Abstract
Sperm cryopreservation offers potential for long-term storage of genetic resources. However, the current protocols for zebrafish Danio rerio are cumbersome and poorly reproducible. Our objective was to facilitate adoption of cryopreservation by streamlining methods from sperm collection through thawing and use. First, sperm activation was evaluated, and motility was completely inhibited when osmolality of the extender was ≥ 295 to 300 mOsmol/kg. To evaluate cryoprotectant toxicity, sperm were incubated with dimethyl sulfoxide (DMSO), N, N-dimethyl acetamide (DMA), methanol, or glycerol at 5, 10, and 15% concentrations. Based on motility, DMSO, DMA, and methanol (≤ 10%) were less toxic; therefore, sperm were cryopreserved using these cryoprotectants at cooling rates of 10 and 20 °C/min. The highest motility (mean ± SD) (35 ± 23%; P ≤ 0.0001) and fertility (13 ± 8%; P ≤ 0.001) in thawed sperm were obtained with the combination of 8% methanol and a cooling rate of 10 °C/min. Further evaluations of 8% methanol and 10 °C/min were performed with males from populations with high (2.05 ± 0.24) and low (1.18 ± 0.12) body condition (P = 0.0001). Motility of thawed sperm from the two populations was 38 ± 16% and 78 ± 10% (P = 0.0001), and fertilization was 6 ± 6% and 33 ± 20% (P = 0.0001). These values were positively related with body condition factor. Overall, this study simplified and standardized sperm cryopreservation, and established a protocol using French straws as a freezing container and an extender without powdered milk. This protocol can be readily adapted for high-throughput application using automated equipment, and motility and fertility comparable to previous reports were obtained. Male variability and sperm quality remain important considerations for future work, especially in mutant and inbred lines.
Keywords: Sperm cryopreservation, In vitro fertilization, Zebrafish, Danio rerio
1. Introduction
Sperm cryopreservation has been studied in more than 200 fish species with a variety of protocols [1,2]. However, few studies have addressed aquarium fishes characterized by small body size and microliter volumes of sperm [3]. Zebrafish Danio rerio is an aquarium fish and a well-established vertebrate model for developmental, genetic, and biomedical research [4]. The average adult body size is 2 to 4 cm, and the sperm volume obtainable by squeezing is ∼1 μl. These features limit the scope of experiments and place constraints on development of procedures for sperm cryopreservation. Currently, there are three reports of protocols for zebrafish sperm cryopreservation. The first protocol was published more than 20 y ago [5], uses 10% methanol as cryoprotectant. This protocol was later adapted and used by genetics laboratories for recovery of specific strains and lines [6,7]; the fertilization level of frozen-thawed sperm was reported to be 28 to 36%. In addition to methanol, N, N-dimethyl acetamide (DMA, 10%) was an effective cryoprotectant for zebrafish sperm in another study (fertilization level of thawed sperm was 9 to 14% [8]).
In these three reports, the containers used for freezing were either glass capillary tubes or cryovials, and freezing was performed on crushed dry ice. All three procedures were complicated, and steps such as freezing rate were not quantified, making it difficult to obtain consistent results in other laboratories [8]. Also, these procedures relied on the use of a specific brand of powdered milk in the extender solution [5,7] which is not readily available worldwide, and also impedes the observation of sperm motility and morphology, especially after thawing. Therefore, further investigation was needed to simplify and standardize sperm cryopreservation, and to identify reproducible procedures and containers to provide consistent cooling and thawing profiles, as well as reliable labeling of samples.
The goals of this study were to simplify and standardize the process for zebrafish sperm cryopreservation, to develop a practical, efficient, and reproducible protocol with potential for high-throughput processing. The specific objectives were to: 1) evaluate sperm motility (percent and duration) across osmotic pressures from 26 to 603 mOsmol/kg; 2) evaluate the toxicity of four cryoprotectants; 3) evaluate the combination of selected cryoprotectants and cooling rates on sperm cryopreservation by estimating the motility and fertility; and 4) demonstrate the contributing effect of body condition factor of males on variability of motility and fertility in thawed sperm.
2. Materials and methods
2.1. Zebrafish
The zebrafish used in this study were obtained from EkkWill Waterlife Resources (Gibsonton, FL, USA) and the Zebrafish International Research Center (ZIRC; Eugene, OR, USA). Males were pigmented wild type (EkkWill), or the AB wild-type line (ZIRC). The fish were maintained in aquaria (1 fish/L) with water flow recirculated through an upwelling bead filter at 28 °C, and fed twice daily with commercial flakes (Aquatic Eco-system, Apopka, FL, USA) and live Artemia larvae grown from cyst (INVE Group; Grantsville, UT, USA). The filter system was back-flushed weekly. The photoperiod was set at 14 h light:10 h dark. Guidelines from the Institutional Animal Care and Use Committees of Louisiana State University were followed for animal care in this study.
2.2. Sperm collection
Sperm were collected by crushing of dissected testis. Briefly, zebrafish were anesthetized in 0.01% tricaine methane sulfonate (MS-222, Western Chemical Inc., Ferndale, WA, USA), rinsed in fresh water, and blotted with a paper towel to dry the body. Before dissection, standard length (from snout tip to the base of the caudal fin) and body weight were measured. The testes were removed and separated from surrounding lipid tissues by use of dissecting microscope (10-x magnification), and transferred to 1.5-mL centrifuge tubes for weighing. Hanks’ balanced salt solution at an osmolality of 300 mOsmol/kg (HBSS300: 0.137 M NaCl, 5.4 mM KCl, 1.3 mM CaCl2, 1.0 mM MgSO4, 0.25 mM Na2HPO4, 0.44 mM KH2PO4, 4.2 mM NaHCO3, and 5.55 mM glucose, pH = 7.2) was used as extender unless other osmolalities were prepared for experiments by adjusting the water volume. Hereafter, HBSS with a specific osmolality such as 300 mOsmol/kg is abbreviated as “HBSS300”.
The sperm were suspended by gently crushing the testis in HBSS and sperm suspensions were held on ice until use. In our protocol development experiments (Experiments I-III), we controlled sperm numbers by use of dilution ratio (specified in each section) with HBSS300 (testis weight (mg):HBSS volume (uL)). In the protocol evaluation experiment (Experiment IV), we estimated sperm concentration for each male by duplicate hemocytometer counts to verify that the concentrations were within an effective working range (1 to 9 × 108 cells/mL) based on our previous studies.
2.3. Motility estimation
To estimate motility, 1 μL of sperm suspension was placed on a glass slide, and 20 μL of distilled water were added to activate the sperm. The motility was observed at 200-x magnification using a dark-phase microscope (Optiphot 2, Nikon Inc., Garden City, NY, USA). The motility was expressed as the percentage of sperm which moved actively in a forward direction; sperm that vibrated in place were not counted as motile. For each sample, the sperm motility was estimated at least two times with three fields observed each time.
2.4. Egg collection for in vitro fertilization
For in vitro fertilization, zebrafish eggs were collected at ZIRC by stripping. The females were anesthetized in 0.01% MS-222 , rinsed in fresh water, blotted dry on a paper towel (excess water will cause swelling of the eggs and prevent fertilization), and were compressed gently along the belly with damp (not wet) fingers in a sterile Petri dish, and returned to fresh water for recovery. Good quality eggs (yellow and translucent) were used in this study, and fresh control sperm from donor males was used to evaluate egg quality during fertilization trials.
Within 5 min after collection, eggs from one or two females were pooled and separated into two groups for in vitro fertilization with control fresh sperm collected by crushing dissected testis from several males in HBSS300 (> 75 eggs) and thawed sperm (> 100 eggs). Before fertilization, frozen sperm samples in 0.25-mL French straws were thawed in a water bath at 40 °C for 5 s, and the straw ends were clipped to release the sperm suspension onto the eggs in the Petri dish. Fresh water with a volume of seven times that of the sperm suspension was added to activate the gametes; the time of addition of the water was recorded as the fertilization time. After 30 min, the fertilized eggs were washed and placed into fresh culture medium (0.0137 M NaCl, 0.54 mM KCl, 1.3 mM CaCl2, 1.0 mM MgSO4, 0.025 mM Na2HPO4, 0.044 mM KH2PO4, and 0.42 mM NaHCO3, pH = 7.2) and placed in a 28 °C incubator. The fertilization rate was determined as the percentage of developing embryos divided by the total number of eggs. Observations were made at late blastula (sphere stage: smooth and approximately spherical shape) to the middle gastrula period (shield stage: when accumulated cells show up at one position along the germ ring) [4] at 4 to 6 h after fertilization.
2.5. Measurement of plasma osmolality
Blood was collected from the severed caudal peduncle with a heparinized capillary tube, and samples from five or six individuals were combined in a 0.5-mL microcentrifuge tube. Plasma was separated from the blood samples by centrifugation at 13,250 × g for 10 min, and a volume of 10 μL of supernatant (plasma) was collected to measure the osmolality with a vapor pressure osmometer (Model 5520, Wescor Inc., Logan, UT, USA).
2.6. Experiment I: Sperm motility in various osmolalities of HBSS
Testes from six males were individually crushed in HBSS300 with a volume of 10 times the testis weight. A graded series of HBSS dilutions of 13 osmolalities (from 26 to 603 mOsmol/kg at intervals of ∼50 mOsmol/kg) was made by mixing the HBSS at an osmolality of 900 mOsmol/kg with double-distilled water. An aliquot (2 μL) of sperm suspension were mixed with 30 μL of HBSS at each osmolality, and the motility was observed immediately by dark-phase microscopy at 200-x magnification. The percent motility and duration of motility were recorded. After the sperm stopped moving, 10 μL of the mixture were removed for measurement of the osmolality with a vapor pressure osmometer.
2.7. Experiment II: Evaluation of the toxicity of four cryoprotectants
Three replicates were produced with pooled sperm from the testes of four males in each replicate. Sperm suspensions were collected by crushing testes in HBSS300 with a volume 25 times the testis weight. Double-strength dilutions in HBSS300 of DMSO, methanol, DMA, and glycerol were mixed with the sperm suspensions to yield final concentrations of 5, 10, and 15%, and the mixtures were incubated on ice. The toxicity of these cryoprotectants was measured by monitoring sperm motility at incubation times of 5, 15, 30, 45, 60, 75, and 90 min. One aliquot of sperm suspension from each replicate was held on ice for monitoring of motility at 90 min.
2.8. Experiment III: Evaluation of motility and fertility of thawed sperm frozen with various combinations of cryoprotectants and cooling rates
Based on the results of the previous experiment, and to compare with previous publications (e.g. [5]), DMA (8%), DMSO (8%), and methanol (4, and 8%) were chosen for further study as cryoprotectants, and sperm were frozen with cooling rates of 10 and 20 °C/min. Testes from five individual males were used for sperm collection in each experimental group. After collection in HBSS300 by crushing of the testis in a volume of 25 times the testis weight, the sperm suspensions were mixed with pre-made double-strength mixtures of HBSS300 and cryoprotectants to yield final concentrations of 8% DMA, 8% DMSO, 8% methanol, and 4% methanol. During 10 min of equilibration at 4 °C with the cryoprotectants, the sperm samples (125 μL) were loaded into 250-μL French straws (IMV International, Minneapolis, MN, USA), and cooled from 5 to -80 °C at 10 or 20 °C per min in a programmable freezer (Kryo 10 series II; Planer Products, Sunbury-on-Thames, UK). The straws were transferred to liquid nitrogen for storage. After storage for 1 wk, the samples were thawed at 40 °C for 5 s. The motility was estimated within 30 s, and the fertility of thawed sperm with 8% DMA and 8% methanol were tested by in vitro fertilization (as described above) by shipping to ZIRC as coded samples for blind analysis.
2.9. Experiment IV: Evaluation of the combination of methanol (8%) with a cooling rate of 10 °C/min using males from two populations with different body condition factors
Based on the results of the previous experiment, the use of 8% methanol as cryoprotectant and a cooling rate of 10 °C/min were selected for further trials. In this experiment, individual males from Population 1 (n = 10) and Population 2 (n = 20) with different body condition factors were tested to evaluate the selected protocol. For each male, the gonadosomatic index was calculated as the percentage of testis weight to the whole body weight, and the body condition factor was calculated as the percentage of body weight (g) to the cube of the body length (cm). Sperm suspensions were collected from individual males by crushing of the testes in HBSS300, and the concentrations were estimated by use of hemocytometer counts. The range of sperm concentrations was 1.08 to 6.30 × 108 sperm/mL (mean ± SD: 2.78 ± 1.51 × 108) in Population 1, and 1.00 to 4.46 × 108 sperm/ml (2.44 ± 1.01 × 108) in Population 2. After mixing with an equal volume of HBSS-methanol (16%), and equilibrating for 10 min, the sperm samples (240 μL) were loaded in 250-μL French straws, cooled from 5 to -80 °C with a rate of 10 °C/min in the programmable freezer, and transferred into liquid nitrogen for storage. After 1 wk of storage, frozen samples were shipped to ZIRC for fertility testing by in vitro fertilization as coded samples for blind analysis. Egg quality was evaluated for each trial by use of fresh sperm (control) collected from ZIRC strain AB males. These males were not a part of this study. To standardize the values between the two trials, fertilization of thawed sperm was expressed as a percentage of the control value.
2.10. Data analysis
Data were analyzed using SYSTAT version 11 (Systat Software Inc., CA, USA). Treatment effects were tested by using either a two-sample Student’s t-test, ANOVA, or repeated-measures ANOVA. Percentage data were arcsine-transformed before analysis. The significance level was set at P < 0.05.
3. Results
3.1. Activation of sperm motility in HBSS at varioius osmolalities
The activation of sperm motility decreased as the osmotic pressure of HBSS increased over the range of 20 to 603 mOsomol/kg (Fig. 1 A). As osmotic pressure increased from 224 to 262 mOsmol/kg, the motility activation was decreased from 70 to 9%, and then decreased to < 1% at 288 mOsmol/kg. At ≥ 300 mOsmol/kg, sperm motility was completely inhibited. Plasma osmolality from blood samples was 296 ± 8 mOsmol/kg (n = 5).
Fig. 1.
The motility (top panel) and swimming duration (bottom panel) of sperm from zebrafish Danio rerio suspended in 13 osmolalites of Hanks’ balanced salt solution (HBSS). Each point represents the mean ± SD of samples from six fish.
The swimming time of activated sperm was different across these osmotic pressures (Fig. 1 B). The swimming duration of activated sperm increased from 72 to 567 s as osmotic pressure increased from 40 to 284 mOsmol/kg.
3.2. Toxicity estimation of various cryoprotectants
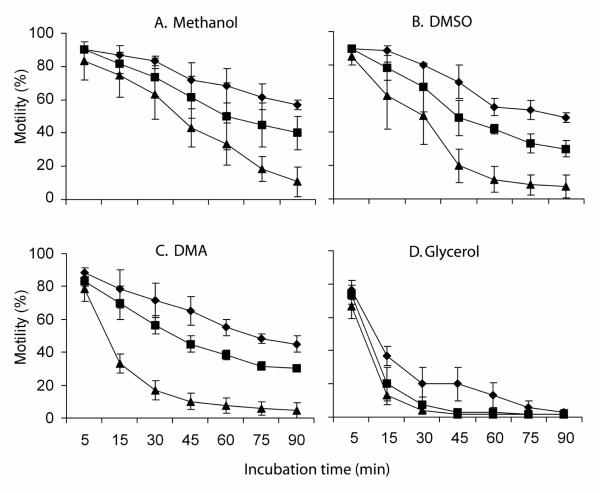
The motility of fresh sperm before incubation with cryoprectants was 90 to 95% in the three replicates. Sperm motility decreased with the concentration of cryoprotetant (5, 10, and 15%) and incubation time (5 to 90 min) for all four cryoprotectants (methanol, DMSO, DMA, and glycerol) (Fig. 2). At 15 min, motility remained above 70% at 5 and 10% of the tested cryoprotectants except for glycerol. Of the four cryoprotectants, glycerol had the greatest reduction in sperm motility; after 15 min, the motility decreased from 95% to 13 to 27% (P ≤ 0.007), and after 30 min, motility was 5 to 15% (Fig. 2 D). For the fresh sperm held without addition of cryoprotectants, the motility was unchanged (90 to 95%) after being held on ice for 90 min.
Fig. 2.
The toxicity of four different cryoprotectants, dimethyl sulfoxide (DMSO) (A), methanol (B), N, N-dimethyl acetamide (DMA) (C), and glycerol (D) at final concentrations of 5, 10, and 15% when incubated with sperm from zebrafish in Hanks’ balanced salt solution at an osmolality of 300 mOsmol/kg. Each point represents the mean ± SD of three replicates (four males in each replicate).
3.3. Motility and fertility of thawed sperm cryopreserved with various cryoprotectants at different cooling rates
Before mixing with the cryoprotectants, fresh sperm samples had similar initial motility (P ≥ 0.629). After cryopreservation with methanol (4% and 8%), DMSO (8%), and DMA (8%), the motility after thawing was significantly affected by cryoprotectant, cooling rate, and their interaction (repeated-measures ANOVA; P ≤ 0.035; Fig. 3). Post-thaw motility of sperm cryopreserved in 8% methanol was higher than that in the other cryoprotectants at a cooling rate of 10 °C/min (P ≤ 0.0001), but not at a rate of 20 °C/min, in which 8% DMA yielded the highest motility (P ≤ 0.001). When 8% methanol was used as cryoprotectant, a cooling rate of 10 °C/min yielded higher motility (35%) than did 20 °C/min (1%) (P = 0.006); and 20 °C/min yielded higher motility (13%) than did 10 °C/min (3%) when 8% DMA was used as cryoprotectant (P = 0.006).
Fig. 3.
Motility (mean ± SD) of fresh sperm before addition of cryoprotectants (white bar) and thawed sperm (black bar) from individual zebrafish (n = 5) after cryopreservation using methanol (4 and 8%), dimethyl sulfoxide (DMSO, 8%), or N, N-dimethyl acetamide (DMA, 8%) as cryoprotectant at a cooling rate of 10 °C/min (top panel) or 20 °C/min (bottom) from 5 to -80 °C.
In vitro fertilization with thawed sperm indicated that higher motility resulted in higher fertilization (Table 1). Sperm cryopreserved with 8% methanol at a cooling rate of 10 °C/min showed higher fertilization level (10 ± 8%) than that at 20 °C/min (0%) (P = 0.005), and higher than that at 10 °C/min with 8% DMA (1 ± 1%) (P = 0.031). However, fertilization of sperm cryopreserved with 8% DMA at a cooling rate of 20 °C/min (4 ± 5%) showed no difference with that of sperm cryopreserved at 10°C/min (1 ± 1%) (P = 0.589).
Table 1.
Motility and In vitro fertilization (mean ± SD; minimum-maximum) of thawed sperm from individual zebrafish Danio rerio (n = 5) after cryopreservation using N, N-dimethyl acetamide (DMA, 8%) and methanol (8%) as cryoprotectants with a cooling rate of 10 or 20°C/min from 5 to -80 °C.
| Cooling rate (°C/min) |
Sperm motility (%) |
In vitro fertilization |
|||
|---|---|---|---|---|---|
| Before freezing |
After thawing | Event success* |
Fertilization (%) |
||
| Control | -- | 90 | -- | 1 in 1 | 65 |
| DMA | 10 | 95 ± 3 (90-98) | 3 ± 2 (1-6) | 2 in 5 | 1 ± 1 (0-1) |
| 20 | 95 ± 3 (90-98) | 13 ± 6 (5-20) | 3 in 5 | 4 ± 5 (0-13) | |
| Methanol | 10 | 94 ± 4 (90-98) | 35 ± 23 (15-60) | 4 in 5 | 10 ± 8 (0-21) |
| 20 | 93 ± 5 (85-98) | 1 ± 1 (0-1) | 0 in 5 | 0 | |
Values of equal or more than 1% based on embryonic development observed at 4 to 6 hr after fertilization
3.4. Evaluation of the combination of methanol (8%) with a cooling rate of 10°C/min using males from two populations with high and low body condition factors
On average, the gonadosomatic index (GSI) from the individual males in population 1 (0.93 ± 0.09%) was lower than that of population 2 (2.32 ± 0.60%; P = 0.0001), and the same pattern was observed for the body condition factor of the males (P = 0.0001; Fig. 4). After collection, the fresh sperm from both populations had the same level of motility (91 ± 3% vs 93 ± 3%) before mixing with cryoprotectant for freezing (P = 0.067). In Population 1, the motility of thawed sperm of the 10 fish was 38 ± 16% (minimum to maximum; 10 to 60%), and the thawed sperm from these 10 males yielded fertilization (mean ± SD: 6 ± 6%) ranging from 0 to 18%. In Population 2, thawed sperm from the 20 males tested yielded an average motility of 78 ± 10% (50 to 90%), and an average fertilization of 33 ± 20 % ranging from 5% to 81% (Fig. 4). Each of these values were higher than that the corresponding values from the first population (P ≤ 0.0001). The eggs from the females used for cryopreserved sperm yielded similar fertilization levels in the two population trials (54 ± 36% vs 77 ± 19%; P = 0.1088; Fig. 4) when fertilized with control fresh sperm from ZIRC strain AB. Because no efforts were made to standardize the sperm numbers between thawed samples and the fresh samples (used only to test egg quality), no statistical comparison was made between the fertilization values of thawed and fresh samples.
Fig. 4.
Comparison of male zebrafish from Population 1 (white bars; n = 10) and Population 2 (black bars; n = 20) used to evaluate sperm cryopreservation with 8% methanol as cryoprotectant and a cooling rate of 10 °C/min. Comparison of the gonadosomatic index (GSI), body condition factor, motility of sperm before freezing (initial motility) and after thawing (thawed motility), fertility of thawed sperm (thawed fertility) expressed as percentage of fertilization produced by control sperm from ZIRC strain AB with the same eggs used for thawed sperm.
4. Discussion
An understanding of sperm activation and motility is necessary to formulate extender solutions, and to enable refrigerated storage and shipping of samples prior to cryopreservation. The motility of zebrafish sperm decreased as the osmolality of the extender solution increased, and was completely inhibited at osmolalities of 300 mOsomol/kg and above. This agreed with the plasma osmolality (296 ± 8 mOsmol/kg), and is typical for the sperm of most freshwater fishes [9-11]. Fish sperm are usually immotile in the testis [10], and motility is controlled by factors such as osmolality, ions, temperature, and pH [9,12,13]. Osmolality is the dominant factor in most species studied. In general, motility of sperm from freshwater fish is initiated by hypotonic solutions, and motility of sperm from marine fish is initiated by hypertonic solutions [10]. A third modality is where motility is initiated when osmolality is isotonic to that of the plasma, a condition that has been described for live-bearing fishes [14]. This characteristic of sperm activation control by osmolality provides opportunities to extend the working lifetime of sperm by suspension in a buffer (extender solution) with suitable osmolality, and is especially useful in sperm cryopreservation and artificial fertilization.
After activation, sperm can swim from seconds to minutes. The duration of motility varies among species, and can be affected by osmolality, temperature, and pH [9,13]. In this study, the activated sperm were found to swim longer in higher osmolalities,which agrees with observations made in other species [9]. This result could be useful in artificial fertilization if duration of motility is important for sperm-egg interaction and fertilization.
For conventional sperm cryopreservation, cryoprotectants are necessary for protection against freezing damage due to intracellular ice crystal formation. In this study, the effect of four cryoprotectants on motility was tested for pre-freeze incubation (equilibration). Glycerol is widely used as a cryoprotectant in sperm cryopreservation [15]. However, it reduced motility of zebrafish sperm within 15 min, indicating acute toxicity and a lack of suitability as a cryoprotectant. The other three cryoprotectants tested, DMSO, DMA and methanol, also showed toxicity to sperm. However, within 15 min, motility remained ∼70% when concentrations of 5 or 10% were used, which indicated sufficient time for permeation of the cryoprotection and sample processing (e.g., straw filling) before freezing. Therefore, in the subsequent freezing trial, these cryoprotectants with concentrations of <10% (i.e. 4 or 8%) were used as candidates for cryopreservation.
During freezing and thawing, sperm cells experience challenges such as formation of intracellular ice crystals, excessive dehydration and consequent concentration of solutes, or rapid osmotic swelling, leading to conditions that can be lethal. Thus, optimization of cooling rates, specific extenders, containers, and cryoprotectants are important, and this process is usually dictated by the interaction of these parameters. Previous studies of zebrafish sperm were not able to quantify the cooling rate because the freezing was performed by placement of cryovials or capillary tubes on crushed dry ice [5,7,8]. In the present study, the effect of cooling rate with three cryoprotectants was tested using a programmable freezer and standardized plastic straws. Motility and fertility of thawed sperm were used as indices for evaluation of the effects of cooling rate, cryoprotectant, and their interactions on sperm cryopreservation. The results demonstrated that different combinations of cryoprotectants and cooling rates can provide variable protection to sperm cells during cryopreservation.
Dimethyl sulfoxide is generally considered as a common and effective cryoprotectant for cryopreservation of fish sperm [16,17] and cell lines [18]. In the present study, DMSO had a limited protective effect for zebrafish sperm under the conditions tested (4 and 8% with cooling rates of 10 and 20 °C/min), resulting in low motility of thawed sperm. Methanol and DMA appeared to be suitable cryoprotectants for use with zebrafish sperm, consistent with previous reports [5,8]. However, different cooling rates were required for methanol and DMA to obtain protection for sperm during freezing.
Cooling rate is an important factor in sperm cryopreservation; it affects the osmotic balance of intracellular and extracellular solutions during freezing. When the cooling rate is slow, osmotic equilibrium is maintained, freezable water leaves the cell, and death can be caused by excessive dehydration; conversely, when the cooling rate is fast, little or no freezable water leaves the cell, and large intracellular crystals can form, causing damage to the cell. Ideally, a balanced situation allows survival when the cooling rate is fast enough to minimize the time of exposure to concentrated solutions and yet is slow enough to minimize the amount of intracellular ice below a damaging level. This balance can be determined empirically, and varies based on the cryoprotectants used and the physiology of sperm. For zebrafish sperm, the present study indicated that the combinations of 8% methanol with 10 °C/min had the best protective effects for cells during freezing when followed by rapid thawing.
In sperm cryopreservation, considerable variation in the viability of cryopreserved sperm after thawing exists among species and individuals [19], such as in boar [20], sea bream Sparus aurata [21], and Pacific oyster Crassostrea gigas [22]. In fresh sperm of mammals, birds, fishes, and insects, apparently normal samples display differential capability of fertilizing eggs [23]. In this study, cryopreserved sperm of individual males from two different populations had significantly different motility and fertility after thawing, although the sperm motility of the two populations was not different before freezing. The reasons for the variation in the susceptibility of spermatozoa to injury could lie in differences that have been identified in sperm quality such as membrane integrity and functionality, adenosine triphosphate (ATP) content, and mitochondrial functionality [21]. Recent studies suggest that there is a genetic basis for variation in thawed sperm quality, and also suggest that molecular technologies could identify markers linked to genes influencing this variation [24,25]. In the present study, gonadosomatic index and body condition factor were recorded and are suggested as indicators for evaluating zebrafish sperm quality for cryopreservation. This agrees with studies about fertility of fresh sperm which concluded that large relative testis size and sperm production capacity were linked to sperm competition ability [23,26]. However, in zebrafish further investigation is necessary to develop more effective indicators for evaluating sperm quality or genetic background in relation to cryopreservation. At present, care should be taken to ensure that males used for sperm collection are provided an ample and balanced diet to ensure good body condition. In addition, estimation of motility at collection may not be a direct indicator of sperm quality with respect to cryopreservation especially in sperm of undernourished males. Differences in males that are not observable in motility and fertility of fresh sperm may become evident after cryopreservation.
Standardization of protocols for sperm cryopreservation is necessary for reproducible results, and also helps to quantify the relationships among the multiple factors in the process of protocol development. For example, the use of French straws as a freezing container in sperm cryopreservation offers the following potential benefits: commercial availability, automated or semi-automated sample filling, permanent labeling with detailed information, choice of colors, ease of storage and retrieval, minimal space requirements, and reproducible thermal properties for efficient freezing and thawing. In addition to the choice of freezing container, other opportunities for improving standardization of cryopreservation include the control of sperm concentration, extender composition and quality, and the reporting of methods and results. Of these, control of sperm concentration is probably most important for ensuring that treatment effects and the results of various studies can be compared effectively [27].
In summary, this study simplified and standardized the conditions for zebrafish sperm cryopreservation, and provided a practical protocol which employs a standard freezing container and eliminates of the use of powdered milk which was included in all the previous protocols. With this protocol fertilization comparable to previous methods was obtained from thawed sperm. The optimized protocol is as follows: crushing of the testis in a volume of 25 to 40 times of testis weight (yielding a sperm concentration of 1 to 9 × 108 cell/mL) in HBSS with an osmolality of 300 mOsmol/kg; mixing of sperm suspension with an equal volume of pre-made 16% methanol in HBSS300 (yielding a final concentration of 8% methanol); incubating the mixture for no more than 10 min; loading 240 μL of sperm suspension into 250-μL French straws; freezing of the samples at a cooling rate of 10 °C/min from 5 °C to -80 °C; transferring of the frozen samples into liquid nitrogen for storage; thawing of the samples at 40 °C for 5 s; adding the thawed sperm onto eggs; adding water with a volume of seven times of sperm suspension to activate the gametes; changing into zebrafish embryo media (10% HBSS300), and evaluating embryonic development at 4 to 6 h after fertilization.
Future studies should address the use of stripped sperm (for non-lethal sample collection) and identification of the major factors contributing to male variability including evaluation of mutant and inbred lines. Overall, efforts should be focused on development of a high-throughput platform for cryopreservation, because at present there are thousands of zebrafish lines worldwide that require preservation (www.zfin.org). This process will require simultaneous development of database capabilities, rules and agreements for labeling and coding, standard practices for quality evaluation, and establishment of mechanisms for sample transport among locations. This would also require development of robust biosecurity provisions to address the potential for transfer of pathogens or other contaminants in frozen samples.
Acknowledgements
This work was supported in part by funding from USPHS grant P-40-RR17072 from the National Center for Research Resources, the U.S. Department of Agriculture, and the National and Louisiana Sea Grant College Programs. We thank M. Hagedorn and W. Burnside for discussion and assistance. This manuscript has been approved for publication by the Director of the Louisiana Agricultural Experiment Station as number 06-11-0383.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Tiersch TR. Introduction. In: Tiersch TR, Mazik PM, editors. Cryopreservation in Aquatic Species. World Aquaculture Society; Baton Rouge, LA: 2000. pp. xix–xxvi. [Google Scholar]
- [2].Leung LKP, Jamieson BGM. Live preservation of fish gametes. In: Jamieson BGM, editor. Fish evolution and systematics: evidence for spermatozoa. Cambridge University Press; Cambridge, UK: 1991. pp. 245–69. [Google Scholar]
- [3].Tiersch TR. Cryopreservation in aquarium fishes. Mar Biotechnol. 2001;3:S212–23. doi: 10.1007/s10126001-0044-z. [DOI] [PubMed] [Google Scholar]
- [4].Westerfield M. The Zebrafish Book, A Guide for the Laboratory Use of Zebrafish Danio rerio. University of Oregon Press; Eugene, OR: 2000. pp. 1.1–11.93. [Google Scholar]
- [5].Harvey B, Kelley RN, Ashwoodsmith MJ. Cryopreservation of zebra fish spermatozoa using methanol. Can J Zool. 1982;60:1867–70. [Google Scholar]
- [6].Walker C, Streisinger G. Freezing sperm. In: Westerfield M, editor. The Zebrafish Book, A Guide for the Laboratory Use of Zebrafish Danio rerio. University of Oregon Press; Eugene, OR: 2000. pp. 7.32–7.34. [Google Scholar]
- [7].Draper BW, McCallum CM, Scout JL, Slade AJ, Moens CB. A high-throughput method for identifying N-Ethyl-N-Nitrosourea (ENU)-induced point mutations in zebrafish. In: Detrich HW III, Westerfield M, Zon LI, editors. The zebrafish genomics, and informatics. Volume 77 of Methods in Cell Biology. Elsevier Press; San Diego: 2004. pp. 91–112. [DOI] [PubMed] [Google Scholar]
- [8].Morris JP, Berghmans S, Zahrieh D, Neuberg DS, Kanki JP, Look AT. Zebrafish sperm cryopreservation with N,N-dimethylacetamide. Biotechniques. 2003;35:956–68. doi: 10.2144/03355st03. [DOI] [PubMed] [Google Scholar]
- [9].Alavi SMH, Cosson J. Sperm motility in fishes. (II) Effects of ions and osmolality: A review. Cell Biol Int. 2006;30:1–14. doi: 10.1016/j.cellbi.2005.06.004. [DOI] [PubMed] [Google Scholar]
- [10].Morisawa M, Suzuki K. Osmolality and potassium-ion - their roles in initiation of sperm motility in teleosts. Science. 1980;210:1145–7. doi: 10.1126/science.7444445. [DOI] [PubMed] [Google Scholar]
- [11].Morisawa M, Suzuki K, Shimizu H, Morisawa S, Yasuda K. Effects of osmolality and potassium on motility of spermatozoa from fresh-water cyprinid fishes. J Exp Biol. 1983;107:95–103. doi: 10.1242/jeb.107.1.95. [DOI] [PubMed] [Google Scholar]
- [12].Cosson J. The ionic and osmotic factors controlling motility of fish spermatozoa. Aquacult Int. 2004;12:69–85. [Google Scholar]
- [13].Alavi SMH, Cosson J. Sperm motility in fishes. I. Effects of temperature and pH: a review. Cell Biol Int. 2005;29:101–10. doi: 10.1016/j.cellbi.2004.11.021. [DOI] [PubMed] [Google Scholar]
- [14].Yang H, Hazelwood L, Walter RB, Tiersch TR. Effect of osmotic immobilization on refrigerated storage and cryopreservation of sperm from a viviparous fish, the green swordtail Xiphophorus helleri. Cryobiology. 2006;52:209–18. doi: 10.1016/j.cryobiol.2005.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Royere D, Barthelemy C, Hamamah S, Lansac J. Cryopreservation of spermatozoa: A 1996 review. Hum Reprod Update. 1996;2:553–9. doi: 10.1093/humupd/2.6.553. [DOI] [PubMed] [Google Scholar]
- [16].Lahnsteiner F, Berger B, Horvath A, Urbanyi B, Weismann T. Cryopreservation of spermatozoa in cyprinid fishes. Theriogenology. 2000;54:1477–98. doi: 10.1016/s0093-691x(00)00469-6. [DOI] [PubMed] [Google Scholar]
- [17].Billard R, Cosson J, Noveiri SB, Pourkazemi M. Cryopreservation and short-term storage of sturgeon sperm, a review. Aquaculture. 2004;236:1–9. [Google Scholar]
- [18].Zhang T, Rawson DM. Studies on cryopreservation of luc gene transfected bluegill sunfish fibroblast cell line. Cryoletters. 2002;23:191–6. [PubMed] [Google Scholar]
- [19].Thurston LM, Watson PF, Holt WV. Semen cryopreservation: A genetic explanation for species and individual variation. Cryoletters. 2002;23:255–62. [PubMed] [Google Scholar]
- [20].Holt WV, Medrano A, Thurston LM, Watson PE. The significance of cooling rates and animal variability for boar sperm cryopreservation: insights from the cryomicroscope. Theriogenology. 2005;63:370–82. doi: 10.1016/j.theriogenology.2004.09.018. [DOI] [PubMed] [Google Scholar]
- [21].Cabrita E, Robles V, Cunado S, Wallace JC, Sarasquete C, Herraez MP. Evaluation of gilthead sea bream, Sparus aurata, sperm quality after cryopreservation in 5 ml macrotubes. Cryobiology. 2005;50:273–84. doi: 10.1016/j.cryobiol.2005.02.005. [DOI] [PubMed] [Google Scholar]
- [22].Dong Q, Huang C, Eudeline B, Tiersch TR. Systematic factor optimization for cryopreservation of shipped sperm samples of diploid Pacific oysters, Crassostrea gigas. Cryobiology. 2005;51:176–97. doi: 10.1016/j.cryobiol.2005.06.007. [DOI] [PubMed] [Google Scholar]
- [23].Holt WV, Van Look KJW. Concepts in sperm heterogeneity, sperm selection and sperm competition as biological foundations for laboratory tests of semen quality. Reproduction. 2004;127:527–35. doi: 10.1530/rep.1.00134. [DOI] [PubMed] [Google Scholar]
- [24].Watson PF. Recent developments and concepts in the cryopreservation of spermatozoa and the assessment of their post-thawing function. Reprod Fert Develop. 1995;7:871–91. doi: 10.1071/rd9950871. [DOI] [PubMed] [Google Scholar]
- [25].Holt WV. Basic aspects of frozen storage of semen. Anim Reprod Sci. 2000;62:3–22. doi: 10.1016/s0378-4320(00)00152-4. [DOI] [PubMed] [Google Scholar]
- [26].Harcourt AH, Harvey PH, Larson SG, Short RV. Testis weight, body-weight and breeding system in primates. Nature. 1981;293:55–7. doi: 10.1038/293055a0. [DOI] [PubMed] [Google Scholar]
- [27].Dong Q, Huang C, Tiersch TR. Control of sperm concentration is necessary for standardization of sperm cryopreservation in aquatic species: evidence from sperm agglutination in oysters. Cryobiology. 2007;54:87–98. doi: 10.1016/j.cryobiol.2006.11.007. [DOI] [PubMed] [Google Scholar]