Abstract
Little is known about the natural history of drug dependence. This article describes the development and predictors of DSM-IV nicotine dependence in adolescence when tobacco use is initiated. In a two-stage design, a survey was administered to 6th-10th graders in the Chicago Public Schools to select a cohort of adolescents. Household interviews were conducted with adolescents five times and with one parent (predominantly mothers) three times over two years. The analytical sample includes 353 youths, who started using tobacco within 12 months preceding Wave 1 or between Waves 1-5. Survival analysis estimated latency to individual DSM-IV nicotine dependence criteria and the full dependence syndrome. Twenty-five percent of youths experienced the syndrome within 23 months of tobacco use onset. Tolerance, impaired control and withdrawal were experienced most frequently. Youths who experienced full dependence experienced their first symptom faster after tobacco use onset than those who experienced only one criterion through the end of the observation period. Cox proportional hazards models estimated the importance of time-constant and time-varying sociodemographic, tobacco and other drug use, parental and peer smoking, social psychological and biological risk factors for experiencing the first criterion and the full syndrome. Pleasant initial sensitivity to tobacco and number of cigarettes smoked the prior month predicted both outcomes. Parental dependence predicted the full syndrome. Significant covariates were generally the same across gender and racial/ethnic subgroups. The predictive significance of the initial smoking experience and parental dependence highlight the potential importance of genetic factors in the etiology of nicotine dependence.
1. Introduction
Most of our knowledge about substance dependence derives from clinical studies of individuals in treatment or epidemiological studies of the general adult population. The majority are cross-sectional studies in which individuals are identified much after they have become dependent. Identifying the period of risk for onset of dependence and understanding the nature of the transition from onset to dependence is crucial to the development of prevention and intervention programs. Tobacco use provides a useful model for the developmental study of the transition to drug dependence, since many drugs of abuse share the same neurobiological processes (Hyman et al., 2006). Smoking onset takes place in adolescence; initiation is mostly complete by the late teens, with 90% of smokers reporting that they first tried smoking by the age of 18; early onset is related to chronic use and nicotine dependence in adulthood; and tobacco is the most addictive of recreational substances, with the exception of heroin (Breslau et al., 1993a; Breslau and Peterson, 1996; Chabrol et al., 2000; Chassin et al., 1996; Flint and Novotny, 1998; Giovino, 1999; Giovino et al., 1995; Johnson et al., 2004; Kandel, 2003; Kandel and Chen, 2000; Kandel and Yamaguchi, 1993; Substance Abuse and Mental Health Services Administration, 2005).
Prospective longitudinal data are needed starting in adolescence to study the developmental course of nicotine dependence. Adolescents experience symptoms of nicotine dependence (Centers for Disease Control, 1994; Colby et al., 2000; DiFranza et al., 2000, 2002; Fergusson et al., 1996; Kandel et al., 2005; Karp et al., 2006; McNeill et al., 1986; Nelson and Wittchen, 1998; O'Loughlin et al., 2002, 2003, 2004; Prokhorov et al., 1998, 2001, 2005; Rojas et al., 1998; Sledjeski et al., 2007; Stanton, 1995), although crude rates of dependence are lower among adolescents than adults (Andreski and Breslau, 1993; Anthony et al., 1994; Heishman et al., 1997; Kandel, 2003; Kandel et al., 1997; Kandel and Chen, 2000). Age-specific comparisons based on cross-sectional samples document that rates increase rapidly throughout adolescence up to age 18 when they stabilize (Kandel, 2003; Nelson and Wittchen, 1998).
In contrast to cigarette smoking, relatively little research has been conducted on the natural history of nicotine dependence and its predictors. Most prospective studies have not measured dependence, have not followed youths at closely enough spaced intervals to obtain adequate data on patterns of behavior and their predictors, have not assessed the timing and latency of the transition from experimental to dependent smoking, nor have they examined subgroup differences, in particular race/ethnic differences (Chassin et al., 1996; Choi et al., 1997; Fergusson et al., 1996; McNeill, 1991; Stanton, 1995; Stanton et al., 1991). Three recent exceptions include the 3-year follow-up by DiFranza et al. (2000, 2002) in two small Massachusetts cities of 7th graders contacted every three months, the 6-year follow-up by O'Loughlin et al. (Gervais et al., 2006; Karp et al., 2005, 2006; O'Loughlin et al., 2004) in Canada with a similar design of a cohort of 7th graders contacted every three months, and the 4-year annual follow-up of 9th graders in Northern Virginia by Audrain-McGovern et al. (2004a,b, 2007). In each study, dependence was measured differently. DiFranza et al. (2000, 2002) used the HONC (Wheeler et al., 2004), O'Loughlin et al. (2002) the HONC and an ICD-10 based measure, Audrain-McGovern et al. (2007) used the mFTQ (Prokhorov et al., 1998). These studies have also focused on different aspects of the transition to nicotine dependence. DiFranza et al. (2002) examined latency from the onset of monthly smoking, defined as having smoked on two or more days within a two-month period, to the onset of the first symptom of HONC dependence while O'Loughlin and colleagues (Gervais et al., 2006) examined the latency from the onset of the first cigarette puffed to the first onset of selected ICD-10 dependence symptoms as well as full dependence. Both Karp et al. (2006) and Audrain-McGovern (2007) examined predictors of full dependence.
Latency from onset of smoking to dependence varies across studies, reflecting in part the different definitions of onset of smoking. DiFranza et al. (2002) reported that 50% of youths experienced their first HONC symptom within 54 days of the onset of monthly smoking, which occurred on average 486 days after the onset of smoking (DiFranza et al., 2002, p. 2). O'Loughlin and her colleagues (Gervais et al., 2006), who applied survival methods to determine the latency to various smoking behaviors after the first cigarette puffed, found that 25% of adolescent smokers experienced withdrawal within 11 months and full nicotine dependence within 40.6 months. The subjective experiences of “mental” and “physical” addiction were reported within a much shorter period of time, on average 2.5 months for mental addiction and 5.4 months for physical addiction.
1.1. Correlates and predictors of nicotine dependence
In 2000, in a comprehensive review of cross-sectional and longitudinal studies, Mayhew et al. (2000) concluded that very few factors have been identified as unique predictors of transitions to onset, experimental smoking, regular smoking, or dependence. Since then, several studies have examined predictors of dependence in adolescence and early adulthood. We only consider prospective studies of adolescents that restricted their analyses to samples of smokers to ensure that predictors are unique to dependence and do not characterize smoking more generally. Thus, we do not review studies where those dependent were compared to all those not dependent, including subjects who never smoked (Elkins et al., 2006; Fergusson et al., 1996; Patton et al., 2005).
Factors identified as constituting risks for nicotine dependence in adolescence, once having smoked, include sociodemographic characteristics; history and extensiveness of smoking; other substance use; exposure to smokers in the proximate social environment, i.e., parents and peers; individual characteristics, such as psychiatric disorders, delinquency, and novelty seeking; and biological factors, such as initial sensitivity to nicotine, exposure to prenatal smoking, nicotine metabolism, in addition to genetic vulnerability.
Rates of nicotine dependence are higher among whites than minorities and among females than males among adolescents (Andreski and Breslau, 1993; Breslau et al., 1994, 2001; DiFranza et al., 2002; Kandel, 2003; Kandel and Chen, 2000; Kandel et al., 1997; O'Loughin et al., 2002) and young adults (Hu et al., 2006). In a sample of young adults, higher education, school enrollment and part-time employment were associated with lower rates of lifetime dependence (Hu et al., 2006).
Extensiveness of use predicts the onset and progression of dependence, and is associated with higher rates of dependence (Audrain-McGovern et al., 2007; Kandel and Chen, 2000; Karp et al., 2006; O'Loughlin et al., 2002, 2003). Earlier age at onset of smoking a whole cigarette (Audrain-McGovern et al., 2007; Breslau et al., 1994) and a shorter latency between onset and daily smoking are associated with higher rates of lifetime dependence (Hu et al., 2006). Use of substances other than tobacco, in particular marijuana, increases the risk of nicotine dependence (Audrain-McGovern et al., 2007; Timberlake et al., 2006).
Besides extensiveness of smoking, the best documented feature of nicotine dependence is its comorbidity with psychiatric disorders, especially depressive mood, anxiety, disruptive and personality disorders (Dierker et al., 2001; DiFranza et al., 2004b; Isensee et al., 2003; O'Loughlin et al., 2002; Rojas et al., 1998; Sonntag et al., 2000). This association among adolescents replicates findings among adults (Breslau et al., 1991, 1993b, 1994, 2004; John et al., 2004; Kendler et al., 1993; Kessler, 2004). Less conventional behavior, such as delinquency, and personality factors, such as neuroticism and novelty seeking, are associated with higher rates of lifetime and current dependence (Breslau et al., 1994; Hu et al., 2006).
The association between parental smoking and child nicotine dependence is inconsistent across studies. Two studies reported that offspring had an increased risk of becoming regular smokers or nicotine dependent from adolescence to early adulthood when their mothers had ever smoked, had ever been daily smokers or dependent on nicotine (Hu et al., 2006; Lieb et al., 2003), although Lieb et al. (2003) found no association between maternal dependence with child dependence at baseline. Audrain-McGovern et al. (2007) found no association between household (parents and siblings) smoking and child dependence. In the absence of genetic assessment, parental dependence may index a genetic liability as well as a role model for smoking. As regards peers, association with smoking peers increases the risk of dependence (Audrain-McGovern et al., 2007; Hu et al., 2006).
Physiological factors also constitute important risk factors. A critical risk for nicotine dependence may derive from individual differences in sensitivity to nicotine resulting from genetic influences or intrauterine environmental exposure (Eissenberg and Balster, 2000; Madden et al., 1999; Perkins et al., 1996; Pomerleau, 1995; Pomerleau et al., 1998). The most sensitive individuals, who initially experience more positive or both more positive and negative effects, may be most likely to become dependent. They are more sensitive to the reinforcing effects of nicotine and develop tolerance more rapidly (Pomerleau et al., 1998). Several studies have now confirmed that initial pleasant smoking experiences are associated with subsequent dependence in adolescence (Audrain-McGovern et al., 2007; DiFranza et al., 2004a; Hu et al., 2006). However, DiFranza et al. (2004a) also found that the negative experience of nausea increased subsequent dependence, although other negative experiences, such as coughing and bad taste, reduced the risk of dependence. The initial sensitivity model is supported by genetic, biobehavioral human, and animal studies (Collins and Marks, 1991; Marks et al., 1991; Niaura et al., 2001; Overstreet, 1995; West and Russell, 1988).
Another risk factor may be prenatal maternal smoking, which predicts offspring nicotine dependence in adolescence (Lieb et al., 2003) and adulthood (Buka et al., 2003). Prenatal exposure may have direct effects expressed as an induced biological vulnerability to the addictive properties of nicotine (Abreu-Villace et al., 2004; Benwell et al., 1988; Collins and Marks, 1989) and indirect effects manifested through nicotine induced behavioral problems during childhood, e.g., hyperactivity, conduct disorder (Fergusson et al., 1998; Fried, 1989; Griesler et al., 1998; Milberger et al., 1996; Orlebeke et al., 1997; Richardson and Tizabi, 1994; Vaglenova et al., 2004; Wakschlag et al., 1997; Weissman et al., 1999; Williams et al., 1998). These behaviors are well-documented risk factors for delinquency and substance use (Moffitt, 1993), particularly smoking (Barkley et al., 1990; Brown et al., 1996; Kollins et al., 2005; Lynskey and Fergusson, 1995) and nicotine dependence (Breslau et al., 1993a; Storr et al., 2004). Other biological factors, such as nicotine metabolism and genetic vulnerability, which constitute risk factors for dependence are not investigated in our study and are not discussed further.
This study describes the development of nicotine dependence in adolescence at the criterion and syndrome levels as defined by DSM-IV, as well as the risk factors for the transition to dependence following onset of tobacco use. We address three questions: (1) What is the time lag between the onset of tobacco use and the onset of individual criteria and the full syndrome of nicotine dependence in adolescence? (2) Does latency vary in different gender and racial/ethnic subgroups? (3) What factors affect the rates of transition to dependence? The analyses are based on a longitudinal cohort of recent adolescent tobacco users drawn from a school sample with closely spaced assessments, measures of tobacco use and symptoms of nicotine dependence, and putative risk and protective factors. These factors cover sociodemographic, social-psychological, parental and peer smoking, and biological domains. We have also examined level of pubertal development, a factor associated with smoking that has not been examined for dependence. Early pubertal development is positively associated with earlier smoking onset and higher rates of experimental and daily smoking, particularly among females (Bratberg et al., 2005; Harrell et al., 1998; Lanza and Collins, 2002; Martin et al., 2002; Wilson et al., 1994). The restriction to an analytical sample of smokers allows for the identification of risk and protective factors specific to nicotine dependence.
We tested the following hypotheses. The rate of transition from experimental smoking to nicotine dependence in adolescence will be higher among: (1) females than males; (2) whites than minorities; (3) those with high initial sensitivity to tobacco; (4) those who smoke extensively; (5) those who use other substances; (6) those with high levels of depression, anxiety and conduct problems; (7) those with a nicotine dependent parent; (8) those exposed to prenatal maternal smoking; (9) those at higher stage of pubertal development.
2. Methods
2.1. Sample
The analyses are based on five waves of interviews with a subsample from a multi-ethnic longitudinal cohort of 1,039 6th-10th graders from the Chicago Public Schools (CPS) and one of parents, preferably their mothers. A two-stage design was implemented to select efficiently the target sample for follow-up. In Phase I (spring 2003), 15,763 students in grades 6-10 were sampled from 43 public schools in the CPS. The sample was designed to provide approximately equal numbers of adolescents among the three major ethnic groups: non-Hispanic white, non-Hispanic African American, and Hispanic. Because of the ethnic distribution in the CPS, largely Hispanic schools were excluded and schools with large numbers of non-Hispanic white students were oversampled. The resulting sample is representative of each racial/ethnic group from the CPS, except for Hispanics from largely Hispanic high schools and whites with Polish speaking parents. Schools were divided into eight segments. Students were administered a brief questionnaire through eight surveys staggered over four months; the completion rate for the survey was 83.1%. Responses were used to select a target sample of 1,236 youths: 1,106 tobacco users who reported having started to use tobacco in the prior 12 months and 130 non-tobacco users susceptible to start smoking, divided as evenly as possible among non-Hispanic whites, non-Hispanic African Americans and Hispanics. Susceptible non-smokers satisfied 2 of 3 criteria as per Pierce et al. (1996): (a) might try smoking a cigarette soon; did not answer “definitely not” to whether (b) would smoke if a friend offered them a cigarette; or (c) will be smoking cigarettes in one year. Whites and African Americans who had started to use tobacco 0-12 months earlier and Hispanics who had started 0-6 months earlier were selected with certainty; Hispanics who started 7-12 months earlier were sampled at a 25% rate, because there was a larger number of Hispanics than of other race/ethnic groups in the sample schools. The onset of tobacco use was based on a question that asked students “How long has it been since you FIRST tried or used a tobacco product?”, with the coded responses ranging from “I first tried within the last 3 months, 4-6 months ago, 7-12, 13-18, 19-24 and more than 24 months ago.” Of the tobacco users in the school survey (N=4,363), 1,623 (37.2%) reported having started to use within the last 12 months; 1,106 were selected for the target sample. Another 751 (17.2%) reported having started 13-24 months ago and 1,989 (45.6%) started more than 24 months ago. Youths who had started to use within the last 12 months were older at onset of tobacco use than those who had initiated tobacco use more than 24 months earlier and lighter users than those who initiated more than 12 months earlier. Those who started using tobacco within the last 12 months were on average 12.2 years old at onset (S.D.=2.1) compared with 12.3 years (S.D.=1.8) for those who started 13-24 months ago and 10.7 years (S.D.=2.0) for those who started more than 24 months earlier. Of these groups, 13.6%, 19.2% and 25.2%, respectively, had ever smoked more than 25 cigarettes.
In Phase II, on average 9 weeks after each school survey, 1,039 (84.1%) of 1,236 targeted youths and one parent (272 white, 343 African American, 424 Hispanic) agreed to participate in the longitudinal follow-up consisting of three annual computerized household interviews with youths and parents, each about 90 minutes long (Waves 1, 3, 5), and two short bi-annual interviews (20 minutes long) with youths six months after Waves 1 and 3 (Waves 2, 4). In 902 (86.8%) families, mothers were the participating parent (870 biological, 21 adoptive, 11 step or foster); 58 respondents were fathers; 79 were other parental figures, such as grandmothers. Data were collected from 2/03 - 10/03 at Wave 1; 8/03 - 3/04 at Wave 2; 2/04 - 10/04 at Wave 3; 8/04 - 3/05 at Wave 4; and 2/05 - 10/05 at Wave 5. The average interval between waves was 6.0-6.3 months; range=3-10 months. Completion rates at each successive wave were 96% of the Wave 1 sample (N=1000, 996, 999, 1001, Waves 2-5). Hispanics who had started to use tobacco 7-12 months earlier were given a weight of 4, since they were sampled at the rate of 25%. All Hispanic tobacco users were rescaled to the unweighted number who were interviewed.
To maximize participants' trust in the confidentiality of their responses, attempts were made to interview parent and child simultaneously by two different interviewers on the same day. At Wave 1, in 874 families, adolescents and parents were interviewed on the same day; of those, 510 were interviewed simultaneously by different interviewers. The field work was conducted by the National Opinion Research Center (NORC) at the University of Chicago.
2.2. Human subject procedures
Passive parental consent was obtained for the school survey and active consent for the household interviews; adolescent assent was obtained for the school and household interviews. The interviewers emphasized that all answers and results would be kept completely confidential and would not be communicated to anyone, including the adolescents' parents or teachers. All procedures for obtaining parental consent and youth assent were approved by the Institutional Review Boards of the New York State Psychiatric Institute, Columbia University, and of NORC.
2.3. Data collection
Annual household computer assisted personal interviews were conducted with adolescents and one parent (mothers 86.8%). Tobacco use patterns were ascertained for cigarettes, cigars, pipes, bidis, kreteks, and smokeless tobacco (chewing tobacco, snuff, dip). The adolescent interview included a tobacco use history chart that obtained detailed monthly information on patterns of smoking (i.e., number of days had smoked and the average number of cigarettes smoked per day in each month) for the 12 months preceding the Wave 1 interview and for the intervals since each prior interview at Waves 2-5. A unique component of the chart included the ascertainment of specific DSM-IV dependence symptoms on a monthly basis in the intervals between successive waves as of Wave 1. At Wave 1, respondents were asked to report the onset month of each DSM-IV symptom experienced in the prior 12 months.
2.4. Definitions of variables
2.4.1. Nicotine dependence
Nicotine dependence was measured as per the Diagnostic and Statistical Manual of Mental Disorder, fourth Edition (DSM-IV) (American Psychiatric Association, 1994), because of its clearly delineated domain structure and its extensiveness of use.1 The specific scale that we used was developed for adolescents and young adults by the Tobacco Etiology Research Network (Dierker et al., 2007; Sledjeski et al., 2007). The scale has high internal reliability and concurrent, predictive and incremental validity. In a sample of college students, Cronbach's α was .75, item-total consistency ranged from .89 to .91, and higher levels of DSM dependence predicted continued smoking, higher levels of quantity and frequency of smoking, and shorter periods of abstinence from smoking over time (Sledjeski et al., 2007). The 11-item scale measured specific symptoms, which defined the seven DSM-IV dependence criteria experienced in connection with the specific tobacco products used by respondents (See also Kandel et al., 2005). The computerized interviews personalized the questions to specify the products used by each respondent (see Appendix A). The seven criteria were tolerance, withdrawal, impaired control, unsuccessful attempts to quit, great deal of time spent using tobacco, neglect important activities, and use despite physical or psychological problems (α=.84). The question about withdrawal asked about 12 specific symptoms; 3 were sham items included to check on the reliability of respondents' answers. These 3 items and a fourth item about craving were excluded from the scoring. The withdrawal criterion was met when respondents checked at least four of the eight valid symptoms. In all, 33.3% reported one valid withdrawal symptom, 11.0% reported one sham symptom, and only 2.2% reported only a sham symptom. Full dependence was defined when at least three criteria were met within a 12-month period; onset was dated as of the onset month of the third criterion.
2.4.2. Covariates
Time-constant (TC) and time-varying (TV) variables were included in the models. Time-varying variables were measured at each wave following onset of tobacco use up to the onset of the first dependence criterion, with the exception of number of cigarettes smoked. This variable was measured on a monthly basis prior to the first dependence criterion. With the exception of two parental variables, the data were reported by adolescents.
2.4.2.1. Sociodemographic Variables
TC - Race/ethnicity: non-Hispanic white; non-Hispanic African American; and Hispanic.
TC - Gender: male; female.
2.4.2.2. Tobacco and Drug Use History
TC - Other tobacco products (smokeless tobacco, cigars, pipes, bidis, kreteks) used before cigarettes: None used before cigarettes; used before cigarettes; used only tobacco products other than cigarettes.
TC - Onset age of tobacco use: 10-13; 14-17 years. Based on birth date, and month and year used first tobacco product.
TC - Initial sensitivity to tobacco use (modified Pomerleau et al., 1998). Measured experiences associated with first tobacco use. Dizziness and rush/buzz symptoms were differentiated into pleasurable and unpleasurable experiences. Two scales averaged the scores of component items: (1) pleasant symptoms (pleasant sensations, relaxation, pleasurable dizziness, pleasurable rush or buzz (α=.77)); (2) unpleasant symptoms (unpleasant sensations, nausea, unpleasurable dizziness, unpleasurable rush or buzz, coughing, heart pounding, headache, bad taste (α=.80)). Each symptom coded 1=none to 4=intense experience.
TV - Number of cigarettes smoked the month prior to the onset of the first criterion. Calculated as the product of the number of days smoked each month (frequency) and number of cigarettes smoked on days smoked (quantity). Both variables were recoded to mid-point values before multiplication. Frequency of cigarette use: Tobacco users who had never smoked cigarettes or who did not smoke in a particular month were coded 0; smoked 1-2 days=1.5; 3-5 days=4; 6-9 days=7.5; 10-19 days=15; 20-29 days=25; 30 days=30. Daily quantity smoked on days smoked: One or two puffs=0.5; 1 cigarette=1; 2-5 cigarettes=3; 6-15 cigarettes=10; 16-25 cigarettes=20; 26-35 cigarettes=30; more than 35 cigarettes=40 per day smoked in the month.
TC - Alcohol use before tobacco use onset: 1=yes; 0=no.
TC - Marijuana use before tobacco use onset: 1=yes; 0=no.
TC - Blunt use before tobacco use onset: 1=yes; 0=no.
2.4.2.3. Smoking by Parents, Siblings and Peers
TC - Participating parent's lifetime smoking and dependence (W1): Self-reported by interviewed parent (307 mothers, 24 fathers, 22 others): never smoked cigarettes; ever smoked but never DSM-IV nicotine dependent; lifetime DSM-IV nicotine dependent.
TC - Biological mother's prenatal smoking (W1): Reported by interviewed parent: 1=yes; 0=no. A dummy variable was included for 22 missing cases.
TV - Perceived siblings' ever smoked (W1, W3): 1=sibling ever smoked cigarettes; 0=none.
TV - Perceived peer smoking (W1, W3): 1=at least one close friend currently smoked cigarettes; 0=none.
2.4.2.4. Psychosocial Variables
Measured at Wave 1 or wave prior to the first dependence criterion
TV - Depressive symptoms scale (W1-W4): Average of 12 4-point items (Gadow et al., 2002). The scale measures DSM-IV criteria for major depressive disorder and dysthymia (American Psychiatric Association, 1994) in the last 30 days. Youths rated their behaviors (trouble falling or staying asleep, trouble concentrating, tired/no energy, eating a lot, sleeping a lot, skipped meals/ate little) and feelings (grouchy/cranky, unhappy/sad, did not feel like doing anything, did not act like self, felt things never work out right, felt could not do things as well as others) in the last 30 days on a 4-point scale (0=never, 1=sometimes, 2=often, 3=very often). The suicide ideation item was removed from the original 13-item scale because of human subject concerns. Average score ranged from 0-3 (α=.87). A dummy variable indexing high depression was created for those who scored in the upper 25% of the distribution (scores >.90).
TV - Anxiety disorder diagnostic screen (W1, W3): Scored positive in the last 12 months for DSM-IV social phobia (2 symptoms) or generalized anxiety disorder (3 out of 4 symptoms) on the DISC predictive scale (DPS v4.32, Lucas et al., 2001) (α=.63).
TV - Conduct disorder diagnostic screen (W1, W3): Scored positive for DSM-IV conduct disorder on the DISC predictive scale if met at least 3 out of 8 symptoms in the last 12 months (DPS v4.32, Lucas et al., 2001) (α=.62).
TV - Novelty seeking (W1, W3): Based on Cloninger's Tridimensional Personality Questionnaire (Wills et al., 1998). Average score of nine 5-point items: 1=not at all true; 2=a little true; 3=somewhat true; 4=pretty true; 5=very true. The items were: Try things just for fun, look for something exciting, can get people to believe lies, do things based on how feel at the moment, get excited and lose control, like when people can do whatever they want, follow instincts, can stretch the truth and change interests a lot (α=0.87).
TV - Academic performance (W1, W3): Grades on last report card: 1=D or lower; 2=half C or mostly C; 3=half B or mostly B; 4=half A or mostly A. A dummy variable was included for 7 missing cases.
TV - Pubertal development (W1, W3) (Petersen et al., 1988): Average score of five items on self-reported changes on gender relevant characteristics. Items common to both genders were growth spurt in height, body hair, and skin change. Facial hair and voice change were asked of boys only; breast change and menarche were asked of girls only. Items were scored 1=no development; 2=beginning development; 3=additional development; 4=development already past (α=.77), except for menarche scored 1=no, 4=yes.
2.5. The Follow-Up Cohort
2.5.1. Participation in the follow-up household interviews
Participants (N=1,039) and non-participants (N=197) in the household interviews were compared on sociodemographic characteristics and school reports of tobacco behavior. Non-participants did not differ on age or gender; however, a higher proportion of non-Hispanic whites than non-Hispanic African Americans declined to participate. Non-participants were more likely than participants to report in school having ever smoked (92.8% vs. 85.0%, p<.01), to have smoked more extensively (11.1% versus 6.3% had ever smoked 100 or more cigarettes, p<.05), and to have met criteria for DSM-IV nicotine dependence (31.1% vs. 24.4%), although the last difference was not statistically significant.
2.5.2. Inconsistencies in reporting
There were many discrepancies between the school and household smoking reports (Griesler et al., submitted). Of the youths who had reported lifetime tobacco use in school (N=922), 213 denied at Wave 1 in the household having ever used tobacco. There were further discrepancies in the age of tobacco use onset. In the household interviews, adolescents were asked the specific date (month, year) of first use of each tobacco product ever used. Time since onset was calculated as the difference between the earliest date of use of any product and the interview date. Only 281 (39.6%) of the 709 adolescents who had reported in school having started using tobacco in the prior 12 months were identified as having started to use within the prior year based on the more precise (month/year of onset) ascertainment in the household interviews; 428 were estimated to have started to smoke more than one year before Wave 1. Of these, 75 started within 12 months prior to the school survey but more than 12 months prior to the household interview because of the time lag between the two data collections. Those who denied having used tobacco in the household interviews were, according to their school reports, younger, more likely to be African Americans, lighter smokers and less likely to meet criteria for the full DSM dependence syndrome than those who admitted use in the household. The youths (N=353), who in the household were estimated to have started using tobacco more than a year prior to the household interview even though they had been selected because they had reported in the school having started to use tobacco within the prior 12 months, were more likely to be males, African Americans, heavier smokers and to have experienced more DSM-IV dependence symptoms than those who were correctly assessed as having started using tobacco within the prior 12 months. The group (N=75) who no longer fell within the 12 month interval because of the time lag between the school and household data collections did not differ from those correctly classified. Thus, those who denied having used tobacco in the household were lighter users than those who admitted use. By contrast, those who were reclassified as having started to use more than 12 months earlier were heavier users than those who remained classified as having started within the last 12 months.
The cumulative impact of non-participation and inconsistent reporting resulted in a sample that was biased towards the exclusion of tobacco users, especially heavier users.
2.5.3. Analytical sample
The analytical sample included 353 youths, who were identified in the household at Wave 1 or after Wave 1 as having started using any tobacco product within the 12 months preceding Wave 1 (N=286) or who started to use tobacco between Waves 1 and 5 (N=67), and could time the onset of their first symptom. Nineteen additional youths could not do so and were excluded. Smoking only one or two puffs qualified cigarette smokers for inclusion in the sample. Of those who started within the prior 12 months, 43 started within the last 3 months, 76 started 4-6 months earlier, 84 started 7-9 months earlier, and 83 started 10-12 months earlier.
2.6. Statistical analysis
Detailed monthly histories through Wave 5 were available for up to 35 months. Survival analysis modeled the intervals from the onset of tobacco use to the first, second and third DSM-IV criterion, and to each of the seven specific criteria. Cox proportional hazards models estimated the association between the covariates and the transition to the first criterion and full DSM dependence (third criterion experienced within a 12-month period). For each covariate, the resulting hazard ratio represents its effect on the risk of experiencing the event for individuals with different exposures or values on the covariate. Hazard ratios below 1 denote negative effects, those above 1 denote positive effects. To facilitate comparison of effects across continuous variables, metric regression coefficients were multiplied by their standard deviations to obtain standardized regression coefficients. To evaluate gender and racial/ethnic differences in predictors, models were rerun for each subgroup separately. Significant multivariate predictors for each group were then entered as interaction terms in models estimated on the total sample.
3. Results
3.1. Descriptive analyses
At Wave 1, adolescents in the analytical sample were on average 14.0 years old (S.D.=1.3), range 11 to 17 years; 42.7% were male, 57.3% were female. The racial/ethnic distribution was non-Hispanic white (29.1%), non-Hispanic African American (26.8%) and Hispanic (44.1%). The overwhelming majority of youths had smoked cigarettes (95.7%). Other products used were cigars (36.9%), smokeless tobacco (3.9%), kreteks (3.1%), pipes (3.2%) and bidis (1.9%). The average age of tobacco use onset was 14.3 years (S.D.=1.3), range 10 to 17 years. These youths were light users. By Wave 5, 26.9% of the smokers had ever smoked less than one cigarette, 8.9% had ever smoked a whole cigarette, 18.3% had smoked 2-5 cigarettes, 11.4% 6-15, 7.4% 16-25, 7.9% 26-99 and 19.2% 100 or more cigarettes; 10.1% had ever smoked daily. In any one month, a very small percentage of smokers (4.2%) reported smoking 30 days in that month; another 3.4% reported smoking 20-29 days. Those who smoked daily smoked an average of 9.5 cigarettes per day.
By Wave 5, 52.5% of tobacco users had experienced at least one criterion of DSM-IV nicotine dependence, 39.7% two or more criteria, 26.2% three or more criteria (Table 1). Of those who had experienced one criterion, 42.8% had already done so by Wave 1; 26.7% of those who met three criteria had also done so by Wave 1. Observed full dependence rates were similar among males and females, and significantly lower among Hispanics (18.9%) than non-Hispanic African Americans (35.5%, p<.01). Tolerance and impaired control were the criteria experienced most frequently (36.9% each), followed closely by withdrawal (29.7%). The prevalences of the other criteria were much lower: 16.9% for unsuccessful attempt to quit, 13.2% for using despite negative consequences. Youths reported symptoms after a short period of cigarette smoking and some reported the symptoms although they did not smoke in the month preceding onset of symptoms. Thus, youths reported tolerance having smoked on average for 27 days after the onset of smoking; 27.2% did so after having smoked only 1 or 2 days. The percentages of smokers reporting tolerance as a function of the number of cigarettes smoked prior to the onset of tolerance (or up to the last observation point, if censored) were as follows: among those who reported smoking zero cigarettes (20.2%); less than 1 cigarette (67.4%); 1-2 cigarettes (41.2%); 3-5 (46.6%); 6-15 (78.9%); 16-29 (74.3%); 30-50 (75.9%); 51-100 (82.1%); and over 100 cigarettes (85.3%). Corresponding percentages for withdrawal were 36.8%, 62.7%, 29.2%, 36.2%, 44.9%, 75.3%, 85.5%, 67.1% and 61.0%. There are seeming inconsistencies at the very lowest levels of consumption and a sharp increase in the experience of criteria after smoking more than 3-5 cigarettes for tolerance and more than 6-15 cigarettes for withdrawal. Thereafter, rates remain at essentially the same levels for tolerance, and decline for withdrawal after 30-50 cigarettes. Rates of specific criteria were very similar across the three ethnic groups, although fewer Hispanics reported tolerance and unsuccessful efforts to quit than non-Hispanic whites and non-Hispanic African Americans. Among those with symptoms, 27.0% experienced the first criterion the same month as the onset of tobacco use, 5.8% the first month following onset, and 7.1% the second month following onset.
Table 1.
Distribution of Lifetime DSM-IV Nicotine Dependence Criteria by Wave 5 Among All New Tobacco Users, and by Gender and Race/Ethnicity (W1-W5, n=353)
| Total Sample
% |
Males
% |
Females
% |
Whites
% |
African Americans
% |
Hispanics
% |
|
|---|---|---|---|---|---|---|
| Number of DSM-IV criteria | ||||||
| Only one | 12.8 | 14.8 | 11.3 | 16.8 | 9.7 | 12.1 |
| Only two | 13.5 | 12.9 | 13.9 | 12.9 | 16.1 | 12.3 |
| Three or more | 26.2 | 21.7 | 29.6 | 28.7c,d | 35.5c | 18.9d |
| None | 47.5 | 50.6 | 45.2 | 41.6 | 38.7 | 56.7 |
| Type of DSM-IV criterion | ||||||
| Tolerance | 36.9 | 37.9 | 36.1 | 41.6c | 44.1 c | 29.4d |
| Impaired control | 36.9 | 33.7 | 39.2 | 38.6c,d | 50.5 c | 27.4d |
| Withdrawal | 29.7 | 24.0 a | 33.9b | 31.7 | 33.3 | 26.2 |
| Unsuccessful attempts to quit | 16.9 | 12.4 | 20.2 | 20.8c | 24.7 c | 9.4d |
| Use despite negative consequences | 13.2 | 8.9 a | 16.4b | 13.9 | 14.0 | 12.3 |
| Time spent using | 12.9 | 13.8 | 12.3 | 13.9 | 12.9 | 12.3 |
| Neglect activities | 5.9 | 7.2 | 5.0 | 5.9 | 8.6 | 4.2 |
| Total n | (353) | (150) | (203) | (101) | (93) | (159) |
Note: Groups with different superscripts are significantly different at p<0.05.
3.2. Survival analysis: Latency to the onset of dependence
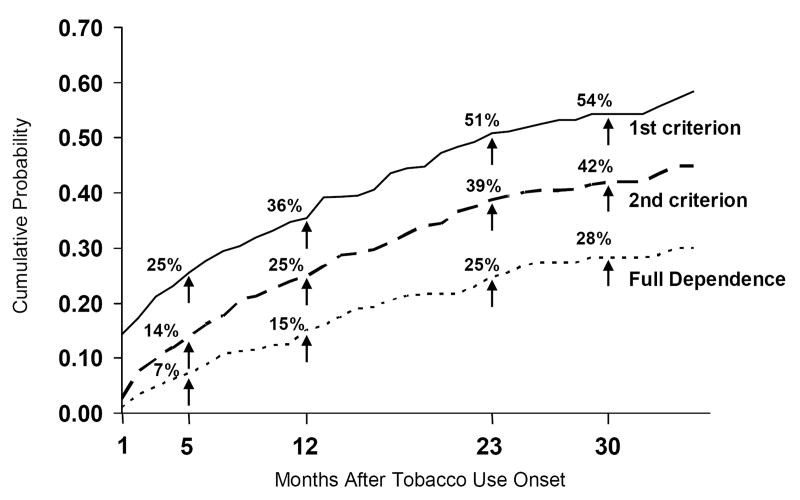
Survival analysis estimated that 25% of tobacco users would experience the first criterion within 5 months, 35.5% within 12 months, and 50.8% within 23 months of onset. Twenty-five percent would experience the second criterion within 12 months and the full dependence syndrome (three criteria) within 23 months of having started to use tobacco (Figure 1). The risk of experiencing the first DSM-IV criterion was much higher within three months after onset of smoking than at any other time period. Among those who experienced one criterion, 24.6% experienced full dependence (i.e., three criteria) within three months after the onset of the first criterion and 45.5% within 12 months. As of the 13th month, an additional 12.8% transitioned to full dependence within the next 12 months. Progression to the second and third criteria was much faster than progression to the first. Among those who had experienced three criteria, 46.5% were estimated to have experienced their first criterion within the first three months following onset of tobacco use, whereas 75.5% were estimated to have experienced a second criterion within three months of the first, and 68.0% a third criterion within three months of the second one. In addition, the latency to the first criterion from onset of tobacco use was almost twice as long among those who only experienced one criterion ( =11.2 months, S.D.=9.5 months) as among those who went on to experience three criteria ( =6.3 months, S.D.=7.6 months).
Figure 1.
Cumulative Distribution Function of the Onset of the First DSM-IV Nicotine Dependence Criterion and Full DSM-IV Dependence Syndrome After Tobacco Use Onset (New Tobacco Users W1-W5, N=353)
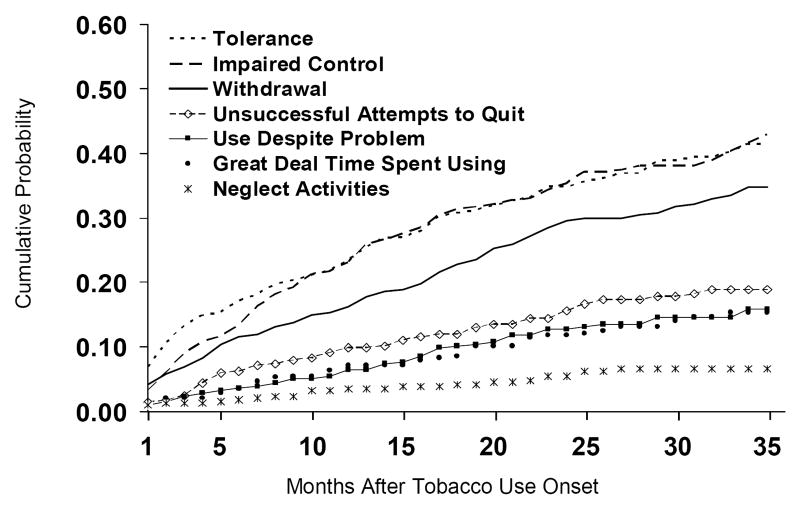
Tolerance, impaired control and withdrawal were the three criteria experienced most frequently (Figure 2). The latency period for impaired control was slightly longer than for tolerance, but the rates of transition were the same for both criteria as of the tenth month following onset of tobacco use. Throughout the observation period, tolerance was experienced at higher rates than withdrawal. Among the specific withdrawal symptoms, the most prevalent were felt frustrated, angry (22.8%), felt restless, impatient (17.2%), felt irritable (14.3%), felt sad, blue, depressed (13.3%), felt tense, anxious (12.6%), difficulty concentrating (12.2%), didn't sleep well (12.0%).
Figure 2.
Cumulative Distribution Function of the Onset of Each DSM-IV Criterion of Nicotine Dependence After First Tobacco Use After Tobacco Use Onset (New Tobacco Users W1-W5, N=353)
3.3. Covariates and predictors of transitions
Nineteen variables that measured sociodemographic characteristics, tobacco use patterns, use of other drugs, social psychological factors, and smoking by parents and peers were included in Cox proportional hazards models to evaluate correlates and predictors of the transition to the first DSM-IV dependence criterion and to the full dependence syndrome (3 criteria within a 12 month period) among new tobacco users. The distributions of the variables appear in Table 2. The results of the multivariate analysis appear in Table 3.
Table 2.
Distributions of Covariates in the Proportional Hazards Models on the First DSM-IV Nicotine Dependence Criterion and Full DSM-IV Dependence Syndrome (W1-W5 New Tobacco Users, n=353)
| Covariates | Statistics |
|---|---|
| Race/ethnicity | |
| White (%) | 29.1 |
| African American (%) | 26.8 |
| Hispanic (%) | 44.1 |
| Male (vs. female) (%) | 42.7 |
| Other tobacco use | |
| No other tobacco use before first cigarette (%) | 89.0 |
| Other tobacco use before cig (%) | 6.7 |
| Other tobacco only (%) | 4.3 |
| Onset age tobacco use >=14 (%) | 61.8 |
| Number of cigs smoked in the month prior to 1st DSM criterion (M (S.D.)) | 11.38 (39.57) |
| Alcohol use before tobacco use onset (%) | 40.1 |
| Marijuana use before tobacco use onset (%) | 20.9 |
| Blunt use before tobacco use onset (%) | 15.9 |
| Initial sensitivity to tobacco | |
| Pleasant experiences (M (S.D.)) | 1.42 (0.51) |
| Unpleasant experiences (M (S.D.)) | 1.59 (0.50) |
| Parent DSM-IV dependencea | |
| Never smoked (%) | 30.0 |
| Smoked, never dependent (%) | 48.2 |
| Ever dependent (%) | 21.8 |
| Maternal prenatal smokinga (%) | 17.3 |
| Peer smokingb (%) | 51.1 |
| Siblings smokedb (%) | 40.8 |
| Depressive symptoms (Upper 25%)c (%) | 32.1 |
| Anxiety disorder positive screenb (%) | 26.8 |
| Conduct disorder positive screenb (%) | 9.7 |
| Novelty seekingb (M (S.D.)) | 2.58 (0.77) |
| Academic performanceb (M (S.D.)) | 2.74 (0.84) |
| Pubertal developmentb (M (S.D.)) | 3.10 (0.57) |
Reported by interviewed parent.
Measured at Wave 1, or the Wave prior to the onset month of the 1st DSM-IV criterion (W3).
Measured at Wave 1, or the Wave prior to the onset month of the 1st DSM-IV criterion (W2, 3, or 4).
Table 3.
Predictors of First DSM-IV Criterion of Nicotine Dependence and Full DSM-IV Nicotine Dependence Syndrome (Proportional hazards model) (W1-W5 New Tobacco Users, n=353)a
| Predictors | A. First DSM-IV Criterion | B. Full DSM-IV Dependence Syndrome | ||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | |||||
| Hazard Ratio | 95% CI | Hazard Ratio | 95% CI | Hazard Ratio | 95% CI | Hazard Ratio | 95% CI | |
| Race/ethnicity (vs. white) | ||||||||
| African American | 1.20 | 0.82-1.74 | 1.02 | 0.69-1.51 | 1.32 | 0.81-2.16 | 1.05 | 0.61-1.82 |
| Hispanic | 0.69* | 0.49-0.98 | 0.68 * | 0.47-0.99 | 0.62 † | 0.37-1.03 | 0.66 | 0.38-1.17 |
| Male (vs. female) | 0.85 | 0.64-1.14 | 0.78 | 0.56-1.09 | 0.73 | 0.47-1.12 | 0.54 * | 0.33-0.90 |
| Other tobacco use | ||||||||
| (vs. no other tobacco use before first cig) | ||||||||
| Other tobacco use before cig | 1.99** | 1.22-3.25 | 1.34 | 0.77-2.31 | 2.14 * | 1.16-3.93 | 1.52 | 0.77-3.02 |
| Other tobacco only | 0.57 | 0.21-1.55 | 0.41 | 0.16-1.22 | 0.34 | 0.05-2.46 | 0.20 | 0.03-1.46 |
| Onset age tobacco use >=14 (vs. <14) | 1.19 | 0.88-1.59 | 1.31 | 0.85-2.02 | ||||
| N. cig smoked prior monthbc | 1.51*** | 1.37-1.66 | 1.43 *** | 1.27-1.61 | 1.49*** | 1.35-1.65 | 1.52*** | 1.33-1.75 |
| Alcohol use before tobacco use onset | 1.30† | 0.97-1.74 | 1.65 * | 1.09-2.49 | 1.54 † | 0.98-2.41 | ||
| Marijuana use before tobacco use onset | 1.46* | 1.03-2.08 | 0.80 | 0.51-1.26 | 1.90 ** | 1.21-2.98 | 1.05 | 0.61-1.82 |
| Blunt use before tobacco use onset | 2.13*** | 1.49-3.01 | 1.65 * | 1.05-2.59 | 1.32 | 0.78-2.25 | ||
| Initial sensitivity to tobacco | ||||||||
| Pleasant experiencesc | 1.60*** | 1.42-1.80 | 1.39 *** | 1.19-1.63 | 1.95*** | 1.67-2.29 | 1.73*** | 1.42-2.11 |
| Unpleasant experiencesc | 1.08 | 0.94-1.24 | 1.14 | 0.95-1.36 | ||||
| Parent DSM-IV dependenced | ||||||||
| (vs. never smoked) | ||||||||
| Smoked, never dependent | 1.06 | 0.75-1.51 | 1.06 | 0.72-1.57 | 1.73 † | 0.98-3.06 | 1.79 † | 0.97-3.28 |
| Ever dependent | 1.73** | 1.17-2.57 | 1.45 | 0.92-2.27 | 2.83 *** | 1.54-5.19 | 2.39* | 1.20-4.74 |
| Maternal prenatal smokingd | 0.89 | 0.59-1.34 | 1.00 | 0.56-1.80 | ||||
| Peer smokingbe | 1.52** | 1.13-2.04 | 1.23 | 0.90-1.69 | 1.60 * | 1.06-2.41 | 1.14 | 0.71-1.82 |
| Siblings smokedbe | 1.39* | 1.04-1.87 | 1.14 | 0.83-1.56 | 1.12 | 0.74-1.68 | ||
| Depressive symptoms (Upper 25%)bf | 1.43* | 1.08-1.94 | 1.23 | 0.79-1.60 | 1.60 * | 1.06-2.41 | 1.16 | 0.68-1.97 |
| Anxiety disorder positive screenbe | 1.26 | 0.92-1.72 | 1.60 * | 1.06-2.41 | 1.57† | 0.94-2.61 | ||
| Conduct disorder positive screenbe | 2.69*** | 1.82-3.98 | 1.43 | 0.91-2.25 | 2.34** | 1.41-3.89 | 1.08 | 0.60-1.95 |
| Novelty seekingbce | 1.30** | 1.11-1.52 | 1.11 | 0.93-1.32 | 1.21 | 0.96-1.53 | ||
| Academic performancebce | 0.89† | 0.76-1.04 | 0.89 | 0.73-1.08 | ||||
| Pubertal developmentbce | 0.98 | 0.84-1.15 | 1.02 | 0.79-1.32 | ||||
p<.10
p<.05
p<01
p<.001
Multivariate models exclude 8 cases in Model A, 4 cases in Model B because of missing data.
Time-varying before onset of first DSM criterion.
Based on standardized scores for continuous covariates.
Reported by interviewed parent.
Measured at Waves 1 or the Wave prior to the onset month of the 1st DSM-IV criterion (W3).
Measured at Waves 1, or the Wave prior to the onset month of the 1st DSM-IV criterion (W2, 3, or 4).
3.3.1. Correlates and predictors of latency to the first DSM-IV criterion
Twelve factors were statistically significant correlates or predictors of the first DSM-IV criterion at the univariate level (Table 3, Panel A). The most significant factors that increased the risk of experiencing at least one criterion were being positive on the conduct disorder screen, blunt use prior to any tobacco use, other tobacco use prior to cigarette use, number of cigarettes smoked the prior month, initial pleasant experiences upon first tobacco use, having a parent dependent on nicotine and friends who smoke. Additional significant positive correlates included novelty seeking, depressive symptoms, sibling ever smoked, and marijuana use prior to tobacco use. Being Hispanic reduced the risk and provided some protective effect. With control for other covariates, four factors remained significant: number of cigarettes smoked the month prior to the onset of the first criterion, initial pleasant experiences upon first tobacco use, and blunt use prior to tobacco use increased the risk of experiencing symptoms of dependence; being Hispanic reduced the risk. It is of interest to note that conduct disorder remained significant when both novelty seeking and initial pleasant sensitivity were removed from the multivariate model (data not presented).
3.3.2. Correlates and predictors of the full DSM-IV dependence syndrome
Ten factors were statistically significant correlates or predictors of the full DSM-IV dependence syndrome at the univariate level (Table 3, Panel B). The most significant factors were having a parent who had ever been dependent on nicotine, a positive diagnostic screen for conduct disorder, initial pleasant experiences with first tobacco use, marijuana use prior to first tobacco use, use of other tobacco products prior to cigarette use, and number of cigarettes smoked the month prior to experiencing the first criterion (of the three criteria experienced within a 12-month period). Other significant covariates included alcohol use prior to the onset of tobacco use, peer smoking, high depressive symptoms and being positive on the anxiety disorder screen. With control for all covariates, four factors were statistically significant. Factors that increased the risk were parental dependence, initial pleasant experiences, and number of cigarettes smoked the month prior to the first dependence criterion; being male became significant (negative).
Essentially the same factors were significant at the univariate level for the initial criterion and the full dependence syndrome, although the effect of pleasant experiences appeared to be stronger for full dependence than the first criterion. With control for other covariates, two factors, number of cigarettes smoked the month prior to the onset of the first criterion and initial pleasant experiences, were common predictors of the first criterion and of the full dependence syndrome. Parental dependence and being female predicted the full syndrome but not the first criterion. Blunt use before tobacco use (positive) and being Hispanic (negative) predicted onset of the first criterion but not full dependence. There were striking differences in the level of cigarette consumption preceding the first criterion among those who went on to experience only one criterion throughout the 35-month observation period (M=4.4 cigarettes during the preceding month, S.D.=13.0 cigarettes) and those who subsequently went on to experience three criteria (M=33.1 cigarettes during the preceding month, S.D.=70.3 cigarettes). In the month immediately prior to the third criterion, the mean monthly consumption increased by one third to 43.4 cigarettes per month (S.D.=79.0).
3.3.3. Gender specific predictors
To evaluate potential gender differences in predictors, the models were rerun for each gender separately. Significant multivariate predictors for each group were then entered as interaction terms in models estimated on the total sample. There were few statistically significant interactions: one interaction for the transition to the first criterion and two interactions for full dependence. For males, but not for females, using other tobacco products before the first cigarette was a significant predictor of the first criterion, and parental smoking, whether or not dependent, was a predictor of full dependence. For females only, being positive on the anxiety disorder screen was a significant risk factor of full dependence.
3.3.4. Racial/ethnic specific predictors
The same approach was used to identify race/ethnic specific predictors of dependence as was used for gender. The use of other tobacco products before cigarettes predicted the transition to the first criterion for minorities, especially African Americans. Number of cigarettes smoked prior to the first criterion was a significant predictor of full dependence for all race/ethnic groups, but the association was statistically stronger for Hispanics (Hazards ratio=2.40) than for non-Hispanic whites (Hazards ratio=1.36, difference significant at p<0.01).
4. Conclusion
We have presented novel findings regarding the development of nicotine dependence and the factors associated with the transition to dependence in adolescence, the period in the life cycle of initiation to tobacco use. A school-based sample of young adolescents, who reported in school having started to use tobacco within the prior 12 months, were interviewed in their homes five times over two years at six month intervals; one parent, predominantly mothers, was interviewed three times. More than half the sample of adolescents experienced one criterion of DSM-IV nicotine dependence; 26% met full criteria for dependence. Survival analysis estimated the rate and the latency of the onset of dependence following the onset of tobacco use. Among these young tobacco users, 21% were estimated to have experienced their first criterion within 3 months of tobacco use onset, 36% within 12 months; 25% were estimated to experience the full syndrome within 23 months. Among those who experienced three criteria, progression to the second and third criteria was faster than progression to the first criterion. The transition rates to full dependence in this sample are much faster than those reported by Gervais et al. (2006). Comparison with DiFranza et al. (2002)'s sample is not possible because latencies are presented as of the onset of monthly smoking (defined as having smoked twice in a two-month period) rather than as of the onset of any use and survival analysis was not implemented. Differences in diagnostic criteria and in the measures could explain some of the differences observed between the studies. Thus, craving was included in ICD-10 but not DSM-IV, and the items in each measure also differed somewhat. In addition, cultural differences between the U.S. and Canada might be a contributing explanatory factor, although the nature of the relevant cultural differences remains to be explored. Tolerance, impaired control and withdrawal were the three most commonly experienced criteria. It is noteworthy that these youths, who were not familiar with the addiction nomenclature, most frequently mentioned the symptoms that constitute the physiological components of addiction. That very few youths endorsed the sham withdrawal symptoms is some evidence for the validity of their answers.
However, symptoms, such as tolerance, were reported at very low levels of consumption or even when adolescents stated not smoking the month preceding onset of the symptom. Adolescents' reports of symptoms at very low levels of consumption have also been observed for nicotine by O'Loughlin et al. (2003) and for cannabis by Chen and Anthony (2003) and DiFranza et al. (2000). These seemingly anomalous patterns raise questions about the youths' potential misinterpretation of the questions about symptoms of dependence. The patterns may also be partially explained by physiological factors. McGehee and his colleagues reported that in the rat a single nicotine exposure increased dopamine levels in the mesolimbic reward system for hours (Mansvelder et al, 2003). However, the findings also challenge the definition of symptoms of dependence in a very young population of drug users. As stressed by Chen and Anthony (2003) with respect to marijuana dependence, there are age-related biases in the reporting of drug-related experiences. Early-onset cannabis users reported many more dependence problems and at much lower levels of cannabis use than late-onset users. This was not due only to greater sensitivity to the effects of cannabis, but to the over-reporting of negative experiences associated with cannabis use. In addition, early-onset cannabis smokers answered the tolerance items differently from late onset smokers. The interpretation and meaning of experiences of dependence may be subjectively different among adolescents and adults. In a personal communication (4/10/07), Anthony concludes that “[For early-onset cannabis smokers], the usual definition of a symptom (observable manifestation of an underlying pathology) clearly is not met, and this has provoked us to substitute the term ‘clinical features’ or just ‘features’ associated with cannabis dependence”. Methodological and nosological issues related to the measurement of drug dependence need to be further addressed in the field.
The present results support the hypothesis that the quality of the initial tobacco experience affects the subsequent smoking careers of smokers and provide new understanding of important aspects of that initial experience. With control for other covariates, initial pleasant sensitivity and extensiveness of smoking were among the two most important common predictors of the rapidity of the transitions to the first dependence criterion and to full dependence. Initial sensitivity was more important for the full syndrome than the first criterion. The “sensitivity” model posits that the most sensitive individuals, who initially experience both more positive and negative effects and at a lower dose, are those most likely to become dependent. These individuals are more sensitive to the reinforcing effects of nicotine, they develop tolerance more rapidly and are more likely to continue smoking than less sensitive individuals (Pomerleau, 1995; Pomerleau et al., 1998). However, it is not always clear from the theoretical discussions whether sensitivity refers to the increased experience of multiple symptoms, whether negative or positive, or only of positive symptoms. Our results clearly indicate that experiencing pleasant symptoms is the determining factor. This conclusion is strengthened by the fact that we differentiated the ambiguous items of dizziness and rush or buzz into “pleasant' and “unpleasant”. These findings confirm an increasing body of research on adolescents that suggests that pleasant experiences associated with initial tobacco use greatly accelerate the transition to nicotine dependence (Audrain-McGovern et al., 2007; DiFranza et al., 2004a; Hu et al., 2006). Extensiveness of smoking prior to the first dependence criterion appears to be a very important prognostic indicator of increasing dependence. Those who met criteria for full dependence smoked much more extensively prior to their first symptom than those whose symptoms would not increase over time. The consumption of those who would transition to full dependence increased after the first criterion.
Tolerance and dependence may partially result from pre-existing individual biobehavioral differences in sensitivity to nicotine rather than only from extensive smoking (DiFranza et al., 2004a; Pomerleau, 1995; Shiffman, 1991). This process may underlie substance dependence more generally. Indeed, positive experiences associated with early cannabis use have been found to be strong predictors of later dependence, whereas negative experiences were not (Fergusson et al., 2003). Pleasurable experiences associated with initial use of a substance may be a strong prognostic factor of later dependence on that substance. Furthermore, the use of other tobacco products prior to cigarettes and of other substances (alcohol, marijuana, and blunts) prior to tobacco predict nicotine dependence. This observation highlights the fact that the use of multiple drugs increases the addictive potential of an individual drug, in this case nicotine.
Prenatal maternal smoking and adolescent pubertal status had no zero order effects. The absence of pubertal effects may be due to the fact that most of the sample was in the later stages of puberty or that puberty affects smoking initiation but not dependence. The impact of prenatal maternal smoking may manifest itself later on in offspring smoking career. With control for other covariates, parental nicotine dependence predicted the full dependence syndrome but not the first criterion, the associations between nicotine dependence and psychological or behavioral factors, such as novelty seeking, depression and conduct problems were no longer significant either for the first criterion or the full syndrome. Most of the same factors predicted nicotine dependence across gender and race/ethnic subgroups.
Human genetic epidemiological studies and animal studies document that genetic factors contribute to smoking initiation, age of initiation, persistence of smoking and dependence, with heritability about 50% for initiation and 70% for dependence (Heath et al., 1993, 1999; Kendler et al., 1999; Vink et al., 2005). Genetic factors may be mediated by individual differences in initial sensitivity to nicotine, novelty seeking, psychopathology, in particular conduct disorder and depression, intelligence and SES (Gilbert and Gilbert, 1995; Heath and Madden, 1995; Overstreet, 1995; Perkins et al., 1996; Pomerleau, 1995). The significance of initial sensitivity and parental dependence in predicting the nicotine dependence syndrome highlight the potential importance of inferred genetic factors in the etiology of nicotine dependence.
The findings presented support to a certain extent the validity of the monthly reports, although other results document also the unreliability of the data. Thus, there is a striking difference in the reported extensiveness of smoking the month prior to the experience of the first criterion among those who subsequently reported only one criterion through the end of the observation period and those who went on to experience two additional criteria. Furthermore, average monthly cigarettes increased by one-third the month preceding the third criterion. In addition, latency between the onset of tobacco use and the first criterion was longer for those who experienced only one criterion than for those who went on to experience three criteria.
The limitations of the study must be acknowledged. Because of our sample selection criteria, factors related to non-participation of the school sample in the follow-up cohort, and inconsistencies in reports of having ever smoked (Griesler et al., submitted) and of age of tobacco use onset provided in school and in the household, the resulting sample includes lighter smokers than the target school population. The psychosocial data are not prospective for many of the subjects in the study, since a high proportion had already started to use tobacco within the twelve months preceding the initial interview and many had experienced their first dependence symptom prior to that interview. Selecting a multiethnic sample of non-tobacco users who will eventually begin to use tobacco and develop symptoms of dependence within the time frame of a relatively short follow-up requires an extremely large sample. In this study, we sampled close to 16,000 students to achieve a target sample of 1,236 cases and a participating cohort of 1,039 youths, from whom we identified the analytical sample of 353 new tobacco users. The assessments of tobacco use, symptoms of dependence and timing of various tobacco use behaviors rely on respondents' self-reports and are subject to errors of recall, interpretation, and denial. These limitations are inherent to research that must rely on self-report information provided by subjects, especially young respondents (Griesler et al., submitted). In addition, as per Dierker et al. (2007), we did not use Criterion A, “daily use of nicotine for several weeks”, to define a sample eligible for answering questions about withdrawal. This criterion assumes that daily smoking is a prerequisite for the symptom, an assumption that is probably not correct for adolescents (DiFranza et al., 2000). For instance, we found that of those defined as being nicotine dependent (as per DSM-IV) in the National Household Survey on Drug Abuse 2000 (based on NHSDA 2000, SAMHSA 2002), 14.5% among adolescents 12-17 years old but 3.6% among adults 18 and over had never smoked daily. Inclusion of Criterion A would have lowered slightly the rate of withdrawal and of dependence and might have affected the results on the timing and onset of withdrawal and dependence.
Within these limitations, a strength of the study is the assessment of DSM-IV symptoms of dependence in a community sample of adolescents who were light smokers. The data that we have presented provide unique understanding of the time after onset of tobacco use when young tobacco users are most at risk for experiencing symptoms of nicotine dependence and which young tobacco users are most at risk for becoming dependent.
Supplementary Material
Acknowledgments
This research was partially supported by research grants DA12697 from NIDA/NCI and ALF CU51672301A1 from the American Legacy Foundation (Denise Kandel, principal investigator), and a Research Scientist Award (DA00081) from the National Institute on Drug Abuse to Denise Kandel. The authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Portions of this paper were presented at the 12th Annual Meeting of the Society for Research on Nicotine and Tobacco, Orlando, Florida, February 2006. We wish to acknowledge our debt to the reviewers of Drug and Alcohol Dependence. We have benefited greatly from their valuable comments on earlier versions of the manuscript.
Appendix A. Nicotine Dependence Measure as per the Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (based on Dierker et al., 2007)
Coding for all items is 1=yes; 2=no. In some questions, 3=does not apply, was allowed. Specific tobacco product used in the last 12 months, as reported by respondent earlier in the interview, were plugged into the question.
The next questions are about some problems or experiences you may have had over the last 12 months because of [SMOKING TOBACCO PRODUCTS/SMOKING TOBACCO PRODUCTS OR CHEWING SMOKELESS TOBACCO/CHEWING SMOKELESS TOBACCO].
| A. | Over time, did you find that you could [SMOKE/SMOKE OR CHEW/CHEW] more without feeling nauseated or dizzy? (Tolerance) | |
| B. | Compared to when you first started [SMOKING/SMOKING OR CHEWING/CHEWING], did you need to [SMOKE/SMOKE OR CHEW/CHEW] more in order to feel satisfied or get the same effect? (Tolerance) | |
| C. | Did you ever have times when you stopped, cut down, or went without [SMOKING/SMOKING OR CHEWING/CHEWING] for a period of time and then experienced the following: a. A strong need or urge to [SMOKE/SMOKE OR CHEW/CHEW]; b. Felt irritable; c. Difficulty concentrating; d. Felt sad, blue or depressed; e. Felt frustrated or angry; f. Muscle aches*; g. Felt restlessness or impatient; h. Increased appetite or weight gain; i. Increased heart rate*; j. Nausea or vomiting*; k. Felt tense or anxious; l. Didn't sleep well. [As per DSM-IV, item a was not used in the definition of withdrawal.] * Sham items. | |
| D. | Did you ever have times when you [SMOKED/SMOKED OR CHEWED/CHEWED] to keep from feeling bad? (Withdrawal) | |
| E. | Did you ever have times when you [SMOKED/SMOKED OR CHEWED/CHEWED], even though you promised yourself you wouldn't? (Impaired control) | |
| F. | Were there ever times when you [SMOKED/SMOKED OR CHEWED/CHEWED] more frequently or for more days in a row than you intended? (Impaired control) | |
| G. | Were there times when you tried to stop or cut down on your [SMOKING/SMOKING OR CHEWING/CHEWING] and found that you were not able to do so? (Unsuccessful attempts to quit) | |
| H. | Did you ever have periods of several days or more when you [CHAIN-SMOKED, THAT IS, STARTED ANOTHER [DISPLAY PRODUCTS SMOKED]/CHAIN-SMOKED, THAT IS, STARTED ANOTHER [DISPLAY PRODUCTS SMOKED], OR STARTED ANOTHER CHEW/STARTED ANOTHER CHEW] as soon as you had finished one? (Great deal of time spent using) | |
| I. | Did you ever have a period of a month or longer when you gave up or greatly reduced important activities—like sports, school, work, or spending time with friends and family so you could [SMOKE/SMOKE OR CHEW/CHEW]? (Neglect activities) | |
| J. | Did your tobacco use ever cause you any physical problems like coughing, difficulty breathing, lung trouble or problems with your heart or blood pressure? (Use despite negative consequences) - (Screen item for J.a) | |
| J.a | Did you continue to [SMOKE/SMOKE OR CHEW/CHEW] even though you knew that [SMOKING/SMOKING OR CHEWING/CHEWING] was causing you physical problems or making them worse? (Use despite negative consequences) | |
| K. | Did your tobacco use ever cause you any emotional problems like irritability, nervousness, restlessness, difficulty concentrating, or depression? (Use despite negative consequences) - (Screen item for K.a) | |
| K.a | Did you continue to [SMOKE/SMOKE OR CHEW/CHEW] even though you knew that [SMOKING/SMOKING OR CHEWING/CHEWING] was causing you emotional problems or making them worse? (Use despite negative consequences) |
Footnotes
Additional background for this report is provided as Supplementary Material by accessing the online version of this paper at http://dx.doi.org by entering doi:xxxxxxx.
Additional background for this report is provided as Supplementary Material by accessing the online version of this paper at http://dx.doi.org by entering doi:xxxxxxx.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abreu-Villaca Y, Seidler FJ, Tate CA, Cousins MM, Slotkin TA. Prenatal nicotine exposure alters the response to nicotine administration in adolescence: Effects on cholinergic systems during exposure and withdrawal. Neuropsychopharmacology. 2004;29:879–890. doi: 10.1038/sj.npp.1300401. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Association; Washington: 1994. [Google Scholar]
- Andreski P, Breslau N. Smoking and nicotine dependence in young adults: Differences between blacks and whites. Drug Alcohol Depend. 1993;32:119–125. doi: 10.1016/0376-8716(93)80004-x. [DOI] [PubMed] [Google Scholar]
- Anthony JC, Warner LA, Kessler RC. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances and inhalants: Basic findings from the National Comorbidity Survey. Exp Clin Psychopharmacol. 1994;2:244–268. [Google Scholar]
- Audrain-McGovern J, Koudsi NA, Rodriguez D, Wileyto EP, Shields PG, Tyndale RF. The role of CYP2A6 in the emergence of nicotine dependence in adolescents. Pediatrics. 2007;119:e264–e274. doi: 10.1542/peds.2006-1583. [DOI] [PubMed] [Google Scholar]
- Audrain-McGovern J, Lerman C, Wileyto EP, Rodriguez D, Shields PG. Interacting effects of genetic predisposition and depression on adolescent smoking progression. Am J Psychiatry. 2004a;161:1224–1230. doi: 10.1176/appi.ajp.161.7.1224. [DOI] [PubMed] [Google Scholar]
- Audrain-McGovern J, Rodriguez D, Tercyak KP, Cuevas J, Rodgers K, Patterson F. Identifying and characterizing adolescent smoking trajectories. Cancer Epidemiol Biomarkers Prev. 2004b;13:2023–2034. [PubMed] [Google Scholar]
- Barkley R, Fischer M, Edelbrock C, Smallish L. The adolescent outcome of hyperactive children diagnosed by research criteria: I. An 8-year prospective follow-up study. J Am Acad Child Adol Psychiatry. 1990;29:546–557. doi: 10.1097/00004583-199007000-00007. [DOI] [PubMed] [Google Scholar]
- Benwell MD, Balfour DJ, Anderson JD. Evidence that tobacco smoking increases the density of (-) -[3H] nicotine binding sites in human brain. J Neurochem. 1988;50:1243–1247. doi: 10.1111/j.1471-4159.1988.tb10600.x. [DOI] [PubMed] [Google Scholar]
- Bratberg GH, Nilsen TI, Holmen TL, Vatten LJ. Sexual maturation in early adolescence and alcohol drinking and cigarette smoking in late adolescence: a prospective study of 2,129 Norwegian girls and boys. Eur J Pediatr. 2005;164:621–625. doi: 10.1007/s00431-005-1721-0. [DOI] [PubMed] [Google Scholar]
- Breslau N, Fenn N, Peterson EL. Early smoking initiation and nicotine dependence in a cohort of young adults. Drug Alcohol Depend. 1993a;33:129–137. doi: 10.1016/0376-8716(93)90054-t. [DOI] [PubMed] [Google Scholar]
- Breslau N, Kilbey MM, Andreski P. Nicotine dependence, major depression, and anxiety in young adults. Arch Gen Psychiatry. 1991;48:1069–1074. doi: 10.1001/archpsyc.1991.01810360033005. [DOI] [PubMed] [Google Scholar]
- Breslau N, Kilbey MM, Andreski P. Nicotine dependence and major depression: new evidence from a prospective investigation. Arch Gen Psychiatry. 1993b;50:31–35. doi: 10.1001/archpsyc.1993.01820130033006. [DOI] [PubMed] [Google Scholar]
- Breslau N, Kilbey MM, Andreski P. DSM-III-R nicotine dependence in young adults: prevalence, correlates and associated psychiatric disorders. Addiction. 1994;89:743–754. doi: 10.1111/j.1360-0443.1994.tb00960.x. [DOI] [PubMed] [Google Scholar]
- Breslau N, Novak SP, Kessler RC. Psychiatric disorders and stages of smoking. Biol Psychiatry. 2004;55:69–76. doi: 10.1016/s0006-3223(03)00317-2. [DOI] [PubMed] [Google Scholar]
- Breslau N, Peterson EL. Smoking cessation in young adults: Age at initiation of cigarette smoking and other suspected influences. Am J Public Health. 1996;86:214–220. doi: 10.2105/ajph.86.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Lewinsohn PM, Seeley JR, Wagner EF. Cigarette smoking, major depression, and other psychiatric disorders among adolescents. J Am Acad Child Adolec Psychiatry. 1996;35:1602–1610. doi: 10.1097/00004583-199612000-00011. [DOI] [PubMed] [Google Scholar]
- Buka SL, Shenassa ED, Niaura R. Elevated risk of tobacco dependence among offspring of mothers who smoked during pregnancy: A 30-year prospective study. Am J Psychiatry. 2003;160:1978–1984. doi: 10.1176/appi.ajp.160.11.1978. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Reasons for tobacco use and symptoms of nicotine withdrawal among adolescents and young tobacco users: United States, 1993. MMWR Morb Mortal Wkly Rep. 1994;43:745–750. [PubMed] [Google Scholar]
- Chabrol H, Faury R, Mullet E, Callahan S, Weigelt A, Labrousse F. Étude de la dépendence nicotinique chez 342 adolescents fumeurs. Arch Pédiatr. 2000;7:1064–1071. doi: 10.1016/s0929-693x(00)00314-6. [DOI] [PubMed] [Google Scholar]
- Chassin LA, Presson CC, Rose JS, Sherman SJ. The natural history of cigarette smoking from adolescence to adulthood: Demographic predictors of continuity and change. Health Psychol. 1996;15:478–484. doi: 10.1037//0278-6133.15.6.478. [DOI] [PubMed] [Google Scholar]
- Choi WS, Patten CA, Gillin JC, Kaplan RM, Pierce JP. Cigarette smoking predicts development of depressive symptoms among U.S. adolescents. Ann Behav Med. 1997;19:42–50. doi: 10.1007/BF02883426. [DOI] [PubMed] [Google Scholar]
- Colby SM, Tiffany ST, Shiffman S, Niaura RS. Are adolescent smokers dependent on nicotine? A review of the evidence. Drug Alc Dep. 2000;59:S83–S95. doi: 10.1016/s0376-8716(99)00166-0. [DOI] [PubMed] [Google Scholar]
- Collins AC, Marks MJ. Chronic nicotine exposure and nicotine receptors: Influence of genetic factors. Prog Brain Res. 1989;79:137–146. [PubMed] [Google Scholar]
- Collins AC, Marks MJ. Progress towards the development of animal models of smoking related behaviors. J Addict Dis. 1991;10:109–126. doi: 10.1300/J069v10n01_08. [DOI] [PubMed] [Google Scholar]
- Dierker LC, Avenevoli S, Merikangas KR, Flaherty BP, Stolar M. Association between psychiatric disorders and the progression of tobacco use behaviors. J Am Acad Child Adolesc Psychiatry. 2001;40:1159–1167. doi: 10.1097/00004583-200110000-00009. [DOI] [PubMed] [Google Scholar]
- Dierker LC, Donny E, Tiffany S, Colby SM, Perrine N, Clayton RR. The association between cigarette smoking and DSM-IV nicotine dependence among first year college students. Drug Alcohol Depend. 2007;86:106–14. doi: 10.1016/j.drugalcdep.2006.05.025. [DOI] [PubMed] [Google Scholar]
- DiFranza JR, Rigotti NA, McNeill AD, Ockene JK, Savageau JA, St Cyr D, Coleman M. Initial symptoms of nicotine dependence in adolescents. Tob Control. 2000;9:313–319. doi: 10.1136/tc.9.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFranza JR, Savageau JA, Fletcher K, Fletcher K, Ockene JK, McNeill AD, Coleman M, Wood C. Development of symptoms of tobacco dependence in youths: 30 month follow up data from the DANDY study. Tob Control. 2002;11:228–235. doi: 10.1136/tc.11.3.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFranza JR, Savageau JA, Fletcher K, Ockene JK, Rigotti NA, McNeill AD, Coleman M, Wood C. Recollections and repercussions of the first inhaled cigarette. Addict Behav. 2004a;29:261–272. doi: 10.1016/j.addbeh.2003.08.002. [DOI] [PubMed] [Google Scholar]
- DiFranza JR, Savageau JA, Rigotti NA, Ockene JK, McNeill AD, Coleman M, Wood C. Trait anxiety and nicotine dependence in adolescents A report from the DANDY study. Addict Behav. 2004b;29:911–919. doi: 10.1016/j.addbeh.2004.02.021. [DOI] [PubMed] [Google Scholar]
- Eissenberg T, Balster RL. Initial tobacco use episodes in children and adolescents: Current knowledge, future directions. Drug Alc Dep. 2000;59:S41–S60. doi: 10.1016/s0376-8716(99)00164-7. [DOI] [PubMed] [Google Scholar]
- Elkins IJ, King SM, McGue M, Iacono WG. Personality traits and the development of nicotine, alcohol, and illicit drug disorders: prospective links from adolescence to young adulthood. J Abnorm Psychol. 2006;115:26–39. doi: 10.1037/0021-843X.115.1.26. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Lynskey MT, Madden PAF. Early reactions to cannabis predict later dependence. Arch Gen Psychiatry. 2003;60:1033–1039. doi: 10.1001/archpsyc.60.10.1033. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Lynskey MT, Horwood LJ. Comorbidity between depressive disorders and nicotine dependence in a cohort of 16-year-olds. Arch Gen Psychiatry. 1996;53:1043–1047. doi: 10.1001/archpsyc.1996.01830110081010. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Woodward LJ, Horwood LJ. Maternal smoking during pregnancy and psychiatric adjustment in late adolescence. Arch Gen Psychiatry. 1998;55:721–727. doi: 10.1001/archpsyc.55.8.721. [DOI] [PubMed] [Google Scholar]
- Flint AJ, Novotny TE. Trends in black/white differences in current smoking among 18- to 24-year-olds in the United States, 1983-1993. Am J Prev Med. 1998;14:19–24. doi: 10.1016/s0749-3797(97)00009-3. [DOI] [PubMed] [Google Scholar]
- Fried PA. Cigarettes and marijuana: Are there measurable long-term neurobehavioral effects? Neurotoxicol. 1989;10:577–584. [PubMed] [Google Scholar]
- Gadow KD, Sprafkin J, Carlson G, Schneider J, Nolan EE, Mattison RE, Rundberg-Rivera V. A DSM-IV-referenced, adolescent self-report rating scale. J Am Acad Child Adolesc Psychiatry. 2002;41:671–679. doi: 10.1097/00004583-200206000-00006. [DOI] [PubMed] [Google Scholar]
- Gervais A, O'Loughlin J, Meshefedjian G, Bancej C, Tremblay M. Milestones in the natural course of cigarette use onset in adolescents. CMAJ. 2006;175:255–261. doi: 10.1503/cmaj.051235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert DG, Gilbert BO. Personality, psychopathology, and nicotine response a mediators of the genetics of smoking. Behav Gen. 1995;25:133–147. doi: 10.1007/BF02196923. [DOI] [PubMed] [Google Scholar]
- Giovino G. Epidemiology of tobacco use among U.S. adolescents. Nicotine Tob Res. 1999;1:S31–S40. doi: 10.1080/14622299050011571. [DOI] [PubMed] [Google Scholar]
- Giovino G, Henningfield JE, Tomar SL, Escobedo LG, Slade J. Epidemiology of tobacco use and dependence. Epid Rev. 1995;17:48–65. doi: 10.1093/oxfordjournals.epirev.a036185. [DOI] [PubMed] [Google Scholar]
- Griesler PC, Kandel DB, Davies M. Maternal smoking in pregnancy, child behavior problems, and adolescent smoking. J Res Adol. 1998;8:159–185. [Google Scholar]
- Griesler PC, Kandel DB, Schaffran C, Hu MC, Davies M. Recanting of smoking among adolescents: a comparison of reports in school and in household. 2006 doi: 10.1093/poq/nfn016. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell JS, Bangdiwala SI, Deng S, Webb JP, Bradley C. Smoking initiation in youth: the roles of gender, race, socioeconomics, and developmental status. J Adolesc Health. 1998;23:271–279. doi: 10.1016/s1054-139x(98)00078-0. [DOI] [PubMed] [Google Scholar]
- Heath AC, Cates R, Martin NG, Meyer J, Hewitt JK, Neale MC, Eaves LJ. Genetic contribution to risk of smoking initiation: Comparisons across birth cohorts and across cultures. J Subst Abuse. 1993;5:221–246. doi: 10.1016/0899-3289(93)90065-j. [DOI] [PubMed] [Google Scholar]
- Heath AC, Kirk KM, Meyer JM, Martin NG. Genetic and social determinants of initiation and age at onset of smoking in Australian twins. Behav Genet. 1999;29:395–407. doi: 10.1023/a:1021670703806. [DOI] [PubMed] [Google Scholar]
- Heath AC, Madden AF. Genetic influences on smoking behavior. In: Turner JR, Cardon LR, Hewitt JK, editors. Behavior Genetic Approaches in Behavioral Medicine. Plenum Press; New York: 1995. pp. 45–66. [Google Scholar]
- Heishman SJ, Kozlowski LT, Henningfield JE. Nicotine addiction: Implications for public health policy. J Soc Issues. 1997;53:13–33. [Google Scholar]
- Hu MC, Davies M, Kandel DB. Epidemiology and correlates of daily smoking and nicotine dependence among young adults in the United States. Am J Pub Health. 2006;96:299–308. doi: 10.2105/AJPH.2004.057232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Isensee B, Wittchen HU, Stein MB, Höfler M, Lieb R. Smoking increases the risk of panic. Arch Gen Psychiatry. 2003;60:692–700. doi: 10.1001/archpsyc.60.7.692. [DOI] [PubMed] [Google Scholar]
- John U, Meyer C, Rumpf HJ, Hapke U. Smoking, nicotine dependence and psychiatric comorbidity--a population-based study including smoking cessation after three years. Drug Alcohol Depend. 2004;76:287–295. doi: 10.1016/j.drugalcdep.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Johnson E, Rhee SH, Chase GA, Breslau N. Comorbidity of depression with levels of smoking: An exploration of the shared familial risk hypothesis. Nicotine Tob Res. 2004;6:1029–1038. doi: 10.1080/14622200412331324901. [DOI] [PubMed] [Google Scholar]
- Kandel DB. The natural history of smoking and nicotine dependence. Proceedings of Symposium on Addictions: Impact on Canada; Ottawa, Ontario, Canada: Royal Society of Canada; 2003. [Google Scholar]
- Kandel DB, Chen K. Extent of nicotine dependence and smoking in the United States: 1991-1993. Nicotine Tob Res. 2000;2:263–274. doi: 10.1080/14622200050147538. [DOI] [PubMed] [Google Scholar]
- Kandel DB, Chen K, Warner LA, Kessler RC, Grant B. Prevalence and demographic correlates of symptoms of last year dependence on alcohol, nicotine, marijuana and cocaine in the US population. Drug Alcohol Depend. 1997;44:11–29. doi: 10.1016/s0376-8716(96)01315-4. [DOI] [PubMed] [Google Scholar]
- Kandel DB, Schaffran C, Griesler P, Samuolis J, Davies M, Galanti MR. On the measurement of nicotine dependence in adolescence: comparisons of the FTND and a DSM-IV based scale. J Pediatr Psychol. 2005;30:319–332. doi: 10.1093/jpepsy/jsi027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel DB, Yamaguchi K. From beer to crack: Developmental patterns of involvement in drugs. Am J Public Health. 1993;83:851–855. doi: 10.2105/ajph.83.6.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp I, O'Loughlin J, Hanley J, Tyndale RF, Paradis G. Risk factors for tobacco dependence in adolescent smoking. Tob Control. 2006;15:199–204. doi: 10.1136/tc.2005.014118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp I, O'Loughlin J, Paradis G, Hanley J, DiFranza J. Smoking trajectories of adolescent novice smokers in a longitudinal study of tobacco use. Ann Epidemiol. 2005;15:445–452. doi: 10.1016/j.annepidem.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, MacLean CJ, Heath AC, Eaves LJ, Kessler RC. Smoking and major depression: a causal analysis. Arch Gen Psychiatry. 1993;50:36–43. doi: 10.1001/archpsyc.1993.01820130038007. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Sullivan P, Corey LA, Gardner CO, Prescott CA. A population-based twin study in women of smoking initiation and nicotine dependence. Psychol Med. 1999;29:299–308. doi: 10.1017/s0033291798008022. [DOI] [PubMed] [Google Scholar]
- Kessler RC. The epidemiology of dual diagnosis. Biol Psychiatry. 2004;56:730–737. doi: 10.1016/j.biopsych.2004.06.034. [DOI] [PubMed] [Google Scholar]
- Kollins SH, McClernon J, Fuemmeler BF. Association between smoking and Attention-Deficit/Hyperactivity Disorder symptoms in a population-based sample of young adults. Arch Gen Psychiatry. 2005;62:1142–1147. doi: 10.1001/archpsyc.62.10.1142. [DOI] [PubMed] [Google Scholar]
- Lanza ST, Collins LM. Pubertal timing and the onset of substance use in females during early adolescence. Prev Sci. 2002;3:69–82. doi: 10.1023/a:1014675410947. [DOI] [PubMed] [Google Scholar]
- Lieb R, Schreier A, Pfister H, Wittchen HU. Maternal smoking and smoking in adolescents: a prospective community study of adolescents and their mothers. Eur Addict Res. 2003;9:120–130. doi: 10.1159/000070980. [DOI] [PubMed] [Google Scholar]
- Lucas CP, Zhang H, Fisher PW, Shaffer D, Regier DA, Narrow WE, Bourdon K, Dulcan MK, Canino G, Rubio-Stipec M, Lahey BB, Friman P. The DISC Predictive Scales (DPS): Efficiently screening for diagnoses. J Am Acad Child Adolesc Psychiatry. 2001;40:443–449. doi: 10.1097/00004583-200104000-00013. [DOI] [PubMed] [Google Scholar]
- Lynskey MT, Fergusson DM. Childhood conduct problems, attention deficit behaviors, and adolescent alcohol, tobacco, and illicit drug use. J Abnorm Child Psychol. 1995;23:281–302. doi: 10.1007/BF01447558. [DOI] [PubMed] [Google Scholar]
- Madden PAF, Heath AC, Pedersen NL, Kaprio J, Koskenvo MJ, Martin NG. The genetics of smoking persistence in men and women: a multicultural study. Behav Genet. 1999;29:423–431. doi: 10.1023/a:1021674804714. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2003;33:905–919. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Campbell SM, Romm E, Collins AC. Genotype influences the development of tolerance to nicotine in the mouse. J Pharmacol Exp Ther. 1991;259:392–402. [PubMed] [Google Scholar]
- Martin CA, Kelly TH, Rayens MK, Brogli BR, Brenzel A, Smith WJ, Omar HA. Sensation seeking, puberty, and nicotine, alcohol, and marijuana use in adolescence. J Am Acad Child Adolesc Psychiatry. 2002;41:1495–1502. doi: 10.1097/00004583-200212000-00022. [DOI] [PubMed] [Google Scholar]
- Mayhew KP, Flay BR, Mott JA. Stages in the development of adolescent smoking. Drug Alcohol Depend. 2000;59:S61–S81. doi: 10.1016/s0376-8716(99)00165-9. [DOI] [PubMed] [Google Scholar]
- McNeill AD. The development of dependence on smoking in children. Br J Addict. 1991;86:589–592. doi: 10.1111/j.1360-0443.1991.tb01813.x. [DOI] [PubMed] [Google Scholar]
- McNeill AD, West RJ, Jarvis M, Jackson P, Bryant A. Cigarette withdrawal symptoms in adolescent smokers. Psychopharmacology. 1986;90:533–536. doi: 10.1007/BF00174074. [DOI] [PubMed] [Google Scholar]
- Milberger S, Biederman J, Faraone SV, Chen L, Jones J. Is maternal smoking during pregnancy a risk factor for attention deficit hyperactivity disorder in children? Am J Psychiatry. 1996;153:1138–1142. doi: 10.1176/ajp.153.9.1138. [DOI] [PubMed] [Google Scholar]
- Moffitt TE. Adolescent-limited and life-course-persistent antisocial behavior: A developmental taxonomy. Psychol Rev. 1993;100:674–701. [PubMed] [Google Scholar]
- Nelson CB, Wittchen HU. Smoking and nicotine dependence: Results from a sample of 14- to 24-year-olds in Germany. Eur Addict Res. 1998;4:42–49. doi: 10.1159/000018929. [DOI] [PubMed] [Google Scholar]
- Niaura R, Shadel WG, Goldstein MG, Hutchison KE, Abrams DB. Individual differences in responses to the first cigarette following overnight abstinence in regular smokers. Nicotine Tob Res. 2001;3:37–44. doi: 10.1080/14622200020032088. [DOI] [PubMed] [Google Scholar]
- O'Loughlin J, DiFranza J, Tarasuk J, Meshefedjian G, McMillan-Davey E, Paradis G, Tyndale RF, Clarke P, Hanley J. Assessment of nicotine dependence symptoms in adolescents: A comparison of five indicators. Tob Control. 2002;11:354–360. doi: 10.1136/tc.11.4.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Loughlin J, DiFranza J, Tyndale RF, Meshefedjian G, McMillan-Davey E, Clarke PB, Hanley J, Paradis G. Nicotine-dependence symptoms are associated with smoking frequency in adolescents. Am J Prev Med. 2003;25:219–225. doi: 10.1016/s0749-3797(03)00198-3. [DOI] [PubMed] [Google Scholar]
- O'Loughlin J, Paradis G, Kim W, DiFranza J, Meshefedjian G, McMillan-Davey E, Wong S, Hanley J, Tyndale RF. Genetically decreased CYP2A6 and the risk of tobacco dependence: a prospective study of novice smokers. Tob Control. 2004;13:422–428. doi: 10.1136/tc.2003.007070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlebeke JF, Knol DL, Verhulst FC. Increase in child behavior problems resulting from maternal smoking in pregnancy. Arch Environ Health. 1997;52:317–321. doi: 10.1080/00039899709602205. [DOI] [PubMed] [Google Scholar]
- Overstreet DH. Differential effects of nicotine in inbred and selectively bred rodents. Behav Genet. 1995;2:179–185. doi: 10.1007/BF02196926. [DOI] [PubMed] [Google Scholar]
- Patton GC, Coffey C, Carlin JB, Sawyer SM, Lynskey M. Reverse gateways? Frequent cannabis use as a predictor of tobacco initiation and nicotine dependence. Addiction. 2005;100:1518–1525. doi: 10.1111/j.1360-0443.2005.01220.x. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Benowitz NL, Henningfield J, Newhouse P, Pomerleau O, Swan G. Conference summary: Society for Research on Nicotine and Tobacco. Addiction. 1996;91:129–144. doi: 10.1046/j.1360-0443.1996.91112915.x. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: reliability, validity, and initial norms. J Youth Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Pierce JP, Choi WS, Gilpin EA, Farkas AJ. Validation of susceptibility as a predictor of which adolescents take up smoking in the United States. Health Psychol. 1996;15:355–361. doi: 10.1037//0278-6133.15.5.355. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF. Individual differences in sensitivity to nicotine: Implications for genetic research on nicotine dependence. Behav Genet. 1995;25:161–177. doi: 10.1007/BF02196925. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Pomerleau CS, Namenek RJ. Early experience with tobacco among women smokers, ex-smokers, and never smokers. Addiction. 1998;93:595–599. doi: 10.1046/j.1360-0443.1998.93459515.x. [DOI] [PubMed] [Google Scholar]
- Prokhorov AV, Hudmon KS, de Moor CA, Kelder SH, Conroy JL, Ordway N. Nicotine dependence, withdrawal symptoms, and adolescents' readiness to quit smoking. Nicotine Tob Res. 2001;3:151–155. doi: 10.1080/14622200110043068. [DOI] [PubMed] [Google Scholar]
- Prokhorov AV, Hudmon KS, Cinciripini PM, Marani S. “Withdrawal symptoms” in adolescents: a comparison of former smokers and never-smokers. Nicotine Tob Res. 2005;7:909–913. doi: 10.1080/14622200500266114. [DOI] [PubMed] [Google Scholar]
- Prokhorov AV, Koehly LM, Pallonen UE, Hudmon KS. Adolescent nicotine dependence measured by the modified Fagerstrom Tolerance Questionnaire at two time points. J Child Adol Subst Abuse. 1998;7:35–47. [Google Scholar]
- Richardson SA, Tizabi Y. Hyperactivity in the offspring of nicotine-treated rats: Role of the mesolimbic and nigrostriatal dopaminergic pathways. Pharmacol Biochem Behav. 1994;47:331–337. doi: 10.1016/0091-3057(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Rojas NL, Killen JD, Haydel KF, Robinson TN. Nicotine dependence among adolescent smokers. Arch Pediatr Adol Med. 1998;152:151–156. doi: 10.1001/archpedi.152.2.151. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Refining models of dependence: variations across persons and situations. Br J Addict. 1991;86:611–615. doi: 10.1111/j.1360-0443.1991.tb01817.x. [DOI] [PubMed] [Google Scholar]
- Sonntag H, Wittchen HU, Höfler M, Kessler RC, Stein MB. Are social fears and DSM-IV social anxiety disorder associated with smoking and nicotine dependence in adolescents and young adults? Eur Psychiatry. 2000;15:67–74. doi: 10.1016/s0924-9338(00)00209-1. [DOI] [PubMed] [Google Scholar]
- Sledjeski EM, Dierker LC, Costello D, Shiffman S, Donny E, Flay BR Tobacco Etiology Research Network (TERN) Predictive validity of four nicotine dependence measures in a college sample. Drug Alcohol Depend. 2007;87:10–19. doi: 10.1016/j.drugalcdep.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Stanton WR. DMSII-R Tobacco dependence and quitting during late adolescence. Addict Behav. 1995;20:595–603. doi: 10.1016/0306-4603(95)00019-9. [DOI] [PubMed] [Google Scholar]
- Stanton WR, Silva PA, Oei TPS. Change in children's smoking from age 9 to age 15 years: the Dunedin study. Public Health. 1991;105:425–433. doi: 10.1016/s0033-3506(05)80612-2. [DOI] [PubMed] [Google Scholar]
- Storr CL, Reboussin BA, Anthony JC. Early childhood misbehavior and the estimated risk of becoming tobacco-dependent. Am J Epidemiol. 2004;160:126–130. doi: 10.1093/aje/kwh184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, Office of Applied Studies. National Household Survey on Drug Abuse, 2000 [Computer file]. ICPSR03262-v1. Inter-University Consortium for Political and Social Research; 2002. 10/11/2002. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2004 National Survey on Drug Use and Health: National Findings. Office of Applied Studies; Rockville, MD: 2005. [Google Scholar]
- Timberlake DS, Haberstick BC, Hopfer CJ, Bricker J, Sakai JT, Lessem JM, Hewitt JK. Progression from marijuana use to daily smoking and nicotine dependence in a national sample of U.S. adolescents. Drug Alcohol Dep. 2006 Dec 13; doi: 10.1016/j.drugalcdep.2006.11.005. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Vaglenova J, Birru S, Pandiella NM, Breese CR. An assessment of the long-term developmental and behavioral teratogenicity of prenatal nicotine exposure. Behav Brain Res. 2004;150:159–170. doi: 10.1016/j.bbr.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Vink JM, Willemsen G, Boomsma DI. Heritability of smoking initiation and nicotine dependence. Behav Genet. 2005;35:397–406. doi: 10.1007/s10519-004-1327-8. [DOI] [PubMed] [Google Scholar]
- Wakschlag LS, Lahey BB, Loeber R, Green SM, Gordon RA, Leventhal BL. Maternal smoking during pregnancy and the risk of conduct disorder in boys. Arch Gen Psychiatry. 1997;54:670–676. doi: 10.1001/archpsyc.1997.01830190098010. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Warner V, Wickramaratne P, Kandel DB. Maternal smoking during pregnancy and psychopathology in offspring followed to adulthood. J Am Acad Child Adolesc Psychiatry. 1999;38:892–899. doi: 10.1097/00004583-199907000-00020. [DOI] [PubMed] [Google Scholar]
- West RJ, Russell MAH. Loss of acute nicotine tolerance and severity of cigarette withdrawal. Psychopharmacology. 1988;94:563–565. doi: 10.1007/BF00212856. [DOI] [PubMed] [Google Scholar]
- Wheeler KC, Fletcher KE, Wellman RJ, DiFranza JR. Screening adolescents for nicotine dependence: The hooked on nicotine checklist. J Adolescent Health. 2004;35:225–230. doi: 10.1016/j.jadohealth.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Williams GM, O'Callaghan M, Najman JM, Bor W, Andersen MJ, Richards DUC. Maternal cigarette smoking and child psychiatric morbidity: A longitudinal study. Pediatrics. 1998;102:e11. doi: 10.1542/peds.102.1.e11. [DOI] [PubMed] [Google Scholar]
- Wills TA, Windle M, Cleary SD. Temperament and novelty seeking in adolescent substance use: Convergence of dimensions of temperament with constructs from Cloninger's theory. J Pers Soc Psychol. 1998;74:387–406. doi: 10.1037//0022-3514.74.2.387. [DOI] [PubMed] [Google Scholar]
- Wilson DM, Killen JD, Hayward C, Robinson TN, Hammer LD, Kraemer HC, Varady A, Taylor CB. Timing and rate of sexual maturation and the onset of cigarette and alcohol use among teenage girls. Arch Pediatr Adolesc Med. 1994;148:789–795. doi: 10.1001/archpedi.1994.02170080019004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.