Abstract
Entheses are sites where tendons, ligaments, joint capsules or fascia attach to bone. Inflammation of the entheses (enthesitis) is a well‐known hallmark of spondyloarthritis (SpA). As entheses are associated with adjacent, functionally related structures, the concepts of an enthesis organ and functional entheses have been proposed. This is important in interpreting imaging findings in entheseal‐related diseases. Conventional radiographs and CT are able to depict the chronic changes associated with enthesitis but are of very limited use in early disease. In contrast, MRI is sensitive for detecting early signs of enthesitis and can evaluate both soft‐tissue changes and intraosseous abnormalities of active enthesitis. It is therefore useful for the early diagnosis of enthesitis‐related arthropathies and monitoring therapy. Current knowledge and typical MRI features of the most commonly involved entheses of the appendicular skeleton in patients with SpA are reviewed. The MRI appearances of inflammatory and degenerative enthesopathy are described. New options for imaging enthesitis, including whole‐body MRI and high‐resolution microscopy MRI, are briefly discussed.
Entheses are sites where tendons, ligaments, joint capsules or fascia attach to bone1 providing a mechanism for reducing stress at the bony interface.2 In general, entheses dissipate biomechanical stress and in doing so are thought to be subjected to repeated micro‐trauma.3 Inflammation of the entheses is called enthesitis, and insertional disorders in general are termed enthesopathies. In addition to the well‐recognised association with the spondyloarthritides (SpA), enthesitis can also be associated with endocrinological, metabolic, traumatic and degenerative conditions.4
Two types of entheses can be distinguished by their structure and location: the fibrous and the fibrocartilaginous type.5 Fibrous entheses are typical of the metaphyses and diaphyses of long bones, but most entheses are fibrocartilaginous, and these are the ones that are affected in SpA2—for example, the sites of tendon insertions into the epiphyses of long bones.6 MRI studies of sites of enthesitis have shown that carriage of the HLA‐B27 gene is associated with the degree of MRI peri‐entheseal bone marrow oedema.7,8 Therefore, MRI studies in man may be directly relevant for understanding disease pathogenesis. The purpose of this article is to focus on the diagnostic role of MRI in the appendicular skeleton.
Despite the widespread understanding of an enthesis as being the junction between the tendon or ligament and the bone, a new concept of an “enthesis organ” has been proposed.2,9 The basis of this concept is the fact that the enthesis is associated with other structures adjacent to it that are functionally related.2 The idea is best illustrated by the Achilles tendon insertion where, in addition to the insertional fibrocartilage, there are further fibrocartilage layers covering the surface of the bone and the anterior surface of the tendon.5 Other components of the Achilles enthesis are the retrocalcaneal bursa, a synovial‐covered fat pad and the adjacent calcaneal bone.5,9,10 All these structures are involved in normal enthesis function and contribute to protecting the enthesis during locomotion and may be involved in enthesis inflammation. The anatomical concept of the enthesis organ is directly related to the pathophysiology of SpA, as enthesitis in SpA is associated with diffuse pathological changes in the connective tissue and underlying bone in the immediate vicinity of the insertion.11 The location at which tendons wrap around bony pulleys shares strong anatomical, biomechanical and histological similarities to entheses, so that another type of enthesis, the “functional enthesis”, has been proposed.2,6 According to this concept, the fibrocartilage layer covering the bone at sites where tendons change direction can thus be considered equivalent to the fibrocartilage in an enthesis organ.2
Knowledge of these anatomical concepts is essential for the interpretation of MRI in SpA. Firstly, inflammation may be quite diffuse and not confined to the insertional region only, so the MRI changes may be diffuse. Secondly, MRI abnormalities may be present at sites that are not true insertions but nevertheless represent the same pathophysiological process.
The need to diagnose SpA early has been increasingly recognised. Conventional radiography as well as CT detect the more chronic changes in enthesitis, are not suited to early disease, and will not be addressed in this review. In contrast, both ultrasound and MRI have been shown to be sensitive for detecting early signs of enthesitis in patients with SpA.8,12,13,14,15,16 However, only the latter can fully evaluate both soft‐tissue changes and intraosseous abnormalities of active enthesitis. As stated, enthesitis or enthesopathy can be associated with several disease processes. Despite a high reported sensitivity of MRI in detecting enthesitis, it lacks specificity.8,13,17 Thus, differentiating between the different aetiologies of enthesitis on the basis of their MRI characteristics is difficult and not always possible. These aspects will be covered in this review.
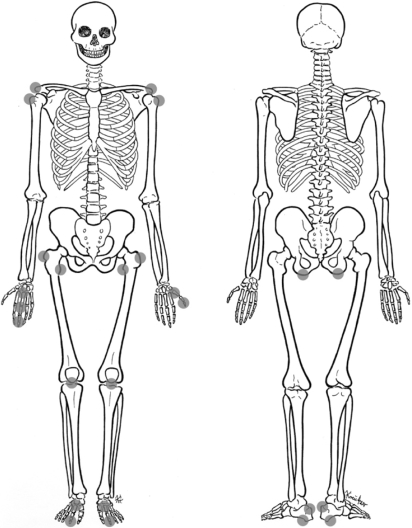
The MRI features of axial skeleton entheses have already been reviewed,18,19 but, to date, no comprehensive review of the MRI appearances of appendicular skeleton entheses has been published. The purpose of this review article is to describe the MRI features of active enthesitis of the most commonly involved entheses of the appendicular skeleton (fig 1) in patients with SpA. The MRI characterising features of SpA‐associated enthesitis in contrast with other common causes of enthesitis will be presented in correlation with the newly proposed concepts of the enthesis organ and functional entheses. Emerging MRI methods for the assessment of enthesitis including whole‐body MRI and high‐resolution MRI will also be discussed. The data presented summarise updated literature and our own personal experience.
Figure 1 Most commonly affected appendicular entheseal sites in spondyloarthritis.
Technical parameters of MRI of the entheses
MRI scans can be performed using a high‐field (⩾1.0 T) or a low‐field (<0.5 T) magnet depending on the size of the organ scanned.
The recommended MRI pulse sequences for enthesitis evaluation based on our personal experiences are:
T1‐weighted spin‐echo sequence (for depiction of bone, fat, muscles, ligaments and tendons)
T2‐weighted turbo spin‐echo sequence with fat suppression or short tau inversion recovery (STIR) sequence (for depiction of oedema, joint effusion, bursitis and tenosynovitis)
T1‐weighted spin‐echo sequence with fat suppression after contrast material administration (for depiction of osteiitis, tendonitis and bursitis). The depiction of entheseal pathology only by STIR sequences may not be sufficient in mild disease with minute changes, where the application of paramagnetic contrast agents is recommended.
The normal tendon on MR images has homogeneous low signal intensity in all sequences. When evaluating MR images for enthesitis one should evaluate:
thickness and signal intensity of tendons and ligaments
peri‐enthesal soft tissues for swelling or oedema
adjacent bone marrow to detect oedema, best appreciated as high signal in fat suppressed sequences
adjacent bone for erosions (cortical bone defects and contour irregularities) and enthesophytes (extensions of marrow contents isointense to the medullary bone), both best appreciated on T1‐weighted sequences
additional findings in adjacent structures (joint or bursal fluid for example).
Dedicated low‐field MRI devices with a field strength around 0.2 T were introduced in the 1990s for imaging peripheral joints. These devices offer better patient comfort at reduced overall cost.20,21 Studies evaluating diagnostic quality of these devices were performed predominantly in patients with rheumatoid arthritis to detect erosions and synovitis,20,22 but some early data on the detection of enthesitis are also available.23 A major drawback of low‐field MRI devices is the missing option of frequency‐selective fat suppression to detect bone marrow oedema—a key feature of enthesitis. Alternatively, inversion recovery sequences or image subtraction of unenhanced and contrast‐enhanced sequences can be used with varying success. The use of Dixon algorithm‐based sequences may provide better options in the future,24 as these sequences produce unequivocal water‐fat signal decomposition even in the presence of field inhomogeneity. At present, the use of low‐field‐strength MRI devices for the detection of enthesitis cannot be recommended as a routine modality because of the lack of controlled studies investigating peripheral manifestations of psoriatic arthritis (PsA), ankylosing spondylitis (AS) and other enthesis‐associated diseases.
Advances in MRI technology
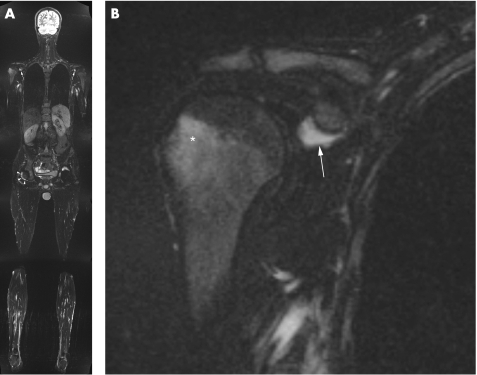
Two MRI techniques, whole‐body MRI and high‐resolution MRI, have recently been introduced and have a promising role in imaging enthesitis. The development of multi‐channel scanners using accelerated imaging techniques (parallel imaging), a whole‐body surface coil system, and a freely moving table with a total coverage of 205 cm in head‐to‐feet direction enables imaging of the entire body in a single head‐to‐toe scan in a relatively short period of time. Whole‐body MRI can be used to detect multiple sites of enthesitis in patients with SpA in one single imaging session25 (fig 2).
Figure 2 A 25‐year‐old man with ankylosing spondylitis. A 1.5 T whole‐body MRI coronal STIR sequence (A) showing bilateral subcoracoid bursitis (arrows) and effusion (arrowheads) in the right hip joint (left hip joint obscured by metal artifacts due to total hip arthroplasty). A zoomed view of the right shoulder (B) shows intense bone marrow oedema in the greater tuberosity (asterisk) and subcoracoid bursitis (arrow).
A second development has been the use of high‐resolution MRI for the assessment of enthesitis. Conventional MRI is limited at certain insertions because of low spatial resolution and low water content of entheses. This is especially the case in small joints such as finger joints. Various groups have used some form of high‐resolution MRI to study finger joints, but these were mainly performed on custom‐built scanners.26,27,28,29 Recent developments in high‐resolution MRI have allowed the use of specialised “microscopy coils”, which are small surface coils that can be used with commercially available clinical MRI scanners.17,30 This technique provides a displayed pixel dimension of 80–100 μm, and therefore can offer a new insight into the small entheses of the hand.
Upper extremities
Shoulder
The humeral tuberosities as well as acromial and clavicular insertions of the deltoid muscle are shoulder entheses that may be involved in SpA. Differentiating between inflammatory enthesitis of the shoulder and degenerative or mechanical enthesitis which are commonly found on MRI is not always possible. In shoulder disease associated with AS, a dominant feature of enthesitis is prominent bone marrow oedema adjacent to the insertion of the supraspinatus (fig 2) and within the acromion at the origin of the deltoid.9,13 Intense entheseal bone marrow oedema, particularly in the acromion and in association with erosions at the greater tuberosity was found to be the most specific sign in AS shoulders.13 Enthesitis of the shoulder is a common finding in certain age groups and can result from multiple aetiologies; the most common one is mechanical, or degenerative, enthesitis. Of note, degenerative enthesopathy may also be associated with significant adjacent bone marrow oedema.31 Thus, the presence of intense entheseal bone marrow oedema in the absence of significant injury may suggest the presence of AS or SpA, but cannot exclude mechanical enthesitis.
Elbow
Enthesitis of the elbow is evident in SpA but is probably much less common than mechanically or degenerative related disease. Scarpa et al32 report on two patients with PsA who had enthesitis of the olecranon and of the proximal radius at the bicipital tendon insertion site. Another report describes enthesitis of the lateral epicondyle which appears several months before the onset of psoriatic disease.33 MRI of these patients revealed oedema of the common extensor tendon. From our personal experience with whole‐body MRI, unilateral or bilateral elbow effusions are not uncommon findings in patients with AS, and the relationship between such findings and enthesitis needs yet to be evaluated.
Hand
The hand consists of many entheses, among them the insertions of the flexor and extensor tendons at the phalanges. SpA commonly involves the hand in the form of finger synovitis and dactylitis.34,35,36 MRI studies demonstrate that enthesitis and extracapsular changes are common adjacent to synovial joints in SpA.37 Because of the smaller size of the finger joints and the involved synovitis, direct involvement of the entheses of the fingers is hard to demonstrate.
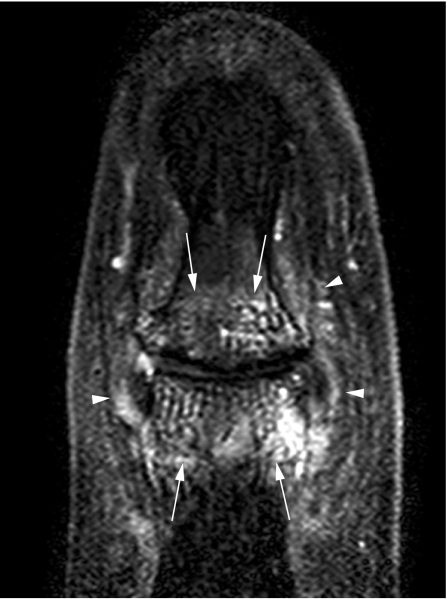
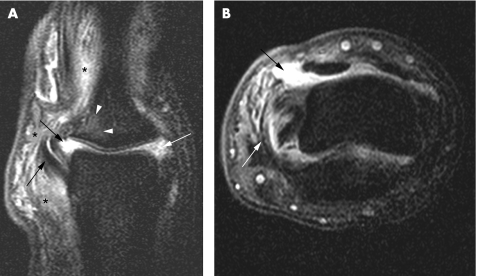
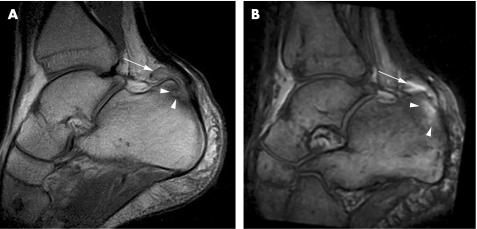
In a study comparing rheumatoid arthritis and PsA, extracapsular and peri‐entheseal changes were noted to be more common in the latter, implying the involvement of entheses in the pathogenesis of psoriatic disease.37 There is a dispute about the involvement of the entheses of the fingers and their role in the inflammation process of the hand of patients with SpA, specifically those with dactylitis. In entheses such as the extensor tendons of the interphalangeal joints, the tendons replace the joint capsule dorsally,38 then an articular hyaline cartilage is present instead of periosteal fibrocartilage, and the joint cavity becomes the equivalent of a bursa.2 As a result, it has been argued that the bone adjacent to the enthesis should be evaluated as well as part of the enthesis organ (fig 3).9,10 It has also been suggested that flexor tendons of the hand should be regarded as functional entheses and that the dactylitis seen in patients with PsA is related to the enthesitis concept.9 To test this hypothesis, the insertions of the flexor and extensor digitorum tendons in 11 fingers with dactylitis of patients with PsA were studied by conventional MRI.36 Soft‐tissue oedema was observed in 46%, but bone marrow oedema was noted in none. Tenosynovitis was noted in all tendons. It was concluded that dactylitis is due to flexor tenosynovitis and not to enthesitis.36 However, bone marrow oedema may be overlooked and not easily detected because of limitations in spatial resolution of conventional 1.5 T MRI of small joints.10 Indeed, in a recent study of the distal interphalangeal joint using a high‐resolution microscopy MRI coil, bone marrow oedema was located near the insertions of the collateral ligaments emanating from the enthesis.30 This was especially common in patients with PsA (figs 4 and 5) in contrast with patients with osteoarthritic disease (supplementary fig 1, which can be found at http://ard.bmjjournals.com/supplemental).30 In this study, ligament and tendon changes were common not only in patients with PsA but also in osteoarthritic patients. However, these structures in the osteoarthritis group showed much less inflammation and enabled good differentiation between the two groups at the population level. Figure 4 and supplementary fig 1 show the similarities between inflammatory and degenerative enthesopathy in the hand.
Figure 3 A 23‐year‐old woman with psoriatic arthritis and dactylitis. A 1.5 T sagittal T1‐weighted fat‐suppressed sequence after contrast injection using a high‐resolution 23 mm diameter microscopy MRI coil shows increased signal of the extensor tendon (arrow) corresponding to a site at which fibrocartilage formation is known to occur within the fused extensor tendon–joint capsule. This is a type of functional enthesis. In addition, distal interphalangeal joint synovitis (arrowheads) is seen, and enhancement around the nail bed is evident (asterisk).
Figure 4 A 40‐year‐old woman with psoriatic arthritis and distal interphalangeal joint disease. A 1.5 T coronal high‐resolution T1‐weighted fat‐suppressed sequence after contrast injection. The diffuse pattern of peri‐articular bone marrow oedema (arrows) as well as peri‐entheseal soft‐tissue oedema (arrowheads) is seen.
Figure 5 A 23‐year‐old woman with a 6‐month history of dactylitis of the thumb. 1.5 T coronal (A) and axial (B) T1‐weighted fat‐suppressed sequences after contrast injection using a high‐resolution microscopy coil placed under the interphalangeal joint. Significant unilateral collateral ligament involvement (white arrow) with associated bone marrow oedema at the enthesis (white arrowheads). In addition, interphalangeal joint synovitis (black arrows) and peri‐entheseal soft‐tissue oedema (black asterisks) are seen.
In preliminary work, we used high‐resolution MRI to assess whether enthesitis is an integral feature of dactylitis. As shown in figs 3 and 5, which were obtained from a patient with dactylitis, enthesitis is indeed present. To what extent small‐joint poly‐enthesitis is a feature of dactylitis has not yet been resolved.
Lower extremities
The entheses of the lower extremities are more often involved in SpA than those of the upper extremities, and heel enthesitis is the most common followed by enthesitis of the patella and the tibial tubercle.39,40
Hip
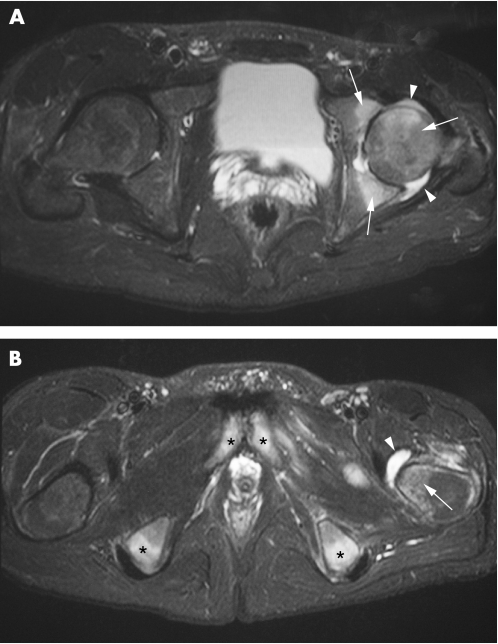
Hip joint involvement in patients with SpA is common, and early involvement is said to be a marker for severe disease.41 Clinical and radiographic studies have suggested that this involvement is, however, more of an arthritis type rather than enthesitis‐based.42 The entheses of the hip include the muscle attachments to the greater and lesser trochanters as well as the origin of the adductor muscles at the pubic and ischial bones. These sites are best appreciated on coronal MR images (supplementary fig 2). We have noted that patients with active SpA may have florid hip osteitis/enthesitis possibly as part of the enthesis organ (fig 6). Hip involvement in SpA may progress more rapidly than the rate of progression of synovial‐based disease such as rheumatoid arthritis. Indeed the patient reported in fig 6 required total hip replacement within 6 months of disease onset. The prognostic value of diffuse MRI osteitis/enthesitis in synovial joint disease has yet to be determined, but is likely to be of immense value in the hip joint.
Figure 6 A 20‐year‐old HLA‐B27‐positive man with raised C‐reactive protein and hip pain for 3 months due to hip enthesitis, initially misinterpreted as infectious disease. Total hip replacement was necessary 6 months after disease onset. A 1.5 T transverse STIR sequence (A) showing extensive bone marrow oedema (white arrows) in the femoral head and acetabulum as well as hip joint effusion (white arrowheads). (B) Same sequence more caudally shows bursitis (black arrowhead) and enthesis‐related bone marrow oedema at the lesser femoral trochanter (black arrow), ischial tubercles (white asterisks) and pubic bones (black asterisks).
Knee
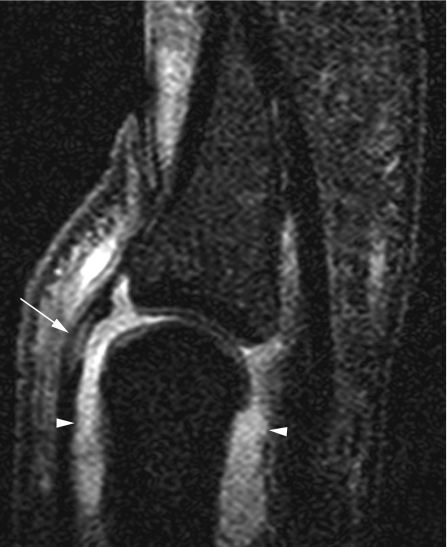
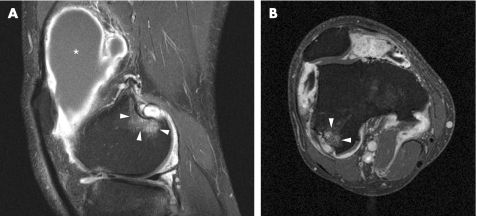
Often involved entheses in SpA are the insertion of the quadriceps tendon at the upper patellar pole and the insertions of the patellar ligament at the lower patellar pole and the tibial tubercle.35,43 To a lesser extent, femoral condyle muscle insertions are also involved (fig 7). In a study comprising 10 patients with early SpA and 10 with early rheumatoid arthritis, peri‐entheseal soft‐tissue oedema was demonstrated in all patients with 10 SpA but in only four of those with arthritis.12 Bone marrow oedema at entheseal sites was seen in six of the patients with SpA, whereas no enthesis‐related bone marrow oedema was detected in the 10 with rheumatoid arthritis. Thus, early differentiation of those patients who would develop AS versus rheumatoid arthritis seems possible. One should note that, in adolescence, enthesitis of the patellar ligament can be confused with traction apophysitis (Osgood–Schlatter disease).44 Further studies are needed to determine whether entheseal‐related bone marrow oedema is prognostically relevant.
Figure 7 A 27‐year‐old man with ankylosing spondylitis. 1.5 T sagittal (A) and axial (B) fat‐suppressed T1‐weighted sequences after contrast injection of the knee. Localised bone marrow oedema at the posterior aspect of the lateral femoral condyle (arrowheads) at the insertion site of the lateral collateral ligament and the origins of the lateral head of the gastrocnemius and the popliteus muscles is depicted. A large suprapatellar effusion along with inflamed and thickened synovial membrane is also present (asterisk).
Hindfoot
SpA often affects the hindfoot, with the calcaneus being the most involved bone.14,45 Both the Achilles tendon and the plantar fascia attach to the calcaneus. Focal erosions at the insertion of the Achilles tendon and enthesopathy in both the Achilles and plantar fascia insertions were observed radiographically in 33–58% of cases.46
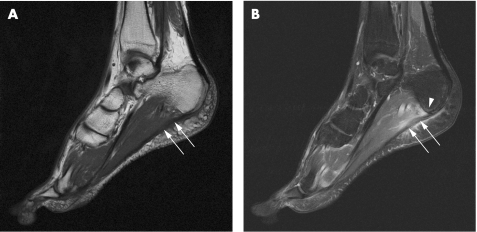
In 40–50% of cases of plantar fasciitis, a plantar calcaneal enthesophyte is associated.14 In a group of 17 patients with SpA and 11 controls, mechanically induced and inflammatory enthesitis had been shown to have similar MRI appearances with soft tissue and bone marrow oedema at the plantar fascia insertion8 (fig 8). However, patients with SpA showed more severe bone marrow oedema, and its degree correlated with the presence of HLA‐B27, suggesting that the effect of the HLA‐B27 gene may be mediated in the bone adjacent to entheseal insertions. From the clinical perspective, however, it was difficult to differentiate between non‐HLA‐B27‐related SpA and pure mechanically induced enthesopathy.
Figure 8 Plantar fasciitis in a 27‐year‐old man with reactive arthritis. A 1.5 T sagittal T1‐weighted sequence of the hindfoot (A) shows a thickened plantar fascia with increased signal intensity (arrows). A fat‐suppressed T1‐weighted sequence after contrast injection (B) shows enhancement of the plantar fascia (arrows), surrounding soft‐tissue oedema, and minimal calcaneal bone marrow oedema (arrowhead).
The thickness of the Achilles tendon in patients with SpA and severe enthesitis is significantly increased.47 Swelling of the tendon is not limited to the insertion, but often extends some centimetres proximally.40 The normal flattened appearance of the tendon is lost and a round configuration as well as an increased signal intensity may be evident.16 Additional findings in Achilles tendon enthesitis are retrocalcaneal bursitis, subcutaneous oedema and calcaneal bone marrow oedema16,35,40,47 (fig 9). This pattern of involvement can be viewed as part of the Achilles enthesis organ.9,10 These additional findings are also described in exercise‐induced tendinopathy and thus cannot always reliably help to differentiate between the two.48 Haglund's syndrome, a mechanically induced inflammation of the retrocalcaneal bursa, supracalcaneal bursa and Achilles tendon, should also be regarded in the differential diagnosis.49
Figure 9 Achilles tendon enthesitis in an 18‐year‐old man with juvenile spondyloarthritis. A 0.2 T sagittal native T1‐weighted spin‐echo sequence (A) and T1‐weighted three‐dimensional gradient‐echo sequence after contrast injection (B) of the hindfoot (two different sections) showing irregular Achilles tendon with increased signal intensity. In addition, retrocalcaneal bursitis (arrow), a calcaneal erosion (black arrowheads) and localised calcaneal bone marrow oedema (white arrowheads) are seen.
Midfoot
The talonavicular, calcaneocuboideal and intertarsal joints all are part of the midfoot.
These midfoot joints, connected by a supporting network of tendons and ligaments, perform only minute gliding movements (arthrodia). The midfoot fibrocartilagenous joints share the same histology and biomechanics as insertions proper.2 Thus, like the sacroiliac joint, the midfoot joints can be regarded as functional entheses. Involvement of these entheses is designated tarsitis and is characterised by bone marrow oedema just as other SpA‐related entheses (supplementary fig 3). Tarsitis occurs especially in juvenile forms of SpA in up to 88% of cases50,51 and correlates with poor prognosis. However, diffuse bone marrow oedema may occur in a similar pattern and is a feature of severe degenerative joint disease, especially neuropathic joint disease.
Toes
Many entheses involve the toes, among which are the insertion of the plantar aponeurosis on the base of the fifth metatarsal bone and on the metatarsal heads.43 As in the fingers, here the involvement of the flexor tendons in dactylitis can also be considered as enthesitis as part of the functional enthesis concept. Toe dactylitis, commonly seen in PsA, is evident in 23% of SpA cases,52 but may be under‐represented.53 A study of 12 sausage‐like toes of patients with SpA using conventional MRI showed that toe dactylitis is predominantly due to flexor tenosynovitis and that extensor tenosynovitis may also be present.54 However, unlike for the hand, the functional enthesis theory could not yet been validated by high‐resolution MRI at this site.
Summary
Enthesitis is a well‐known hallmark of SpA, which recently gained special attention because of its potential role in the disease pathogenesis. MRI is highly sensitive for active enthesitis and depicts not only the enthesis itself but also associated findings such as soft‐tissue involvement and bone marrow oedema. Although bone marrow oedema is a prominent feature of enthesitis, it is not universally seen in all enthesopathies. Extensive and diffuse patterns of bone marrow oedema are more closely related to inflammatory enthesitis, as shown in the hip. When soft‐tissue involvement occurs in a synovial joint, synovitis may mask some, if not all, MRI features of enthesitis. Still, differentiation between the different causes of enthesitis (ie, inflammatory, mechanical, metabolic) is only reliably possible in the context of the available clinical information. Despite these limitations, MRI represents a significant advance for the early diagnosis of enthesitis‐related arthropathies and for monitoring therapy that targets entheseal inflammation.
Three supplementary figures can be found at http://ard.bmj.com/supplemental.
Copyright © 2007 BMJ Publishing Group and European League Against Rheumatism
Supplementary Material
Abbreviations
AS - ankylosing spondylitis
PsA - psoriatic arthritis
SpA - spondyloarthritis
STIR - short tau inversion recovery
Footnotes
Competing interests: None.
Three supplementary figures can be found at http://ard.bmj.com/supplemental.
References
- 1.Francois R J, Eulderink F, Bywaters E G. Commented glossary for rheumatic spinal diseases, based on pathology. Ann Rheum Dis 199554615–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin M, McGonagle D. The anatomical basis for disease localisation in seronegative spondyloarthropathy at entheses and related sites. J Anat 2001199503–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benjamin M, Toumi H, Suzuki D, Redman S, Emery P, McGonagle D. Microdamage and altered vascularity at the enthesis‐bone interface provides an anatomic explanation for bone involvement in the HLA‐B27‐associated spondylarthritides and allied disorders. Arthritis Rheum 200756224–233. [DOI] [PubMed] [Google Scholar]
- 4.Resnick D, Niwayama G. Entheses and enthesopathy. Anatomical, pathological, and radiological correlation. Radiology 19831461–9. [DOI] [PubMed] [Google Scholar]
- 5.Rufai A, Ralphs J R, Benjamin M. Structure and histopathology of the insertional region of the human Achilles tendon. J Orthop Res 199513585–593. [DOI] [PubMed] [Google Scholar]
- 6.Benjamin M, Ralphs J R. Fibrocartilage in tendons and ligaments: an adaptation to compressive load. J Anat 1998193(Pt 4)481–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braun J, Khan M A, Sieper J. Enthesitis and ankylosis in spondyloarthropathy: what is the target of the immune response? Ann Rheum Dis 200059985–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGonagle D, Marzo‐Ortega H, O'Connor P, Gibbon W, Pease C, Reece R.et al The role of biomechanical factors and HLA‐B27 in magnetic resonance imaging‐determined bone changes in plantar fascia enthesopathy. Arthritis Rheum 200246489–493. [DOI] [PubMed] [Google Scholar]
- 9.McGonagle D, Marzo‐Ortega H, Benjamin M, Emery P. Report on the Second International Enthesitis Workshop. Arthritis Rheum 200348896–905. [DOI] [PubMed] [Google Scholar]
- 10.McGonagle D. Imaging the joint and enthesis: insights into pathogenesis of psoriatic arthritis. Ann Rheum Dis 200564(Suppl 2)ii58–ii60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGonagle D, Khan M A, Marzo‐Ortega H, O'Connor P, Gibbon W, Emery P. Enthesitis in spondyloarthropathy. Curr Opin Rheumatol 199911244–250. [DOI] [PubMed] [Google Scholar]
- 12.McGonagle D, Gibbon W, O'Connor P, Green M, Pease C, Emery P. Characteristic magnetic resonance imaging entheseal changes of knee synovitis in spondylarthropathy. Arthritis Rheum 199841694–700. [DOI] [PubMed] [Google Scholar]
- 13.Lambert R G, Dhillon S S, Jhangri G S, Sacks J, Sacks H, Wong B.et al High prevalence of symptomatic enthesopathy of the shoulder in ankylosing spondylitis: deltoid origin involvement constitutes a hallmark of disease. Arthritis Rheum 200451681–690. [DOI] [PubMed] [Google Scholar]
- 14.Erdem C Z, Sarikaya S, Erdem L O, Ozdolap S, Gundogdu S. MR imaging features of foot involvement in ankylosing spondylitis. Eur J Radiol 200553110–119. [DOI] [PubMed] [Google Scholar]
- 15.Kamel M, Eid H, Mansour R. Ultrasound detection of knee patellar enthesitis: a comparison with magnetic resonance imaging. Ann Rheum Dis 200463213–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamel M, Eid H, Mansour R. Ultrasound detection of heel enthesitis: a comparison with magnetic resonance imaging. J Rheumatol 200330774–778. [PubMed] [Google Scholar]
- 17.Tan A L, Grainger A J, Tanner S F, Shelley D M, Pease C, Emery P.et al High‐resolution magnetic resonance imaging for the assessment of hand osteoarthritis. Arthritis Rheum 2005522355–2365. [DOI] [PubMed] [Google Scholar]
- 18.Hermann K G, Althoff C E, Schneider U, Zühlsdorf S, Lembcke A E, Hamm B.et al Magnetic resonance imaging of spinal changes in patients with spondyloarthritis and correlation with conventional radiography. RadioGraphics 200525559–570. [DOI] [PubMed] [Google Scholar]
- 19.Hermann K G, Bollow M. Magnetic resonance imaging of the axial skeleton in rheumatoid disease. Best Pract Res Clin Rheumatol 200418881–907. [DOI] [PubMed] [Google Scholar]
- 20.Savnik A, Malmskov H, Thomsen H S, Bretlau T, Graff L B, Nielsen H.et al MRI of the arthritic small joints: comparison of extremity MRI (0.2 T) vs high‐field MRI (1.5 T). Eur Radiol 2001111030–1038. [DOI] [PubMed] [Google Scholar]
- 21.Peterfy C G. Is there a role for extremity magnetic resonance imaging in routine clinical management of rheumatoid arthritis? J Rheumatol 200431640–644. [PubMed] [Google Scholar]
- 22.Ejbjerg B J, Narvestad E, Jacobsen S, Thomsen H S, Ostergaard M. Optimised, low cost, low field dedicated extremity MRI is highly specific and sensitive for synovitis and bone erosions in rheumatoid arthritis wrist and finger joints: comparison with conventional high field MRI and radiography. Ann Rheum Dis 2005641280–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eshed I, Althoff C E, Feist E, Minden K, Schink T, Hamm B.et al MRI evaluation of the hindfoot in patients with spondyloarthritides: comparison of low‐field and high‐field strength units [abstract]. Skeletal Radiol 200635418–483. [DOI] [PubMed] [Google Scholar]
- 24.Wohlgemuth W A, Roemer F W, Bohndorf K. Short tau inversion recovery and three‐point Dixon water‐fat separation sequences in acute traumatic bone fractures at open 0.35 tesla MRI. Skeletal Radiol 200231343–348. [DOI] [PubMed] [Google Scholar]
- 25.Hermann K G A, Sieper J, Rudwaleit M, Eshed I, Hamm B, Althoff C E. Axial and peripheral manifestation in spondyloarthritis: a whole body MRI approach [EULAR abstract]. Ann Rheum Dis 200665692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis A R, Nolan M J, Hodgson R J, Benjamin M, Ralphs J R, Archer C W.et al High resolution magnetic resonance imaging of the proximal interphalangeal joints. Correlation with histology and production of a three‐dimensional data set. J Hand Surg [Br] 199621488–495. [DOI] [PubMed] [Google Scholar]
- 27.Wong E C, Jesmanowicz A, Hyde J S. High‐resolution, short echo time MR imaging of the fingers and wrist with a local gradient coil. Radiology 1991181393–397. [DOI] [PubMed] [Google Scholar]
- 28.Fry M E, Jacoby R K, Hutton C W, Ellis R E, Phil M, Pittard S.et al High‐resolution magnetic resonance imaging of the interphalangeal joints of the hand. Skeletal Radiol 199120273–277. [DOI] [PubMed] [Google Scholar]
- 29.Gasson J, Gandy S J, Hutton C W, Jacoby R K, Summers I R, Vennart W. Magnetic resonance imaging of rheumatoid arthritis in metacarpophalangeal joints. Skeletal Radiol 200029324–334. [DOI] [PubMed] [Google Scholar]
- 30.Tan A L, Grainger A J, Tanner S F, Emery P, McGonagle D. A high‐resolution magnetic resonance imaging study of distal interphalangeal joint arthropathy in psoriatic arthritis and osteoarthritis: are they the same? Arthritis Rheum 2006541328–1333. [DOI] [PubMed] [Google Scholar]
- 31.McCauley T R, Disler D G, Tam M K. Bone marrow edema in the greater tuberosity of the humerus at MR imaging: association with rotator cuff tears and traumatic injury. Magn Reson Imaging 200018979–984. [DOI] [PubMed] [Google Scholar]
- 32.Scarpa R, Ames P R, della Valle G, Lubrano E, Oriente P. A rare enthesopathy in psoriatic oligoarthritis. Acta Derm Venereol Suppl (Stockh) 199418674–75. [PubMed] [Google Scholar]
- 33.Taylor P W, Stoecker W. Enthesitis of the elbow in psoriatic arthritis. J Rheumatol 1997242268–2269. [PubMed] [Google Scholar]
- 34.McQueen F, Lassere M, Ostergaard M. Magnetic resonance imaging in psoriatic arthritis: a review of the literature. Arthritis Res Ther 20068207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barozzi L, Olivieri I, De Matteis M, Padula A, Pavlica P. Seronegative spondylarthropathies: imaging of spondylitis, enthesitis and dactylitis. Eur J Radiol. 1998;27(Suppl 1)S12–S17. [DOI] [PubMed]
- 36.Olivieri I, Salvarani C, Cantini F, Scarano E, Padula A, Niccoli L.et al Fast spin echo‐T2‐weighted sequences with fat saturation in dactylitis of spondylarthritis. No evidence of entheseal involvement of the flexor digitorum tendons. Arthritis Rheum 2002462964–2967. [DOI] [PubMed] [Google Scholar]
- 37.Jevtic V, Watt I, Rozman B, Kos‐Golja M, Demsar F, Jarh O. Distinctive radiological features of small hand joints in rheumatoid arthritis and seronegative spondyloarthritis demonstrated by contrast‐enhanced (Gd‐DTPA) magnetic resonance imaging. Skeletal Radiol 199524351–355. [DOI] [PubMed] [Google Scholar]
- 38.Lewis A R, Ralphs J R, Kneafsey B, Benjamin M. Distribution of collagens and glycosaminoglycans in the joint capsule of the proximal interphalangeal joint of the human finger. Anat Rec 1998250281–291. [DOI] [PubMed] [Google Scholar]
- 39.Olivieri I, Padula A, Pierro A, Favaro L, Oranges G S, Ferri S. Late onset undifferentiated seronegative spondyloarthropathy. J Rheumatol 199522899–903. [PubMed] [Google Scholar]
- 40.Olivieri I, Barozzi L, Padula A, De Matteis M, Pierro A, Cantini F.et al Retrocalcaneal bursitis in spondyloarthropathy: assessment by ultrasonography and magnetic resonance imaging. J Rheumatol 1998251352–1357. [PubMed] [Google Scholar]
- 41.Amor B, Santos R S, Nahal R, Listrat V, Dougados M. Predictive factors for the longterm outcome of spondyloarthropathies. J Rheumatol 1994211883–1887. [PubMed] [Google Scholar]
- 42.Michet C J, Mason T G, Mazlumzadeh M. Hip joint disease in psoriatic arthritis: risk factors and natural history. Ann Rheum Dis 2005641068–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olivieri I, Barozzi L, Padula A. Enthesiopathy: clinical manifestations, imaging and treatment. Baillieres Clin Rheumatol 199812665–681. [DOI] [PubMed] [Google Scholar]
- 44.Olivieri I, Padula A, Giasi V, Scarano E. Enthesitis of spondylarthritis can masquerade as Osgood‐Schlatter disease by radiographic findings. Arthritis Rheum 200349147–148. [DOI] [PubMed] [Google Scholar]
- 45.Resnick D. Patterns of peripheral joint disease in ankylosing spondylitis. Radiology 1974110523–532. [DOI] [PubMed] [Google Scholar]
- 46.Resnick D, Feingold M L, Curd J, Niwayama G, Goergen T G. Calcaneal abnormalities in articular disorders. Rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, and Reiter syndrome. Radiology 1977125355–366. [DOI] [PubMed] [Google Scholar]
- 47.Olivieri I, Gemignani G, Bini C, Grassi L, Pasero G. Diffuse Achilles tendon thickening in juvenile onset seronegative HLA‐B27 positive spondyloarthropathy. J Rheumatol 198815381–382. [PubMed] [Google Scholar]
- 48.Haims A H, Schweitzer M E, Patel R S, Hecht P, Wapner K L. MR imaging of the Achilles tendon: overlap of findings in symptomatic and asymptomatic individuals. Skeletal Radiol 200029640–645. [DOI] [PubMed] [Google Scholar]
- 49.Tuite M J. MR imaging of the tendons of the foot and ankle. Semin Musculoskelet Radiol 20026119–131. [DOI] [PubMed] [Google Scholar]
- 50.Burgos‐Vargas R, Pacheco‐Tena C, Vazquez‐Mellado J. A short‐term follow‐up of enthesitis and arthritis in the active phase of juvenile onset spondyloarthropathies. Clin Exp Rheumatol 200220727–731. [PubMed] [Google Scholar]
- 51.Bollow M, Hermann K G A, Biedermann T, Sieper J, Schöntube M, Braun J. Very early spondyloarthritis: where the inflammation in the sacroiliac joints starts. Ann Rheum Dis 2005641644–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eulry F, Diamano J, Launay D, Tabache F, Lechevalier D, Magnin J. Sausage‐like toe and heel pain: value for diagnosing and evaluating the severity of spondyloarthropathies defined by Amor's criteria. A retrospective study in 161 patients. Joint Bone Spine 200269574–579. [DOI] [PubMed] [Google Scholar]
- 53.Bezza A, Niamane R, Amine B, El Maghraoui A, Bensabbah R, Hajjaj‐Hassouni N. Involvement of the foot in patients with psoriatic arthritis. A review of 26 cases. Joint Bone Spine 200471546–549. [DOI] [PubMed] [Google Scholar]
- 54.Olivieri I, Barozzi L, Pierro A, De Matteis M, Padula A, Pavlica P. Toe dactylitis in patients with spondyloarthropathy: assessment by magnetic resonance imaging. J Rheumatol 199724926–930. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.