Abstract
Dendritic cells (DC) are potent antigen-presenting cells that are essential for initiating adaptive immune responses. Residing within the airway mucosa, pulmonary DC continually sample the antigenic content of inhaled air and migrate to draining lymph nodes, where they present these antigens to naive T cells. The migratory patterns of pulmonary DC are highly dependent upon inflammatory conditions in the lung. Under steady-state, or non-inflammatory, conditions, pulmonary DC undergo slow but constitutive migration to draining lymph nodes, where they remain for several days and confer antigen-specific tolerance. With the onset of pulmonary inflammation, airway DC trafficking increases dramatically, and these cells rapidly accumulate within draining lymph nodes. However, within a few days, the number of airway-derived DC in lymph nodes stabilizes or declines, even in the face of ongoing pulmonary inflammation. Here, we summarize current understanding of the molecular and cellular mechanisms underlying pulmonary DC trafficking to the lymph node and the recruitment of DC precurors to the lung. It is hoped that an improved understanding of these mechanisms will lead to novel DC-mediated therapeutic strategies to treat immune-related pulmonary disease.
Keywords: lung, dendritic cells, lymph nodes
INNATE IMMUNITY TRIGGERS DENDRITIC CELL MATURATION AND ADAPTIVE IMMUNE RESPONSES
Innate immune responses provide a front-line defense against invading pathogens. In addition to this function, the innate immune system also triggers adaptive immune responses, which are required to eradicate invading organisms. Although these innate and adaptive immune responses are essential for vertebrate survival, they must be under strict regulatory control to avoid unnecessary immune-related damage to critical organs. This is particularly important in the lung, an organ that samples approximately 10,000 L of air every day and is exposed to a wide variety of particles and macromolecules. To maintain efficient gas exchange, the lung's mucosal immune system must remain tolerant to a multitude of innocuous airborne particles, yet be capable of generating robust effector immune responses to inhaled pathogens.
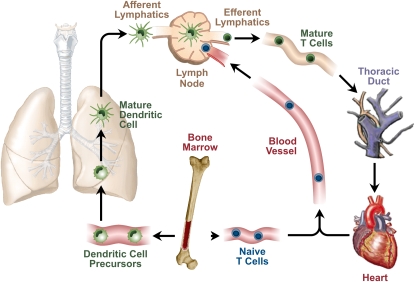
Dendritic cells (DC) develop from bone marrow–derived precursor cells that travel in the bloodstream to lymphoid tissues and the epithelia of body surfaces, such as the skin, gut, and airway. After arriving in these tissues, the precursor cells undergo a maturation step and acquire the ability to take up antigens (Figure 1). After this antigen uptake, DC migrate to draining lymph nodes, where they present airway-derived antigens to naive T cells (1, 2). This DC migration event is necessary because naive T cells do not reside within peripheral tissues, but circulate continuously between the blood and secondary lymphoid tissues, such as the spleen and lymph nodes. After arrival in the lymph node, DC move about within T cell areas, making transient contact with a large number of naive T cells and more sustained contact with those that recognize antigens presented on the DC surface (3). Through this series of events, DC provide a dynamic representation of the antigenic environment within the lung to a large number of potentially responsive, naive T cells. Those cells that recognize the presented antigen subsequently differentiate into regulatory, helper, or effector T cells that can enter peripheral tissue to assist in adaptive immune responses.
Figure 1.
Life cycle of pulmonary dendritic cells (DC). DC precursors exit the bone marrow and travel in the circulation to the lung, where they establish residence. Under steady-state conditions, pulmonary DC migrate constitutively to draining thoracic lymph nodes and present pulmonary antigens to naive T cells.
The immunologic consequences of antigen presentation in draining lymph nodes depend largely on inflammatory conditions within the airway. Under steady-state conditions, inert antigens within the airway generally invoke tolerogenic responses (4–6). This antigen-specific tolerance is not simply the absence of an effector immune response, but is an active process requiring T cell proliferation and differentiation (7). Upon antigen presentation by tolerogenic DC, naive T cells acquire regulatory properties (Treg cells), and are able to suppress responses to antigens even when they are administered together with powerful adjuvants. A widely-held consensus is that tolerogenic signals are conferred by antigens presented by immature DC. However, most immature DC do not take up antigen well and are not very motile, and at least some tolerogenic DC display markers often associated with maturation (8). Thus, the phenotypic nature of tolerogenic DC remains a matter of some debate (9). Nonetheless, tolerogenic responses to harmless inhaled antigens are clearly important to prevent unnecessary and harmful inflammatory responses in the lung, and the breakdown of this tolerance is regarded as an important event in the development of allergic asthma.
In addition to remaining tolerant to harmless airborne antigens, vertebrates must also generate rapid and effective immune responses to inhaled pathogens. An increasing body of evidence suggests that DC recognize pathogens through innate mechanisms encoded in the germ line. For example, individual members of the Toll-like receptor (TLR) family signal in response to various pathogen-associated molecular patterns (PAMPs) derived from a variety of organisms, including bacteria, viruses, and fungi. Thus, TLR4 recognizes endotoxin, or LPS from gram-negative bacteria, TLR3 recognizes single-standed DNA associated with viruses, and TLR9 recognizes unmethylated CpG motifs found in bacterial DNA. Recognition of these PAMPs by DC triggers their maturation, mobilization, and capacity to prime naive T cells to become effector cells that will, in turn, clear the pathogen from the host (reviewed in Reference 10).
Active infections of the lung are relatively rare, but this organ is constantly exposed to at least some level of proinflammatory stimuli in the form of PAMPs and nonbiological environmental agents, such as ozone and airborne particulate matter. The effect of these agents on pulmonary immunity depends to a large extent on their levels. Thus, when endotoxin-free ovalbumin is delivered to the airway of mice, they do not become sensitized to this protein. However, when small amounts of endotoxin are coadministered together with the ovalbumin, mice develop antigen-specific Th2 immune responses. When delivered to the airway together with high levels of endotoxin, ovalbumin elicits Th1 responses (11). The adjuvant activity of LPS probably resides, at least in part, to its ability to increase display of costimulatory molecules, such as CD80 and CD86, on the surface of DC (reviewed in Reference 10). In addition to these changes, the tempo and amplitude of DC trafficking to draining lymph nodes also increase dramatically during inflammation. The trafficking of leukocytes, including DC, is governed largely by a family of small chemotactic molecules known as chemokines. However, compared with our knowledge of costimulatory molecule regulation, comparatively little is known of the molecular mechanisms that govern pulmonary DC trafficking to lymph nodes, and it is unclear whether regulation of this trafficking controls the nature of adaptive immune responses in the lung.
PULMONARY DC SUBSETS
Pulmonary DC are not a homogeneous set of cells; rather, they can be classified based on several criteria, including anatomical location and display of cell surface markers, such as CD11b and CD8α (12). Immunostaining of the rat trachea has revealed that two types of DC can be distinguished by their morphology and proximity to the airway lumen (13). DC having the classic dendriform morphology and high levels of major histocompatibility complex (MHC) class II are found within the epithelium of the airway wall (13, 14). However, these cells comprise only 20% of total airway mucosal DC population. The remaining 80% lie beneath the epithelium, and represent a heterogeneous population. Some of these cells have a dendriform morphology, and might represent mature, antigen-bearing DC that are trafficking to draining lymph nodes. Other cells within the supepithelial region are smaller with a more rounded morphology, and likely represent immature cells in transit from the blood to the airway mucosa. Phenotypic analyses have shown that the intraepithelial DC have endocytic activity, whereas the subepithelial cells do not (13). Despite their differences in morphology and activities, these two major DC types display similar cell surface molecules, further supporting the notion that they represent different stages of maturation rather than distinct lineages.
In addition to differences in their maturation stages, pulmonary DC also exhibit functional differences, depending on their anatomical location and display levels of cell surface molecules. DC having high levels of the DC marker, CD11c, are found in both the airway and parenchyma of the mouse lung. Compared with their counterparts in the lung parenchyma, CD11chi cells in the airway are more endocytic, are more efficient at presenting peptides to naive T cells, and have a shorter half-life (15). In addition to this CD11chi DC, a short-lived CD11cneg mDC is also found in the airway mucosa, but not in the parenchyma. Neither airway nor parenchymal DC of naive mice can present whole protein to T cells without further maturation in vitro, or trafficking to lymph nodes in vivo.
PULMONARY DC TRAFFICKING TO LYMPH NODES UNDER STEADY-STATE CONDITIONS
The trafficking of antigen-bearing pulmonary DC to draining lymph nodes is a critical initial step in adaptive immune responses. Detailed studies of this trafficking event require the ability to distinguish “resident” DC in the lymph node from cells that have migrated there from the lung. This is most commonly achieved through the use of carboxyfluoroscein succinimidyl ester (CFSE). When this fluorescent dye is delivered to the trachea, cells exposed to the airway will take up the dye and retain it for several days. CFSE+ DC migrating from the airway to the lymph nodes can therefore be identified and distinguished from resident DC using flow cytometry or fluorescent microscopy. CFSE staining can be used to study pulmonary DC trafficking in the absence of deliberate antigen challenge. One day after CFSE treatment, approximately 2% of cells within draining lymph nodes are CFSE+ (16). These CFSE+ DC have higher levels of CD40, CD80, CD86, and MHC class II than the resident lymph node DC that do not contain CFSE. This observation raises the question of whether CFSE treatment causes DC maturation and consequent increased display of these molecules, or whether these cells had already matured before their staining by CFSE. The first possibility suggests that CFSE staining of the airway might not be an appropriate model to study DC trafficking under steady-state conditions. The latter possibility suggests that some cells having cell surface characteristics of immunogenic DC undergo constitutive trafficking from the airway—even under steady-state conditions that are widely believed to confer tolerogenic, not immunogenic, responses.
The use of fluorescently labeled proteins provides a means to discriminate between DC that have taken up antigen and those that have not. Vaermaelen and coworkers first used fluorescein isothiocyanate–conjugated ovalbumin to formally demonstrate that DC take up antigen in the airway and subsequently migrate to draining lymph nodes (17). The allergen-bearing cells within the lymph node were identified as being CD11cmed–hi/MHC class IIhi cells, whereas DC displaying lower levels of MHC class II did not contain antigen. These types of experiments are designed to mimic steady-state conditions in the airway, but, as with CFSE-based experiments, one cannot exclude the possibility that the reagents or procedures used perturb the maturation status or trafficking patterns of airway DC. This concern is underscored by the findings that even low levels of LPS can confer sensitizing conditions in the airway (11), presumably by altering the function of airway DC.
It has long been known that mice exposed to aerosolized ovalbumin become tolerized to that allergen, as evidenced by their inability to subsequently become sensitized by intraperitoneal injections with allergen complexed to alum (18), a commonly used method of allergic sensitization. Hintzen and colleagues recently demonstrated that mice lacking the chemokine receptor, CCR7, do not undero allergen-specific tolerance after exposure to aerosolized ovalbumin (19). Moreover, after airway delivery of fluorescently labeled ovalbumin previously treated to remove endotoxin, allergen-bearing DC of wild-type mice, but not CCR7-deficient mice, migrate to draining lymph nodes (19). This finding is consistent with the work of Jakubzick and colleagues, who showed that mice lacking the chemokine ligands of CCR7 have a reduced capacity to transport latex particles from the lung to draining lymph nodes (20). Therefore, under these conditions, CCR7 is an important receptor for pulmonary DC trafficking. It is currently unclear whether this same chemokine receptor, or a different one, is important for pulmonary DC trafficking under other conditions, including those that promote allergen sensitization (11). Interestingly, resident DC are present in lymph nodes of CCR7-deficient mice (19), suggesting that these DC migrate from the lung or from the blood by a CCR7-independent pathway.
PULMONARY DC TRAFFICKING TO LYMPH NODES DURING ALLERGIC INFLAMMATION
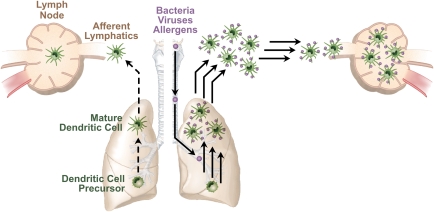
During ongoing allergic inflammation, the total number of DC in the lung, airway, and draining lymph nodes are increased, as are levels of costimulatory molecules displayed on these cells (21). In addition, the speed of migration and the number of allergen-bearing DC trafficking to lymph nodes is also increased (21) (Figure 2). In the face of continued allergen challenge, this dramatic increase in allergen-bearing DC trafficking might be expected to accelerate ongoing immune responses indefinitely; however, this does not happen. When sensitized mice are challenged daily with allergen, the intensity of pulmonary inflammation reaches a maximum after approximately 7 to 10 days, and declines thereafter (22). There are several possibilities that might account for this eventual decline in allergic pulmonary inflammation. First, the life span of airway-derived DC in the lymph nodes of mice with inflamed lungs is much shorter than that of mice with uninflamed lungs. This decreased life span might be related to levels of the antiapoptotic gene, Bcl-2, which rise in DC shortly (24 h) after their arrival in the lymph node, but decline to baseline values by 48 hours after arrival (21). If so, other genes must also contribute to this phenomenon, because Bcl-2 levels at 48 hours after arrival do not decline to levels below that seen in noninflamed lymph nodes, where DC survive much longer. A second possibility is that excessive stimulation of T cells by DC densities might induce T-cell death or exhaustion, as has been seen in vitro (23) and in vivo (24, 25). A third possibility is that, although these DC display high levels of MHC class II, they are actually tolerogenic. Finally, conditions within lymph nodes that drain inflamed tissues may promote the activation of Treg cells, even in the presence of antigen-presenting DC, which might, under other circumstances, be immunogenic. The latter two hypotheses are not mutually exclusive, and are both supported by the finding that tolerogenic DC interact with the inhibitory receptors, programmed death-1 (PD-1) and cytotoxic lymphocyte-associated antigen-4, on T cells, and that blocking these interactions interferes with tolerance (26).
Figure 2.
Pulmonary DC trafficking is increased by innate immune stimuli. Antigen-bearing DC migration increases in both speed and volume after pulmonary exposure to a variety of innate immune–stimulating agents in the environment. This leads to an initial increase in the number of airway-derived DC in the lymph node, but this increase is short lived.
Not all allergen-bearing DC in the lung traffic to draining lymph nodes. In mice sensitized for a Th2 response to Leishmania, substantial numbers of DC-presenting Leishmania analogue of the receptors of activated C kinase (LACK) peptides can be found in the airways for up to 8 weeks after LACK antigen exposure (27). These LACK-presenting airway DC express high levels of costimulatory molecules on their cell surface, suggesting that these cells might contribute to the chronic Th2 airway inflammation characteristic of allergic asthma. It is not yet known whether these allergen-bearing DC fail to migrate to lymph nodes, because they do not have the chemokine receptor(s) required for trafficking.
PULMONARY DC TRAFFICKING TO LYMPH NODES AFTER BACTERIAL CHALLENGE
Challenge of rats with heat-killed Moraxella catarrhalis induces a transient change in the morphology of the submucosal population of DC. The initial rounded appearance of these cells is replaced by the dendriform appearance normally associated with more mature DC. This morphologic change is accompanied by an increase in levels of maturation markers, but these cells do not acquire the endocytic activity or the ability to support antigen-specific T-cell proliferation (13). Therefore, morphologic appearance and display of cell surface molecules on pulmonary DC in bacteria-challenged mice do not necessarily predict their maturity as measured by the more stringent criterion of T-cell proliferation.
In contrast to most airway DC, antigen-bearing DC in the lymph node support antigen-specific T-cell proliferation, and this ability is augmented by innate immune signals. Thus, in the absence of bacterial challenge, DC within draining lymph nodes of rats treated with ovalbumin stimulate T-cell proliferation relatively poorly, but this ability is increased in animals treated with ovalbumin together with heat-killed bacteria (13). The ability of DC in the lymph node, but not in the airway, to support T-cell proliferation suggests that pulmonary DC might continue their maturation process en route to the lymph node. Alternatively, fully mature, antigen-presenting DC might leave the lung and begin their migration to draining lymph nodes immediately upon acquiring this capability.
The lack of T-cell stimulatory ability of airway DC from animals treated with both allergen and heat-killed bacteria is not an inherent property of these cells. Airway DC of mice that that have been previously sensitized can stimulate T cells upon challenge with that allergen. Moreover, this T cell–stimulating activity does not require an innate immune stimulus, such as heat-killed bacteria (13). Therefore, sensitization must modify pulmonary DC, possibly through their interaction with allergen-specific T cells. This T cell–stimulating activity of airway DC is seen immediately after allergen challenge, but not at 24 hours after challenge. Conversely, T-cell stimulatory ability is seen in lymph node DC by 24 hours, but not at 2 hours after challenge. The simplest explanation for these findings is that virtually all of the DC in the airway of sensitized and challenged mice migrate to draining lymph nodes within hours of allergen challenge.
PULMONARY DC TRAFFICKING TO LYMPH NODES AFTER VIRAL CHALLENGE
As seen during ongoing allergic pulmonary inflammation or after treatment with heat-killed bacteria, viral infection also increases the rate of pulmonary DC trafficking to draining lymph nodes. In mice receiving CFSE followed by infection with influenza virus, the number of airway-derived DC represent approximately 7% of total lymph node DC, as opposed to only 2% under steady-state conditions (16). Unlike what is seen under steady-state conditions, this percentage continues to rise in influenza-infected animals, and reaches a maximum of 18% at 18 hours after infection. At or near this point, the percentage of airway-derived cells in the lymph node begins to decline rapidly, falling to 8% by 24 hours, and returning to 2% by 48 hours after infection. Interestingly, this decline occurs despite an ongoing viral infection in the lung and a continuing increase in the total number of CD11c+ cells in the lung. Moreover, a second viral challenge fails to elicit a second wave of airway DC accumulation in draining lymph nodes. Although this rapid reduction in airway-derived DC within the draining lymph node might be due to reduced trafficking, it seems possible that at least some of this decline might also result from a decreased life span of DC in lymph nodes of virus-infected lungs. Whatever the mechanism, it is notable that this decline in airway-derived DC is seen during both ongoing allergic inflammation and virus-induced inflammation (16, 21).
RECRUITMENT OF DC PRECURSORS TO THE LUNG UNDER STEADY-STATE CONDITIONS
The above described departure of mature DC from the lung to draining lymph nodes—even under steady-state conditions—suggests that these cells must be replaced at a similar rate to maintain homeostasis. Studies of CFSE-stained airway DC have shown that the turnover rates of these cells depend on their anatomic location and cell surface markers. The turnover rate of DC in the upper airway is much greater than that of their counterparts in the lung parenchyma. Whereas approximately 80% of the upper airway DC are replaced by new cells within 18 hours, only 12% of parenchymal DC are replaced within 9 days (16). These differences in turnover rates are also associated with display levels of CD11c. Upper airway DC display moderate levels of CD11c, whereas most lower airway DC have high expression of this surface molecule. However, the lower airway also contains DC with moderate levels of CD11c, and these cells appear to turnover more quickly than those with high levels of CD11c. Thus, moderate levels of CD11c may define airway DC in both the upper and lower airways, whereas DC displaying higher levels of this marker might reside within the lung parenchyma.
Stumbles and coworkers have shown that an antagonist to the chemokine receptors, CCR1 and CCR5, reduces baseline numbers of rat tracheal intraepithelial DC by approximately 50%. Assuming that this antagonist was fully effective, these data suggest that, under steady-state conditions, there are at least two pathways to recruit DC precursors: one that depends on CCR1 and/or CCR5, and one that does not. It is possible that the latter mechanism involves the chemokine receptor, CX3CR1. This receptor is highly expressed on a population of monocytes that are recruited to noninflamed tissues, including the lung, and, in CX3CR1-deficient mice, monocyte recruitment is markedly reduced in uninflamed tissue, but not in inflamed tissue (28). Because monocytes can differentiate into DC, CX3CR1-directed monocyte recruitment might be important in maintaining DC homeostasis under steady-state conditions.
RECRUITMENT OF DC PRECURSORS TO THE LUNG AFTER BACTERIAL CHALLENGE
The rate of DC accumulation in the lung increases markedly after challenge with heat-killed bacteria (29). However, unlike a wave of incoming neutrophils, which rapidly moves into the airway lumen, the DC precursors remain within the epithelium during the acute inflammatory response and assume the dendriform morphology typical of mature airway DC. During the 48-hour period after bacterial challenge, many of these DC migrate to draining lymph nodes. Interestingly, no such responses are seen after similar bacterial challenges to the epidermis or peritoneum, suggesting that the requirements for DC trafficking in the lung are different from those of other tissues. The CCR1/CCR5 antagonist blocks an even higher proportion of this bacteria-induced DC recruitment to the lung than that seen under steady-state conditions. This finding does not exclude a function for other chemokine receptors in recruiting DC precursors to the lung during bacteria-induced inflammation, however, because different receptors might be sequentially required for DC precursor recruitment, or for the recruitment of other types of DC precursors.
RECRUITMENT OF DC PRECURSORS TO THE LUNG AFTER ANTIGENIC CHALLENGE
Airway exposure to inert protein antigens, such as ovalbumin, can elicit rapid DC accumulation in the lung, and the extent of this accumulation is markedly increased if the animals have been previously sensitized (14). Apparently, DC precursor recruitment during allergen challenge differs mechanistically from the recruitment after a bacterial stimulus because the former is not blocked by the CCR1/CCR5 antagonist (30). Some of this difference might be due to the presence of antigen-specific T cells in the lung that can modify the responses of DC to antigen. During such secondary immune responses, the number of mature DC in the lung correlates with levels of inflammation, and with levels of various chemokines and cytokines (22, 31). However, it is difficult to determine whether increased levels of pulmonary DC lead to increased inflammation or whether the converse is true.
Osterholzer and colleagues studied chemokine receptor requirements for DC recruitment to the lung after their sensitization and challenge with sheep red blood cells (32). In this study, mice lacking the chemokine receptor, CCR2, had fewer DC in the lung parenchyma and airway at 3 days after challenge than similarly challenged wild-type mice. By contrast, mice lacking CCR6 had normal numbers of DC in the lung, but fewer DC in the airway compared with wild-type mice. These findings suggest that, in this model, CCR2 directs DC precursors from the blood to the lung interstitium, whereas CCR6 directs transit of DC from the interstitium to the airway. The requirements of CCR1 and CCR5 were not addressed in this study of secondary immune responses, but, as noted previously, these receptors were dispensable for DC recruitment to the lung after secondary immune responses to ovalbumin (30). It is possible, therefore, that during secondary immune responses in the lung, CCR2 and CCR6 are important for DC recruitment, whereas CCR1 and CCR5 fulfill this function under steady-state conditions and after innate immune stimuli.
RECRUITMENT OF DC PRECURSORS TO THE LUNG AFTER VIRAL CHALLENGE
The number of mature pulmonary DC is increased after infection with influenza virus (33), sendai virus (30, 34), or respiratory syncytial virus (RSV) (35). However, the nature of immunologic responses to these pathogens depends on the individual virus. For example, RSV induces a Th2-type response and eosinophil recruitment to the lung, whereas influenza infection leads to a Th1-type response. It is not yet clear whether different viruses also have different effects on DC trafficking. With this caveat in mind, the CCR1/CCR5 antagonist fails to block the influx of DC to the lung after infection with Sendai virus (30). This finding contrasts with the efficacy of this antagonist in blocking bacteria-induced DC recruitment (30), and suggests that different chemokine ligands might be induced by bacterial and viral infections. It would be helpful to know whether CCR6 and CCR2 also function to recruit DC to the lung in the virus-infected lung, as they appear to do in sheep red blood cell–challenged lung.
In RSV-infected mice, the large increases in mature pulmonary DC outlast acute infection (35), and might explain the enhancement of subsequent responses to allergen exposure associated with RSV infections. This increase in allergen sensitivity is not restricted to viruses that promote Th2 responses, however, because mice previously challenged with influenza virus, which induces a Th1 response, are also more sensitive to challenge with allergen (33).
Recruitment of DC precursors from the blood to the lung has been widely regarded as the primary means of replenishing the pulmonary DC population after an inflammatory challenge. However, a recent study demonstrated that RSV infection reduces the number of DC precursors in the lung and prevents the increase in mature DC that is normally seen after challenge with viral infection or LPS (36). These findings call into question the notion that increases in mature pulmonary DC seen after inflammatory challenge is due primarily to recruitment of DC precursors that subsequently undergo maturation. Additional studies will be required to determine whether the findings in this study are relevant to other types of inflammatory challenges of the lung.
FUTURE DIRECTIONS
The critical role of DC in immune responses suggests that the ability to manipulate DC migration might have therapeutic potential for some pulmonary diseases. In the case of allergic asthma, inhibiting the traffic of immunogenic DC to lymph nodes while augmenting the traffic of tolerogenic DC might reduce allergic inflammation. Before this potential can be realized, however, additional information is required regarding the molecular pathways that direct DC trafficking during different physiologic settings. CCR7 is an important chemokine receptor for tolerogenic responses in the mouse lung, but the molecular pathways directing immunogenic pulmonary DC to lymph nodes have not been described, and there are virtually no data available at present regarding trafficking requirements for pulmonary DC in humans. The ability to alter recruitment of DC precursors from the blood to the lung might also have therapeutic benefit. Enhancing this recruitment might increase the strength of immune responses in individuals with recurring viral infections, whereas inhibiting DC recruitment might be beneficial for individuals with asthma. Clearly, as our understanding of pulmonary DC trafficking becomes more developed, there will be an increased likelihood of successfully exploiting DC migration for therapeutic intervention.
Supported by the intramural research program of the National Institutes of Health (NIH), National Institute of Environmental Health Sciences (D.N.C.), and by NIH grants HL54450 and HL56389 (K.B.).
Conflict of Interest Statement: D.N.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. K.B. will receive €1,000 in 2007 for speaking at a conference sponsored by Boehriner-Ingelheim.
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature 1998;392:245–252. [DOI] [PubMed] [Google Scholar]
- 2.Guermonprez P, Valladeau J, Zitvogel L, Thery C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu Rev Immunol 2002;20:621–667. Epub 2001 Oct 4. [DOI] [PubMed] [Google Scholar]
- 3.Mempel TR, Henrickson SE, Von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature 2004;427:154–159. [DOI] [PubMed] [Google Scholar]
- 4.Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med 2002;196:1627–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, Ravetch JV, Steinman RM, Nussenzweig MC. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med 2001;194:769–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol 2003;21:685–711. Epub 2001 Dec 19. [DOI] [PubMed] [Google Scholar]
- 7.Hugues S, Boissonnas A, Amigorena S, Fetler L. The dynamics of dendritic cell–T cell interactions in priming and tolerance. Curr Opin Immunol 2006;18:491–495. Epub 2006 Jun 12. [DOI] [PubMed] [Google Scholar]
- 8.Albert ML, Jegathesan M, Darnell RB. Dendritic cell maturation is required for the cross-tolerization of CD8+ T cells. Nat Immunol 2001;2:1010–1017. [DOI] [PubMed] [Google Scholar]
- 9.Reis e Sousa C. Dendritic cells in a mature age. Nat Rev Immunol 2006;6:476–483. [DOI] [PubMed] [Google Scholar]
- 10.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol 2004;5:987–995. [DOI] [PubMed] [Google Scholar]
- 11.Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, Toll-like receptor 4–dependent T helper cell type 2 responses to inhaled antigen. J Exp Med 2002;196:1645–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wikstrom ME, Batanero E, Smith M, Thomas JA, von Garnier C, Holt PG, Stumbles PA. Influence of mucosal adjuvants on antigen passage and CD4+ T cell activation during the primary response to airborne allergen. J Immunol 2006;177:913–924. [DOI] [PubMed] [Google Scholar]
- 13.Jahnsen FL, Strickland DH, Thomas JA, Tobagus IT, Napoli S, Zosky GR, Turner DJ, Sly PD, Stumbles PA, Holt PG. Accelerated antigen sampling and transport by airway mucosal dendritic cells following inhalation of a bacterial stimulus. J Immunol 2006;177:5861–5867. [DOI] [PubMed] [Google Scholar]
- 14.Holt PG. Pulmonary dendritic cells in local immunity to inert and pathogenic antigens in the respiratory tract. Proc Am Thorac Soc 2005;2:116–120. [DOI] [PubMed] [Google Scholar]
- 15.Holt PG, Haining S, Nelson DJ, Sedgwick JD. Origin and steady-state turnover of class II MHC-bearing dendritic cells in the epithelium of the conducting airways. J Immunol 1994;153:256–261. [PubMed] [Google Scholar]
- 16.Legge KL, Braciale TJ. Accelerated migration of respiratory dendritic cells to the regional lymph nodes is limited to the early phase of pulmonary infection. Immunity 2003;18:265–277. [DOI] [PubMed] [Google Scholar]
- 17.Vermaelen KY, Carro-Muino I, Lambrecht BN, Pauwels RA. Specific migratory dendritic cells rapidly transport antigen from the airways to the thoracic lymph nodes. J Exp Med 2001;193:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sedgwick JD, Holt PG. Induction of IgE-isotype specific tolerance by passive antigenic stimulation of the respiratory mucosa. Immunology 1983;50:625–630. [PMC free article] [PubMed] [Google Scholar]
- 19.Hintzen G, Ohl L, Del Rio ML, Rodriguez-Barbosa JI, Pabst O, Kocks JR, Krege J, Hardtke S, Forster R. Induction of tolerance to innocuous inhaled antigen relies on a CCR7-dependent dendritic cell–mediated antigen transport to the bronchial lymph node. J Immunol 2006;177:7346–7354. [DOI] [PubMed] [Google Scholar]
- 20.Jakubzick C, Tacke F, Llodra J, van Rooijen N, Randolph GJ. Modulation of dendritic cell trafficking to and from the airways. J Immunol 2006;176:3578–3584. [DOI] [PubMed] [Google Scholar]
- 21.Vermaelen K, Pauwels R. Accelerated airway dendritic cell maturation, trafficking, and elimination in a mouse model of asthma. Am J Respir Cell Mol Biol 2003;29(3 Pt 1):405–409. Epub 2003 Apr 17. [DOI] [PubMed] [Google Scholar]
- 22.Hollingsworth JW, Whitehead GS, Lin KL, Nakano H, Gunn MD, Schwartz DA, Cook DN. TLR4 signaling attenuates ongoing allergic inflammation. J Immunol 2006;176:5856–5862. [DOI] [PubMed] [Google Scholar]
- 23.Langenkamp A, Casorati G, Garavaglia C, Dellabona P, Lanzavecchia A, Sallusto F. T cell priming by dendritic cells: thresholds for proliferation, differentiation and death and intraclonal functional diversification. Eur J Immunol 2002;32:2046–2054. [DOI] [PubMed] [Google Scholar]
- 24.Gallimore A, Glithero A, Godkin A, Tissot AC, Pluckthun A, Elliott T, Hengartner H, Zinkernagel R. Induction and exhaustion of lymphocytic choriomeningitis virus–specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I–peptide complexes. J Exp Med 1998;187:1383–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med 1998;188:2205–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Probst HC, McCoy K, Okazaki T, Honjo T, van den Broek M. Resting dendritic cells induce peripheral CD8+ T cell tolerance through PD-1 and CTLA-4. Nat Immunol 2005;6:280–286. Epub 2005 Jan 30. [DOI] [PubMed] [Google Scholar]
- 27.Julia V, Hessel EM, Malherbe L, Glaichenhaus N, O'Garra A, Coffman RL. A restricted subset of dendritic cells captures airborne antigens and remains able to activate specific T cells long after antigen exposure. Immunity 2002;16:271–283. [DOI] [PubMed] [Google Scholar]
- 28.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 2003;19:71–82. [DOI] [PubMed] [Google Scholar]
- 29.McWilliam AS, Nelson D, Thomas JA, Holt PG. Rapid dendritic cell recruitment is a hallmark of the acute inflammatory response at mucosal surfaces. J Exp Med 1994;179:1331–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stumbles PA, Strickland DH, Pimm CL, Proksch SF, Marsh AM, McWilliam AS, Bosco A, Tobagus I, Thomas JA, Napoli S, et al. Regulation of dendritic cell recruitment into resting and inflamed airway epithelium: use of alternative chemokine receptors as a function of inducing stimulus. J Immunol 2001;167:228–234. [DOI] [PubMed] [Google Scholar]
- 31.Whitehead GS, Wang T, Degraff LM, Card JW, Lira SA, Graham GJ, Cook DN. The chemokine receptor D6 has opposing effects on allergic inflammation and airway reactivity. Am J Respir Crit Care Med 2007;175:243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Osterholzer JJ, Ames T, Polak T, Sonstein J, Moore BB, Chensue SW, Toews GB, Curtis JL. CCR2 and CCR6, but not endothelial selectins, mediate the accumulation of immature dendritic cells within the lungs of mice in response to particulate antigen. J Immunol 2005;175:874–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dahl ME, Dabbagh K, Liggitt D, Kim S, Lewis DB. Viral-induced T helper type 1 responses enhance allergic disease by effects on lung dendritic cells. Nat Immunol 2004;5:337–343. Epub 2004 Feb 15. [DOI] [PubMed] [Google Scholar]
- 34.McWilliam AS, Napoli S, Marsh AM, Pemper FL, Nelson DJ, Pimm CL, Stumbles PA, Wells TN, Holt PG. Dendritic cells are recruited into the airway epithelium during the inflammatory response to a broad spectrum of stimuli. J Exp Med 1996;184:2429–2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beyer M, Bartz H, Horner K, Doths S, Koerner-Rettberg C, Schwarze J. Sustained increases in numbers of pulmonary dendritic cells after respiratory syncytial virus infection. J Allergy Clin Immunol 2004;113:127–133. [DOI] [PubMed] [Google Scholar]
- 36.Wang H, Peters N, Laza-Stanca V, Nawroly N, Johnston SL, Schwarze J. Local CD11c+ MHC class II–precursors generate lung dendritic cells during respiratory viral infection, but are depleted in the process. J Immunol 2006;177:2536–2542. [DOI] [PubMed] [Google Scholar]