Abstract
Mice lacking matrix metalloproteinase-3 (MMP-3; stromelysin-1) demonstrated significantly less injury than their normal counterparts following formation of IgG-containing immune complexes in the alveolar wall or in the wall of the peritoneum. Likewise, mice lacking MMP-3 demonstrated less lung injury following intra-tracheal instillation of the chemotactic cytokine macrophage inhibitory protein-2 (MIP-2) than did mice with MMP-3. There was a relationship between tissue injury (evidenced histologically) and accumulation of anti-laminin 111 immuno-reactive material in the bronchoalveolar lavage (BAL) or peritoneal lavage (PL) fluid. There was also a relationship between tissue injury and influx of neutrophils into the BAL or PL fluid. Taken together, these data demonstrate an important role for MMP-3 in acute inflammatory tissue injury.
INTRODUCTION
The patho-physiology of acute lung inflammation is not completely understood. Experimental studies in rodents have demonstrated the importance of reactive oxygen species generated by resident alveolar macrophages and neutrophils recruited from the circulation (Johnson and Ward, 1974; Johnson and Ward, 1981). In vitro studies have shown that cellular elements of the lung parenchyma, especially vascular endothelial cells, are important oxidant targets (Varani et al., 1985; Gannon et al., 1987).
Matrix metalloproteinases (MMPs) also contribute to the tissue injury observed in acute lung inflammation. Several clinical studies have shown that levels of neutrophil-derived MMPs are elevated in various chronic (Vignola et al., 1988; Hayashi et al., 1996; Hayashi et al., 1997; Ohno et al., 1997; Dahlen et al., 1999) and acute (Ricou et al., 1996; Sweet et al., 2001; Cederqvist et al., 2001; Suga et al., 2000; Choi et al., 2002; Hartog et al., 2003) lung diseases. In experimental animals, Intra-alveolar instillation of recombinant tissue inhibitor of metalloproteianses-2 (TIMP-2) reduces acute lung injury (Mulligan et al., 1993; Gibbs et al., 1999) while neutralization of endogenous MMP inhibitor activity in the lung increases tissue damage in the same experimental models (Gipson et al., 1999). Although these studies support a role for MMPs (as a family of degradative enzymes) in acute lung injury, it is difficult to distinguish among the MMPs based on the use of inhibitors since neither the natural MMP inhibitors nor the synthetic agents that have been developed are sufficiently specific to distinguish among various MMPs that may be phlogistic.
The use of rodents with selective gene-deletions has allowed for the role of specific MMPs to be evaluated in various disease processes. Our past studies have demonstrated that experimental lung injury is reduced in rodents lacking stromelysin-1 (MMP-3) (i.e., MMP-3 -/- mice) as compared to their normal counterparts (Warner et al., 2001). MMP-3 is a broadly-active enzyme with capacity to degrade a number of non-collagenous components of the extracellular matrix and to further degrade interstitial collagen molecules that have been cut by one of the mammalian collagenases (Shapiro, 1998; Nelson, 2000). A number of past studies have implicated MMP-3 in the pathogenesis of rheumatoid arthritis (Okada et al., 1989; Okada, 1992; Kanemoto et al., 1996; van Meurs et al., 1996), and studies by Wang et al. (Wang et al., 1999) using MMP-3 -/- mice demonstrated a role in delayed type hypersensitivity. How MMP-3 contributes to the development of acute tissue injury is not fully understood. Here we demonstrate that neutrophil-mediated tissue damage is reduced at two different sites (lung and peritoneal wall / mesentery) in mice lacking MMP-3 as compared to their normal counterparts. There is a direct relationship between reduced tissue injury (evidenced histologically), a reduction in vascular wall damage, and reduced accumulation of neutrophils in the lavage fluid.
MATERIALS AND METHODS
Mice
The MMP-3 -/- mice and their wild-type littermates were obtained commercially from Taconic Laboratories (Germantown, NY). These animals were originally developed from a donor strain of 129/SvEV ES cell implanted into a C57Bl/6J recipient (Mudgett, et al., 1998). Control C57BL6J mice (MMP-3 +/+) of the same age, strain and sex were used as controls. All mice used in these experiments were approximately 25 grams in weight, were between 3-4 months of age, and were maintained in a germ-free environment. All experiments were performed in compliance with the standards set by the University of Michigan’s Committee for the Use and Care of Animals.
IgG immune complex injury in lung
MMP-3 +/+ and MMP-3 -/- mice were anesthetized with an intra-peritoneal injection of ketamine hydrochloride (100 mg/kg) (Fort Dodge Animal Health, Fort Dodge, IA) plus xylazine (20 mg/kg) (Ben Venue Laboratories, Bedford, OH). Briefly, rabbit anti-bovine serum albumin (BSA) antibody (ICN; Aurora, OH) was instilled intra-tracheally (60 μl total volume), and BSA (0.5 mg per mouse in 0.5 ml of PBS) was injected intravenously. Four hours later, animals were sacrificed. Bronchoalveolar lavage (BAL) fluid was collected for analysis as indicated below. Tissue was fixed in 10% buffered formalin en bloc and used for histology. This is a well-established model of acute inflammatory lung injury in rodents (Johnson and Ward, 1974; Johnson and Ward, 1981; Mulligan et al., 1993; Gibbs et al., 1999; Gipson et al., 1999; Warner et al., 2001).
IgG immune complex injury in the peritoneum
MMP-3 +/+ and MMP-3 -/- mice were anesthetized as above. IgG immune complexes were formed in the peritoneal wall/mesentery by intra-peritoneal injection of anti-BSA (250 μl volume) and intravenous BSA (0.5 mg per mouse in 0.5 ml of PBS). Animals were sacrificed 4-hours post-challenge. Peritoneal lavage (PL) fluid was collected for analysis and peritoneal wall tissue and mesentery were fixed in 10% buffered formalin.
MIP-2-mediated injury in lung
MMP-3 +/+ and MMP-3 -/- mice were anesthetized as indicated above. Macrophage inhibitory protein - 2 (MIP-2) (Sigma Chemical Co.) (250 ng in 60 μl of saline) was instilled into the lungs at time-zero and animals sacrificed four hours later and analyzed as described above.
Histology
Formalin-fixed tissue from lung or peritoneum was stained with hematoxylin and eosin (H&E) and examined by light microscopy.
BAL fluid and PL fluid collection
BAL fluid was collected from the animals after sacrifice by inserting an 18 gauge needle into the trachea above the point of bifurcation. Lungs were sequentially washed with three 800-μl aliquots of PBS. After the first aliquot was recovered from the lungs, cells were pelletted and the supernatant fluid saved for analysis. The second and third washes were combined with the cell pellet from the first wash and cell counts were performed on the total recovered cells. PL fluid was collected from the animals after sacrifice using a 18 gauge needle inserted through the peritoneal wall. The peritoneum was sequentially washed with three 5-ml aliquots of PBS. After the first aliquot was recovered from the peritoneum, cells were pelletted and the supernatant fluid saved for analysis. The second and third washes were combined with the cell pellet from the first wash and cell counts were performed on the total recovered cells.
BAL and PL Fluid Cell Counts
Total cells recovered from the BAL and PL fluids were washed 2 times with PBS. Total cell counts were performed using a hemacytometer on an inverted tissue culture microscope. Cells were then spotted onto glass slides using a Cytospin 3 microfuge (Shandon Scientific Ltd., Astmoor, England) and stained with H&E. Neutrophil numbers were determinee sample.
Gelatin zymography
SDS-PAGE substrate-embedded enzymography (zymography) was carried out by a modification of the method of Heussen and Dowdle (Heussen and Dowdle, 1980). Briefly, SDS-PAGE gels were prepared for mini gels from 30:1 acrylamide : bis with the final concentrations of the other components of the gels being: Tris-HCl at pH 8.8 (325 mM), SDS (0.1%), ammonium persulfate (0.05%), and TEMED (0.05%). Gelatin (1 mg/ml) was incorporated into the gel at the time of casting. The gels were routinely 7.5% acrylamide. Denatured but non-reduced samples and standards were then electrophoresed into the gels at constant voltage of 150 V in an ice bath under non-reducing conditions. When the dye fronts reached a point approximately 0.5 cm from the bottom, the gels were removed and subjected to the following wash protocol: (2x) 15 minutes in 50 mM Tris buffer (containing 1 mM Ca2+ and 0.5 mM Zn2+) with 2.5% Triton X-100; (1x) 5 minutes in Tris buffer alone; and finally overnight in Tris buffer with 1% Triton X-100. The gels were stained the following morning with Coumassie Brilliant Blue 250-R. Following destaining, zones of enzyme activity were detected as regions of negative staining. The zymograms were digitized and converted to negative images. Quantification was accomplished by determining the number of pixels in the negative images. Volumes of 5-15 μl of undiluted culture fluid were normally used for these assays, with zones of activity being proportional to the quantity of culture fluid used. Gelatin zymography is useful for detection of MMP-2 (72-kD gelatinase A) and MMP-9 (92-kD gelatinase B) in biological fluids.
Immunoblot analysis for laminin in BAL and PL fluid
BAL and PL fluid samples were separated by SDS-PAGE under denaturing and reducing conditions and transferred to nitrocellulose membranes. After blocking with a 5% nonfat milk solution in Tris-buffered saline with 0.1% Tween (TTBS) at 4°C overnight, membranes were incubated for one hour at room temperature with a rabbit polyclonal antibody reactive with mouse laminin 111 (LM 111; α1β1γ1 in the recent classification scheme [Aumailley et al., 2005]). The polyclonal antibody (Chemicon; Temicula, CA), was diluted 1:1000 in 0.5% nonfat milk/0.1% TTBS. Thereafter, the membranes were washed with TTBS and bound antibody detected using the Phototope-HRP Western blot detection kit (Cell Signaling Technologies, Inc.; Beverly, MA). Images were scanned, digitized and quantified. In addition to the rabbit polyclonal antibody, monoclonal antibodies to laminin 111 A chain (α1 chain) and laminin 111 B chain (β1 chain) were also used. Both were obtained from Chemicon.
RESULTS
Alveolar wall damage in MMP-3 +/+ and MMP-3 -/- mice
In the first set of experiments, lung injury was induced in MMP-3 +/+ and MMP-3 -/- mice via the intra-alveolar wall formation of IgG-containing immune complexes or following intra-tracheal instillation of MIP-2. Four hours post-induction, lung injury was assessed. Consistent with previous findings (Warner et al., 2001), MMP-3 +/+ mice challenged with immune complexes demonstrated severe lung injury as evidenced histologically, while significantly less tissue injury was observed in MMP-3 -/- animals challenged under the same conditions (Figure 1, Panel A).
Figure 1.
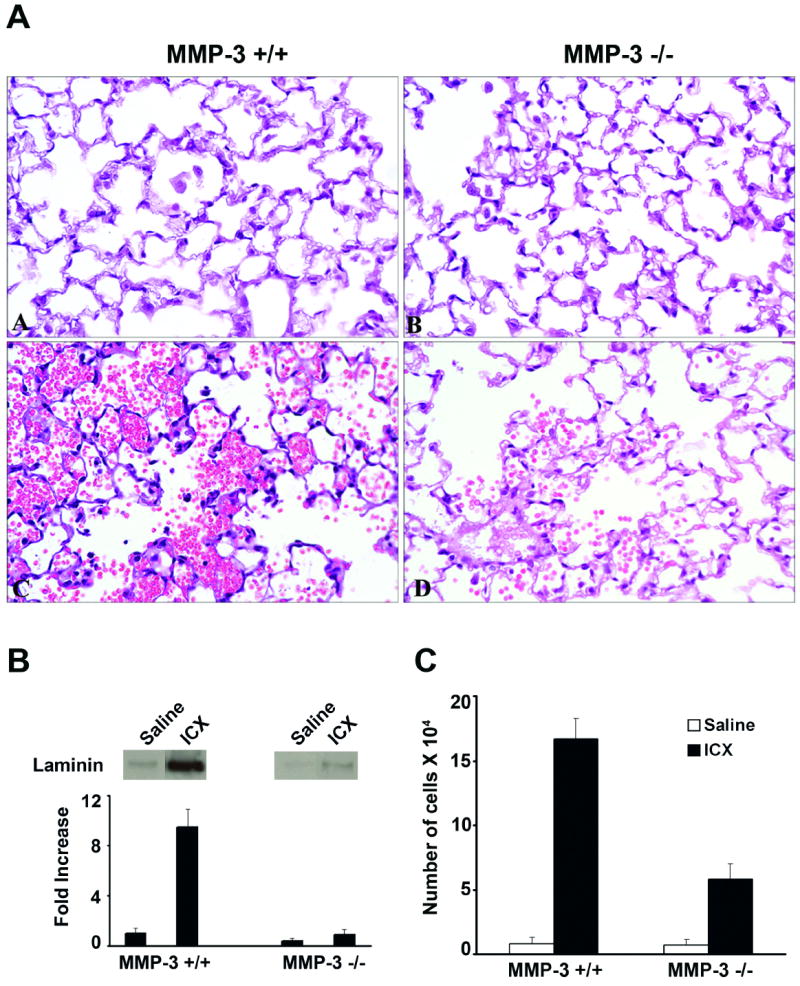
Tissue injury, vascular wall damage and neutrophil accumulation in immune complex-challenged MMP-3 +/+ and MMP-3 -/- mice. Panel A: Histological evidence of lung injury in MMP-3 +/+ and MMP-3 -/- mice four hours after formation of IgG-containing immune complexes in the alveolar wall. Tissue damage is indicated by the presence of red blood cells and fibrin in the alveolar space. There is significantly more damage in the lungs of MMP-3 +/+ mice (section C) than in lungs of MMP-3 -/- mice (section D). There is essentially no lung damage in saline-challenged mice of either strain (sections A and B). Sections are hematoxylin and eosin stained (X160). Panel B: Anti-laminin 111 Immunoreactivity in BAL fluid of MMP-3 +/+ and MMP-3 -/- mice four hours after formation of IgG-containing immune complexes in the alveolar wall. Values represent means and standard deviations based on 8 animals per group. Statistical significance of the differences was assessed using ANOVA followed by paired-group comparisons. Differences between MMP-3 +/+ and corresponding MMP-3 -/- values were significant at p<0.05 level. Representative bands are shown in the inserts above the quantified material. Panel C: Neutrophils in BAL fluid of MMP-3 +/+ and MMP-3 -/- mice four hours after formation of IgG-containing immune complexes in the alveolar wall. Values represent means and standard deviations based on 8 animals per group. Statistical significance of the differences was assessed using ANOVA followed by paired-group comparisons. Differences between MMP-3 +/+ and corresponding MMP-3 -/- values were significant at p<0.05 level.
When MIP-2 was instilled into the airway, there was also clear histological evidence of injury. Although the degree of lung damage was considerably less in both mouse strains than seen with immune complex challenge, injury was more severe in MMP-3 +/+ mice than in MMP-3 -/- mice (Figure 2, Panel A). When saline was instilled, no significant injury was observed in either mouse strain (Figure 1, Panel A).
Figure 2.
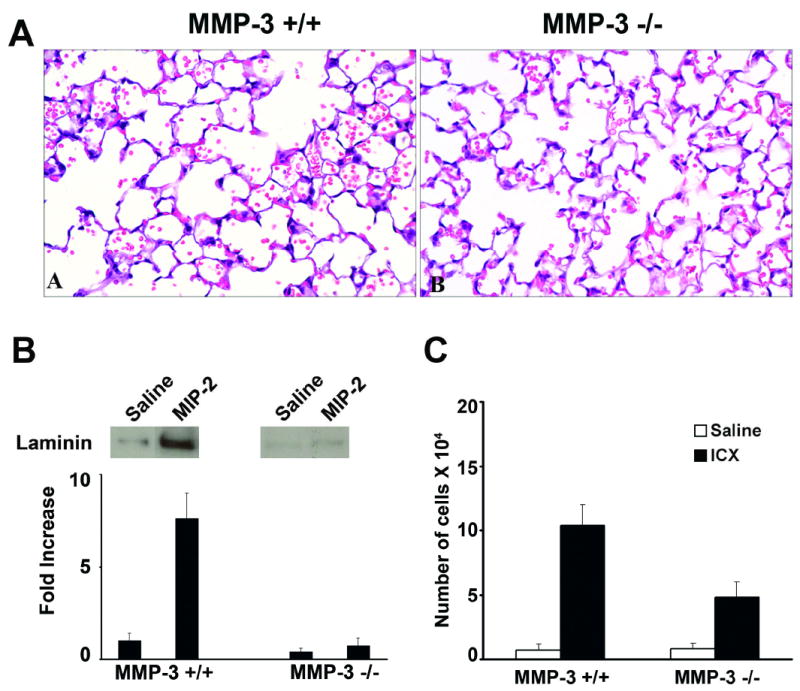
Tissue injury, vascular wall damage and neutrophil accumulation in MIP-2 - challenged MMP-3 +/+ and MMP-3 -/- mice. Panel A: Histological evidence of lung injury in MMP-3 +/+ and MMP-3 -/- mice four hours after intra-tracheal instillation of MIP-2. Tissue damage is indicated by the presence of red blood cells and fibrin in the alveolar space. There is significantly more damage in the lungs of MMP-3 +/+ mice (section A) than in lungs of MMP-3 -/- mice (section B). Sections are hematoxylin and eosin stained (X160). Panel B: Anti-laminin 111 Immunoreactivity in BAL fluid in BAL fluid of MMP-3 +/+ and MMP-3 -/- mice four hours after intra-tracheal instillation of MIP-2. Values represent means and standard deviations based on 8 animals per group. Statistical significance of the differences was assessed using ANOVA followed by paired-group comparisons. Differences between MMP-3 +/+ and corresponding MMP-3 -/- values were significant at p<0.05 level. Representative bands are shown in the inserts above the quantified material. Panel C: Neutrophils in BAL fluid of MMP-3 +/+ and MMP-3 -/- mice four hours after intra-tracheal instillation of MIP-2. Values represent means and standard deviations based on 8 animals per group. Statistical significance of the differences was assessed using ANOVA followed by paired-group comparisons. Differences between MMP-3 +/+ and corresponding MMP-3 -/- values were significant at p<0.05 level.
In addition to assessing injury histologically, we also assessed immuno-reactive laminin in BAL fluid from challenged mice. In both the immune complex-challenged mice and MIP-2 – challenged mice, there was significantly more immuno-reactive material released into the BAL fluid of the MMP-3 +/+ mice than into the BAL fluid of the MMP-3 -/- mice (Figures 1 and 2, Panel B). The western blots shown in Figures 1 and 2, were obtained using a rabbit polyclonal anti-mouse laminin antibody for detection. When monoclonal antibodies to laminin 111 α1 chain and murine laminin 111 β1 chain were used, detectable immuno-reactive material was obtained with both antibodies.
In addition to assessing anti-laminin 111 immuno-reactivity, the same BAL fluid samples were assessed for MMP-2 and MMP-9 by gelatin zymography. There was a substantial increase in levels of both enzymes in the BAL fluid from animals challenged with either immune complexes or MIP-2 relative to saline-treated controls. On the other hand, there was little difference between the MMP-3 +/+ and MMP-3 -/- mice (not shown). Most likely, this reflects the fact that parenchymal cells in the airways (along with neutrophils recruited from the circulation) are a source of these enzymes (Mulligan et al., 1993; Gibbs et al., 1999).
Using the same BAL fluid specimens, the number of neutrophils in the alveolar space was determined. With saline as challenge, there was minimal influx of neutrophils into the alveolar space and no significant difference between the two strains of mice (Table 1). With immune complex-challenge or with the chemotactic stimulus MIP-2, the number of intra-alveolar neutrophils increased significantly in both mouse strains as compared to untreated or saline-treated controls. With both stimuli, significantly more cells were recovered in the BAL fluid of MMP-3 +/+ mice than in BAL fluid of their MMP-3 – deficient counterparts (Figures 1 and 2, Panel C). Thus, there was a good correlation between histological evidence of tissue damage, damage to the vascular wall, and neutrophil-influx into the BAL fluid.
Table 1.
Recruitment of neutrophils into BAL fluid and PL fluid of MMP-3 +/+ and MMP-3 -/- mice by saline.
| MMP-3 +/+ | MMP-3 -/- | |
|---|---|---|
| BAL | ||
| No treatment | 3.1 ± 1.0 | 3.3 ± 1.0 |
| Saline | 3.8 + 1.3 | 3.9 ± 2.2 |
| PL | ||
| No treatment | 1.2 ± 0.3 | 2.2 ± 0.3 |
| Saline | 1.9 ± 0.9 | 2.9 ± 0.9 |
Values shown represent the total numbers of neutrophils per mouse recovered in BAL or PL fluid ± standard deviations. For BAL studies, values are times 103 and are based on 12 animals per group. For PL studies, values are times 105 and are based on 6 animals per group.
Peritoneal wall/mesentery damage in MMP-3 +/+ and MMP-3 -/- mice
In addition to assessing lung injury, MMP-3 +/+ and MMP-3 -/- mice were also examined for damage to the peritoneal wall/mesentery following formation of immune complexes in the peritoneal wall/mesentery. Gross and histological examination of the peritoneal wall and mesentery provided no evidence of injury in either strain of mouse following saline injection (not surprisingly). In the immune complex-challenged mice, areas along the peritoneal surface where neutrophils were migrating into the peritoneal space could be seen. Such areas were significantly less prevalent in the MMP-3 -/- mice than in MMP-3 +/+ controls, and fewer neutrophils were seen at those sites in MMP-3 -/- mice than in controls. The upper and middle histological sections presented in Figure 3 demonstrate the peritoneal wall surface of mice exposed to intra-peritoneal saline (upper sections) or mice following formation of immune complexes in the peritoneal wall (middle sections). Additionally, small neutrophil-rich abcesses could also be seen in the peritoneal wall and mesenteries of MMP-3 +/+ mice (lower-left section, arrow). Unlike in the lung where histological evidence of injury was widespread, injury in the peritoneal wall and mesentery was focal in nature. Such areas of neutrophil accumulation were not seen in MMP-3 -/- mice, although we could identify collections of neutrophils within the small vessels (lower-right section, arrow).
Figure 3.
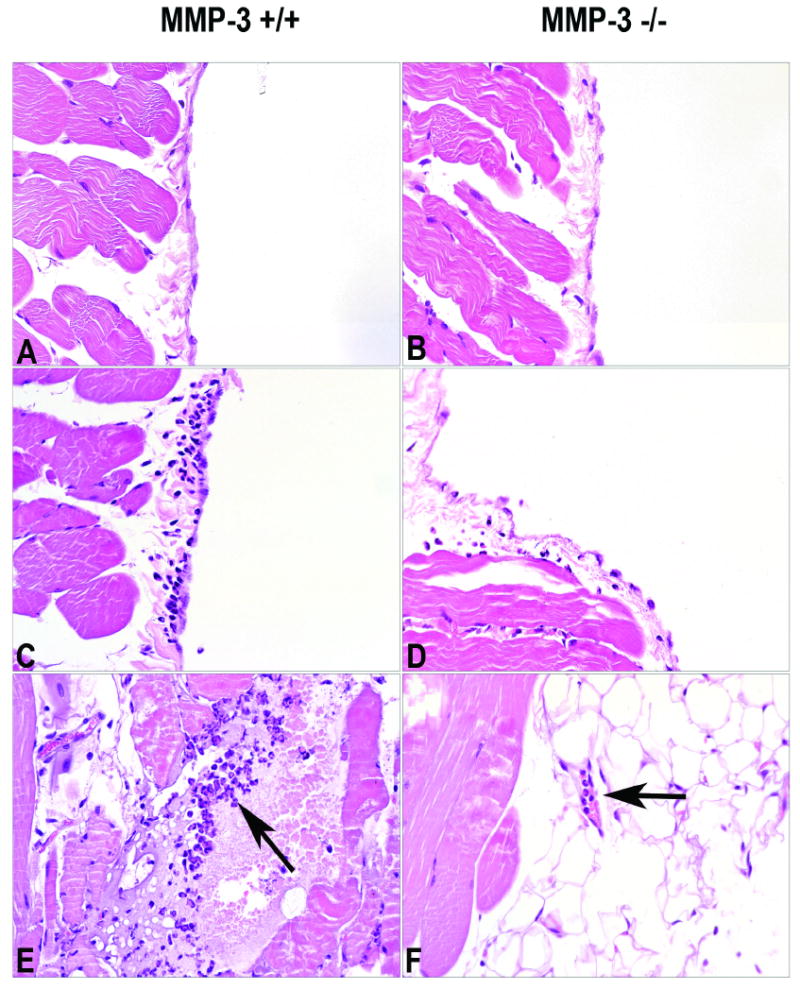
Histological evidence of peritoneal wall/mesentery injury in MMP-3 +/+ and MMP-3 -/- mice four hours after formation of IgG-containing immune complexes in the peritoneal wall/mesentery Sections A and B: Peritoneal wall from saline-injected animals. There is essentially no injury. Sections C and D: Peritoneal wall from immune complex-challenged animals. Focal areas of neutrophil accumulation along the peritoneal wall can be seen in both strains of mice. In MMP-3 +/+ mice, these areas are more wide spread and contain more neutrophils than similar areas in MMP-3 -/- mice. Section E: A neutrophil-rich lesion can be seen in the peritoneal wall of a MMP-3 +/+ mouse (arrow). Section F: In this MMP-3 -/- mouse neutrophils can be seen in a mesenteric vessel, but there is no evidence of extravascular neutrophils (arrow). Sections are hematoxylin and eosin stained (X160).
Just as in the lung, Immuno-reactive laminin (i.e., reactivity with the rabbit polyclonal antibody to laminin 111) in PL fluid correlated with tissue damage. There was little immuno-reactive material in the lavage fluid of saline-challenged animals of either strain, while immuno-reactivity was readily detectable in the lavage fluid of animals challenged with immune complexes. As expected, the levels were higher in the MMP-3 +/+ mice than in MMP-3 -/- mice (Figure 4, Panel A). In addition to assessing PL fluid for anti-laminin 111 immuno-reactive material, MMP-2 and MMP-9 levels were also assessed. Consistent with the results from the lung studies, increased levels of both enzymes were observed in the immune complex-challenged animals relative to saline-treated controls, but there was only a modest difference between the two groups of mice (Figure 4, Panel B).
Figure 4.
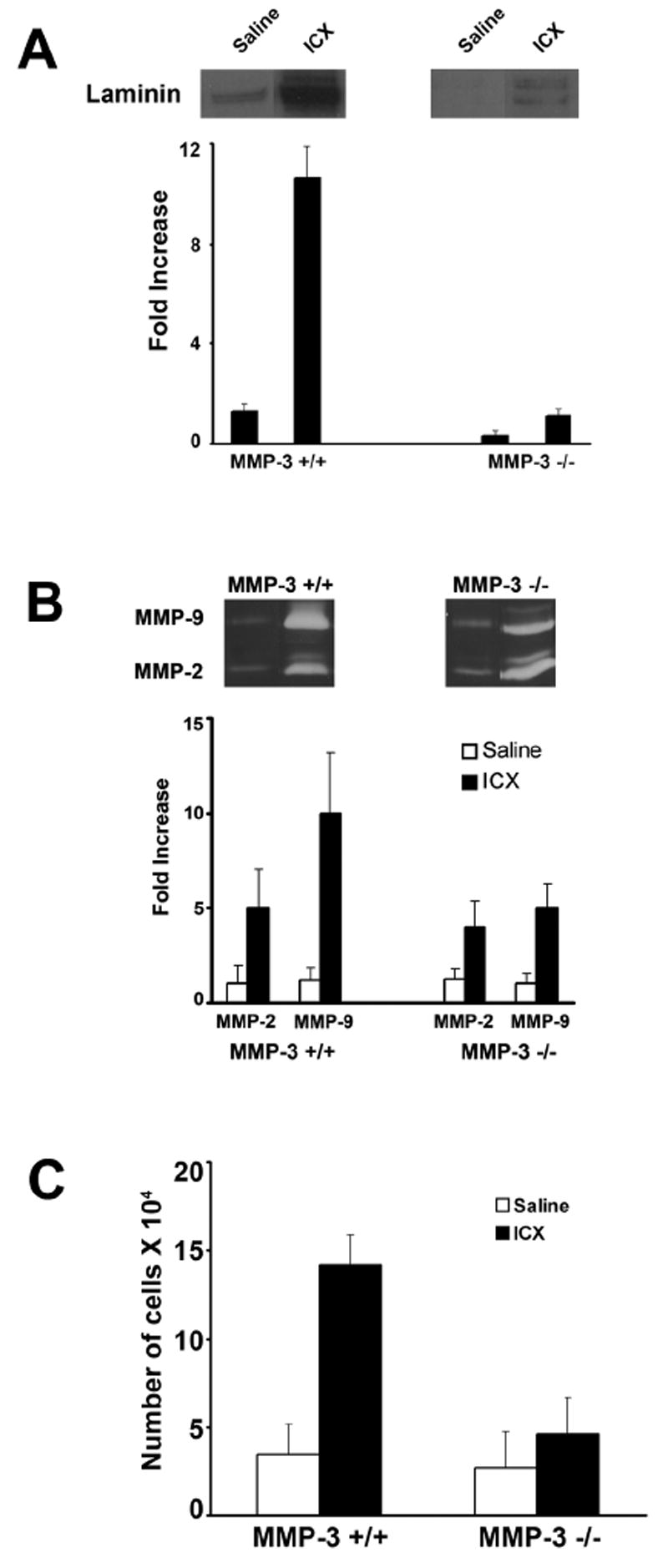
Panel A: Anti-laminin 111 Immunoreactivity in PL fluid of MMP-3 +/+ and MMP-3 -/- mice four hours after formation of IgG-containing immune complexes in the peritoneal wall/mesentery. Values represent means and standard deviations based on 6 animals per group. Statistical significance of the differences was assessed using ANOVA followed by paired-group comparisons. Differences between MMP-3 +/+ and corresponding MMP-3 -/- values were significant at p<0.05 level. Representative bands are shown in the inserts above the quantified material. Panel B: MMP-2 and MMP-9 levels in PL fluid of MMP-3 +/+ and MMP-3 -/- mice four hours after formation of IgG-containing immune complexes in the peritoneal wall/mesentery. Values represent means and standard deviations based on 3-4 animals per group. Representative bands are shown in the inserts. Panel C: Neutrophils in PL fluid of MMP-3 +/+ and MMP-3 -/- mice four hours after formation of IgG-containing immune complexes in the peritoneal wall/mesentery. Values represent means and standard deviations based on 6 animals per group. Statistical significance of the differences was assessed using ANOVA followed by paired-group comparisons. Differences between MMP-3 +/+ and corresponding MMP-3 -/- values were significant at p<0.05 level.
PL fluid from saline- and immune complex-challenged mice was examined for neutrophil accumulation. When saline was injected into the peritoneal space, the number of recovered neutrophils was small in both mouse strains and there was no significant difference between the two strains. The number of recovered cells was, however, greater than the number recovered from untreated control animals (Table 1). When immune complexes were formed in the peritoneal wall/mesentery, large numbers of neutrophils accumulated in the PL fluid. Consistent with the less severe tissue damage in MMP-3 -/- mice, there were fewer cells in the PL fluid from these animals than in controls (Figure 4, Panel C). Thus, similar to what was observed in the lung, there was a good correlation between histological evidence of tissue damage, damage to the peritoneal wall, and neutrophil influx into the PL fluid.
DISCUSSION
In the present study we compared tissue injury at two sites (alveolar wall and peritoneal wall/mesentery) in mice lacking MMP-3 and their normal counterparts. At both sites, the degree of tissue injury was significantly reduced in mice lacking MMP-3 as compared to the controls. The finding that MMP-3 contributes to tissue injury in situations where neutrophils are an important component of the patho-physiology is consistent with previous findings in experimental models of arthritis (Okada et al., 1989; Okada, 1989; Kanemoto, 1996; van Meurs et al., 1999), in a model of delayed type hypersensitivity (Wang et al., 1999) and in our own past studies using a pulmonary injury model (Warner et al., 2001).
How MMP-3 contributes to tissue damage in the lung or peritoneal wall/mesentery is not fully understood. One thing is certain; at both sites, fewer neutrophils from MMP-3 -/- mice than from their normal counterparts crossed the vascular wall into the extravascular space. A number of past in vitro and in vivo studies have suggested that none of the major families of proteolytic enzymes are required for neutrophil transmigration across the vessel wall. Huber and Weiss (1989) showed that neutrophil migration across an in vitro vessel wall construct could not be blocked with either serine proteinase or metalloproteinase inhibitors. Although this “negative” finding could be explained by a failure of the high molecular weight natural inhibitors to access the protected site, a subsequent study by Mackarel et al. (2000) came to a similar conclusion based on the use of synthetic low molecular weight proteinase inhibitors. In another study, Betsuyaku et al (1999) demonstrated that neutrophils from mice deficient in MMP-9 were as effective as their normal counterparts in migrating into lung, peritoneum or skin. Three different stimuli were used in their study, depending on tissue site, with comparable results. Finally, Conway et al. (1995) reported that adjuvant arthritis in rats could be significantly attenuated in animals receiving a synthetic MMP inhibitor systemically. The same treatment was reported to have only minimal effect on neutrophil accumulation in the synovium. Based on these observations, it has been suggested that neutrophil migration across the vascular wall into a nidus of inflammation does not depend, a priori, on MMP-mediated damage to the vessel wall.
An alternative explanation might be that MMPs modulate the environment – for example, by generating (through proteolytic cleavage) chemotactic peptides from complement-cascade proteins or from extracellular matrix molecules. Additionally, MMPs can function by stimulating cytokine generation in macrophages (or other resident cells) at the site of the developing inflammation, cleave the active peptides from the cell surface, process inactive precursors into their active forms or degrade and clear active chemotactic moieties and / or inhibitory peptides. All of these effects could be expected to alter neutrophil recruitment into sites of the developing inflammatory nidus. A recent review by Parks, Wilson and Lopez-Boado has addressed these issues in detail (Parks et al., 2004).
Superficially, our data would seem to be inconsistent with these prior studies. Our studies showed that even when a preformed, potent, neutrophil chemotactic was instilled into the lungs, neutrophil accumulation in mice lacking MMP-3 was reduced as compared to what was observed in wild-type animals. Thus, it would seem that MMP-3 must be doing something more than promoting an environment conducive to neutrophil recruitment. It is well-established from in vitro studies that MMP-3 has the capacity to degrade many of the extracellular matrix components found in the basement membrane (Shapiro, 1998; Nelson et al., 2000). It could be hypothesized that MMP-3 directly damages the connective tissue component of the vessel wall and / or the interstitium. It must be cautioned, however, that there is no compelling evidence that this occurs in vivo, and it is difficult to extrapolate from in vitro matrix damage to what might occur in vivo. It also must be remembered that acute inflammation consists of a series of amplification loops – i.e., more neturophils recruited into a tissue, the more tissue damage; and the more tissue damage, the more recruitment. Thus, by the time acute lung injury is “clinically” and histologically apparent, it is virtually impossible to distinguish a precise sequence of events. Which of the multiple possible mechanisms is key to understanding how MMP-3 contributes to neutrophil recruitment into the lung and acute lung injury will have to await additional studies.
An important issue is the relationship between findings in rodent tissue injury models and inflammatory tissue damage in humans. As a way to begin addressing this issue, we recently analyzed BAL fluid samples from 28 acute lung injury (ALI) / acute respiratory distress syndrome (ARDS) patents for a number of MMPs. In all of the ALI / ARDS patients, there were high levels of MMP-2, MMP-8 and MMP-9, but in only a subset of patients was there evidence of MMP-1 and MMP-3. However, in those patients with high levels of BAL MMP-1 and MMP-3 in addition to the other enzymes, overall mortality was higher (83% mortality versus 32%). Disease severity by a number of additional criteria was also greater in the patients with detectable MMP-1 and MMP-3 than in patients without these enzymes (Fligiel et al., 2006). Thus, clinical studies as well as studies in the experimental rodent models suggest that MMPs including MMP-3 may play an important patho-physiological role.
Acknowledgments
This study was supported in part by grant HL70979 from the USPHS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aumailley M, Bruckner-Tuderman L, Carter WG, et al. A simplified laminin nomenclature. Matrix Biol. 2005;24:326–332. doi: 10.1016/j.matbio.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Betsuyaku T, Shipley JM, Liu Z, Senior RM. Neutrophil emigration in the lungs, peritoneum, and skin does not require gelatinase B. Am J Respir Cell Mol Biol. 1999;20:1303–1309. doi: 10.1165/ajrcmb.20.6.3558. [DOI] [PubMed] [Google Scholar]
- Cederqvist K, Sorsa T, Tervahartiala T, Maisi P, Reunanen K, Lassus P, Andersson S. Matrix metalloproteinases-2, -8, and –9 and TIMP-2 in tracheal aspirates from preterm infants with respiratory distress. Pediatrics. 2001;108:686–692. doi: 10.1542/peds.108.3.686. [DOI] [PubMed] [Google Scholar]
- Choi KH, Lee HB, Jeong MY, Rhee YK, Chung MJ, Kwak YG, Lee YC. The role of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in crypogenic organizing pneumonia. Chest. 2002;121:1478–1485. doi: 10.1378/chest.121.5.1478. [DOI] [PubMed] [Google Scholar]
- Conway JG, Wakefield JA, Brown RH, Marron BE, Sekut L, Stimpson SA, McElroy A, Menius A, Jeffreys JJ, Clark RL, McGeehan GM, Connolly KM. Inhibition of cartilage and bone destruction in adjuvant arthritis in the rat by a matrix metalloproteinase inhibitor. J Exp Med. 1995;182:449–457. doi: 10.1084/jem.182.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlen G, Shute J, Howarth P. Immunohistochemical localisation of the matrix metalloproteinases MMP-3 and MMP-9 within the airways in ashtma. Thorax. 1999;7:590–596. doi: 10.1136/thx.54.7.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fligiel SEG, Standiford T, Fligiel SEG, Strieter RW, Tashkin D, Warner RL, Johnson KJ, Varani J. Matrix metalloproteinases (MMPs) and MMP Inhibitors in acute lung inflammation. Human Pathol. 2006;37:422–430. doi: 10.1016/j.humpath.2005.11.023. [DOI] [PubMed] [Google Scholar]
- Gannon DE, Varani J, Phan SH, Ward JH, Kaplan J, Till GO, Simon RG, Ryan US, Ward PA. Source of iron in neutrophil-mediated killing of endothelial cells. Lab Invest. 1987;57:37–44. [PubMed] [Google Scholar]
- Gibbs DF, Shanley TP, Warner RL, Murphy HS, Varani J, Johnson KJ. The role of matrix metalloproteinases in models of macrophage-dependent acute lung injury: evidence for the alveolar macrophage as a source of the proteinases. Am J Respiratory Cell Molec Biol. 1999;20:1145–1154. doi: 10.1165/ajrcmb.20.6.3482. [DOI] [PubMed] [Google Scholar]
- Gipson TS, Bless NM, Shanley TP, Crouch LD, Bleavins MR, Younkin EM, Sarma V, Gibbs DF, Tefera W, McConnell PC, Mueller WT, Johnson KJ, Ward PA. Regulatory role of endogenous protease inhibitors in acute lung inflammatory injury. J Immunol. 1999;162:3653–3661. [PubMed] [Google Scholar]
- Harlan JM, Schwartz BR, Reidy MA. Activated neutrophils disrupt endothelial monolayer integrity by an oxygen radical-independent mechanism. Lab Invest. 1985;52:141–145. [PubMed] [Google Scholar]
- Hartog CM, Wermelt JA, Sommerfeld CO, Eichler W, Dalhoff K, Braun J. Pulmonary matrix metalloproteinase excess in hospital-acquired pneumonia. Am J Respir Crit Care Med. 2003;167:593–598. doi: 10.1164/rccm.200203-258OC. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Rush WL, Travis WD, Liotta LA, Stetler-Stevenson WG, Ferrans VJ. Immunohistochemical study of matrix metalloproteinases and their tissue inhibitors in pulmonary langerhan’s cell granulomatosis. Arch Pathol Lab Med. 1997;121:930–937. [PubMed] [Google Scholar]
- Hayashi T, Stetler-Stevenson WG, Fleming M. Immunohistochemical study of metalloproteinases and their tissue inhibitors in the lungs of patients with diffuse alveolar damage and idiopathic pulmonary fibrosis. Am J Pathol. 1996;149:531–548. [PMC free article] [PubMed] [Google Scholar]
- Henson PM, Johnson RB. Tissue injury in inflammation: oxidants, proteinases and cationic proteins. J Clin Invest. 1987;79:674–699. doi: 10.1172/JCI112869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heussen C, Dowdle EB. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Analyt Biochem. 1980;102:196–202. doi: 10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
- Huber AR, Weiss SJ. Disruption of the subendothelial basement membrane during neutrophil diapedesis in an in vitro construct of a blood vessel wall. J Clin Invest. 1989;83:1122–1136. doi: 10.1172/JCI113992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KJ, Ward PA. Acute immunologic pulmonary alveolitis. J Clin Invest. 1974;54:349–357. doi: 10.1172/JCI107770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KJ, Ward PA. Role of oxygen metabolites in immune complex injury of lung. J Immunol. 1981;126:2355–2359. [PubMed] [Google Scholar]
- Kanemoto M, Hukuda S, Komiya Y, Katsuura A, Nishioka J. Immunohistochemical study of matrix metalloproteinase 3 and tissue inhibitor of metalloproteinase-1 in human intervetebral discs. Spine. 1996;21:1–8. doi: 10.1097/00007632-199601010-00001. [DOI] [PubMed] [Google Scholar]
- Mackarel AJ, Russell KJ, Brady CS, FitzGerald MX, O’Connor CM. Interleukin-8 and leukotriene-B(4), but not formylmethionyl leucyl phenylalanine, stimulate CD18-independent migration of neutrophils across human pulmonary artery endothelial cells in vitro. Am J Respir Cell Mol Biol. 2000;23:154–161. doi: 10.1165/ajrcmb.23.2.3853. [DOI] [PubMed] [Google Scholar]
- Mulligan MS, Desrochers PE, Chinnaiyan AM, Gibbs DF, Varani J, Johnson KJ, Weiss SJ. In vivo suppression of immune complex-alveolitis by secretory leukoproteinase inhibitor and tissue inhibitor of metalloproteinases-2. Proc Nat Acad Sci (USA) 1993;90:11523–1527. doi: 10.1073/pnas.90.24.11523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudgett JS, Hutchinson NI, Chartrain MA, Forsyth AJ, McDonnel J, Singel II, Bayne EK, Flanagan J, Kawka D, Shen CF, Stevens K, Chen H, Trumbauer M, Visco DM. Susceptibility of stromelysin 1-deficient mice to collagen-induced arthritis and cartilage destruction. Arthritis Rheum. 1998;41:110–121. doi: 10.1002/1529-0131(199801)41:1<110::AID-ART14>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Murphy HS, Warner RL, Bakopoulos N, Dame MK, Varani J, Ward PA. Endothelial cell determinants of susceptibility to neutrophil-mediated killing. Shock. 1999;12:111–117. doi: 10.1097/00024382-199908000-00004. [DOI] [PubMed] [Google Scholar]
- Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM. Matrix metalloproteinases: biological activity and clinical implications. J Clin Oncol. 2000;18:1135–1149. doi: 10.1200/JCO.2000.18.5.1135. [DOI] [PubMed] [Google Scholar]
- Nilhand S, Cremer A, Fluck J, Eble JA, Krieg T, Solberg S. Conraction-dependent apoptosis of normal dermal fibroblasts. J Invest Dermatol. 2001;116:686–692. doi: 10.1046/j.1523-1747.2001.01342.x. [DOI] [PubMed] [Google Scholar]
- Ohno I, Ohtani H, Nitta Y, Suzuki J, Hoshi H, Honma M, Isoyama S, Tanno Y, Tamura G, Yamauchi K, Nagura H, Shirato K. Eosinophils as a source of metalloproteinase 9 in asthmatic airway inflammation. Am J Respir Cell Molec Biol. 1997;16:212–219. doi: 10.1165/ajrcmb.16.3.9070604. [DOI] [PubMed] [Google Scholar]
- Okada Y. Localization of matrix metalloproteinase 3 (stromelysin) in osteoarthritic cartilage and synovium. Lab Invest. 1992;66:1069–1073. [PubMed] [Google Scholar]
- Okada Y, Takeuchi N, Tomita K, Nakanishi I, Nagase H. Immunolocalisation of matrix metalloproteinase 3 (stromelysin) in rheumatoid synovioblasts (B cells): correlation with rheumatoid arthritis. Ann Rheum Dis. 1989;48:645–653. doi: 10.1136/ard.48.8.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nature Reviews / Immunology. 2004;4:617–629. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- Phan SH, Gannon DE, Varani J, Ryan US, Ward PA. Xanthine oxidase activity in rat pulmonary artery endothelial cells and its alteration by activated neutrophils. Am J Path. 1989;134:1201–1212. [PMC free article] [PubMed] [Google Scholar]
- Ricou B, Nicod L, Lacraz S, Welgus HG, Suter PM, Dayer JM. Matrix metalloproteinases and TIMP in acute respiratory distress syndrome. Am J Respir Crit Care Med. 1996;154:346–352. doi: 10.1164/ajrccm.154.2.8756805. [DOI] [PubMed] [Google Scholar]
- Shapiro SD. Matrix metalloproteinase degradation of extracellular matrix: biological consequences. Current Opin Cell Biol. 1998;10:602–608. doi: 10.1016/s0955-0674(98)80035-5. [DOI] [PubMed] [Google Scholar]
- Suga M, Iyonaga K, Okamoto T, Gushima Y, Miyakawa H, Akaike T, Ando M. Characteristic elevation of matrix metalloproteinase activity in idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2000;162:1949–1956. doi: 10.1164/ajrccm.162.5.9906096. [DOI] [PubMed] [Google Scholar]
- Sweet DG, McMahon KJ, Curley AE, O’Connor CM, Halliday HL. Type I collagenases in bronchoalveolar lavage fluid from preterm babies at risk of developing chronic lung disease. Arch Dis Child Fetal Neonatal Ed. 2001;84:F168–F171. doi: 10.1136/fn.84.3.F168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian B, Lessan K, Kahm J, Kleidon J, Henke C. bi integrin regulates fibroblast viability during collagen matrix contraction through a phosphatidylinositol 3-kinase/Akt/protein kinase B signaling pathway. J Biol Chem. 2004;277:24667–24675. doi: 10.1074/jbc.M203565200. [DOI] [PubMed] [Google Scholar]
- van Meurs J, van Lent P, Holthuysen A, Lambrou D, Bayne E, Singer I, van den Berg W. Active matrix metalloproteinases are present in cartilage during immune complex-mediated arthritis: a pivotal role for stromelysin-1 in cartilage destruction. J Immunol. 1999;163:5633–5639. [PubMed] [Google Scholar]
- Varani J, Fligiel SEG, Till GO, Kunkel RG, Ryan US, Ward PA. Pulmonary endothelial cell killing by human neutrophils: Possible involvement of hydroxyl radical. Lab Invest. 1985;53:656–663. [PubMed] [Google Scholar]
- Varani J, Ginsburg I, Schuger L, Gibbs DF, Bromberg J, Johnson KJ, Ryan US, Ward PA. Endothelial cell killing by neutrophils: Synergistic interaction of oxygen products and proteases. Am J Path. 1989;135:435–438. [PMC free article] [PubMed] [Google Scholar]
- Varani J, Phan SH, Gibbs DF, Ryan US, Ward PA. H2O2-mediated cytotoxicity of rat pulmonary endothelial cells: Changes in ATP and purine products and effects of protective interventions. Lab Invest. 1990;63:683–687. [PubMed] [Google Scholar]
- Varani J, Taylor CG, Dame M, Ward PA. Human umbilical vein endothelial cell killing by activated neutrophils: Loss of sensitivity to injury is accompanied by decreased iron content during in vitro culture and is restored with exogenous iron. Lab Invest. 1992;66:708–714. [PubMed] [Google Scholar]
- Vignola A, Riccobono L, Mirabella A. Sputum: TIMP-1 to MMP-9 ratio correlates with airflow obstruction in asthma and COPD. Am J Respir Crit Care Med. 1988;158:1945–1950. doi: 10.1164/ajrccm.158.6.9803014. [DOI] [PubMed] [Google Scholar]
- Wang M, Qin X, Mudgett JS, Ferguson TA, Senior RM, Welgus HG. Matrix metalloproteinase deficiencies affect contact hypersensitivity: Stromelysin-1 deficiency prevents the response and gelatinase B deficiency prolongs the response. Proc Nat Acad Sci USA. 1999;96:6885–6889. doi: 10.1073/pnas.96.12.6885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner RL, Beltran L, Younkin E, Varani J, Johnson KJ. Role of stromelysin 1 and gelatinase B in experimental lung injury. Amer J Resp Cell Molec Biol. 2001;24:1–8. doi: 10.1165/ajrcmb.24.5.4160. [DOI] [PubMed] [Google Scholar]


