Abstract
The COLIA1 Sp1 polymorphism has been associated with bone mineral density (BMD) and fracture. A promoter polymorphism, -1997 G/T, also has been associated with BMD. In this study, we examined whether these polymorphisms alone and in the form of haplotypes influence bone parameters and fracture risk in a large population-based cohort of elderly Caucasians. We determined the COLIA1 -1997 G/T (promoter) and Sp1 G/T (intron) polymorphisms in 6,280 individuals and inferred haplotypes. Femoral neck BMD and BMD change were compared across COLIA1 genotypes at baseline and follow-up (mean 6.5 years). We also investigated the relationship between the COLIA1 polymorphisms and incident nonvertebral fractures, which were recorded during a mean follow-up period of 7.4 years. Vertebral fractures were assessed by radiographs on 3,456 genotyped individuals. Femoral neck BMD measured at baseline was 3.8% lower in women carrying two copies of the T-Sp1 allele (P for trend = 0.03). No genotype dependent differences in BMD loss were observed. In women homozygous for the T allele of the Sp1 polymorphism, the risk of fragility fracture increased 2.3 times (95% confidence interval 1.4–3.9, P = 0.001). No such association was observed with the promoter polymorphism. In men, no association with either the Sp1 or the -1997 G/T promoter polymorphism was seen with BMD or fracture. High linkage disequilibrium (LD; D′ = 0.99, r2 = 0.03) exists between the two studied polymorphisms. We observed three haplotypes in our population: haplotype 1 (Gpromoter–Gintron) frequency (f) = 69%, haplotype 2 (Gpromoter–Tintron) f = 17.6%, and haplotype 3 (Tpromoter–Gintron) f = 13.4%. Haplotype 2 was associated with a 2.1-fold increased risk of fragility fracture in women (95% confidence interval 1.2–3.7, P = 0.001). We confirm that the COLIA1 Sp1 polymorphism influences BMD and the risk of fracture in postmenopausal Caucasian women. In contrast, we found no independent effect of the -1997 G/T promoter polymorphism on BMD or fracture.
Keywords: Bone, Osteoporosis, Bone mineral density, Fracture, COLIA1
Introduction
Osteoporosis is a multifactorial disease with both genetic and environmental determinants. It is characterized by a reduction in bone mineral density (BMD) and microarchitectural deterioration of bone tissue, which leads to an increased risk of fracture in later life [1, 2]. Being a predictor of bone fragility and susceptibility to fracture, BMD is used for the diagnosis of osteoporosis [3, 4]. The risk of fracture is dependent not only on BMD but also on geometry, architecture, material properties, and mass distribution [5].
The skeletal determinants of osteoporotic fracture risk such as BMD, bone loss, and bone geometry are all subject to strong genetic influences [2, 6, 7]. It has been estimated from twin studies that 60-80% of the variance in BMD is attributable to genetic factors [8, 9]. Several genes are thought to be involved in the pathogenesis of osteoporosis. Collagen type I alpha 1 (COLIA1) is one of the most prominent candidate genes, which has been consistently associated with osteoporosis in different populations [10–12]. COLIA1 encodes the alpha 1 chain of collagen type I, which is the most abundant structural protein in the bone matrix; rare mutations in this gene cause osteogenesis imperfecta, a mendelian disorder presenting with moderate to severe bone fragility [13, 14].
Previously, Grant et al. [15] identified a relatively common guanine to thymidine (G→T) polymorphism in the first intron of COLIA1. This polymorphism affects one of the binding sites of the transcription factor Sp1 and results in increased expression of collagen type I alpha 1 in bone matrix in T carriers [11] .We and others have shown that the T allele is associated with osteoporosis, lower BMD [10, 15], and increased fracture risk [10, 12, 16, 17]. Moreover, in a very large prospective meta-analysis of individual data, we observed that the Sp1 polymorphism in the COLIA1 gene is associated with reduced BMD and incident vertebral fractures independent of BMD [18]. In addition to the Sp1 polymorphism, Garcia-Giralt et al. [19] described two polymorphisms within the COLIA1 promoter region: -1997 G/T and -1663 indelT. The study showed in a small cohort of postmenopausal Spanish women that the T allele of the -1997 G/T polymorphism was significantly associated with a 7.5% decreased lumbar spine BMD and a 12% decreased femoral neck BMD. Furthermore, they analyzed compound genotypes including three polymorphic sites. However, this small study of the promoter polymorphisms in women investigated the relation in form of compound genotypes, was not extended to haplotypes of promoter and Sp1 polymorphisms, and most importantly, did not analyze fractures, the clinically most relevant end point of osteoporosis.
Therefore, we investigated the influence of both COLIA1 polymorphisms independently and in the form of haplotypes in relation to baseline femoral neck BMD, change in BMD with follow-up, and risk of vertebral and nonvertebral fractures in a large population-based cohort of elderly Caucasian men and women.
Materials and Methods
Study Population
This study was embedded in the Rotterdam Study, a population–based cohort study in which all residents of the Rotterdam suburb Ommoord aged 55 years and older were invited to take part. The design of the study has been described elsewhere [20]. Written informed consent was obtained from all participants, and the Medical Ethics Committee of the Erasmus Medical Center approved the study. Baseline data collection was conducted in January 1990 and June 1993, while two follow-up assessments were performed between July 1993 and January 1996 and from July 1996 until December 1999. A total of 7,983 subjects participated in the study (response rate 78%), and for the present study, we examined 6,280 individuals who were genotyped.
Study Design
This study was performed in three steps. In the first step, we performed a cross-sectional analysis where we examined the relation between the genotype and baseline BMD (n = 5,737). In the second step, we performed a longitudinal analysis to study change in BMD between baseline and the second follow-up (mean 6.5 ± standard deviation [SD] 0.6 years, n = 2,670). In the third step, we looked at the relation between COLIA1 polymorphisms and the risk of incident fracture. We studied incidence of nonvertebral fracture (mean follow-up 7.4 ± 3.3 years, n = 6,280) and vertebral fractures assessed by radiographs both at baseline (1990–1993) and at follow-up visit’ between 1997 and 1999, thoracolumbar radiographs of the spine were available for 3,469 individuals in a mean follow-up of 6.4 years.
Measurements
BMD (g/cm2) of the hip and L2-L4 of the lumbar spine were measured by dual-energy X-ray absorptiometry (DXA) using a Lunar DPX densitometry (DPX-L) (Lunar Radiation, Madison, WI) and reanalyzed with DPX-IQ software, under standard protocols. Methods, quality assurance, accuracy, and precision issues of the DXA measurements have been described previously [21]. The relative change of BMD from baseline was estimated as the difference in BMD between assessment periods divided by the BMD at baseline.
Height (cm) and weight (kg) were measured with a stadiometer at the initial examination, in standing position wearing indoor clothes without shoes. Body mass index (BMI) was computed as weight divided by height (kg/cm2).
Fracture Follow-up
Information on incident nonvertebral fractures was collected from baseline (1990–1993) until January 1, 2002 (mean follow-up ± SD 7.4 ± 3.3 years, n = 6,280).
Nonvertebral fracture events were retrieved from computerized records of the general practitioners (GPs) in the research area. Research physicians regularly followed participant information in GP records outside the research area and made an independent review, encoding all reported events. Subsequently, a medical expert in the field reviewed all coded events for final classification. We excluded fractures that were considered nonosteoporotic, i.e., caused by cancer and high trauma, including fractures of the hand, foot, skull, and face. Subsequently, we analyzed separately “fragility” fractures, which were defined as any fracture of the hip, pelvis, or proximal humerus that had occurred with minimal trauma at older age (mean >75 years).
Vertebral Fracture Assessment
Both at baseline and at first follow-up, between 1997 and 1999, thoracolumbar radiographs of the spine were obtained. The follow-up radiographs were available for 3,456 individuals, who survived an average of 6.4 (SD = 0.4) years after the baseline visit and who were still able to come to our research center. All follow-up radiographs were scored for the presence of vertebral fracture by the McCloskey/Kanis method, as described previously [22].
Genotyping
Genomic DNA was extracted from samples of peripheral venous blood according to standard procedures. Genomic DNA (1–2 ng) was dispensed into 384-well plates using a Sciclone ALH3000 pipetting robot (Caliper, Mountain View, CA). Genotypes were determined using the Taqman allelic discrimination assay. The Assay-by-Design service (http://www.appliedbio-systems.com) was used to set up a Taqman allelic discrimination assay for the COL1PR-1997 polymorphism (primers: Fw, GCCTCCGGAGGGTGTCA, Rv, AAGGAGAGCAATTCTTACAGGTGTCT; probes: FAM-CCTGAGGGATGGAA-MGB, VIC-CCTGAAGGATGGAAG-MGB. The polymerase chain reaction (PCR) mixture included 1–2 ng of genomic DNA in a 2 μL volume and the following reagents: FAM and VIC probes (200 nM), primers (0.9 μM), and 2× Taqman PCR master mix (ABgene, Epsom, UK). Reagents were dispensed in a 384-well plate using the Deerac Equator NS808 (Deerac Fluidics, Dublin, Ireland). PCR cycling was performed in 384-well PCR plates in an ABI 9700 system (Applied Biosystems, Foster City, CA) and consisted of initial denaturation for 15 minutes at 95°C, 40 cycles with denaturation of 15 seconds at 95°C, and annealing and extension for 60 seconds at 60°C. Results were analyzed by the ABI Taqman 7900HT using the sequence detection system 2.22 software (Applied Biosystems). To confirm the accuracy of genotyping results, 332 (5%) randomly selected samples were regenotyped with the same method. No inconsistencies were observed. For Sp1 (intron1) an assay was set up using Primer Express Software version 2.0 (Applied Biosystems, Foster city, CA). Forward and reverse primer sequences were 5′-GTTGTCTAGGTGCTGGAGGTT-3′ and 5′-GGCGAGGGAGGAGAGAAGG-3′. The PCR mixture included 5 ng of genomic DNA in a 4 μL volume and the following reagents: FAM-CCCGCCCACATTCCCTGG-MGB probes (250 nM), TET-CCCGCCCCCATTCCCTGG-MGB probe (500 nM), primers (300 nM), 2× Taqman PCR master mix (Applied Biosystems). PCR cycling was performed in 384-well PCR plates in the ABI 9700 PCR system and consisted of initial denaturation for 15 minutes at 95°C, 40 cycles with denaturation of 15 seconds at 95°C, and annealing and extension for 60 seconds at 60°C. Results were analyzed by the ABI Taqman 7900HT using the sequence detection system 2.1 software (Applied Biosystems). To confirm the accuracy of the genotyping results, 332 randomly selected samples were genotyped for a second time with the same method. All polymorphisms had an error rate lower than 1%.
Statistical Analysis
Hardy-Weinberg equilibrium of the COLIA1 polymorphism genotypes was tested using the GENEPOP package [23]. Linkage disequilibrium (LD) between each pair of alleles at both polymorphic loci was calculated as D′ and r2 [24].
We stratified all analyses by gender, considering peak bone mass, changes in BMD, and fractures, following age- and sex-specific patterns. Baseline BMD and BMD rate of change were compared across COLIA1 polymorphisms using univariate analysis of variance (ANOVA). Corrections were made for age and BMI. Trend analysis assuming an underlying additive genetic model was done for the presence of zero, one, or two copies of the associated allele, incorporating the genotype variable as a continuous term in a general linear regression model. For the analysis of nonvertebral fracture follow-up data, we computed the incidence rates of fracture among genotypes and used Cox’s proportional hazards model, adjusting for age and BMI to estimate risk of fracture. For vertebral fractures, odd ratios with 95% confidence intervals (95% CIs) were calculated using logistic regression models since no data on the exact time of occurrence could be determined.
We used HaploStats (available at http://www.cran.r-project.org/) to estimate the frequency of inferred haplotypes and investigate the association of haplotypes with BMD and the risk of fractures. We restricted the analysis to haplotypes with an inferred frequency of more than 0.02. The first haplotype, which was most frequent, was used as reference.
Significant P values were 0.05 or lower. Finally, model assumptions were verified and model residuals checked for goodness-of-fit. SPSS 11.0 (SPSS, Chicago, IL) was used for the analyses.
Results
Allele and genotype frequencies of the -1997 G/T and Sp1 polymorphisms were in Hardy-Weinberg equilibrium (P = 0.61 and P = 0.10, respectively). General characteristics of the study population at baseline and follow-up are shown in Table 1.
Table 1.
General characteristics of the study population at baseline and second follow-up
| Women | Men | |||
|---|---|---|---|---|
| Baseline | Second follow-up | Baseline | Second follow-up | |
| Number | n = 3,374 | n = 1,724 | n = 2,452 | n = 1,287 |
| Age (years) | 68.3 ± 8.2 | 72.7 ± 6.8 | 67.6 ± 7.7 | 72.2 ± 6.5 |
| Height (cm) | 161.1 ± 6.8 | 160.6 ± 6.4 | 174.6 ± 6.8 | 174.0 ± 6.7 |
| Weight (kg) | 69.3 ± 11.4 | 70.3 ± 12.2 | 78.2 ± 10.8 | 79.5 ± 11.3 |
| BMI (kg/m2) | 26.7 ± 4.1 | 27.2 ± 4.4 | 25.6 ± 3.0 | 26.3 ± 3.2 |
| FN-BMD (g/cm2)a | 0.83 ± 0.14 | 0.80 ± 0.13 | 0.92 ± 0.14 | 0.90 ± 0.14 |
| Lumbar spine BMD (g/cm2) | 1.03 ± 0.18 | - | 1.16 ± 0.20 | - |
| FN-BMD change | n = 1,527 | n = 1,143 | ||
| FN-BMD change (relative % of baseline year)b | −0.84 ± 1.09 | - | ||
Values are means ± SD. Anthropometric measurement based on 5,826 individuals at baseline and 3,011individuals at follow-up
aBMD measurements based on 5,737 individual at baseline and 2,670 individual at second follow-up
bFemoral neck (FN) BMD change was measured between second follow-up and baseline. Second follow-up measurements were performed on average 6.5 (SD = 0.6) years after baseline
Baseline BMD by COLIA1 Genotypes
In both genders, age, height, weight, and BMI did not differ significantly between genotypes for the -1997 G/T and Sp1 polymorphisms (data not shown). Table 2 shows the BMD values according to COLIA1 genotypes in men and women. Femoral neck BMD was 3.8% lower (mean difference = 0.03 g/cm2, P = 0.09) in women who were homozygous carriers of the Sp1 T allele compared to noncarriers, with evidence for an allele dose effect (P for trend = 0.03) (Table 2). No association was found between the -1997 promoter polymorphism and lumbar spine BMD or femoral neck BMD in men or women (Table 2). We did not observe any significant association between the Sp1 and -1997 promoter polymorphisms with changes in BMD during follow-up in men or women (Table 2).
Table 2.
BMD measurements by COLIA1 genotypes at baseline and follow-up
| Promoter -1997 G/T | Intron 1 Sp1 G/T | |||||||
|---|---|---|---|---|---|---|---|---|
| GG | GT | TT | P* | GG | GT | TT | P* | |
| Men | n = 1,663 | n = 494 | n = 37 | n = 1,484 | n = 655 | n = 55 | ||
| Femoral neck (g/cm2) | 0.92 ± 0.13 | 0.92 ± 0.14 | 0.90 ± 0.14 | 1.00 | 0.92 ± 0.14 | 0.92 ± 0.14 | 0.90 ± 0.12 | 0.45 |
| Lumbar spine (g/cm2) | 1.16 ± 0.19 | 1.17 ± 0.20 | 1.15 ± 0.22 | 0.96 | 1.17 ± 0.19 | 1.16 ± 0.20 | 1.16 ± 0.20 | 0.91 |
| Number | n = 668 | n = 233 | n = 16 | n = 606 | n = 293 | n = 18 | ||
| FN-BMD change (relative % of baseline year) | −0.46 ± 0.91 | −0.36 ± 0.93 | −0.38 ± 0.60 | 0.62 | −0.39 ± 0.93 | −0.56 ± 0.87 | −0.30 ± 0.82 | 0.01 |
| Women | n = 2,157 | n = 710 | n = 53 | n = 1,971 | n = 858 | n = 91 | ||
| Femoral neck (g/cm2) | 0.83 ± 0.14 | 0.84 ± 0.13 | 0.85 ± 0.13 | 0. 22 | 0.83 ± 0.13 | 0.82 ± 0.14 | 0.80 ± 0.14 | 0.09** |
| Lumbar spine (g/cm2) | 1.03 ± 0.18 | 1.04 ± 0.18 | 1.01 ± 0.18 | 0.69 | 1.04 ± 0.18 | 1.04 ± 0.19 | 1.01 ± 0.20 | 0.52 |
| Number | n = 820 | n = 298 | n = 24 | n = 773 | n = 340 | n = 29 | ||
| FN-BMD change (relative % of baseline year) | −0.84 ± 1.11 | −0.83 ± 1.01 | −0.78 ± 1.03 | 0.85 | −0.85 ± 1.08 | −0.80 ± 1.04 | −0.74 ± 1.49 | 0.55 |
Values are expressed as mean ± SD. Adjustments for age and BMI. Femoral neck (FN) BMD change was measured between second follow-up and baseline
*P for ANOVA. **For trend linear regression: P = 0.03
Risk of Fracture by COLIA1 Genotypes
The relation between risk of fracture and COLIA1 Sp1 polymorphism is shown in Table 3. Women with two copies of the T allele of the Sp1 polymorphism had a 2.3 times higher risk of fragility fracture (95% CI 1.4-3.9, P = 0.001). Adjustment for femoral neck BMD did not essentially modify the association. A similar association was observed in men who were homozygous carriers of the Sp1 T allele, which was borderline significant (risk ratio = 2.3, 95% CI 0.9–5.8, P = 0.07). For the -1997 promoter polymorphism no association was found with any type of fracture in either men or women (Table 3).
Table 3.
Risk of fractures by COLIA1 genotypes
| COLIA1 genotypes | Types of fracture | Event (%) | Risk ratio (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| GG | GT | TT | GG (reference) | GT vs. reference | TT vs. reference | ||
| Men | |||||||
| Promoter -1997 G/T | |||||||
| Nonvertebral | 147/1952 (7.5) | 49/592 (8.3) | 1/46 (2.2) | 1.0 (reference) | 3.18 (0.45–22.74) | 3.44 (0.47–29.94) | |
| Fragility | 72/1952 (3.7) | 14/592 (2.4) | 1/46 (1.8) | 1.0 (reference) | 1.46 (0.20–10.55) | 0.94 (0.12.7.14) | |
| Vertebral | 100/1056 (9.5) | 35/340 (10.3) | 3/28 (10.7) | 1.0 (reference) | 1.07 (0.71–1.61) | 1.23 (0.36–4.18) | |
| Intron 1 Sp1 G/T | |||||||
| Nonvertebral | 91/1254 (7.3) | 39/574 (6.8) | 4/44 (9.1) | 1.0 (reference) | 0.97 (0.71–1.33) | 1.40 (0.65–3.00) | |
| Fragility | 36/1254 (2.9) | 14/574 (2.4) | 2/44 (4.5) | 1.0 (reference) | 0.94 (0.58–1.51) | 2.34 (0.94–5.85) | |
| Vertebral | 68/719 (9.5) | 31/350 (8.9) | 2/22 (9.1) | 1.0 (reference) | 0.83 (0.55–1.24) | 1.59 (0.60–4.22) | |
| Women | |||||||
| Promoter -1997 G/T | |||||||
| Nonvertebral | 528/2721 (19.4) | 182/879 (20.7) | 8/63 (12.7) | 1.0 (reference) | 1.53 (0.76–3.09) | 1.66 (0.82–3.36) | |
| Fragility | 216/2721 (7.9) | 73/879 (8.3) | 4/63 (6.3) | 1.0 (reference) | 1.13 (0.42–3.05) | 1.19 (0.44–3.26) | |
| Vertebral | 159/1320 (12.0) | 52/445 (11.7) | 5/35 (14.3) | 1.0 (reference) | 0.98 (0.70–1.37) | 1.31 (0.49–3.45) | |
| Intron 1 Sp1 G/T | |||||||
| Nonvertebral | 279/1626 (17.2) | 121/710 (17.0) | 17/76 (22.4) | 1.0 (reference) | 1.05 (0.90–1.23) | 1.34 (0.92–1.23) | |
| Fragility | 98/1626 (6.0) | 42/710 (5.9) | 10/76 (13.2) | 1.0 (reference) | 1.01 (0.78–1.31) | 2.33 (1.39–3.87) | |
| Vertebral | 98/891 (11.0) | 51/401 (12.7) | 3/34 (8.8) | 1.0 (reference) | 1.21 (0.88–1.65) | 1.37 (0.56–3.35) | |
Adjustments for age and BMI
Haplotype Analysis
High LD exists between the -1997 G/T and Sp1 polymorphisms, as assessed by D′ measure (D′ = 0.99, r2 = 0.03). We observed in our population three -1997 G/T, Sp1 G/T haplotype alleles (Fig. 1): haplotype 1 (Gpromoter–GIntron), 69.0%; haplotype 2 (Gpromoter–TIntron), 17.6%; and haplotype 3 (Tpromoter–GIntron), 13.4%. Haplotype 4 (Tpromoter–TIntron) was not present (Fig. 1). The strong LD between the -1997 G/T and Sp1 G/T polymorphism and the virtual nonexistence of haplotype 4 suggests there is absence of ancestral recombination in the region. We observed a borderline significant association (P = 0.06) between haplotype 2 (Gpromoter–TIntron) and femoral neck BMD in women. Women who carried haplotype 2 (Gpromoter–TIntron) had lower (−0.01 mg/cm2) femoral neck BMD compared with those who carried haplotype 1 (Gpromoter–GIntron).
Fig. 1.
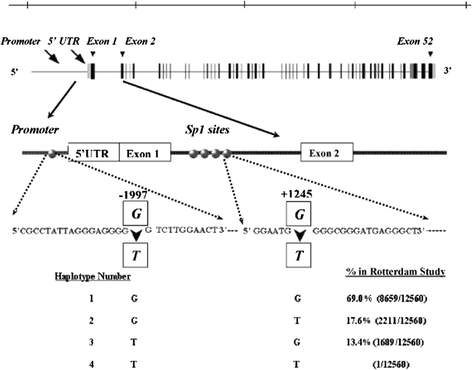
Schematic representation of the COLIA1 gene with the structural-1997 G/T polymorphism in the promoter region and G/T Sp1 polymorphism at binding site, with observed haplotype frequencies in the Rotterdam Study
The relation between risk of fracture and COLIA1 haplotypes is shown in Table 4. Women with haplotype 2 (Gpromoter–TIntron) had a 2.1 times higher relative risk of fragility fracture (P = 0.03); in men the increase in risk was 2.0 (P = 0.31). These results were essentially unchanged after adjustment for femoral neck BMD. For haplotype 3 (Tpromoter–GIntron) we found no association with any type of fracture in either men or women.
Table 4.
Risk of fracture by COLIA1 haplotypes
| Types of fracture | Men, OR (95% CI) | Women, OR (95% CI) | ||||
|---|---|---|---|---|---|---|
| Haplotype 1 | Haplotype 2 | Haplotype 3 | Haplotype 1 | Haplotype 2 | Haplotype 3 | |
| Nonvertebral | 1 (reference) | 1.29 (0.55–3.02) | 0.00 (0.00-∞) | 1 (reference) | 1.31 (0.83–2.07) | 0.61 (0.29–1.29) |
| Fragility | 1 (reference) | 2.01 (0.71–5.67) | 0.00 (0.00-∞) | 1 (reference) | 2.12 (1.23–3.66) | 0.80 (0.29–2.23) |
| Vertebral | 1 (reference) | 1.64 (0.62–4.31) | 1.19 (0.35–4.00) | 1 (reference) | 1.23 (0.51–2.95) | 1.31 (0.50–3.42) |
Adjustments for age and BMI. Haplotype 1, (Gpromoter–GIntron); haplotype 2, (Gpromoter–TIntron); haplotype 3, (Tpromoter–GIntron)
Discussion
In this large population-based study, we found that the Sp1 polymorphism influences the risk of fragility fracture in elderly women, with a similar yet not significant effect in men. Similarly, women homozygous for the T allele had 3.8% lower BMD at baseline. The -1997 G/T polymorphism showed no independent effect on fracture risk or BMD levels in both genders. The haplotype analysis showed an association with BMD and fracture in women, which appeared to be driven by the effect of the Sp1 polymorphism.
A study in Spain [19] showed that the -1997 G/T polymorphism located in the promoter region of the COLIA1 gene was associated with BMD in postmenopausal women of Spanish origin. In addition, analysis of compound genotypes of the three studied polymorphisms (-1997 G/T, Sp1, and -1663 indelT) suggested that the lowest value for BMD corresponded to GG homozygous at -1997 and heterozygous at the other two loci. Furthermore, in another report, the same group observed a possible functional mechanism for the -1997 G/T polymorphism [25]. Our population-based study suggests there is no independent effect of the -1997 polymorphism on BMD and the risk of fractures.
Recently, Stewart et al. [26] examined the three polymorphisms of the COLIA1 gene in forms of haplotypes in postmenopausal women. In contrast with our study, they observed an association between reduced BMD values and the promoter –1997G/T polymorphism. Since the promoter -1997G/T polymorphism is in strong LD with the Sp1 polymorphism, the observed association of haplotype 2 is driven by the SP1 polymorphism.
We showed that the association between fragility fractures and Sp1 polymorphism is significant only in women. We also found that the association between fragility fracture and the Sp1 polymorphism was independent of femoral neck BMD. A possible explanation for an increased risk of fracture is the different number of fractures between men and women. There are a higher number of fractures in women compared to men (13.2% in women and 4.5% in men). This suggests that other underlying biological mechanisms beyond BMD levels, such as the role of microarchitecture and composition of mineral crystals in bone tissue, might explain the increased fracture risk [11, 27, 28]. Biomechanical testing of bone samples from heterozygous individuals with the GT genotype showed reduced bone strength compared to the homozygous GG genotype and a slight reduction in mineralization of bone [11]. Presence of the T allele in the COLIA1 Sp1 binding site leads to an abnormal relative level of COLIA1/COLIA2, which may reduce bone quality and quantity [11]. Accordingly, we assume that a weaker network of abnormal collagen cross-linking may generate a three-dimensional unstable condition that may be responsible for its relatively greater risk of fragility fracture in elderly women homozygous for the Sp1 T allele. Similarly, it is likely that the Sp1 polymorphism drives these associations since evidence of functionality of this polymorphism has been reported previously.
In the Genetic Markers for Osteoporosis (GENOMOS) Study, which is the largest study examining the Sp1 polymorphism (n = 20,786) in relation to osteoporosis, an association between the Sp1 polymorphism and a 1.3 times incident risk of vertebral fractures was also observed. An effect of the -1997 promoter polymorphism due to power limitations cannot be fully excluded and should be subject to study in a larger population like that of GENOMOS.
Our present study has some limitations. Survival bias may play a role if individuals who were lost to follow-up were associated to genotype. Considering this selection bias, a possible relationship of the COLIA1 polymorphisms with changes in BMD cannot be fully excluded.
In conclusion, we observed an increased risk of fragility fractures in women carriers of the COLIA1 Sp1 T allele. In contrast, the -1997 G/T polymorphism by itself appears to have no influence on fracture or BMD in postmenopausal women, though the role of power limitations cannot be excluded.
Acknowledgement
We thank B. Basdew and W. Hugens for help in genotyping and data management. This work was supported by the Netherlands Organization for Scientific Research (014–93–015) and the European GENOMOS project (QL46-CT-2002–02629) and was part of N. Y.’s MSc Training Program in Genetic Epidemiology at the Netherlands Institute for Health Sciences.
References
- 1.Kanis JA, Melton LJ 3rd, Christiansen C, Johnston CC, Khaltaev N (1994) The diagnosis of osteoporosis. J Bone Miner Res 9:1137–1141 [DOI] [PubMed]
- 2.Cummings SR, Kelsey JL, Nevitt MC, O’Dowd KJ (1985) Epidemiology of osteoporosis and osteoporotic fractures. Epidemiol Rev 7:178–208 [DOI] [PubMed]
- 3.Hui SL, Slemenda CW, Johnston CC Jr (1988) Age and bone mass as predictors of fracture in a prospective study. J Clin Invest 81:1804–1809 [DOI] [PMC free article] [PubMed]
- 4.Nguyen T, Sambrook P, Kelly P, Jones G, Lord S, Freund J, Eisman J (1993) Prediction of osteoporotic fractures by postural instability and bone density. BMJ 307:1111–1115 [DOI] [PMC free article] [PubMed]
- 5.McCreadie BR, Goldstein SA (2000) Biomechanics of fracture: is bone mineral density sufficient to assess risk? J Bone Miner Res 15:2305–2308 [DOI] [PubMed]
- 6.Arden NK, Baker J, Hogg C, Baan K, Spector TD (1996) The heritability of bone mineral density, ultrasound of the calcaneus and hip axis length: a study of postmenopausal twins. J Bone Miner Res 11:530–534 [DOI] [PubMed]
- 7.Slemenda CW, Turner CH, Peacock M, Christian JC, Sorbel J, Hui SL, Johnston CC (1996) The genetics of proximal femur geometry, distribution of bone mass and bone mineral density. Osteoporos Int 6:178–182 [DOI] [PubMed]
- 8.Pocock NA, Eisman JA, Hopper JL, Yeates MG, Sambrook PN, Eberl S (1987) Genetic determinants of bone mass in adults. A twin study. J Clin Invest 80:706–710 [DOI] [PMC free article] [PubMed]
- 9.Gueguen R, Jouanny P, Guillemin F, Kuntz C, Pourel J, Siest G (1995) Segregation analysis and variance components analysis of bone mineral density in healthy families. J Bone Miner Res 10:2017–2022 [DOI] [PubMed]
- 10.Uitterlinden AG, Burger H, Huang Q, Yue F, McGuigan FE, Grant SF, Hofman A, van Leeuwen JP, Pols HA, Ralston SH (1998) Relation of alleles of the collagen type Ialpha1 gene to bone density and the risk of osteoporotic fractures in postmenopausal women. N Engl J Med 338:1016–1021 [DOI] [PubMed]
- 11.Mann V, Hobson EE, Li B, Stewart TL, Grant SF, Robins SP, Aspden RM, Ralston SH (2001) A COL1A1 Sp1 binding site polymorphism predisposes to osteoporotic fracture by affecting bone density and quality. J Clin Invest 107:899–907 [DOI] [PMC free article] [PubMed]
- 12.Mann V, Ralston SH (2003) Meta-analysis of COL1A1 Sp1 polymorphism in relation to bone mineral density and osteoporotic fracture. Bone 32:711–717 [DOI] [PubMed]
- 13.Byers PH (1990) Brittle bones-fragile molecules: disorders of collagen gene structure and expression. Trends Genet 6:293–300 [DOI] [PubMed]
- 14.Rauch F, Glorieux FH (2004) Osteogenesis imperfecta. Lancet 363:1377–1385 [DOI] [PubMed]
- 15.Grant SF, Reid DM, Blake G, Herd R, Fogelman I, Ralston SH (1996) Reduced bone density and osteoporosis associated with a polymorphic Sp1 binding site in the collagen type I alpha 1 gene. Nat Genet 14:203–205 [DOI] [PubMed]
- 16.Keen RW, Woodford-Richens KL, Grant SF, Ralston SH, Lanchbury JS, Spector TD (1999) Association of polymorphism at the type I collagen (COL1A1) locus with reduced bone mineral density, increased fracture risk, and increased collagen turnover. Arthritis Rheum 42:285–290 [DOI] [PubMed]
- 17.Langdahl BL, Ralston SH, Grant SF, Eriksen EF (1998) An Sp1 binding site polymorphism in the COLIA1 gene predicts osteoporotic fractures in both men and women. J Bone Miner Res 13:1384–1389 [DOI] [PubMed]
- 18.Ralston SH, Uitterlinden AG, Brandi ML, Balcells S, Langdahl BL, Lips P, Lorenc R, Obermayer-Pietsch B, Scollen S, Bustamante M, Husted LB, Carey AH, Diez-Perez A, Dunning AM, Falchetti A, Karczmarewicz E, Kruk M, van Leeuwen JP, van Meurs JB, Mangion J, McGuigan FE, Mellibovsky L, del Monte F, Pols HA, Reeve J, Reid DM, Renner W, Rivadeneira F, van Schoor NM, Sherlock RE, Ioannidis JP (2006) Large-scale evidence for the effect of the COLIA1 Sp1 polymorphism on osteoporosis outcomes: the GENOMOS study. PLoS Med 3:e90 [DOI] [PMC free article] [PubMed]
- 19.Garcia-Giralt N, Nogues X, Enjuanes A, Puig J, Mellibovsky L, Bay-Jensen A, Carreras R, Balcells S, Diez-Perez A, Grinberg D (2002) Two new single-nucleotide polymorphisms in the COL1A1 upstream regulatory region and their relationship to bone mineral density. J Bone Miner Res 17:384–393 [DOI] [PubMed]
- 20.Hofman A, Grobbee DE, de Jong PT, van den Ouweland FA (1991) Determinants of disease and disability in the elderly: the Rotterdam Elderly Study. Eur J Epidemiol 7:403–422 [DOI] [PubMed]
- 21.Burger H, van Daele PL, Algra D, van den Ouweland FA, Grobbee DE, Hofman A, van Kuijk C, Schutte HE, Birkenhager JC, Pols HA (1994) The association between age and bone mineral density in men and women aged 55 years and over: the Rotterdam Study. Bone Miner 25:1–13 [DOI] [PubMed]
- 22.McCloskey EV, Spector TD, Eyres KS, Fern ED, O’Rourke N, Vasikaran S, Kanis JA (1993) The assessment of vertebral deformity: a method for use in population studies and clinical trials. Osteoporos Int 3:138–147 [DOI] [PubMed]
- 23.Raymond MRF (1995) GENEPOP population genetics. Software for exact tests and ecumenicism. Heredity 86:248–249
- 24.Stephens M, Smith NJ, Donnelly P (2001) A new statistical method for haplotype reconstruction from population data. Am J Hum Genet 68:978–989 [DOI] [PMC free article] [PubMed]
- 25.Garcia-Giralt N, Enjuanes A, Bustamante M, Mellibovsky L, Nogues X, Carreras R, Diez-Perez A, Grinberg D, Balcells S (2005) In vitro functional assay of alleles and haplotypes of two COL1A1-promoter SNPs. Bone 36:902–908 [DOI] [PubMed]
- 26.Stewart TL, Jin H, McGuigan FE, Albagha OM, Garcia-Giralt N, Bassiti A, Grinberg D, Balcells S, Reid DM, Ralston SH (2006) Haplotypes defined by promoter and intron 1 polymorphisms of the COLIA1 gene regulate bone mineral density in women. J Clin Endocrinol Metab 91:3575–3583 [DOI] [PubMed]
- 27.Looker AC, Beck TJ (2004) Maternal history of osteoporosis and femur geometry. Calcif Tissue Int 75:277–285 [DOI] [PubMed]
- 28.Genant HK, Block JE, Steiger P, Glueer CC, Smith R (1987) Quantitative computed tomography in assessment of osteoporosis. Semin Nucl Med 17:316–333 [DOI] [PubMed]


