Abstract
Proinflammatory cytokines secreted by memory CD8+ and CD4+ T cells are thought to play a direct role in the pathogenesis of dengue virus infection by increasing vascular permeability and thereby inducing the pathophysiologic events associated with dengue hemorrhagic fever and dengue shock syndrome. Severe disease is frequently observed in the setting of secondary infection with heterologous dengue virus serotypes, suggesting a role for cross-reactive memory T cells in the immunopathogenesis of severe disease. We used a large panel of well-characterized dengue virus-specific CD8+ T-cell clones isolated from Pacific Islanders previously infected with dengue virus 1 to examine effector memory function, focusing on a novel dominant HLA-B*5502-restricted NS5329-337 epitope, and assessed T-cell responses to stimulation with variant peptides representing heterologous serotypes. Variant peptides were differentially recognized by dengue virus 1-specific effector CD8+ cytotoxic T lymphocytes (CTL) in a heterogeneous and clone-specific manner, in which cytolytic function and cytokine secretion could be enhanced, diminished, or abrogated compared with cognate peptide stimulation. Dengue virus-specific CTL stimulated with cognate and variant peptides demonstrated a cytokine response hierarchy of gamma IFN (IFN-γ) > tumor necrosis factor alpha (TNF-α) > interleukin-2 (IL-2), and a subset of clones also produced IL-4 and IL-6. Individual clones demonstrated greater avidity for variant peptides representing heterologous serotypes, including serotypes previously encountered by the subject, and IFN-γ and TNF-α secretion was enhanced by stimulation with these heterologous peptides. Altered antiviral T-cell responses in response to stimulation with heterologous dengue virus serotypes have implications for control of virus replication and for disease pathogenesis.
Dengue viruses are mosquito-borne, enveloped RNA viruses which form their own antigenic complex within the Flaviviridae family, separate from the Japanese encephalitis and tick-borne encephalitis serocomplexes. There are four antigenically distinct viruses: dengue virus 1 (Den-1), Den-2, Den-3, and Den-4, all of which can cause dengue fever (DF), an acute febrile illness usually presenting with rash, headaches, myalgia, arthralgia, and leukopenia (10). A subset of infected individuals may develop more severe disease characterized by plasma leakage and hemorrhagic manifestations (dengue hemorrhagic fever [DHF]). There are four grades of DHF, the most severe of which are associated with dengue shock syndrome (DSS), which is characterized by circulatory failure and hypovolemic shock. In the absence of treatment DSS is associated with high mortality, particularly in children. An estimated 50 to 100 million cases of DF and hundreds of thousands of cases of DHF occur each year (32). There are no approved dengue virus vaccines available.
The pathogenesis of DHF/DSS is not well understood, but epidemiological observations suggest that severe disease occurs more frequently in the setting of secondary infection with heterologous dengue virus serotypes (11, 25). Ennis and coworkers (15, 21) have proposed a model for the immunopathogenesis of DHF/DSS in which dengue virus-specific memory T cells from a previous infection are activated by infected monocytes or macrophages via major histocompatibility complex (MHC)-restricted presentation of heterologous dengue virus epitopes to produce cytokines, including gamma interferon (IFN-γ), which upregulate expression of cell surface FcγR. Infection of monocytes is subsequently enhanced when low-affinity, nonneutralizing antibodies to dengue virus, persisting from the previous infection, facilitate uptake of virus via attachment to FcγR (12). In turn, T-cell activation is enhanced, and secretion of proinflammatory cytokines and other vasodilatory molecules, which mediate development of vascular leakage, is increased.
Cross-reactive memory T cells and their role in mediating immunoprotective or immunopathologic responses have been intensively studied in several viral systems in recent years (2, 3, 26, 27, 34). Existing immunity to one virus can modulate immune responses to infection with a second, unrelated virus by activation of circulating memory T cells via MHC-restricted presentation of peptide epitopes derived from the second virus. Such cross-reactive epitopes comply with the binding motif for the relevant MHC molecule and share several amino acids at critical positions of the T-cell determinant. Since memory T cells have lower activation thresholds (4, 33) than naïve T cells, they may therefore be preferentially activated during secondary infection with heterologous viruses. Memory T cells stimulated with heterologous virus variant peptides may display altered function compared with stimulation with cognate epitope peptide.
Although the four dengue virus serotypes are antigenically and genotypically distinct, varying by up to 30% at the nucleotide level, there is sufficient amino acid sequence homology within antigenically relevant regions of the viral proteins to potentially allow cross-recognition by memory T cells generated in response to previous infection with heterologous dengue viruses. Thus, cross-reactive T-cell responses in secondary infection may fail to control virus replication because of the intrinsically lower avidity of memory cells primed during a first infection for altered epitope peptides from the secondary virus.
We examined dengue virus-specific T-cell cross-reactivity in the setting of naturally acquired infection in individuals exposed to dengue viruses in Hawaii and elsewhere in the Pacific. Although dengue epidemics occur frequently throughout the Pacific region, this is the first report of dengue virus-specific T-cell responses in Pacific Islanders. HLA frequencies in Polynesians, Melanesians, and Micronesians, the three major Pacific groups, may differ from those in Caucasian and Asian populations, in whom dengue virus-specific T-cell responses have been most studied, and there are no data on cell-mediated responses to dengue virus infection in the Pacific population. We synthesized a panel of peptides based on the consensus sequence of the dominant dengue virus strain circulating in Hawaii during an epidemic of Den-1 in 2001-2002, the first known dengue epidemic in almost 60 years (7), and used this panel to identify immunodominant dengue virus-specific responses in Pacific Islanders and to examine T-cell cross-reactivity at the clonal level.
MATERIALS AND METHODS
Study subjects and blood samples.
Blood samples were obtained from 12 individuals living in Hawaii who were infected with Den-1 in 2001-2002 and who experienced DF (7, 13). The case definition for laboratory-positive recent dengue virus infection was defined as a person who had dengue virus isolated from serum or a positive dengue virus immunoglobulin M antibody test result (7). Subjects were consented for collection of 100 ml of blood at each visit. Peripheral blood mononuclear cells (PBMC), isolated by Ficoll-Hypaque density gradient centrifugation from blood samples obtained 29 to 44 months after infection, were stored in liquid nitrogen for up to 2 years prior to analysis. HLA class 1 typing was performed on all samples, using standard serological techniques, and sequence-based molecular typing was performed on selected samples (Atria Genetics, CA). The study protocol was approved by the Institutional Review Board of the University of Hawaii at Manoa, and written informed consent was obtained from all participants prior to collection of blood.
Neutralization assay.
To confirm previous dengue virus infection in selected subjects, a 90% plaque reduction serum neutralization test was performed as previously described (24). Briefly, serum samples were diluted at twofold dilutions starting at 1:10 in OPTI-MEM medium (Gibco) supplemented with 2% fetal bovine serum (FBS), incubated with each of the four dengue virus serotypes for a final input dose of 100 PFU, and added to six-well plates containing Vero cell monolayers. Back-titrations of each virus were included to confirm 90% plaque reduction. An overlay of 1% agarose in M199 medium (Gibco) was added, and plates were incubated at 37°C for 10 days. A second agarose overlay containing 0.004% Neutral Red was added, and plaques were visualized and counted within 3 days.
Viruses and peptides.
In a previous study of the molecular epidemiology of Den-1 in Hawaii (13), dengue viruses were isolated from stored acute-phase samples collected at the time of the epidemic in 2001. Briefly, serum or plasma collected from symptomatic individuals during acute infection was inoculated onto C6/36 cells. Viral RNA was extracted from low-passage supernatants, reverse transcribed to cDNA, and amplified by PCR. Phylogenetic analysis of full-length E gene sequences showed that most (15/16) viruses fell within a single homogeneous group representing the dominant epidemic strain of Den-1 circulating in Hawaii in 2001. In order to generate a peptide library to be used to study Den-1-specific T-cell responses, the full-length genomes of four representative isolates were sequenced using primer sets designed to amplify overlapping regions of the genome (22; unpublished data). Amino acid sequence similarity for the full-length genome was between 99.9 and 100%, indicating that highly homogeneous Den-1 viruses circulated in Hawaii in 2001. Sequences were deposited in GenBank, and the accession numbers are DQ672560, DQ672561, DQ672562, and DQ672563. A consensus deduced amino acid sequence was generated by alignment of the four full-length genomes, and a set of 476 overlapping peptides spanning the full-length E, NS3, NS4A, NS4B, and NS5 genes was synthesized (PepSets; Mimotopes, Melbourne, Australia). Peptides were 15 amino acids long, overlapping by 10. Fine mapping of the identified 15- to 20-mer epitopes was conducted using 9-amino-acid-long peptide truncations (Mimotopes). Variant peptides, representing heterologous dengue virus serotype sequences corresponding to the epitopes of interest, were synthesized as 9-mer homologs of the mapped epitope peptide (Mimotopes).
IFN-γ ELISPOT assay.
PBMC were thawed, washed, and resuspended to a final concentration of 2 × 106/ml in RPMI 1640 medium supplemented with 10% FBS (R-10 medium; Gemini Bio-Products, West Sacramento, CA). Enzyme-linked immunospot (ELISPOT) assays were performed in 96-well plates (Millipore Corp.) coated with mouse anti-human IFN-γ antibody (clone 1-D1K; Mabtech AB, Sweden). PBMC were plated at 2 × 105 cells/well and exposed to peptides at a final concentration of 5 μg/ml. Peptides were screened in 48 pools, most of which contained 10 peptides each; 1 pool for each gene contained between 3 and 7 peptides. All peptide pools were screened in duplicate wells. Each plate included two negative control wells which contained cells but no peptides and two positive control wells which included cells plus phytohemagglutinin (Sigma) at a final concentration of 5 μg/ml. When responses were detected for pooled peptides, PBMC were tested against the individual peptides within the particular pool in duplicate wells. Plates were incubated at 37°C for 16 to 20 h, washed, and then incubated with biotinylated mouse anti-human IFN-γ monoclonal antibody (clone 7-B6-1; Mabtech AB, Sweden), labeled with streptavidin-peroxidase, and subsequently developed using fresh peroxidase substrate buffer (Vector Laboratories, Burlingame, CA). Spots were quantified using a digital reader (Cellular Technologies Limited, Cleveland, OH). Dengue virus-specific T-cell precursor frequency was expressed as spot-forming units (SFU) per million PBMC. Background activity (spots present in negative control wells) was subtracted from test wells and was always less than 30 SFU/million PBMC. A positive response was defined as being greater than twice the average of the negative control wells. In certain assays, CD8+ cells were positively selected by antibody-coated magnetic beads (StemCell Technologies, Vancouver, BC, Canada) and were plated at the same final concentration of 2 × 105 cells/well.
Target cells.
B-lymphoblastoid cell lines (BLCL) were derived from all subjects by transformation of PBMC with Epstein-Barr virus. Briefly, 20 × 106 PBMC were suspended in 10 ml R-20, in the presence of cyclosporine A at a final concentration of 0.5 μg/ml, and 10 ml of supernatant from the marmoset cell line B95-8 was added. The culture was maintained in 5% CO2 at 37°C for up to 4 weeks to allow transformation to occur. BLCL were cryopreserved in liquid nitrogen in aliquots of 10 × 106 cells until needed. For cytotoxicity assays, BLCL were labeled with 3.7 MBq Na251CrO4 (Perkin-Elmer, Wellesley, MA) overnight at 37°C, washed twice, and suspended at a final concentration of 3 × 104 to 5 × 104 cells/ml R-10.
Isolation of Den-1-specific T-cell lines and clones.
T-cell lines specific for NS5326-340 were derived by stimulation of PBMC with IFN-γ ELISPOT-mapped cognate peptide. Autologous BLCL were used as antigen-presenting cells. A total of 5 × 106 BLCL were suspended in R-10, in minimal volume, incubated with 10 μg peptide for 90 min, and irradiated at 12,000 rads in an X-ray irradiator (Faxitron X-ray Corporation, Wheeler, IL). The cells were washed and then placed in culture with 10 × 106 patient PBMC and allogeneic irradiated feeder PBMC at a final concentration of 1 × 106/ml in R-10 medium containing 50 U/ml interleukin-2 (IL-2; Cell Sciences, Canton, MA). Cells were restimulated every 10 to 14 days. T-cell lines were screened for specificity and lytic ability in 51Cr release cytotoxicity assays. Den-1-specific T-cell lines were cloned by limiting dilution cell culture at inputs varying between 0.3 and 50 cells/well; T cells were added to wells containing 1 × 105 irradiated allogeneic feeder PBMC in AIM-V medium (Invitrogen) containing 10% FBS, IL-2, and peptide-pulsed BLCL. Cultures were restimulated every 10 to 14 days, and proliferating wells were expanded into 48-well and then 6-well tissue culture plates. Once specific T-cell lines were derived, they were maintained by restimulation with the anti-CD3 monoclonal antibody 12F6 (kindly provided by Johnson Wong, Massachusetts General Hospital, MA).
Cytotoxicity assay.
51Cr-release cytotoxicity assays were performed in 96-well plates using peptide-pulsed BLCL as target cells. Peptides were added at various concentrations to 1 × 106 to 2 × 106 BLCL in minimal volume, incubated for 90 min at 37°C, and then suspended at a final volume of 1 × 106 cells/ml. Effector T cells were suspended in R-10 medium at a final concentration of 3 × 105 to 5 × 105/ml, and 100 μl was added to wells containing 3 × 103 to 5 × 103 51Cr-labeled target cells for a final effector/target ratio of 10:1 for screening and ratios of 20:1 to 0.25:1 for dose-response experiments. Plates were centrifuged at 200 × g for 5 min and then incubated at 37°C for 5 h. A 30-μl aliquot of supernatant was collected onto Lumaplates (Perkin-Elmer, Wellesley, MA), and 51Cr activity was measured in a Topcount gamma counter (Perkin-Elmer). Specific lysis was calculated as follows: [(experimental release − minimum release)/(maximum release − minimum release)] × 100. Assays were performed in duplicate.
HLA restriction analysis.
To identify the HLA molecule used by subjects 2, 3, and 7 to present the NS5329-337 KPWDVIPMV epitope, a mismatched target restriction assay was performed. 51Cr-labeled allogeneic BLCL expressing a single HLA molecule in common with the autologous BLCL were pulsed with peptide and included in 51Cr-release cytotoxicity assays as targets. Effector T cells were added at a final effector/target ratio of 10:1, and specific lysis was determined as described above.
Measurement of cytokine responses.
IFN-γ, tumor necrosis factor alpha (TNF-α), IL-2, IL-4, IL-6, and IL-10 were simultaneously detected using the Th1/Th2 cytokine bead array kit from BD Biosciences (San Jose, CA) according to the manufacturer's protocol. Dengue virus-specific T-cell clones were incubated with BLCL pulsed with cognate and variant peptides in R-10 medium for 5 h, and supernatant was harvested and stored at −80°C for future analysis. After thawing, 50 μl of supernatant was added to 50 μl of a cocktail of capture beads and detector antibodies and 50 μl of phycoerythrin-labeled detection reagent. The tubes were incubated at room temperature for 3 h in the dark and then washed to remove excess unbound detector antibody. The samples were then resuspended in 300 μl of wash buffer before acquisition on a dual laser FACSCalibur flow cytometer. The data were analyzed using the cytokine bead array software (BD Biosciences). Standard curves were generated for each cytokine using the mixed cytokine standard provided with the kit, and the concentration of each cytokine in the supernatant was determined by interpolation from the appropriate standard curve. The minimum and maximum quantifiable levels for each cytokine were 6 pg/ml and 2,000 pg/ml, respectively.
T-cell receptor analysis.
Cellular RNA was extracted from 50 × 104 to 1 × 106 cells using the PicoPure RNA isolation kit (Arcturus, Mountain View, CA) according to the manufacturer's instructions. RNA was reverse transcribed using the SuperScript III first-strand system (Invitrogen Life Technologies) in a volume of 20 μl and primed with oligo(dT) primers. T-cell receptor (TCR) Vβ usage was determined using a panel of 24 Vβ family-specific 5′ primers and a common Cβ 3′ primer (9). PCR amplification consisted of an initial denaturation step of 95°C for 60 s, followed by 35 cycles of 95°C for 60 s, 60°C for 55 s, and 72°C for 90 s, and terminating at 72°C for 10 min. The TCR Vβ gene usage was identified by the presence of a single PCR product from the panel of 24 TCR Vβ and TCR Cβ primer reactions (16).
RESULTS
Identification of T-cell determinants in the Den-1 NS3, NS4A, NS4B, and NS5 genes.
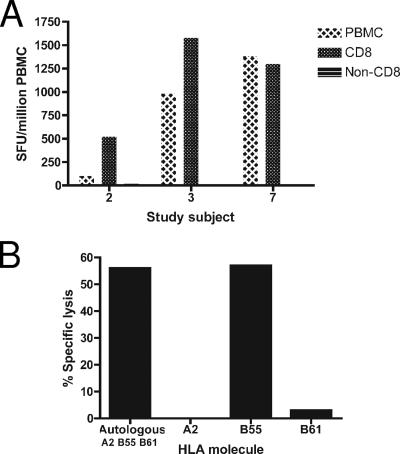
In order to measure the breadth and magnitude of Dengue virus-specific memory T-cell responses, PBMC obtained from 12 subjects 29 to 44 months after infection with Den-1 in 2001 were tested in an IFN-γ ELISPOT assay against a series of 476 synthetic peptide antigens spanning the Den-1 Hawaii 2001 E, NS3, NS4A, NS4B, and NS5 genes. Responses directed against NS3, NS4B, and NS5 peptides were identified in seven subjects, at up to 1,470 SFU/million PBMC (Table 1). One subject responded to four antigens, in the NS5 (NS5291-310) and NS3 (NS361-80, NS3231-245, NS3496-515) genes. Two subjects responded to two antigens each in NS3 and NS5 (NS3216-230 and NS5326-340) and NS3 and NS4B (NS3526-540 and NS4B50-70). Responses to NS3 were detected in four of seven subjects; one (NS3526-540) was novel. Three novel T-cell determinants at NS5291-310, NS5326-340, and NS5376-395 were identified in five of seven subjects. The epitope at NS5326-340, which reproducibly produced an IFN-γ response ranging from 60 to 1,470 SFU/million PBMC, was present in three individuals. The responding cells were confirmed to be CD8+ T cells in ELISPOT assays in which positively selected CD8+ T cells were tested in parallel with CD8-depleted PBMC (Fig. 1A).
TABLE 1.
T-cell epitopes in Den-1
| Subject no.a | HLA | Epitope location | Sequence | SFU/million PBMC |
|---|---|---|---|---|
| 1 | A2,11; B35,62; C7,8 | NS5 291-310b,d | WHYDEDNPYKTWAYHGSYEV | 38-55 |
| NS3 496-515b | LDNINTPEGIIPALFEPERE | 33-380 | ||
| NS3 61-80 | QGKRLEPSWASVKKDLISYG | 73 | ||
| NS3 231-245 | EMAEALKGMPIRYQT | 313 | ||
| 2 | A2,24; B55,62; C1,9 | NS5 326-340c,d | LLTKPWDVIPMVTQI | 60-110 |
| 3 | A3,24; B51,55; C1 | NS5 326-340c,d | LLTKPWDVIPMVTQI | 110-970 |
| NS3 216-230 | KLRTLVLAPTRVVAS | 225 | ||
| 4 | A2,32; B35,62; C7,10 | NS5 376-395b,d | TAKWLWGFLSRNKKPRICTR | 37 |
| 5 | A1,30; B27,61; C1,5 | NS3 526-540d | LRGEARKTFVELMRR | 350 |
| NS4B 56-70d | TIENTTANISLTAIA | 40 | ||
| 6 | A2,23; B44,62; C4,9 | NS3 71-85 | SVKKDLISYGGGWRF | 65 |
| 7 | A2; B55,61; C1 | NS5 326-340c,d | LLTKPWDVIPMVTQI | 1,130-1,178 |
With the exception of subject 4, all subjects were Pacific Islanders.
The epitope is likely located in the overlapping 10-mer peptide (underlined); equal responses were detected to two adjacent 15-mer peptides.
The epitope was fine mapped to the 9-mer peptide KPWDVIPMV located at NS5 329-337.
Novel epitope.
FIG. 1.
The Den-1 NS5329-337 CD8+ T-cell epitope is restricted by HLA-B*5502. Responses to the epitope were CD8 dependent; depletion of CD8+ T cells with antibody-labeled magnetic beads abrogated the IFN-γ ELISPOT response, and only the PBMC and CD8+ fractions were positive (A). T-cell lines generated by stimulation of PBMC collected up to 5 years after infection with ELISPOT-mapped cognate peptide were cytolytic. Den-1 NS5329-337-specific T cells lysed autologous peptide-pulsed BLCL in a 5-hour 51Cr-release assay. All three subjects who responded to the epitope shared the molecule HLA B*5502, which was confirmed to be the restricting element in mismatched target cytotoxicity assays. Target cells were pulsed with cognate peptide and were either autologous BLCL or donor BLCL which matched at only one allele. Strong lytic responses were only seen for the autologous cell line and for the donor cell line expressing HLA B55 (B). A representative experiment shows responses measured for subject 3.
Definition of an immunodominant HLA B*5502-restricted NS5329-337 epitope.
The three patients in whom the NS5326-340 T-cell determinant was identified shared the MHC class 1 molecule HLA B*5502. This molecule was confirmed to be the restricting element in mismatch cytotoxicity assays (Fig. 1B). To define the precise location of the epitope within the 15-mer NS5326-340 region, truncated peptides were designed to conform with the binding motif for the MHC superfamily B7, of which HLA B55 is a member (29). In this motif the anchor residue at position 2 is required to be P; the anchor at position 9 may be one of eight amino acids, A, I, M, L, V, F, W, or Y. Four peptides of 9 or 10 amino acids were synthesized, deleted at the N or the C terminus, and tested in cytotoxicity and IFN-γ ELISPOT assays. Only one peptide, designated KP9, contained the required anchors at positions 2 (P) and 9 (V), and this was expected to act as the optimum determinant. PBMC and NS5-specific T-cell lines tested by IFN-γ ELISPOT and cytotoxicity assays, respectively, demonstrated strong responses to this peptide and not to the other three, confirming our predicted outcome and defining the exact position of the HLA B55-restricted epitope as NS5329-337 KPWDVIPMV. These data are summarized in Table 2.
TABLE 2.
Definition of a T-cell epitope at Den-1 NS5 329-337
| Peptide truncationa | Sequence | CTL response
|
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IFN-γ ELISPOTb | 51Cr releasec | |||||||||||||||||||||
| NS5 321-335 | N | G | V | V | R | L | L | T | K | P | W | D | V | I | P | 6 | 0 | |||||
| NS5 326-340 | L | L | T | K | P | W | D | V | I | P | M | V | T | Q | I | 164 | 28 | |||||
| NS5 326-335 | L | L | T | K | P | W | D | V | I | P | 0 | 2 | ||||||||||
| NS5 328-336 | T | K | P | W | D | V | I | P | M | 1 | 9 | |||||||||||
| NS5 329-337 | K | P | W | D | V | I | P | M | V | 281 | 29 | |||||||||||
| NS5 331-338 | W | D | V | I | P | M | V | T | 3 | 5 | ||||||||||||
Peptide truncations were designed to conform with the binding motif for the MHC superfamily B7, of which HLA B55 is a member. In this motif the anchor at position 2 is required to be proline (P). The optimum T-cell determinant is located at positions 329 to 337.
Mean number of SFU/million PBMC.
Percent specific lysis.
Cross-reactivity of NS5329-337-specific T cells for variant peptides representing heterologous dengue viruses.
A 49-amino-acid-long region including 15 amino acids upstream and downstream of the Den-1 NS5329-337 KPWDVIPMV epitope sequence was entered into a BLAST search of the GenBank database. Several homologs of the Den-1 NS5329-337 epitope (Tables 3 and 4) were identified. Four variants of Den-2 (D2-1, D2-2, D2-3, and D2-4) and one variant of Den-3 (D3) were identified. All Den-1 sequences were identical to the epitope (D1 KP9), as were all Den-4 sequences. Nine-mer peptides representing the variant sequences were synthesized and used to investigate serotype cross-reactivity.
TABLE 3.
Recognition of altered peptide ligands: subject 2a
| Peptide
|
% Specific lysis by clone
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variant | Sequence | 2H1 | 9C8 | 7E6 | 1C12 | 4B4 | 9C12 | 3G5 | 2B10 | 8A10 | 9A2 | 9E1 | 1C10 | 3H12 | 6G8 | 8G3 | 8H2 |
| D1 KP9 | KPWDVIPMV | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| D2-1 | KPWDIIPMV | 91 | 0 | 101 | 100 | 181 | 101 | 94 | 101 | 14 | 97 | 94 | 84 | 87 | 57 | 26 | 76 |
| D2-2 | KPWDVVPMV | 52 | 132 | 124 | 121 | 229 | 122 | 95 | 99 | 127 | 122 | 94 | 84 | 95 | 97 | 84 | 91 |
| D2-3 | KPWDVLPMV | 100 | 102 | 85 | 8 | 184 | 120 | 87 | 89 | 96 | 3 | 11 | 93 | 62 | 97 | 94 | 10 |
| D2-4 | KPWDVLPTV | 94 | 56 | 124 | 22 | 17 | 120 | 70 | 8 | 118 | 0 | 6 | 20 | 54 | 77 | 9 | 6 |
| D3 | KPWDVVPTV | 24 | 132 | 114 | 99 | 178 | 122 | 106 | 75 | 109 | 77 | 66 | 25 | 118 | 103 | 28 | 53 |
Altered peptide ligands representing heterologous dengue viruses are differentially recognized by Den-1 NS5329-337-specific CTL clones. Bolded letters indicate the variant amino acid(s). CTL clones generated by specific stimulation with the cognate peptide KP9 were tested against a panel of altered peptide ligands representing heterologous serotypes in a 5-h 51Cr release assay. Peptides were included at a final concentration of 5 μg/ml. Results are expressed as a percentage of specific lysis induced by cognate D1 KP9 epitope peptide.
TABLE 4.
Recognition of altered peptide ligands: subject 3a
| Peptide
|
% Specific lysis by clone
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variant | Sequence | 1C11 | 1A11 | 10A9 | 1D4 | 2C9 | 9F1 | 10G12 | 10C9 | 1B4 | 10G4 | 1G10 | 1H2 | 6C11 | 9F5 |
| D1 KP9 | KPWDVIPMV | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| D2-1 | KPWDIIPMV | 124 | 120 | 59 | 58 | 27 | 35 | 35 | 27 | 37 | 44 | 88 | 89 | 64 | 61 |
| D2-2 | KPWDVVPMV | 94 | 113 | 98 | 98 | 12 | 78 | 25 | 12 | 24 | 107 | 88 | 84 | 91 | 98 |
| D2-3 | KPWDVLPMV | 118 | 120 | 105 | 115 | 97 | 55 | 97 | 97 | 93 | 77 | 69 | 85 | 82 | 89 |
| D2-4 | KPWDVLPTV | 2 | 0 | 117 | 100 | 91 | 33 | 96 | 91 | 87 | 99 | 75 | 94 | 50 | 100 |
| D3 | KPWDVVPTV | 0 | 0 | 88 | 94 | 3 | 29 | 8 | 3 | 5 | 97 | 100 | 14 | 3 | 104 |
Altered peptide ligands representing heterologous dengue viruses are differentially recognized by Den-1 NS5329-337-specific CTL clones. Bolded letters indicate variant amino acid(s). CTL clones generated by specific stimulation with the cognate peptide KP9 were tested against a panel of altered peptide ligands representing heterologous serotypes in a 5-h 51Cr release assay. Peptides were included at a final concentration of 5 μg/ml. Results are expressed as a percentage of specific lysis induced by cognate D1 KP9 epitope peptide.
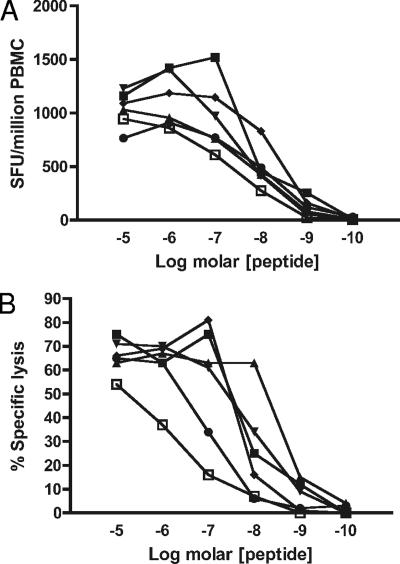
All variant peptides produced high-frequency T-cell responses when tested against PBMC from the three Den-1-immune subjects by direct ex vivo IFN-γ ELISPOT, even at peptide concentrations which were likely to be representative of concentrations achieved in vivo (Fig. 2A). High-avidity responses were also measured for T-cell lines derived from each subject by stimulation with cognate KP9 peptide and tested in 51Cr release cytotoxicity assays against target cells presenting cognate and variant peptides (Fig. 2B), confirming that within the polyclonal population of Den-specific cells there was a high degree of recognition for these variant peptide ligands. The agreement between the ex vivo IFN-γ assay and data derived from cultured T-cell lines confirmed the immunodominant nature of T-cell responses to the NS5329-337 epitope and its variant ligands.
FIG. 2.
Den-1-infected individuals mount effector T-cell responses to variant epitope peptides representing heterologous serotypes. Analysis of polyclonal T-cell populations indicates there is a high degree of cross-reactivity between cognate Den-1 KP9 NS5329-337 epitope peptide and variant peptides representing heterologous dengue viruses. High-avidity responses were measured by direct ex vivo IFN-γ ELISPOT of whole-fraction PBMC (A) and for T-cell lines derived by stimulation of PBMC with cognate NS5329-337 KPWDVIPMV peptide (B) and tested in 51Cr release assays, in which target cells were autologous BLCL pulsed with NS5329-337 epitope peptide KP9 (▪) or variant peptides representing heterologous dengue viruses D2-1 (▴), D2-2 (▾), D2-3 (⧫), D2-4 (•), and D3 (□). Similar data were obtained for subjects 2, 3, and 7; representative experiments from subject 7 are shown.
Because of the high degree of cross-reactivity for heterologous epitope peptides seen in subjects 2, 3, and 7, plaque reduction serum neutralization tests were conducted to identify serological evidence of previous dengue virus infection. Although flavivirus serum neutralization test results should always be interpreted with caution, titers of ≥1:320 for Den-2 in all three subjects suggested prior infection with this serotype, and this was supported by their reports of experiencing dengue-like illness during an epidemic of Den-2 in French Polynesia in 1996 (6). Titers for Den-1 were 1:160 for subject 2 and ≥1:320 for subjects 3 and 7. Titers between 1:40 and 1:160 for Den-3 and Den-4 were measured for all three subjects, and this either represents dengue virus infection before 1996 or cross-reactivity among dengue virus serotypes in the neutralization assay.
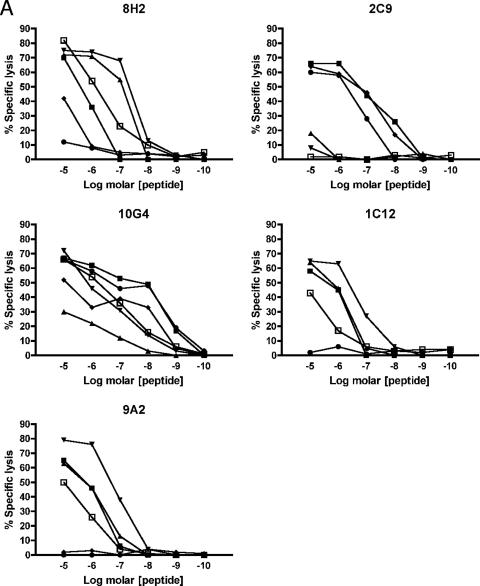
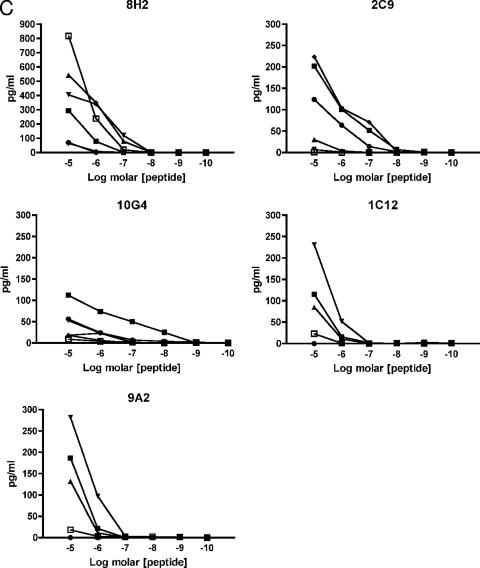
We then sought to examine T-cell cross-reactivity at the clonal level and examined the ability of NS5329-337-specific CTL clones to recognize altered or variant peptide ligands representing heterologous dengue virus serotypes. Clones differentially lysed BLCL targets presenting variant peptides with specific lysis ranging between 0 and 100% while concurrently lysing targets presenting the cognate Den-1 K9 peptide with high efficiency (Tables 3 and 4). Two patterns of recognition were noted: the clones either recognized all variant peptides presented to them and lysed target cells efficiently or differentially recognized the variants and showed abrogated or diminished lysis for at least one peptide. Variant peptides induced diminished or abrogated recognition in a clone-specific manner, which did not necessarily predict diminished responses for other clones. Recognition of virus variants could be abrogated by V→I substitution at position 5, I→V or L at position 6, and/or M→T at position 8 of the epitope. Substitution of one or more residues was not necessarily sufficient in itself to guarantee altered recognition of the pMHC complex by all clones. In addition, responses were subject specific. For example, although the D2-4 I6L and M8T substitutions of the peptide nonamer significantly diminished cytolysis for almost half of all clones for subject 3, the same phenomenon was not observed for clones isolated from subject 2. Similarly, the D3 I6V and M8T substitutions significantly reduced cytolysis for half of the clones isolated from subject 2 but not subject 3 clones. Titration curves generated for five clones isolated from the two subjects showed differential responses to the panel of variant peptide ligands (Fig. 3), including high-avidity responses for variants representing heterologous virus serotypes previously encountered by the subjects. These data show that within each individual there is a population of dengue virus-specific T cells with a spectrum of functional phenotypes and suggest that within this population, memory cells primed by previous dengue virus infection can be preferentially activated.
FIG. 3.
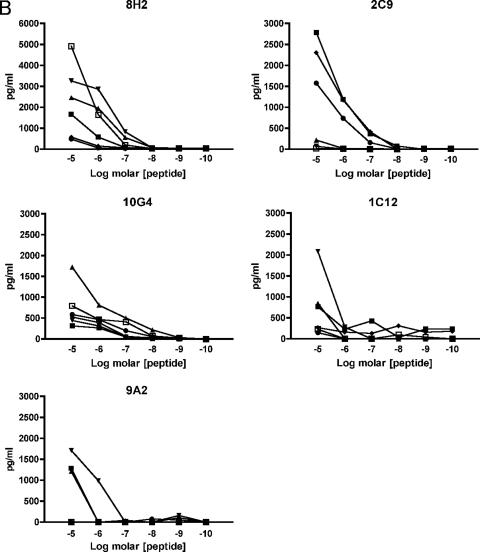
Differential functional avidities of Den-1-specific T-cell clones for altered peptide ligands representing heterologous and previously encountered serotypes. Den-1 NS5329-337-specific CD8+ T-cell clones were incubated with 51Cr-labeled autologous transformed B cells pulsed with cognate D1 epitope peptide KP9 (▪) and variant peptides D2-1 (▴), D2-2 (▾), D2-3 (⧫), D2-4 (•), and D3 (▪). Peptide titration curves suggest that functional avidity for heterologous serotypes may be enhanced compared to responses for cognate epitope peptides (A). Secretion of the proinflammatory cytokines IFN-γ (B) and TNF-α (C) was also differentially altered when clones were stimulated with altered peptide ligands, particularly D2-2 (▾) and D3 (□), compared with cognate KP9 Den-1 peptide (□). Clones 10G4 and 2C9 were isolated from subject 2; clones 8H2, 1C12, and 9A2 were isolated from subject 3.
Since the target pMHC complex was identical for both subjects, our findings of altered target cell recognition suggested that variation in the TCR binding site for the pMHC complex (5, 8, 23) contributed to the altered effector phenotype. CDR3 sequencing of representative clones from subject 3 confirmed that distinct clonotypes were present (Table 5), highlighting the role of T-cell receptor diversity in determining effector function and immunodominance.
TABLE 5.
T-cell receptor Vβ usage of Den-1 NS5329-337-specific CTL clonesa
| Clone | Vβ family | CDR3 sequence | No. of amino acids |
|---|---|---|---|
| 7E6 | 7 | CASS QDPGMNTEAFF | 13 |
| 9C8 | 13 | CASS RAGTGRYSGNTLYF | 15 |
| 8A10 | 1 | CASS PQGGGAQETQYF | 16 |
| 1C12 | 22 | CASS EETLPSNEKLFF | 16 |
| 4B4 | 1 | CASS AYGQGGEKLFF | 13 |
| 9C12 | 10 | CASS RKSGQADTQYF | 15 |
TCR clonotypes of representative CTL clones with distinct cytolytic responses to stimulation with cognate and variant peptides are shown.
Den-1-specific CTL clones stimulated by NS5329-337 variant ligands differentially secrete Th1 and Th2 cytokines.
Since we observed marked differences in cytolytic activity for effector cells presented with cognate and variant peptide ligands, we sought to determine whether cytokine secretion profiles were similarly altered at the single-epitope level. To define the cytokines produced by Den-1-specific CTL, clones isolated from subjects 2 and 3 were stimulated with autologous BLCL presenting the cognate and variant peptides, and protein concentration in the cell culture supernatants was determined using a flow cytometry-based cytokine bead array for the Th1/Th2 cytokines IFN-γ, TNF-α, IL-2, IL-4, IL-6, and IL-10. A hierarchy of cytokine expression was observed. All clones from subjects 2 and 3 produced IFN-γ in response to stimulation with the cognate Den-1 KP9 peptide (Table 6; Fig. 3 and 4); however, IFN-γ secretion was abrogated or significantly diminished in 14 of 17 clones stimulated with the variants D2-2, D2-4, and D3. All clones isolated from subject 2 also produced TNF-α in response to stimulation with cognate D1 KP9, although this was not the case for subject 3 clones, of which only two of eight secreted TNF-α in response to cognate KP9 peptide. IL-2 secretion was significantly reduced for a number of clones, from both subjects. Overall, when all peptides were considered, most clones secreted IFN-γ, followed by TNF-α, then IL-2 (Table 6; Fig. 4). IL-4 and IL-6 production could be measured in a smaller number of clones: 7/17 clones produced IL-4, and 2/17 clones produced IL-6, at low concentrations. No IL-10 secretion was observed for any clones stimulated with any peptides.
TABLE 6.
Den-1 NS5329-337-specific CTL clones differentially secrete Tc1 and Tc2 cytokines on stimulation with cognate Den-1 KP9 peptide and altered peptide ligands representing heterologous dengue virusesa
| Subject and clone | Peptide | Cytolysis (%)b | Response (pg/ml culture supernatant)
|
|||||
|---|---|---|---|---|---|---|---|---|
| IFN-γ | TNF-α | IL-2 | IL-4 | IL-6 | ||||
| Subject 2 | ||||||||
| 1H2 | KP9 | 100 | >2,000 | 252 | 8 | 10 | −c | |
| D2-1 | 89 | 1,964 | 120 | 10 | 10 | − | ||
| D2-2 | 84 | 838 | 43 | 22 | − | − | ||
| D2-3 | 85 | 2,241 | 157 | 11 | 6 | − | ||
| D2-4 | 94 | 1,177 | 115 | 8 | − | − | ||
| D3 | 14 | 66 | − | − | − | − | ||
| 6C11 | KP9 | 100 | >2,000 | 109 | − | − | − | |
| D2-1 | 64 | 237 | 9 | − | − | − | ||
| D2-2 | 91 | 626 | 22 | − | − | − | ||
| D2-3 | 82 | 1,233 | 36 | − | − | − | ||
| D2-4 | 50 | 155 | 8 | − | − | − | ||
| D3 | 3 | 63 | − | − | − | − | ||
| 1D4 | KP9 | 100 | >2,000 | 1,498 | 197 | 171 | − | |
| D2-1 | 58 | 99 | 11 | 89 | − | − | ||
| D2-2 | 98 | >2,000 | 1,239 | 298 | 126 | − | ||
| D2-3 | 115 | >2,000 | 1,143 | 197 | 129 | − | ||
| D2-4 | 100 | >2,000 | 1,913 | 298 | 182 | − | ||
| D3 | 94 | >2,000 | 1,732 | 325 | 150 | − | ||
| 1B4 | KP9 | 100 | >2,000 | 139 | 43 | 8 | − | |
| D2-1 | 37 | 111 | 15 | 79 | − | − | ||
| D2-2 | 24 | 31 | − | 79 | − | − | ||
| D2-3 | 93 | >2,000 | 212 | 141 | 8 | − | ||
| D2-4 | 87 | 1,182 | 90 | 119 | − | − | ||
| D3 | 5 | 21 | − | 43 | − | − | ||
| 10G12 | KP9 | 100 | >2,000 | 153 | 87 | − | − | |
| D2-1 | 35 | 236 | 33 | 114 | − | − | ||
| D2-2 | 25 | 25 | − | 103 | − | − | ||
| D2-3 | 97 | 1,800 | 156 | 98 | − | − | ||
| D2-4 | 96 | 1,205 | 84 | 109 | − | − | ||
| D3 | 8 | − | − | 120 | − | − | ||
| 2C9 | KP9 | 100 | >2,000 | 202 | − | 8 | − | |
| D2-1 | 27 | 220 | 30 | − | − | − | ||
| D2-2 | 12 | 70 | 7 | − | − | − | ||
| D2-3 | 97 | >2,000 | 224 | 19 | 14 | − | ||
| D2-4 | 91 | 1,582 | 124 | 12 | − | − | ||
| D3 | 3 | 21 | − | − | − | − | ||
| Subject 3 | ||||||||
| 7E6 | KP9 | 100 | 188 | − | − | − | − | |
| D2-1 | 101 | 156 | − | − | − | − | ||
| D2-2 | 124 | 120 | 45 | − | − | − | ||
| D2-3 | 85 | 104 | − | − | − | − | ||
| D2-4 | 124 | 90 | − | − | − | − | ||
| D3 | 114 | 108 | − | − | − | − | ||
| 1C12 | KP9 | 100 | >2,000 | − | 164 | − | − | |
| D2-1 | 100 | >2,000 | − | 154 | − | − | ||
| D2-2 | 121 | >2,000 | − | 91 | − | − | ||
| D2-3 | 8 | − | − | − | − | |||
| D2-4 | 22 | − | − | − | − | |||
| D3 | 99 | 1,535 | − | − | − | − | ||
| 4B4 | KP9 | 100 | 1,668 | − | − | − | − | |
| D2-2 | 229 | >2,000 | − | − | 479 | − | ||
| D2-1 | 181 | >2,000 | − | − | − | − | ||
| D2-3 | 184 | 953 | − | − | − | |||
| D2-4 | 17 | 107 | − | − | 44 | − | ||
| D3 | 178 | 452 | − | − | − | − | ||
| 9C12 | KP9 | 100 | >2,000 | − | − | − | − | |
| D2-1 | 101 | >2,000 | − | − | − | − | ||
| D2-2 | 122 | >2,000 | − | − | − | − | ||
| D2-3 | 120 | 1,440 | − | − | − | − | ||
| D2-4 | 120 | 813 | − | − | − | − | ||
| D3 | 122 | 1,730 | − | − | − | − | ||
| 2B10 | KP9 | 100 | >2,000 | 206 | 225 | 34 | 196 | |
| D2-1 | 101 | >2,000 | 138 | 413 | 38 | 286 | ||
| D2-2 | 99 | >2,000 | 1,653 | − | 363 | − | ||
| D2-3 | 89 | >2,000 | − | 54 | − | 55 | ||
| D2-4 | 8 | 137 | − | − | − | − | ||
| D3 | 75 | 1,516 | − | 39 | − | − | ||
| 1C10 | KP9 | 100 | >2,000 | 1,870 | 239 | 189 | 70 | |
| D2-1 | 84 | >2,000 | 1,154 | 77 | 117 | 67 | ||
| D2-2 | 84 | >2,000 | 800 | 35 | 85 | 64 | ||
| D2-3 | 93 | >2,000 | 980 | 93 | 106 | 58 | ||
| D2-4 | 20 | >2,000 | 51 | − | − | 62 | ||
| D3 | 25 | >2,000 | 44 | − | − | 49 | ||
Peptides were added at a final concentration of 5 μg/ml. Supernatants were also tested for IL-10; results for IL-10 were always below the limit of detection.
Cytolysis is reported as a percentage of the cognate KP9 peptide epitope lysis value, corrected to 100%.
−, below detectable limits of the assay.
FIG. 4.
Den-1 NS5329-337-specific CD8+ T-cell clones demonstrate a cytokine phenotype hierarchy of IFN-γ > TNF-α > IL-2 when stimulated with cognate and altered peptide ligands. Cell lines were incubated with peptide-pulsed autologous BLCL at a ratio of 10:1 for 5 h, and harvested supernatants were tested for cytokine production by flow cytometric cytokine bead array. Seventeen clones were analyzed. Results are expressed as the percent total clones analyzed secreting the relevant cytokines. All clones produced IFN-γ on stimulation with cognate peptide, and responses were variably reduced in response to variant peptide ligands. The Tc2 cytokines IL-4 and IL-6 were secreted by a subset of clones which also produced Tc1 cytokines.
DISCUSSION
Epidemics of dengue-like illnesses have been recorded in the Pacific region for more than 100 years. Beginning in 2000 and continuing through 2007, a series of major Den-1-associated DF and DHF epidemics occurred in south and western Pacific nations. Dengue virus-specific CTL epitopes have not been defined in Polynesians or other Pacific Islanders, in whom the most frequent HLA molecules may differ from those commonly found in Asian and Caucasian populations (28). We therefore designed this study of dengue virus serotype cross-reactivity, in the setting of naturally acquired infection in Pacific Islanders, without making any initial assumptions regarding HLA and immunodominance. We analyzed Den-1-specific responses in individuals infected in Hawaii in 2001-2002, during the first known dengue epidemic in more than 60 years, using a panel of synthetic peptides based on the consensus amino acid sequence of four Den-1 strains circulating in Hawaii at the time. We identified several novel T-cell epitopes in the viral NS3, NS4B, and NS5 and used one NS5 antigen, identified in 3 of 12 subjects and restricted by HLA-B55, a molecule expressed frequently in Pacific Islander populations, to examine cellular immunity at the clonal level and to define effector cell function associated with cross-reactive Den-specific T-cell responses. We previously (13) sequenced the full-length E gene of 16 viruses isolated during the Hawaii dengue outbreak of 2001-2002 and found that almost all (15/16) isolates, including the four included in the present study, showed 99.9% similarity with a reference Tahitian strain of Den-1. Based on these data we were confident that the consensus amino acid sequence we utilized for our peptide synthesis was truly representative of the predominant Den-1 strain circulating in Hawaii in 2001-2002, introduced from French Polynesia.
We focused our analysis of dengue virus-specific effector cell function on responses to the viral NS5 and specifically to a novel Den-1 nonameric peptide epitope in the nuclear localization sequence of the NS5 gene, since this was the strongest and most immunodominant response identified in the subjects we analyzed. To our knowledge, this is the first report of T-cell responses directed at NS5 epitopes. The NS5 gene encodes the viral RNA-dependent RNA polymerase and is highly conserved across all four serotypes, consistent with its central role in virus replication. Our findings demonstrate that T-cell epitopes within this gene are highly cross-reactive and can reactivate dengue virus-specific memory T cells generated in response to previous infection with heterologous serotypes. Most studies of dengue virus-specific T-cell-mediated immunity have focused on responses to the viral NS3. In the present study we have shown that dengue virus-specific effector T-cell responses are more broadly directed than previously reported, and our results suggest that in naturally acquired infection the host cell-mediated immune response may be heterogeneous and directed at an array of epitopes encoded by several regions of the genome.
Altered peptide ligands are variants of defined MHC class I- or MHC class II-restricted T-cell epitopes which induce alterations in effector cell function, compared to responses induced by the cognate epitope peptide. Subtle changes in the epitope sequence, by as little as a single amino acid, can result in dramatic differences in cytokine secretion phenotype and proliferation and anergy (5, 8, 20, 30). We found that T-cell clones specific for Den-1 NS5329-337 KPWDVIPMV showed distinct patterns of recognition for variant peptides representing heterologous dengue viruses and that cytolytic function and cytokine secretion could be altered in a clone-dependent manner. Single amino acid substitutions within the epitope altered secretion patterns of cytokines, including IFN-γ, TNF-α, IL-2, IL-4, and IL-6, for individual clones. Our findings of altered cytokine production induced by stimulation with altered peptide ligands representing heterologous viruses demonstrate that the functional phenotype of Den-specific memory T cells can be dramatically modified and that individual cytokines can be turned on or off depending on the nature of the pMHC-TCR contact and subsequent signaling events. During a secondary dengue virus infection, it is therefore plausible that the breadth and nature of the cytokine response is determined by factors including specificity of preexisting memory T cells, the secondary infecting virus, and HLA type. An association between these factors and pathogenesis of DHF/DSS has been proposed by others (17-19, 31). Reduced or abrogated cytolytic and cytokine function resulting from activation by heterologous, secondary virus would directly impact the ability of the infected host to control viral replication. Enhanced functional avidity resulting in increased production of proinflammatory cytokines, including TNF-α, could contribute to pathophysiologic events directly affecting vascular permeability (1).
Our results demonstrate considerable functional heterogeneity among dengue virus-specific T-cell clones. A polyclonal population of dengue virus-specific CD8+ T cells was detected at high frequency by ex vivo IFN-γ ELISPOT, several years after infection, and analysis at the clonal level showed that this population was composed of a heterogeneous mixture of epitope-specific T cells with distinct cytokine secretion patterns and cytolytic function. Functional avidity varied among clones, and our finding that Den-1-specific T-cell responses may be biased towards other serotypes, including serotypes encountered many years previously, provides further support for the concept of heterologous immunity (26, 27, 34) and original antigenic sin in memory T-cell responses (14, 19).
Peptide-specific modification of lytic function and cytokine production presumably resulted from altered engagement of the individual TCR with the variant peptide-MHC complex; as expected, distinct clonally distributed TCRs were engaged by the variant peptide-MHC complexes. No single amino acid substitution in the NS5329-337 epitope abolished or diminished cytolysis or cytokine production for all clones analyzed. Alteration in function was highly clone and peptide dependent, and these findings suggest a predominant role for the TCR in determining functional outcome. Amino acid changes in the variant peptides were subtle and yet induced significant changes in phenotype, suggesting that TCR engagement of variant peptides induced different signals than the cognate peptide to result in a change in effector function and that different activation thresholds, dependent on antigen concentration, were required for the individual variant peptide-MHC-TCR complexes before T-cell function was elicited. The observed differences in structural avidity among several clones, which corresponded directly to altered cytolysis and production of IFN-γ and TNF-α, support this.
In summary, we have used a large panel of well-characterized T-cell clones to demonstrate that CD8+ Den-specific memory T cells can be activated by an array of altered peptide ligands representing heterologous dengue viruses to induce strong cross-reactive cytolytic and cytokine responses. The polyclonal, heterogeneous Den-specific memory T-cell population is capable of responding to a range of antigens; however, at the clonal level the altered, skewed phenotype induced by stimulation with variant peptides and the enhanced structural avidity that may be measured for previously encountered antigens may not necessarily be protective but may contribute to pathogenesis of dengue virus infection. In addition, we have extended the findings of other groups, who have studied dengue virus-specific T-cell responses in Caucasian and Asian populations, to Pacific Islanders.
Acknowledgments
We thank George Hui for helpful discussions and for critical review of the manuscript and Frank Ennis and Alan Rothman for helpful advice.
This work was made possible by grant P20RR018727 from the National Center for Research Resources of the National Institutes of Health.
Footnotes
Published ahead of print on 11 July 2007.
REFERENCES
- 1.Abe, Y., S. Sekiya, T. Yamasita, and F. Sendo. 1990. Vascular hyperpermeability induced by tumor necrosis factor and its augmentation by IL-1 and IFN-γ is inhibited by selective depletion of neutrophils with a monoclonal antibody. J. Immunol. 145:2902-2907. [PubMed] [Google Scholar]
- 2.Bashyam, H. S., S. Green, and A. L. Rothman. 2006. Dengue virus-reactive CD8+ T cells display quantitative and qualitative differences in their response to variant epitopes of heterologous virus serotypes. J. Immunol. 176:2817-2824. [DOI] [PubMed] [Google Scholar]
- 3.Chen, H. D., A. E. Fraire, I. Joris, M. A. Brehm, R. M. Welsh, and L. K. Selin. 2001. Memory CD8+ T cells in heterologous antiviral immunity and immunopathology in the lung. Nat. Immunol. 2:1067-1076. [DOI] [PubMed] [Google Scholar]
- 4.Cho, B. K., C. Wang, S. Sugawa, H. N. Eisen, and J. Chen. 1999. Functional differences between memory and naive CD8 T cells. Proc. Natl. Acad. Sci. USA 96:2976-2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Magistris, M. T., J. Alexander, M. Coggeshall, A. Altman, F. C. A. Gaeta, H. M. Grey, and A. Sette. 1992. Antigen analog-major histocompatibility complexes act as antagonists of the T cell receptor. Cell 68:625-634. [DOI] [PubMed] [Google Scholar]
- 6.Deparis, X., B. Murgue, C. Roche, O. Cassar, and E. Chungue. 1998. Changing clinical and biological manifestations of dengue during the dengue-2 epidemic in French Polynesia in 1996/1997: description and analysis in a prospective study. Trop. Med. Int. Health 3:859-865. [DOI] [PubMed] [Google Scholar]
- 7.Effler, P. V., L. Pang, P. Kitsutani, V. Vorndam, M. Nakata, T. Ayers, J. Elm, T. Tom, P. Reiter, J. G. Rigau-Perez, J. M. Hayes, K. Mills, M. Napier, G. G. Clark, D. J. Gubler, et al. 2005. Dengue fever, Hawaii, 2001-2002. Emerg. Infect. Dis. 11:742-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evavold, B. D., and P. M. Allen. 1991. Separation of IL-4 production from Th cell proliferation by an altered T cell receptor ligand. Science 252:1308-1310. [DOI] [PubMed] [Google Scholar]
- 9.Genevee, C., A. Diu, J. Nierat, A. Caignard, P. Y. Dietrich, L. Ferradini, S. Roman-Roman, F. Triebel, and T. Hercend. 1992. An experimentally validated panel of subfamily-specific oligonucleotide primers (V alpha 1-w29/V beta 1-w24) for the study of human T cell receptor variable V gene segment usage by polymerase chain reaction. Eur. J. Immunol. 22:1261-1269. [DOI] [PubMed] [Google Scholar]
- 10.Gubler, D. J. 1998. Dengue and dengue hemorrhagic fever. Clin. Microbiol. Rev. 11:480-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guzman, M. G., G. Kouri, J. Bravo, M. Soler, and E. Martinez. 1991. Sequential infection as a risk factor for dengue hemorrhagic fever/dengue shock syndrome (DHF/DSS) during the 1981 dengue hemorrhagic Cuban epidemic. Mem. Inst. Oswaldo Cruz 86:367. [DOI] [PubMed] [Google Scholar]
- 12.Halstead, S. B. 1988. Pathogenesis of dengue: challenges to molecular biology. Science 239:476-481. [DOI] [PubMed] [Google Scholar]
- 13.Imrie, A., Z. Zhao, S. N. Bennett, P. Kitsutani, M. Laille, and P. V. Effler. 2006. Molecular epidemiology of dengue in the Pacific: introduction of two distinct strains of dengue virus type 1 into Hawaii. Ann. Trop. Med. Parasitol. 100:327-336. [DOI] [PubMed] [Google Scholar]
- 14.Klenerman, P., and R. M. Zinkernagel. 1998. Original antigenic sin impairs cytotoxic T lymphocyte responses to viruses bearing variant epitopes. Nature 394:482-485. [DOI] [PubMed] [Google Scholar]
- 15.Kurane, I., A. L. Rothman, P. G. Livingston, S. Green, S. J. Gagnon, J. Janus, B. L. Innis, S. Nimmannitya, A. Nisalak, and F. A. Ennis. 1994. Immunopathologic mechanisms of dengue hemorrhagic fever and dengue shock syndrome. Arch. Virol. (Suppl.). 9:59-64. [DOI] [PubMed] [Google Scholar]
- 16.Lefranc, M. P., and G. Lefranc. 2001. The T cell receptor facts book. Academic Press, San Diego, CA.
- 17.Leitmeyer, K. C., D. W. Vaughn, D. M. Watts, R. Salas, I. Villalobos, de Chacon, C. Ramos, and R. Rico-Hesse. 1999. Dengue virus structural differences that correlate with pathogenesis. J. Virol. 73:4738-4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loke, H., D. B. Bethell, C. X. Phuong, M. Dung, J. Schneider, N. J. White, N. P. Day, J. Farrar, and A. V. Hill. 2001. Strong HLA class I-restricted T cell responses in dengue hemorrhagic fever: a double-edged sword? J. Infect. Dis. 184:1369-1373. [DOI] [PubMed] [Google Scholar]
- 19.Mongkolsapaya, J., W. Dejnirattisai, X. Xiao-Ning, S. Vasanawathana, N. Tangthawornchaikul, A. Chairunsri, S. Sawasdivorn, T. Duangchinda, T. Dong, S. Rowland-Jones, P. Yenchitsomanus, A. McMichael, P. Malasit, and G. Screaton. 2003. Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nat. Med. 9:921-927. [DOI] [PubMed] [Google Scholar]
- 20.Pfeiffer, C., J. Stein, S. Southwood, H. Ketelaar, A. Sette, and K. Bottomly. 1995. Altered peptide ligands can control CD4 T lymphocyte differentiation in vivo. J. Exp. Med. 181:1569-1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rothman, A. L., and F. A. Ennis. 1999. Immunopathogenesis of dengue hemorrhagic fever. Virology 257:1-6. [DOI] [PubMed] [Google Scholar]
- 22.Rozen, S., and H. J. Skaletsky. 2000. Primer3 on the WWW for general users and for biologist programmers, p. 365-386. In S. Krawetz and S. Misener (ed.), Bioinformatics methods and protocols: methods in molecular biology. Humana Press, Totowa, NJ. [DOI] [PubMed]
- 23.Rudolph, M. G., R. L. Stanfield, and I. A. Wilson. 2006. How TCRs bind MHCs, peptides, and coreceptors. Annu. Rev. Immunol. 24:419-466. [DOI] [PubMed] [Google Scholar]
- 24.Russell, P. K., A. Nisalak, P. Sukhavachana, and S. Vivona. 1967. A plaque reduction test for dengue virus neutralizing antibodies. J. Immunol. 99:285-290. [PubMed] [Google Scholar]
- 25.Sangkawibba, N., S. Rojanasuphot, S. Ahandrik, S. Viriyapongse, S. Jatansen, V. Salitul, B. Phanthumachinda, and S. B. Halstead. 1984. Risk factors in dengue shock syndrome: a prospective epidemiological study in Rayong, Thailand. I. The 1980 outbreak. Am. J. Epidemiol. 120:653-659. [DOI] [PubMed] [Google Scholar]
- 26.Selin, L. K., S. R. Nahill, and R. M. Welsh. 1994. Cross-reactivities in memory cytotoxic T lymphocyte recognition of heterologous viruses. J. Exp. Med. 179:1933-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Selin, L. K., S. M. Varga, I. C. Wong, and R. M. Welsh. 1998. Protective heterologous antiviral immunity and enhanced immunopathogenesis mediated by memory T cell populations. J. Exp. Med. 188:1705-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Serjeantson, S. W. 1989. HLA genes and antigens, p. 120-173. In A. V. Hill and S. W. Serjeantson (ed.), The colonization of the Pacific: a genetic trail. Oxford University Press, Oxford, United Kingdom.
- 29.Sette, A., and J. Sidney. 1999. Nine major HLA class I supertypes account for the vast preponderance of HLA-A and -B polymorphism. Immunogenetics 50:201-212. [DOI] [PubMed] [Google Scholar]
- 30.Sloan-Lancaster, J., B. D. Evavold, and P. M. Allen. 1993. Induction of T-cell anergy by altered T-cell-receptor ligand on live antigen-presenting cells. Nature 363:156-159. [DOI] [PubMed] [Google Scholar]
- 31.Stephens H. A., R. Klaythong, M. Sirikong, D. W. Vaughn, S. Green, S. Kalayanarooj, T. P. Endy, D. H. Librarty, A. Nisalak, B. L. Innis, A. L. Rothman, F. A. Ennis, and D. Chandanayingyong. 2002. HLA-A and -B allele associations with secondary dengue virus infections correlate with disease severity and the infecting viral serotype in ethnic Thais. Tissue Antigens 60:309-318. [DOI] [PubMed] [Google Scholar]
- 32.Stephenson, J. R. 2005. Understanding dengue pathogenesis: implications for vaccine design. Bull. W. H. O. 83:308-314. [PMC free article] [PubMed] [Google Scholar]
- 33.Veiga-Fernandes, H., U. Walter, C. Bourgeois, A. McLean, and B. Rocha. 2000. Response of naive and memory CD8+ T cells to antigen stimulation in vivo. Nat. Immunol. 1:47-53. [DOI] [PubMed] [Google Scholar]
- 34.Welsh, R. M., and L. K. Selin. 2002. No one is naive: the significance of heterologous T-cell immunity. Nat. Rev. Immunol. 2:417-426. [DOI] [PubMed] [Google Scholar]