kanthasamy et al., 2003; Kaul et al., 2003; Yang et al., 2004; Kitazawa et al., 2005), we further examined whether PKCδ has any physiological role in regulating dopamine (DA) synthesis. Herein, we report a novel functional interaction between PKCδ and TH in which PKCδ negatively regulates TH activity and dopamine synthesis by enhancing PP2A activity.
Materials and Methods
Chemicals.
Rottlerin, recombinant PKCδ protein, NSD-1015 (3-hydroxy-benzyl hydrazine), okadaic acid, dibutyryl cAMP, protease mixture, ATP, protein A-Sepharose, protein G-Sepharose, and anti-β-actin antibody were obtained from Sigma-Aldrich (St. Louis, MO); purified PP2A and PP2Ac enzyme were purchased from Upstate Biotechnology (Chicago, IL). Mouse tyrosine hydroxylase antibody, PhosphoTH-ser40, and ser31 antibodies were purchased from Chemicon (Temecula, CA); the rabbit polyclonal antibody for tyrosine hydroxylase was obtained from Calbiochem (La Jolla, CA) Bioscience (King of Prussia, PA). Rabbit PKCδ antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA); mouse PKCδ antibody and PP2Ac antibody were obtained from BD Biosciences (San Jose, CA). Anti-rabbit and anti-mouse secondary antibodies and the ECL chemiluminescence kit were purchased from GE Healthcare (Piscataway, NJ). Alexa 488-conjugated anti-rabbit/mouse, Cy3-conjugated anti-rabbit/mouse antibody, and Hoechst 33342 were purchased from Invitrogen (Eugene, OR). [γ-32P]ATP was purchased from PerkinElmer (Boston, MA). The Serine/Threonine Phosphatase Assay kit was purchased from Promega (Madison, WI); the AMAXA Nucleofector kit was obtained from Amaxa (AMAXA, Cologne, Germany). The Bradford protein assay kit was purchased from Bio-Rad (Hercules, CA). RPMI (Roswell Park Memorial Institute) media, fetal bovine serum, l-glutamine, penicillin, and streptomycin were purchased from Invitrogen (Gaithersburg, MD).
Animal studies.
Six- to 8-week-old C57Bl/6 mice and PKCδ knock-out mice weighing 25–30 g were housed in standard conditions: constant temperature (22 ± 1°C), humidity (relative, 30%), and a 12 h light/dark cycle with free access to food and water. PKCδ knock-out animals were kindly provided by Dr. Keiichi Nakayama's laboratory (Division of Cell Biology, Department of Molecular and Cellular Biology, Medical Institute of Bioregulation, Kyushu University, Fukuoka, Japan). We obtained a pair of male and female heterozygous PKCδ (+/−) C57 black mice from Dr. Nakayama's laboratory and established a breeding colony in our animal facility. We then genotyped animals in our colony as per the protocol described previously (Miyamoto et al., 2002). Briefly, genomic DNA was isolated from tails of 3- to 4-week-old mice and then subjected to PCR followed by electrophoresis. The genotype of the animals was confirmed using the molecular size of PCR products: PKCδ naive (+/+), 900 bp; PKCδ (+/−), 900 and 600 bp; and PKCδ knock-out (−/−), 600 bp. The animals and protocol procedures were approved and supervised by the Committee on Animal Care at Iowa State University.
Cell culture models.
PC12 and differentiated N27 cells were cultured as described previously (Adams et al., 1996; Anantharam et al., 2002; Kaul et al., 2003). Briefly, cells were grown in RPMI 1640 medium containing 10% fetal bovine serum, 2 mm l-glutamine, 50 U of penicillin, and 50 μg/ml streptomycin. Cells were maintained in a humidified atmosphere of 5% CO2 at 37°C. N27 cells were differentiated with 2 mm dibutyryl cAMP for 3–5 d and then used for experiments described below. Primary mesencephalic neuronal cultures were prepared from the ventral mesencephalon of gestational 16- to 18-d-old mice embryos as described previously (Yang et al., 2004). Mesencephalic tissues were dissected and maintained in ice-cold Ca2+-free HBSS and then dissociated in HBSS solution containing trypsin-EDTA (0.25%) for 20 min at 37°C. The dissociated cells were then plated at equal density (0.5 × 106 cells) in 30-mm-diameter tissue culture wells precoated with poly-d-lysine (1 mg/ml). Cultures were maintained in a chemically defined medium consisting of neurobasal medium fortified with B-27 supplements, l-glutamine (500 μm), penicillin (100 IU/ml), and streptomycin (100 μg/ml) (Invitrogen). The cells were maintained in a humidified CO2 incubator (5% CO2, 37°C) for 24 h and then treated with cytosine arabinoside (10 μm) for 24 h to inhibit glial cell proliferation. Half of the culture medium was replaced every 2 d. Approximately 6- to 7-d-old cultures were used for experiments.
Transfection of PKCδK376R gene in N27 cells and primary mesencephalic neurons.
Plasmid pPKCδK376R-V5 encodes the loss of function PKCδ-V5 epitope-tagged mutant protein; K376R refers to the mutation of the lysine residue at position 376 to arginine in the catalytic site, resulting in inactivation of the kinase. Plasmid pLacZ-V5 encodes the β-galactosidase protein alone with a V5-epitope and is used as a vector control. N27 cells stably expressing PKCδK376R-V5 (herein referred to as PKCδ-DN cells) and LacZ alone-expressing cells (LacZ cells) were cultured as described previously (Kitazawa et al., 2005). PKCδ-DN or LacZ expressing N27 cells were identified by immunostaining of the C-terminal V5 epitope on expressed proteins and were differentiated with 2 mm dibutyryl cAMP before experiments. PKCδ-DN and LacZ were also transiently expressed in mouse primary mesencephalic neurons using an AMAXA Nucleofector kit (AMAXA). As described above, primary mesencephalic neurons were prepared from the midbrain of 16- to 18-d-old mice embryos. After digestion with trypsin-EDTA-HBSS, the primary neurons were homogenously resuspended with transfection buffer provided with the kit to a final concentration of 4–5 × 106 neurons/100 μl and mixed with 2 μg of plasmid DNA encoding either PKCδ-DN-V5 or LacZ-V5. Electroporation was performed with an AMAXA Nucleofector instrument as per the manufacturer protocol. The transfected neurons were then transferred to 24-well plates containing poly-d-lysine- and laminin-coated coverslips. After 24 h, the primary neurons were fixed and used for immunocytochemistry. Transfection efficiency was >75% as determined by immunostaining of V5 expression.
Design, synthesis, and transfection of small interfering RNA.
Small interfering RNAs (siRNAs) were prepared by an in vitro transcription method as described previously (Yang et al., 2004). Initially, siRNA target sites specific to rat PKCδ mRNA (gene identifier: 18959249), as determined by blast analysis, were chosen. One nonspecific siRNA (NS-siRNA) was also chosen based on random sequence. For each siRNA, sense and antisense templates were designed based on each target sequence and partial T7 promoter sequence (Donze and Picard, 2002): for PKCδ-siRNA, sense, 5′-AACTGTTTGTGAATTTGCCTTCCTGT CTC-3′; antisense, 5′-AAAAGGCAAATTCACAAACAGCCTGTCTC-3′; for NS-siRNA, sense, 5′-AATTCTCACACTTCGGAGAACCTGTCTC-3′; antisense, 5′-AAGTTCTCCG AAGTGTGAGAACCTGTCTC-3′. All template oligonucleotides were chemically synthesized and PAGE purified. In vitro transcription, annealing, and purification of siRNA duplexes were performed using the protocol supplied with the silencer siRNA construction kit (Ambion, Austin, TX). Briefly, ∼2 μg of each single-strand (ss) transcription template was first annealed with the T7 promoter and filled in by Klenow DNA polymerase to form double-strand transcription templates. For preparation of each siRNA duplex, transcription reactions were first performed with separated antisense and sense templates using the T7 RNA polymerase provided with the kit and then annealed to form siRNA duplexes. Then, the siRNA duplex was treated with DNase and RNase to remove the extra nucleotides of transcribed siRNA to meet the structural 3′UU overhang and 5′ phosphate requirement (Elbashir et al., 2001). N27 cells (50–70% confluence) and primary mesencephalic neurons were transfected with siRNA duplexes by using an AMAXA Nucleofector kit (AMAXA) as described in our previous study (Yang et al., 2004).
Treatment paradigm.
PC12 cells, differentiated N27 dopaminergic cells, and primary mesencephalic neurons were exposed to 1–10 μm rottlerin for the duration of the experiment. DMSO (0.01%) was used as vehicle control. PKCδ-DN- and Lac Z-expressing N27 cells were treated only with 0.01% DMSO. For measurement of TH activity in rottlerin-treated cultures, cells were exposed to 2 mm NSD-1015 for 1 h before rottlerin treatment. Untreated or vehicle-treated cells were used as control samples. We derived the concentrations of rottlerin and okadaic acid used in this study based on previously published literature. Rottlerin inhibits PKCδ kinase activity with a Ki of 3–6 μm, whereas PKCα, β, γ, ε, and λ Ki values are at least 5–10 times higher (Gschwendt, 1999; Davies et al., 2000; Way et al., 2000; Soltoff, 2001). In our previous study, we showed 1–10 μm rottlerin dose-dependently attenuated kinase activity to a greater extent (Anantharam et al., 2002). We used 5 μm rottlerin in the study, which was lower than Ki values of other PKC isoforms. Additionally, we used various genetic approaches such as RNA interference (RNAi), dominant-negative mutant, and knock-out approaches to validate the results obtained with rottlerin treatment. We used 2 μm okadaic acid to completely inhibit PP2A activity in dopaminergic cells, because previous studies demonstrated IC50 value ranges from 0.1 to 1 μm for PP2A activity in cell culture models (Favre et al., 1997; Schonthal, 1998).
Western blotting.
Cell and brain lysates containing equal amounts of protein were loaded in each lane and separated on a 10–12% SDS-PAGE gel as described previously (Kaul et al., 2003). After the separation, proteins were transferred to nitrocellulose membrane, and nonspecific binding sites were blocked by treating with 5% nonfat dry milk powder. The membranes were then treated with primary antibodies directed against PKCδ (rabbit polyclonal or mouse monoclonal for PKCδ knock-out studies, 1:2000 dilution), TH (rabbit polyclonal or mouse monoclonal, 1:1000), phospho TH-ser40 (rabbit polyclonal, 1:1000), phospho TH-ser31 TH (rabbit polyclonal, 1:1000), or PP2Ac (mouse monoclonal, 1:1000). The primary antibody treatments were followed by treatment with secondary HRP-conjugated anti-rabbit or anti-mouse IgG (1:2000) for 1 h at room temperature (RT). Secondary antibody-bound proteins were detected using GE Healthcare's ECL kit. To confirm equal protein loading, blots were reprobed with a β-actin antibody (1:5000 dilution). Western blot images were captured with a Kodak (Rochester, NY) 2000 MM imaging system, and data were analyzed using 1D Kodak imaging analysis software.
Coimmunoprecipitation.
Immunoprecipitation studies were conducted to determine the association properties between PKCδ, TH, and PP2A and were performed as described in our previous study (Kaul et al., 2005). Briefly, PC12, N27 cells, or substantia nigral tissue from PKCδ (+/+) or PKCδ (−/−) mouse brain were washed with ice-cold Ca2+-free PBS saline and resuspended in a lysis buffer (25 mm HEPES, pH 7.5, 20 mm β-glycerophosphate, 0.1 mm sodium orthovanadate, 0.1% Triton X-100, 0.3 m NaCl, 1.5 mm MgCl2, 0.2 mm EDTA, 0.5 mm DTT, 10 mm NaF, and protease inhibitor mixture). The suspension was kept on ice for 30 min and then centrifuged at 10,000 × g for 5 min. The supernatants were collected and used for immunoprecipitation. Extracts containing ∼200 μg of total protein were immunoprecipitated overnight at 4°C with 5–20 μg of anti-PKCδ (rabbit polyclonal), anti-TH (mouse monoclonal), anti-PP2Ac (mouse monoclonal) antibodies, rabbit IgG, or mouse IgG. Mouse monoclonal anti-PKCδ antibody was used for PKCδ (−/−) brain tissue. PKCδ blocking peptide (50 μg) was used to neutralize PKCδ antibody by incubating for 1 h and used as a negative control. The immunoprecipitates were then adsorbed onto Protein A- or G-Sepharose for 1 h at 4°C. The Sepharose-bound antigen-antibody complexes were washed three times with PBS to remove unbound proteins. For association studies, samples were mixed with 2× SDS-PAGE loading buffer and boiled for 5 min, and then proteins were separated on SDS-PAGE and subjected to Western blot as described previously.
32P-Phosphorylation assays.
To determine whether PKCδ can directly phosphorylate PP2A, we used recombinant PKCδ in the in vitro phosphorylation assays. Immunoprecipitations were performed as described previously (Kaul et al., 2003). Briefly, PP2Ac was immunoprecipitated from N27 cell lysates using mouse monoclonal PP2Ac antibody. The samples were then incubated with protein G-Sepharose, and the immunoprecipitates and recombinant PP2Ac were used in the in vitro phosphorylation assays. Recombinant PKCδ was resuspended in 2× kinase buffer (40 mm Tris, pH 7.4, 20 mm MgCl2, 20 μm ATP, 2.5 mm CaCl2), and the reaction was started by adding 20 μl of reaction buffer containing immunoprecipitated or recombinant PP2Ac and 5 μCi of [γ-32P] ATP (4500 Ci/mm). To inhibit PKCδ, recombinant PKCδ protein was preincubated with 5 μm rottlerin for 15 min before the reaction. After incubation for 10 min at 30°C, the reaction was terminated by addition of 2× SDS-gel loading buffer and separated by SDS-PAGE. PP2Ac and histone H1 phosphorylated bands were detected using a Personal Molecular Imager (FX model; Bio-Rad) and quantified with Quantity One 4.2.0 software. Histone H1 substrate was used as a positive control for PKCδ kinase activity.
PP2A assay.
To determine PP2A phosphatase activity, we used the Serine/Threonine Phosphatase Assay kit from Promega. In cell-free assay, 20 ng of recombinant PP2A enzyme in PP2A reaction buffer (250 mm imidazole, pH 7.2, 1 mm EGTA, 0.1% β-mercaptoethanol, 5 μl of peptide substrate, 0.5 mg/ml BSA) was incubated with 20 ng of recombinant PKCδ protein in the presence or absence of 5 μm rottlerin. Okadaic acid (2 μm) was used as a positive control. Reaction was started by adding PKCδ reaction buffer containing 40 mm Tris, pH 7.4, 20 mm MgCl2, 20 μm ATP, 2.5 mm CaCl2, 50 μg/ml phosphatidylserine, and 4.0 μm dioleoylglycerol. After 1 h of incubation, molybdate dye (double strength) was added to the reaction mixture. For in vivo PP2A activity measurement, N27 cells and substantia nigral tissue from mouse brain were homogenized in lysis buffer (25 mm Tris-HCl, 10 mm β-mercaptoethanol, 2 mm EDTA, protease inhibitor) supplied with the kit. After centrifugation, the supernatants were used for measurement of PP2 activity by incubating equal volumes of the substrate and PP2A reaction buffer for 1 h. PP2A activity was determined by measuring the amount of free phosphate generated in a reaction by measuring the absorbance of a molybdate:malachite green:phosphate complex at 600 nm using a Spectramax plate reader (Molecular Devices, Union City, CA). The effective range for the detection of phosphate released in this assay is 100–4000 pmol of phosphate.
Tyrosine hydroxylase activity.
TH enzyme activity was measured by the modified method of Hayashi et al. (1988) in which DOPA levels are quantified as an index of TH activity after inhibition of DOPA decarboxylase with the decarboxylase inhibitor NSD-1015 (Hayashi et al., 1988). Briefly, cells were incubated with Krebs-HEPES buffer, pH 7.4, containing 2 mm NSD-1015 at 37°C for 30 min and then subjected to the treatment paradigm as described previously. After treatment, cells were collected and resuspended in antioxidant solution, sonicated, and centrifuged, and DOPA levels in the supernatants were measured by HPLC detection as described below.
Measurements of DA and its metabolites.
DA and dihydroxyphenyl acetic acid (DOPAC) levels in PC12, N27 cells, and brain striatal tissues were determined by HPLC with electrochemical detection (EC); samples were prepared as described previously (Kitazawa et al., 2001; Sun et al., 2006). Briefly, neurotransmitters were extracted from samples using 0.1 m perchloric acid containing 0.05% Na2EDTA and 0.1% Na2S2O5. The extracts were filtered in 0.22 μm spin tubes, and 20 μl of the samples was loaded for analysis. DA and DOPAC were separated isocratically by a reversed-phase column with a flow rate of 0.7 ml/min. An HPLC system (ESA, Bedford, MA) with an ESA automatic sampler (model 542) was used for these experiments. The EC system consisted of an ESA coulochem model 5100A with a microanalysis cell model 5014A and a guard cell model 5020 (ESA). The peak areas of standard DA and DOPAC were compared with that of samples. The DA and DOPAC levels in the samples were measured and expressed as nanograms per milligrams of protein, and retention times for DA and DOPAC were 5.8–7.7 min and 4.7–5.5 min, respectively.
Immunohistochemical staining of brain slices.
Mice were perfused with 4% paraformaldehyde after anesthesia with ketamine, and then the brain was cut on a microtome into 20 μm sections. Sections from substantia nigra pars compacta were treated for immunofluorescence staining. Brain sections were first blocked with 5% normal goat serum containing 0.4% BSA and 0.2% Triton X-100 in PBS for 20 min and then incubated with antibodies directed against PKCδ (rabbit polyclonal, 1:500 dilution) and TH (mouse monoclonal, 1:500 dilution) overnight at 4°C followed by incubation with either Alexa 488-conjugated (green, 1:1000) or Cy3-conjugated (red, 1:1000) secondary antibody for 1 h at RT. Secondary antibody treatments were followed by incubation with Hoechst 33342 (10 μg/ml) for 3 min at RT to stain the nucleus. Then the slices were mounted on a slide and viewed under a Nikon inverted fluorescence microscope (model TE-2000U); images were captured with a SPOT digital camera (Diagnostic Instruments, Sterling Heights, MI).
Immunocytochemical staining in cell cultures.
Immunostaining of PKCδ, TH, P-TH-ser40, and P-TH-ser31 was performed in PC12, N27, and primary mesencephalic neurons. Cells were grown on poly-l-lysine-coated glass coverslips. After treatment, the cells were fixed with 4% paraformaldehyde and processed for immunocytochemical staining. First, nonspecific sites were blocked with 5% normal goat serum containing 0.4% BSA and 0.2% Triton X-100 in PBS for 20 min. Cells and primary neurons were then incubated with antibodies directed against PKCδ (mouse monoclonal, 1:500 dilution), TH (rabbit polyclonal, 1:500 dilution), and P-TH-ser40 (rabbit polyclonal, 1:500 dilution) overnight at 4°C followed by incubation with either Alexa 488-conjugated (green, 1:1000) or Cy3-conjugated (red, 1:1000) secondary antibody for 1 h at RT. Secondary antibody treatments were followed by incubation with Hoechst 33342 (10 μg/ml) for 3 min at room temperature to stain the nucleus. Then the coverslips containing stained cells were washed with PBS, mounted on a slide, and viewed under a Nikon inverted fluorescence microscope (model TE-2000U); images were captured with a SPOT digital camera (Diagnostic Instruments).
Data analysis.
Data analysis was performed using Prism 4.0 software (GraphPad Software, San Diego, CA). Data were first analyzed using one-way ANOVA, and then Bonferroni's posttest was performed to compare all treatment groups, and differences with p < 0.05 were considered significant.
Results
PKCδ physically associates with TH
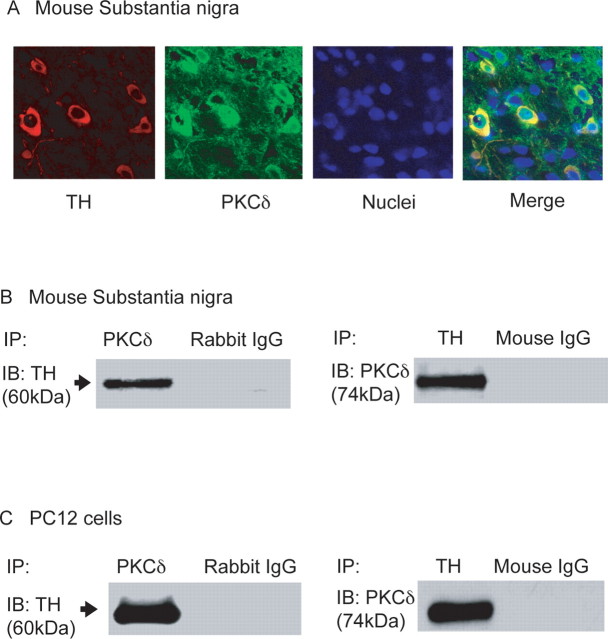
First we examined the level of PKCδ expression in nigral dopaminergic neurons. Double immunostaining of mouse nigral tissues with PKCδ and TH showed a strong colocalization of these proteins (Fig. 1A). To further confirm their possible interaction, we performed coimmunoprecipitation and reverse immunoprecipitation studies. As shown in Figure 1B, immunoprecipitation with PKCδ antibody followed by immunoblotting with TH antibody showed a clear association of TH with PKCδ in mouse substantia nigral lysate (Fig. 1B). In reverse immunoprecipitation analysis, TH antibody was used for immunoprecipitation, and PKCδ antibody was used for immunoblotting. The right panel in Figure 1B shows a PKCδ band in TH immunoprecipitates from mouse brain, indicating an association between these two proteins. Rabbit IgG and mouse IgG immunoprecipitates were used as negative controls. These results clearly indicate that PKCδ physically associates with TH in the dopaminergic neuronal system.
Figure 1.
PKCδ associates with TH in mouse brain and PC12 cells. A, Immunohistochemical analysis. Mouse brain was cut to 20 μm thickness at the level of the substantia nigra and stained with PKCδ polyclonal antibody (Ab) (1:500 dilution) and TH monoclonal Ab (1:500 dilution), followed by incubation with either Alexa 488-conjugated (green; 1:1000) or Cy3-conjugated (red; 1:1000) secondary antibody. Hoechst 33342 (10 μg/ml) was used to stain the nucleus. Red, TH; green, PKCδ; blue, nucleus. B, PKCδ (74 kDa) coimmunoprecipitated with TH in mouse substantia nigra. PKCδ was immunoprecipitated (IP) from mouse substantia nigra lysates by using PKCδ polyclonal Ab (1:100) and immunoblotted (IB) with anti-TH (1:100). In reverse immunoprecipitation studies, TH was immunoprecipitated with mouse monoclonal anti-TH antibody (1:100) and immunoblotted with PKCδ. TH (60 kDa) coimmunoprecipitated with PKCδ in mouse substantia nigra. Similarly, PKCδ (74 kDa) coimmunoprecipitated with TH in mouse substantia nigra. C, PKCδ also coimmunoprecipitated with TH in PC12 cells, and PKCδ was immunoprecipitated (IP) from PC12 cell lysates by using PKCδ polyclonal Ab (1:100) and immunoblotted (IB) with anti-TH (1:100). In reverse immunoprecipitation studies, TH was immunoprecipitated with mouse monoclonal anti-TH antibody (1:100) and immunoblotted with PKCδ. TH (60 kDa) coimmunoprecipitated with PKCδ in PC12 cell lysates. Similarly, PKCδ (74 kDa) coimmunoprecipitated with TH in PC12 cell lysates. Rabbit IgG and mouse IgG were used as negative controls.
To further determine the functional relationship between PKCδ and TH, we used a PC12 model, which has been used extensively for posttranslational regulation of TH (Haycock, 1989, 1990). First, we performed immunoprecipitation studies to verify that PKCδ interacts with TH in this cell model. Similar to nigral tissue, immunoprecipitation and reverse immunoprecipitation studies showed a clear association and interaction between PKCδ and TH (Fig. 1C). These results not only confirmed the PKCδ and TH interaction in dopamine producing cells but also indicated the usefulness of PC12 cells for examining the role of PKCδ in the regulation of TH function.
PKCδ inhibition enhances TH activity
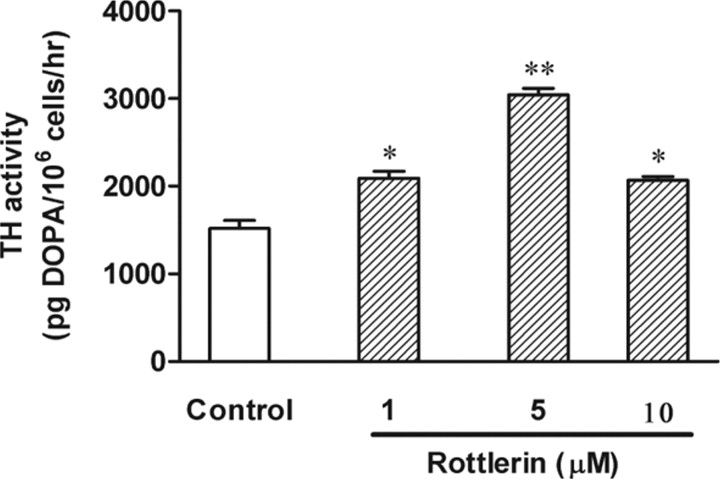
Because PKCδ colocalized with TH, we examined whether PKCδ has any influence on TH activity. As a first step, we determined TH activity under conditions of PKCδ inhibition using various doses of the PKCδ-specific inhibitor rottlerin. TH activity was measured by determining DOPA levels after inhibition of DOPA decarboxylase with the enzyme inhibitor NSD-1015, as described in Materials and Methods. PC12 cells were exposed to 2 mm NSD-1015 for 1 h before treatment with the PKCδ-specific inhibitor rottlerin for 3 h. We used 1–10 μm rottlerin in the present study, because we previously showed this dose range effectively inhibits PKCδ activity (Anantharam et al., 2002). As shown in Figure 2, rottlerin treatment significantly increased intracellular DOPA levels, indicating increased TH activity after PKCδ inhibition. Treatment with 1, 5, and 10 μm rottlerin increased TH activity to 2091.09 ± 80, 3045 ± 75, and 2067 ± 44 pg DOPA/106 cells/h, respectively, compared with the control level of 1519 ± 91 pg DOPA/106 cells/h. Together, these results suggest that inhibition of PKCδ activation results in increased TH activity.
Figure 2.
PKCδ inhibition enhances TH activity. PC12 cells were incubated with the DOPA decarboxylase inhibitor NSD-1015 (2 mm) for 1 h before treatment with the PKCδ inhibitor rottlerin (1–10 μm). DMSO (0.01%) was used as vehicle control. After 3 h of rottlerin treatment, cells were lysed and extracts were used for determining l-DOPA levels by HPLC. Rottlerin treatment increased l-DOPA levels, indicating enhanced TH activity. The data represent mean ± SEM of six to eight individual measurements. Asterisks (*p < 0.05; **p < 0.01) indicate significant differences between rottlerin-treated cells and control cells.
Effect of PKCδ inhibition on TH phosphorylation
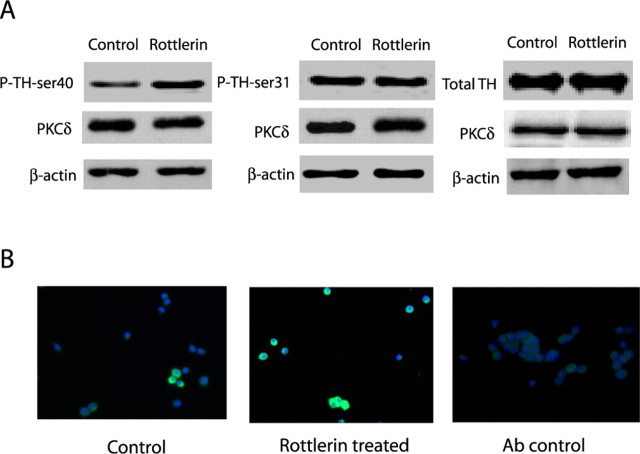
Because inhibition of PKCδ resulted in enhanced TH activity, we examined whether PKCδ has any effect on the phosphorylation status of TH. It is well established that TH activity can be regulated by phosphorylation of multiple serine residues (Campbell et al., 1986; Mitchell et al., 1990), and serine phosphorylation at positions 31 and 40 has been suggested to play a key role in TH activation and increased dopamine biosynthesis (Haycock, 1990). We measured the extent of TH-ser31 and TH-ser40 phosphorylation in immunoblots using phospho-specific antibodies directed against TH-ser31 and TH-ser40. As shown in Figure 3A, the level of TH-ser40 phosphorylation was significantly enhanced in rottlerin-treated cells (5 μm rottlerin for 3 h), whereas the level of TH-ser31 phosphorylation was unaltered. Similarly, the levels of total TH and PKCδ were unaltered in rottlerin-treated PC12 cells compared with 0.01% DMSO control-treated cells, indicating that rottlerin treatment does not alter the expression of TH and PKCδ. Nitrocellulose membranes were reprobed with β-actin antibody, and the density of the 43 kDa β-actin band was identical in all lanes, confirming equal protein loading. Densitometric analysis of the 60 kDa P-TH-ser40 band in Figure 3A revealed a threefold increase in phosphorylation compared with vehicle-treated cells, whereas there was no increase in protein levels of P-TH-ser31 and TH. TH-ser40 phosphorylation was also confirmed in immunofluorescence measurements. After treatment with 5 μm rottlerin for 3 h, PC12 cells were fixed and processed for immunofluorescence staining of P-TH-ser40 using Alex 488 secondary antibody. An increase in bright green immunofluorescence-positive cells was observed in rottlerin-treated cells (Fig. 3B), but only a weak staining was observed in vehicle-treated cells, indicating that PKCδ inhibition results in increased phosphorylation of TH-ser40. No staining was observed in cells stained with Alexa 488 alone. Cell count analysis of images using MetaMorph image analysis revealed that rottlerin treatment induced the increase in P-TH-ser40-stained cells by 333% compared with vehicle-treated cells. These results demonstrate that PKCδ inhibition results in enhanced TH phosphorylation specifically at ser40.
Figure 3.
PKCδ inhibition enhances TH-ser40 phosphorylation. A, Western blot analysis. PC12 cells were exposed to rottlerin (5 μm) or 0.01% DMS0 (vehicle control) for 3 h. Cell extracts were prepared and separated by SDS-PAGE and transferred to nitrocellulose membrane. Phospho-specific antibodies directed against P-TH-ser40 and P-TH-ser31, and antibodies directed against TH and PKCδ were used for immunoblotting. To confirm equal protein loading in each lane, the membranes were reprobed with β-actin antibody. The immunoblots were visualized using the GE Healthcare ECL detection agents. Densitometric analysis of 60 kDa TH and P-TH-ser40 and P-TH-ser31 bands represents the mean ± SEM from three separate experiments (**p < 0.01). B, Immunocytochemistry. PC12 cells were grown on poly-l-lysine-coated coverslips and then exposed to either 5 μm rottlerin or 0.01% DMS0 for 3 h. After treatment, the cells were fixed and immunostained for P-TH-ser40 followed by staining with Alexa 488-conjugated antibody (green), as described in Materials and Methods. For a negative control, cells were also immunostained with Alexa 488 without primary antibody staining. Immunostained cells were mounted and viewed under a Nikon TE2000 fluorescence microscope, and images were captured with a SPOT digital camera. For in situ quantitative analysis of P-TH-ser40 levels, fluorescence immunoreactivity in cells was measured from six different areas in each group using MetaMorph image analysis software, and data were analyzed with Prism software. Experiments were repeated three times, and representative images are presented.
Effect of loss of function PKCδ mutant on TH activity and DA synthesis in mesencephalic dopaminergic neuronal cells
Although PC12 cells are a good model to study TH function, they are non-neuronal cells derived from adrenal pheochromocytoma. Therefore, to further determine whether PKCδ alters TH activity in neuronal cells, we used immortalized rat mesencephalic dopaminergic neuronal cells (N27 cells), which are a homogenous population of TH-positive neuronal cells that synthesize and release dopamine after differentiation (Clarkson et al., 1999; Zhou et al., 2000). Also, the N27 dopaminergic cell line is easily transfectable and convenient for establishing stable cell lines compared with hard-to-transfect PC12 cells. In recent years, this neuronal cell line has been recognized by many investigators, including us, as a highly useful cell culture model for studying degenerative mechanisms in Parkinson's disease (Clarkson et al., 1999; Kaul et al., 2003, 2005; Miranda et al., 2004; Peng et al., 2005).
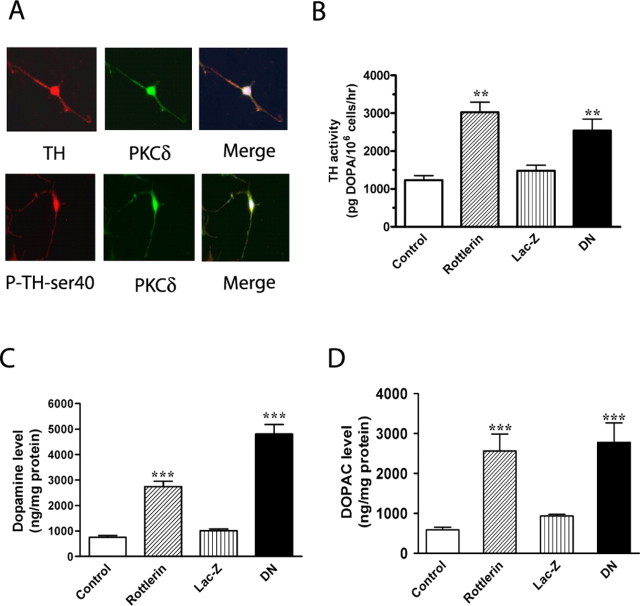
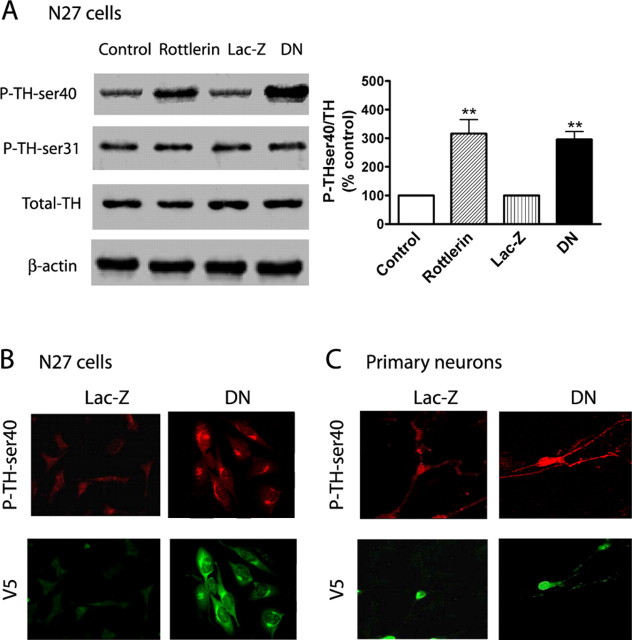
First, we examined whether PKCδ interacts with TH in N27 dopaminergic cells in a manner similar to that observed in PC12 cells and mouse nigral tissue (Fig. 4A). As shown in Figure 4A, double immunostaining studies with differentiated N27 cells showed colocalization of PKCδ with TH and P-TH-ser40, further supporting the previous results of association of PKCδ with TH observed in PC12 cells. Next, we examined whether PKCδ inhibition would alter TH activity and dopamine in N27 cells. For these studies, we used genetic approaches in addition to PKCδ pharmacological inhibitor studies. We used N27 cells stably expressing a loss of function PKCδ dominant-negative mutant (PKCδ-DN) established in our laboratory (Kaul et al., 2003; Kitazawa et al., 2005). N27 cells were exposed to 2 mm NSD-1015 for 1 h before a 3 h treatment with the PKCδ-specific inhibitor rottlerin (5 μm) or 0.01% DMSO and then measured the DOPA levels as a measure of TH activity. We also measured DOPA levels in PKCδ-DN expressing N27 cells and Lac-Z-expressing N27 cells (vector control). As shown in Figure 4B, DOPA levels were significantly higher in rottlerin-treated cells compared with vehicle-treated cells and were in agreement with the data obtained in PC12 cells (Fig. 2). Also, TH activity was significantly higher in PKCδ-DN mutant-expressing cells compared with LacZ-expressing cells (Fig. 4B). The TH activity was 1231 ± 120 and 3025 ± 267 pg DOPA/106 cells/h in vehicle- and rottlerin-treated cells and 1483 ± 146 and 2539 ± 307 pg DOPA/106 cells/r in LacZ- and PKCδ-DN-expressing cells, respectively (Fig. 4B).
Figure 4.
PKCδ inhibition regulates TH activity and DA synthesis in N27 dopaminergic neuronal cells. A, In situ colocalization of TH and PKCδ. Differentiated N27 cells were grown on poly-l-lysine-coated coverslips and processed for double immunostaining with anti-PKCδ monoclonal and anti-TH polyclonal antibodies or anti-PKCδ monoclonal and anti-P-TH-ser40 polyclonal antibodies, as described in Materials and Methods. B, TH activity in PKCδ inhibitor rottlerin-treated cells and PKCδ-DN cells. Differentiated N27 cells stably expressing LacZ or PKCδ-DN were pretreated with 2 mm NSD-1015 for 1 h and lysed, and extracts were used for determining l-DOPA levels by HPLC. Also, cell extracts from N27 cells incubated with 2 mm NSD-1015 for 1 h before treatment with 0.01% DMSO (vehicle control) or rottlerin (5 μm for 3 h) were also used for measuring DOPA levels. C, D, DA synthesis. Cell extracts from N27 cells stably expressing LacZ and PKCδ-DN, or treated with rottlerin (5 μm for 3 h), were used for determining DA and DOPAC levels by HPLC. The data represent a mean ± SEM of six to eight individual measurements. Asterisks (**p < 0.01; ***p < 0.001) indicate significant differences between rottlerin-treated cells and vehicle control cells, or between LacZ- and PKCδ-DN-expressing cells.
An increase in TH activity should result in an increase in dopamine synthesis and, therefore, we measured the levels of cellular DA by HPLC in N27 cells expressing PKCδ-DN and in rottlerin-treated N27 cells. Vehicle-treated N27 cells and Lac-Z-expressing N27 cells were used as controls. Steady-state DA levels were significantly increased in rottlerin-treated and PKCδ-DN mutant-expressing N27 cells compared with vehicle-treated and LacZ-expressing cells, respectively (Fig. 4C). The DA level in rottlerin treated-cells was 2748 ± 209 ng/mg protein compared with 754 ± 73 ng/mg protein in vehicle-treated N27 cells, a 3.7-fold increase. Similarly, DA levels in PKCδ-DN mutant-expressing cells were 4806 ± 373 ng/mg protein compared with 1015 ± 73 ng/mg protein in LacZ-expressing cells, a 4.8-fold increase (Fig. 4C). Thus, both inhibition of baseline PKCδ activity with rottlerin and loss of function PKCδ-DN mutant increased DA levels in the dopaminergic cells. To determine whether increased DA levels in the rottlerin-treated and PKCδ-DN-expressing cells were attributable to enhanced TH activity or to reduced degradation of DA to its major metabolite DOPAC by monoamine oxidase, we measured DOPAC levels. As shown in Figure 4D, DOPAC levels were also significantly increased by fourfold in rottlerin-treated cells and by threefold in PKCδ-DN-expressing cells, compared with the vehicle-treated and LacZ-expressing N27 cells, indicating that the dopamine degradation pathway is not altered during rottlerin treatment. Together, these data suggest that PKCδ inhibition increases DA synthesis in dopaminergic neuronal cells as a result of increased TH activation.
PKCδ negatively modulates TH-ser40 phosphorylation
Because TH activity and dopamine synthesis were enhanced in N27 cells expressing PKCδ-DN and in rottlerin-treated N27 cells, we further examined whether PKCδ inhibition increases the phosphorylation status of TH in N27 dopaminergic neuronal cells. As shown in Figure 5A, the levels of TH-ser40 phosphorylation were significantly enhanced in rottlerin-treated N27 cells, whereas the level of TH-ser31 phosphorylation was not altered compared with vehicle-treated cells. Also, the level of total TH was unaltered in rottlerin-treated N27 cells. Similarly, the TH-ser40 phosphorylation level was also significantly enhanced in PKCδ-DN-expressing cells compared with LacZ-expressing cells, whereas TH-ser31 phosphorylation and total TH levels were unaltered. Nitrocellulose membranes were reprobed with β-actin antibody, and the density of the 43 kDa β-actin band was identical in all lanes, confirming equal protein loading. Densitometric analysis of the P-TH-ser40 (60 kDa) band revealed a threefold to fourfold increase in rottlerin-treated and PKCδ-DN-expressing cells compared with DMSO and LacZ-expressing cells. We also confirmed TH-ser40 phosphorylation by immunofluorescence measurements in N27 cells. N27 cells stably expressing PKCδ-DN-V5 and LacZ-V5 were fixed and double immunostained for antibodies directed against P-TH-ser40 and V5-epitope in PKCδ-DN-V5 and LacZ-V5 constructs. Primary antibody staining was followed by Cy3-labeled secondary antibody against TH-ser40 and Alexa 488-labeled secondary antibody against the V5-epitopes. As shown in Figure 5B, a bright red immunofluorescence staining was observed in PKCδ-DN-expressing cells compared with a very weak staining in Lac-Z-expressing cells, suggesting that PKCδ inhibition resulted in enhanced phosphorylation of TH at ser40. Both the LacZ- and PKCδ-DN-expressing cells were stained for vector fusion protein V5 (Fig. 5B, green), demonstrating the expression level of the constructs. Analysis of fluorescent intensity for TH-ser40 immunostaining revealed a 2.5-fold increase in PKCδ-DN-expressing cells compared with Lac-Z-expressing cells (Fig. 5B). Collectively, these results demonstrate that PKCδ inhibition results in enhanced TH phosphorylation at ser40 in N27 dopaminergic neuronal cells.
Figure 5.
Effect of PKCδ-DN and rottlerin on TH-ser40 phosphorylation. A, Western blot. Cell extracts from rottlerin-treated, PKCδ-DN, and LacZ-expressing N27 cells were subjected to Western blot analysis, as described in Materials and Methods. P-TH-ser40, P-TH-ser31, and TH levels were detected using appropriate phospho-specific and TH antibodies. Densitometric analysis of 60 kDa P-TH-ser40, P-TH-ser31, and TH bands are shown next to the Western blot image. The data represent the mean ± SEM from three separate experiments (**p < 0.01). B, TH-ser40 phosphorylation in stably expressing PKCδ-DN and LacZ N27 cells. C, Primary mesencephalic cultures transiently expressing PKCδ-DN and LacZ constructs. Briefly, N27 cells and primary mesencephalic neurons expressing PKCδ-DN and LacZ (these constructs express V5 fusion protein) were cultured and processed for double immunostaining of P-TH-ser40 and V5 using specific antibodies. Stained cells were mounted on slides and viewed under a Nikon TE2000 fluorescence microscope, and images were captured with a SPOT digital camera, as described in Materials and Methods. P-TH-ser40 levels were quantitatively analyzed from six different areas in each group using MetaMorph image analysis. Experiments were repeated three times, and representative images are shown. Asterisks (**p < 0.01) indicate significant differences between LacZ- and PKCδ-DN-expressing cells.
To further confirm the regulatory role of PKCδ in TH phosphorylation in the dopaminergic system, we examined the effect of PKCδ inhibition on TH-ser40 phosphorylation in primary dopaminergic neurons obtained from the ventral mesencephalon of embryonic day 16 (E16) to E18 mouse embryos. Primary mesencephalic cultures were transfected with plasmids coding for the loss of function PKCδ-DN mutant and LacZ using the Amaxa nucleofector system. Twenty-four hours after transfection, primary neurons were processed for immunohistochemical analysis using TH-ser40 and V5-epitope antibodies in PKCδ-DN mutant and LacZ cells. As shown in Figure 5C, immunochemical staining revealed that the extent of TH-ser40 phosphorylation was significantly enhanced in primary neurons transfected with the PKCδ-DN mutant compared with LacZ-transfected primary neurons. V5 staining was used for identification of transfected cells. Fluorescent intensity analysis of TH-ser40 immunostaining showed ∼2.5 times more fluorescence in primary neurons expressing the PKCδ-DN mutant compared with Lac-Z-expressing neurons. These results confirm that PKCδ negatively modulates TH-ser40 phosphorylation in primary dopaminergic neurons.
Enhanced TH-ser40 phosphorylation and DA synthesis in PKCδ siRNA-transfected dopaminergic neuronal cells
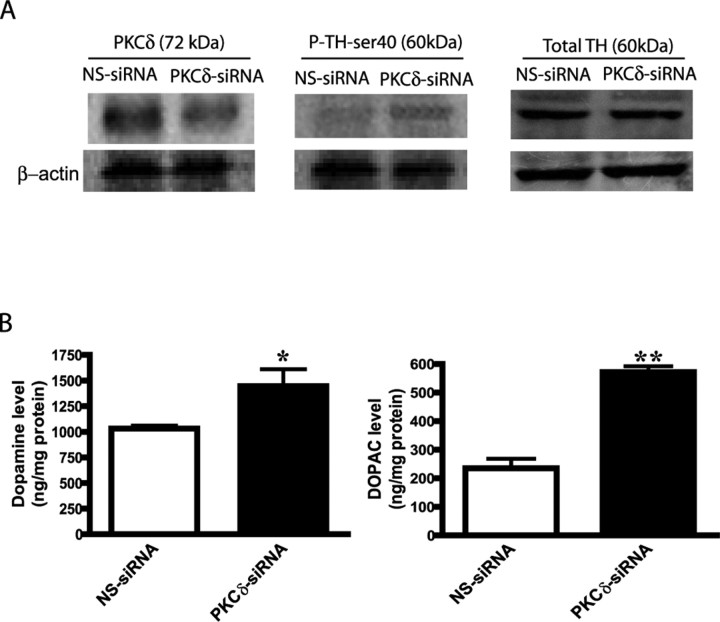
To further substantiate the regulatory role of TH activity and DA synthesis by PKCδ, we used an RNAi approach. We recently developed PKCδ siRNAs that specifically suppress PKCδ expression, but not the expression of PKCε, a novel isoform most closely and phylogenetically related to PKCδ (Yang et al., 2004). The siRNAs also did not produce any cytotoxic effect in N27 dopaminergic cells. In this experiment, we measured TH-ser40 phosphorylation, and DA and DOPAC levels after siRNA mediated suppression of PKCδ expression in N27 cells. Figure 6A shows a significant suppression of endogenous PKCδ expression in PKCδ siRNA-transfected cells compared with NS-siRNA-transfected N27 cells. A 60% reduction in PKCδ expression was observed in PKCδ-siRNA-transfected cells as measured by Western blot analysis. Importantly, TH-ser40 phosphorylation levels were also significantly higher in PKCδ-siRNA-transfected cells compared with NS-siRNA-transfected N27 cells, whereas the total TH levels were similar in both PKCδ-siRNA- and NS-siRNA-transfected cells. Densitometric analysis of P-TH-ser40 (60 kDa band) revealed a twofold increase in PKCδ-siRNA-transfected cells compared with NS-siRNA-transfected N27 cells (Fig. 6A). Reprobing of the nitrocellulose membranes with β-actin antibody showed the density of the 43 kDa β-actin band to be identical in all lanes, confirming equal protein loading. Next, we measured DA and DOPAC levels in siRNA-transfected N27 cells by HPLC. As shown in Figure 6B, DA and DOPAC levels were significantly higher in PKC-siRNA-transfected cells compared with NS-siRNA-transfected N27 cells. A 45% increase in the DA level and a twofold increase in DOPAC were noted in PKCδ-siRNA-transfected cells compared with NS-siRNA-transfected N27 cells. Together, these data further substantiate that PKCδ negatively regulates TH-ser40 phosphorylation and DA synthesis in dopaminergic neuronal cells.
Figure 6.
Effect of RNAi-mediated knockdown of PKCδ on P-TH-ser40 levels and DA synthesis. A, Western blot of TH-ser40 phosphorylation. Briefly, N27 cells were transfected with PKCδ-siRNA and NS-siRNA, and then extracts from siRNA-transfected N27 cells were subjected to Western blot analyses of PKCδ, P-TH-ser40, and total TH. Densitometric analysis of the 74 kDa native PKCδ band and 60 kDa P-TH-ser40 and TH bands were normalized to β-actin levels and are plotted below their respective Western blots. B, DA synthesis. Cell extracts from PKCδ-siRNA and NS-siRNA-transfected N27 cells were used for determining DA and DOPAC levels by HPLC. The data represent a mean ± SEM of six to eight individual measurements. Asterisks (*p < 0.05 and **p < 0.01) indicate significant differences between PKCδ-siRNA- and NS-siRNA-transfected N27 cells.
Increased TH-ser40 phosphorylation in primary mesencephalic dopaminergic neurons from PKCδ knock-out (−/−) mice
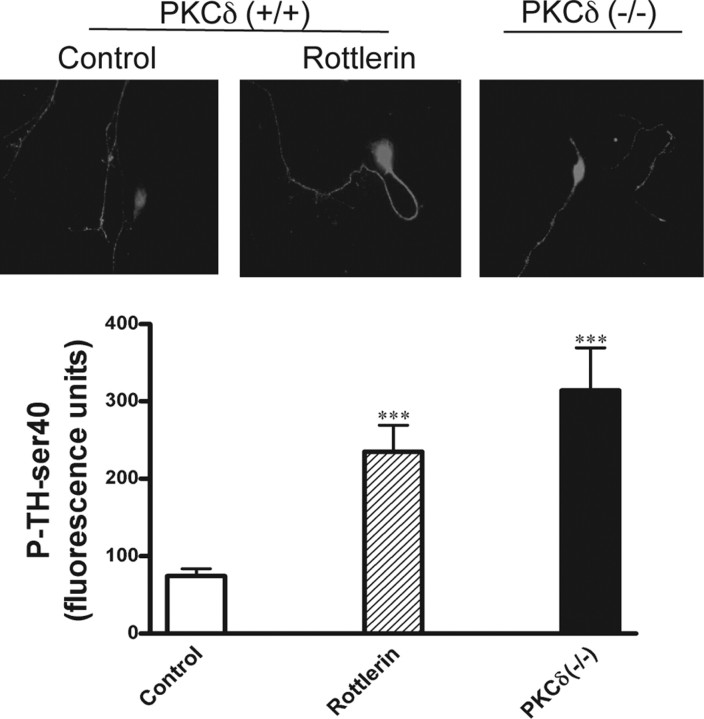
We also extended the TH phosphorylation studies to primary mesencephalic dopaminergic neurons derived from naive and PKCδ knock-out (−/−) E16–18 mouse embryos (Miyamoto et al., 2002). The level of TH-ser40 phosphorylation in nigral dopaminergic neurons in PKCδ (+/+) and PKCδ (−/−) mice was compared by immunostaining. The baseline TH-ser40 phosphorylation levels were significantly higher in untreated primary neurons obtained from PKCδ (−/−) mice compared with untreated PKCδ (+/+) mice (Fig. 7). Fluorescent intensity measurements revealed that the TH-ser40 level was threefold higher in PKCδ (−/−) dopaminergic neurons compared with PKCδ (+/+) mesencephalic neurons. In addition to the knock-out studies, we tested the effect of the PKCδ inhibitor rottlerin on TH-ser40 phosphorylation in the PKCδ (+/+) primary neuronal cultures. The cultures were treated with 5 μm rottlerin for 3 h, and then the level of TH-ser40 phosphorylation was measured. As shown in Figure 7, rottlerin treatment increased TH-ser40 phosphorylation in PKCδ (+/+) primary dopaminergic neurons. The fluorescent intensity was increased twofold in rottlerin-treated dopaminergic neurons compared with dopaminergic neurons. Together, these results confirm that suppression of PKCδ increases TH-ser40 phosphorylation in dopaminergic neurons.
Figure 7.
P-TH-ser40 levels in primary mesencephalic cultures from PKCδ knock-out (−/−) animals. Primary mesencephalic neurons were cultured on laminin-coated coverslips from PKCδ (−/−) and PKCδ (+/+) E16–E18 pups. Primary cultures from PKCδ (+/+) animals were also incubated with 5 μm rottlerin for 3 h. Cells were fixed and processed for P-TH-ser40 immunocytochemistry. Cy3-conjugated secondary antibody was used for visualization of P-TH-ser40 and viewed under a Nikon TE2000 fluorescence microscope. P-TH-ser40 levels were quantitatively analyzed from six different areas in each group using MetaMorph image analysis. Asterisks (***p < 0.001) indicate significant differences from controls. Experiments were repeated three times, and representative images are shown.
Effect of PP2A inhibition on TH activity and TH-ser40 phosphorylation
The increased TH-ser40 phosphorylation resulting from PKCδ inhibition suggested that PKCδ may affect dephosphorylation of TH under normal conditions. Previous studies have demonstrated that PP2A is a major serine phosphatase that mediates the dephosphorylation of TH, in particular TH-ser40, resulting in the inactivation of TH (Vrana and Roskoski, 1983; Haavik et al., 1989). Because both of these proteins negatively regulate TH, we hypothesize that PKCδ may work through PP2A to reduce dephosphorylation of TH-ser40 and TH activity. This would explain why inhibition of PKCδ increases TH-ser 40 phosphorylation, TH activity, and dopamine synthesis.
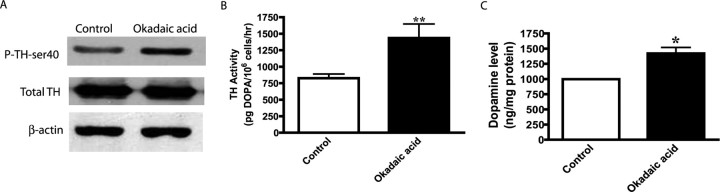
To test this novel hypothesis, we first examined whether PP2A regulates TH activity and TH-ser40 phosphorylation in our dopaminergic model system. N27 cells were incubated with the PP2A inhibitor okadaic acid (2 μm) for 2 h, and then TH-ser40 phosphorylation and TH activity were measured. As shown in Figure 8A, Western blot analysis revealed an increase in baseline TH-ser40 phosphorylation levels in okadaic acid-treated cells compared with untreated cells. The levels of total TH were similar in okadaic acid-treated and untreated cells, suggesting that an increase in the TH-ser40 phosphorylation level is not a result of increased TH expression. We also measured TH activity as well as dopamine content in okadaic acid-treated cells. HPLC measurement revealed a significant increase in TH activity (Fig. 8B) and DA level (Fig. 8C) in okadaic acid–treated cells. TH activity was increased from 826 ± 64 DOPA pg/106 cells/h in control cells to 1434 ± 21 DOPA pg/106 cells/h in okadaic acid-treated cells. The dopamine levels increased from 997 ± 30 ng/mg protein in untreated cells to 1421 ± 10 ng/mg protein in okadaic acid-treated cells. Together, these results suggest that PP2A regulates TH activity and dopamine synthesis in dopaminergic neurons.
Figure 8.
Effect of PP2A inhibition on TH-ser40 phosphorylation, TH activity, and dopamine levels. A, Western blot analysis of P-TH-ser40. N27 cells were treated with 0.01% DMSO (vehicle control) or 2 μm okadaic acid for 2 h, and then cell lysates were subjected to immunoblot for total TH, P-TH-ser40, and β-actin. B, Effect of okadaic acid on TH activity. C, Dopamine content. N27 cells were treated with 2 μm okadaic acid for 2 h. To determine TH activity, N27 cells were incubated with a DOPA decarboxylase inhibitor (2 mm NSD-1015 for 1 h) before okadaic acid treatment. After treatment, cells were lysed and neurochemicals were extracted for determining l-DOPA and dopamine levels by HPLC. The data represent the mean ± SEM from three separate experiments. Asterisks (*p < 0.05; **p < 0.01) indicate significant differences between untreated and okadaic acid-treated N27 cells.
Association and phosphorylation of PP2A by PKCδ
To determine the interaction between PKCδ and PP2A, we first examined whether PP2A is physically associated with PKCδ. Immunoprecipitation studies were conducted in cell culture as well as in mouse substantia nigral tissue lysate to determine the possible association. Immunoprecipitation studies revealed PP2Ac immunoreactivity in PKCδ immunoprecipitates from PKCδ (+/+) mouse substantia nigra lysates (Fig. 9A) and N27 cell lysates (Fig. 9C). Similarly, in reverse immunoprecipitation analysis, the PP2Ac immunoprecipitates showed PKCδ immunoreactivity. The nigral lysate from PKCδ (−/−) mice was used as a negative control. No PP2Ac immunoreactivity was observed in PKCδ immunoprecipitates from PKCδ (−/−) mouse substantia nigra lysates (Fig. 9B), demonstrating the specificity of immunoprecipitation studies. In addition, immunoprecipitated samples using preadsorbed PKCδ antibody with blocking peptide showed substantially reduced immunoreactivity to PP2Ac, indicating the specificity of PKCδ antibody used in the study. Rabbit IgG and mouse IgG immunoprecipitates were used as additional negative controls in these experiments. Collectively, these results indicate a physical association between PP2Ac and PKCδ in a dopaminergic cell line as well as in substantia nigra.
Figure 9.
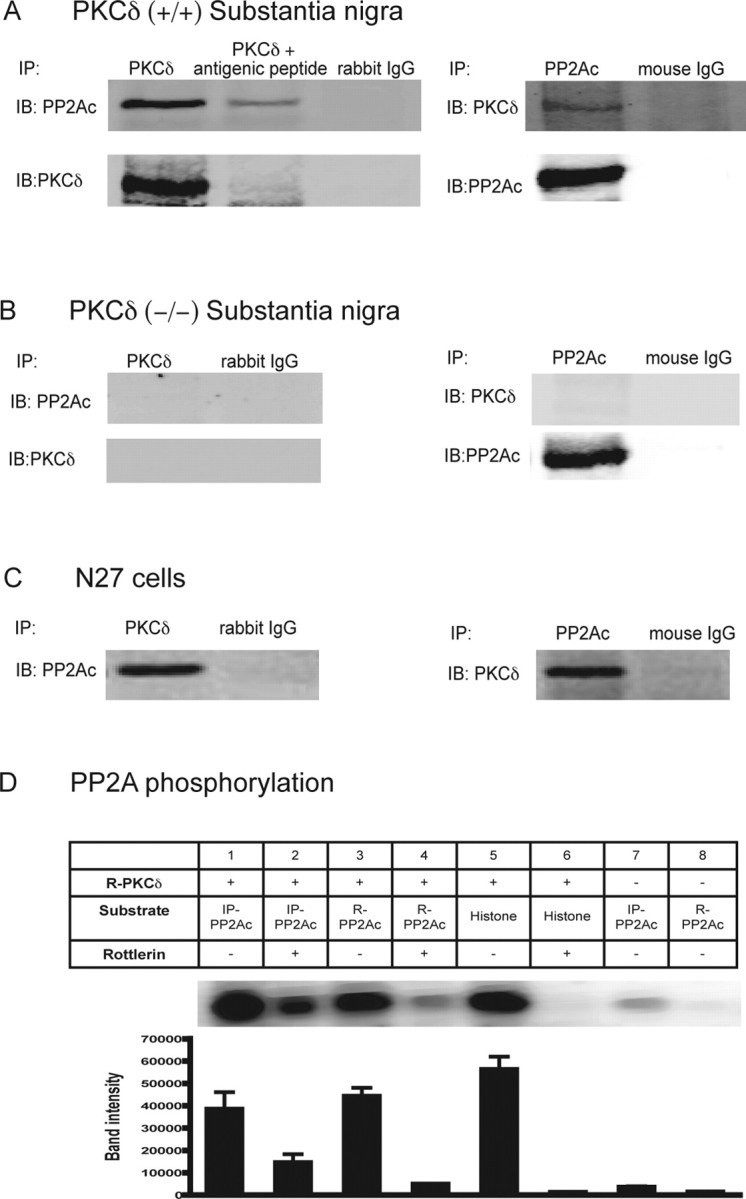
PKCδ associates with PP2Ac and modulates PP2A activity. Immunoprecipitation assays: A, PKCδ (+/+) mouse substantia nigra; B, PKCδ (−/−) mouse substantia nigra; and C, N27 cells. Anti-PKCδ polyclonal antibody was used for immunoprecipitation (IP) and immunoblotted (IB) with mouse monoclonal PP2Ac antibody. In reverse immunoprecipitation studies, anti-PP2Ac mouse monoclonal antibody was used for immunoprecipitation followed by immunoblotting with rabbit polyclonal PKCδ antibody. Anti-PKCδ polyclonal antibody was coincubated with antigenic blocking peptide in the immunoprecipitation studies as a negative control. Rabbit IgG and mouse IgG were also used as negative controls. D, In vitro kinase assay, PKCδ phosphorylates PP2Ac. The top panel is a representative autoradiography showing [32P] phosphorylation of the immunoprecipitated PP2Ac (IP-PP2Ac) and recombinant PP2Ac (R-PP2Ac) by recombinant PKCδ (R-PKCδ). PP2Ac immunoprecipitates from N27 cell homogenates represent endogenous PP2Ac. Recombinant PKCδ protein was purchased from Sigma. Immunoprecipitation and phosphorylation assays were performed as described in Materials and Methods. The quantitative data in the bottom panel represent the mean ± SEM from three assays. Histone H1 was used as positive control substrate, and for negative controls, no substrate was added.
Next, we determined whether physical association of PP2Ac with PKCδ involves direct phosphorylation of PP2Ac by PKCδ. We performed phosphorylation studies using immunoprecipitated PP2Ac from N27 cells and pure recombinant PP2Ac protein as substrate. As shown in Figure 9D, both immunoprecipitated and recombinant PP2Ac were effectively phosphorylated by recombinant pure PKCδ as determined by 32P-in vitro kinase assays (Fig. 9D, lanes 1, 3). Histone H1 was used as a positive control substrate in phosphorylation assays (Fig. 9D, lane 5). To further confirm that PKCδ directly phosphorylates PP2Ac, and not another PP2Ac-associated kinase, recombinant PKCδ was preincubated with rottlerin for 15 min before the addition of 32P-ATP in the in vitro kinase assays. Rottlerin strongly reduced direct phosphorylation of immunoprecipitated and recombinant PP2Ac by PKCδ (Fig. 9D, lanes 2, 4). In the positive control, incubation with rottlerin also blocked the direct phosphorylation of histone by PKCδ (Fig. 9D, lane 6). Immunoprecipitated and recombinant PP2Ac samples incubated with 32P-ATP without PKCδ were used as negative controls (Fig. 9D, lanes 7 and 8). A weak band was observed in PP2Ac immunoprecipitates, which may be attributed to the endogenously associated PKCδ (Fig. 9D, lane 7). Collectively, these data indicate that PKCδ associates with and phosphorylates PP2Ac.
Because PP2Ac can be phosphorylated by PKCδ, we examined whether PKCδ phosphorylation activates or inactivates PP2A enzyme activity. We first checked the possibility in a cell-free system. Purified PP2A was incubated with recombinant PKCδ protein in the presence or absence of rottlerin, and PP2A phosphatase activity was determined. The PP2A inhibitor okadaic acid was used as a positive control. As shown in Figure 10A, incubation of PP2A with PKCδ strongly enhanced PP2A activity, which was attenuated by rottlerin. For the in vivo studies, we determined PP2A phosphatase activity in rottlerin-treated N27 cells and in N27 cells expressing the loss of function PKCδ-DN mutant. PP2A activity was significantly reduced in rottlerin-treated cells compared with vehicle-treated N27 cells (Fig. 10B). Similarly, PP2A activity was also significantly reduced in PKCδ-DN-mutant-expressing cells compared with LacZ-expressing N27 cells (Fig. 10B), indicating that suppression of PKCδ kinase activity reduces the enzymatic activity of endogenous PP2A. However, total PP2A level was unchanged in rottlerin-treated or PKCδ-DN cells (Fig. 10C), indicating that the decreased PP2A activity is not attributable to reduced PP2A protein levels. Taken together with the phosphorylation studies, these data suggest that PKCδ phosphorylates PP2Ac and enhances PP2A activity.
Figure 10.
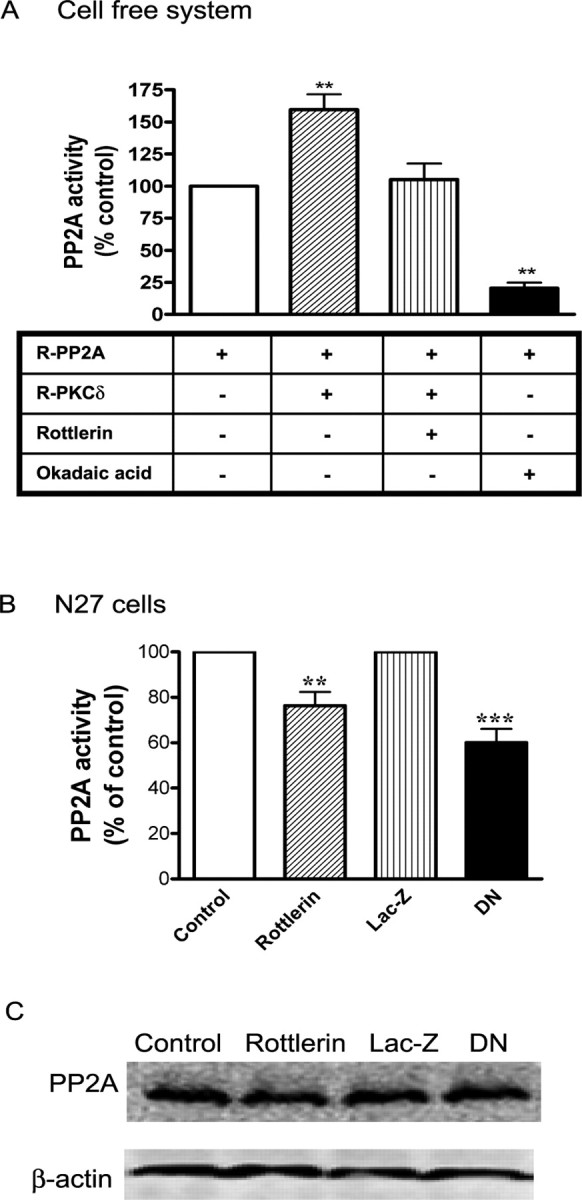
Effect of PKCδ inhibition on PP2A activity. A, Cell-free system. Recombinant PP2A enzyme (R-PP2A) was incubated with recombinant PKCδ (R- PKCδ) in the presence or absence of 5 μm rottlerin for 1 h. PP2A enzyme activity was measured by using a serine/threonine phosphatase assay kit from Promega. Okadaic acid (2 μm) was used as a positive control to inhibit PP2A activity. B, N27 cells. N27 cells were treated with 0.01% DMSO (vehicle control) or 3 μm rottlerin for 3 h, or expressed LacZ or PKCδ-DN. The data represent a mean ± SEM of four to six individual measurements. Asterisks (**p < 0.01; ***p < 0.001) indicate significant differences between either rottlerin-treated and vehicle control cells or LacZ- and PKCδ-DN-expressing N27 cells. C, Effect of PKCδ inhibition on PP2A protein level. Cell lysates from control, rottlerin-treated N27 cells, or LacZ and PKCδ-DN-expressing N27 cells were used to determine PP2A protein level by Western blot analysis.
Regulation of PP2A activity, TH-ser40 phosphorylation, and DA synthesis in PKCδ (−/−) knock-out animals
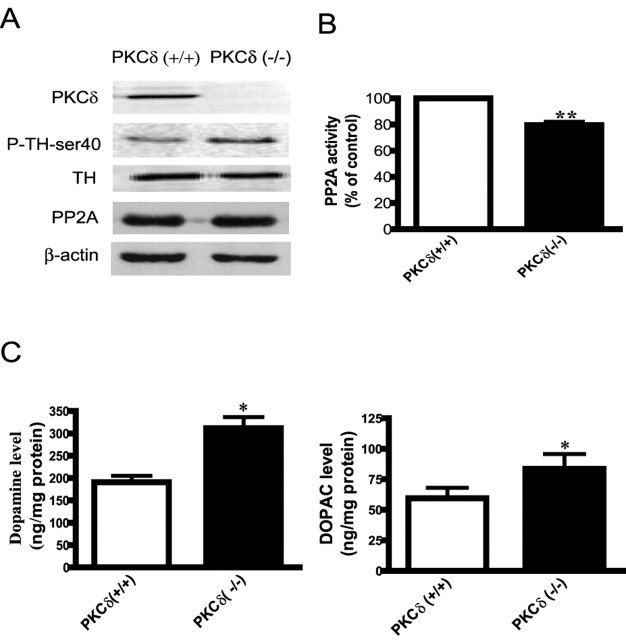
Additional validation of the role of PKCδ in the regulation of TH activity and DA synthesis was sought by extending these studies to PKCδ (−/−) knock-out animals. As shown in Figure 11A, a lack of PKCδ expression in PKCδ (−/−) animals was confirmed in Western blots; 74 kDa native PKCδ protein was present only in the brain lysates from PKCδ (+/+) mice but not from the PKCδ (−/−) mice. Next, we examined TH-ser40 phosphorylation status in PKCδ (−/−) animals. Determination of TH-ser40 phosphorylation in substantia nigra brain tissue revealed a significantly higher level in PKCδ (−/−) animals compared with PKCδ (+/+) animals, whereas the total TH levels were similar in the substantia nigra of these animals (Fig. 11A). The density of the 43 kDa β-actin band was identical in all lanes, indicating equal protein loading.
Figure 11.
Increased TH-ser40 phosphorylation, dopamine levels, and PP2A activity in PKCδ (−/−) knock-out animals. A, Western blot analysis of P-TH-ser40. Substantia nigral lysates from PKCδ (−/−) knock-out and PKCδ (+/+) naive animals were subjected to immunoblotting of PKCδ, P-TH-ser40, and total TH. B, PP2A activity. PP2A activity was measured in substantia nigra homogenates obtained from PKCδ (+/+) and PKCδ (−/−) animals. The data represent a mean ± SEM from four to six animals. Asterisks (**p < 0.01) indicate significant differences between PKCδ (+/+) and PKCδ (−/−) animals. C, Neurotransmitter levels. DA and DOPAC levels were determined in the striatal extracts of PKCδ (−/−) and PKCδ (+/+) animals by HPLC. The data represent a mean ± SEM from 6 to 10 animals. Asterisks (*p < 0.05) indicate significant differences between PKCδ (−/−) and PKCδ (+/+) animals.
To determine whether PKCδ influences PP2A activity in vivo, we compared PP2A enzyme activity in the substantia nigra of PKCδ (+/+) and PKCδ (−/−) animals. PP2A activity in the substantia nigra lysates from PKCδ (−/−) mice was significantly lower than in PKCδ (+/+) mice (Fig. 11B), supporting our findings from cell culture studies that PKCδ positively influences PP2A activity in nigral neurons.
We also compared DA and DOPAC levels between PKCδ (−/−) animals and PKCδ (+/+) animals. HPLC analysis revealed that striatal DA and DOPAC levels were significantly higher in the striatum of PKCδ (−/−) animals compared with PKCδ (+/+) animals (Fig. 11C). DA levels were determined to be 311.7 ± 24.88 ng/mg protein in PKCδ (−/−) compared with 190 ± 14.24 ng/mg protein in PKCδ (+/+) animals, an increase of 60%. Similarly, striatal DOPAC levels were estimated to be 83.64 ± 12.02 ng/mg protein in PKCδ (−/−) compared with 59.41 ± 8.697 ng/mg protein in PKCδ (+/+) animals, an increase of 40%. Together, these in vivo data further demonstrate that PKCδ negatively regulates TH activity, resulting in reduced TH phosphorylation and DA synthesis.
Discussion
In the present study, we systematically characterized the regulation of TH by a member of the novel class of the protein kinase C family, PKCδ, and demonstrated the following: (1) PKCδ is highly expressed in nigral dopaminergic neurons; (2) suppression of PKCδ activity by kinase inhibitors, dominant-negative mutants, or RNAi-mediated knockdown increases TH-ser40 phosphorylation, TH activity, and dopamine levels; (3) PKCδ phosphorylates PP2A to promote dephosphorylation of TH-ser40, and inhibition of PKCδ attenuates PP2A activity, resulting in elevated TH-ser40 phosphorylation, TH activity, and dopamine levels; and (4) TH activity and dopamine levels are enhanced in PKCδ (−/−) knock-out animals. These results were obtained using both cellular and molecular biological approaches in multiple cell culture models, including PC12 cells, N27 mesencephalic dopaminergic cells, and primary mesencephalic neurons, as well as in animal models, including a knock-out model. Collectively, to our knowledge, this is the first report describing a negative regulation of the rate-limiting enzyme of the dopamine synthetic pathway, TH, by PKCδ via modulation of PP2A activity.
Phosphorylation is a key posttranslational mechanism to regulate TH activity. Phosphorylation of serine residues at 8, 19, 31, and 40 can activate TH, resulting in enhanced dopamine synthesis (Campbell et al., 1986; Haycock, 1990; Mitchell et al., 1990; Lindgren et al., 2001; McCulloch et al., 2001; Dunkley et al., 2004). A number of kinases, including PKC, PKA, CaMKII, and mitogen-activated protein kinase, have been shown to phosphorylate one or more of these sites to increase TH activity, depending on the cell type. Because we found a high expression of PKCδ in nigral dopaminergic neurons (Fig. 1), we initially hypothesized that PKCδ might phosphorylate TH to increase its activity. To test this hypothesis, we used the PKCδ inhibitor rottlerin to inhibit the kinase and anticipated that inhibition of PKCδ would result in inhibition of TH activity. Surprisingly, we observed a dose-dependent increase in TH activity and dopamine levels in cells treated with the PKCδ inhibitor rottlerin. To further confirm this observation, PKCδ-dominant-negative mutant and siRNAs were used for PKCδ inhibition, which also caused increased TH activity and dopamine levels in dopaminergic cells. Determination of TH-ser40 phosphorylation under conditions of PKCδ inhibition further revealed an enhanced TH-ser40 phosphorylation with no significant change in phosphorylation of TH-ser31. Of the different phosphorylation sites, Ser40 is the major regulatory site contributing to increased TH activity and dopamine synthesis in vivo (Meligeni et al., 1982; Waymire et al., 1991; Daubner et al., 1992; Wu et al., 1992; McCulloch et al., 2001). Studies using PC12 cells either responsive or nonresponsive to cAMP stimuli demonstrated that PKA is an important kinase in TH-ser40 phosphorylation (Wilson et al., 1996; Salvatore et al., 2001). Recently, Kobori et al. (2004) reported that glial cell line-derived neurotrophic factor increases TH-ser31 and TH-ser40 phosphorylation, which contributes to enhanced dopamine synthesis in mesencephalic cultures. Together, phosphorylation of the serine residues also appears to depend on cell types and stimuli.
General PKC increases ser-40 phosphorylation and TH activity (Cahill et al., 1989; Haycock, 1990, 1993; Haycock and Haycock, 1991; Waymire et al., 1991; Bobrovskaya et al., 1998); however, the effect of PKC subtypes has never been explored. At the present time, 12 different isoforms of PKC have been identified and grouped into three major classes (Gschwendt, 1999; Dempsey et al., 2000; Maher, 2001; Kanthasamy et al., 2003). The conventional class of PKC isoforms PKC (α, βI, βII, and γ require diacylglycerol (DAG) and Ca2+ for activation; the novel PKC isoforms PKCδ, ε, μ, η, and θ require only DAG but not Ca2+ for activation; and the atypical PKCs, PKC τ, λ, and ζ require neither Ca2+ nor DAG for activation). The isoform-specific physiological functions of each subtype of PKCs are yet to be characterized in the CNS. The recently available, more specific pharmacological inhibitors, genetic mutants, and siRNAs specific for subtypes of isoforms are extremely useful in characterizing functional significance of PKC isoforms. Our results of enhanced TH activity by pharmacological inhibitors and siRNA in three different cell culture models clearly demonstrate that PKCδ can negatively regulate TH activity.
An increase in TH-ser40 phosphorylation is normally mediated by either activation of a kinase responsible for the serine phosphorylation or by blocking the activity of the phosphatase responsible for dephosphorylation of ser40. Because we found an increase in TH-ser40 phosphorylation and TH activity under the condition of PKCδ inhibition (pharmacological inhibitor, dominant-negative mutant, siRNA studies) (Figs. 2–6), we hypothesized that PKCδ attenuates TH function by enhancing phosphatase activity. Haavik et al. (1989) demonstrated that PP2A is the major serine/threonine phosphatase that regulates >90% of the dephosphorylation of TH at Ser40. In their study, okadaic acid treatment dramatically increased TH phosphorylation and TH activity, establishing PP2A as an important regulator of both TH activity and Ser40 phosphorylation. Recently, Leal et al. (2002) demonstrated that PP2A dephosphorylated TH-ser40 at twice the rate compared with TH-ser31 and TH-ser19, suggesting a preferential dephosphorylation of TH-ser40 by PP2A. Additionally, Lindgren et al. (2001) showed that PP2A inhibitor treatment in rat striatal slices did not increase TH phosphorylation at Ser31, whereas TH-ser40 phosphorylation was readily increased, indicating that the TH-ser40 site may be highly regulated by phosphorylation/dephosphorylation reactions compared with TH-ser31. Furthermore, among these three phosphorylated serine residues, TH-ser40 mainly contributed to tyrosine hydroxylase activation and dopamine synthesis in vivo (Ramsey et al., 1996). Thus, consistent with previous studies, our results indicate that TH ser40 is predominantly regulated by phosphorylation/dephosphorylation.
An active PP2A enzyme consists of a heterotrimer of the structural A subunit, a catalytic C subunit, and a regulatory B subunit (Dobrowsky and Hannun, 1993; Sontag et al., 1995; McCright et al., 1996; Ruvolo et al., 2002). The exact nature of the physical association and dynamic regulation of TH, PKCδ, and PP2A are yet to be characterized; however, recent literature provides some information regarding this interaction. PKCδ, TH, or PP2A have been shown recently to physically associate with each other, as well as other putative chaperone proteins such as α-synuclein and 14-3-3 (Ostrerova et al., 1999; Kleppe et al., 2001; Srivastava et al., 2002; Kjarland et al., 2006). Recently, Peng et al. (2005) demonstrated that a functional interaction between α-synuclein and PP2A can regulate TH phosphorylation and TH activity. Srivastava et al. (2002) reported a physical interaction between PKCδ and PP2A in NIH3T3 cells, and that dephosphorylation of PKCδ by PP2A results in its inactivation. In our recent study, we showed that α-synuclein interacts with PKCδ and regulates its activity after neurotoxic insults (Kaul et al., 2005). Therefore, in the dopaminergic system, the physical and functional association between PKCδ, TH, and PP2A could be facilitated and/or regulated by chaperone proteins such as α-synuclein (Kaul et al., 2005; Peng et al., 2005) and 14-3-3 (Ostrerova et al., 1999; Kleppe et al., 2001; Kjarland et al., 2006). Nevertheless, additional studies are required in both in vitro and in vivo model systems to elucidate the dynamics of physical and functional regulation of PKCδ and PP2A in regulation of TH activity.
In the present study, we show that PKCδ and PP2Ac physically associate in dopaminergic neuronal cell lysates as well as in mouse brain substantia nigra lysates. This interaction of PKCδ with PP2Ac may stimulate PP2A activity. To determine whether the physical association is accompanied by a functional interaction, we measured PP2A activity under conditions where PKCδ activity was inhibited. We found that PP2A activity was significantly decreased, without altering the PP2A protein levels, by the PKCδ inhibitor rottlerin. Basal PP2A enzymatic activity was also significantly reduced in dopaminergic cells stably expressing loss of function kinase inactive PKCδ-DN mutant compared with LacZ cells. Furthermore, substantia nigra of PKCδ (−/−) mice showed significantly lower basal PP2A activity compared with naive animals, indicating that PKCδ can augment PP2A activity. Additionally, in vitro kinase assays also revealed that PKCδ can phosphorylate PP2Ac. Together, these data suggest that physical association accompanied by PP2Ac phosphorylation by PKCδ results in PP2A activation. PP2A activity can be effectively regulated by phosphorylation. Many studies have shown that phosphorylation of PP2A at Tyr307 reduces its activity (Chen et al., 1992), indicating that Tyr307 is a key negative regulatory phosphorylation site. PP2A could also be phosphorylated at serine/threonine sites (Guo and Damuni, 1993), but the specific PKC isoforms involved in the PP2A phosphorylation at various sites of PP2A are yet to be characterized. Following the observation that PKCδ can phosphorylate PP2Ac to increase activity, we further examined whether enhanced PP2A activity reduces TH-ser40 phosphorylation and TH activity. Treatment with the PP2A inhibitor okadaic acid increased the TH-ser40 level and enhanced TH activity, suggesting that PP2A effectively regulates TH activity and ser40 phosphorylation. Our results are in agreement with a recent study showing attenuation of TH activity and TH-ser40 phosphorylation by PP2A (Peng et al., 2005). Collectively, our data on PKCδ and PP2A suggest that PKCδ negatively regulates TH-ser40 phosphorylation and TH activity via increased PP2A activity by direct phosphorylation of PP2A.
Regulation of TH activity and DA levels is critical for normal dopaminergic neurotransmission in the CNS. Excessive DA production may not only alter neurotransmission but may also contribute to neuronal cell death through increased oxidative stress (Hoyt et al., 1997; Luo et al., 1998). In this regard, we wish to point out that PKCδ can be activated by at least two independent mechanisms in neuronal cells. These include membrane translocation and caspase-3-dependent proteolytic cleavage (Kikkawa et al., 2002; Brodie and Blumberg, 2003; Kanthasamy et al., 2003). Of the two activation mechanisms, PKCδ activated by membrane translocation after tonic stimulation by lipid activators contributes to cell survival and proliferation (Kikkawa et al., 2002; Kanthasamy et al., 2003; Jackson and Foster, 2004). As demonstrated in our recent studies, another form of activation is caspase-3-dependent proteolytic cleavage of native PKCδ into regulatory and catalytic fragments resulting in persistent activation during exposure to neurotoxic agents such as 1-methyl-4-phenylpyridinium, methylcyclopentadienyl manganese tricarbonyl, manganese, or dieldrin (Anantharam et al., 2002; Kaul et al., 2003; Latchoumycandane et al., 2005). This form of proteolytic activation contributes to apoptotic cell death of dopaminergic neurons (Kanthasamy et al., 2003). In the case of a lipid activator, 12-O-tetradecanoylphorbol-13-acetate induced membrane translocation of native PKCδ but did not induce apoptotic cell death in dopaminergic cell lines (our unpublished observations). Furthermore, recent studies from our laboratory and others have shown that, unlike the native 74 kDa PKCδ, the 41 kDa catalytically active PKCδ fragment, after proteolytic cleavage, is targeted to various subcellular organelles, including the mitochondria (Majumder et al., 2000) and nucleus (DeVries et al., 2002), to activate apoptotic cell death signaling molecules. These results suggest that native and cleaved PKCδ have different substrate profiles. In this study, we examined the effect of native intact PKCδ on PP2A activity and TH regulation under normal conditions, but we did not study the effect of neurotoxicant-induced proteolytically activated PKCδ. Apparently, a high expression of PKCδ in nigral dopaminergic neurons has dual functions. In normal situations, PKCδ phosphorylates PP2A to increase the phosphatase activity, resulting in enhanced dephosphorylation of TH-ser40, which eventually leads to decreased TH activity and DA synthesis (summarized in Fig. 12). This negative regulation may be important for maintaining optimal dopamine levels. In addition to the function of regulating dopamine synthesis, under the condition of enhanced oxidative stress induced by neurotoxic insults, PKCδ serves as a key downstream proapoptotic effector of caspase-3, resulting in proteolytic activation of the kinase, which contributes to cell death (Anantharam et al., 2002; Kanthasamy et al., 2003; Kaul et al., 2003; Yang et al., 2004).
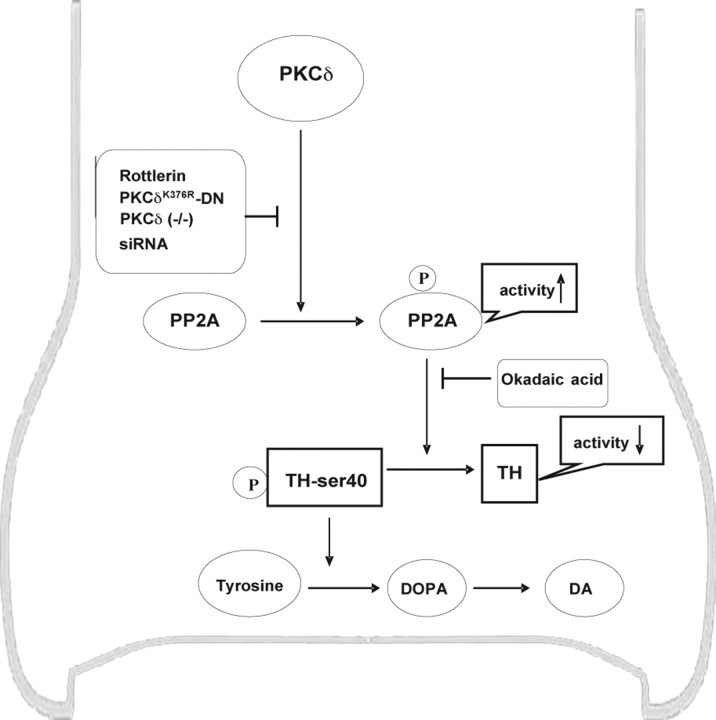
Figure 12.
A schematic depiction of TH regulation by PKCδ in dopaminergic cells. PKCδ enhances PP2A activity by phosphorylation of PP2Ac. Increased PP2A activity decreases TH phosphorylation at site ser40, which leads to inactivation of TH and reduction of its activity and an ultimate decrease in dopamine synthesis. Inhibition of PKCδ by rottlerin, PKCδ-DN mutant, PKCδ KO, or PKCδ siRNA suppresses PP2A activity resulting from a decreased phosphorylation of PP2A. The okadaic acid inhibits the PP2A phosphatase activity, thereby preventing the dephosphorylation of P-TH-ser40 and enhancing the TH enzymatic activity. In conclusion, our data suggest that PKCδ negatively regulates TH via PP2A.
In conclusion, we provide novel evidence that PKCδ negatively regulates TH activity and DA synthesis via activation of PP2A, and we suggest that PKCδ-mediated regulation of TH may have important implications in neurological dopaminergic system disorders, such as Parkinson's disease.
Footnotes
This work was supported by National Institutes of Health Grants NS 38644 and NS45133. This study was included in the pending United States patent application ISURF 03411.
References
- Adams FS, La Rosa FG, Kumar S, Edwards-Prasad J, Kentroti S, Vernadakis A, Freed CR, Prasad KN. Characterization and transplantation of two neuronal cell lines with dopaminergic properties. Neurochem Res. 1996;21:619–627. doi: 10.1007/BF02527762. [DOI] [PubMed] [Google Scholar]
- Albert KA, Wu WC, Nairn AC, Greengard P. Inhibition by calmodulin of calcium/phospholipid-dependent protein phosphorylation. Proc Natl Acad Sci USA. 1984;81:3622–3625. doi: 10.1073/pnas.81.12.3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anantharam V, Kitazawa M, Wagner J, Kaul S, Kanthasamy AG. Caspase-3-dependent proteolytic cleavage of protein kinase Cdelta is essential for oxidative stress-mediated dopaminergic cell death after exposure to methylcyclopentadienyl manganese tricarbonyl. J Neurosci. 2002;22:1738–1751. doi: 10.1523/JNEUROSCI.22-05-01738.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobrovskaya L, Cheah TB, Bunn SJ, Dunkley PR. Tyrosine hydroxylase in bovine adrenal chromaffin cells: angiotensin II-stimulated activity and phosphorylation of Ser19, Ser31, and Ser40. J Neurochem. 1998;70:2565–2573. doi: 10.1046/j.1471-4159.1998.70062565.x. [DOI] [PubMed] [Google Scholar]
- Brodie C, Blumberg PM. Regulation of cell apoptosis by protein kinase c delta. Apoptosis. 2003;8:19–27. doi: 10.1023/a:1021640817208. [DOI] [PubMed] [Google Scholar]
- Bunn SJ, Saunders HI. Staurosporine inhibits inositol phosphate formation in bovine adrenal medullary cells. Eur J Pharmacol. 1995;290:227–236. doi: 10.1016/0922-4106(95)00082-8. [DOI] [PubMed] [Google Scholar]
- Cahill AL, Horwitz J, Perlman RL. Phosphorylation of tyrosine hydroxylase in protein kinase C-deficient PC12 cells. Neuroscience. 1989;30:811–818. doi: 10.1016/0306-4522(89)90172-3. [DOI] [PubMed] [Google Scholar]
- Campbell DG, Hardie DG, Vulliet PR. Identification of four phosphorylation sites in the N-terminal region of tyrosine hydroxylase. J Biol Chem. 1986;261:10489–10492. [PubMed] [Google Scholar]
- Chen J, Martin BL, Brautigan DL. Regulation of protein serine-threonine phosphatase type-2A by tyrosine phosphorylation. Science. 1992;257:1261–1264. doi: 10.1126/science.1325671. [DOI] [PubMed] [Google Scholar]
- Clarkson ED, Edwards-Prasad J, Freed CR, Prasad KN. Immortalized dopamine neurons: a model to study neurotoxicity and neuroprotection. Proc Soc Exp Biol Med. 1999;222:157–163. doi: 10.1046/j.1525-1373.1999.d01-126.x. [DOI] [PubMed] [Google Scholar]
- Daubner SC, Lauriano C, Haycock JW, Fitzpatrick PF. Site-directed mutagenesis of serine 40 of rat tyrosine hydroxylase. Effects of dopamine and cAMP-dependent phosphorylation on enzyme activity. J Biol Chem. 1992;267:12639–12646. [PubMed] [Google Scholar]
- Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey EC, Newton AC, Mochly-Rosen D, Fields AP, Reyland ME, Insel PA, Messing RO. Protein kinase C isozymes and the regulation of diverse cell responses. Am J Physiol Lung Cell Mol Physiol. 2000;279:L429–L438. doi: 10.1152/ajplung.2000.279.3.L429. [DOI] [PubMed] [Google Scholar]
- DeVries TA, Neville MC, Reyland ME. Nuclear import of PKCdelta is required for apoptosis: identification of a novel nuclear import sequence. EMBO J. 2002;21:6050–6060. doi: 10.1093/emboj/cdf606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrowsky RT, Hannun YA. Ceramide-activated protein phosphatase: partial purification and relationship to protein phosphatase 2A. Adv Lipid Res. 1993;25:91–104. [PubMed] [Google Scholar]
- Donze O, Picard D. RNA interference in mammalian cells using siRNAs synthesized with T7 RNA polymerase. Nucleic Acids Res. 2002;30:e46. doi: 10.1093/nar/30.10.e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkley PR, Bobrovskaya L, Graham ME, von Nagy-Felsobuki EI, Dickson PW. Tyrosine hydroxylase phosphorylation: regulation and consequences. J Neurochem. 2004;91:1025–1043. doi: 10.1111/j.1471-4159.2004.02797.x. [DOI] [PubMed] [Google Scholar]
- Elbashir SM, Lendeckel W, Tuschl T. RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev. 2001;15:188–200. doi: 10.1101/gad.862301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre B, Turowski P, Hemmings BA. Differential inhibition and posttranslational modification of protein phosphatase 1 and 2A in MCF7 cells treated with calyculin-A, okadaic acid, and tautomycin. J Biol Chem. 1997;272:13856–13863. doi: 10.1074/jbc.272.21.13856. [DOI] [PubMed] [Google Scholar]
- Funakoshi H, Okuno S, Fujisawa H. Different effects on activity caused by phosphorylation of tyrosine hydroxylase at serine 40 by three multifunctional protein kinases. J Biol Chem. 1991;266:15614–15620. [PubMed] [Google Scholar]
- Gschwendt M. Protein kinase C delta. Eur J Biochem. 1999;259:555–564. doi: 10.1046/j.1432-1327.1999.00120.x. [DOI] [PubMed] [Google Scholar]
- Guo H, Damuni Z. Autophosphorylation-activated protein kinase phosphorylates and inactivates protein phosphatase 2A. Proc Natl Acad Sci USA. 1993;90:2500–2504. doi: 10.1073/pnas.90.6.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haavik J, Schelling DL, Campbell DG, Andersson KK, Flatmark T, Cohen P. Identification of protein phosphatase 2A as the major tyrosine hydroxylase phosphatase in adrenal medulla and corpus striatum: evidence from the effects of okadaic acid. FEBS Lett. 1989;251:36–42. doi: 10.1016/0014-5793(89)81424-3. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Miwa S, Lee K, Koshimura K, Kamel A, Hamahata K, Fujiwara M. A nonisotopic method for determination of the in vivo activities of tyrosine hydroxylase in the rat adrenal gland. Anal Biochem. 1988;168:176–183. doi: 10.1016/0003-2697(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Haycock JW. Quantitation of tyrosine hydroxylase, protein levels: spot immunolabeling with an affinity-purified antibody. Anal Biochem. 1989;181:259–266. doi: 10.1016/0003-2697(89)90240-6. [DOI] [PubMed] [Google Scholar]
- Haycock JW. Phosphorylation of tyrosine hydroxylase in situ at serine 8, 19, 31, and 40. J Biol Chem. 1990;265:11682–11691. [PubMed] [Google Scholar]
- Haycock JW. Multiple signaling pathways in bovine chromaffin cells regulate tyrosine hydroxylase phosphorylation at Ser19, Ser31, and Ser40. Neurochem Res. 1993;18:15–26. doi: 10.1007/BF00966919. [DOI] [PubMed] [Google Scholar]
- Haycock JW, Haycock DA. Tyrosine hydroxylase in rat brain dopaminergic nerve terminals. Multiple-site phosphorylation in vivo and in synaptosomes. J Biol Chem. 1991;266:5650–5657. [PubMed] [Google Scholar]
- Haycock JW, Ahn NG, Cobb MH, Krebs EG. ERK1 and ERK2, two microtubule-associated protein 2 kinases, mediate the phosphorylation of tyrosine hydroxylase at serine-31 in situ. Proc Natl Acad Sci USA. 1992;89:2365–2369. doi: 10.1073/pnas.89.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt KR, Reynolds IJ, Hastings TG. Mechanisms of dopamine-induced cell death in cultured rat forebrain neurons: interactions with and differences from glutamate-induced cell death. Exp Neurol. 1997;143:269–281. doi: 10.1006/exnr.1996.6374. [DOI] [PubMed] [Google Scholar]
- Jackson DN, Foster DA. The enigmatic protein kinase Cdelta: complex roles in cell proliferation and survival. FASEB J. 2004;18:627–636. doi: 10.1096/fj.03-0979rev. [DOI] [PubMed] [Google Scholar]
- Kanthasamy AG, Kitazawa M, Kaul S, Yang Y, Lahiri DK, Anantharam V, Kanthasamy A. Proteolytic activation of proapoptotic kinase PKCdelta is regulated by overexpression of Bcl-2: implications for oxidative stress and environmental factors in Parkinson's disease. Ann NY Acad Sci. 2003;1010:683–686. doi: 10.1196/annals.1299.125. [DOI] [PubMed] [Google Scholar]
- Kaul S, Kanthasamy A, Kitazawa M, Anantharam V, Kanthasamy AG. Caspase-3 dependent proteolytic activation of protein kinase C delta mediates and regulates 1-methyl-4-phenylpyridinium (MPP+)-induced apoptotic cell death in dopaminergic cells: relevance to oxidative stress in dopaminergic degeneration. Eur J Neurosci. 2003;18:1387–1401. doi: 10.1046/j.1460-9568.2003.02864.x. [DOI] [PubMed] [Google Scholar]
- Kaul S, Anantharam V, Kanthasamy A, Kanthasamy AG. Wild-type alpha-synuclein interacts with pro-apoptotic proteins PKCdelta and BAD to protect dopaminergic neuronal cells against MPP+-induced apoptotic cell death. Brain Res Mol Brain Res. 2005;139:137–152. doi: 10.1016/j.molbrainres.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Kikkawa U, Matsuzaki H, Yamamoto T. Protein kinase C delta (PKC delta): activation mechanisms and functions. J Biochem (Tokyo) 2002;132:831–839. doi: 10.1093/oxfordjournals.jbchem.a003294. [DOI] [PubMed] [Google Scholar]
- Kitazawa M, Anantharam V, Kanthasamy AG. Dieldrin-induced oxidative stress and neurochemical changes contribute to apoptopic cell death in dopaminergic cells. Free Radic Biol Med. 2001;31:1473–1485. doi: 10.1016/s0891-5849(01)00726-2. [DOI] [PubMed] [Google Scholar]
- Kitazawa M, Anantharam V, Yang Y, Hirata Y, Kanthasamy A, Kanthasamy AG. Activation of protein kinase C delta by proteolytic cleavage contributes to manganese-induced apoptosis in dopaminergic cells: protective role of Bcl-2. Biochem Pharmacol. 2005;69:133–146. doi: 10.1016/j.bcp.2004.08.035. [DOI] [PubMed] [Google Scholar]
- Kjarland E, Keen TJ, Kleppe R. Does isoform diversity explain functional differences in the 14-3-3 protein family? Curr Pharm Biotechnol. 2006;7:217–223. doi: 10.2174/138920106777549777. [DOI] [PubMed] [Google Scholar]
- Kleppe R, Toska K, Haavik J. Interaction of phosphorylated tyrosine hydroxylase with 14-3-3 proteins: evidence for a phosphoserine 40-dependent association. J Neurochem. 2001;77:1097–1107. doi: 10.1046/j.1471-4159.2001.00318.x. [DOI] [PubMed] [Google Scholar]
- Kobori N, Waymire JC, Haycock JW, Clifton GL, Dash PK. Enhancement of tyrosine hydroxylase phosphorylation and activity by glial cell line-derived neurotrophic factor. J Biol Chem. 2004;279:2182–2191. doi: 10.1074/jbc.M310734200. [DOI] [PubMed] [Google Scholar]
- Latchoumycandane C, Anantharam V, Kitazawa M, Yang Y, Kanthasamy A, Kanthasamy AG. Protein kinase Cdelta is a key downstream mediator of manganese-induced apoptosis in dopaminergic neuronal cells. J Pharmacol Exp Ther. 2005;313:46–55. doi: 10.1124/jpet.104.078469. [DOI] [PubMed] [Google Scholar]
- Leal RB, Sim AT, Goncalves CA, Dunkley PR. Tyrosine hydroxylase dephosphorylation by protein phosphatase 2A in bovine adrenal chromaffin cells. Neurochem Res. 2002;27:207–213. doi: 10.1023/a:1014880403970. [DOI] [PubMed] [Google Scholar]
- Lee KY, Lew JY, Tang D, Schlesinger DH, Deutch AY, Goldstein M. Antibodies to a synthetic peptide corresponding to a Ser-40-containing segment of tyrosine hydroxylase: activation and immunohistochemical localization of tyrosine hydroxylase. J Neurochem. 1989;53:1238–1244. doi: 10.1111/j.1471-4159.1989.tb07420.x. [DOI] [PubMed] [Google Scholar]
- Lindgren N, Xu ZQ, Herrera-Marschitz M, Haycock J, Hokfelt T, Fisone G. Dopamine D(2) receptors regulate tyrosine hydroxylase activity and phosphorylation at Ser40 in rat striatum. Eur J Neurosci. 2001;13:773–780. doi: 10.1046/j.0953-816x.2000.01443.x. [DOI] [PubMed] [Google Scholar]
- Luo Y, Umegaki H, Wang X, Abe R, Roth GS. Dopamine induces apoptosis through an oxidation-involved SAPK/JNK activation pathway. J Biol Chem. 1998;273:3756–3764. doi: 10.1074/jbc.273.6.3756. [DOI] [PubMed] [Google Scholar]
- Maher P. How protein kinase C activation protects nerve cells from oxidative stress-induced cell death. J Neurosci. 2001;21:2929–2938. doi: 10.1523/JNEUROSCI.21-09-02929.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder PK, Pandey P, Sun X, Cheng K, Datta R, Saxena S, Kharbanda S, Kufe D. Mitochondrial translocation of protein kinase C delta in phorbol ester-induced cytochrome c release and apoptosis. J Biol Chem. 2000;275:21793–21796. doi: 10.1074/jbc.C000048200. [DOI] [PubMed] [Google Scholar]
- McCright B, Rivers AM, Audlin S, Virshup DM. The B56 family of protein phosphatase 2A (PP2A) regulatory subunits encodes differentiation-induced phosphoproteins that target PP2A to both nucleus and cytoplasm. J Biol Chem. 1996;271:22081–22089. doi: 10.1074/jbc.271.36.22081. [DOI] [PubMed] [Google Scholar]
- McCulloch RI, Daubner SC, Fitzpatrick PF. Effects of substitution at serine 40 of tyrosine hydroxylase on catecholamine binding. Biochemistry. 2001;40:7273–7278. doi: 10.1021/bi010546d. [DOI] [PubMed] [Google Scholar]
- McTigue M, Cremins J, Halegoua S. Nerve growth factor and other agents mediate phosphorylation and activation of tyrosine hydroxylase. A convergence of multiple kinase activities. J Biol Chem. 1985;260:9047–9056. [PubMed] [Google Scholar]
- Meligeni JA, Haycock JW, Bennett WF, Waymire JC. Phosphorylation and activation of tyrosine hydroxylase mediate the cAMP-induced increase in catecholamine biosynthesis in adrenal chromaffin cells. J Biol Chem. 1982;257:12632–12640. [PubMed] [Google Scholar]
- Miranda M, Sorkina T, Grammatopoulos TN, Zawada WM, Sorkin A. Multiple molecular determinants in the carboxyl terminus regulate dopamine transporter export from endoplasmic reticulum. J Biol Chem. 2004;279:30760–30770. doi: 10.1074/jbc.M312774200. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Hardie DG, Vulliet PR. Site-specific phosphorylation of tyrosine hydroxylase after KCl depolarization and nerve growth factor treatment of PC12 cells. J Biol Chem. 1990;265:22358–22364. [PubMed] [Google Scholar]
- Miyamoto A, Nakayama K, Imaki H, Hirose S, Jiang Y, Abe M, Tsukiyama T, Nagahama H, Ohno S, Hatakeyama S, Nakayama KI. Increased proliferation of B cells and auto-immunity in mice lacking protein kinase Cdelta. Nature. 2002;416:865–869. doi: 10.1038/416865a. [DOI] [PubMed] [Google Scholar]
- Ostrerova N, Petrucelli L, Farrer M, Mehta N, Choi P, Hardy J, Wolozin B. α-Synuclein shares physical and functional homology with 14-3-3 proteins. J Neurosci. 1999;19:5782–5791. doi: 10.1523/JNEUROSCI.19-14-05782.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X, Tehranian R, Dietrich P, Stefanis L, Perez RG. Alpha-synuclein activation of protein phosphatase 2A reduces tyrosine hydroxylase phosphorylation in dopaminergic cells. J Cell Sci. 2005;118:3523–3530. doi: 10.1242/jcs.02481. [DOI] [PubMed] [Google Scholar]
- Ramsey AJ, Hillas PJ, Fitzpatrick PF. Characterization of the active site iron in tyrosine hydroxylase. Redox states of the iron. J Biol Chem. 1996;271:24395–24400. doi: 10.1074/jbc.271.40.24395. [DOI] [PubMed] [Google Scholar]
- Ruvolo PP, Clark W, Mumby M, Gao F, May WS. A functional role for the B56 alpha-subunit of protein phosphatase 2A in ceramide-mediated regulation of Bcl2 phosphorylation status and function. J Biol Chem. 2002;277:22847–22852. doi: 10.1074/jbc.M201830200. [DOI] [PubMed] [Google Scholar]
- Salvatore MF, Waymire JC, Haycock JW. Depolarization-stimulated catecholamine biosynthesis: involvement of protein kinases and tyrosine hydroxylase phosphorylation sites in situ. J Neurochem. 2001;79:349–360. doi: 10.1046/j.1471-4159.2001.00593.x. [DOI] [PubMed] [Google Scholar]
- Schonthal AH. Role of PP2A in intracellular signal transduction pathways. Front Biosci. 1998;3:D1262–D1273. doi: 10.2741/A361. [DOI] [PubMed] [Google Scholar]
- Soltoff SP. Rottlerin is a mitochondrial uncoupler that decreases cellular ATP levels and indirectly blocks protein kinase Cdelta tyrosine phosphorylation. J Biol Chem. 2001;276:37986–37992. doi: 10.1074/jbc.M105073200. [DOI] [PubMed] [Google Scholar]
- Sontag E, Nunbhakdi-Craig V, Bloom GS, Mumby MC. A novel pool of protein phosphatase 2A is associated with microtubules and is regulated during the cell cycle. J Cell Biol. 1995;128:1131–1144. doi: 10.1083/jcb.128.6.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava J, Goris J, Dilworth SM, Parker PJ. Dephosphorylation of PKCdelta by protein phosphatase 2Ac and its inhibition by nucleotides. FEBS Lett. 2002;516:265–269. doi: 10.1016/s0014-5793(02)02500-0. [DOI] [PubMed] [Google Scholar]
- Sun F, Anantharam V, Zhang D, Latchoumycandane C, Kanthasamy A, Kanthasamy AG. Proteasome inhibitor MG-132 induces dopaminergic degeneration in cell culture and animal models. Neurotoxicology. 2006;27:807–815. doi: 10.1016/j.neuro.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Tachikawa E, Tank AW, Weiner DH, Mosimann WF, Yanagihara N, Weiner N. Tyrosine hydroxylase is activated and phosphorylated on different sites in rat pheochromocytoma PC12 cells treated with phorbol ester and forskolin. J Neurochem. 1987;48:1366–1376. doi: 10.1111/j.1471-4159.1987.tb05673.x. [DOI] [PubMed] [Google Scholar]
- Vrana KE, Roskoski R., Jr Tyrosine hydroxylase inactivation following cAMP-dependent phosphorylation activation. J Neurochem. 1983;40:1692–1700. doi: 10.1111/j.1471-4159.1983.tb08144.x. [DOI] [PubMed] [Google Scholar]
- Way KJ, Chou E, King GL. Identification of PKC-isoform-specific biological actions using pharmacological approaches. Trends Pharmacol Sci. 2000;21:181–187. doi: 10.1016/s0165-6147(00)01468-1. [DOI] [PubMed] [Google Scholar]
- Waymire JC, Craviso GL, Lichteig K, Johnston JP, Baldwin C, Zigmond RE. Vasoactive intestinal peptide stimulates catecholamine biosynthesis in isolated adrenal chromaffin cells: evidence for a cyclic AMP-dependent phosphorylation and activation of tyrosine hydroxylase. J Neurochem. 1991;57:1313–1324. doi: 10.1111/j.1471-4159.1991.tb08296.x. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM, Schmunk GA, Shannak K, Haycock JW, Kish SJ. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med. 1996;2:699–703. doi: 10.1038/nm0696-699. [DOI] [PubMed] [Google Scholar]
- Wu J, Filer D, Friedhoff AJ, Goldstein M. Site-directed mutagenesis of tyrosine hydroxylase. Role of serine 40 in catalysis. J Biol Chem. 1992;267:25754–25758. [PubMed] [Google Scholar]
- Yang Y, Kaul S, Zhang D, Anantharam V, Kanthasamy AG. Suppression of caspase-3-dependent proteolytic activation of protein kinase C delta by small interfering RNA prevents MPP+-induced dopaminergic degeneration. Mol Cell Neurosci. 2004;25:406–421. doi: 10.1016/j.mcn.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Zhou W, Hurlbert MS, Schaack J, Prasad KN, Freed CR. Overexpression of human alpha-synuclein causes dopamine neuron death in rat primary culture and immortalized mesencephalon-derived cells. Brain Res. 2000;866:33–43. doi: 10.1016/s0006-8993(00)02215-0. [DOI] [PubMed] [Google Scholar]