Abstract
Although other imaging techniques, such as magnetic resonance imaging and computer tomography, are becoming more and more important in cardiology, two-dimensional echocardiography is still the most used technique in clinical cardiology. Quantification of left ventricular function and dimensions is important because therapeutic strategies, for example implanting an ICD after myocardial infarction, are based on ejection fraction measurements. Because of the sometimes low quality of echocardiographic images we started to use an ultrasound contrast agent and in this article we describe our experiences with SonoVue, a second-generation contrast agent, over a threeyear period in the Thoraxcentre. (Neth Heart J 2007;15:55-60.)
Keywords: echocardiography, sonoVue, clinical practice
Two-dimensional echocardiography is the main diagnostic technique for most cardiac diagnoses. Although considerable technical improvements have been achieved during the last decades, poor acoustic windows are still an important limiting factor of transthoracic echocardiography. Ultrasound contrast agents that traverse the pulmonary circulation and opacify the left cardiac chambers may overcome this limitation and have proven to be important for the assessment of left ventricular (LV) border detection and function.1,2 An overview of the use of ultrasound contrast agents was published in this journal in 1998.3,4 SonoVue, a commercially available contrast agent, has been used in the Netherlands since 2001. In this article we report our SonoVue experience during a three-year period from 2002 to 2004.
Contrast agent
SonoVue (Bracco, Milan, Italy) is based on stabilised sulphur hexafluoride microbubbles surrounded by a phospholipid shell with a mean size of 2.5 μm.5 After mixing with saline, a manual process that takes less than one minute (figure 1), a suspension is obtained with SonoVue microbubbles in a concentration of 1 to 5 × 108 per ml. This suspension should be injected intravenously in a straight access through a three-way stopcock (to avoid destruction of the microbubbles) as a bolus (0.5 ml with additional 0.25 ml injections when necessary) followed by saline injection or as a continuous infusion by a dedicated pump delivered by the Bracco company. In our institute we only use bolus injections. It should be emphasised that it is important to constantly shake the suspension to keep the microbubbles soluble. For virtually all indications 5 ml of SonoVue is sufficient; simple questions (LV ejection fraction) can often be answered with only half this volume in 15 minutes.
Figure 1.

SonoVue file including syringe and needle.
Echocardiography
Transthoracic echocardiography was performed with the Philips Sonos 5500 (Philips, Best, the Netherlands) system with a S3 transducer in the second harmonic contrast-imaging mode. Transmit/receive frequency was 1.6/3.2 MHz and the mechanical index was 0.4 for endocardial border detection and 0.1 for myocardial perfusion imaging. Mechanical index reflects the normalised energy to which a target (such as a microbubble) is exposed in an ultrasound field. Normally this index ranges from 0.1 to 2.0. For myocardial perfusion studies power modulation imaging was used. With this technique, differences in acoustic properties of microbubbles and tissue result inselective enhancement of microbubble-generated reflections but suppression of reflections from cardiac tissues. To visualise myocardial contrast bubble replenishment with real-time power modulation imaging, at peak contrast intensity the microbubbles in the myocardium were first destroyed with flash imaging with high a mechanical index (1.6).
The basic assumption underlying power modulation imaging is that the reflective properties of cardiac structures, unlike those of microbubbles, are mostly linear.6 Power modulation uses this assumption by transmitting repeated pulses of different intensities in the same direction (figure 2). Two consecutive pulses of identical shape but twofold difference in amplitude would result in identical reflections from the heart, other than the expected twofold difference in amplitude. The smaller pulse is then multiplied by 2 and subtracted from the larger one, resulting in a zero signal. When reflected bythe nonlinear microbubbles, the same two pulses would differ from each other not only in amplitude but also in their shape. Amplifying the smaller pulse and subtracting it from the larger one would result ina nonzero signal. The amplitude of this signalis colour-coded and displayed in an overlay over the grey-scale image.
Figure 2.

Upper panel shows myocardial reflection and lower panel microbubble reflection in power modulation mode (also see text for explanation). From: Mor-Avi V, et al. Circulation 2001;104:352-7.
Real-time power modulation imaging was started after flash imaging with a high mechanical index (1.6) to destroy the microbubbles in the myocardium at peak contrast intensity in order to explore artefacts and to visualise myocardial contrast bubble replenishment.
Contrast applications
During the three-year period, 289 contrast studies were performed in 241 patients (48 patients underwent two studies because of a research protocol).7 As indicated in the next sections and in table 1, SonoVue was used for many different indications.
Table 1.
Overview of SonoVue contrast echocardiography applications.
| Application | Patients | Reference |
|---|---|---|
| Research – completed | ||
|
96 | 5 |
|
20 | 16 |
| Research – ongoing | ||
|
96 | |
|
29 | |
|
14 | |
|
34 | |
|
8 | |
|
43 | |
| Routine use | ||
|
50 | |
|
25 | |
|
12 |
*The same patients as in reference 5.
LV ejection fraction
As seen in figure 3, echo contrast improves endocardial border detection and therefore makes LV wall motion analysis8 and LV ejection fraction assessment9,10 more reliable. This improvement is reflected by an increase in intra- and inter-observer agreement of LV ejection fraction and volumes.9,10 In a recently completed study in our centre, LV ejection fraction inter-observer variability decreased from 16.9% with second harmonic imaging to 7.0% with contrast imaging (unpublished data). Currently, we are using contrast for LV ejection fraction in several research protocols and in routine patients in whom accurate assessment of LV ejection fraction is necessary because of potential changes in clinical decision-making. Wellknown examples of this latter include initiation of chemotherapy and implantation of an internal cardiac defibrillator.11
Figure 3.
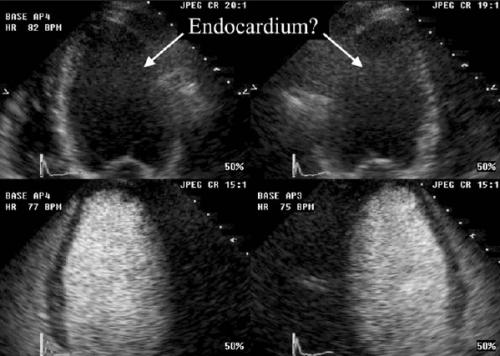
Visualisation of endocardial border in four- and three-chamber images without (upper images) and with (lower images) contrast.
Myocardial perfusion
Contrast myocardial perfusion reflects total myocardial capillary blood volume. One of the most promising applications of this new modality is in patients with acute myocardial infarction. We and others have shown that intact myocardial perfusion of infarcted myocardium assessed by contrast echocardiography can predict functional recovery in time.12,13 In addition, contrast echocardiography contributes to a better measurement of interventricular septal thickness and the combination of wall thickness >11 mm in the infarct region and intact perfusion is an excellent predictor of functional improvement.7
Another application of myocardial perfusion is in patients with hyperthrophic cardiomyopathy undergoing percutaneous transluminal septal myocardial ablation. During this procedure a localised chemical septal myocardial infarction is induced by injection of ethanol into one or more septal branches. By infusion of echo contrast the specific septal branch that supplies that part of the hypertrophied septum believed to produce LV outflow tract obstruction can be easily identified. In at least 10% of the procedures the echo contrast images change the selection of the septal branch.14,15
Noncompaction cardiomyopathy
Noncompaction cardiomyopathy is a relatively rare congenital, unclassified cardiomyopathy that is characterised by excessively prominent trabecular meshwork and deep intertrabecular recesses, best evidenced by colour Doppler flow.16 Currently, we are studying the value of contrast echocardiography in these noncompaction patients. As seen in figure 4, we have described one patient in the literature in whom flow in the intertrabecular recesses could only be detected with the use of SonoVue contrast.17
Figure 4.

Intertrabecular recesses filling with contrast (*) and apical mobile thrombus in a patient with noncompaction cardiomyopathy.
Right ventricular function and morphology
Echocardiographic visualisation of the right ventricle remains a true challenge for the clinician. More assessment of right ventricular dimension and function may be particularly useful in patients with congenital heart disease. In one study we demonstrated that in particular in the near-field images right ventricular contrast imaging gives a significantly better visibility of the endocardial border (figure 5).18 In addition, better identification of right ventricular trabeculation was possible, which may be important in the differentiation of right ventricular hypertrophy from marked trabeculation.
Figure 5.

Endocardial border detection of right ventricle without (left) and with contrast (right).
More recently, we started a research protocol in patients with a suspicion of arrhythmogenic right ventricular cardiomyopathy. According to existing guidelines echocardiography should be part of the diagnostic work-up. For the diagnosis of arrhythmogenic right ventricular cardiomyopathy visualisation of the right ventricle is important, but often difficult in routine echocardiography. We are currently examining whether contrast echocardiography can help in the visualisation of right ventricular aneurysms, (segmental) right ventricular dilatation and (regional) right ventricular hypokinesia, all criteria for the diagnosis of this cardiac disease.19
Stress echocardiography
The use of echo contrast improves interobserver agreement and diagnostic accuracy of dobutamine stress echocardiography, in particular in patients with suboptimal image quality.8,20,21 The limited use of contrast in stress echocardiography in our centre is mainly due to the confidence of the observer during the described time period in its interpretation quality and to a lesser extent SonoVue costs. Because 3D real-time stress echocardiography suffers from a significant decrease in image quality compared with conventional 2D imaging we are convinced that contrast may play an even more important role in this new stress modality. At the moment the role of contrast in 3D real-time stress echocardiography is one of our main research interests.
Differentiation of extra- and intracardiac structures
Contrast echocardiography may also be helpful in the identification of cavities of unknown origin. Since newer contrast agents such as SonoVue pass the pulmonary circulation, the microbubbles can reach all intracardiac cavities. If opacification with SonoVue is limited to the cardiac chambers, the cavity of interest (figure 6) has no luminal connection to a cardiac chamber and is thus diagnosed as located extracardiacally.
Figure 6.

Patient with echinococcus cyst; contrast image shows no connection with left ventricle.
Left ventricular thrombus detection
LV thrombus detection may have important implications with regard to the use of anticoagulant therapy. With conventional echocardiography it is sometimes difficult to image well-defined apical details. As seen in figure 4, besides the noncompaction cardiomyopathy, contrast echocardiography may also be of great help in the correct interpretation of the presence or absence of an apical LV thrombus.
Safety
During the described three-year study period, two of the 241 patients (1%) experienced mild hypotension, sinus tachycardia and skin flushing most likely caused by an allergic reaction to SonoVue. Both patients had received SonoVue for the second time and were successfully treated with intravenous clemastine and hydrocortisone. Before this study period we used SonoVue in a role-in phase in a patient to enhance endocardial border detection with dobutamine-stress echocardiography. He developed extensive skin erythema and anaphylactic shock with a decrease in blood pressure from 150/70 to 70/30 mmHg. After intravenously administered clemastine, hydrocortisone and volume, the patient rapidly recovered and was discharged the next day. This adverse event was reported to the European Medicines Agency (EMEA). In 2004 several serious side effects with SonoVue were also reported by others22 and in a postmarketing analysis of 157,838 SonoVue studies 19 cases of severe (0.0012%) and three cases of fatal adverse events (0.002%) were described (http://www.emea.eu.int/ humandocs/Humans/EPAR/sonovue/sonovue.htm). All three patients with a fatal outcome had severe coronary artery disease and their clinical situation was far from stable. The allergic reactions may have been caused by the sulphur hexafluoride gas or the shell component polyethylene glycol (macrogel 4000).23,24 Subsequently, in May 2004 the EMEA recommended not to use SonoVue as an ultrasound agent in cardiology. After a review of the cases by the Committee for Human Medicinal Products in November 2004 the recommendations changed and the use of SonoVue was again allowed in cardiac ultrasound. However, SonoVue is still contraindicated in patients with recent unstable cardiac symptoms, a recent (<7 days) coronary intervention, class III and IV heart failure or serious arrhythmias. An allergic reaction should always be anticipated and antiallergic drugs should be available in addition to standard resuscitation equipment. It is also recommended to keep the patient under medical supervision during and for at least 30 minutes following the infusion of SonoVue.
Limitations of SonoVue contrast
As described before, SonoVue may have important side effects, which makes the attendance of a physician mandatory. Contrast echocardiography needs a different pre-setting of the echo machine (mechanical index, second harmonic imaging). Another limitation for the widespread use of SonoVue is costs because there is only a small reimbursement for contrast echocardiography.
Conclusion
When detailed morphological and/or quantitative information of the heart is needed echo contrast agents such as SonoVue may provide better and more reliable results. Imaging of myocardial perfusion will be a main area for future applications, but in our opinion it is not applicable for routine practice at this moment. Costs and safety issues will most likely determine the clinical future of SonoVue.
References
- 1.Hundley WG, Kizilbash AM, Afridi I, Franco F, Peshock RM, Grayburn PA. Administration of an intravenous perfluorocarbon contrast agent improves echocardiographic determination of left ventricular volumes and ejection fraction: comparison with cine magnetic resonance imaging. J Am Coll Cardiol 1998;32:1426- 32. [DOI] [PubMed] [Google Scholar]
- 2.Kasprzak JD, Paelinck B, Ten Cate FJ, et al. Comparison of native and contrast-enhanced harmonic echocardiography for visualization of left ventricular endocardial border. Am J Cardiol 1999;83:211-7. [DOI] [PubMed] [Google Scholar]
- 3.Sieswerda GT, Kamp O, Visser CA. The use of contrast agents in echocardiography. Part I: History, Principles and Developments in Agents and Ultrasound Technology. Neth Heart J 1998;5:583-8. [Google Scholar]
- 4.Sieswerda GT, Kamp O, Visser CA. The use of contrast agents in echocardiography. Part II: clinical indications and experimental applications. Neth Heart J 1998;5:648-57. [Google Scholar]
- 5.Schneider M. SonoVue, a new ultrasound contrast agent. Eur Radiol 1999;9(Suppl 3):S347-8. [DOI] [PubMed] [Google Scholar]
- 6.Mor-Avi V, Caiani EG, Collins KA, Korcarz CE, Bednarz JE, Lang RM. Combined assessment of myocardial perfusion and regional left ventricular function by analysis of contrast-enhanced power modulation images. Circulation 2001;104:352-7. [DOI] [PubMed] [Google Scholar]
- 7.Biagini E, Galema TW, Schinkel AF, Vletter WB, Roelandt JR, Ten Cate FJ. Myocardial wall thickness predicts recovery of contractile function after primary coronary intervention for acute myocardial infarction. J Am Coll Cardiol 2004;43:1489-93. [DOI] [PubMed] [Google Scholar]
- 8.Dolan MS, Riad K, El-Shafei A, et al. Effect of intravenous contrast for left ventricular opacification and border definition on sensitivity and specificity of dobutamine stress echocardiography compared with coronary angiography in technically difficult patients. Am Heart J 2001;142:908-15. [DOI] [PubMed] [Google Scholar]
- 9.Malm S, Frigstad S, Sagberg E, Larsson H, Skjaerpe T. Accurate and reproducible measurement of left ventricular volume and ejection fraction by contrast echocardiography: a comparison with magnetic resonance imaging. J Am Coll Cardiol 2004;44:1030- 5. [DOI] [PubMed] [Google Scholar]
- 10.Thomson HL, Basmadjian AJ, Rainbird AJ, et al. Contrast echocardiography improves the accuracy and reproducibility of left ventricular remodeling measurements: a prospective, randomly assigned, blinded study. J Am Coll Cardiol 2001;38:867-75. [DOI] [PubMed] [Google Scholar]
- 11.Moss AJ, Hall WJ, Cannom DS, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med 1996;335:1933-40. [DOI] [PubMed] [Google Scholar]
- 12.Rocchi G, Kasprzak JD, Galema TW, de Jong N, Ten Cate FJ. Usefulness of power Doppler contrast echocardiography to identify reperfusion after acute myocardial infarction. Am J Cardiol 2001;87:278-82. [DOI] [PubMed] [Google Scholar]
- 13.Sieswerda GT, Klein LJ, Kamp O, et al. Quantitative evaluation of myocardial perfusion in patients with revascularized myocardial infarction: comparison between intravenous myocardial contrast echocardiography and 99mTc-sestamibi single photon emission computed tomography. Eur J Echocardiogr 2004;5:41-50. [DOI] [PubMed] [Google Scholar]
- 14.Faber L, Seggewiss H, Gleichmann U. Percutaneous transluminal septal myocardial ablation in hypertrophic obstructive cardiomyopathy: results with respect to intraprocedural myocardial contrast echocardiography. Circulation 1998;98:2415-21. [DOI] [PubMed] [Google Scholar]
- 15.Faber L, Seggewiss H, Welge D, et al. Echo-guided percutaneous septal ablation for symptomatic hypertrophic obstructive cardiomyopathy: 7 years of experience. Eur J Echocardiogr 2004;5:347- 55. [DOI] [PubMed] [Google Scholar]
- 16.Jenni R, Oechslin E, Schneider J, Attenhofer Jost C, Kaufmann PA. Echocardiographic and pathoanatomical characteristics of isolated left ventricular non-compaction: a step towards classification as a distinct cardiomyopathy. Heart 2001;86:666-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Laat LE, Galema TW, Krenning BJ, Roelandt JR. Diagnosis of non-compaction cardiomyopathy with contrast echocardiography. Int J Cardiol 2004;94:127-8. [DOI] [PubMed] [Google Scholar]
- 18.van den Bosch AE, Meijboom FJ, McGhie JS, Roos-Hesselink JW, Ten Cate FJ, Roelandt JR. Enhanced visualisation of the right ventricle by contrast echocardiography in congenital heart disease. Eur J Echocardiogr 2004;5:104-10. [DOI] [PubMed] [Google Scholar]
- 19.McKenna WJ, Thiene G, Nava A, et al. Diagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Task Force of the Working Group Myocardial and Pericardial Disease of the European Society of Cardiology and of the Scientific Council on Cardiomyopathies of the International Society and Federation of Cardiology. Br Heart J 1994;71:215-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mathias W, Jr. Arruda AL, Andrade JL, Filho OC, Porter TR. Endocardial border delineation during dobutamine infusion using contrast echocardiography. Echocardiography 2002;19:109-14. [DOI] [PubMed] [Google Scholar]
- 21.Ten Cate FJ. Usefulness of ultrasound contrast for image enhancement during stress echocardiography. Echocardiography 2002; 19(7 Pt 2):621-5. [PubMed] [Google Scholar]
- 22.de Groot MC, van Zwieten-Boot BJ, van Grootheest AC. [Severe adverse reactions after the use of sulphur hexafluoride (SonoVue) as an ultrasonographic contrast agent]. Ned Tijdschr Geneeskd 2004;148:1887-8. [PubMed] [Google Scholar]
- 23.Fisher AA. Immediate and delayed allergic contact reactions to polyethylene glycol. Contact Dermatitis 1978;4:135-8. [DOI] [PubMed] [Google Scholar]
- 24.Dewachter P, Mouton-Faivre C. Anaphylaxis to macrogol 4000 after a parenteral corticoid injection. Allergy 2005;60:705-6. [DOI] [PubMed] [Google Scholar]


