Abstract
Cranial placodes are ectodermal regions that contribute extensively to the vertebrate peripheral sensory nervous system. The development of the ophthalmic trigeminal (opV) placode, which gives rise only to sensory neurons of the ophthalmic lobe of the trigeminal ganglion, is a useful model of sensory neuron development. While key differentiation processes have been characterized at the tissue and cellular levels, the signaling pathways governing opV placode development have not. Here, we tested in chick whether the canonical Wnt signaling pathway regulates opV placode development. By introducing a Wnt reporter into embryonic chick head ectoderm, we show that the canonical pathway is active in Pax3+ opV placode cells as, or shortly after, they are induced to express Pax3. Blocking the canonical Wnt pathway resulted in the failure of targeted cells to adopt or maintain an opV fate, as assayed by the expression of various markers including Pax3, FGFR4, Eya2, and the neuronal differentiation markers Islet1, neurofilament and NeuN, although, surprisingly, it led to upregulation of Neurogenin2, both in the opV placode and elsewhere in the ectoderm. Activating the canonical Wnt signaling pathway, however, was not sufficient to induce Pax3, the earliest specific marker of the opV placode. We conclude that canonical Wnt signaling is necessary for normal opV placode development, and propose that other molecular cues are required in addition to Wnt signaling to promote cells toward an opV placode fate.
Keywords: ophthalmic trigeminal placode, Wnt, Pax3, FGFR4, Eya2, Ngn2, sensory neurogenesis
Introduction
In vertebrates, neurogenic placodes, along with a subpopulation of neural crest cells, contribute sensory neurons to the developing cranial ganglia. While some neurogenic placodes, such as the otic and olfactory placodes, give rise to several non-neuronal and neuronal derivatives, the trigeminal and epibranchial (geniculate, petrosal, and nodose) placodes give rise exclusively to sensory neurons (reviewed in Baker and Bronner-Fraser, 2001; Schlosser, 2006). The development of these solely neurogenic placodes provides an advantageous model for understanding the stepwise process of neurogenesis. In the case of the trigeminal ganglion (the sensory ganglion of cranial nerve V), sensory neurons derive from neural crest cells and two placodes, the ophthalmic trigeminal (opV) and maxillomandibular trigeminal (mmV) placodes. Each placode contributes neurons to the distal region of its respective ganglionic lobe, while the neural crest contributes the proximal neurons as well as glial cells (D'Amico-Martel and Noden, 1983; reviewed in Baker and Bronner-Fraser, 2001; Schlosser, 2006). Until the last decade, research on trigeminal placode induction was not well advanced, primarily due to the lack of molecular markers for placodal epithelia (Noden, 1993). Stark et al. (1997) identified the paired-domain/homeodomain transcription factor Pax3 as the earliest known gene expressed in the avian opV placode. Using Pax3 as a marker, the tissue interactions involved in induction and specification of opV placode cells were identified. It was shown that a diffusible signal(s) from the neural tube induces Pax3 expression in the placode (Stark et al., 1997). Explant and grafting experiments subsequently were performed to better characterize the timing and tissue interactions involved in the competence, induction, and specification of the opV placode (Baker et al., 1999). It was shown that the Pax3-inducing signal is present throughout most of the dorsal neural tube, and that early in development the entire head ectoderm is competent to form Pax3+ opV placode cells (Baker et al., 1999). Similar experiments showed that Pax3 expression in opV placode ectoderm correlates with specification and commitment to a Pax3+, cutaneous sensory neuron fate (Baker et al., 1999; Baker and Bronner-Fraser, 2000; Baker et al., 2002). While these studies revealed the cellular and tissue interactions needed for opV placode formation, the signaling molecules involved in this process were not determined. While other research has shown, for example, that epibranchial placode induction in chick and zebrafish requires BMPs and FGFs originating from the pharyngeal endoderm and cephalic mesoderm (Begbie et al., 1999; Holzschuh et al., 2005; Nechiporuk et al., 2005, 2007; Nikaido et al., 2007; Sun et al., 2007), and that FGF and Wnt signaling are required for the initial development and maintenance of the otic placode (see Ladher et al., 2000, 2005; Martin and Groves, 2006; Ohyama et al., 2006), such molecules have not yet been identified for the trigeminal placodes.
Because it was previously shown that opV placode-inducing activity is present in the dorsal neural tube, we aimed to identify candidate inducing molecules expressed in this tissue. Multiple Wnt family members, such as Wnt-1, 2, -3a, and -4, can be detected throughout the neural tube in early chick embryos (Hollyday et al., 1995; Marcelle et al., 1997). For example, Wnt-1 expression begins as early as the 3-somite stage (ss) in the dorsal neural tube. By the 10ss, expression is found throughout the midbrain, hindbrain, and the future spinal cord. By the 14ss, expression is restricted to a narrow region in the dorsal midline encircling the midbrain/hindbrain boundary, directly adjacent to the opV placode. Furthermore, the Wnt receptors Frizzled-2 (Fz-2) and Fz-7 are expressed in a pattern consistent with a potential role in receiving an opV placode-inducing signal. By the 3ss, Fz-7 is strongly expressed in cranial ectoderm, later becoming restricted to the presumptive trigeminal placode, persisting through the 25ss. Fz-2 also begins to be faintly expressed at the 3ss in the cranial ectoderm, and by the 7-10ss becomes broadly expressed in cranial ectoderm, again persisting through the 25ss (Stark et al., 2000). Correlating evidence from other vertebrate models also suggests that Wnt signaling is a candidate for opV placode induction. In Wnt-1 null and Wnt-1/Wnt-3a double knockout mice, the opV nerve is considerably reduced, whereas Wnt-3a null mice have normal opV branching (Ikeya et al., 1997). Furthermore, trigeminal neurons expand rostrally in zebrafish headless mutants, in which canonical Wnt signaling is over-activated (Kim et al., 2000). Considering both the functional data from the mouse and zebrafish, and the Wnt/Fz expression data in the chick, we hypothesized that Wnt signaling is involved in opV placode cell specification and/or later development.
Wnt/Frizzled signaling has been shown to activate three separate intracellular pathways. Activation of the canonical cascade leads to cytoplasmic stabilization of β-catenin which translocates to the nucleus and associates with TCF/LEF transcription factors. The binding of this complex leads to canonical Wnt target gene expression and can lead to determination of cell fate and differentiation (Veeman et al., 2003). The non-canonical Wnt pathways include the planar cell polarity pathway, which is involved in cytoskeletal rearrangements, and the Ca2+ pathway which has been shown to be involved in cell migration and heart development (Huelsken and Behrens, 2002; Kuhl, 2004). Because genetic evidence strongly suggests that Wnt-1 and Wnt-3a signal via the β-catenin pathway, we focused our efforts on investigating canonical Wnt signaling in opV placode development. Using in ovo electroporation to disrupt or activate the canonical Wnt signaling pathway in opV placode ectoderm we show that blocking canonical Wnt signaling disrupts all aspects of opV placode differentiation, including expression of the opV placode markers Pax3, FGF receptor 4 and Eya2, as well as for delamination and neuronal differentiation. However, canonical Wnt signaling is not sufficient to induce Pax3 in competent ectoderm, suggesting a more complex process requiring multiple signals for opV placodal cell fate determination.
Materials and Methods
Expression reagents
A modified red fluorescent protein (RFP) Wnt reporter electroporation construct, made by replacing the LacZ gene of the TOPGAL construct (DasGupta and Fuchs, 1999) with nuclear RFP, was a kind gift from Dr. Andy Groves (House Ear Institute, Los Angeles). Dominant active human β-catenin and dominant negative human TCF4 (Tetsu and McCormick, 1999) inserted into the pCIG vector with IRES-nuclear localized green fluorescent protein (GFP), as well as the pCIG-GFP control vector were a kind gift from Dr. Andy McMahon (Megason and McMahon, 2002). These expression constructs were prepared for electroporation by resuspending at a concentration of 4-8μg/μl in water with fast green added for visualization.
In ovo electroporation of chicken embryos
Fertilized chicken (Gallus gallus) eggs were obtained from local farms and incubated to the desired stage in a humidified incubator at 38°C. The DNA constructs described above were electroporated into 2-14 somite stage (ss) chicken embryos using standard electroporation techniques, where electrodes were placed on either side of the embryo, or (most often) using a vertical electroporation method, where the reference electrode was placed underneath the embryo through a small hole made outside the area opaca, and the driving electrode was placed directly above the area of interest (BTX 820 electroporator from Genetronics: five 10ms pulses of 10V each, one second gap between each pulse; or TSS20 Ovodyne electroporator from Intracel Ltd, Shepreth, UK: four 50ms pulses of 4 V each, one second gap between each pulse).
Foil barrier experiments
Following electroporation in 3-6 somite stage embryos, as described above, a slit between the ectoderm and the neural folds was made using a glass needle. A tantalum foil barrier was then positioned in the slit using fine forceps. Barriers were made by cutting 7.5 μm thick tantalum foil (Goodfellow #TA000280) into pieces of approximately 250×350 μm, which were then shaped using fine forceps (Stark et al., 1997). Embryos were collected after 24 hours and prepared for cryosectioning and immunohistochemical staining.
In situ hybridization
Chicken Pax3 (Matsunaga, et al., 2001), FGFR4 (Marcelle et al., 1995), Eya2 (Litsiou et al., 2005; kind gift of Dr Andrea Streit) and Ngn2 (Perez et al., 1999; kind gift of Prof. David Anderson) digoxigenin-labeled RNA probes were synthesized as described by Henrique et al. (1995).
For whole-mount in situ hybridization (Pax3), embryos were incubated for 12-48 hours after electroporation before being fixed in 4% formaldehyde and processed for whole-mount in situ hybridization according to Henrique et al. (1995).
For in situ hybridization on wax sections (FGFR4, Eya2, Ngn2), embryos were fixed in modified Carnoy's solution (60% ethanol, 11.1% formaldehyde, 10% glacial acetic acid), washed in diethylpyrocarbonate (DEPC)-treated phosphate-buffered saline (PBS), dehydrated into methanol, cleared in Histosol (National Diagnostics), embedded in paraffin wax (Raymond Lamb) and sectioned at 8μm with a rotary microtome (Microm). Sections were mounted in distilled water on Superfrost® Plus slides (VWR), and dried overnight at 37°C. In situ hybridization was carried out using a Boekel slide incubator according to Etchevers et al. (2001), except that the slides were not treated with proteinase K, the first two post-hybridization washes were in pre-warmed 50% formamide, 1×SSC, 0.1% Tween-20, and the color reaction solution contained 5% polyvinyl alcohol (Sigma).
Immunohistochemistry and analysis
The following primary antibodies were used: Pax3 (mouse IgG2a; Baker et al., 1999), Pax2 (rabbit; Zymed/Invitrogen), Pax6 (mouse IgG1; Developmental Studies Hybridoma Bank: DSHB), Islet-1 (mouse IgG2b; DSHB), neurofilament (mouse IgG1 DSHB), NeuN (mouse IgG1; Chemicon), and GFP (rabbit; Molecular Probes/Invitrogen). The Developmental Studies Hybridoma Bank (DSHB) was developed under the auspices of the NICHD and is maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA 52242. Appropriately matched Alexa488-, Alexa546- or Alexa594-conjugated goat anti-mouse or goat anti-rabbit secondary antibodies were obtained from Molecular Probes/Invitrogen; biotinylated goat anti-mouse secondary antibodies were obtained from Southern Biotech.
For immunohistochemistry on cryosections, embryos were embedded in gelatin and cryosectioned to generate 10-12 μm sections of the area of interest. Sections were mounted on Superfrost® Plus glass slides and the gelatin removed by treating the slides in phosphate-buffered saline (PBS) at 37°C for 15-20 minutes. The slides were incubated overnight at 4°C in primary antibody, diluted in antibody buffer (PBS, 0.1% bovine serum albumen, 0.1% Triton-X100), followed by incubation for 1-2 hours at room temperature in secondary antibodies diluted in antibody buffer. When biotinylated secondary antibody was used, slides were further incubated for 1 hour at room temperature in Alexa350-conjugated NeutrAvidin (Molecular Probes/Invitrogen) diluted 1:100 in PBS. Three 5-10 minute washes in PBS followed each incubation. Slides were mounted in Fluoromount G (SouthernBiotech). Sections were analyzed using epifluorescent microscopy; photographs from different channels were superimposed using Adobe Photoshop or Olympus Microsuite to observe overlapping expression.
Quantitative analysis
For quantitative analysis of the various markers used after electroporation, two approaches were used. To count Pax3+ targeted cells and analyze the contribution of targeted cells to the opV ganglion on cryosections of electroporated embryos, five random sections from the opV (Pax3+) placode/ganglion region of each embryo were analyzed to minimize variability and bias. Cells positive for Pax3, GFP, or both were determined manually or using Olympus Microsuite software to identify cells with minimum color thresholds. In the second approach, used to count targeted cells after in situ hybridization on wax sections for FGFR4 followed by immunostaining for Pax3, Islet1 and GFP (when staining was more variable and not all sections were positive for all three molecular markers), all targeted cells were counted in the opV placode (Pax3+ and/or FGFR4+) region. The percentage of cells positive for each marker was calculated for each embryo, and statistical analysis performed, with p-values calculated using Student's t-test to compare the standard means of control and experimental samples.
Results
Canonical Wnt signaling is active in Pax3+ opV placode cells
In chick embryos, Pax3 mRNA expression is first seen by in situ hybridization in ectoderm cells immediately adjacent to the midbrain-level neural folds at the 4-somite stage (ss) (Stark et al., 1997), while robust Pax3 protein expression is first detectable by immunohistochemistry at the 7ss in a similar location, after which the domain of Pax3 expression expands laterally into the surface ectoderm away from the neural folds (Baker et al., 1999). In order to determine whether or not canonical Wnt signaling is active in Pax3+ opV placode cells, we co-electroporated a GFP control construct (pCIG-GFP) together with a modified RFP version of the TOPGAL Wnt reporter (containing three multimerized LEF/TCF consensus binding sites) into the surface head ectoderm of 2-11ss chick embryos, encompassing the trigeminal placode region adjacent to the midbrain and rostral hindbrain. Nuclear RFP should only be expressed in cells in which the canonical Wnt signaling pathway is active. Preliminary results in embryos collected 24 hours after electroporation (26-29ss) clearly showed RFP+ cells in the opV placode domain (data not shown). Because RFP is very stable, it was also necessary to analyze embryos at earlier timepoints in order to determine whether the Wnt pathway is active during the early stages of placode induction and throughout opV placode development. Embryos were electroporated between the 2-8ss (n=20) with both the RFP Wnt reporter construct and the control GFP construct, and sacrificed 10-12 hours post-electroporation (10-16ss). Whole-mount embryos showed only a subset of targeted cells (GFP expression) with activated canonical Wnt signaling (RFP expression; Fig. 1A-C), indicating that Wnt signaling is only active in a subset of cranial ectoderm cells, which appear to be predominantly in the dorsal ectoderm. RFP expression was visible in a fairly broad region of dorsal head ectoderm adjacent to the midbrain and rostral hindbrain (Fig. 1B,C), correlating well with known domains of frizzled (Wnt receptor) expression (Stark et al., 2000). However, canonical Wnt activity was not restricted to opV placodal ectoderm, since RFP was observed generally in dorsal cranial ectoderm, relatively close to the neural tube. Electroporated embryos were subsequently cryosectioned and immunostained for Pax3, which showed canonical Wnt signaling activity in many, though not all, GFP+/Pax3+cells (Fig. 1D-K). While we did observe a few RFP+/Pax3- cells in the opV placode region, the overall pattern suggests that canonical Wnt signaling is active in opV placode ectoderm even at early stages of opV placode development.
Figure 1.

Canonical Wnt signaling is active in most Pax3+ ophthalmic trigeminal (opV) placode cells. (A-C) A 14ss chick embryo, 12 hours after co-electroporation into cranial surface ectoderm at the 6ss with GFP (green: A, C) and with a Wnt reporter construct that expresses RFP in response to canonical Wnt signaling (red: B, C). Canonical Wnt signaling can be seen in a medial subset of electroporated cells (i.e., always relatively close to the neural tube), extending from the rostral midbrain to the caudal most extent of electroporation (mid-caudal hindbrain). mb, midbrain. (D-G) Transverse section through the opV placode region of a 15ss embryo, 10 hours after electroporation at the 8ss with the RFP Wnt reporter and GFP, showing single-channel (D-F) and merged (G) images of GFP expression (green), RFP/canonical Wnt activity (red) and Pax3 expression (blue). Only a medial subset of all GFP+ cells are RFP+, i.e., responding to canonical Wnt signaling. (H-K) Higher-power view of boxed regions in D-G, showing single-channel (H-I) and merged (K) images of GFP expression (green), RFP/canonical Wnt activity (red) and Pax3 expression (blue). Arrowheads show examples of Pax3+ opV placode cells in which canonical Wnt signaling is active. mb, midbrain; nt, neural tube; ph, pharynx.
Canonical Wnt signaling is required for Pax3 expression in the opV placode
Explant and grafting experiments have previously shown that significant numbers of cells in midbrain-level opV placode ectoderm are specified and/or committed to express Pax3 from the 8ss onwards (Baker et al., 1999), and that Pax3 expression in opV placode ectoderm correlates with commitment to a Pax3+ cutaneous sensory neuron fate (Baker and Bronner-Fraser, 2000; Baker et al., 2002). To determine if canonical Wnt signaling is necessary for opV placode cell development, we electroporated dominant negative Tcf4 (DN-Tcf; Megason and McMahon, 2002), which acts as a constitutive repressor of canonical Wnt targets due to its inability to bind β-catenin, into cranial ectoderm of 2-6ss embryos (i.e. before significant Pax3 specification/commitment; Baker et al., 1999). Gene expression from the promoter can typically be observed within 2-3 hours after electroporation (Nakamura et al., 2004), indicating that the stage at which Wnt signaling is inhibited likely ranges from about the 4-8ss. GFP was electroporated as a control. Embryos collected 12 and 24 hours post-electroporation, at about the 12ss and 20ss respectively, were prepared for immunohistochemistry as described, and sections through the opV placode region were analyzed in order to characterize GFP+ targeted cells within the opV placode domain ectoderm.
In GFP-electroporated control embryos, targeted ectoderm appeared similar to untargeted ectoderm. Within the opV placode domain, GFP+ targeted cells included many Pax3+ cells as well as several Pax3- cells (Fig. 2A-D). Embryos displaying obviously unhealthy tissue morphology were excluded from the data set, and only embryos where DAPI-stained nuclei were intact without any apparent degradation were included. The same stringent criteria were used for DN-Tcf electroporated experimental embryos, where DN-Tcf was expressed concomitantly with GFP. In these experimental embryos, a dramatic loss of GFP+/Pax3+ cells was observed, confirming that expression of DN-Tcf resulted in cell-autonomous loss of Pax3 expression. Untargeted cells within the placode domain frequently expressed Pax3 (Fig. 2E-H). To quantify these observations, Pax3+, GFP+ and coexpressing cells were counted from five random sections through the placode domain of each electroporated embryo (in order to compensate for variation in targeting efficiency, and to avoid experimental bias), and the percentage of targeted cells in the placode domain co-expressing Pax3 was calculated for each embryo, and statistical analysis performed, with p-values calculated using Student's t-test to compare the standard means of control and experimental samples. In GFP-electroporated control embryos targeted at the 2-6ss (n=5) and allowed to develop for 12 hours to about the 12ss, a mean of 61.5% of targeted cells (s.d. +/- 6.3; 250 total GFP+ cells) from within the placode domain of each embryo also expressed Pax3. In DN-Tcf-electroporated embryos (n=6), however, the mean was dramatically reduced to 4.0% of targeted cells (s.d. +/- 3.5; 258 total GFP+ cells), a difference that is highly statistically significant compared to controls (p<0.0001; Fig.2I). Similarly, DN-Tcf-electroporated embryos allowed to develop 24 hours (to approximately the 20ss) showed a highly statistically significant reduction when compared to controls. A mean of 60.4% of targeted cells (s.d. +/- 18.2; 280 total GFP+ cells) per control embryo (n=8), also expressed Pax3, versus a mean of 3.5% (s.d. +/- 2.5; 430 total GFP+ cells) in DN-Tcf-electroporated embryos (n=10, p<0.0001; Fig. 2A-I). Therefore, blocking canonical Wnt signaling in the opV placode by electroporating DN-Tcf at the 2-6ss led to a highly statistically significant reduction of Pax3 expression in targeted cells, detectable at both 12 and 24 hours after electroporation.
Figure 2.

Blocking canonical Wnt signaling leads to loss of Pax3 in the opV placode. (A) Transverse section through the opV placode region of a ∼20ss embryo, 24 hours after electroporation at the 2-6ss with the GFP control vector (green) and immunostaining for Pax3 (red) to identify opV placode cells. (B-D) Single-channel (B,C) and merged (D) images showing a magnified view of the boxed region in A. Cells targeted with the control plasmid express GFP (green, arrowheads), and continue to express high levels of Pax3 (red, arrows; overlapping cells shown in yellow in panel D). (E) Transverse section through the opV placode region of a ∼22ss embryo, 24 hours after electroporation at the 2-6ss with dominant negative Tcf4 (DN-Tcf; GFP+ cells in green) and immunostaining for Pax3 (red). (F-H) Single-channel (F,G) and merged (H) images showing a magnified view of the boxed region in E. GFP+ cells targeted with DN-Tcf show a cell-autonomous loss of Pax3 (arrowheads); adjacent untargeted cells express Pax3 at normal levels (arrow). (I) Histograms showing the percentage of targeted cells expressing Pax3 in control embryos (blue) and DN-Tcf-electroporated embryos (yellow). Embryos electroporated at the 2-6ss were allowed to develop for 12 or 24 hours to about the 12ss and 20ss respectively; embryos electroporated at the 10-14ss were collected after 12 hours at about the 20ss. Sample size for each experiment is indicated. The dramatic reduction in Pax3 expression in DN-Tcf-electroporated embryos compared to controls is highly statistically significant at all timepoints (p<0.0001; *). The reduction in Pax3+ cells in embryos electroporated at the 10-14ss shows a highly statistically significant reduction compared to control embryos (p<0.0001), however a less dramatic reduction compared to embryos electroporated with DN-Tcf at the 2-6ss was observed (p<0.05; **).
To investigate the effects of blocking Wnt signaling in opV placode cells later in development (i.e. after significant numbers of opV placode cells are specified and/or committed to express Pax3), 10-14ss embryos were electroporated as described, and allowed to develop for 12 hours to about the 20-22ss, the same stage as the 2-6ss embryos described above, which were allowed to develop for 24 hours. In GFP-electroporated control embryos (n=6), a mean of 61.9% of targeted cells (s.d. +/- 4.1; 426 total GFP+ cells) in each embryo expressed Pax3, similar to younger control embryos. Although many cells in the targeted region are already expressing Pax3 and/or committed to express Pax3 at the time of electroporation, the mean percentage of targeted cells expressing Pax3 was unexpectedly reduced in DN-Tcf electroporated embryos (n=6), to only 14.6% (s.d. +/- 8.8; 246 total GFP+ cells), a difference highly significant from controls (p<0.0001; Fig. 2I). This indicates that canonical Wnt signaling is necessary to maintain Pax3 expression in the opV placode. Interestingly, this reduction was not as dramatic as that observed in embryos electroporated at the 2-6ss, before significant numbers of cells are committed to express Pax3 (3.5% compared to 14.6% coexpressing cells after 12 hours; statistically significant, p<0.05), pointing to potential differences in the effects of blocking Wnt signaling before and after cell specification.
Canonical Wnt signaling is not sufficient to induce Pax3 in the opV placode
To determine if Wnt signaling is sufficient to induce the opV placode cell fate in cranial ectoderm cells, we electroporated a dominant-active form of β-catenin (DA-βcat), which is resistant to proteolysis and therefore constitutively activates the Wnt pathway (Megason and McMahon, 2002), into head and rostral trunk ectoderm of 2-11ss embryos. After electroporation, embryos were allowed to develop for 24 hours to about the 20ss before being photographed for GFP expression, and prepared for in situ hybridization or immunohistochemistry as described.
Whole-mount in situ hybridization for Pax3 revealed that the Pax3 expression domain did not expand concomitant with DA-βcat misexpression, even when competent ectoderm adjacent to rhombomeres 2-4 (Baker et al., 1999) was targeted (Fig. 3B, C). To ensure that the DA-βcat construct is indeed activating the Wnt pathway, DA-βcat was co-electroporated with the RFP Wnt reporter. Embryos electroporated with DA-βcat and Wnt reporter showed a near complete overlap of GFP and RFP expression. (n=6; Fig. 3E). Embryos co-electroporated with the control GFP and RFP Wnt reporter (n=6; Fig. 3D) produced only limited overlapping expression, indicating that DA-βcat successfully activated the Wnt pathway. To confirm that DA-βcat did not increase Pax3 expression, and to determine whether DA-βcat misexpression biased cells within the placode domain towards Pax3 expression, sections through the opV placode and adjacent regions were analyzed to identify Pax3+ cells and GFP/DA-βcat expressing cells within the ectoderm. Misexpression of DA-βcat in competent cranial ectoderm did not result in an increase in the number of targeted cells expressing Pax3 when compared to controls, neither within the placode domain (Fig. 4A-H) nor in sections through adjacent ectoderm (data not shown). In control embryos (n=10), the mean percentage per embryo of GFP+ opV placode cells that expressed Pax3 was 66.4% (s.d. +/- 10.8; 1000 total GFP+ cells), while in experimental embryos, the mean was effectively the same, at 68.3% (s.d. +/- 7.7; 830 total GFP+ cells; Fig. 4I). Therefore, no significant difference between control and experimental embryos was observed after ectopically activating Wnt signaling in the opV placode domain.
Figure 3.

Canonical Wnt signaling does not expand Pax3 expression outside the opV placode region. (A) In situ hybridization for Pax3, showing normal Pax3 expression in the opV placode. (B-C) 23ss embryo, 24 hours after electroporation at the 5-9ss with dominant active β-catenin (DA-βcat), showing a similar pattern of Pax3 expression as the control (B) even after broad ectodermal targeting with DA-βcat, visualized by epifluorescent microscopy for GFP (C). (D,E) Positive control showing that DA-βcat activates the Wnt pathway in the opV placode region. (D) Transverse section through the head region of a ∼20ss embryo, 24 hours after co-electroporation with the GFP control vector (green) and the Wnt RFP reporter (red) at the 5-9ss. A restricted pattern of Wnt activity (RFP expression) is seen, with many targeted green cells not activating the Wnt pathway. (E) Transverse section through the head region of a ∼23ss embryo, 24 hours after co-electroporation with DA-βcat (green) and the Wnt RFP reporter (red) at the 5-9ss. Nearly all of the targeted cells are also expressing RFP, confirming that the DA-βcat construct is indeed activating the Wnt pathway. (ov-otic vesicle)
Figure 4.

Activating Wnt signaling does not increase the number of Pax3+ cells in the opV placode. (A) Transverse section through the opV placode of a ∼25ss embryo, 24 hours after electroporation with the GFP control vector (green) at the 5-9ss, immunostained for Pax3 (red). (B-D) Magnified view of boxed region in A showing single-channel (B,C) and merged (D) images. Cells targeted with control vector express GFP (arrowheads). Many cells within the placode domain also express high levels of Pax3 (overlapping cells shown in yellow in D; flanked by arrow/arrowhead). (E) Transverse section through the opV placode of a ∼25ss embryo, 24 hours after electroporation with dominant active β-catenin (DA-βcat; green) at the 5-9ss, immunostained for Pax3 (red). (F-H) Magnified view of boxed region in E showing single-channel (F,G) and merged (H) images. GFP+ cells targeted with DA-βcat are prevalent (arrowheads), as are cells expressing high levels of Pax3 (arrows; overlapping cells shown in yellow in H; flanked by arrow/arrowhead). (I) Histogram showing the percentage of targeted ectodermal cells expressing Pax3 in control embryos and DA-βcat-electroporated embryos. The difference between the two is not statistically significant.
To rule out the possibility that endogenous placode induction cues were masking the effect of DA-βcat misexpression (so as to test conclusively that Wnt signaling is not sufficient to induce Pax3), we blocked signaling from the neural tube using tantalum foil barriers (Stark et. al., 1997) and misexpressed DA-βcat in adjacent ectoderm to look for rescue of Pax3 expression (Fig. 5; n=15). We did not observe a significant rescue of Pax3 in targeted ectoderm, supporting the conclusion that canonical Wnt signaling is not sufficient for opV placode induction. Since we had now shown that Wnt signaling was required for Pax3 expression, but could not induce it on its own, we next examined more carefully the effects of DN-Tcf on opV placode cell differentiation.
Figure 5.

Activating Wnt signaling does not rescue loss of Pax3 in opV placode ectoderm after blocking the Pax3-inducing signal from the brain. (A) ∼20ss embryo collected 24 hours after being electroporated at the 2-6ss with dominant active β-catenin (DA-βcat) and in which, immediately after electroporation, a tantalum foil barrier was placed unilaterally between the neural tube and adjacent ectoderm to block the diffusible Pax3-inducing signal from the neural tube. (B) Same embryo as in A, under epifluorescence microscopy following removal of the barrier, showing the extent of the GFP/DA-βcatenin expression domain. (C) Section through the opV placode region showing GFP (green) and Pax3 expression (red). A dramatic reduction in the number of Pax3-expressing opV placode cells lateral to the barrier was seen, as shown previously (Stark et al., 1997); there was no rescue by DA-β-catenin, as evidenced by the many GFP+/Pax3-negative cells lateral to the barrier. (White line indicates barrier location. ov, otic vesicle)
Canonical Wnt signaling is required for opV placode cell delamination and contribution to the ganglion
To assess opV ganglion formation after blocking canonical Wnt signaling, we electroporated DN-Tcf into 2-6ss embryos, and allowed them to develop for 48 hours to about the 35ss, when the ganglion is becoming well-defined. Embryos were analyzed by counting the number of GFP+/Pax3+ cells in each embryo compared to the total number of Pax3+ cells (targeted and untargeted) in the ganglion on five randomly-selected sections through the opV placode/ganglion region of each embryo. (Although some neural crest cells express Pax3 at very low levels, opV placodal Pax3 expression is much higher and thus easily distinguishable from neural crest expression in migrating cells and in the ganglion.) This analysis allowed us to determine the proportion of all Pax3+ cells in the ganglion that co-expressed GFP in control and experimental embryos. Qualitative analysis of these sections showed that after control GFP electroporation, many GFP+ cells contributed to the ganglion (Fig. 6A), while after DN-Tcf electroporation, nearly all of the GFP+/DN-Tcf expressing cells remained in the ectoderm, with no contribution to the opV ganglion (Fig. 6B). Because of the near absence of GFP+ ganglionic cells in DN-Tcf electroporated embryos, cell counts were performed differently, where all Pax3+ cells were counted, and the percentage of those cells expressing GFP was compared between control and experimental embryos: in control embryos (n=5), the mean percentage per embryo of Pax3+ ganglionic cells that co-expressed GFP was 47.9% (s.d. +/- 5.2; 1158 total Pax3+ cells), while in DN-Tcf-electroporated embryos (n=3), the mean was only 1.1% (s.d. +/- 0.3; Fig. 6C; 285 total Pax3+ cells). Thus, electroporating DN-Tcf into the opV placode at the 2-6ss led to a highly statistically significant reduction in the contribution of targeted cells to the ganglion (p<0.005; Fig. 6C). Importantly, though targeted cells in experimental embryos did not contribute to the ganglion, and did not express Pax3, the opV ganglion still contained many Pax3+/GFP- placode cells (Fig. 6B; an average of 95 Pax3+ cells per DN-Tcf electroporated embryo, compared to an average of 193 Pax3+ cells per control embryo), indicating that untargeted ectoderm retained placodal competence at early stages.
Figure 6.

Blocking canonical Wnt signaling prevents targeted cells from delaminating and contributing to the opV ganglion. (A) Transverse section through opV ganglion region of a ∼35ss embryo, 48 hours after electroporation at the 2-6ss with the GFP control vector (green), immunostained for Pax3 (red). GFP+ cells contribute substantially to the opV ganglion, with numerous cells expressing both GFP and Pax3 (yellow). (B) Transverse section through opV ganglion region of a ∼35ss embryo, 48 hours after electroporation at the 2-6ss with DN-Tcf (green) and immunostained for Pax3 (red). Virtually all GFP+ cells remain in the ectoderm, do not express Pax3, and do not contribute to the ganglion, though a significant number of untargeted Pax3+ ganglionic cells are present. (C) Histogram showing the percentage of Pax3+ opV ganglion cells that were also targeted (GFP+) in control (n=5) versus DN-Tcf embryos (n=3). The dramatic reduction in the contribution of DN-Tcf-targeted cells to the ganglion is highly statistically significant (p<0.005). (D) Transverse section through opV ganglion region of a ∼35ss embryo, 36 hours after electroporation at the 10-14ss with the GFP control vector (green), immunostained for Pax3 (red). Targeted cells contribute substantially to the opV ganglion, with numerous cells expressing both GFP and Pax3 (yellow). (E) Transverse section through opV ganglion region of a ∼35ss embryo, 36 hours after electroporation at the 10-14ss with DN-Tcf (green), immunostained for Pax3 (red). DN-Tcf-targeted cells remain in the ectoderm and do not express Pax3. Unlike in embryos targeted earlier in development (as in B), very few untargeted Pax3+ cells are present in the ganglion. (F) Histogram showing the percentage of Pax3+ cells within the ganglion co-expressing GFP in control (n=5) versus DN-Tcf embryos (n=7). The dramatic reduction in the contribution of DN-Tcf-targeted cells to the ganglion is statistically significant (p<0.02).
We showed above that DN-Tcf electroporation at the 10-14ss, when significant numbers of cells in midbrain-level opV placode ectoderm are already specified and committed to express Pax3, results in a dramatic loss of Pax3-expressing ectoderm cells within 12 hours. To investigate ganglion development under these conditions we electroporated DN-Tcf into the head ectoderm of 10-14ss embryos, which were allowed to develop for 30-36 hours to about the 35ss, when the ganglion becomes well defined. We found that blocking Wnt signaling in older embryos, where cells are apparently already expressing and/or committed to express Pax3, not only led to the loss of Pax3 expression but also to the failure of targeted cells to contribute to the ganglion (Fig. 6E). Again, all Pax3+ cells were counted, and the percentage of those cells expressing GFP was compared between control and experimental embryos and statistical analysis performed, with p-values calculated using Student's t-test as described. In control embryos (n=5), the mean percentage per embryo of Pax3+ cells in the ganglion that co-expressed GFP was 39.0% (s.d. +/- 18.8; 700 total Pax3+ cells; Fig. 6D,F), while in experimental embryos (n=7), the mean was only 2.5% (s.d. +/- 3.7; 175 total Pax3+ cells; Fig. 6E,F). (In separate experiments, a similarly significant reduction was seen in the percentage of targeted cells that had delaminated from the ectoderm 24 hours after electroporation of 10-14ss embryos; data not shown.) Thus, electroporating DN-Tcf into the opV placode at the 10-14ss led to a statistically significant reduction in the contribution of targeted cells to the ganglion (p<0.02). Importantly, we also observed that the overall placodal contribution to the opV ganglion was significantly reduced, with a dramatic reduction in the total number of Pax3+ cells (targeted or untargeted) contributing to the ganglion (an average of 25 Pax3+ cells per DN-Tcf electroporated embryo, compared to an average of 193 Pax3+ cells per control embryo). This effect was in contrast to what was seen after electroporating DN-Tcf at the 2-6ss, where untargeted cells were prevalent in the ganglion (50% compared to controls when targeting at the 2-6ss; 15% compared to controls when targeting at the 10-14ss).
Canonical Wnt signaling is required for opV placode differentiation
As described above, blocking canonical Wnt signaling in the opV placode region leads to the loss of Pax3 expression, and the failure of targeted cells to delaminate from the ectoderm and contribute to the opV ganglion. The next question was whether DN-Tcf targeted opV placode cells indeed fail to differentiate as opV neurons, or whether they differentiate normally but simply fail to delaminate. To answer this, we analyzed expression of both the opV placode-specific marker FGF receptor 4 (FGFR4, previously known as FREK; Marcelle et al., 1995), which is expressed a few hours after Pax3 in opV placode cells (Stark et al., 1997), and the early neuronal differentiation marker Islet1 (Ericson et al, 1992; Mulder et al., 1995). We performed in situ hybridization for FGFR4 on sections of embryos electroporated at 5-6ss and allowed to develop for 24 hours (to the 21-27ss), followed by immunostaining for Pax3, Islet1, and GFP (native GFP fluorescence is lost after in situ hybridization). Due to the variability in staining from in situ hybridization and analyzing multiple markers, all GFP+ cells in the opV placode region of each embryo were counted from well-stained sections as described, resulting in several hundred GFP+ cells being counted for both experimental (DN-Tcf) and control (GFP) embryos. A minimum of six individual placodes/ganglia were analyzed for each marker (placodes/ganglia with targeted cells on both sides of the same embryo were counted independently). We found that DN-Tcf expressing opV placode cells, as well as being significantly less likely to express Pax3 and delaminate from the ectoderm, were significantly less likely to express the opV placode-specific marker FGFR4, 24 hours after electroporation at the 5-6ss (Fig. 7). In GFP-electroporated embryos, the mean percentage per placode of targeted cells that co-expressed FGFR4 was 26.9% (s.d. +/- 12.3; 305 total GFP+ cells; n=6 from 5 embryos). In DN-Tcf-electroporated embryos, the mean per placode was only 3.7% (s.d. +/- 4.1; 473 total GFP+ cells; n=7 from 4 embryos), a highly statistically significant reduction (p<0.0005; Student's t-test).
Figure 7.

Blocking canonical Wnt signaling blocks FGFR4 expression in opV placode cells. (A-D) Single-channel (A-C) and merged (D) images of a transverse section at the level of the opV placode in a 20ss embryo in which the control GFP plasmid had been electroporated into cranial surface ectoderm at the 5ss, followed by in situ hybridization on sections for FGFR4 (C) and immunostaining for GFP (green, A) and Pax3 (red, B). Arrowheads indicate GFP-electroporated cells expressing both FGFR4 and Pax3. (E-H) Single-channel (E-G) and merged (H) images of a transverse section at the level of the opV placode in a 27ss embryo in which DN-Tcf had been electroporated into cranial surface ectoderm at the 6ss, followed by in situ hybridization on sections for FGFR4 (G) and immunostaining for GFP (green, E) and Pax3 (red, F). Arrows indicate examples of DN-Tcf-targeted (GFP+) opV placode cells that do not express either FGFR4 or Pax3; interestingly, two of these cells have delaminated. All of the Pax3+ cells that have delaminated, many of which are also detectably FGFR4, are untargeted.
The transcription factor Islet1, which is expressed early in the differentiation of various neuronal subtypes (Ericson et al, 1992; Mulder et al., 1995) is expressed in the opV placode from the 17ss, and marks differentiating opV neurons both in the ectoderm and in the opV ganglion. We found that blocking canonical Wnt signaling led to a significant reduction in the proportion of targeted cells expressing Islet1 24 hours after electroporation. In GFP-electroporated embryos, the mean percentage per placode/ganglion of targeted cells that co-expressed Islet1 was 14.5% (s.d. +/- 13.8; 449 total GFP+ cells; n=7 from 6 embryos). In DN-Tcf-electroporated embryos, the mean was only 4.7% (s.d. +/- 7.7; 844 total GFP+ cells; n=11 from 7 embryos), a statistically significant reduction (p<0.05, Student's t-test; Fig. 10A-J).
Figure 10.
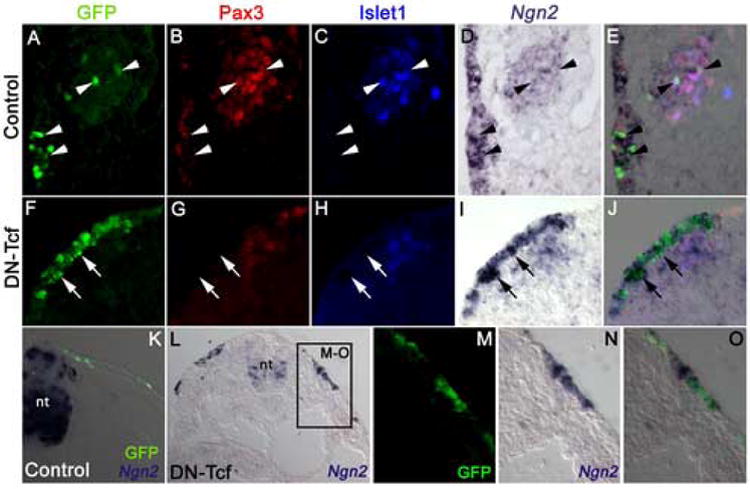
Blocking canonical Wnt signaling blocks neurogenesis in opV placode-derived cells but upregulates Ngn2 expression, both in opV placode cells and other regions of ectoderm, including trunk ectoderm. (A-E) Single-channel (A-D) and merged (E) images of a transverse section at the level of the opV placode in a 26ss embryo in which the control GFP vector had been electroporated into cranial surface ectoderm at the 7ss, followed by in situ hybridization on sections for Ngn2 (D) and immunostaining for GFP (green, A), Pax3 (red, B) and the early neuronal differentiation marker Islet1 (blue, C). Arrowheads indicate examples of GFP-targeted opV placode cells that express both Ngn2 and Pax3 in the ectoderm, and Ngn2, Pax3 and Islet1 in the adjacent opV placode-derived ganglion. (F-J) Single-channel (F-I) and merged (J) images of a transverse section at the level of the opV placode in a 26ss embryo in which DN-Tcf had been electroporated into cranial surface ectoderm at the 6ss, followed by in situ hybridization on sections for Ngn2 (I) and immunostaining for GFP (green, F), Pax3 (red, G) and Islet1 (blue, H). Strong Ngn2 expression, but no Pax3 expression, is seen in all DN-Tcf-targeted cells in the ectoderm (arrows indicate examples), immediately ventral to Pax3+ untargeted ectoderm cells. Some untargeted Pax3+Islet1+ neurons, many of which are also detectably Ngn2+, have delaminated from the ectoderm. (K) Merged view of a transverse section through the trunk of a 28ss embryo in which the control GFP vector had been electroporated into surface ectoderm at the 10ss, followed by in situ hybridization on sections for Ngn2 and immunostaining for GFP (green), showing normal Ngn2 expression in the neural tube (nt) but no staining in the surface ectoderm. (L) Transverse section through the trunk of a 19ss embryo in which DN-Tcf had been electroporated into surface ectoderm at the 10ss, followed by in situ hybridization on sections for Ngn2, showing ectopic Ngn2 expression in surface ectoderm as well as the normal Ngn2 expression pattern in the neural tube (nt). (M-O) Single-channel (M, N) and merged (O) magnified views of boxed region in L, showing Ngn2 expression (N) in Dn-Tcf-targeted cells (green, M). The Ngn2 staining is so strong in some cells that it has masked the GFP immunofluorescence. (nt, neural tube)
Older embryos electroporated with DN-Tcf between the 10-14ss and collected at approximately the 35ss, during ganglion condensation, were also analyzed for the presence of neuronal differentiation markers normally found in opV ganglion cells (neurofilament, and the neuron-specific neuronal antigen NeuN (Mullen et al., 1992). As in previous experiments, DN-Tcf targeted GFP cells remained in the ectoderm, and although the neuronal markers were still found in untargeted cells in the opV ganglion, none of the targeted cells (in all embryos analyzed) expressed NeuN (0/5 embryos) or neurofilament (0/4 embryos; Fig. 8A,B).
Figure 8.

OpV placode cells targeted with DN-Tcf do not differentiate as neurons and do not express other placodal markers. (A-D) Transverse sections through the opV ganglion of embryos electroporated with DN-Tcf between the 10-14ss and allowed to develop to approximately the 35ss. The majority of targeted GFP+ cells remained in the ectoderm and did not delaminate or contribute to the opV ganglion. They did not express either (A) the post-mitotic neuronal antigen NeuN; (B) the neuronal marker neurofilament; (C) the otic and epibranchial placode marker Pax2; (D) or the lens placode marker Pax6.
Therefore, blocking canonical Wnt signaling in opV placodal ectoderm by electroporation of DN-Tcf leads not only to a significant reduction in Pax3 expression, delamination and contribution to the ganglion, but also to a significant reduction both in the expression of the downstream opV placode-specific marker FGFR4 and in neuronal differentiation, as assessed by Islet1, NeuN, and neurofilament expression.
Blocking canonical Wnt signaling does not upregulate markers of other placodes
Throughout all stages and time points tested, the great majority of DN-Tcf targeted cells remain in the ectoderm. To address the possibility that these cells may be adopting a different placodal fate, we analyzed DN-Tcf electroporated embryos for the expression of Pax2, which marks the epibranchial as well as the otic placodes in the chick (Baker and Bronner-Fraser, 2000), and Pax6, which marks the lens placode (see Bhattacharyya et al., 2004). Immunohistochemistry on sections through the head region show that DN-Tcf targeted cells did not upregulate Pax2 (0/4 embryos) or Pax6 (0/6 embryos) in any of the embryos tested (Fig. 8C,D).
Canonical Wnt signaling is required to maintain expression of Eya2 in the opV placode
We also investigated whether blocking the Wnt signaling pathway affects markers expressed generally in all cranial placodes. In the chick, Eya2 is expressed in the preplacodal domain (a horseshoe-shaped region of ectoderm surrounding the anterior neural plate at the neurula stage; Litsiou et al., 2005) and is maintained in placode derivatives, including cranial sensory ganglia (Mishima and Tomarev, 1998). Expression of Eya2 in the chick preplacodal domain at neurula stages requires inhibition of canonical Wnt signaling (Litsiou et al., 2005), so it might be predicted that blocking Wnt signaling at later stages might upregulate Eya2 expression in the opV placode. We used in situ hybridization on sections of electroporated embryos, followed by immunostaining for Pax3, Islet and GFP, to investigate this possibility. We found that 24 hours after electroporating 6ss embryos with the DN-Tcf construct (n=3), targeted cells within the opV placode region appeared to downregulate Eya2 as compared to their untargeted neighbors (Fig. 9). Although only qualitative, these results do not support the hypothesis that blocking canonical Wnt signaling at early somite stages prevents opV placode cells from progressing beyond a pre-placodal state. Instead, they suggest that canonical Wnt signaling is required to maintain the expression not only of Pax3 itself, and the downstream opV placode-specific marker FGFR4, but also of the pan-placodal marker Eya2. Overall, these results suggest that canonical Wnt signaling is required to maintain all aspects of the opV placode fate.
Figure 9.

Blocking canonical Wnt signaling blocks expression of the pan-placodal marker Eya2 in opV placode cells. (A) Merged image of a transverse section at the level of the opV placode in a 19ss embryo in which GFP had been electroporated into cranial surface ectoderm at the 5ss, followed by in situ hybridization on sections for Eya2 and immunostaining for GFP (green). (B-D) Single-channel (B,C) and merged images (D) of higher-power view of boxed region in A. Arrowheads show examples of GFP-targeted ectoderm cells expressing Eya2. (E) Merged image of a transverse section at the level of the opV placode in an 18ss embryo in which DN-Tcf had been electroporated into cranial surface ectoderm at the 6ss, followed by in situ hybridization on sections for Eya2 and immunostaining for GFP (green). (F-H) Single-channel (F,G) and merged images (H) of higher-power view of boxed region in E. Arrowheads delimit region of Eya2 expression in untargeted ectodermal cells, bounded both dorsally and ventrally by Eya2-negative DN-Tcf-targeted ectoderm. (nt, neural tube; ph, pharynx)
Blocking canonical Wnt signaling upregulates Neurogenin2 expression in all embryonic ectoderm
We also used in situ hybridization on sections of electroporated embryos, followed by immunostaining for Pax3, Islet and GFP, to assess the effect of blocking canonical Wnt signaling on the expression of Neurogenin2 (Ngn2), a proneural transcription factor expressed early in the neurogenic cascade in specific subsets of sensory neurons (Fode et al., 1998; Ma et al., 1998). Ngn2 begins to be expressed in the opV placode from as early as the 10ss in the chick, where it has been described as a specific marker for opV placode-derived neurons, with Ngn1 being expressed in all other neurogenic placodes (Begbie et al., 2002). However, at later stages than those analyzed by Begbie et al. (2002), we found that Ngn2 is expressed in all placode-derived neurons in the chick (data not shown), so it is not in fact a specific marker for opV placode neurons, but rather a more general marker for placode-derived sensory neurons.
In stark contrast to the downregulation of opV placode markers (Pax3, FGFR4, Eya2) and neuronal differentiation markers (Islet1, neurofilament and NeuN) that we previously saw after electroporating DN-Tcf, we found that, 24 hours after electroporating DN-Tcf at the 5-7ss (n=5), seemingly all targeted cells dramatically upregulated expression of Ngn2 (Fig. 10 F-J). No effect was seen on Ngn2 expression in the opV placode region of a control embryo electroporated with GFP (Fig. 10A-E). Intriguingly, the upregulation of Ngn2 after DN-Tcf expression was not confined to the opV placodal area, nor even to cranial ectoderm, since a similar upregulation was seen even in trunk ectoderm (Fig. 10L-O), where electroporation of GFP alone had no effect (n=5). These results are perhaps even more surprising in light of the failure of DN-Tcf-targeted cells to differentiate as neurons, given that Ngn2 misexpression (using retroviruses) in the chick trunk has previously been shown to lead to neuronal differentiation even in non-ectodermal cells (Perez et al., 1999).
Discussion
Canonical Wnt signaling is necessary, but not sufficient, for adoption of an opV placode cell fate
The combined work of Stark et al. (1997) and Baker et al. (1999), through various barrier, grafting and explant experiments, supported a model for ophthalmic trigeminal (opV) placode induction in which all cranial ectoderm is initially competent to express Pax3 in response to a diffusible inducing signal from the dorsal neural tube, with spatiotemporal changes in competence contributing to the restriction of Pax3 expression in the opV placode adjacent to the midbrain and rostral hindbrain. Over a 36-hour period from the 4-somite stage (ss) onwards, cells in the opV placode domain upregulate first Pax3, then FGFR4 and Ngn2, delaminate from the ectoderm and differentiate as sensory neurons in the condensing ophthalmic lobe of the trigeminal ganglion (Stark et al., 1997; Baker et al., 1999; Begbie et al., 2002). Pax3 expression is maintained at high levels in opV placode-derived neurons until some point between E4.5 and E5.5 (Baker et al., 2002).
Until now, the signaling pathways required for opV placode development were unknown, although a possible role for Wnt signaling was suggested by reduction of the opV nerve in Wnt-1 null (though not Wnt-3a null) mice (Ikeya et al., 1997), and a rostral expansion of trigeminal neurons in zebrafish mutants in which the canonical Wnt pathway is over-activated (Kim et al., 2000). The dorsal neural tube expresses Wnt-1, Wnt-3a and Wnt4 (Hollyday et al., 1995; Marcelle et al., 1997), and Wnt receptors (frizzleds) are present in cranial ectoderm (Stark et al., 2000). The RFP Wnt reporter results presented here show that in the chick embryo, canonical Wnt signaling is active in dorsal cranial ectoderm near the neural tube, including Pax3+ opV placode cells.. Blocking canonical Wnt signaling before the 8ss, i.e., before significant numbers of ectoderm cells are specified and committed to express Pax3 (Baker et al., 1999), disrupts opV placodal Pax3 expression:. Regardless of how long embryos were allowed to develop after electroporation (12, 24, or 48 hours), dramatically fewer targeted cells expressed Pax3 or delaminated in embryos electroporated with dominant negative Tcf4 (DN-TCF) compared to controls.. This is likely due to either a failure of targeted cells to upregulate Pax3 (loss of induction), or a rapid downregulation of initial Pax3 expression (immediate loss of maintenance).
In addition to the observed loss of Pax3 and failure of targeted cells to delaminate, blocking canonical Wnt signaling before the 8ss also led to a clear reduction in expression of the opV placode-specific marker FGFR4 (expressed after Pax3 in the opV placode; Stark et al., 1997), the general placodal marker Eya2 (expressed at neurula stages in preplacodal ectoderm and maintained in placode-derived cells; see Litsiou et al., 2005; Bailey and Streit, 2006), and the early neuronal differentiation marker Islet1. In addition, expression of the later neuronal differentiation markers neurofilament and NeuN was never observed in targeted cells. Taken together, these data support the hypothesis that canonical Wnt signaling is required for opV placode cell differentiation. However, activation of canonical Wnt signaling with dominant active β-catenin (DA-β-cat) had no effect on the number of targeted cells expressing Pax3 in the ectoderm, either within the opV placode domain or in nearby ectoderm, indicating that Wnt signaling alone is not sufficient to induce Pax3. Even when a barrier was placed between the neural tube and ectoderm to block endogenous inductive signaling, activation of canonical Wnt signaling was not able to rescue Pax3 expression. Hence, although canonical Wnt signaling is required for adoption of an opV placode fate, it does not induce Pax3, the earliest marker for the opV placode.
Canonical Wnt signaling is necessary to maintain an opV placode fate
From the 8ss, a substantial number of ectoderm cells at the level of the midbrain are specified and committed to express Pax3 and to adopt a Pax3+ cutaneous sensory neuron fate, as determined by explant and heterotopic grafting experiments (Baker et al., 1999, 2002; Baker and Bronner-Fraser, 2000). We found that electroporating DN-Tcf at the 10-14ss, when a significant number of opV placode cells already express Pax3, also resulted in the downregulation of Pax3 and the failure of targeted cells to delaminate, just as was observed in embryos targeted at the 2-6ss. By inhibiting canonical Wnt signaling late, therefore, we were able to alter the fate of opV placode cells that were already apparently committed (in heterotopic grafting experiments) to express Pax3 and adopt an opV placode cell fate. Hence, canonical Wnt signaling is required to maintain Pax3 expression, and also to allow delamination and contribution to the ophthalmic lobe of the trigeminal ganglion.
Given that Wnt signaling is required to maintain Pax3 expression in opV placode cells, how then can we explain the previous specification and commitment data obtained through explant and heterotopic grafting experiments? One might predict from the data presented here that Pax3+ opV ectoderm cells would lose Pax3 expression in neutral culture conditions (specification test), and possibly also after grafting to a heterotopic location (commitment test, in this case, grafting over the lateral plate mesoderm or to the nodose placode; Baker et al., 1999, 2002; Baker and Bronner-Fraser, 2000), unless canonical Wnt signals were also present in the grafted location. There are at least two possible explanations for the maintenance of Pax3 expression in the specification test. First, Wnts may be expressed in the explanted ectoderm itself, thus autocrine signaling would maintain Pax3 expression. Second, Wnt signaling may be required to maintain Pax3 expression in the presence of alternative specification cues in the cranial environment, so in explant culture, Wnt signals would no longer be required to maintain Pax3 expression. Future experiments aimed at examining the expression of Wnt family members, and the response of cranial ectoderm tissue to various candidate signaling factors, will resolve this question.
Although DN-Tcf-targeted cells remained in the ectoderm and did not express Pax3 in either the early (2-6ss) or late (10-14ss) Wnt inhibition experiments, there was a significant reduction in the total number of untargeted Pax3+ placode cells contributing to the ganglion after electroporation at later stages, relative to the significant compensation seen after electroporation at early stages. This suggests that, after DN-Tcf electroporation at the 10-14ss, neighboring untargeted cells have either lost competence to express Pax3, or that the Pax3-inducing signal is no longer present when Wnt activity is blocked. Some support for the latter hypothesis is provided by previous grafting experiments, which suggested that the Pax3-inducing signal at the midbrain level may decline after the 8ss (Baker et al., 1999). This is well before the stage at which Wnt signaling is blocked in this study, which is likely to be 2-3 hours after electroporation, perhaps at the 12-16ss. Conversely, sufficient inducing activity must be present (perhaps from the rostral hindbrain or midbrain/hindbrain junction) through at least the 12ss, since previous ablation experiments showed that when presumptive opV placodal ectoderm in 12ss embryos was removed and embryos were allowed to develop to a stage of ganglion condensation, opV placode cells were still present, albeit reduced in number, with the majority of remaining cells condensing external to the ganglion (Stark et al., 1997). Whether due to a loss of competence or a loss of the inducing signal, our results suggest that much of the cellular plasticity of opV and nearby cranial ectoderm is lost by a few hours after the 10-14ss..
Upregulation of Ngn2 in response to DN-Tcf
The observation that Ngn2, which is expressed at some stage in all placode-derived neurons, is dramatically upregulated in all embryonic ectoderm (including trunk ectoderm) after DN-Tcf electroporation may seem contradictory to other data presented here. A simple model may include a regulatory loop between Pax3 and Ngn2. A recent study has revealed a dynamic interplay between Pax6 and Ngn2 in the stepwise process of neurogenesis in the spinal cord (Bel-Vialar et al., 2007). Their data showed that Pax6 acts as one player in upregulating Ngn2 expression, and subsequent high levels of Ngn2 initiate the downregulation of Pax6, which is necessary for neuronal differentiation. In contrast, Pax3 expression is maintained for several days in differentiated opV placode-derived neurons (Baker et al., 2002), so a similar model (i.e., Ngn2-mediated downregulation of Pax3) cannot apply during normal opV placode development.
An alternative scenario is the direct regulation of Ngn2 by the Wnt pathway. Several studies indicate that the regulation of Neurogenin gene expression by β-catenin/Tcf is context-specific. Upstream regulatory regions of Ngn1, for example, have known binding sites for Lef/Tcf, and the β-catenin/Tcf complex has been shown to bind these regulatory elements (Hirabayashi et al., 2004). One study showed that while Lef/Tcf activation sites were confirmed in the promoter regions of neurogenins, β-catenin itself is likely able to bind the Ngn1 promoter region directly, independent of Lef/Tcf (Israsena et al., 2004). Additional studies have concluded that, depending on the presence of cofactors such as FGF2, and on the state of cellular differentiation, Wnt signaling can enhance cell proliferation, promote differentiation, or inhibit differentiation of neuronal precursor cells (Hirabayashi et al., 2004; Muroyama et al., 2004). It has also been shown that antagonizing Wnt signaling upregulates Ngn2, and that under various cellular contexts, β-catenin/Tcf may act as a repressor or an activator of Ngn1 (Aubert et al., 2002; Kubo et al., 2005). Our results support these and other observations describing the complex interplay between various signal transduction pathways that converge during the several stages of cellular differentiation during neurogenesis. While introducing DN-Tcf clearly downregulated most developmental markers of the opV placode, Ngn2 was unexpectedly upregulated even in the absence of further neuronal differentiation. The molecular mechanisms underlying this result warrant future study.
Proposed models for the involvement of canonical Wnt signaling in opV placode development
Since canonical Wnt signaling is not sufficient for opV placode induction, is it possible that it functions only in specified cells as a general mechanism for maintaining or further promoting placodal cell fate? One possible model is that canonical Wnt signaling acts as a downstream “lock-in” step for Pax3+ cranial ectoderm cells, stabilizing Pax3 expression and enabling cells to adopt their specified fate. This is similar to what may be happening in cranial ectoderm cells in the otic placode, where it has been shown that initial otic placode development requires combined Wnt and Fgf signals (Ladher et al., 2000, 2005). Clarifying these results, Ohyama et al. (2006) proposed a model wherein Fgf signaling specifies a domain of Pax2 expressing cells that must subsequently receive Wnt signaling to adopt an otic placode fate. Wnt signaling in otic placode development also has an important later function, along with Sonic Hedgehog, in patterning (reviewed by Brigande et al., 2000; Fekete and Wu, 2002; Ohyama et al., 2006). This is also consistent with the model proposed by Arias and Hayward (2006), in which Wnt signaling stabilizes and maintains gene expression during cell fate transitions and other developmental processes, enabling the adoption of specific cell fates. The data presented here support a model requiring multiple signals, since we show that canonical Wnt signaling is required for adoption and maintenance of the opV placode cell fate, but is not itself sufficient to induce competent cells toward that same fate.
Alternatively, one cannot rule out Wnts playing a dual role in opV placode cell specification. In this model, the dorsal neural tube emits a canonical Wnt signal, in conjunction with other signal(s), such as FGF or BMP, which act together to induce or specify competent ectoderm to become Pax3+ opV placode cells. Subsequently Wnts, either from within the cranial ectoderm or adjacent tissue, act to maintain Pax3 expression and the opV placode cell fate until cellular differentiation. This is supported somewhat by the result that only 12 hours after electroporation of 2-6ss embryos, Pax3 is not present in DN-Tcf electroporated cells. When considering the timing of normal Pax3 specification, loss of maintenance would be occurring nearly at the same time as initial Pax3 expression. Similar multi-step requirements for Wnt signaling occur in other developmental systems, such as in the developing somites, where canonical and non-canonical Wnt signals function throughout the several steps of myogenesis (Marcelle et al., 1997; Schmidt et al., 2000, 2004; Linker et al., 2003, 2005; Geetha-Loganathan et al., 2006), and have been shown to regulate Pax3 activity directly (Brunelli et al., 2007). Regardless of the interpretation of these results, a requirement for canonical Wnt signaling in opV placode development helps explain the previous observation that Wnt-1-deficient mice have a reduced opV nerve (Ikeya et al., 1997).
Conclusions
The stepwise process of cell fate determination is often a complex process of cell-cell signaling and continued fate restriction. While the tissue interactions required for opV placode development have been known for some time through classical embryological experiments, the molecules involved in the process have eluded discovery. We have demonstrated that canonical Wnt signaling is required for cells to adopt the opV placode cell fate, and must be maintained for their continued differentiation. Canonical Wnt signaling however is not sufficient, since activating this signal transduction pathway alone does not promote cells toward an opV placode fate. The specification of these cells, therefore, requires additional signals, which remain elusive. Future studies will aim to identify the additional molecular cues that must be involved in opV placode induction and differentiation.
Acknowledgments
We thank Dr. Andy McMahon for generously providing the DN-Tcf and DA-βcat pCIG vectors. Thanks to Dr. Andy Groves for the RFP Wnt reporter electroporation construct, to Dr. Andrea Streit for the Eya2 clone, and to Prof. David Anderson for the Ngn2 clone. Thanks to Dr. Jeff Barrow for helpful suggestions on this research, and for review of the manuscript. Thanks to the many undergraduate students who contributed to this work, especially Roland Goode, Tyler Prestwich, Michael Secrist and Trent Clifton. This research was supported by the following sources: NIH/NICHD grant #5R03HD041470-02 and #1R01HD046475-01 to M.R.S.; BYU/ORCA mentored research grant to M.R.S.; a Peterhouse Research Studentship to C.D.; a March of Dimes Basil O'Connor Award 5-FY04-195 and an Isaac Newton Trust award to C.V.H.B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arias AM, Hayward P. Filtering transcriptional noise during development: concepts and mechanisms. Nat Rev Genet. 2006;7:34–44. doi: 10.1038/nrg1750. [DOI] [PubMed] [Google Scholar]
- Aubert J, Dunstan H, Chambers I, Smith A. Functional gene screening in embryonic stem cells implicates Wnt antagonism in neural differentiation. Nat Biotechnol. 2002;20:1240–5. doi: 10.1038/nbt763. [DOI] [PubMed] [Google Scholar]
- Bailey AP, Streit A. Sensory organs: making and breaking the pre-placodal region. Curr Top Dev Biol. 2006;72:167–204. doi: 10.1016/S0070-2153(05)72003-2. [DOI] [PubMed] [Google Scholar]
- Baker CVH, Stark MR, Marcelle C, Bronner-Fraser M. Competence, specification and induction of Pax-3 in the trigeminal placode. Development. 1999;126:147–56. doi: 10.1242/dev.126.1.147. [DOI] [PubMed] [Google Scholar]
- Baker CVH, Bronner-Fraser M. Establishing neuronal identity in vertebrate neurogenic placodes. Development. 2000;127:3045–56. doi: 10.1242/dev.127.14.3045. [DOI] [PubMed] [Google Scholar]
- Baker CVH, Bronner-Fraser M. Vertebrate cranial placodes I. Embryonic induction. Dev Biol. 2001;232:1–61. doi: 10.1006/dbio.2001.0156. [DOI] [PubMed] [Google Scholar]
- Baker CVH, Stark MR, Bronner-Fraser M. Pax3-expressing trigeminal placode cells can localize to trunk neural crest sites but are committed to a cutaneous sensory neuron fate. Dev Biol. 2002;249:219–36. doi: 10.1006/dbio.2002.0767. [DOI] [PubMed] [Google Scholar]
- Begbie J, Brunet JF, Rubenstein JL, Graham A. Induction of the epibranchial placodes. Development. 1999;126:895–902. doi: 10.1242/dev.126.5.895. [DOI] [PubMed] [Google Scholar]
- Begbie J, Ballivet M, Graham A. Early steps in the production of sensory neurons by the neurogenic placodes. Mol Cell Neurosci. 2002;21:502–11. doi: 10.1006/mcne.2002.1197. [DOI] [PubMed] [Google Scholar]
- Bel-Vialar S, Medevielle F, Pituello F. The on/off of Pax6 controls the tempo of neuronal differentiation in the developing spinal cord. Dev Biol. 2007;305:659–673. doi: 10.1016/j.ydbio.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Bailey AP, Bronner-Fraser M, Streit A. Segregation of lens and olfactory precursors from a common territory: cell sorting and reciprocity of Dlx5 and Pax6 expression. Dev Biol. 2004;271:403–14. doi: 10.1016/j.ydbio.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Brigande JV, Kiernan AE, Gao X, Iten LE, Fekete DM. Molecular genetics of pattern formation in the inner ear: do compartment boundaries play a role? Proc Natl Acad Sci. 2000;97:11700–6. doi: 10.1073/pnas.97.22.11700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunelli S, Relaix F, Baesso S, Buckingham M, Cossu G. Beta catenin-independent activation of MyoD in presomitic mesoderm requires PKC and depends on Pax3 transcriptional activity. Dev Biol. 2007;304:604–14. doi: 10.1016/j.ydbio.2007.01.006. [DOI] [PubMed] [Google Scholar]
- D'Amico-Martel A, Noden DM. Contributions of placodal and neural crest cells to avian cranial peripheral ganglia. Am J Anat. 1983;166:445–68. doi: 10.1002/aja.1001660406. [DOI] [PubMed] [Google Scholar]
- DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–68. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- Ericson J, Thor S, Edlund T, Jessell TM, Yamada T. Early stages of motor neuron differentiation revealed by expression of homeobox gene Islet-1. Science. 1992;256:1555–60. doi: 10.1126/science.1350865. [DOI] [PubMed] [Google Scholar]
- Etchevers HC, Vincent C, Le Douarin NM, Couly GF. The cephalic neural crest provides pericytes and smooth muscle cells to all blood vessels of the face and forebrain. Development. 2001;128:1059–68. doi: 10.1242/dev.128.7.1059. [DOI] [PubMed] [Google Scholar]
- Fekete DM, Wu DK. Revisiting cell fate specification in the inner ear. Curr Opin Neurobiol. 2002;12:35–42. doi: 10.1016/s0959-4388(02)00287-8. [DOI] [PubMed] [Google Scholar]
- Fode C, Gradwohl G, Morin X, Dierich A, LeMeur M, Goridis C, Guillemot F. The bHLH protein NEUROGENIN 2 is a determination factor for epibranchial placode-derived sensory neurons. Neuron. 1998;20:483–94. doi: 10.1016/s0896-6273(00)80989-7. [DOI] [PubMed] [Google Scholar]
- Geetha-Loganathan P, Nimmagadda S, Huang R, Christ B, Scaal M. Regulation of ectodermal Wnt6 expression by the neural tube is transduced by dermomyotomal Wnt11: a mechanism of dermomyotomal lip sustainment. Development. 2006;133:2897–904. doi: 10.1242/dev.02464. [DOI] [PubMed] [Google Scholar]
- Henrique D, Adam J, Myat A, Chitnis A, Lewis J, Ish-Horowicz D. Expression of a Delta homologue in prospective neurons in the chick. Nature. 1995;375:787–90. doi: 10.1038/375787a0. [DOI] [PubMed] [Google Scholar]
- Hirabayashi Y, Itoh Y, Tabata H, Nakajima K, Akiyama T, Masuyama N, Gotoh Y. The Wnt/beta-catenin pathway directs neuronal differentiation of cortical neural precursor cells. Development. 2004;131:2791–801. doi: 10.1242/dev.01165. [DOI] [PubMed] [Google Scholar]
- Hollyday M, McMahon JA, McMahon AP. Wnt expression patterns in chick embryo nervous system. Mech Dev. 1995;52:9–25. doi: 10.1016/0925-4773(95)00385-e. [DOI] [PubMed] [Google Scholar]
- Holzschuh J, Wada N, Wada C, Schaffer A, Javidan Y, Tallafuss A, Bally-Cuif L, Schilling TF. Requirements for endoderm and BMP signaling in sensory neurogenesis in zebrafish. Development. 2005;132:3731–42. doi: 10.1242/dev.01936. [DOI] [PubMed] [Google Scholar]
- Huelsken J, Behrens J. The Wnt signalling pathway. J Cell Sci. 2002;115:3977–8. doi: 10.1242/jcs.00089. [DOI] [PubMed] [Google Scholar]
- Ikeya M, Lee SM, Johnson JE, McMahon AP, Takada S. Wnt signalling required for expansion of neural crest and CNS progenitors. Nature. 1997;389:966–70. doi: 10.1038/40146. [DOI] [PubMed] [Google Scholar]
- Israsena N, Hu M, Fu W, Kan L, Kessler JA. The presence of FGF2 signaling determines whether beta-catenin exerts effects on proliferation or neuronal differentiation of neural stem cells. Dev Biol. 2004;268:220–31. doi: 10.1016/j.ydbio.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Kim CH, Oda T, Itoh M, Jiang D, Artinger KB, Chandrasekharappa SC, Driever W, Chitnis AB. Repressor activity of Headless/Tcf3 is essential for vertebrate head formation. Nature. 2000;407:913–6. doi: 10.1038/35038097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo F, Takeichi M, Nakagawa S. Wnt2b inhibits differentiation of retinal progenitor cells in the absence of Notch activity by downregulating the expression of proneural genes. Development. 2005;132:2759–70. doi: 10.1242/dev.01856. [DOI] [PubMed] [Google Scholar]
- Kuhl M. The WNT/calcium pathway: biochemical mediators, tools and future requirements. Front Biosci. 2004;9:967–74. doi: 10.2741/1307. [DOI] [PubMed] [Google Scholar]
- Ladher RK, Anakwe KU, Gurney AL, Schoenwolf GC, Francis-West PH. Identification of synergistic signals initiating inner ear development. Science. 2000;290:1965–7. doi: 10.1126/science.290.5498.1965. [DOI] [PubMed] [Google Scholar]
- Ladher RK, Wright TJ, Moon AM, Mansour SL, Schoenwolf GC. FGF8 initiates inner ear induction in chick and mouse. Genes Dev. 2005;19:603–13. doi: 10.1101/gad.1273605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linker C, Lesbros C, Stark MR, Marcelle C. Intrinsic signals regulate the initial steps of myogenesis in vertebrates. Development. 2003;130:4797–807. doi: 10.1242/dev.00688. [DOI] [PubMed] [Google Scholar]
- Linker C, Lesbros C, Gros J, Burrus LW, Rawls A, Marcelle C. beta-Catenin-dependent Wnt signalling controls the epithelial organisation of somites through the activation of paraxis. Development. 2005;132:3895–905. doi: 10.1242/dev.01961. [DOI] [PubMed] [Google Scholar]
- Litsiou A, Hanson S, Streit A. A balance of FGF, BMP and WNT signalling positions the future placode territory in the head. Development. 2005;132:4051–62. doi: 10.1242/dev.01964. [DOI] [PubMed] [Google Scholar]
- Ma Q, Chen Z, del Barco Barrantes I, de la Pompa JL, Anderson DJ. neurogenin1 is essential for the determination of neuronal precursors for proximal cranial sensory ganglia. Neuron. 1998;20:469–82. doi: 10.1016/s0896-6273(00)80988-5. [DOI] [PubMed] [Google Scholar]
- Marcelle C, Wolf J, Bronner-Fraser M. The in vivo expression of the FGF receptor FREK mRNA in avian myoblasts suggests a role in muscle growth and differentiation. Dev Biol. 1995;172:100–14. doi: 10.1006/dbio.1995.0008. [DOI] [PubMed] [Google Scholar]
- Marcelle C, Stark MR, Bronner-Fraser M. Coordinate actions of BMPs, Wnts, Shh and noggin mediate patterning of the dorsal somite. Development. 1997;124:3955–63. doi: 10.1242/dev.124.20.3955. [DOI] [PubMed] [Google Scholar]
- Martin K, Groves AK. Competence of cranial ectoderm to respond to Fgf signaling suggests a two-step model of otic placode induction. Development. 2006;133:877–87. doi: 10.1242/dev.02267. [DOI] [PubMed] [Google Scholar]
- Matsunaga E, Araki I, Nakamura H. Role of Pax3/7 in the tectum regionalization. Development. 2001;128:4069–77. doi: 10.1242/dev.128.20.4069. [DOI] [PubMed] [Google Scholar]
- Megason SG, McMahon AP. A mitogen gradient of dorsal midline Wnts organizes growth in the CNS. Development. 2002;129:2087–98. doi: 10.1242/dev.129.9.2087. [DOI] [PubMed] [Google Scholar]
- Mishima N, Tomarev S. Chicken Eyes absent 2 gene: isolation and expression pattern during development. Int J Dev Biol. 1998;42:1109–15. [PubMed] [Google Scholar]
- Mulder H, Leckstrom A, Uddman R, Ekblad E, Westermark P, Sundler F. Islet amyloid polypeptide (amylin) is expressed in sensory neurons. J Neurosci. 1995;15:7625–32. doi: 10.1523/JNEUROSCI.15-11-07625.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–11. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- Muroyama Y, Kondoh H, Takada S. Wnt proteins promote neuronal differentiation in neural stem cell culture. Biochem Biophys Res Commun. 2004;313:915–21. doi: 10.1016/j.bbrc.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Katahira T, Sato T, Watanabe Y, Funahashi J. Gain- and loss-of-function in chick embryos by electroporation. Mech Dev. 2004;121:1137–43. doi: 10.1016/j.mod.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Nechiporuk A, Linbo T, Raible DW. Endoderm-derived Fgf3 is necessary and sufficient for inducing neurogenesis in the epibranchial placodes in zebrafish. Development. 2005;132:3717–30. doi: 10.1242/dev.01876. [DOI] [PubMed] [Google Scholar]
- Nechiporuk A, Linbo T, Poss KD, Raible DW. Specification of epibranchial placodes in zebrafish. Development. 2007;134:611–23. doi: 10.1242/dev.02749. [DOI] [PubMed] [Google Scholar]
- Nikaido M, Doi K, Shimizu T, Hibi M, Kikuchi Y, Yamasu K. Initial specification of the epibranchial placode in zebrafish embryos depends on the fibroblast growth factor signal. Dev Dyn. 2007;236:564–71. doi: 10.1002/dvdy.21050. [DOI] [PubMed] [Google Scholar]
- Noden DM. Spatial integration among cells forming the cranial peripheral nervous system. J Neurobiol. 1993;24:248–61. doi: 10.1002/neu.480240210. [DOI] [PubMed] [Google Scholar]
- Ohyama T, Mohamed OA, Taketo MM, Dufort D, Groves AK. Wnt signals mediate a fate decision between otic placode and epidermis. Development. 2006;133:865–75. doi: 10.1242/dev.02271. [DOI] [PubMed] [Google Scholar]
- Perez SE, Rebelo S, Anderson DJ. Early specification of sensory neuron fate revealed by expression and function of neurogenins in the chick embryo. Development. 1999;126:1715–28. doi: 10.1242/dev.126.8.1715. [DOI] [PubMed] [Google Scholar]
- Schlosser G. Induction and specification of cranial placodes. Dev Biol. 2006;294:303–51. doi: 10.1016/j.ydbio.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Tanaka M, Munsterberg A. Expression of (beta)-catenin in the developing chick myotome is regulated by myogenic signals. Development. 2000;127:4105–13. doi: 10.1242/dev.127.19.4105. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Stoeckelhuber M, McKinnell I, Putz R, Christ B, Patel K. Wnt 6 regulates the epithelialisation process of the segmental plate mesoderm leading to somite formation. Dev Biol. 2004;271:198–209. doi: 10.1016/j.ydbio.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Stark MR, Sechrist J, Bronner-Fraser M, Marcelle C. Neural tube-ectoderm interactions are required for trigeminal placode formation. Development. 1997;124:4287–95. doi: 10.1242/dev.124.21.4287. [DOI] [PubMed] [Google Scholar]
- Stark MR, Biggs JJ, Schoenwolf GC, Rao MS. Characterization of avian frizzled genes in cranial placode development. Mech Dev. 2000;93:195–200. doi: 10.1016/s0925-4773(00)00263-x. [DOI] [PubMed] [Google Scholar]
- Sun SK, Dee CT, Tripathi VB, Rengifo A, Hirst CS, Scotting PJ. Epibranchial and otic placodes are induced by a common Fgf signal, but their subsequent development is independent. Dev Biol. 2007;303:675–86. doi: 10.1016/j.ydbio.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–6. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell. 2003;5:367–77. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]


