Abstract
Numerous randomized trials have been published investigating the effectiveness of treatments for non-specific low-back pain (LBP) either by trials comparing interventions with a no-treatment group or comparing different interventions. In trials comparing two interventions, often no differences are found and it raises questions about the basic benefit of each treatment. To estimate the effect sizes of treatments for non-specific LBP compared to no-treatment comparison groups, we searched for randomized controlled trials from systematic reviews of treatment of non-specific LBP in the latest issue of the Cochrane Library, issue 2, 2005 and available databases until December 2005. Extracted data were effect sizes estimated as Standardized Mean Differences (SMD) and Relative Risk (RR) or data enabling calculation of effect sizes. For acute LBP, the effect size of non-steroidal anti-inflammatory drugs (NSAIDs) and manipulation were only modest (ES: 0.51 and 0.40, respectively) and there was no effect of exercise (ES: 0.07). For chronic LBP, acupuncture, behavioral therapy, exercise therapy, and NSAIDs had the largest effect sizes (SMD: 0.61, 0.57, and 0.52, and RR: 0.61, respectively), all with only a modest effect. Transcutaneous electric nerve stimulation and manipulation had small effect sizes (SMD: 0.22 and 0.35, respectively). As a conclusion, the effect of treatments for LBP is only small to moderate. Therefore, there is a dire need for developing more effective interventions.
Keywords: Effect size, Low-back pain, Placebo, Systematic review, Randomized controlled trial
Introduction
Non-specific low-back pain (LBP) is by most physicians considered as a recurring, benign, and self-limiting condition, but for patients it is a painful and disabling experience for which they frequently demand treatment. Several treatments are available for LBP, such as analgesics, non-steroidal anti-inflammatory drugs (NSAIDs), exercise, behavioral therapy, spinal manipulation, and acupuncture. Numerous randomized trials have been published investigating the effectiveness of treatments for non-specific LBP. However, there are important differences in how these trials have been conducted: Many trials assess the effect of combinations of different interventions; some trials compare interventions with no treatment or placebo, whereas others compare different interventions for non-specific LBP. In trials comparing two interventions, there is often no difference found between groups and it remains unclear if any of the interventions are effective or not and it raises questions about the basic benefit of each treatment.
Several systematic reviews have been published addressing the question of the effect of a particular treatment for LBP. Conclusions from many of these reviews are based on qualitative analysis. This type of analysis uses various levels of evidence (from “strong” to no “evidence”) regarding the effectiveness of a treatment taking into account the participants, interventions, controls, outcomes, and methodological quality of the original studies [80]. Quantitative analysis, on the other hand, is a statistical approach involving pooling data (meta-analysis) that provides an overall effect estimate, which allow direct comparing between effects of different treatments.
The objective of the present study was to synthesize the results of randomized controlled trials (RCT) for common LBP treatments comparing the interventions to placebo/sham or no-treatment comparison groups, to estimate a pooled effect size for each treatment, and compare them with each other.
Methods
Study selection
We searched for RCT from systematic reviews of treatment of acute and chronic non-specific LBP in the latest issue of the Cochrane Library, issue 2, 2005 and used Medline/Pubmed, Embase, Cinhal, and Amed to search for additional papers limiting the search from the time of the last search in each Cochrane review until December 2005. The following terms were used for the search: LBP (Mesh) or LBP (tw), placebo effect (Mesh). In addition, RCT keywords were used for the search: “LBP” and the name of the treatment of current interest (i.e., exercise, manipulation, behavioral treatment, NSAIDs, and acupuncture) [80].
Low-back pain was defined as pain located below the scapulas and above the cleft of the buttocks [81] and non-specific LBP was defined as back pain not attributable to a recognizable, known specific pathology (i.e., infection, tumors, osteoporosis, fracture, structural deformity, inflammatory disorder, radicular syndrome, or cauda equina syndrome) [87]. Three criteria defined relevant trials: (1) the trials should compared the treatment to a no-treatment comparison group, (2) the trials should investigate an unselected and general population, and (3) the treatment should be practiced and be available in several countries.
The no-treatment comparison groups included subjects receiving placebo, sham treatments, no treatment, or those on a waiting list. Waiting-list implied that the patients were waiting for a treatment for their back pain. Placebo was defined as a medicine which has no inherent pertinent pharmacologic activity, but which is effective by virtue of the factor of suggestion attendant upon its administration. Sham treatment was defined as procedures where medical personal goes through the motions without actually performing the treatment such as sham acupuncture for acupuncture or detuned Transcutaneous electric nerve stimulation (TENS). No treatment implied that the patients were not prescribed any drugs by the physician or recommended any treatment or home exercises for their back pain from any other medical personnel. They could, however, receive a booklet with advice about daily activities.
We looked separately at trials investigating acute and subacute/chronic LBP. Acute LBP was defined as duration of pain less than 6 weeks and the subacute/chronic condition more than 6 weeks.
Outcome measures
Outcomes were self-reported pain intensity and self-reported physical functioning. In the LBP literature, several outcome measures have been used to assess the construct of pain intensity [for example, 10 cm or 100 mm visual analogue scale (VAS), McGill Pain Questionnaire, and numeric (11, 21, or 101 points) rating scale (NRS)] [86]. LBP-specific functioning can also be measured with various instruments [for example, Oswestry Disability Index (0–100), Quebec Back Pain Disability Scale (0–100), and the 24-point Roland Morris Disability Questionnaire] [44]. We used the standardized mean difference (SMD) to estimate the treatment effect of the individual trials for similar constructs. This will allow direct comparison of studies, which used different measures of sufficiently similar constructs [19].
Data extraction
Data were extracted from the included trials separately both for acute and subacute/chronic LBP, and also for short-term (assessment closest to 6 weeks after randomization) and long-term (assessment between 6 and 12 months after randomization) follow-up. We extracted effect sizes estimated as SMD and Relative Risk (RR) or data for calculation of effect size. Cohen categorized effect size values as small (ES: 0.2–0.5), moderate (ES: 0.5–0.8), and large (ES: >0.8) [16] but it is uncertain how this applies to the field of LBP.
We calculated effect size from either continuous (mean, SD, and confidence interval) or dichotomous variables (number of patients with good/excellent response to treatment). For continuous variables effect size was calculated as SMD, which is defined as the differences in outcome measures between two groups divided by the SD of the of the control group, the SD of the treatment group, or the pooled SD [19, 41]. For dichotomous data the effect size was calculated as RR, where RR is the risk of an event in the treatment group divided by the risk of the event in the comparison group. If no data were available for estimating effect size, the author of the trial was contacted by E-mail, and if data were provided, these trials were also included. If variance data were not reported as SDs, they were calculated from the trial data using standard error of the mean (SE) or 95% confidence intervals. If variance data were not reported, SDs from other relevant studies were used, i.e., studies concerning the same treatment and the same condition (acute or chronic). The pooled SD of the treatment effects for each group from relevant studies was calculated using the following formula [19]:
 |
where n is the number of participants in each treatment group, N the total number and SD1 and SD2 are standard deviations for the intervention group and control group, respectively [19]. The percentage of the SDpooled of the mean difference of change from relevant studies was used in studies with missing variance data [25].
The quality of the included trials was reported as assessed by the authors of the systematic Cochrane reviews. For the original articles published after the reviews, one of the authors of the present study (AK) assessed the quality according to the 11-item criteria list recommended in the method guidelines for systematic reviews of the Cochrane Back Review Group [80]. Some reviews used another criteria based on a list consisting of three items [38].
Analysis
A quantitative meta-analysis was performed in which the effect sizes (SMDs and RR) were pooled using a random effect model.
We assessed statistical heterogeneity using I2 statistics and confidence intervals [34]. We present the effect sizes separately for dichotomous and continuous variables. For continuous variables, the pooled effect sizes for the treatments for the acute and chronic condition are presented. In addition, the effect sizes for the individual studies for each treatment for the chronic condition and short-term follow-up are presented.
Review Manager, Version 4.2 for Windows (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2003) was used for the analyses.
Results
We included seven reviews from the latest issue of the Cochrane Library, issue 2, 2005, from which we included 41 trials of 228 (Tables 1, 2). An additional six trials were identified from the updated search in Pubmed/Medline, Embase, Cinhal, and Amed. Table 1 shows the number of trials included from the systematic reviews and the updated search. About 20% of the trials were included, ranging from 4 to 50%. In most cases, the reason for not including a trial was because one type of intervention was compared to another intervention and not to a no-treatment group.
Table 1.
The total number of trials included in the systematic reviews from the latest issue of the Cochrane Library, issue 2, 2005, the number of these trials included in the present review for the acute and the chronic condition according to the inclusions criteria, and the number of included trials from the updated literature search
| Treatment | Search for trials until | Number of included trials in the Cochrane review | Number of included trials in the present study | Percentage of included trials in the present study | Number of included trials from the updated search for acute LBP | ||
|---|---|---|---|---|---|---|---|
| Acute LBP | Chronic LBP | Acute LBP | Chronic LBP | ||||
| Acupuncture for dry-needling for LBP [24] | February 2003 | 35 | 7 | 20 | |||
| Behavioral treatment chronic LBP [83] | October 2003 | 20 | 7 | 35 | |||
| Exercise therapy for LBP [81] | October 2004 | 49 | 4 | 6 | 20 | 1 | |
| Muscle relaxants for non-specific LBP, Benzodiazepines/non benzodiazepines [85] | October 2001 | 30 | 3 | 2 | 17 | 1 | |
| Non-steroidal anti-inflammatory drugs for LBP [84] | September 1998 | 51 | 2 | 4 | 1 | 3 | |
| Spinal manipulative therapy for LBP [3] | January 2000 | 39 | 3 | 5 | 21 | ||
| Transcutaneous electric nerve stimulation (TENS) for chronic LBP [43] | April 2005 | 4 | 2 | 50 | |||
LBP low-back pain
Table 2.
Reasons for not including trials listed in the reviews from the Cochrane Library, issue 2, 2005 and reason for not including trials comparing treatment with no-treatment group in the updated search
| Review from the Cochrane Library, issue 2, 2005 | No trials or only one compared intervention with a no-treatment group (n = 9) [4, 23, 28, 31, 33, 35, 52, 56, 59, 68, 82, 90] |
| A selective population were included (n = 1) [39] | |
| The availability of the treatment outside Spain is unknown (guideline December-04) (n = 1) [79] | |
| All trials are published in other reviews (n = 3) [30, 67, 81] | |
| The diagnoses in the trials were other than non-specific low-back pain (n = 2) [27, 58] | |
| Updated search, trials with a no-treatment comparison group | Included both patients with neck and back pain (n = 1) [48] |
| No trials or only one compared intervention with a no-treatment group (n = 3) [48] | |
| Patients in no-treatment group received co-interventions (n = 3) [47, 58, 67] |
Acute low-back pain
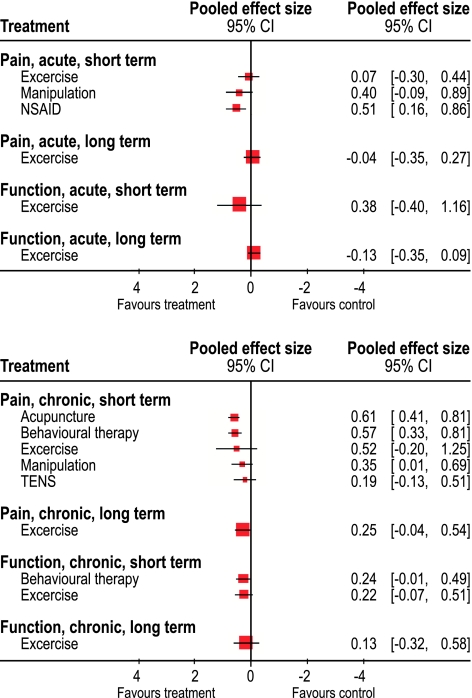
Table 3 describes the studies, which evaluated the effectiveness of treatments for acute LBP. The effect sizes of the individual studies and the pooled effect sizes are presented in Tables 4 and 5 and in Fig. 1. Unless otherwise noted, the effect sizes presented are calculated from continuous outcome measures. We included four studies on exercise therapy [13, 26, 50, 54], which were published between 1993 and 2005: the quality was high in two studies and low in two. Regarding short-term follow-up, the pooled effect sizes for pain relief and function were 0.07 (95% CI: −0.30 to 0.44) and 0.38 (95% CI: −0.40 to 1.16), respectively. For long-term follow-up, the corresponding figures were −0.04 (95% CI: −0.35 to 0.27) and −0.13 (95% CI: −0.35 to 0.09) (Table 4 and Fig. 1). Moreover, these studies were characterized by a high degree of heterogeneity (I2 = 69.1%).
Table 3.
Description of the included studies for acute LBP
| Treatment | Author, reference | Year | Number of patients | Method quality | Comparative group | Outcome measurement | |
|---|---|---|---|---|---|---|---|
| Pain | Function | ||||||
| Exercise | Faas et al. [26] | 1993 | 473 | 9 | Sham treatment | VAS (0–85 point) | Loss of mobility dimension |
| Malmivaara et al. [50] | 1995 | 186 | 9 | No treatment | VAS (0–10 cm) | Oswestry disability Index (0–100) | |
| Chok et al. [13] | 1999 | 54 | 4 | No treatment | VAS (0–100 mm) | Roland Morris disability questionnaire | |
| Mayer et al. [54] | 2005 | 50 | 5 | No treatment | VAS (0–6 point) | Roland Morris disability questionnaire | |
| Manipulation | Bergquist-Ullman and Larsson et al. [8] | 1977 | 198 | 3 | Placebo | Pain index: range 0–70 | Ten items, four point scale |
| Glover et al. [29] | 1974 | 84 | 6 | Sham-detuned short wave diathermy | Pain (% relief) | ||
| Postacchini et al. [63] | 1988 | 147 | 3 | Placebo gel | Pain four point scale | Ten items | |
| NSAID | Babej-Dolle et al. [5] | 1994 | 260 | 9 | Placebo injection | VAS (0–100 mm) | |
| Szpalski et al. [71] | 1994 | 73 | 7 | Placebo injection | VAS (0–100 mm) | ||
| Dreiser et al. [18] | 2003 | 372 | 6 | Placebo | VAS (0–100 mm) | ||
| Muscle relaxants (non-benzodiapines) | Lepisto et al. [47] | 1979 | 30 | 8 | Placebo | Pain four points scale | |
| Barrata et al. [6] | 1982 | 117 | 6 | Placebo | Number of patients who improved in pain | ||
| Berry et al. [9] | 1988 | 112 | 7 | Placebo | VAS (0–100 mm) | ||
| Tuzun et al. [78] | 2003 | 149 | 9 | Placebo | VAS (0–100 mm) | ||
VAS visual analogue scale
Table 4.
Effect sizes (SMR) for acute LBP for pain and function
| Treatment | Author | Acute pain. short-term | Acute pain. long-term | Acute function. short-term | Acute function. long-term | ||||
|---|---|---|---|---|---|---|---|---|---|
| SMD | CI | SMD | CI | SMD | CI | SMD | CI | ||
| Exercise | Faas et al. [26] | 0.03 | [−0.19–0.25] | 0.10 | [−0.12 to 0.32] | −0.11 | [−0.33 to 0.11] | −0.10 | [−0.32 to 0.12] |
| Malmivaara et al. [50] | −0.44 | [−0.81 to −0.08] | −0.28 | [−0.64 to 0.09] | −0.52 | [−0.15 to −0.89] | −0.33 | [−0.70 to 0.33] | |
| Chok et al. [13] | 0.49 | [−0.06 to 1.03] | 0.39 | [−0.16 to 0.93] | 2.02 | [2.69 to 1.36] | 0.18 | [−0.36 to 0.71] | |
| Mayer et al. [54] | 0.39 | [−0.17 to 0.95] | 0.33 | [−0.89 to 0.22] | |||||
| Pooled effect size | 0.07 | [−0.30 to 0.44] | −0.04 | [−0.35 to 0.27] | 0.38 | [−0.40 to 1.16] | −0.13 | [−0.35 to 0.09] | |
| Manipulation | Glover et al. [29] | 0.01 | [−1.05 to 1.07] | ||||||
| Bergquist-Ullman and Larsson [8] | 0.18 | [−0.52 to 0.89] | |||||||
| Postacchini et al. [63] | 0.85 | [0.09 to 1.61] | |||||||
| Pooled effect size | 0.40 | [−0.09 to 0.89] | |||||||
| NSAID | Babej-Dolle et al. [5] | 0.84 | [0.52 to 1.15] | ||||||
| Szpalski et al. [71] | 0.20 | [−0.27 to 0.67] | |||||||
| Dreiser et al. [18] | 0.41 | [0.13 to 0.69] | |||||||
| Pooled effect size | 0.51 | [0.16 to 0.86] | |||||||
LBP low-back pain, SMD standardized mean difference, CI 95% confidence interval
Table 5.
Effect sizes (relative risk) for acute LBP for pain
| Treatment | Authors, references | Relative risk | CI |
|---|---|---|---|
| Non-benzodiazepines | Lepisto et al. [47] | 0.67 | [0.13 to 3.44] |
| Barrata et al. [6] | 0.56 | [0.42 to 0.75] | |
| Berry et al. [9] | 0.68 | [0.38 to 1.24] | |
| Tuzun et al. [78] | 0.41 | [0.28 to 0.60] | |
| Pooled effect size | 0.52 | [0.42 to 0.65] |
LBP low-back pain, CI 95% confidence interval
Fig. 1.
The pooled effect sizes for treatments for acute (at the top) and chronic low-back pain (at the bottom) for pain and function, short- and long-term follow-up, presented in a Forest plot
We included three studies on spinal manipulation [8, 29, 63], which were published from 1974 to 1988: one was of medium and two were of low quality. Measures of variance were missing in all three studies, and therefore we used SDs from other RCT concerning manipulation in patients with acute LBP [36, 88]. The pooled SDs of these two studies were 85 and 140% of the mean difference, respectively. For the calculations of effect size, we chose a SD of 112.5% of the difference in mean. We also performed a sensitivity analysis with SD values of 85 and 140% of the mean, applied on the studies without measurements of variations [8, 29, 63]. The pooled effect sizes for short-term pain-relief were 0.50 with SD at 85%, 0.33 with SD at 140%, and 0.40 (95% CI: −0.09 to 0.89) with SD at 112.5 (Table 4 and Fig. 1). The studies were homogeneous (I2 = 10.7%).
Three studies on NSAIDs were included [5, 18, 71], which were published from 1994 to 2003: one of high quality and two of moderate quality. The pooled effect size for short-term pain relief was 0.51 (95% CI: 0.16 to 0.86) (Table 4 and Fig. 1), but the studies exhibited a high degree of heterogeneity (I2 = 67.8%).
Four studies on muscle relaxants comparing non-benzodiazepines with placebo were included [6, 9, 47, 78]. They were published from 1979 to 2003: two were of high quality and two moderate quality. The pooled effect size was calculated from dichotomous variable, and was for pain short-term follow-up 0.52 (95% CI: 0.42 to 0.65) (Table 5). The studies were homogenous (I2 = 0.0).
Chronic low-back pain
Table 6 describes the included studies for evaluation of the treatments for chronic LBP, the effect sizes are presented in Tables 6 and 7, in Figs. 1 and 2. We included six studies on exercise therapy [45, 64, 65, 69, 76, 91], which were published from 1981 to 2000: one was of medium and five of low quality. The pooled effect size was 0.52 (95% CI: −0.21 to 1.25) for short-term pain relief, and 0.25 (95% CI: −0.04 to 0.54) for long-term pain relief. For short- and long-term improvement in function, the effect sizes were 0.22 (95% CI: −0.07 to 0.51) and 0.13 (95% CI: −0.32 to 0.58), respectively. The studies had a high degree of heterogeneity (I2 = 88.5%).
Table 6.
Effect sizes (SMR) for chronic LBP for pain and function
| Treatment | Author, reference | Chronic pain, short-term | Chronic pain, long-term | Chronic function, short-term | Chronic function, long-term | ||||
|---|---|---|---|---|---|---|---|---|---|
| SMD | CI | SMD | CI | SMD | CI | SMD | CI | ||
| Exercise | Zylbergold et al. [91] | 1.03 | [0.08 to 1.97] | 0.29 | [−0.59 to 1.17] | ||||
| Turner et al. [76] | 0.37 | [−0.21 to −0.94] | −0.02 | [−0.59 to 0.55] | |||||
| Risch et al. [65] | 2.31 | [1.61 to 3.01] | 0.92 | [0.35 to 1.49] | |||||
| Soukup et al. [69] | 0.06 | [−0.41 to 0.53] | 0.06 | [−0.41 to 0.53] | 0.05 | [−0.42 to 0.52] | −0.25 | [−0.72 to 0.23] | |
| Preyde et al. [64] | 0.05 | [−0.51 to 0.62] | 0.59 | [0.01 to 1.17] | 0.01 | [−0.56 to 0.58] | 0.17 | [−0.40 to 0.74] | |
| Kuukkanen et al. [45] | −0.48 | [−1.00 to 0.05] | 0.20 | [−0.32 to 0.72] | 0.13 | [−0.39 to 0.65] | 0.17 | [−0.40 to 0.74] | |
| Pooled effect size | 0.52 | [−0.21 to 1.25] | 0.25 | [−0.04 to 0.54] | 0.22 | [−0.07 to 0.51] | 0.13 | [−0.32 to 0.58] | |
| Behavioral therapy | Turner et al. [74] | 0.51 | [−0.03 to 1.05] | 1.29 | [0.36 to 2.23] | ||||
| Nouwen et al. [60] | 0.36 | [−0.52 to 1.24] | |||||||
| Stukey et al. [70] | 0.79 | [−0.24 to 1.81] | −0.16 | [−1.14 to 0.82] | |||||
| Turner et al. [75] | 0.61 | [0.12 to 1.10] | 0.06 | [−0.47 to 0.59] | |||||
| Linton et al. [49] | 1.61 | [0.63 to 2.59] | |||||||
| Turner et al. [76] | 0.28 | [−0.29 to 0.85] | 0.13 | [−0.45 to 0.70] | |||||
| Turner et al. [77] | 0.53 | [−0.02 to 1.08] | 0.20 | [−0.33 to 0.73] | |||||
| Pooled effect size | 0.57 | [0.33 to 0.81] | 0.24 | [−0.01 to 0.49] | |||||
| Manipulation | Evans et al. [21] | −0.10 | [−0.79 to 0.60] | ||||||
| Waagen et al. [89] | 0.58 | [−0.34 to 1.51] | |||||||
| Ongley et al. [61] | 0.56 | [0.12 to 1.01] | |||||||
| Postacchini et al. [63] | 0.88 | [0.10 to 1.65] | |||||||
| Triano et al. [73] | 0.06 | [−0.37 to 0.48] | |||||||
| Pooled effect size | 0.35 | [0.01 to 0.69] | |||||||
| TENS | Deyo et al. [17] | 0.11 | [−0.24 to 0.46] | ||||||
| Marchand et al. [53] | 0.60 | [−0.19 to 1.39] | |||||||
| Pooled effect size | 0.19 | [−0.13 to 0.51] | |||||||
| Acupuncture | Coan et al. [14] | 0.91 | [0.33 to 1.50] | ||||||
| Medelson et al. [55] | 0.45 | [0.00 to 0.90] | |||||||
| Thomas et al. [72] | 0.46 | [−0.26 to 1.19] | |||||||
| Carlsson et al. [12] | 0.48 | [−0.15 to 1.12] | |||||||
| Kerr et al. [42] | 0.39 | [−0.20 to 0.98] | |||||||
| Leibing et al. [46] | 0.49 | [0.03 to 0.96] | |||||||
| Molsberger et al. [57] | 0.92 | [0.48 to 1.37] | |||||||
| Pooled effect size | 0.61 | [0.41 to 0.81] | |||||||
LBP low-back pain, SMD standardized mean difference, CI 95% confidence interval
Table 7.
Effect sizes (relative risk) for chronic LBP for pain
| Treatment | Authors, reference | Relative risk | CI |
|---|---|---|---|
| Benzodiazepines | Arbus et al. [2] | 0.77 | [0.64 to 0.94] |
| Salzmann et al. [66] | 0.88 | [0.72 to 1.06] | |
| Pooled effect size | 0.82 | [0.72 to 0.94] | |
| NSAID | Katz et al. [40] | 0.53 | [0.44 to 0.66] |
| Palley et al. [62] | 0.57 | [0.44 to 0.74] | |
| Coats et al. [15] | 0.72 | [0.59 to 0.86] | |
| Pooled effect size | 0.61 | [0.50 to 0.74] |
LBP low-back pain, CI 95% confidence interval
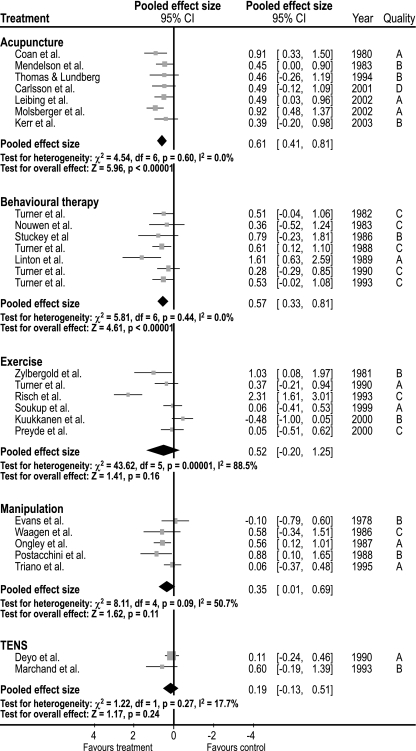
Fig. 2.
The pooled effect sizes for included trials for treatments for chronic low-back pain, for pain presented in a Forest plot
We included seven studies on behavioral treatment [49, 60, 70, 74–77], which were published from 1982 to 1993: one was of high, four of modest quality, and two of low quality. The effect sizes for short-term follow-up for pain relief were 0.57 (95% CI: 0.33 to 0.81) and 0.24 (95% CI: −0.01 to 0.49) for function. The studies were homogenous (I2 = 0.0).
Five studies concerning manipulation were included [21, 61, 63, 73, 89]. The included studies were published from 1978 to 1995: four studies were of modest and one was of low quality. Measures of variance were not available for three studies [21, 63, 89] and, therefore, the variance measurements from the two other studies were used [61, 73]. The pooled SDs of these two studies were 60 and 130% of the mean difference, respectively. For the calculations of effect size, we chose a SD of 95% of the difference in mean. We also performed a sensitivity analysis with SD values of 60 and 130% of the mean, applied on the studies without measurements of variations [21, 63, 89]. The pooled effect sizes for short-term pain-relief were of 0.49 with SD at 60%, 0.19 with SD at 130%, and 0.35 (95% CI: −0.01 to 0.69) with SD at 95% of mean difference. The studies were moderately heterogeneous (I2 = 50.7%).
Two studies [17, 53] were included on Transcutaneous Electrical Nerve Stimulation (TENS) [43], which were published in 1990 and 1993 and were of modest and low quality, respectively. The pooled effect size for short-term pain relief was small 0.19 (95% CI: −0.13 to 0.51). The studies were quite homogeneous (I2 = 17.7%).
We included seven studies in acupuncture [12, 14, 42, 46, 55, 57, 72], which were published from 1980 to 2002 and most were of moderate to high quality. The pooled effect size for short-term pain relief was modest 0.61 (95% CI: 0.41 to 0.81). The studies were homogenous (I2 = 0.0).
We included three studies evaluating the effect of benzodiazepines [2, 66, 85]. They were published in 1990 and 1992 and were considered to be of moderate quality. The effect size was moderate for short-term pain relief RR 0.82 (95% CI: 0.72–0.94) (Table 6). The studies were homogenous (I2 = 0.0).
We considered four studies on the effect of NSAIDs on chronic LBP [10, 15, 40], but one was excluded because there was no data available for estimating effect size [10]. They were published from 2003 to 2004 and were of high quality. The pooled effect size for pain was moderate RR 0.61 (95% CI: 0.50 to 0.74) for short-term follow up.
Discussion
The purpose of the present study was to investigate the effect sizes of treatments for non-specific LBP in randomized controlled studies comparing treatment with no treatment. In general, only a few studies of all the included RCT in the systematic reviews of the Cochrane Library, issue 2, 2005, compared treatments with no treatment (Fig. 1). The results are sobering as there are only modest effect sizes, if any, for short-term pain relief.
For the acute condition the effect size of NSAID and manipulation were only modest, there was no effect of exercise, and function and long-term follow-up were missing from most studies. Our results are in accordance with “the European Guidelines for acute LBP” which recommend NSAID and consideration of a short course of manipulation if the patients do not return to normal activities [20].
For chronic LBP, acupuncture and behavioral therapy had the largest effect size, followed by exercise and NSAID, although, all with only a modest effect. TENS and manipulation had small effect sizes. Only exercise and behavioral therapy measured function, but demonstrated scarcely any effect and data on long-term follow-up were missing.
“The European Guidelines of the management of chronic LBP” [1] recommends behavioral therapy, exercise, and a brief educational intervention, in addition to a brief treatment with NSAID and muscle relaxants. Additionally, they also suggest a short course of manipulation as a treatment option. Although acupuncture is not included in the European guideline’s recommendations, we found that acupuncture had a modest effect size, when compared to placebo or no-treatment groups.
Supervised exercise is recommended as the first line treatment in the management of chronic LBP, without any recommendations on the specific type of exercises [1]. The definition of exercise is wide and defined as “a series of specific movements with the aim of training or developing the body by a routine practice or as physical training to promote good physical health” [32], and accordingly the included studies in the present study concerning exercise contain all kind of exercises and different combinations of exercise programs. We based our results on quantitative analysis, in order to produce a single estimate of a treatment effect [19]. However, to do this in a meaningful way, heterogeneity must be taken into account. Heterogeneity concerns the variation in results across studies, which might be the result of differences in patient selection, type of treatments and combinations of these, durations of treatment, and more. The level of heterogeneity is calculated by chi-square test and is quantified by the I2-value, which describes the percentage of the total variation across studies that are due to heterogeneity rather than chance. We demonstrated a high level of heterogeneity for exercise trials, and although it seems reasonable to compare all kinds of exercise-programs, the limitation is that it is not possible to calculate a pooled effect size. So, for achieving a consistence and homogenous measure of the effect of exercises, it seems suitable to compare studies with the same exercise programs or use advance statistical methods to explore these characteristics [32]. The heterogeneity for manipulation was also high, which might be explained by different manipulations methods and different populations.
It might be surprising that we only found modest effect of the most common treatments for non-specific LBP. For acute LBP, the low-effect sizes might be explained by the fact that it is usually a self-limiting condition with a recovery rate at 90% within 6 weeks [20, 37]. Concerning chronic non-specific LBP, the definition of the diagnosis “chronic non-specific low back pain” is not defined as a clinical entity and diagnosis, but rather a symptom in patients with very different stages of impairment and disability without knowing the specific causes of the pain [1]. This implies that we compare effects of treatments for a condition without a specific diagnostic test, based on patient’s rating of pain, with different unspecific radiological findings and where the prognosis is influenced by psycho–social and work related factors. This is a serious limitation of the study, which may contribute to the modest effect sizes, and it emphasizes the need of defining appropriate sub-classifications of non-specific LBP.
Most RCT for treatments of LBP compare combinations of different interventions with either one intervention or another combination of interventions [4, 7, 51], and only a small part (about 20%) compare treatments with a no-treatment group (Table 1). This is interesting as it would be natural first to investigate the basic benefit of treatments and then compare them to each other. The purpose of the present study was to investigate the basic benefit with the consequence that only a small part of trials from each systematic review would be included (Tables 1, 2) and some treatments in general use would be excluded. Although this limits the generalization of the result, it brings into focus the importance of no-treatment controlled trials for investigating the pure effect of treatments for non-specific low-back pain. However, we are aware of the multitude of difficulties in carrying out no-treatment controlled trials, particularly within surgery.
Although surgery for degenerative conditions affecting the lumbar spine is a common type of treatment, it was excluded from the present study as most RCT compare different surgical techniques. To our knowledge, the only exception is the study by Fritzell et al. [22] comparing lumbar fusion with a no-treatment group. Most patients with chronic low-back pain who are referred to a surgical clinic have been through several non-surgical treatments, inclusive exercises, and they often demand surgical treatment. Hence, it might be a hard task for the physician to persuade these patients to be enrolled into a RCT, since there is a risk they will be randomized to the no-treatment group.
Another limitation is the quality of the included studies. Generally, they ranged from 3 to 7 according to the 11-item criteria list, with a few exceptions of 9 and 11, both for acute and chronic LBP (Tables 3 and 8). In addition, in several studies, mostly concerning manipulation, the variability of the effect estimate was not reported and for this reason we used the SD from other studies. Although, we performed sensitivity analyses, this is a limitation that calls for a cautious interpretation of the results.
Table 8.
Description of the included studies for chronic low-back pain
| Treatment | Author, reference | Year | Number of patients | Method quality | Comparative group | Outcome measurements | |
|---|---|---|---|---|---|---|---|
| Pain | Function | ||||||
| Exercise | Zylbergold et al. [91] | 1981 | 28 | 3 | No treatment | Likert pain scale (five-point) | Likert pain scale (five-point) |
| Turner et al. [76] | 1990 | 96 | 3 | No treatment | McGill pain questionnaire | Sickness impact profile | |
| Risch et al. [65] | 1993 | 54 | 3 | No treatment | West Haven Yale questionnaire | Sickness impact profile | |
| Soukup et al. [69] | 1999 | 120 | 5 | No treatment | Visual Analogue Scale (0–100 mm) | Oswestry disability index | |
| Preyde et al. [64] | 2000 | 98 | 7 | Sham treatment-gel | Present pain index (0–5 points) | Roland Morris disability questionnaire | |
| Kuukkanen et al. [45] | 2000 | 57 | 2 | No treatment | Visual analogue scale (11 points scale) | Oswestry Disability Index | |
| Behavioral therapy | Turner et al. [74] | 1982 | 36 | 3 | Waiting list | Visual analogue scale (0–100 mm) | Sickness impact profile |
| Nouwen et al. [60] | 1983 | 20 | 6 | Waiting list | Pain = duration × intensity | ||
| Stukey et al. [70] | 1986 | 16 | 1 | Placebo EMG | Visual analogue scale (0–100 mm) | Activity (1–7) | |
| Turner et al. [75] | 1988 | 81 | 6 | Waiting list | McGill pain questionnaire | Sickness impact profile | |
| Linton et al. [49] | 1989 | 66 | 8 | Waiting list | Visual analogue scale (0–100 mm) | ||
| Turner et al. [76] | 1990 | 96 | 4 | Waiting list | McGill pain questionnaire | Sickness impact profile | |
| Turner et al. [77] | 1993 | 102 | 5 | Waiting list | McGill pain questionnaire | ||
| Manipulation | Evans et al. [21] | 1978 | 32 | 5 | No treatment | Pain score (four points scale) | |
| Waagen et al. [89] | 1986 | 29 | 5 | Sham manipulation | Visual analogue scale (0–100 mm) | ||
| Ongley et al. [61] | 1987 | 81 | 7 | Sham manipulation | Visual analogue scale (0–100 mm) | Roland and Waddel | |
| Postacchini et al. [63] | 1988 | 160 | 3 | Placebo gel | Pain (four points scale) | Ten items | |
| Triano et al. [73] | 1995 | 86 | 5 | Sham manipulation | Visual analogue scale (0–100 mm) | Oswestry Disability Index | |
| TENS | Deyo et al. [17] | 1990 | 72 | 5 | Sham TENS | Improvement of pain (six points scale) | Sickness impact profile (modified) |
| Marchand et al. [53] | 1993 | 42 | 2 | Sham TENS | Visual analogue scale (0–100 mm) | ||
| Acupuncture | Coan et al. [14] | 1980 | 50 | 2 | No treatment | Visual analogue scale (11 points scale) | Limitation of activity (four point) |
| Mendelson et al. [55] | 1983 | 77 | 5 | Placebo | Visual analogue scale (0–100 mm) | ||
| Thomas and Lundberg et al. [72] | 1994 | 43 | 4 | No treatment | No of words from chart of 83 words describing pain intensity | ||
| Carlsson et al. [12] | 2001 | 51 | 7 | Sham/placebo | Visual analogue scale (0–100 mm) | ||
| Kerr et al. [42] | 2001 | 46 | 4 | Sham/placebo | Visual analogue scale (0–100 mm) | ||
| Leibing et al. [46] | 2002 | 75 | 7 | Sham/placebo | Visual analogue scale (0–10 cm) | Seven areas of activity, 70 questions | |
| Molsberger et al. [57] | 2002 | 186 | 9 | Sham/placebo | Visual analogue scale (0–100 mm) | ||
| NSAID | Katz et al. [40] | 2003 | 461 | 11 | Placebo | Visual analogue scale (0–100 mm) | |
| Coats et al. [15] | 2004 | 293 | 6 | Placebo | Visual analogue scale (0–100 mm) | Roland Morris disability questionnaire | |
| Palley et al. [62] | 2004 | 325 | 11 | Placebo | Visual analogue scale (0–100 mm) | Roland Morris disability questionnaire | |
| Benzodiazepines | Arbus et al. [2] | 1990 | 24 | 6 | Placebo | Pain score 1–5 | |
| Salzmann et al. [66] | 1992 | 83 | 6 | Placebo | Huskisson’s pain scale (night-time/day-time) | ||
Overall, the follow-up in most of the studies was insufficient. Exercise therapy has short and long-term follow-ups both for the acute and the chronic condition, but apart from this treatment there is a lack of long-term follow up. However, it is likely that long-term follow-up would not change the conclusion in current study, as the effect sizes in general were small at short-term follow-up and would most probably not have changed at long-term follow-up.
Outcomes measurements in the field of LBP are pain and function measured by psychometric scales. In the present study, there were several different scales both for pain and function. Although the measurements can be standardized by calculating effect size or by rescaling individual trials outcome for pain and functioning from 0 to 100, comparison of trials would be more easy and precise by using the same scales as recommended by Bombardier et al. [11].
It is common to accept co-interventions in RCT and it might be difficult to carry out RCT not allowing patients to visit other health care providers. However, co-interventions contribute to blur the effects of the treatments in the RCT, so that is the reason for our decision to exclude trials where patients were allowed to have co-interventions [47, 58, 67].
In conclusion, the effect sizes for the pure benefit of treatments of LBP that are compared to no-treatment groups were small to moderate for both the acute and chronic conditions. There was a lack of long-term follow up for pain and function and the quality of the studies were low to moderate. For increasing our knowledge about treatments of non-specific LBP, there is still a need to develop more effective interventions.
Acknowledgment
Dr. Hayden was supported by a Postdoctoral Fellowship Award from the Canadian Institutes of Health Research and the Canadian Chiropractic Research Foundation.
References
- 1.Airaksinen O, Brox JI, Cedraschi C, Hildebrandt J, Klaber-Moffett J, Kovacs F, Mannion AF, Reis S, Staal JB, Ursin H, Zanoli G. Chapter 4. European guidelines for the management of chronic nonspecific low back pain. Eur Spine J. 2006;15(Suppl 2):S192–S300. doi: 10.1007/s00586-006-1072-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arbus L, Fajadet B, Aubert D, Morre M, Goldfinger E. Activity of tetrazepam in low back pain. Clin Trials J. 1990;27:58–67. [Google Scholar]
- 3.Assendelft WJ, Morton SC, Yu EI, Suttorp MJ, Shekelle PG (2004) Spinal manipulative therapy for low back pain. Cochrane Database Syst Rev CD000447 [DOI] [PubMed]
- 4.Aure OF, Nilsen JH, Vasseljen O (2003) Manual therapy and exercise therapy in patients with chronic low back pain: a randomized, controlled trial with 1-year follow-up. Spine 28:525–531; discussion 531–522 [DOI] [PubMed]
- 5.Babej-Dolle R, Freytag S, Eckmeyer J, Zerle G, Schinzel S, Schmeider G, Stankov G. Parenteral dipyrone versus diclofenac and placebo in patients with acute lumbago or sciatic pain: randomized observer-blind multicenter study. Int J Clin Pharmacol Ther. 1994;32:204–209. [PubMed] [Google Scholar]
- 6.Barrata R. A double-blind study of cyclobenzaprine and placebo in the treatment of acute musculoskeletal conditions of the low back pain. Curr Ther Res. 1982;32:646–652. [Google Scholar]
- 7.Bendix AF, Bendix T, Lund C, Kirkbak S, Ostenfeld S. Comparison of three intensive programs for chronic low back pain patients: a prospective, randomized, observer-blinded study with one- year follow-up. Scand J Rehabil Med. 1997;29:81–89. [PubMed] [Google Scholar]
- 8.Bergquist-Ullman M, Larsson U (1977) Acute low back pain in industry. A controlled prospective study with special reference to therapy and confounding factors. Acta Orthop Scand 1–117 [DOI] [PubMed]
- 9.Berry H, Hutchinson D. A multicenter placebo-controlled study in general practice to evaluate the efficacy and safety of tizanidine in acute low-back pain. J Int Med Res. 1988;16:75–82. doi: 10.1177/030006058801600201. [DOI] [PubMed] [Google Scholar]
- 10.Birbara CA, Puopolo AD, Munoz DR, Sheldon EA, Mangione A, Bohidar NR, Geba GP. Treatment of chronic low back pain with etoricoxib, a new cyclo-oxygenase-2 selective inhibitor: improvement in pain and disability—a randomized, placebo-controlled, 3-month trial. J Pain. 2003;4:307–315. doi: 10.1016/S1526-5900(03)00633-3. [DOI] [PubMed] [Google Scholar]
- 11.Bombardier C. Outcome assessments in the evaluation of treatment of spinal disorders: summary and general recommendations. Spine. 2000;25:3100–3103. doi: 10.1097/00007632-200012150-00003. [DOI] [PubMed] [Google Scholar]
- 12.Carlsson CP, Sjolund BH. Acupuncture for chronic low back pain: a randomized placebo-controlled study with long-term follow-up. Clin J Pain. 2001;17:296–305. doi: 10.1097/00002508-200112000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Chok B, Lee R, Latimer J, Tan SB. Endurance training of the trunk extensor muscles in people with subacute low back pain. Phys Ther. 1999;79:1032–1042. [PubMed] [Google Scholar]
- 14.Coan RM, Wong G, Ku SL, Chan YC, Wang L, Ozer FT, Coan PL. The acupuncture treatment of low back pain: a randomized controlled study. Am J Chin Med. 1980;8:181–189. doi: 10.1142/S0192415X80000141. [DOI] [PubMed] [Google Scholar]
- 15.Coats TL, Borenstein DG, Nangia NK, Brown MT. Effects of valdecoxib in the treatment of chronic low back pain: results of a randomized, placebo-controlled trial. Clin Ther. 2004;26:1249–1260. doi: 10.1016/S0149-2918(04)80081-X. [DOI] [PubMed] [Google Scholar]
- 16.Cohen J. Statistical power analysis for the behavioral sciences. New York: Academic; 1977. [Google Scholar]
- 17.Deyo RA, Walsh NE, Martin DC, Schoenfeld LS, Ramamurthy S. A controlled trial of transcutaneous electrical nerve stimulation (TENS) and exercise for chronic low back pain. N Engl J Med. 1990;322:1627–1634. doi: 10.1056/NEJM199006073222303. [DOI] [PubMed] [Google Scholar]
- 18.Dreiser RL, Marty M, Ionescu E, Gold M, Liu JH. Relief of acute low back pain with diclofenac-K 12.5 mg tablets: a flexible dose, ibuprofen 200 mg and placebo-controlled clinical trial. Int J Clin Pharmacol Ther. 2003;41:375–385. doi: 10.5414/cpp41375. [DOI] [PubMed] [Google Scholar]
- 19.Egger M, Smith G, Altman DG. Systematic reviews in health care. Meta-analysis in context. London: BMJ Books; 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.European Commission COST B13 Management Committee (2002) European guidelines for the management of low back pain. Acta Orthop Scand Suppl 73:20–25 [DOI] [PubMed]
- 21.Evans DP, Burke MS, Lloyd KN, Roberts EE, Roberts GM. Lumbar spinal manipulation on trial. Part I—clinical assessment. Rheumatol Rehabil. 1978;17:46–53. doi: 10.1093/rheumatology/17.1.46. [DOI] [PubMed] [Google Scholar]
- 22.Fritzell P, Hagg O, Wessberg P, Nordwall A (2001) 2001 Volvo award winner in clinical studies: lumbar fusion versus nonsurgical treatment for chronic low back pain: a multicenter randomized controlled trial from the Swedish lumbar spine study group. Spine 26:2521–2532; discussion 2532–2524 [DOI] [PubMed]
- 23.Furlan AD, Brosseau L, Imamura M, Irvin E (2002) Massage for low back pain. Cochrane Database Syst Rev CD001929 [DOI] [PubMed]
- 24.Furlan AD, van Tulder MW, Cherkin DC, Tsukayama H, Lao L, Koes BW, Berman BM (2005) Acupuncture and dry-needling for low back pain. Cochrane Database Syst Rev CD001351 [DOI] [PMC free article] [PubMed]
- 25.Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta-analyses can provide accurate results. J Clin Epidemiol. 2006;59:7–10. doi: 10.1016/j.jclinepi.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Faas A, Chavannes AW, Eijk JT, Gubbels JW. A randomized, placebo-controlled trial of exercise therapy in patients with acute low back pain. Spine. 1993;18:1388–1395. [PubMed] [Google Scholar]
- 27.Gibson JN, Grant IC, Waddell G (2000) Surgery for lumbar disc prolapse. Cochrane Database Syst Rev CD001350 [DOI] [PubMed]
- 28.Gibson JN, Waddell G, Grant IC (2000) Surgery for degenerative lumbar spondylosis. Cochrane Database Syst Rev CD001352 [DOI] [PubMed]
- 29.Glover JR, Morris JG, Khosla T. Back pain: a randomized clinical trial of rotational manipulation of the trunk. Br J Ind Med. 1974;31:59–64. doi: 10.1136/oem.31.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guzman J, Esmail R, Karjalainen K, Malmivaara A, Irvin E, Bombardier C (2002) Multidisciplinary bio-psycho-social rehabilitation for chronic low back pain. Cochrane Database Syst Rev CD000963 [DOI] [PubMed]
- 31.Hagen K, Hilde G, Jamtvedt G, Winnem M (2004) Bed rest for acute low-back pain and sciatica. Cochrane Database Syst Rev CD001254 [DOI] [PubMed]
- 32.Hayden JA, Tulder MW, Malmivaara AV, Koes BW. Meta-analysis: exercise therapy for nonspecific low back pain. Ann Intern Med. 2005;142:765–775. doi: 10.7326/0003-4819-142-9-200505030-00013. [DOI] [PubMed] [Google Scholar]
- 33.Heymans M, Tulder M, Esmail R, Bombardier C, Koes B (2004) Back schools for non-specific low-back pain. Cochrane Database Syst Rev CD000261 [DOI] [PMC free article] [PubMed]
- 34.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hilde G, Hagen KB, Jamtvedt G, Winnem M (2002) Advice to stay active as a single treatment for low back pain and sciatica. Cochrane Database Syst Rev CD003632 [DOI] [PubMed]
- 36.Hurley DA, McDonough SM, Dempster M, Moore AP, Baxter GD. A randomized clinical trial of manipulative therapy and interferential therapy for acute low back pain. Spine. 2004;29:2207–2216. doi: 10.1097/01.brs.0000142234.15437.da. [DOI] [PubMed] [Google Scholar]
- 37.Indahl A. Low back pain: diagnosis, treatment, and prognosis. Scand J Rheumatol. 2004;33:199–209. doi: 10.1080/03009740410006916. [DOI] [PubMed] [Google Scholar]
- 38.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 39.Karjalainen K, Malmivaara A, van Tulder M, Roine R, Jauhiainen M, Hurri H, Koes B (2003) Multidisciplinary biopsychosocial rehabilitation for subacute low back pain among working age adults. Cochrane Database Syst Rev CD002193 [DOI] [PubMed]
- 40.Katz N, Ju WD, Krupa DA, Sperling RS, Bozalis Rodgers D, Gertz BJ, Gimbel J, Coleman S, Fisher C, Nabizadeh S, Borenstein D (2003) Efficacy and safety of rofecoxib in patients with chronic low back pain: results from two 4-week, randomized, placebo-controlled, parallel-group, double-blind trials. Spine 28:851–858; discussion 859 [DOI] [PubMed]
- 41.Kazis LE, Anderson JJ, Meenan RF. Effect sizes for interpreting changes in health status. Med Care. 1989;27:S178–S189. doi: 10.1097/00005650-198903001-00015. [DOI] [PubMed] [Google Scholar]
- 42.Kerr DP, Walsh DM, Baxter GD. A study of the use of acupuncture in physiotherapy. Complement Ther Med. 2001;9:21–27. doi: 10.1054/ctim.2000.0422. [DOI] [PubMed] [Google Scholar]
- 43.Khadilkar A, Milne S, Brosseau L, Wells G, Tugwell P, Robinson V, Shea B, Saginur M. Transcutaneous electrical nerve stimulation for the treatment of chronic low back pain: a systematic review. Spine. 2005;30:2657–2666. doi: 10.1097/01.brs.0000188189.21202.0f. [DOI] [PubMed] [Google Scholar]
- 44.Kopec JA. Measuring functional outcomes in persons with back pain: a review of back-specific questionnaires. Spine. 2000;25:3110–3114. doi: 10.1097/00007632-200012150-00005. [DOI] [PubMed] [Google Scholar]
- 45.Kuukkanen T, Malkia E. Effects of a three-month therapeutic exercise programme on flexibility in subjects with low back pain. Physiother Res Int. 2000;5:46–61. doi: 10.1002/pri.183. [DOI] [PubMed] [Google Scholar]
- 46.Leibing E, Leonhardt U, Koster G, Goerlitz A, Rosenfeldt JA, Hilgers R, Ramadori G. Acupuncture treatment of chronic low-back pain—a randomized, blinded, placebo-controlled trial with 9-month follow-up. Pain. 2002;96:189–196. doi: 10.1016/S0304-3959(01)00444-4. [DOI] [PubMed] [Google Scholar]
- 47.Lepisto P. A comparative trial of DS 103–282 and placebo in the treatment of acute skeletal muscle spasms due to disorders of the back. Ther Res. 1979;26:454–459. [Google Scholar]
- 48.Licciardone JC, Stoll ST, Fulda KG, Russo DP, Siu J, Winn W, Swift J., Jr Osteopathic manipulative treatment for chronic low back pain: a randomized controlled trial. Spine. 2003;28:1355–1362. doi: 10.1097/00007632-200307010-00002. [DOI] [PubMed] [Google Scholar]
- 49.Linton SJ, Bradley LA, Jensen I, Spangfort E, Sundell L. The secondary prevention of low back pain: a controlled study with follow-up. Pain. 1989;36:197–207. doi: 10.1016/0304-3959(89)90024-9. [DOI] [PubMed] [Google Scholar]
- 50.Malmivaara A, Hakkinen U, Aro T, Heinrichs ML, Koskenniemi L, Kuosma E, Lappi S, Paloheimo R, Servo C, Vaaranen V, et al. The treatment of acute low back pain—bed rest, exercises, or ordinary activity? N Engl J Med. 1995;332:351–355. doi: 10.1056/NEJM199502093320602. [DOI] [PubMed] [Google Scholar]
- 51.Mannion AF, Junge A, Taimela S, Muntener M, Lorenzo K, Dvorak J. Active therapy for chronic low back pain: part 3. Factors influencing self-rated disability and its change following therapy. Spine. 2001;26:920–929. doi: 10.1097/00007632-200104150-00015. [DOI] [PubMed] [Google Scholar]
- 52.Mannion AF, Muntener M, Taimela S, Dvorak J. A randomized clinical trial of three active therapies for chronic low back pain. Spine. 1999;24:2435–2448. doi: 10.1097/00007632-199912010-00004. [DOI] [PubMed] [Google Scholar]
- 53.Marchand S, Charest J, Li J, Chenard JR, Lavignolle B, Laurencelle L. Is TENS purely a placebo effect? A controlled study on chronic low back pain. Pain. 1993;54:99–106. doi: 10.1016/0304-3959(93)90104-W. [DOI] [PubMed] [Google Scholar]
- 54.Mayer JM, Ralph L, Look M, Erasala GN, Verna JL, Matheson LN, Mooney V. Treating acute low back pain with continuous low-level heat wrap therapy and/or exercise: a randomized controlled trial. Spine J. 2005;5:395–403. doi: 10.1016/j.spinee.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 55.Mendelson G, Selwood TS, Kranz H, Loh TS, Kidson MA, Scott DS. Acupuncture treatment of chronic back pain. A double-blind placebo-controlled trial. Am J Med. 1983;74:49–55. doi: 10.1016/0002-9343(83)91117-8. [DOI] [PubMed] [Google Scholar]
- 56.Mitchell RI, Carmen GM. Results of a multicenter trial using an intensive active exercise program for the treatment of acute soft tissue and back injuries. Spine. 1990;15:514–521. doi: 10.1097/00007632-199006000-00016. [DOI] [PubMed] [Google Scholar]
- 57.Molsberger AF, Mau J, Pawelec DB, Winkler J. Does acupuncture improve the orthopedic management of chronic low back pain—a randomized, blinded, controlled trial with 3 months follow up. Pain. 2002;99:579–587. doi: 10.1016/S0304-3959(02)00269-5. [DOI] [PubMed] [Google Scholar]
- 58.Nelemans PJ, de Bie RA, de Vet HC, Sturmans F (2000) Injection therapy for subacute and chronic benign low back pain. Cochrane Database Syst Rev CD001824 [DOI] [PubMed]
- 59.Niemisto L, Lahtinen-Suopanki T, Rissanen P, Lindgren KA, Sarna S, Hurri H. A randomized trial of combined manipulation, stabilizing exercises, and physician consultation compared to physician consultation alone for chronic low back pain. Spine. 2003;28:2185–2191. doi: 10.1097/01.BRS.0000085096.62603.61. [DOI] [PubMed] [Google Scholar]
- 60.Nouwen A. EMG biofeedback used to reduce standing levels of paraspinal muscle tension in chronic low back pain. Pain. 1983;17:353–360. doi: 10.1016/0304-3959(83)90166-5. [DOI] [PubMed] [Google Scholar]
- 61.Ongley MJ, Klein RG, Dorman TA, Eek BC, Hubert LJ. A new approach to the treatment of chronic low back pain. Lancet. 1987;2:143–146. doi: 10.1016/S0140-6736(87)92340-3. [DOI] [PubMed] [Google Scholar]
- 62.Pallay RM, Seger W, Adler JL, Ettlinger RE, Quaidoo EA, Lipetz R, O’Brien K, Mucciola L, Skalky CS, Petruschke RA, Bohidar NR, Geba GP. Etoricoxib reduced pain and disability and improved quality of life in patients with chronic low back pain: a 3 month, randomized, controlled trial. Scand J Rheumatol. 2004;33:257–266. doi: 10.1080/03009740410005728. [DOI] [PubMed] [Google Scholar]
- 63.Postacchini F, Facchini M, Palieri P. Efficacy of various forms of conservative treatment in low back pain. A comparative study. Neuro Orthop. 1988;6:28–35. [Google Scholar]
- 64.Preyde M. Effectiveness of massage therapy for subacute low-back pain: a randomized controlled trial. CMAJ. 2000;162:1815–1820. [PMC free article] [PubMed] [Google Scholar]
- 65.Risch SV, Norvell NK, Pollock ML, Risch ED, Langer H, Fulton M, Graves JE, Leggett SH. Lumbar strengthening in chronic low back pain patients. Physiologic and psychological benefits. Spine. 1993;18:232–238. doi: 10.1097/00007632-199302000-00010. [DOI] [PubMed] [Google Scholar]
- 66.Salzmann E, Pforringer W, Paal G, et al. Treatment of chronic low-back syndrome with tetrazepamin a placebo controlled double-blind trial. J Drug Dev. 1992;4:219–228. [Google Scholar]
- 67.Schonstein E, Kenny DT, Keating J, Koes BW (2003) Work conditioning, work hardening and functional restoration for workers with back and neck pain. Cochrane Database Syst Rev CD001822 [DOI] [PubMed]
- 68.Sherman KJ, Cherkin DC, Erro J, Miglioretti DL, Deyo RA. Comparing yoga, exercise, and a self-care book for chronic low back pain: a randomized, controlled trial. Ann Intern Med. 2005;143:849–856. doi: 10.7326/0003-4819-143-12-200512200-00003. [DOI] [PubMed] [Google Scholar]
- 69.Soukup MG, Glomsrod B, Lonn JH, Bo K, Larsen S (1999) The effect of a Mensendieck exercise program as secondary prophylaxis for recurrent low back pain. A randomized, controlled trial with 12-month follow-up. Spine 24:1585–1591; discussion 1592 [DOI] [PubMed]
- 70.Stuckey SJ, Jacobs A, Goldfarb J. EMG biofeedback training, relaxation training, and placebo for the relief of chronic back pain. Percept Mot Skills. 1986;63:1023–1036. doi: 10.2466/pms.1986.63.3.1023. [DOI] [PubMed] [Google Scholar]
- 71.Szpalski M, Hayez JP. Objective functional assessment of the efficacy of tenoxicam in the treatment of acute low back pain. A double-blind placebo-controlled study. Br J Rheumatol. 1994;33:74–78. doi: 10.1093/rheumatology/33.1.74. [DOI] [PubMed] [Google Scholar]
- 72.Thomas M, Lundberg T. Importance of modes of acupuncture in the treatment of chronic nociceptive low back pain. Acta Anaesthesiol Scand. 1994;38:63–69. doi: 10.1111/j.1399-6576.1994.tb03839.x. [DOI] [PubMed] [Google Scholar]
- 73.Triano JJ, McGregor M, Hondras MA, Brennan PC. Manipulative therapy versus education programs in chronic low back pain. Spine. 1995;20:948–955. doi: 10.1097/00007632-199504150-00013. [DOI] [PubMed] [Google Scholar]
- 74.Turner JA, Chapman CR. Psychological interventions for chronic pain: a critical review I. Relaxation training and biofeedback. Pain. 1982;12:1–21. doi: 10.1016/0304-3959(82)90167-1. [DOI] [PubMed] [Google Scholar]
- 75.Turner JA, Clancy S. Comparison of operant behavioral and cognitive-behavioral group treatment for chronic low back pain. J Consult Clin Psychol. 1988;56:261–266. doi: 10.1037/0022-006X.56.2.261. [DOI] [PubMed] [Google Scholar]
- 76.Turner JA, Clancy S, McQuade KJ, Cardenas DD. Effectiveness of behavioral therapy for chronic low back pain: a component analysis. J Consult Clin Psychol. 1990;58:573–579. doi: 10.1037/0022-006X.58.5.573. [DOI] [PubMed] [Google Scholar]
- 77.Turner JA, Jensen MP. Efficacy of cognitive therapy for chronic low back pain. Pain. 1993;52:169–177. doi: 10.1016/0304-3959(93)90128-C. [DOI] [PubMed] [Google Scholar]
- 78.Tuzun F, Unalan H, Oner N, Ozguzel H, Kirazli Y, Icagasioglu A, Kuran B, Tuzun S, Basar G. Multicenter, randomized, double-blinded, placebo-controlled trial of thiocolchicoside in acute low back pain. Joint Bone Spine. 2003;70:356–361. doi: 10.1016/S1297-319X(03)00075-7. [DOI] [PubMed] [Google Scholar]
- 79.Urrutia G, Burton AK, Morral A, Bonfill X, Zanoli G (2004) Neuroreflexotherapy for non-specific low-back pain. Cochrane Database Syst Rev CD003009 [DOI] [PMC free article] [PubMed]
- 80.Tulder M, Furlan A, Bombardier C, Bouter L. Updated method guidelines for systematic reviews in the cochrane collaboration back review group. Spine. 2003;28:1290–1299. doi: 10.1097/00007632-200306150-00014. [DOI] [PubMed] [Google Scholar]
- 81.Tulder M, Malmivaara A, Esmail R, Koes B. Exercise therapy for low back pain: a systematic review within the framework of the cochrane collaboration back review group. Spine. 2002;25:2784–2796. doi: 10.1097/00007632-200011010-00011. [DOI] [PubMed] [Google Scholar]
- 82.van Tulder MW, Jellema P, van Poppel MN, Nachemson AL, Bouter LM (2000) Lumbar supports for prevention and treatment of low back pain. Cochrane Database Syst Rev CD001823 [DOI] [PubMed]
- 83.Tulder MW, Ostelo R, Vlaeyen JW, Linton SJ, Morley SJ, Assendelft WJ. Behavioral treatment for chronic low back pain: a systematic review within the framework of the cochrane back review group. Spine. 2000;25:2688–2699. doi: 10.1097/00007632-200010150-00024. [DOI] [PubMed] [Google Scholar]
- 84.van Tulder MW, Scholten RJ, Koes BW, Deyo RA (2000) Non-steroidal anti-inflammatory drugs for low back pain. Cochrane Database Syst Rev CD000396 [DOI] [PubMed]
- 85.van Tulder MW, Touray T, Furlan AD, Solway S, Bouter LM (2003) Muscle relaxants for non-specific low back pain. Cochrane Database Syst Rev CD004252 [DOI] [PMC free article] [PubMed]
- 86.Korff M, Jensen MP, Karoly P. Assessing global pain severity by self-report in clinical and health services research. Spine. 2000;25:3140–3151. doi: 10.1097/00007632-200012150-00009. [DOI] [PubMed] [Google Scholar]
- 87.Waddell G. 1987 Volvo award in clinical sciences. A new clinical model for the treatment of low-back pain. Spine. 1987;12:632–644. doi: 10.1097/00007632-198709000-00002. [DOI] [PubMed] [Google Scholar]
- 88.Wand BM, Bird C, McAuley JH, Dore CJ, MacDowell M, Souza LH. Early intervention for the management of acute low back pain: a single-blind randomized controlled trial of biopsychosocial education, manual therapy, and exercise. Spine. 2004;29:2350–2356. doi: 10.1097/01.brs.0000143619.34308.b4. [DOI] [PubMed] [Google Scholar]
- 89.Waagen G, Haldeman S, Cook G, Lopez D, DeBoer K. Short term trial of chiropractic adjustments for the relief of chronic low back pain. Manual Med. 1986;2:63–67. [Google Scholar]
- 90.Yelland MJ, Mar C, Pirozzo S, Schoene ML, Vercoe P (2004) Prolotherapy injections for chronic low-back pain. Cochrane Database Syst Rev CD004059 [DOI] [PubMed]
- 91.Zylbergold R, Piper M. Lumbar disc disease: comparative analysis of physical therapy. Arch Phys Med Rehabil. 1981;62:176–179. [PubMed] [Google Scholar]