Abstract
The present study is undertaken to investigate the immune response that was induced by the recombinant spike (S) protein from swine-transmissible gastroenteritis virus (TGEV) expressed in mouse mammary cells. A mammary-specific expression vector pEBS containing the full-length cDNA of S gene was constructed and expressed in the mouse mammary cells (EMT6). The recombinant S protein from culture supernatant of transgenic EMT6 was harvested and immunized BALB/c mice. The results demonstrated recombinant S protein was expressed at high levels in mammary cells by Western blotting and enzyme-linked immunosorbent assay (ELISA) detection. The antibody titer in BALB/c mice following immunization with recombinant S protein was detectable after the first immunization. Maximum titers of antibody (8.86 ± 0.19 ng/ml of serum) were attained after the second immunization. In conclusion, the recombinant S protein expressed in mammary cells was able to elicit substantial immunological response against TGEV. This lays the basis for using mammary gland bioreactor generating edible vaccine.
Keywords: TGEV, Spike protein, Immune response, Mammary cells
1. Introduction
Transmissible gastroenteritis coronavirus (TGEV) infects enteric and respiratory tissues of newborn pigs and causes mortality close to 100% (Cavanagh, 1997, Sola et al., 1998). The rapid spread of the transmissible gastroenteritis (TGE) can be prevented only by vaccination. The commercially available vaccines, either inactivated or attenuated, are unable to produce fully protect the piglets (Tuboly et al., 2000). With the development of bioengineering, some attempts have been made to explore novel vaccines against TGEV. As the major inducer of TGEV-neutralizing antibodies, spike (S) protein has been used as the recombinant subunit vaccine candidate antigen. Gomez et al. (1998) and Tuboly et al. (2000) reported S protein could be expressed in plants. However, expression in eukaryotic hosts is required for antigenic determinants that are dependent on glycosylation. Plant cells present differences in protein glycosylation with respect to animal cells that could determine the lose of antigenic determinants in antigens expressed in transgenic plants (Gomez et al., 1998). Glycosylation in plants may differ in the extent of glycosylation, processing, or both of N-linked oligosaccharide side chains (Faye et al., 1993). Furthermore, the complex glycans of plants are often smaller than those of animals, in part due to the absence of sialic acid (Faye et al., 1993). The post-translational processing of protein in mammalian expression system significantly differ from others expression systems, which inevitably causes the difference in the biological activity of the vial antigen.
Mammary gland bioreactor which can provide protection against enteric virus infection for animal immunized by secreting virus antigen protein into their milk is a promising expression system to produce mammalian viral vaccines in an economic and scalable manner. Soler et al. (2005) utilized transgenic rabbit milk as a source of rotavirus antigens. Recombinant secreted rotavirus proteins in milk retained their immunogenicity and their capacity to elicit significant protection against rotavirus infection. As an advantage in relation to other expression systems, it allows post-translational modification of protein. The post-translational modifications with the highest fidelity are essential to produce an efficacious antigen vaccine (Dertzbaugh, 1998).
However, generating transgenic animal is expensive, difficult and time-consuming. The expression analysis of exogenous protein in mammary cell lines provides a rapid and reliable indicator of the expression efficiency of transgenic animal (Donofrio et al., 1996). Hence, the aim of this study was to investigate the immunogenicity of the S protein from TGEV expressed in mouse mammary cells (EMT6).
2. Materials and methods
2.1. Viruses and cells
A TGEV PUR-115 strain (Purchased from China Institute of Veterinary Drug Control) was propagated on the swine testicular (ST) cells (China Institute of Veterinary Drug Control). ST cells were cultured in DMEM (GIBCO BRL) supplemented with 10% fetal bovine serum (FBS).
2.2. Construction of recombinant plasmid pEBS
The viral total RNA was extracted from TGEV strain according to SV 96 Total RNA Isolation System kit instruction (Promega). The RT-PCR was performed using SuperScript RT-PCR System (Invitrogen). The RT primer was 5′-AGTTCGTCAAGTACAGCATCTACGGATGTG-3′. The 4350-bp cDNA fragment of S gene was obtained using a pair of primers (forward, 5′-AGGGTAAGTTGCATTAGAATCATAATGGTA-3′; reverse, 5′-GACCTGTAATGACTCGTAAGTT TAGTTCT-3′), which were designed based on the sequence of TGEV PUR-MAD strain (Almazan et al., 2000). The PCR product was subjected to sequence analysis, and homology was compared with the data in the GenBank. The extend of homology was confirmed to be above 98.5%.
The cDNA fragment of S gene was cloned into pGEM-T (Promega), and 2.8-kb of 5′ regulator sequence of bovine β-casein and 0.6 kb of 3′ flanking sequence of β-casein gene containing ploy (A) additionl signal were added to the up- and downstream of the cDNA, respectively. The expression cassette was excised with SacI and MluI and inserted into expression vector pEGFP-C1 (Clontech) to construct mammary expression vector pEBS. To evaluate transient expression level, the green fluorescence protein (GFP) driven by human cytomegalovirus (CMV) promoter was as a reporter gene for transient expression.
2.3. Transfection and screening of positive clones
Mouse mammary cells EMT6 (Palom et al., 2001) (Purchased from the Fourth Military Medical University of China) were cultured in DMEM supplemented 10% FBS at 37 °C in 5% CO2. 8 × 104 cells were seeded into each well of 24-well plate 1 day prior to transfection. For each well, 0.8 μg pEBS in test group or 0.8 μg pEGFP-C1 in the control group was transfectted using Lipofectamine 2000 reagent (Invitrogen) according to standard protocols. One day after transfection, the cells were examined for the level of transient expression using a fluorescence microscope, trypsinized and seeded onto 6-well tissue culture plate in medium containing 800 μg/ml G418(GIBCO-BRL) to screen positive clones. After 7 days of selection, the clones were isolated and grown separately in the presence of G418 (400 μg/ml) for 14 days. The untransfected cells were used as negative control group.
2.4. Identification of positive cells
2.4.1. PCR analysis
The positive clones were detected by PCR analysis. DNA was extracted from cell clones using Genomic DNA extract kit (TIANGEN). A 512-bp fragment was specifically amplified by PCR. The upper primer, 5′-TGGTTAGGAAATAGATTCTT-3′, anneals to the casein 5′ regulatory sequence. The lower primer, 5′-CTTACGAGTCATTACAGGTC-3′, anneals to the S gene at downstream of the signal peptide.
2.4.2. RT-PCR analysis
Glycoprotein S transcriptional level in cells was analyzed by RT-PCR. Total RNA was isolated from positive cell clones. A 477-bp fragment of the S gene was amplified by RT-PCR using the primers (forward, 5′-TTCGCAATGATAGCAACG-3′; reverse, 5′-ACCACCAAAGGTCTACAAGC-3′). Simultaneously, to rule out the possibility of amplification of contaminant DNA, one group of RNA was treated with 10 units of DNase-free RNase (Promega) for 15 min at 37 °C. The other manipulation was performed as non-treatment group.
2.5. Induced expression recombinant glycoprotein S
The positive clones selected for 3 weeks were incubated at 37 °C in 5% CO2 until 80% confluence. The medium containing 10% FBS was replaced with serum-free DMEM supplemented hydrocortisone (10 μg/ml; Sigma); prolactin (1 μg/ml; Pierce) and insulin (10 μg/ml; Sigma). 48 h later, the culture supernatant was harvested, then proteins were condensed by ultrafilter devices (Millipore) for glycoprotein S expression analysis.
2.6. Detection of recombinant glycoprotein S
2.6.1. ELISA analysis
The concentrated protein from culture supernatant was measured as antigen. ELISA plates were coated with 100 μl specific anti-S protein monoclonal antibody (Abcam, UK) diluted in PBS at 1:10,000, and incubated for 12 h at 4 °C, and then plates were washed and blocked 1 h at 37 °C with 5% fetal bovine serum in PBS containing 0.05% Tween 20. After washing the plates, proteins concentrated from culture supernatant were added to react with the previously adsorbed antibodies in plates during 12 h at 4 °C. Plates were then washed six times with 0.05% Tween 20 in PBS, rabbit anti-S protein antibody obtained after three immunization doses with E. coli expressed S glycoprotein was added and reacted for 1 h at 37 °C. Bound antibody was detected using horseradish peroxidase-conjugated goat anti-rabbit IgG (Sino-American Biotechnology Co.), followed by colour development using O-phenylenediamine dihydrochloride (Sigma) as substrate. Absorbance was measured at 492 nm. Samples considered positive for the S protein gave optical density (OD) at least twice the mean OD obtained with the negative sample.
2.6.2. Western blot analysis
Twenty microliters of the proteins from cell culture supernatant were boiled in sample loading buffer and subjected to SDS-PAGE in 5% polyacrylamide gel. Proteins were transferred electrophoretically onto PVDF membrane (Millipore) where the immunoblots were developed using mouse monoclonal (Abcam, UK) to TGEV at a dilution of 1:1000. Secondary antibody detection was performed and followed by visualization using the BM-Chemiluminescence blotting substrate (Roche).
2.7. Immunization of mice
Eight-week-old female BALB/c mice were obtained from the laboratory animal center of Fourth Military Medical University (Xi’an China), and divided into three groups (15 mice, each). All animal experiments conformed to the Guide for the Care and Use of Experimental Animals. Three groups of mice were immunized orally with expression product of pEBS and pEGFP-C1 or PBS. The proteins from culture supernatant were condensed and dissolved in phosphate buffer salines (PBS). Oral doses of 0.2 ml protein solution or PBS were administered on three consecutive days at days 0, 1 and 2. A booster immunization was given at days 13, 14 and 15, and a second booster immunization was given at days 27, 28 and 29. Sera were collected via tail bleeding. The pre-immunized blood samples were background control.
2.8. Antibody assays
2.8.1. ELISA assay
Mice sera were separated and tested in a TGEV specific ELISA. TGEV infected ST cells monolayer was purified as the antigen in the ELISA. Microtitre plates were coated overnight at 4 °C with purified TGEV. Serum samples were used as primary antibodies. Bound antibodies were detected using horseradish peroxidase conjugated goat anti-mouse IgG (Sino-American Biotechnology Co.). Absorbance was measured at 492 nm. Results were expressed as titers that were determined by expression of the test samples to a standard curve generated by serial dilution of commercially purchased IgG (VMRD, UK) of known titer.
2.8.2. Plaque reduction assay
To detect the neutralization ability of the induced antibodies, immunization serum samples were further measured by plaque reduction assay as described previously (Ho et al., 2005). Differences in the number of plaques formed between treatments were examined for the level of significance by Student's t-test after analysis of variance.
3. Results
3.1. Expression and detection of recombinant glycoprotein S in mammary cells
The recombinant plasmid (Fig. 1 ), carrying a cDNA coding for the full-length glycoprotein S, was obtained by subcloning the corresponding sequence as described in materials and methods.
Fig. 1.

Schematic representation of recombinant plasmid pEBS. CMV Pro: CMV promoter; EGFP: enhance green fluorescence protein reporter gene; CSN2 Pro: bovine β-casein gene promoter; S gene: spike protein gene; PA: bovine β-casein gene 3′ polyadenylation signal.
A strong fluorescent signal was observed at days 1–2 after transfection with pEBS (Fig. 2A) and pEGFP/C1 (Fig. 2C). In negative control, fluorescent signal was not observed (Fig. 2B).
Fig. 2.
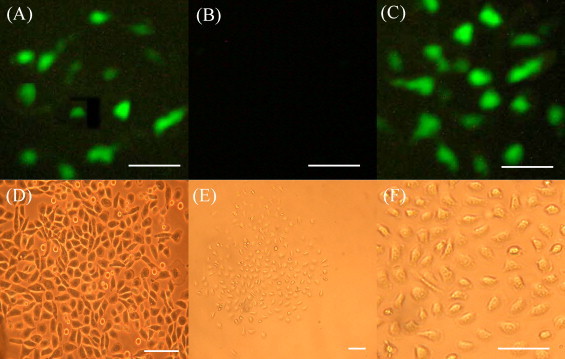
Flueofluence microscope image of positive cell clone (A, B, C and F = 60 μm; D, bar = 40 μm; E, bar = 20 μm). (A) Transfection group of pEBS. (B) Untransfection group. (C) Transfection group of pEGFP/C1. (D) Untransfection EMT-6 cells. (E and F) Positive clone of the transfection group.
The positive cell clones were obtained with selection of G418 (Fig. 2E and F). The presence of the foreign cDNA sequences in cells was screened by PCR analysis (Fig. 3A). RT-PCR analysis showed specific transcription of foreign genes (Fig. 3B).
Fig. 3.
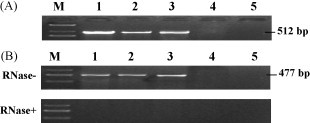
Identification of positive clones. (A) PCR analysis of positive clones. (B) S gene transcription in positive clones analyzed by RT-PCR. Samples were treated with RNase (lower) or not (upper). Lanes 1–3, transfection group of pEBS; lane 4, transfection group of pEGFP/C1; lane 5, untransfection group.
The protein from the culture supernatant of transgenic cell was positive on ELISA (Fig. 4 ). From a titration ELISA using different virus dilutions and specific anti-glycoprotein S antibody, we found that: 1 ml culture supernatant contains a glycoprotein S antigenic mass equivalent to that contained in 2 μg of purified TGEV. A 175 kDa band on membranes blotted indicated that the size of glycoprotein S was as expected (Fig. 5 ).
Fig. 4.

ELISA analysis of recombinant S protein from culture supernatants. The figure shows the mean ± S.E. of the absorbance readings. 1–3, test group. Transfection group of pEGFP/C1 as negative control, purified TGEV virus as positive control. Test groups have higher absorbance than negative control (P < 0.01) and can be up to the level of TGEV. ΛA = absorbance 492 nm.
Fig. 5.

Western blot analysis of recombinant S protein. 1: Purified TGEV virus; 2: untransfection group; 3: transfection group of pEGFP/C1; 4 and 5: transfection group of pEBS; M: protein molecular marker.
3.2. Antibody assays
3.2.1. ELISA assay
The sera concentration of recombinant S protein specific antibody was determined by ELISA. As shown in Fig. 6 , there was no substantial difference in antibody levels between test group and control group prior to oral immunization. All animals that were orally fed with recombinant S protein expressing sera-converted after the second dose (Fig. 6). Elicitation of TGEV specific anti-serum was found to be substantial. A titer of 7.28 ± 0.15 ng/ml of serum of recombinant S protein specific antibody had been attained after the first boost which continued to increase to a level of 8.86 ± 019 ng/ml after the second boost. No significant induction of anti-S protein antibodies was observed in the control groups of mice that received PBS or the empty vector (Fig. 6).
Fig. 6.
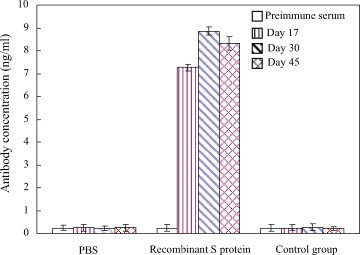
Anti-TGEV S protein antibody titers induced after immunization with recombinant S protein. Sera from three groups of mice immunized orally with recombinant S protein or control group (pEGFP/C1) or PBS were tested by ELISA. Bars represent the mean ELISA titer ± S.E.M. in each group.
3.2.2. Plaque reduction assay
Plaque reduction assays were performed to further determine whether the antibody responses were specific against TGEV glycoprotein S. Results demonstrated that the presence of anti-S protein serum in the culture medium conferred statistically significant neutralizing effects (p < 0.05) on TGEV infection (Fig. 7 ). A nearly 16 ± 0.8% reduction in the number of plaques was consistently observed when plaque reduction assays were carried out using two- to eight-fold diluted sera from recombinant S protein fed mice. The inhibitory effect decreased gradually on further dilutions and reached a level similar to that of the buffer control or the control group at dilutions 1:128 of sera.
Fig. 7.

Inhibition of viral plaque formation by sera from mice fed with recombinant S protein. Maximum reduction in number of plaque, expressed as a percentage of plaques obtained for the negative control samples, using sera collected from mice fed with recombinant S protein was 16 ± 0.8%. Results are mean values and standard errors of triplicates.
4. Discussion
The time and expense involved in generating transgenic animal and then evaluating the transgenic expression pattern is very restrictive. If questions about the ability and efficiency of expression could be asked solely in vitro, rapid progress could be achieved (Whitelaw et al., 1999). Although mammary cells in vitro cannot fully mock the expression milieu in vivo, it is as an efficient model for mammary gland bioreactor of transgenic animal. To investigate the feasibility of mammary gland bioreactor producing efficacious mammalian viral vaccine, in this study, the expression of the S protein from TGEV was tested in mouse mammary cells in vitro.
It is crucial for making mammary gland bioreactor to choose efficient regulator elements. Bovine casein promoter has been widely used for mammary gland-specific expression of foreign genes in transgenic animal. 1.7 kb promoter sequence of the bovine β-casein gene was sufficient for induction of the exogenous gene expression in an in vitro cell culture system (Cerdan et al., 1998, Naruse et al., 2006). The region between −511 and +487 of the β-casein gene promoter has been shown to confer tissue-specific expression in transgenic mice (Rosen, J.M., personal communication). Furthermore, most of the transcription factor response sites or binding elements on the β-casein gene promoter locate at least within the 2.3 kb 5′ of the RNA initiation site (Doppler et al., 1989). In view of these reason, we designed a mammary gland-specific expression vector with 2.8 kb β-casein promoter. In this study, a 175 kDa band corresponding to the expected size of S protein was detected in the culture supernatant of transgenic cells by Western blotting analysis. Our results demonstrated the 2.8 kb 5′ flanking sequence of bovine β-casein could direct specific expression of S gene in mouse mammary cells in vitro.
The hormonal milieu influenced the expression of exogenous S genes in an in vitro mammary cell culture system. Doppler et al. (1989) have shown that it is now possible to use gene transfer methods to study the lactogenic hormone control of milk protein gene expression. A strong induction of chloramphenicol acetyltransferase (CAT) expression by lactogenic hormones was observed in mouse mammary cells (Doppler et al., 1989). This study shows that the combination of hydrocortisone, prolactin and insulin is necessary for induction expression. The maximal induction of S protein was obtained with 10 μg/ml hydrocortisone, 1 μg/ml prolactin and 10 μg/ml insulin at 48 h after induction.
In order to confirm whether the recombinant S protein can induce neutral antibody against TGEV, we orally immunized mice and tested sera by ELISA and plaque reduction assay. Mice immunized with the recombinant S protein sera-converted. We obtained antibody levels similar to the result reported by Ho et al. (2005). Maximum titer of anti-recombinant S protein serum attained 8.86 ± 0.19 ng/ml. Inhibition of viral plaque formation by serum from immunization mice is 14–16%. These results show that S protein is immunogenic. We speculate this is related to the expression system. While a variety of expression vectors have been developed for the efficient synthesis of S protein in other expression systems, in many cases the biological activity of these proteins is impaired because of the failure to process the protein correctly. The mammalian expression system contains the necessary post-translational modification systems requiring for the clevage, phosphorylation and glycosylation of proteins, which should make it possible to efficiently synthesize protein with the high degree of fidelity and secrete biologically important molecules. Therefore, it may be a good choice to express mammalian viral antigen vaccine in mammalian expression systems. Sola et al. (1998) demonstrated that the mouse mammary gland tissue performs the adequate posttranslational processing required for the correct assembly of antibody molecules. The secretion of neutralizing MAbs in the milk of transgenic animals could be applied to improve disease resistance in livestock and to prevent neonatal infections by a number of pathogens for which specific MAbs are available (Sola et al., 1998, Kolb et al., 2001). This approach may lead to the generation of transgenic animals providing lactogenic immunity to their progeny against pathogens.
On the other hand, mice vaccinated with S protein produced the robust immune response against TGEV, which depends on the effective immunization procedure in certain extend. We adopted from the schedule of Ho et al. (2005), which consists of three sets of the successive daily dose of the antigen vaccine. Challacombe et al. found that this pattern of immunization was consistently effective when mice were immunized with oral vaccine.
Certainly, producing vaccine by mammalian cell-based expression system is high-cost and difficultly scalable, so our final goal is to produce anti-virus vaccine in mammary gland bioreactor. Transgenic rabbit expressing S glycoprotein is currently being made by using the same expression cassette. This new system will allow us to test whether the immunity provided by S protein expression in the milk of transgenic rabbit to mice following challenge with TGEV elicit protection. If we determine the feasibility of expressing immunologically active polypeptides in mice, this strategy will provide a new method to protect animal against viral infections of the enteric tract.
5. Conclusion
This study indicated that mammary cells in vitro expressing the recombinant S protein from TGEV elicited a robust immune response against TGEV. This finding lays the first basis for producing TGEV edible vaccine with the mammary gland bioreactor.
Acknowledgements
We thank Drs. Jian-Hong SUN and Ji-Xia Li for the technical assistance. This research was supported by grants from the Chinese National “863” High-Tech Program (Grant No.2004AA213072).
References
- Almazan F., Gonzalez J.M., Penzes Z.l. Engieering the largest RNA virus gene as an infectious bacterial artifical chromosome. Acad. Sci. 2000;97(10):5516–5521. doi: 10.1073/pnas.97.10.5516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh D. Nidovirales: a new order comprising Coronaviridae and Arteriviridae. Arch. Virol. 1997;142:629–633. [PubMed] [Google Scholar]
- Cerdan M.G., Young J.I., Zino E., Falzone T.L., Otero V., Torres H.N., Rubinstein M. Accurate spatial and temporal transgene expression driven by a 3.8-kilobase promoter of the bovine beta-casein gene in the lactating mouse mammary gland. Mol. Reprod. Dev. 1998;49(3):236–245. doi: 10.1002/(SICI)1098-2795(199803)49:3<236::AID-MRD3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Dertzbaugh M.T. Genetically engineered vaccines: an overview. Plasmid. 1998;39:100–113. doi: 10.1006/plas.1997.1329. [DOI] [PubMed] [Google Scholar]
- Donofrio G., Bignetti E., Clark A.J., Whitelaw C.B. Comparable processing of beta-lactoglobulin pre-mRNA in cell culture and transgenic mouse models. Mol. Gen. Genet. 1996;252(4):465–469. doi: 10.1007/BF02173012. [DOI] [PubMed] [Google Scholar]
- Doppler W., Groner B., Ball R.K. Prolactin and glucocorticoid hormones synergistically induce expression of transfected rat beta-casein gene promoter constructs in a mammary cell line. PNAS. 1989;86:104–108. doi: 10.1073/pnas.86.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faye L., Fitchette-Laine A.C., Gomord V., Chekkati A., Delaunay A.M., Driouich A. Detection, biosynthesis and some functions of glycans N-linked to plant secreted proteins. Soc. Exp. Biol. Semin. Ser. 1993;53:213–242. [Google Scholar]
- Gomez N., Carrillo C., Salinas J., Parra F., Borca M.V., Escribano J.M. Expression of immunogenic glycoprotein S polypeptides from transmissible gastroenteritis coronavirus in transgenic plants. Virology. 1998;249:352–358. doi: 10.1006/viro.1998.9315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho P.S., Kwang J., Lee Y.K. Intragastric administration of Lactobacillus casei expressing transmissible gastroentritis coronavirus spike glycoprotein induced specific antibody production. Vaccine. 2005;23:1335–1342. doi: 10.1016/j.vaccine.2004.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb A.F., Pewe L., Webster J., Perlman S., Whitelaw C.B., Siddell S.G. Virus-neutralizing monoclonal antibody expressed in milk of transgenic mice provides full protection against virus-induced encephalitis. J. Virol. 2001;75(6):2803–2809. doi: 10.1128/JVI.75.6.2803-2809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naruse K., Yoo S.K., Kim S.M., Choh Y.J., Lee H.M., Jin D. Analysis of tissue-specific expression of human type II collagen cDNA driven by different sizes of the upstream region of the beta-casein promoter. Biosci. Biotechnol. Biochem. 2006;70(1):93–98. doi: 10.1271/bbb.70.93. [DOI] [PubMed] [Google Scholar]
- Palom Y., Belcourt M.F., Tang L.Q., Mehta S.S., Sartorelli A.C., Pritsos C.A., Pritsos K.L., Rockwell S., Tomasz M. Bioreductive metabolism of mitomycin C in EMT6 mouse mammary tumor cells: cytotoxic and non-cytotoxic pathways, leading to different types of DNA adducts. Biochem. Pharmacol. 2001;61:1517–1529. doi: 10.1016/s0006-2952(01)00609-8. [DOI] [PubMed] [Google Scholar]
- Sola I., Castilla J., Pintado B., Anchez-Morgado J., Whitelaw C.B., Clark A.J., Enjuanes L. Transgenic mice secreting coronavirus neutralizing antibodies into the milk. J. Virol. 1998;72(5):3762–3772. doi: 10.1128/jvi.72.5.3762-3772.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler E., Le Saux A., Guinut F., Passet B., Cohen R., Merle C., Charpilienne A., Fourgeux C., Sorel V., Piriou A., Schwartz-Cornil I., Cohen J., Houdebine L.M. Production of two vaccinating recombinant rotavirus proteins in the milk of transgenic rabbit. Transgenic Res. 2005;14(6):833–844. doi: 10.1007/s11248-005-1771-0. [DOI] [PubMed] [Google Scholar]
- Tuboly T., Yu W., Bailey A., Degrandis S., Du S., Erickson L., Nagy E. Immunogenicity of porcine transmissible gastroenteritis virus spike protein expressed inplants. Vaccine. 2000;18:2023–2028. doi: 10.1016/s0264-410x(99)00525-3. [DOI] [PubMed] [Google Scholar]
- Whitelaw C.B.A., Farini E., Webster J. The changing role of cell culture in the generation of transgenic livestock. Cytotechnology. 1999;31:3–8. doi: 10.1023/A:1008044517150. [DOI] [PMC free article] [PubMed] [Google Scholar]


