Abstract
Background
For many years amitriptyline has been considered one of the reference compounds for the pharmacological treatment of depression. However, new tricyclic drugs, heterocyclic compounds and selective serotonin reuptake inhibitors have been introduced on the market with the claim of a more favourable tolerability/efficacy profile.
Objectives
The aim of the present systematic review was to investigate the tolerability and efficacy of amitriptyline in comparison with the other tricyclic/heterocyclic antidepressants and with the selective serotonin reuptake inhibitors.
Search methods
The Cochrane Collaboration Depression, Anxiety and Neurosis Controlled Trials Register (CCDANCTR‐Studies) was searched on 28‐11‐2005. Reference lists of all included studies were checked.
Selection criteria
Only randomised controlled trials were included. Study participants were of either sex and any age with a primary diagnosis of depression. Included trials compared amitriptyline with another tricyclic/heterocyclic antidepressant or with one of the selective serotonin reuptake inhibitors.
Data collection and analysis
Data were extracted using a standardised form. The number of patients undergoing the randomisation procedure, the number of patients who completed the study and the number of improved patients were extracted. In addition, group mean scores at the end of the trial on Hamilton Depression Scale or any other depression scale were extracted. In the tolerability analysis, the number of patients failing to complete the study and the number of patients complaining of side‐effects were extracted.
Main results
A total number of 194 studies were included in the review. The estimate of the overall odds ratio (OR) for responders showed that more subjects responded to amitriptyline in comparison with the control antidepressant group (OR 1.12 to 95% confidence interval (CI) 1.02 to 1.23, number needed to treat to benefit (NNTB) = 50). The estimate of the efficacy of amitriptyline and control agents on a continuous outcome revealed an effect size which also significantly favoured amitriptyline (Standardised Mean Difference (SMD) 0.13, 95% CI 0.04 to 0.23). Whilst these differences are statistically significant, their clinical significance is less clear. When the efficacy analysis was stratified by drug class, no difference in outcome emerged between amitriptyline and either tricyclic or selective serotonin reuptake inhibitor comparators. The dropout rate in patients taking amitriptyline and control agents was similar; however, the estimate of the proportion of patients who experienced side‐effects significantly favoured control agents in comparison with amitriptyline (OR 0.66, 95% CI 0.59 to 0.74). When the tolerability analysis was stratified by drug class, the dropout rate in patients taking amitriptyline and the selective serotonin reuptake inhibitors significantly favoured the latter (OR 0.84, 95% CI 0.75 to 0.95, number needed to treat to harm (NNTH) = 40). When the responder analysis was stratified by study setting amitriptyline was more effective than control antidepressants in inpatients (OR 1.22, 95% CI 1.04 to 1.42, NNTB = 24), but not in outpatients (OR 1.01, 95%CI 0.88 to 1.17, NNTB = 200).
Authors' conclusions
This present systematic review indicates that amitriptyline is at least as efficacious as other tricyclics or newer compounds. However, the burden of side‐effects in patients receiving it was greater. In comparison with selective serotonin reuptake inhibitors amitriptyline was less well tolerated, and although counterbalanced by a higher proportion of responders, the difference was not statistically significant.
Keywords: Humans; Amitriptyline; Amitriptyline/therapeutic use; Antidepressive Agents, Tricyclic; Antidepressive Agents, Tricyclic/therapeutic use; Depression; Depression/drug therapy; Randomized Controlled Trials as Topic; Selective Serotonin Reuptake Inhibitors; Selective Serotonin Reuptake Inhibitors/therapeutic use
Plain language summary
Amitriptyline for depression
Amitriptyline still has a place in the pharmacological management of depressive episodes. The findings of this systematic review showed that in comparison with control agents, amitriptyline was slightly more effective, but the burden of side‐effects was greater for patients receiving it.
Background
Depression is one of the major mental health problems in primary and secondary healthcare settings. It is associated with marked personal, social and economic morbidity, and creates significant demands on service providers in terms of workload. Treatment is predominantly pharmaceutical or psychological. For many years the tricyclic antidepressant (TCA) amitriptyline has been considered one of the reference compounds for the pharmacological treatment of depression. In the last thirty years, however, new tricyclic drugs, heterocyclic compounds and selective serotonin reuptake inhibitors (SSRIs) fluoxetine, fluvoxamine, sertraline, paroxetine and citalopram have been introduced on to the market (Anon 1993 a). As a result, a large number of antidepressant (AD) drugs are now available (Anon 1993 b; Barbui 1999). These new ADs have been compared with amitriptyline in many randomised controlled trials (RCTs), but evidence of advantages, in terms of tolerability and efficacy has not been clearly provided. Therefore, debate persists as to whether amitriptyline should still be considered the reference AD in the pharmacological management of depression.
Amitriptyline has a wide range of pharmacological actions. In addition to the increased receptor site concentration of noradrenaline and serotonin, that is thought to be relevant for the antidepressant effect, amitriptyline has some affinity for the muscarinic, histaminergic and adrenergic systems (Garattini 1988). This affinity is considered significant for most of the side‐effects of amitriptyline. Other TCAs have been shown to act on similar pathways, though to a lesser extent. From a clinical viewpoint, however, trials comparing amitriptyline to the other tricyclic/heterocyclic drugs have failed to clearly demonstrate that amitriptyline is less well tolerated, even though data suggest patients on amitriptyline complain of more side‐effects than those receiving other TCAs.
Some data indicates amitriptyline is less well tolerated in comparison with SSRIs. Systematic reviews comparing the tolerability of TCAs versus SSRIs in terms of patients who fail to complete the study demonstrated a modest advantage of SSRIs in total discontinuation rates (Anderson 1995). The Canadian Coordinating Office for Health Technology Assessment showed that drop‐out rates due to adverse events were 2% lower for SSRIs (CCOHTA 1997). The comparison of SSRIs with old tricyclics (imipramine and amitriptyline), newer tricyclics (dothiepin, nortriptyline, desipramine, clomipramine and doxepin) and heterocyclic antidepressants (bupropion, mianserin, trazodone, maprotiline, amineptine and nomifensine) showed a lower discontinuation rate of the SSRIs only against amitriptyline and imipramine (Hotopf 1997), thus providing evidence that SSRIs are better tolerated than these two old tricyclic antidepressants.
The evidence of the disadvantage of amitriptyline in terms of dropouts against SSRIs must however be weighed against possible advantages in terms of efficacy. Perry (Perry 1996) reviewed the Hamilton scores in trials comparing TCAs with SSRIs and found TCAs more effective than SSRIs in patients suffering from severe depression with melancholic features. Similar conclusions were reached by Joffe (Joffe 1996), who found a nonsignificant advantage of TCAs against SSRIs in terms of effect size, and by Anderson (Anderson 2000), who showed that TCAs appeared more effective in inpatients but not in outpatients with depression.
The present meta‐analysis, which is the updated Cochrane version of a previously published systematic review (Barbui 2001a) was undertaken to test whether amitryptiline is less well tolerated than tricyclic/heterocyclic drugs and SSRIs, and to investigate whether this disadvantage might be counterbalanced by a better efficacy profile. The possible contribution of study setting on treatment outcome was also investigated.
Objectives
To investigate the tolerability and efficacy of amitriptyline in comparison with tricyclic/heterocyclic AD, other related antidepressants and SSRIs; versus tricyclic/heterocyclic and other related antidepressants; and versus SSRIs.
Methods
Criteria for considering studies for this review
Types of studies
Only randomised controlled trials were included.
Types of participants
Study participants were of either sex and any age with a primary diagnosis of depression. Studies adopting any criteria to define patients suffering from depression were included; in addition, a concurrent diagnosis of another psychiatric disorder was not considered an exclusion criteria. AD trials in depressive patients with a concomitant medical illness were not included in this review.
Types of interventions
Included trials compared amitriptyline with another tricyclic/heterocyclic AD or with one of the SSRIs (fluoxetine, fluvoxamine, sertraline, paroxetine, citalopram).
Types of outcome measures
Efficacy was evaluated using the following outcome measures: (1) number of patients to respond to treatment out of the total number of randomised patients (intention‐to‐treat analysis); (2) group mean scores at the end of the trial on Hamilton Depression Scale (HMD)(Hamilton 1960), or Montgomery Depression Scale (MADRS)(Montgomery 1979), or any depression scale.
Tolerability was evaluated using the following outcome measures: (1) numbers of patients dropping out during the trial as a proportion of the total number of randomised patients; (2) numbers of patients complaining of side‐effects out of the total number of randomised patients.
Search methods for identification of studies
Electronic Searches CCDANCTR‐Studies was searched ‐ 28/11/2005 ‐ using the following terms;
Diagnosis = Depress* or Dysthymi* or "Affective Disorder*" and Intervention = Amitriptyline
Reference lists Reference lists of relevant papers and previous systematic reviews were handsearched for published reports and citations of unpublished research.
Data collection and analysis
Duplicate studies Considerable care was taken to exclude duplicate publications and data from single centres contributing to multi‐centre studies. Data extraction Data were extracted using a standard form.
Dichotomous outcomes Two reviewers independently extracted the number of patients undergoing the randomisation procedure, the number of patients who failed to complete the study and that of patients complaining side‐effects. The number of improved patients was extracted in agreement with the definition of responders adopted in the primary studies. When authors did not report any definition of responders, the number of patients showing a 50% reduction in the HMD or MADRS scale was extracted; if these figures were not available, we extracted the number of patients categorised as "much improved" and "improved" at the Clinical Global Impression (CGI), or the number of patients in the corresponding categories at any other rating scale.
Continuous outcomes The mean scores at endpoint, the standard deviation (SD) or standard error (SE) of these values, and the number of patients included in these analyses, were extracted. Data were extracted from the HMD. If this scale was not available, data were extracted from the MADRS. If both the HMD and MADRS were not available, data were extracted from any other depression scale. When only the SE was reported, it was converted into SD according to Altman 1996.
Statistical analysis
Measures of treatment effect The primary analysis used a fixed effect approach, the Peto Odds Ratio. In addition, a random‐effects estimate, which takes accounts of any additional between‐study variation, was calculated using a moment estimator of the between‐study variance (Der Simonian 1986) as a sensitivity check on the fixed‐effect estimate.
Tolerability data were analysed by calculating the proportion of patients who failed to complete the study and who experienced adverse reactions out of the total number of randomised patients.
A standardised mean difference (SMD) was estimated for each continuous outcome. This measure provides the effect size of the intervention in units of standard deviations. Scores from different outcome scales can be summarized in an overall SMD.
Management of missing data Responders to treatment were calculated including drop‐outs in the analysis, on an intention‐to‐treat basis. When data on drop‐outs were carried forward and included in the efficacy evaluation (Last Observation Carried Forward, LOCF), they were analysed according to the primary studies; when dropouts were excluded from any assessment in the primary studies they were considered as drug failures (both in the amitriptyline and comparator arms). Scores from continuous outcomes were analysed including patients with a final assessment or with a last observation carried forward to the final assessment.
Exploration of heterogeneity and subgroup analyses Heterogeneity of treatment effect between studies was formally tested using the Chi Square statistic. A subgroup and meta‐regression analysis was carried out using the statistical software STATA 7.0. We investigated the extent that study setting predicted size of treatment effect on response and drop‐out. For the purposes of this analysis, studies carried out in psychiatric wards of general hospitals or in psychiatric hospitals and studies that recruited inpatients in these facilities and followed them up on an outpatient basis were considered RCTs in inpatients. Studies carried out in outpatient psychiatric settings (public or private facilities) or among general practice patients or in any other outpatient services were considered RCTs in outpatients.
Results
Description of studies
Design and setting A total number of 227 RCTs met the inclusion criteria; of these, 33 studies were excluded for the reasons listed in the Table of Excluded Studies. Of the 194 remaining studies, 147 RCTs compared amitriptyline with another TCA or related AD, and 47 RCTs compared amitriptyline with one of the SSRIs. In six studies amitriptyline was administered in combination with perphenazine and in two studies in combination with chlordiazepoxide. In two studies a single blind approach was followed, five studies were not blind, and in other seven studies blindness was not clear. The mean and median sample size was 81.7 patients (SD 81.3) and 51 patients, respectively. The mean and median length of follow‐up was 5.2 weeks (SD 2.22) and 5 weeks, respectively. A total of 54 studies was conducted in inpatient psychiatric settings, whilst 70 were conducted in outpatient settings. In the remaining studies patients were enrolled either in both in and outpatient settings (27 studies), or in a general practice setting (24 studies). The setting was unclear in 19 studies. Participants Most of the included trials enrolled patients suffering from major depression. Five studies enrolled patients with bipolar affective disorder during a depressive episode, whilst six and two trials enrolled patients with mixed anxiety‐depressive syndrome and dysthymic disorder, respectively. In about 27% of the trials the Diagnostic Statistical Manual of Mental Disorder (DSM) criteria were followed to make the diagnosis (APA 1980; APA 1994). The Research Diagnostic Criteria (RDC) (Spitzer 1977), the Feighner (Feighner 1972) criteria and the International Classification of Diseases (ICD) (WHO 1974; WHO 1992) criteria were also used. Fifty three per cent of the studies adopted implicit diagnostic criteria. The severity of illness at baseline was measured with the Hamilton Depression Scale (HMD) or the Montgomery scale (MADRS). The study population mostly involved patients aged between 18 and 65, although in some trials patients above 65 were enrolled.
Risk of bias in included studies
A formal quality assessment was performed using the Quality Rating Scale (QRS) (Moncrieff 2001). The rating system is such that the higher the score, the higher is the quality of the trial. Overall QRS scores for the total sample ranged from 8 to 32, out of a maximum of 46. The mean QRS score was 18.0 (SD 3.99), indicating an average medium/low quality of included RCTs. The mean QRS score for studies comparing amitriptyline with other TCA or related ADs was 17.2 (SD 3.71), and the same figure for studies comparing amitriptyline with the SSRIs was 20.3 (SD 3.94), indicating a higher quality in the latter group of studies (z ‐4.36, p < .001).
Of the 194 RCTs included, only 12 reported a method for concealing allocation. In seven of the 12 RCTs the allocation concealment was inadequate, while in the remaining five it was not used. With regard to blindness, in six RCTs blinding was not clear, and in nine RCTs procedure was single blind only. In the remaining RCTs, double blind was clearly indicated.
Effects of interventions
Amitriptyline versus tricyclic/heterocyclic and other related antidepressants and SSRIs Comparison 01: Efficacy of amitriptyline With regards to responders, a total number of 104 RCTs contributed to this analysis, with 7422 patients included (Graph 01.01). The estimate of the overall odds ratio for responders showed that more subjects responded to amitriptyline in comparison with the control AD group (odds ratio (OR) 1.12, 95% confidence interval (CI) 1.02 to 1.23). Test for heterogeneity was performed (Chi2=120.40, df= 103 (p=0.12), I2=14.4%). The Number Needed to Treat to Benefit (NNTB) was 50 subjects. The estimate of the efficacy of amitriptyline and control ADs on continuous measures of depression (Graph 01.04), performed on 2004 and 2015 subjects respectively, included 53 studes and revealed an effect size which also significantly favoured amitriptyline (Standardised Mean Difference (SMD) 0.13, 95% CI 0.04 to 0.23). The test for heterogeneity yielded the following result: Chi2=97.44, df= 152 (p=0.0001), I2=46.6%.
Comparison 02: Tolerability of amitriptyline A total number of 159 RCTs contributed to this analysis, with 14926 patients included. The dropout rate in patients taking amitriptyline and control ADs (analysis 02.01) was similar, yielding an OR of 0.96 (95% CI 0.89 to 1.04; test for heterogeneity: Chi2 =186.02, df=158 (p=0.06), I2 =15.1%). The Number Needed to Treat to Harm (NNTH) was 345 subjects. The estimate of the proportion of patients who experienced side‐effects, performed on 54 studies (5465 patients )(analysis 02.02), significantly favoured control ADs in comparison with amitriptyline (OR 0.66, 95% CI 0.59 to 0.74; test for heterogeneity: Chi2=83.71, df=53 (p=0.005), I2=36.7%). The NNTH was 8 subjects.
Amitriptyline versus tricyclic/heterocyclic and other related antidepressants Comparison 03: Efficacy against tricyclic/heterocyclic and other related antidepressants The analysis of studies that compared amitriptyline with other TCA and related ADs (Graph 03.03), performed on 84 RCTs (5376 subjects), provided an OR for responders of 1.11 (95% CI 0.99 to 1.25), indicating a non‐significant advantage for amitriptyline (NNTB = 50). Test for heterogeneity showed Chi2 = 108.51, df=83 (p=0.03) and I2=23.5%. The estimate of the efficacy of amitriptyline in comparison with other TCA and related ADs on continuous measures of depression (Graph 03.04), performed on 29 RCTs (1360 subjects), revealed an effect size that favoured amitriptyline (SMD 0.18 95% CI 0.00 to 0.35), although this figure did not reach significance. A test for heterogeneity yielded the following results: Chi2=63.20, df=28 (p=0.0002) and I2=55.7%.
Comparison 04: Tolerability against tricyclic/heterocyclic and other related antidepressants (Comparison 04) The dropout rate in patients taking amitriptyline and control TCA or related AD (113 RCTs and 9156 subjects; Graph 04.03) was similar, yielding an OR of 1.07 (95% CI 0.96 to 1.19; test for heterogeneity: Chi2 =124.40, df =112 (p=0.20), I2=10.0%) favouring amitriptyline (NNTB = 71). However, the estimate of the proportion of patients who experienced side‐effects (42 RCTs and 3310 subjects; Graph 04.04) significantly favoured control TCA or related AD over amitriptyline (OR 0.67, 95% CI 0.58 to 0.78; test for heterogeneity: Chi2 = 67.09, df =41 (p=0.006), I2 =38.9%). The NNTH was 9.5 subjects.
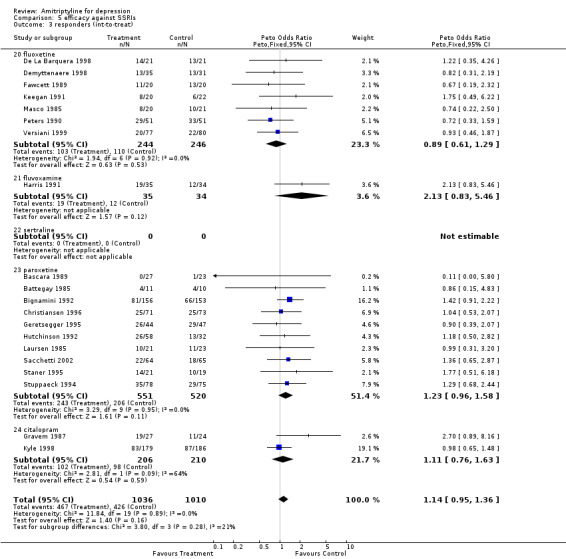
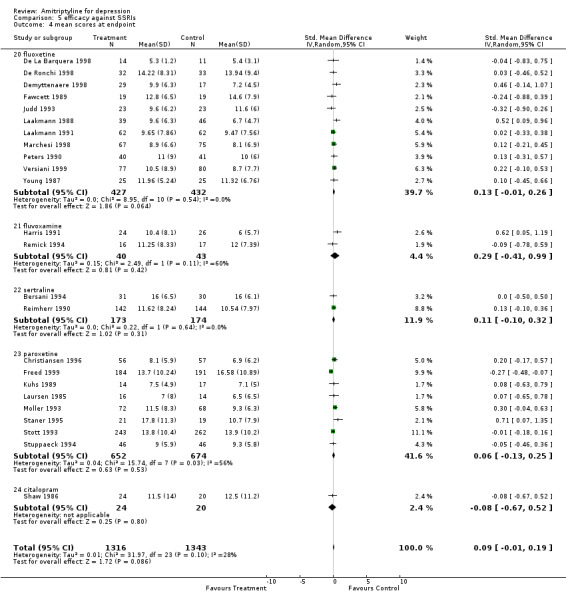
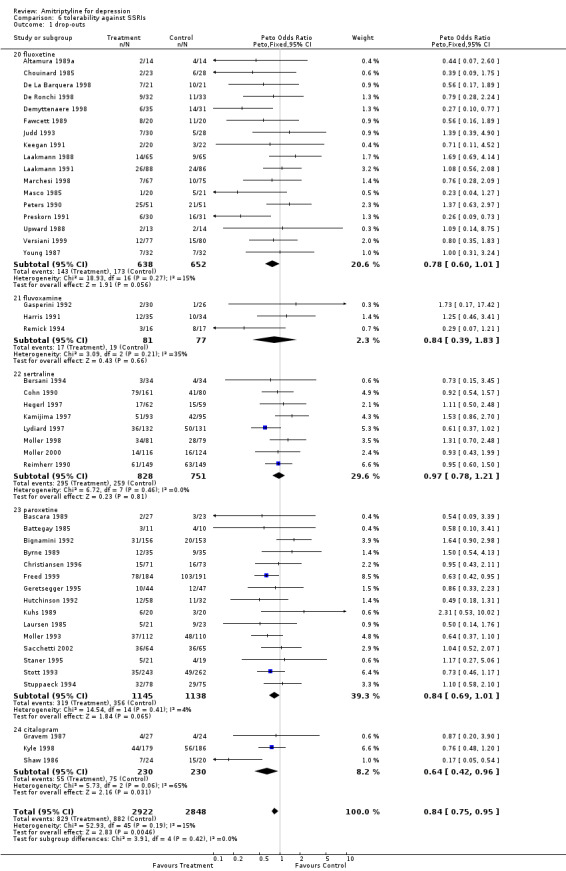
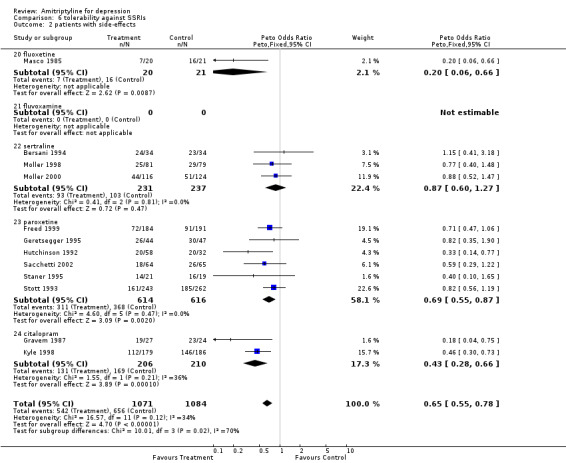
Amitriptyline versus SSRIs Comparison 05: Efficacy against SSRIs The analysis of the 20 studies (2046 subjects) that compared amitriptyline with the SSRIs (Graph 05.03) provided an OR of 1.14 (95% CI 0.95 to 1.36), indicating a non‐significant advantage for amitriptyline (test for heterogeneity: Chi2=11.84, df=19 (p=0.89), I2=0%). The estimate of the efficacy of amitriptyline in comparison with the SSRIs on continuous measures of depression (24 RCTs and 2659 subjects; Graph 05.04) again revealed no statistically significant difference (SMD 0.09, 95% CI ‐0.01 to 0.19; test for heterogeneity: Chi2 =31.97, df =23 (p =0.10), I2 =28.1%). Comparison 06: Tolerability against SSRIs The dropout rate in patients taking amitriptyline and the SSRIs (Graph 06.01), derived from a total number of 46 RCTs (5770 subjects), significantly favoured the latter, yielding an OR of 0.84 (95% CI 0.75 to 0.95; test for heterogeneity: Chi2df =45 (p =0.19), I2 =15.0%) and a NNTH of 40. In addition, the estimate of the proportion of patients who experienced side‐effects (performed on 12 RCTs, with 2155 subjects; Graph 06.02), significantly favoured the SSRIs in comparison with amitriptyline (OR 0.65, 95% CI 0.55 to 0.76; test for heterogeneity: Chi2 =16.57, df =11 (p =0.12), I2 =33.6%). The NNTH was 10 subjects.
Sub‐group analyses Efficacy and tolerability by study setting On treatment response, amitriptyline resulted more effective than control ADs in inpatients (OR 1.22, 95% CI 1.04 to 1.42, NNTB = 24), but not in outpatients (OR 1.01, 95% CI 0.88 to 1.17, NNTB = 200). Among inpatients amitriptyline was significantly more effective than TCAs (OR 1.20, 95% CI 1.02 to 1.42) and non‐significantly more effective than SSRIs (OR 1.30, 95% CI 0.87 to 1.96). Among outpatients no statistically significant differences emerged between amitriptyline and TCAs (OR 0.98, 95% CI 0.81 to 1.17) and between amitriptyline and SSRIs (OR 1.08, 95% CI 0.86 to 1.35).
Amitriptyline was less well tolerated than control ADs in outpatients (OR 0.90, 95% CI 0.81to 0.99, NNTH = 91), but not in inpatients (OR 1.09, 95% CI 0.95 to 1.25, NNTB = 77). Among inpatients no statistically significant differences emerged between amitriptyline and TCAs (OR 1.16, 95% CI 0.97, 1.38) and between amitriptyline and SSRIs (OR 0.99, 95% CI 0.78, 1.24). Among outpatients amitriptyline was significantly less well tolerated in comparison with SSRIs (OR 0.77, 95% CI 0.67 to 0.89) but not in comparison with TCAs (OR 1.03, 95% CI 0.90 to 1.19).
The predictive effect of study setting on responders and dropouts is presented in Table 01. A positive estimate indicates that the factors included in the meta‐regression model predicted a statistically significant advantage (in terms of responders or dropouts) for amitriptyline in comparison with control ADs; a negative estimate indicates that the factors included in the meta‐regression model predicted a statistically significant disadvantage for amitriptyline in comparison with control ADs. In comparison with studies carried out in inpatients, studies carried out in psychiatric outpatient settings predicted a statistically significant disadvantage for amitriptyline over control AD in terms of treatment responders. Given the fact that subgroup analysis should be interpreted with caution, the findings on the differences between in‐patients and out‐patients are exploratory.
Discussion
This present systematic review of clinical trials comparing amitriptyline with all other AD drugs indicates that overall amitriptyline is slightly more efficacious than the pooled results of other TCA or newer compounds. For the efficacy analysis of continuous measures of depression, the SMD significantly favoured amitriptyline over control ADs. However, this analysis only included patients with a final assessment or patients whose efficacy data were carried forward to the final assessment. Thus patients who dropped out of treatment were often excluded. This would have had the effect of favouring amitriptyline. For the analysis of the binary outcome (responders versus non‐reponders) dropouts were assumed to have failed to improve. This conservative approach, which theoretically takes into consideration efficacy and tolerability within the same outcome measure, yielded an odds ratio which indicated a small but significant advantage for amitriptyline over control ADs. When this analysis was stratified by drug class, the effect size was similar, but was no longer statistically significant because of the smaller sample sizes included in each subgroup analysis.
Amitriptyline was less well tolerated than SSRIs, but this disadvantage was counterbalanced by a slightly higher proportion of responders, yielding a no difference in outcome in terms of responders. In practical terms this suggests that the proportion of improved patients after six to eight weeks of therapy is very similar for amitriptyline and the SSRIs, even taking into consideration the higher dropout rate associated with amitriptyline. Despite this, amitriptyline was found to be associated with more side‐effects, as can be seen by the proportion of subjects who complained adverse reactions during the study period, which was significantly higher in comparison with the SSRIs.
Amitriptyline showed a more favourable profile over control agents in inpatients but not in outpatients. Inpatients with depression responded better to amitriptyline than control TCA or SSRI, although the difference in comparison with SSRIs, calculated on a small number of studies, was not statistically significant. In terms of dropouts, amitriptyline was not less well tolerated either in comparison with control TCA or SSRI. These findings are in agreement with those provided by Anderson (Anderson 1998), who showed that among inpatients with depression TCA resulted more effective than SSRIs, and that overall treatment discontinuation rates were not significantly different. In outpatients with depression, in contrast, amitriptyline was not more effective than control agents and resulted less well tolerated. The role of RCT setting was further confirmed by the meta‐regression model, which demonstrated that studies carried out in inpatients were associated with a significant advantage of amitriptyline over control agents in terms of treatment responders. However, no significant associations emerged when treatment dropouts were entered in the model as the dependent variable.
This study has limitations. Firstly, the findings on dropout rates in this meta‐analysis should be interpreted with care, as reasons for dropping out were not given in a high proportion of trials. There are many different reasons for dropping‐out of treatment, and therefore this measure might be an oversimplification of the true tolerability of amitriptyline. Some studies reported the proportion of subjects who discontinued the study because of side‐effects or AD inefficacy or because of other reasons. These figures were not extracted and analysed because reasons for dropping out were given in some studies only, and in many of these studies they were reported for some drop‐out patients only. Secondly, we observed great variability in the overall quality of included studies. The quality rating showed an overall medium/low quality of studies; moreover, RCTs comparing amitriptyline with control TCA or related AD scored significantly lower than RCTs comparing amitriptyline with the SSRIs. A possible explanation of this is that the majority of studies comparing amitriptyline with other TCAs have been published in the 1960s and 1970s, while those comparing amitriptyline with the SSRIs have been published in the last 20 years. Trial methodology has changed during the last four decades, both in terms of design and statistical techniques, and this may explain the difference in the mean quality score between the two groups of studies (Barbui 2001b). Another study limitation is that heterogeneity was investigated by stratifying the analysis according to study setting only; the role of other study characteristics such as length of follow‐up, diagnostic criteria and outcome measures was not assessed. Although this leaves some uncertainty about the role of these independent variables in influencing the overall treatment effect, the analysis was not stratified because we would have inevitably increased the probability of detecting significant difference by chance, but also because we would have decreased the sample size of each analysis, thus increasing the possibility of detecting borderline findings of uncertain clinical relevance.
Authors' conclusions
Implications for practice.
Amitriptyline still has a role in the pharmacological management of depressive episodes. It has the edge in terms of efficacy in comparison with control ADs, but patients have to sustain a great burden of side‐effects. From a clinical viewpoint the intriguing issue is to identify subgroups of subjects who could benefit from amitriptyline therapy. Some data suggest that it should be prescribed to patients suffering from severe depression with melancholia, or to patients admitted to hospital because of the severity of symptoms (Perry 1996; Joffe 1996; Anderson 2000). The present review, by showing a more favourable profile of amitriptyline over control agents in inpatients but not in outpatients, supports this suggestion. On the other side, however, it seems reasonable to suggest the use of the SSRIs in patients at suicide risk, since amitriptyline and other TCAs are less safe when taken in overdose (Spigset 1999). Moreover, the cost of fluoxetine and that of other SSRIs has fallen over the last few years, both in the US and in Europe, making these compounds more cost‐effective. Unfortunately, little evidence is available on the long‐term efficacy and tolerability of amitriptyline versus the SSRIs. Many side‐effects are often experienced at the beginning of treatment, and therefore it might be possible that amitriptyline and other TCAs could be more tolerable in the long‐term. However, no data support this speculation.
Implications for research.
Amitriptyline should still be considered a reference comparator in terms of efficacy in clinical trials of new AD drugs. Future studies, however, should adopt a higher standard in terms of design and statistical analysis. The quality rating has in fact shown that a great proportion of studies does not meet the quality requirements to provide reliable findings, especially in terms of sample size and length of follow‐up. The issue of long‐term treatment of depression with respect to efficacy and tolerability should definitely be addressed, as in real‐life conditions patients with depression receive treatment for more than 6‐12 weeks.
In the light of the increasing use of the SSRIs and newer compounds, future research has to clarify the position of amitriptyline and other old compounds in the pharmacological management of depressive episodes. In particular, large pragmatic RCTs should provide direct evidence on whether amitriptyline is cost‐effective in special patient populations, for example hospitalised patients with severe depression.
What's new
| Date | Event | Description |
|---|---|---|
| 31 October 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 4, 1999 Review first published: Issue 2, 2003
| Date | Event | Description |
|---|---|---|
| 17 May 2007 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We would like to thank the CCDAN Editorial Team for their support, information and advice.
Data and analyses
Comparison 1. overall efficacy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 responders (int‐to‐treat) | 104 | 7422 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.12 [1.02, 1.23] |
| 1.1 amineptine | 1 | 51 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.32 [0.10, 1.04] |
| 1.2 amoxapine | 11 | 601 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.28 [0.91, 1.81] |
| 1.3 clomipramine | 1 | 72 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.42 [0.17, 1.05] |
| 1.4 desipramine | 3 | 146 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.20 [0.62, 2.31] |
| 1.5 dothiepin | 5 | 230 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.54 [0.31, 0.96] |
| 1.6 doxepin | 8 | 331 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.00 [0.63, 1.59] |
| 1.7 imipramine | 9 | 578 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.71 [1.20, 2.43] |
| 1.8 lofepramine | 6 | 376 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.75 [0.49, 1.16] |
| 1.9 maprotiline | 12 | 683 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.90 [0.65, 1.26] |
| 1.10 mianserin | 5 | 242 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.37 [0.82, 2.29] |
| 1.11 minaprine | 2 | 204 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.23 [0.67, 2.27] |
| 1.12 nomifensine | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.13 nortriptyline | 4 | 197 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.36 [0.77, 2.40] |
| 1.14 protriptyline | 1 | 100 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.40 [1.09, 5.26] |
| 1.15 tianeptine | 2 | 565 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.40 [0.95, 2.06] |
| 1.16 trazodone | 8 | 752 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.97 [0.72, 1.30] |
| 1.17 trimipramine | 1 | 26 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.54 [0.11, 2.52] |
| 1.18 viloxazine | 3 | 117 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.76 [0.84, 3.67] |
| 1.19 combination | 2 | 105 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.59 [0.69, 3.71] |
| 1.20 fluoxetine | 7 | 490 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.89 [0.61, 1.29] |
| 1.21 fluvoxamine | 1 | 69 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.13 [0.83, 5.46] |
| 1.22 sertraline | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.23 paroxetine | 10 | 1071 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.23 [0.96, 1.58] |
| 1.24 citalopram | 2 | 416 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.11 [0.76, 1.63] |
| 4 mean scores at endpoint | 53 | 4019 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [0.04, 0.23] |
| 4.1 amineptine | 2 | 89 | Std. Mean Difference (IV, Random, 95% CI) | 0.40 [‐2.78, 3.58] |
| 4.2 amoxapine | 1 | 38 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.54, 0.74] |
| 4.3 clomipramine | 1 | 72 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.70, 0.23] |
| 4.4 desipramine | 2 | 82 | Std. Mean Difference (IV, Random, 95% CI) | 0.42 [‐0.01, 0.86] |
| 4.5 dothiepin | 2 | 93 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.39, 0.42] |
| 4.6 doxepin | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.7 imipramine | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.8 lofepramine | 3 | 111 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.48, 0.47] |
| 4.9 maprotiline | 5 | 139 | Std. Mean Difference (IV, Random, 95% CI) | 0.32 [‐0.06, 0.71] |
| 4.10 mianserin | 3 | 77 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.20, 0.71] |
| 4.11 minaprine | 1 | 58 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.34, 0.69] |
| 4.12 nomifensine | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.13 nortriptyline | 2 | 65 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.63, 0.35] |
| 4.14 protriptyline | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.15 tianeptine | 1 | 211 | Std. Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.09, 0.45] |
| 4.16 trazodone | 4 | 243 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [0.01, 0.54] |
| 4.17 trimipramine | 1 | 34 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.42, 0.93] |
| 4.18 viloxazine | 1 | 48 | Std. Mean Difference (IV, Random, 95% CI) | 0.38 [‐0.19, 0.95] |
| 4.19 combination | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.20 fluoxetine | 11 | 859 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.01, 0.26] |
| 4.21 fluvoxamine | 2 | 83 | Std. Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.41, 0.99] |
| 4.22 sertraline | 2 | 347 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.10, 0.32] |
| 4.23 paroxetine | 8 | 1326 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.13, 0.25] |
| 4.24 citalopram | 1 | 44 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.67, 0.52] |
1.1. Analysis.
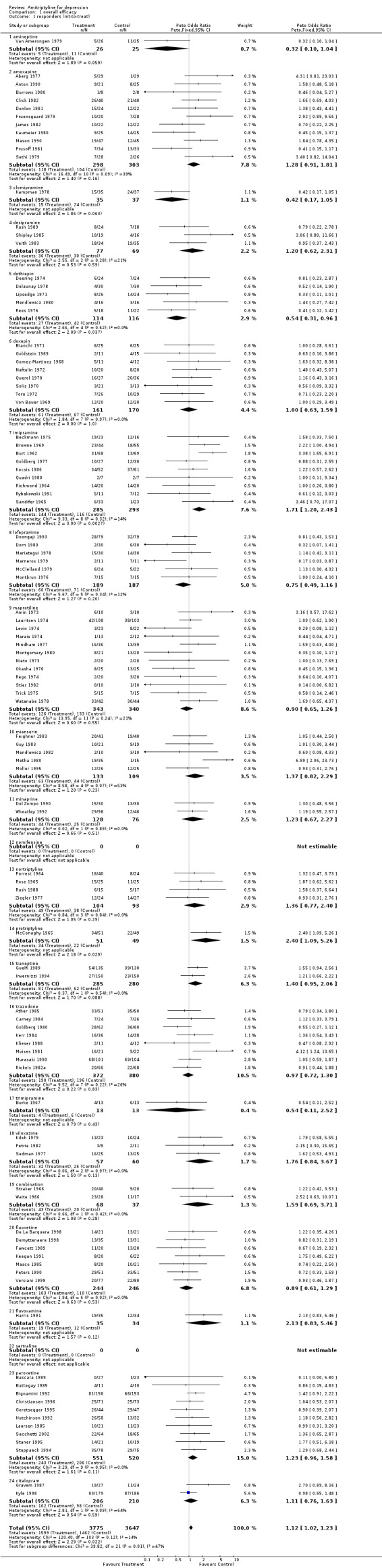
Comparison 1 overall efficacy, Outcome 1 responders (int‐to‐treat).
1.4. Analysis.
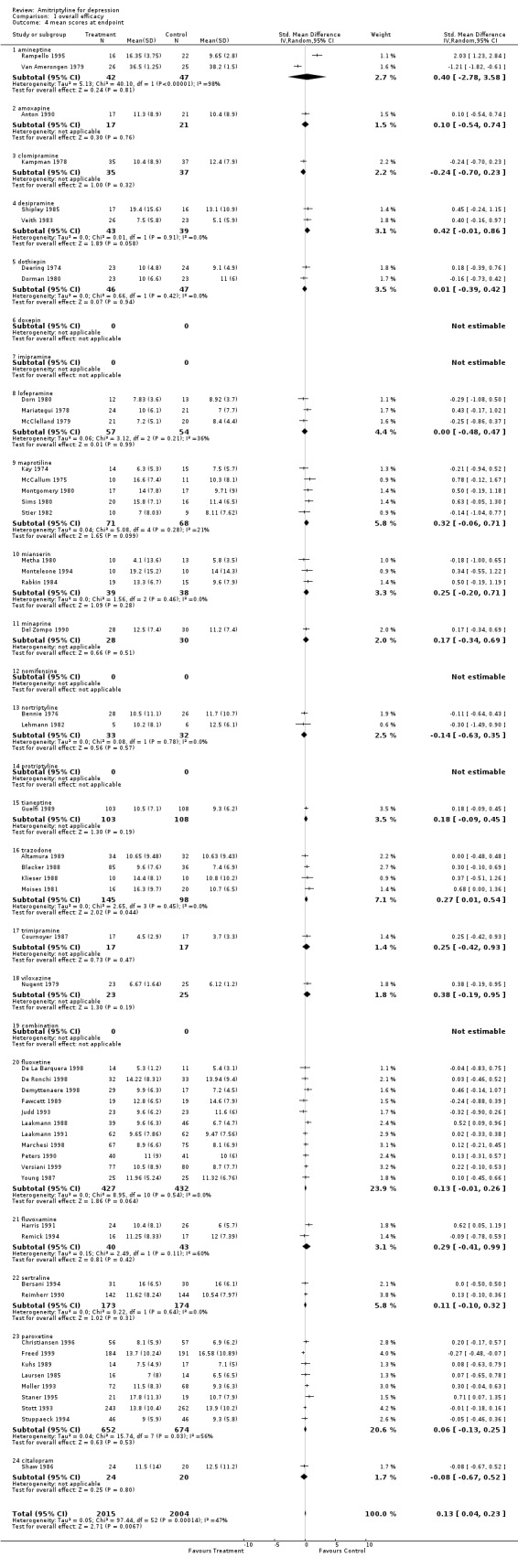
Comparison 1 overall efficacy, Outcome 4 mean scores at endpoint.
Comparison 2. overall tolerability.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 drop‐outs | 174 | 14926 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.96 [0.89, 1.04] |
| 1.1 amineptine | 2 | 95 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.14 [0.38, 3.39] |
| 1.2 amoxapine | 13 | 722 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.09 [0.77, 1.54] |
| 1.3 clomipramine | 3 | 299 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.52 [0.95, 2.41] |
| 1.4 desipramine | 4 | 156 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.72 [0.28, 1.84] |
| 1.5 dothiepin | 8 | 396 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.68 [0.32, 1.47] |
| 1.6 doxepin | 13 | 860 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.84 [0.60, 1.17] |
| 1.7 imipramine | 6 | 349 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.34 [0.71, 2.51] |
| 1.8 lofepramine | 8 | 470 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.85 [0.55, 1.32] |
| 1.9 maprotiline | 18 | 1107 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.97 [0.69, 1.35] |
| 1.10 mianserin | 12 | 787 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.27 [0.91, 1.77] |
| 1.11 minaprine | 3 | 735 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.30 [0.89, 1.90] |
| 1.12 nomifensine | 1 | 29 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.69 [0.04, 12.15] |
| 1.13 nortriptyline | 8 | 467 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.01 [0.56, 1.83] |
| 1.14 protriptyline | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.15 tianeptine | 3 | 694 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.28 [0.89, 1.85] |
| 1.16 trazodone | 11 | 924 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.12 [0.82, 1.51] |
| 1.17 trimipramine | 2 | 115 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.76 [0.30, 1.97] |
| 1.18 viloxazine | 7 | 288 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.32 [0.69, 2.55] |
| 1.19 combination | 6 | 663 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.84 [0.55, 1.28] |
| 1.20 fluoxetine | 17 | 1290 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.78 [0.60, 1.01] |
| 1.21 fluvoxamine | 3 | 158 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.84 [0.39, 1.83] |
| 1.22 sertraline | 8 | 1579 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.97 [0.78, 1.21] |
| 1.23 paroxetine | 15 | 2283 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.84 [0.69, 1.01] |
| 1.24 citalopram | 3 | 460 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.64 [0.42, 0.96] |
| 2 patients with side‐effects | 54 | 5465 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.66 [0.59, 0.74] |
| 2.1 amineptine | 1 | 51 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.46 [0.14, 1.49] |
| 2.2 amoxapine | 3 | 192 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.77 [0.41, 1.44] |
| 2.3 clomipramine | 1 | 72 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.38 [0.55, 3.47] |
| 2.4 desipramine | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.5 dothiepin | 5 | 234 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.51 [0.29, 0.88] |
| 2.6 doxepin | 4 | 168 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.77 [0.39, 1.50] |
| 2.7 imipramine | 2 | 156 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.73 [0.35, 1.50] |
| 2.8 lofepramine | 2 | 62 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.39 [0.14, 1.08] |
| 2.9 maprotiline | 7 | 436 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.59 [0.40, 0.88] |
| 2.10 mianserin | 3 | 266 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.34 [0.20, 0.57] |
| 2.11 minaprine | 3 | 702 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.55 [0.39, 0.80] |
| 2.12 nomifensine | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.13 nortriptyline | 4 | 226 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.51 [0.30, 0.87] |
| 2.14 protriptyline | 1 | 100 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.41 [0.60, 3.33] |
| 2.15 tianeptine | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.16 trazodone | 3 | 428 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.27 [0.87, 1.87] |
| 2.17 trimipramine | 1 | 41 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.51 [0.11, 2.36] |
| 2.18 viloxazine | 1 | 41 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.05 [0.27, 4.12] |
| 2.19 combination | 1 | 135 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.79 [0.34, 1.86] |
| 2.20 fluoxetine | 1 | 41 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.20 [0.06, 0.66] |
| 2.21 fluvoxamine | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.22 sertraline | 3 | 468 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.87 [0.60, 1.27] |
| 2.23 paroxetine | 6 | 1230 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.69 [0.55, 0.87] |
| 2.24 citalopram | 2 | 416 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.43 [0.28, 0.66] |
2.1. Analysis.
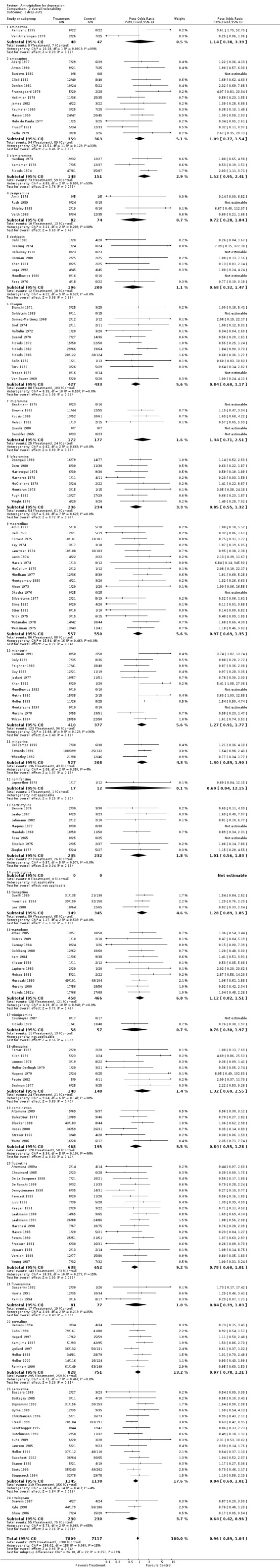
Comparison 2 overall tolerability, Outcome 1 drop‐outs.
2.2. Analysis.
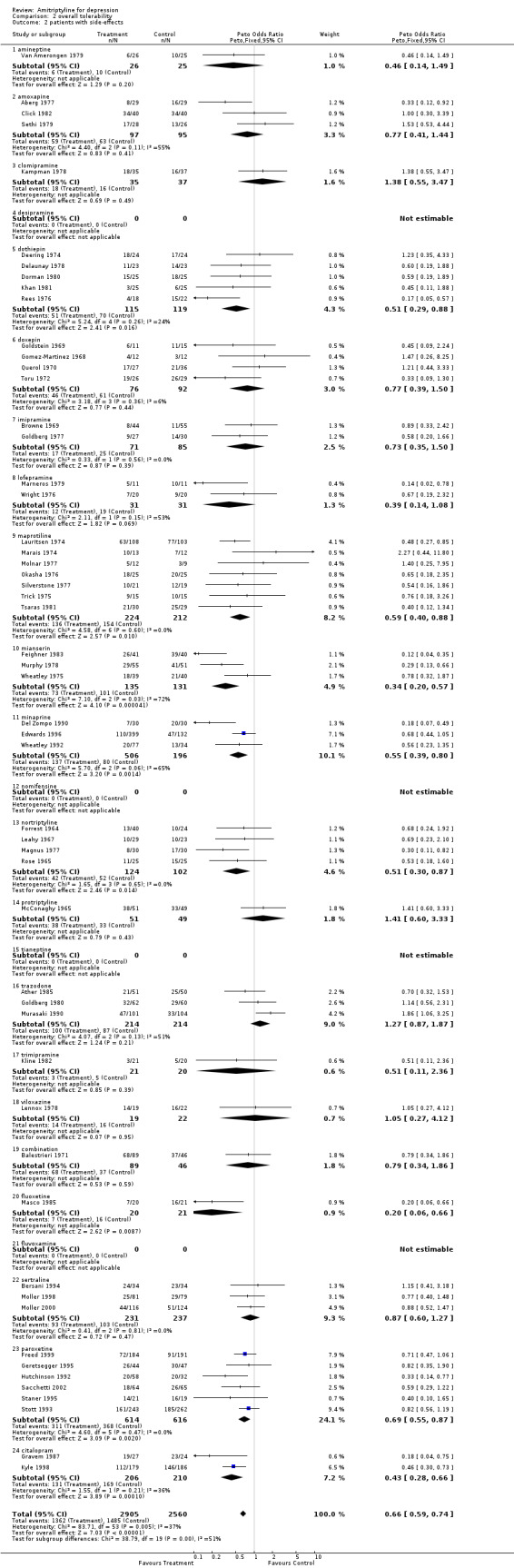
Comparison 2 overall tolerability, Outcome 2 patients with side‐effects.
Comparison 3. efficacy against TCA and related antidepressants.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3 responders (int‐to‐treat) | 84 | 5376 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.11 [0.99, 1.25] |
| 3.1 amineptine | 1 | 51 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.32 [0.10, 1.04] |
| 3.2 amoxapine | 11 | 601 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.28 [0.91, 1.81] |
| 3.3 clomipramine | 1 | 72 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.42 [0.17, 1.05] |
| 3.4 desipramine | 3 | 146 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.20 [0.62, 2.31] |
| 3.5 dothiepin | 5 | 230 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.54 [0.31, 0.96] |
| 3.6 doxepin | 8 | 331 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.00 [0.63, 1.59] |
| 3.7 imipramine | 9 | 578 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.71 [1.20, 2.43] |
| 3.8 lofepramine | 6 | 376 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.75 [0.49, 1.16] |
| 3.9 maprotiline | 12 | 683 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.90 [0.65, 1.26] |
| 3.10 mianserin | 5 | 242 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.37 [0.82, 2.29] |
| 3.11 minaprine | 2 | 204 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.23 [0.67, 2.27] |
| 3.12 nomifensine | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.13 nortriptyline | 4 | 197 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.36 [0.77, 2.40] |
| 3.14 protriptyline | 1 | 100 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.40 [1.09, 5.26] |
| 3.15 tianeptine | 2 | 565 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.40 [0.95, 2.06] |
| 3.16 trazodone | 8 | 752 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.97 [0.72, 1.30] |
| 3.17 trimipramine | 1 | 26 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.54 [0.11, 2.52] |
| 3.18 viloxazine | 3 | 117 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.76 [0.84, 3.67] |
| 3.19 combination | 2 | 105 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.59 [0.69, 3.71] |
| 4 mean scores at endpoint | 29 | 1360 | Std. Mean Difference (IV, Random, 95% CI) | 0.18 [0.00, 0.35] |
| 4.1 amineptine | 2 | 89 | Std. Mean Difference (IV, Random, 95% CI) | 0.40 [‐2.78, 3.58] |
| 4.2 amoxapine | 1 | 38 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.54, 0.74] |
| 4.3 clomipramine | 1 | 72 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.70, 0.23] |
| 4.4 desipramine | 2 | 82 | Std. Mean Difference (IV, Random, 95% CI) | 0.42 [‐0.01, 0.86] |
| 4.5 dothiepin | 2 | 93 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.39, 0.42] |
| 4.6 doxepin | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.7 imipramine | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.8 lofepramine | 3 | 111 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.48, 0.47] |
| 4.9 maprotiline | 5 | 139 | Std. Mean Difference (IV, Random, 95% CI) | 0.32 [‐0.06, 0.71] |
| 4.10 mianserin | 3 | 77 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.20, 0.71] |
| 4.11 minaprine | 1 | 58 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.34, 0.69] |
| 4.12 nomifensine | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.13 nortriptyline | 2 | 65 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.63, 0.35] |
| 4.14 protriptyline | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.15 tianeptine | 1 | 211 | Std. Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.09, 0.45] |
| 4.16 trazodone | 4 | 243 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [0.01, 0.54] |
| 4.17 trimipramine | 1 | 34 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.42, 0.93] |
| 4.18 viloxazine | 1 | 48 | Std. Mean Difference (IV, Random, 95% CI) | 0.38 [‐0.19, 0.95] |
| 4.19 combination | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
3.3. Analysis.
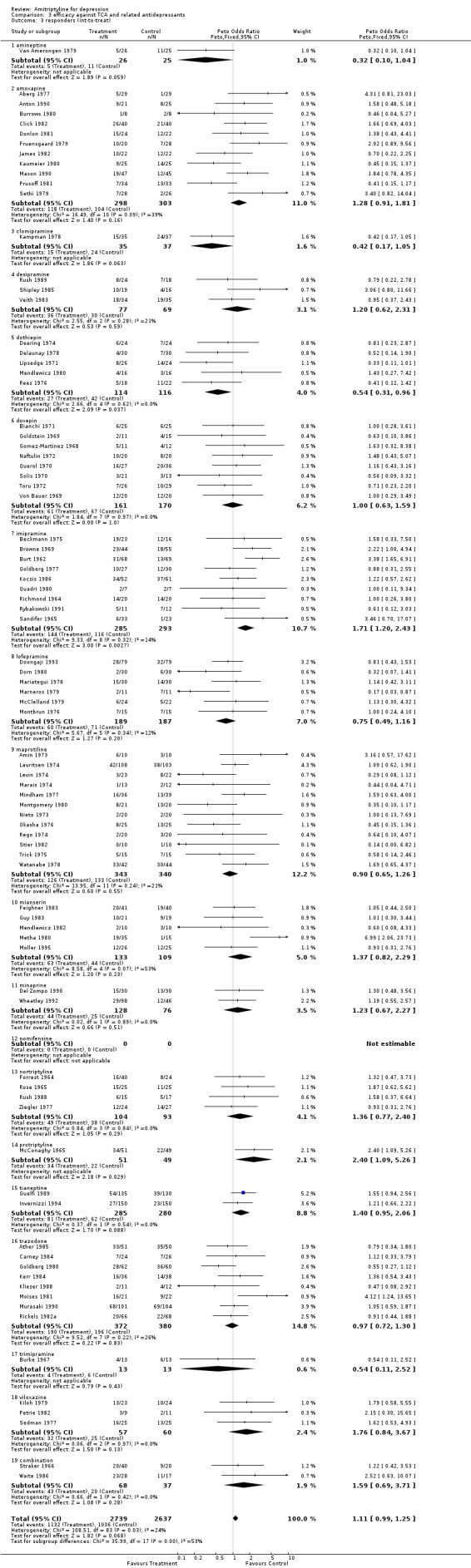
Comparison 3 efficacy against TCA and related antidepressants, Outcome 3 responders (int‐to‐treat).
3.4. Analysis.
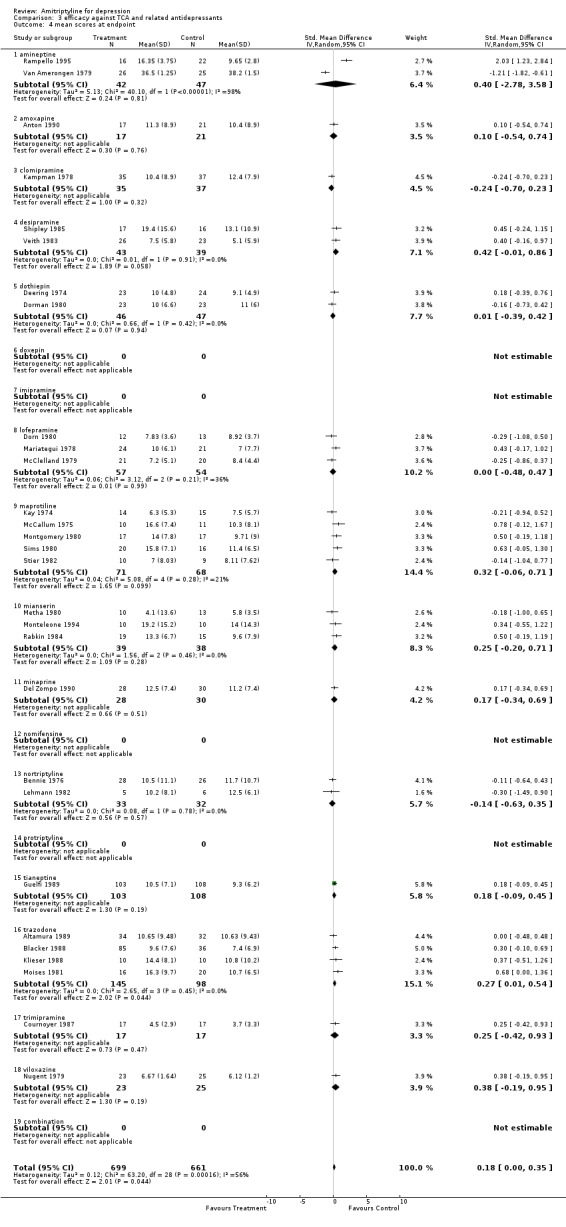
Comparison 3 efficacy against TCA and related antidepressants, Outcome 4 mean scores at endpoint.
Comparison 4. tolerability against TCA and related antidepressants.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3 drop‐outs | 128 | 9156 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.07 [0.96, 1.19] |
| 3.1 amineptine | 2 | 95 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.14 [0.38, 3.39] |
| 3.2 amoxapine | 13 | 722 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.09 [0.77, 1.54] |
| 3.3 clomipramine | 3 | 299 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.52 [0.95, 2.41] |
| 3.4 desipramine | 4 | 156 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.72 [0.28, 1.84] |
| 3.5 dothiepin | 8 | 396 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.68 [0.32, 1.47] |
| 3.6 doxepin | 13 | 860 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.84 [0.60, 1.17] |
| 3.7 imipramine | 6 | 349 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.34 [0.71, 2.51] |
| 3.8 lofepramine | 8 | 470 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.85 [0.55, 1.32] |
| 3.9 maprotiline | 18 | 1107 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.97 [0.69, 1.35] |
| 3.10 mianserin | 12 | 787 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.27 [0.91, 1.77] |
| 3.11 minaprine | 3 | 735 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.30 [0.89, 1.90] |
| 3.12 nomifensine | 1 | 29 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.69 [0.04, 12.15] |
| 3.13 nortriptyline | 8 | 467 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.01 [0.56, 1.83] |
| 3.14 protriptyline | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.15 tianeptine | 3 | 694 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.28 [0.89, 1.85] |
| 3.16 trazodone | 11 | 924 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.12 [0.82, 1.51] |
| 3.17 trimipramine | 2 | 115 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.76 [0.30, 1.97] |
| 3.18 viloxazine | 7 | 288 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.32 [0.69, 2.55] |
| 3.19 combination | 6 | 663 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.84 [0.55, 1.28] |
| 4 patients with side‐effects | 42 | 3310 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.67 [0.58, 0.78] |
| 4.1 amineptine | 1 | 51 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.46 [0.14, 1.49] |
| 4.2 amoxapine | 3 | 192 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.77 [0.41, 1.44] |
| 4.3 clomipramine | 1 | 72 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.38 [0.55, 3.47] |
| 4.4 desipramine | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.5 dothiepin | 5 | 234 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.51 [0.29, 0.88] |
| 4.6 doxepin | 4 | 168 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.77 [0.39, 1.50] |
| 4.7 imipramine | 2 | 156 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.73 [0.35, 1.50] |
| 4.8 lofepramine | 2 | 62 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.39 [0.14, 1.08] |
| 4.9 maprotiline | 7 | 436 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.59 [0.40, 0.88] |
| 4.10 mianserin | 3 | 266 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.34 [0.20, 0.57] |
| 4.11 minaprine | 3 | 702 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.55 [0.39, 0.80] |
| 4.12 nomifensine | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.13 nortriptyline | 4 | 226 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.51 [0.30, 0.87] |
| 4.14 protriptyline | 1 | 100 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.41 [0.60, 3.33] |
| 4.15 tianeptine | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.16 trazodone | 3 | 428 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.27 [0.87, 1.87] |
| 4.17 trimipramine | 1 | 41 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.51 [0.11, 2.36] |
| 4.18 viloxazine | 1 | 41 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.05 [0.27, 4.12] |
| 4.19 combination | 1 | 135 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.79 [0.34, 1.86] |
4.3. Analysis.
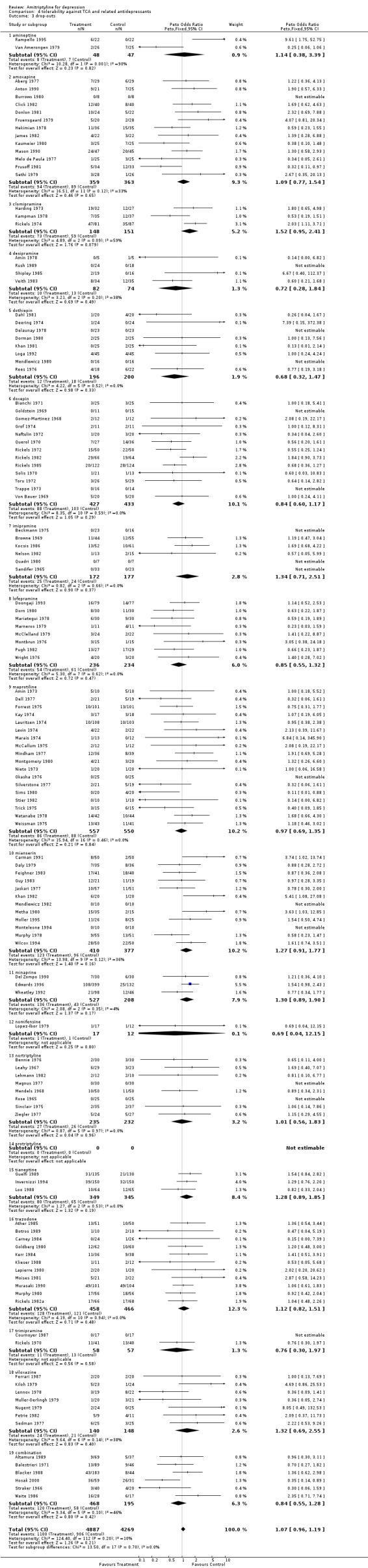
Comparison 4 tolerability against TCA and related antidepressants, Outcome 3 drop‐outs.
4.4. Analysis.
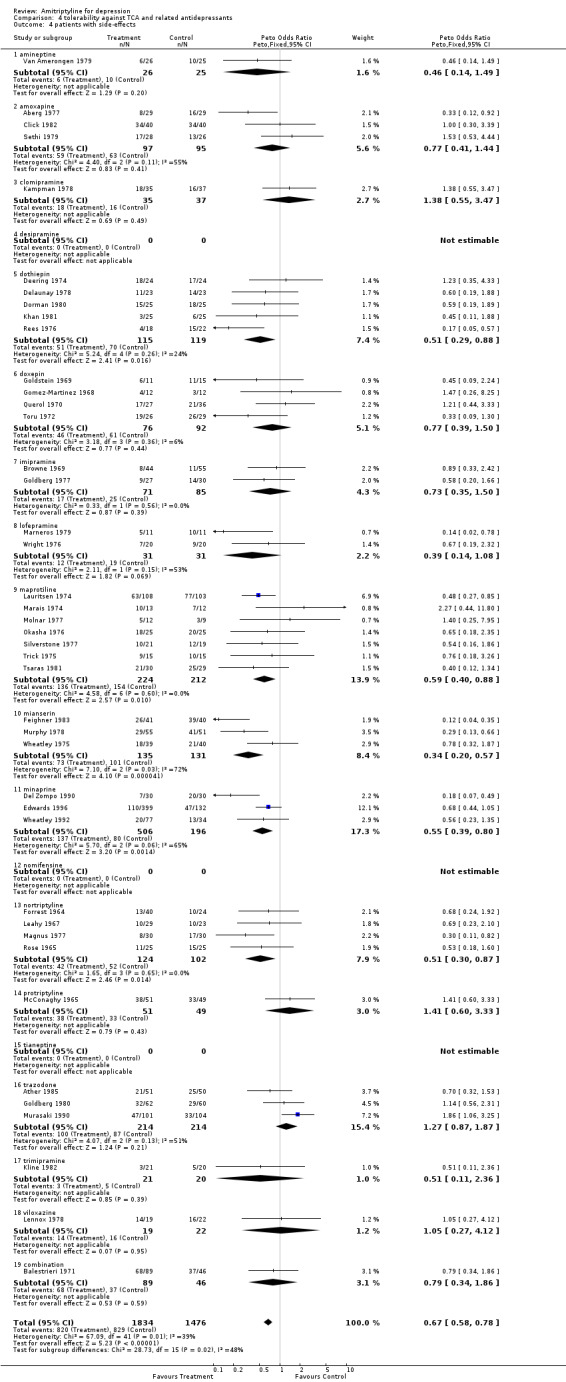
Comparison 4 tolerability against TCA and related antidepressants, Outcome 4 patients with side‐effects.
Comparison 5. efficacy against SSRIs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3 responders (int‐to‐treat) | 20 | 2046 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.14 [0.95, 1.36] |
| 3.20 fluoxetine | 7 | 490 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.89 [0.61, 1.29] |
| 3.21 fluvoxamine | 1 | 69 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.13 [0.83, 5.46] |
| 3.22 sertraline | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.23 paroxetine | 10 | 1071 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.23 [0.96, 1.58] |
| 3.24 citalopram | 2 | 416 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.11 [0.76, 1.63] |
| 4 mean scores at endpoint | 24 | 2659 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.01, 0.19] |
| 4.20 fluoxetine | 11 | 859 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.01, 0.26] |
| 4.21 fluvoxamine | 2 | 83 | Std. Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.41, 0.99] |
| 4.22 sertraline | 2 | 347 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.10, 0.32] |
| 4.23 paroxetine | 8 | 1326 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.13, 0.25] |
| 4.24 citalopram | 1 | 44 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.67, 0.52] |
5.3. Analysis.
Comparison 5 efficacy against SSRIs, Outcome 3 responders (int‐to‐treat).
5.4. Analysis.
Comparison 5 efficacy against SSRIs, Outcome 4 mean scores at endpoint.
Comparison 6. tolerability against SSRIs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 drop‐outs | 46 | 5770 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.84 [0.75, 0.95] |
| 1.20 fluoxetine | 17 | 1290 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.78 [0.60, 1.01] |
| 1.21 fluvoxamine | 3 | 158 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.84 [0.39, 1.83] |
| 1.22 sertraline | 8 | 1579 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.97 [0.78, 1.21] |
| 1.23 paroxetine | 15 | 2283 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.84 [0.69, 1.01] |
| 1.24 citalopram | 3 | 460 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.64 [0.42, 0.96] |
| 2 patients with side‐effects | 12 | 2155 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.65 [0.55, 0.78] |
| 2.20 fluoxetine | 1 | 41 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.20 [0.06, 0.66] |
| 2.21 fluvoxamine | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.22 sertraline | 3 | 468 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.87 [0.60, 1.27] |
| 2.23 paroxetine | 6 | 1230 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.69 [0.55, 0.87] |
| 2.24 citalopram | 2 | 416 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.43 [0.28, 0.66] |
6.1. Analysis.
Comparison 6 tolerability against SSRIs, Outcome 1 drop‐outs.
6.2. Analysis.
Comparison 6 tolerability against SSRIs, Outcome 2 patients with side‐effects.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aberg 1977.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: presence of depression, HMD 25+ and Beck 12+ Age: 49 mean Country: Sweden Setting: inpatients | |
| Interventions | amoxapine versus amitriptyline | |
| Outcomes | responders dropouts patients with side‐effects | |
| Notes | quality rating: 22 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Altamura 1989.
| Methods | Double blind RCT Active treatment: 5 weeks | |
| Participants | Inclusion criteria: DSM III major depression, HMD 17+ Age: 60‐83 Country: Italy Setting: inpatients | |
| Interventions | mianserin versus trazodone versus amitriptyline | |
| Outcomes | mean score at endpoint responders drop‐outs | |
| Notes | quality rating: 17 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Altamura 1989a.
| Methods | Double blind RCT Active treatment: 5 weeks | |
| Participants | Inclusion criteria: DSM III major depression, HMD 18+ Age: 65+ Country: Italy Setting: inpatients | |
| Interventions | fluoxetine versus amitriptyline | |
| Outcomes | dropouts | |
| Notes | quality rating: 16 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Amin 1973.
| Methods | Double blind RCT Active treatment: 8 weeks | |
| Participants | Inclusion criteria: patients with depression Age: 19‐62 Country: Canada Setting: inpatients | |
| Interventions | maprotiline versus amitriptyline | |
| Outcomes | responders dropouts | |
| Notes | quality rating: 17 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Amin 1978.
| Methods | Double blind RCT Active treatment: 3 weeks | |
| Participants | Inclusion criteria: endogenous and neurotic depression Age: 24‐56 Country: Canada Setting: outpatients | |
| Interventions | desipramine versus amitriptyline | |
| Outcomes | dropouts | |
| Notes | quality rating: 13 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Anderson 1972.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: anxiety and mild depression, 25+ on a symptom checklist Age: not clear Country: UK Setting: outpatients | |
| Interventions | nortriptyline+fluphenazine versus amitriptyline+perphenazine | |
| Outcomes | mean score at endpoint | |
| Notes | quality rating: 16 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Anton 1990.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: DSM III major depression with psychotic features | |
| Interventions | amoxapine versus amitriptyline+perphenazine | |
| Outcomes | responders mean score at endpoint drop‐outs | |
| Notes | quality rating: 22 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Ather 1985.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: primary diagnosis of depression, HMD 14+ Age: 59+ Country: UK Setting: in and outpatients | |
| Interventions | trazodone versus amitriptyline | |
| Outcomes | responders drop‐outs patients with side‐effects | |
| Notes | quality rating: 22 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Balestrieri 1971.
| Methods | Double blind RCT Active treatment: 3 weeks | |
| Participants | Inclusion criteria: patients suitable for antidepressant treatment Age: 52.9 mean Country: Italy Setting: inpatients | |
| Interventions | imipramine versus maprotiline versus amitriptyline | |
| Outcomes | drop‐outs patients with side‐effects | |
| Notes | quality rating: 20 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Bascara 1989.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: DSM III R major depression, HMD 18+ Age: 33 average Country: Philipines Setting: not clear | |
| Interventions | paroxetine versus amitriptyline | |
| Outcomes | responders dropouts | |
| Notes | quality rating: 16 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Battegay 1985.
| Methods | Double blind RCT Active treatment: 7 weeks | |
| Participants | Inclusion criteria: DSM III endogenous and reactive depression, HMD 20+ Age: 18‐60 Country: Switzerland Setting: outpatients | |
| Interventions | paroxetine versus amitriptyline | |
| Outcomes | responders dropouts | |
| Notes | quality rating: 19 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Beaini 1980.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: primary depressive illness Age: less than 75 Country: UK Setting: not clear | |
| Interventions | imipramine versus amitriptyline | |
| Outcomes | responders | |
| Notes | quality rating: 13 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Beckmann 1975.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: Feighner primary affective disorder, unipolar Age: 20‐72 Country: US Setting: inpatients | |
| Interventions | imipramine versus amitriptyline | |
| Outcomes | responders drop‐outs | |
| Notes | quality rating: 9 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Bennie 1976.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: patients with anxiety‐depressive states Age: 18‐65 Country: UK Setting: outpatients | |
| Interventions | nortriptyline/fluphenazine versus amitriptyline | |
| Outcomes | mean score at endpoint dropouts | |
| Notes | quality rating: 16 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Bersani 1994.
| Methods | Double blind RCT Active treatment: 8 weeks | |
| Participants | Inclusion criteria: DSM III R major depression, HMD 22+ Age: 21‐69 Country: Italy Setting: outpatients | |
| Interventions | sertraline versus amitriptyline | |
| Outcomes | mean score at endpoint dropouts patients with side‐effects | |
| Notes | quality rating: 15 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Bianchi 1971.
| Methods | Double blind RCT Active treatment: 5 weeks | |
| Participants | Inclusion criteria: Slater and Roth criteria of neurotic and endogenous depression Age: 50 mean Country: Australia Setting: in and outpatients | |
| Interventions | doxepin versus amitriptyline | |
| Outcomes | responders dropouts | |
| Notes | quality rating: 15 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Bignamini 1992.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: DSM III major depression, HMD 18+ Age: 18‐70 Country: Italy Setting: outpatients | |
| Interventions | paroxetine versus amitriptyline | |
| Outcomes | responders dropouts | |
| Notes | quality rating: 19 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Blacker 1988.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: DSM III major depression, HMD 17+ Age: 18‐65 Country: UK Setting: family practice | |
| Interventions | dothiepin versus mianserin versus trazodone versus amitriptyline | |
| Outcomes | mean score at endpoint (trazodone‐amitriptyline) drop‐outs (combination‐amitriptyline) | |
| Notes | quality rating: 23 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Botros 1989.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: primary depressive illness, HMD 17+ Age: 18‐80 Country: UK Setting: outpatients | |
| Interventions | trazodone versus amitriptyline | |
| Outcomes | dropouts | |
| Notes | quality rating: 18 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Browne 1969.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: depressive illness Age: 40‐69 Country: UK Setting: in and outpatients | |
| Interventions | imipramine versus amitriptyline+perphenazine | |
| Outcomes | responders dropouts patients with side‐effects | |
| Notes | quality rating: 16 scale not validated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Burke 1967.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: depressive syndrome requiring hospitalisation Age: 20+ Country: Australia Setting: inpatients | |
| Interventions | trimipramine versus amitriptyline | |
| Outcomes | responders | |
| Notes | quality rating: 16 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Burrows 1980.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: moderate to severe depression requiring hospitalisation Age: 16+ Country: Australia Setting: inpatients | |
| Interventions | amoxapine versus amitriptyline | |
| Outcomes | responders dropouts | |
| Notes | quality rating: 17 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Burt 1962.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: female hospitalised patients with "primary affective alteration" Age: 30‐70 Country: Australia Setting: inpatients | |
| Interventions | imipramine versus amitriptyline | |
| Outcomes | responders | |
| Notes | quality rating: 20 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Byrne 1989.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: endogenous depression, HMD 23+ Age: 18‐65 Country: Belgium, France, UK Setting: inpatients | |
| Interventions | paroxetine versus amitriptyline | |
| Outcomes | dropouts | |
| Notes | quality rating: 17 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Carman 1991.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: DSM III major depression, HMD 17+ Age: 18+ Country: US Setting: outpatients | |
| Interventions | mianserin versus amitriptyline | |
| Outcomes | dropouts | |
| Notes | quality rating: 20 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Carney 1984.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: depression, HMD 14+ Age: not clear Country: Ireland Setting: in and outpatients | |
| Interventions | trazodone versus amitriptyline | |
| Outcomes | responders drop‐outs | |
| Notes | quality rating: 13 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Chouinard 1985.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: RDC major depressive disorder, HMD 20+, Raskin greater than Covi Age: 24‐59 Country: Canada Setting: outpatients | |
| Interventions | fluoxetine versus amitriptyline | |
| Outcomes | dropouts | |
| Notes | quality rating: 20 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Christiansen 1996.
| Methods | Double blind RCT Active treatment: 8 weeks | |
| Participants | Inclusion criteria: patients with depression, HMD 15+ Age: 18‐65 Country: Denmark Setting: family practice | |
| Interventions | paroxetine versus amitriptyline | |
| Outcomes | responders mean score at endpoint dropouts | |
| Notes | quality rating: 21 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Click 1982.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: depressive patients, HMD 25+, Raskin 8+, Zung 50+ Age: 18‐60 Country: US Setting: outpatients | |
| Interventions | amoxapine versus amitriptyline | |
| Outcomes | responders dropouts patients with side‐effects | |
| Notes | quality rating: 15 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Cohn 1990.
| Methods | Double blind RCT Active treatment: 8 weeks | |
| Participants | Inclusion criteria: DSM III R major depressive episode, HMD 18+, Raskin greater than Covi Age: 65+ Country: US Setting: outpatients | |
| Interventions | sertraline versus amitriptyline | |
| Outcomes | dropouts | |
| Notes | quality rating: 16 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Cournoyer 1987.
| Methods | Double blind RCT Active treatment: 3 weeks | |
| Participants | Inclusion criteria: DSM III and RDC criteria major depressive episode, unipolar and bipolar, HMD 20+ Age: 26‐72 Country: Canada Setting: inpatients | |
| Interventions | trimipramine versus amitriptyline | |
| Outcomes | mean score at endpoint dropouts | |
| Notes | quality rating: 18 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Dahl 1981.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: depressive disorder, 'masked depression' Age: 20‐68 Country: Sweden Setting: family practice | |
| Interventions | dothiepin versus amitriptyline | |
| Outcomes | dropouts | |
| Notes | quality rating: 16 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Daly 1979.
| Methods | Double blind RCT Active treatment: 3 weeks | |
| Participants | Inclusion criteria: depression Age: 18‐65 Country: Ireland Setting: inpatients | |
| Interventions | mianserin versus amitriptyline | |
| Outcomes | dropouts | |
| Notes | quality rating: 15 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
De La Barquera 1998.
| Methods | Double‐blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: DSM‐III‐R major depressive disorder, HMD‐21 18+ Age: 18‐65 Country: Mexico Setting: outpatients | |
| Interventions | fluoxetine versus amitriptyline | |
| Outcomes | responders mean score at endpoint dropouts | |
| Notes | quality rating: 22 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
De Ronchi 1998.
| Methods | Double blind RCT Active treatment: 10 weeks | |
| Participants | Inclusion criteria: DSM III R major depressive disorder, HMD 16+ Age: 60+ Country: Italy Setting: inpatients and outpatients | |
| Interventions | fluoxetine versus amitriptyline | |
| Outcomes | mean score at endpoint dropouts | |
| Notes | quality rating: 25 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Deering 1974.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: 'change of mood which exceeded the normal variation in mood by virtue of its severity and duration' Age: 40 average Country: UK Setting: family practice | |
| Interventions | dothiepin versus amitriptyline | |
| Outcomes | responders mean score at endpoint dropouts patients with side‐effects | |
| Notes | quality rating: 19 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Del Zompo 1990.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: DSM III major depression, HMD 16+ Age: 47 mean Country: Italy Setting: outpatients | |
| Interventions | minaprine versus amitriptyline | |
| Outcomes | responders dropouts patients with side‐effects | |
| Notes | quality rating: 23 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Delaunay 1978.
| Methods | Double blind: not clear RCT, allocation concealment may be inadequate Active treatment: 3 weeks | |
| Participants | Inclusion criteria: patients who need antidepressant treatment Age: 18‐75 Country:France Setting: outpatients | |
| Interventions | dosulepine versus amitriptyline | |
| Outcomes | responders dropouts patients with side‐effects | |
| Notes | quality rating: 20 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Dell 1977.
| Methods | Double blind RCT Active treatment: 3 weeks | |
| Participants | Inclusion criteria: depression of sufficient severity as to warrant treatment with antidepressant drugs Age: 45 average Setting: family practice | |
| Interventions | maprotiline versus amitriptyline | |
| Outcomes | dropouts | |
| Notes | quality rating: 19 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Demyttenaere 1998.
| Methods | Double blind RCT Active treatment: 9 weeks | |
| Participants | Inclusion criteria: DSM III R major depression, unipolar, HMD 15+ Age: 18‐60 Country: Belgium Setting: outpatients | |
| Interventions | fluoxetine versus amitriptyline | |
| Outcomes | responders mean score at endpoint dropouts | |
| Notes | quality rating: 20 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Donlon 1981.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: RDC endogenous depression, HMD 25+, Raskin 8+, Zung 50+ Age: 18‐60 Country: US Setting: outpatients | |
| Interventions | amoxapine versus amitriptyline | |
| Outcomes | responders dropouts | |
| Notes | quality rating: 19 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Doongaji 1993.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: DSM III major depression, HMD 20+, CGI 4+ Age: 20‐65 Country: India Setting: in and outpatients | |
| Interventions | lofepramine versus amitriptyline | |
| Outcomes | responders dropouts | |
| Notes | quality rating: 24 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Dorman 1980.
| Methods | Double blind RCT Active treatment: 5 weeks | |
| Participants | Inclusion criteria: patients whose condition was judged appropriate for antidepressant treatment Age: 18‐60 Country: UK Setting: outpatients | |
| Interventions | dothiepin versus amitriptyline | |
| Outcomes | mean scores at endpoint dropouts patients with side‐effects | |
| Notes | quality rating: 21 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Dorn 1980.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: patients with depression Age: 65+ Country: Germany Setting: outpatients | |
| Interventions | lofepramine versus amitriptyline | |
| Outcomes | responders mean score at endpoint dropouts | |
| Notes | quality rating: 17 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Edwards 1996.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: DSM IIIR depression, HMD 17+ Age: 18‐70 Country: UK, Ireland Setting: outpatients | |
| Interventions | minaprine versus amitriptyline | |
| Outcomes | dropouts patients with side‐effects | |
| Notes | quality rating: 32 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Fawcett 1989.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: DSM III major depression, unipolar, HMD 20+ Age: 18+ Country: US Setting: outpatients | |
| Interventions | fluoxetine versus amitriptyline | |
| Outcomes | responders mean score at endpoint dropouts | |
| Notes | quality rating: 21 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Feighner 1983.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: Feighner and RDC criteria for major depressive disorder, HMD 19+ Age: 40 average Country: US Setting: outpatients | |
| Interventions | mianserin versus amitriptyline | |
| Outcomes | responders dropouts patients with side‐effects | |
| Notes | quality rating: 18 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Ferrari 1987.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: DSM III major depression, without psychotic features Age: 18‐70 Country: Italy Setting: inpatients | |
| Interventions | viloxazine versus amitriptyline | |
| Outcomes | dropouts | |
| Notes | quality rating: 17 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Forrest 1964.
| Methods | RCT, not blind Active treatment: 2 weeks | |
| Participants | Inclusion criteria: depressive patients Age:20‐80 Country: UK Setting: in and outpatients | |
| Interventions | nortriptyline versus amitriptyline | |
| Outcomes | responders patients with side‐effects | |
| Notes | quality rating: 14 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Forrest 1975.
| Methods | Double blind RCT Active treatment: 3 weeks | |
| Participants | Inclusion criteria: patients suitable for antidepressant treatment, reactive depression Age: up to 60 Country: UK Setting: family practice | |
| Interventions | maprotiline versus amitriptyline | |
| Outcomes | dropouts | |
| Notes | quality rating: 13 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Freed 1999.
| Methods | Double blind RCT Active treatment: 9 weeks | |
| Participants | Inclusion criteria: patients with MADRS 20+ Age: 19‐85 Country: Australia Setting: family practice | |
| Interventions | paroxetine versus amitriptyline | |
| Outcomes | dropouts | |
| Notes | quality rating: 28 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Fruensgaard 1979.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: endogenous depression Age: 22‐71 Country: Denmark Setting: inpatients | |
| Interventions | amoxapine versus amitriptyline | |
| Outcomes | responders drop‐outs | |
| Notes | quality rating: 13 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Gasperini 1992.
| Methods | Double blind RCT Active treatment: 7 weeks | |
| Participants | Inclusion criteria: DSM III R major depressive episode Age: 51 average Country: Italy Setting: inpatients | |
| Interventions | fluvoxamine versus amitriptyline | |
| Outcomes | dropouts | |
| Notes | quality rating: 17 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Geretsegger 1995.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: DSM III R major depressive episode, HMD 18+ Age: 65+ Country: Germany and Austria Setting: inpatients for at least three weeks | |
| Interventions | paroxetine versus amitriptyline | |
| Outcomes | responders dropouts patients with side‐effects | |
| Notes | quality rating: 19 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Goldberg 1977.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: neurotic depression, HMD 20+, Raskin 7+ Age: 37.8 average Country: US Setting: outpatients | |
| Interventions | imipramine versus amitriptyline | |
| Outcomes | responders patients with side‐effects | |
| Notes | quality rating: 20 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Goldberg 1980.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: New York University criteria of neurotic depression, HMD 18+, Raskin 7+ Age: 18‐60 Country: US Setting: outpatients | |
| Interventions | trazodone versus amitriptyline | |
| Outcomes | responders dropouts patients with side‐effects | |
| Notes | quality rating: 19 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Goldstein 1969.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: two or more target symptoms of anxiety and depression of at least moderate severity Age: 21+ Country: US Setting: outpatients | |
| Interventions | doxepin versus amitriptyline | |
| Outcomes | responders dropouts patients with side‐effects | |
| Notes | quality rating: 15 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Gomez‐Martinez 1968.
| Methods | Double blind RCT, allocation concealment may be inadequate Active treatment: 8 weeks | |
| Participants | Inclusion criteria: depressive state Age: 23‐67 Country: Spain Setting: not clear | |
| Interventions | doxepin versus amitriptyline | |
| Outcomes | responders dropouts patients with side‐effects | |
| Notes | quality rating: 12 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Gravem 1987.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: patients with severe depression Age: 19‐74 Country: Norway Setting: in and outpatients | |
| Interventions | citalopram versus amitriptyline | |
| Outcomes | responders dropouts patients with side‐effects | |
| Notes | quality rating: 20 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Grof 1974.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: Robins criteria of primary depression Age: 25‐65 Country: Canada Setting: in and outpatients | |
| Interventions | doxepin versus amitriptyline | |
| Outcomes | dropouts | |
| Notes | quality rating: 16 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Grof 1977.
| Methods | Double blind RCT Active treatment: 8 weeks | |
| Participants | Inclusion criteria: Feighner criteria of primary depression, HMD 15+ Age: 20‐65 Country: Canada Setting: outpatients | |
| Interventions | nomifensine versus amitriptyline | |
| Outcomes | responders | |
| Notes | quality rating: 16 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Guelfi 1989.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: DSM III dysthymic disorder, MADRS 20+, FDA criteria for anxiety Age: 20‐65 Country: France Setting: outpatients | |
| Interventions | tianeptine versus amitriptyline | |
| Outcomes | responders mean score at endpoint dropouts | |
| Notes | quality rating: 27 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Guy 1983.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: RDC major, minor, intermittent depressive disorder, HMD 19+ Age: 18‐65 Country: US Setting: inpatients | |
| Interventions | mianserin versus amitriptyline | |
| Outcomes | responders dropouts | |
| Notes | quality rating: 24 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Harding 1973.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: primary depression, African or predominantly African origin Age: 15‐70 Country: Jamaica Setting: outpatients | |
| Interventions | clomipramine versus amitriptyline | |
| Outcomes | dropouts | |
| Notes | quality rating: 16 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Harris 1991.
| Methods | Double blind RCT Active treatment: 6 months | |
| Participants | Inclusion criteria: DSM III major depressive episode, HMD 17+ Age: 18‐65 Country: UK Setting: outpatients | |
| Interventions | fluvoxamine versus amitriptyline | |
| Outcomes | responders dropouts | |
| Notes | quality rating: 18 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Hegerl 1997.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: DSM‐III‐R major depressive episode Age: unclear Country: Germany and Hungary Setting: inpatients | |
| Interventions | sertraline versus amitriptyline | |
| Outcomes | dropouts | |
| Notes | quality rating: 14 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Hekimian 1978.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: moderate to severe depressive illness, Raskin 8+ Age: 18‐65 Country: US Setting: outpatients | |
| Interventions | amoxapine versus amitriptyline | |
| Outcomes | dropouts | |
| Notes | quality rating: 20 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Hosak 2000.
| Methods | No blind RCT Active treatment: 6 months | |
| Participants | Inclusion criteria: ICD‐10 depressive episode Age: 44.5 mean Country: Czech Republic Setting: outpatients | |
| Interventions | citalopram versus fluoxetine versus amitriptyline | |
| Outcomes | dropouts | |
| Notes | quality rating:20 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Hutchinson 1992.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: DSM III major depressive episode, HMD 18+ Age: 65+ Country:UK Setting: family practice | |
| Interventions | paroxetine versus amitriptyline | |
| Outcomes | responders dropouts patients with side‐effects | |
| Notes | quality rating: 20 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Invernizzi 1994.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: DSM III depressive disorder, HMD 18+ Age: 18‐70 Country: Italy Setting: in and outpatients | |
| Interventions | tianeptine versus amitriptyline | |
| Outcomes | responders dropouts | |
| Notes | quality rating: 24 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
James 1982.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: primary diagnosis of depression requiring antidepressants, HMD 25+, Beck 12+ Age: 16‐65 Country: Ireland Setting: inpatients | |
| Interventions | amoxapine versus amitriptyline | |
| Outcomes | responders dropouts | |
| Notes | quality rating: 19 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Jaskari 1977.
| Methods | Double blind RCT Active treatment: 3 weeks | |
| Participants | Inclusion criteria: depressive illness Age: 17‐64 Country: Finland Setting: inpatients | |
| Interventions | mianserin versus amitriptyline | |
| Outcomes | dropouts | |
| Notes | quality rating: 16 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Jessel 1981.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: depressive symptomatology in female patients Age: 70 average Country: Germany Setting: inpatients | |
| Interventions | lofepramine versus amitriptyline | |
| Outcomes | responders | |
| Notes | quality rating: 17 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Judd 1993.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: DSM III R major depressive disorder, HMD 17+ Age: 21‐63 Country: Australia Setting: in and outpatients | |
| Interventions | fluoxetine versus amitriptyline | |
| Outcomes | mean score at endpoint dropouts | |
| Notes | quality rating: 20 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Kamijima 1997.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: DSM IV Major Depression, 16+ HMD Age: not clear Country: Japan Setting: in and outpatients | |
| Interventions | sertraline versus amitriptyline | |
| Outcomes | dropouts | |
| Notes | quality rating: 17 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Kampman 1978.
| Methods | Double blind RCT Active treatment: 8 weeks | |
| Participants | Inclusion criteria: clinical depression Age: 18‐66 Country: Finland Setting: outpatients | |
| Interventions | clomipramine versus amitriptyline | |
| Outcomes | responders mean score at endpoint dropouts patients with side‐effects | |
| Notes | quality rating: 21 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Kaumeier 1980.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: depressive illness, HMD 25+, Beck 12+ Age: 50 average Country: Germany Setting: inpatients | |
| Interventions | amoxapine versus amitriptyline | |
| Outcomes | responders dropouts | |
| Notes | quality rating: 20 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Kay 1974.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: depressed women with persistent depressive symptoms of more than one month's duration Age: 18+ Country: Australia Setting: family practice | |
| Interventions | maprotiline versus amitriptyline | |
| Outcomes | mean score at endpoint dropouts | |
| Notes | quality rating: 15 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Keegan 1991.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: DSM III or DIS major depression, unipolar, HMD 20+, Raskin greater than Covi Age: 18‐70 Country: Canada Setting: not clear | |
| Interventions | fluoxetine versus amitriptyline | |
| Outcomes | responders dropout | |
| Notes | quality rating: 20 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Kerr 1984.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: ICD IX primary depressive illness Age: 21‐77 Country: UK Setting: inpatients | |
| Interventions | trazodone versus amitriptyline | |
| Outcomes | responders dropouts | |
| Notes | quality rating: 15 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Khan 1981.
| Methods | Single blind RCT (doctors blind to treatments) Active treatment: 4 weeks | |
| Participants | Inclusion criteria: depressive illness requiring antidepressants Age: 60+ Country: UK Setting: outpatients | |
| Interventions | dothiepin versus amitriptyline | |
| Outcomes | dropouts patients with side‐effects | |
| Notes | quality rating: 14 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Khan 1982.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: severe depressive illness with suicidal ideation Age: 18‐65 Country: UK Setting: inpatients | |
| Interventions | mianserin versus amitriptyline | |
| Outcomes | dropouts | |
| Notes | quality rating: 15 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Kiloh 1979.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: endogenous depression as defined by Slater and Roth, HMD 14‐37 Age: not clear, adults Country: Australia Setting: inpatients and outpatients | |
| Interventions | viloxazine versus amitriptyline | |
| Outcomes | responders dropouts | |
| Notes | quality rating: 15 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Klieser 1988.
| Methods | Double blind RCT Active treatment: 3 weeks | |
| Participants | Inclusion criteria: DSM III major depressive disorder Age: 40 average Country: Germany Setting: inpatients | |
| Interventions | trazodone versus amitriptyline | |
| Outcomes | responders dropouts | |
| Notes | quality rating: 15 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Kline 1982.
| Methods | RCT, but extra patients were placed in case of dropouts Not blind Active treatment: 4 weeks | |
| Participants | Inclusion criteria: neurotic and manic‐depressive patients Age: 21+ Country: US Setting: outpatients | |
| Interventions | trimipramine versus amitriptyline | |
| Outcomes | patients with side‐effects | |
| Notes | quality rating: 11 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Kocsis 1986.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: SADS and RCD diagnosis of major depressive disorder, unipolar and bipolar Age: adults Country: US Setting: inpatients | |
| Interventions | imipramine versus amitriptyline | |
| Outcomes | responders dropouts | |
| Notes | quality rating: 18 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Kuhs 1989.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: DSM III major depression, HMD 18+ Age: 18‐65 Country: Germany Setting: inpatients | |
| Interventions | paroxetine versus amitriptyline | |
| Outcomes | mean score at endpoint dropouts | |
| Notes | quality rating: 14 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Kyle 1998.
| Methods | Double blind RCT Active treatment: 8 weeks | |
| Participants | Inclusion criteria: DSM III R major depression, MADRS 22+, MMSE 24+ Age: 65+ Country: UK Setting: family practice | |
| Interventions | citalopram versus amitriptyline | |
| Outcomes | responders dropouts patients with side‐effects | |
| Notes | quality rating: 27 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Laakmann 1988.
| Methods | Double blind RCT Active treatment: 5 weeks | |
| Participants | Inclusion criteria: depressive syndromes, HMD 17+, Raskin 8+ Age: 19‐74 Country: Germany Setting: outpatients | |
| Interventions | fluoxetine versus amitriptyline | |
| Outcomes | mean score at endpoint dropouts | |
| Notes | quality rating: 20 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Laakmann 1991.
| Methods | Double blind RCT Active treatment: 5 weeks | |
| Participants | Inclusion criteria: depressive patients, HMD 17+, Raskin 8+ Age: 19‐74 Country: Germany Setting: outpatients | |
| Interventions | fluoxetine versus amitriptyline | |
| Outcomes | mean score at endpoint dropouts | |
| Notes | quality rating: 20 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Lapierre 1980.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: Feighner criteria of primary affective disorder, agitated and retarded depression Age: 18‐55 Country: Canada Setting: in and outpatients | |
| Interventions | trazodone versus amitriptyline | |
| Outcomes | dropouts | |
| Notes | quality rating: 12 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Lauritsen 1974.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: patients requiring antidepressants Age: 16+ Country: Sweden, Finland, Holland, Denmark Setting: inpatients | |
| Interventions | maprotiline versus amitriptyline | |
| Outcomes | responders dropouts patients with side‐effects | |
| Notes | quality rating: 17 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Laursen 1985.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: ICD 8 Manic‐depressive psychosis, HMD 15+ Age: 35‐81 Country: Denmark Setting: inpatients with outpatient followup | |
| Interventions | paroxetine versus amitriptyline | |
| Outcomes | responders mean score at endpoint dropouts | |
| Notes | quality rating: 19 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Leahy 1967.
| Methods | Double blind RCT Active treatment: 3 months | |
| Participants | Inclusion criteria: marked and persistent mood depression of at least 2 weeks' duration Age: 25‐70 Country: UK Setting: inpatients | |
| Interventions | nortriptyline versus amitriptyline | |
| Outcomes | dropouts patients with side‐effects | |
| Notes | quality rating: 12 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Lehmann 1982.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: RDC major unipolar depression, HMD 20+ Age: 18‐69 Country: US Setting: inpatients | |
| Interventions | nortriptyline versus amitriptyline | |
| Outcomes | mean score at endpoint dropouts | |
| Notes | quality rating: 16 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Lennox 1978.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: depressed patients requiring antidepressants Age: 18‐68 Country: UK Setting: family practice | |
| Interventions | viloxazine versus amitriptyline | |
| Outcomes | dropouts patients with side‐effects | |
| Notes | quality rating: 18 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Levin 1974.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: patients with depressive illness requiring antidepressants Age: not clear Country: South Africa Setting: inpatients and outpatients | |
| Interventions | maprotiline versus amitriptyline | |
| Outcomes | responders dropouts | |
| Notes | quality rating: 12 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Lipsedge 1971.
| Methods | Double blind RCT Active treatment: 3 months | |
| Participants | Inclusion criteria: primary depressive illness with anxiety Age: 16‐73 Country: UK Setting: outpatients | |
| Interventions | dothiepin versus amitriptyline | |
| Outcomes | responders | |
| Notes | quality rating: 16 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Loga 1992.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: DSM III R recurrent major depression, bipolar depression, depressive neurosis Age: 18‐70 Country: Yugoslavia Setting: inpatients | |
| Interventions | dothiepin versus amitriptyline | |
| Outcomes | dropouts | |
| Notes | quality rating: 19 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Loo 1988.
| Methods | Double blind RCT Active treatment: 8 weeks | |
| Participants | Inclusion criteria: DSM III depressive syndrome or dysthymic disorder and chronic alcoholism, MADRS 20+ Age: 38 average Country: France Setting: in and outpatients | |
| Interventions | tianeptine versus amitriptyline | |
| Outcomes | dropouts | |
| Notes | quality rating: 21 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Lopez‐Ibor 1979.
| Methods | Double blind RCT, allocation concealment may be inadequate Active treatment: 4 weeks | |
| Participants | Inclusion criteria: endogenous depression Age: 30+ Country: Spain Setting: not clear | |
| Interventions | nomifensine versus amitriptyline | |
| Outcomes | dropouts | |
| Notes | quality rating: 16 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Lydiard 1997.
| Methods | Double blind RCT Active treatment: 8 weeks | |
| Participants | Inclusion criteria: DSM III R major depression, HMD 18+, CGI 3+ Age: adults Country: US Setting: outpatients | |
| Interventions | sertraline versus amitriptyline | |
| Outcomes | dropouts | |
| Notes | quality rating: 16 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Magnus 1977.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: depressive patients requiring antidepressants Age: 18‐65 Country: UK Setting: outpatients | |
| Interventions | nortriptyline+fluphenazine versus amitriptyline | |
| Outcomes | dropouts patients with side‐effects | |
| Notes | quality rating: 18 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Marais 1974.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: endogenous depression requiring antidepressants Age: 31‐70 Country: South Africa Setting: in and outpatients | |
| Interventions | maprotiline versus amitriptyline | |
| Outcomes | responders dropouts patients with side‐effects | |
| Notes | quality rating: 17 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Marchesi 1998.
| Methods | Double blind RCT Active treatment: 10 weeks | |
| Participants | Inclusion criteria: DSM III R major depression, HMD 16+ Age: 43 average Country: Italy Setting: outpatients | |
| Interventions | fluoxetine versus amitriptyline | |
| Outcomes | mean score at endpoint dropouts | |
| Notes | quality rating: 29 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Mariategui 1978.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: endogenous and neurotic depression requiring antidepressants Age: 16‐65 Country: Perù Setting: outpatients | |
| Interventions | lofepramine versus amitriptyline | |
| Outcomes | responders mean score at endpoint dropouts | |
| Notes | quality rating: 14 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Marneros 1979.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: Schneider and Spitzer criteria of endogenous depression Age: 24‐65 Country: German Setting: inpatients | |
| Interventions | lofepramine versus amitriptyline | |
| Outcomes | responders dropouts patients with side‐effects | |
| Notes | quality rating: 19 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Masco 1985.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: major depressive illness, HMD 20+, Raskin greater than Covi Age: 51 average Country: US Setting: outpatients | |
| Interventions | fluoxetine versus amitriptyline | |
| Outcomes | responders dropouts patients with side‐effects | |
| Notes | quality rating: 21 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Mason 1990.
| Methods | Double blind RCT Active treatment: 8 weeks | |
| Participants | Inclusion criteria: DSM III major depressive disorder, HMD 20+ Age: 46 average Country: US Setting: inpatients and outpatients | |
| Interventions | amoxapine versus amitriptyline | |
| Outcomes | responders dropouts | |
| Notes | quality rating: 19 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
McCallum 1975.
| Methods | Double blind RCT Active treatment: 3 weeks | |
| Participants | Inclusion criteria: patients with depression Age: 42 average Country: Australia Setting: outpatients | |
| Interventions | maprotiline versus amitriptyline | |
| Outcomes | mean score at endpoint dropouts | |
| Notes | quality rating: 18 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
McClelland 1979.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: ICD endogenous or neurotic depression Age: 55 average Country: UK Setting: inpatients and outpatients | |
| Interventions | lofepramine versus amitriptyline | |
| Outcomes | responders mean score at endpoint dropouts | |
| Notes | quality rating: 20 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
McConaghy 1965.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: patients suffering from depression Age: adults Country: Australia Setting: outpatients | |
| Interventions | protriptyline versus amitriptyline | |
| Outcomes | responders patients with side‐effects | |
| Notes | quality rating: 18 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Melo de Paula 1977.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: patients with depression, HMD 25+, Beck 12+ Age: 48 average Country: Brazil Setting: inpatients | |
| Interventions | amoxapine versus amitriptyline | |
| Outcomes | dropouts | |
| Notes | quality rating: 19 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Mendels 1968.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: patients with depression Age: 54 average Country: US Setting: in and outpatients | |
| Interventions | nortriptyline versus amitriptyline | |
| Outcomes | dropouts | |
| Notes | quality rating: 20 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Mendlewicz 1980.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: Feighner criteria of primary affective disorder, unipolar or bipolar Age: 40 average Country: Belgium Setting: inpatients | |
| Interventions | dothiepin versus amitriptyline | |
| Outcomes | responders dropouts | |
| Notes | quality rating: 15 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Mendlewicz 1982.
| Methods | RCT, not blind (not clear) Active treatment: 4 weeks | |
| Participants | Inclusion criteria: Feighner criteria of primary affective disorder Age: 29‐68 Country: Belgium Setting: inpatients | |
| Interventions | mianserin versus amitriptyline | |
| Outcomes | responders dropouts | |
| Notes | quality rating: 21 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Metha 1980.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: depressive illness requiring antidepressants Age: 41 average Country: UK Setting: family practice | |
| Interventions | mianserin versus amitriptyline | |
| Outcomes | responders mean score at endpoint dropouts | |
| Notes | quality rating: 15 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Mindham 1977.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: depression, patients suitable for treatment with tricyclic antidepressants Age: 19‐75 Country: UK Setting: family practice | |
| Interventions | maprotiline versus amitriptyline | |
| Outcomes | responders dropouts | |
| Notes | quality rating: 17 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Moises 1981.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: ICD and Feighner criteria of primary affective disorder Age: 22‐70 | |
| Interventions | trazodone versus amitriptyline | |
| Outcomes | responders mean score at endpoint dropouts | |
| Notes | quality rating: 16 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Moller 1993.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: DSM III major depressive disorder, HMD 18+ Age: not clear Country: Germany, Hungary Setting: inatients | |
| Interventions | paroxetine versus amitriptyline | |
| Outcomes | mean score at endpoint dropouts | |
| Notes | quality rating: 22 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Moller 1995.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: DSM III major depressive episode, HMD 18+ Age: 18‐65 Country: Germany Setting: inpatients | |
| Interventions | mianserin versus amitriptyline | |
| Outcomes | responders dropouts | |
| Notes | quality rating: 21 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Moller 1998.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: DSM III R major depression, single episode or recurrent, HMD 21+ Age: 18‐75 Country: Germany, Hungary, Czech Republic Setting: inpatients | |
| Interventions | sertraline versus amitriptyline | |
| Outcomes | dropouts patients with side‐effects | |
| Notes | quality rating: 31 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Moller 2000.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | IInclusion criteria: DSM III R major depression, single episode or recurrent, HMD 21+ Age: 18‐75 Country: Germany Setting: outpatients and family practice | |
| Interventions | sertraline versus amitriptyline | |
| Outcomes | dropouts | |
| Notes | quality rating: 27 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Molnar 1977.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: ICD‐8 depression requiring hospitalisation, HMD 15+, MMPI 60‐ Age: 21‐62 Country: Canada Setting: outpatients | |
| Interventions | maprotiline versus amitriptyline | |
| Outcomes | responders patients with side‐effects | |
| Notes | quality rating: 13 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Montbrun 1976.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: patients with depression, Raskin 9+ Age: adults Country: Luxemburg Setting: not clear | |
| Interventions | lofepramine versus amitriptyline | |
| Outcomes | responders dropouts | |
| Notes | quality rating: 12 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Monteleone 1994.
| Methods | RCT Double‐blind (not clear) Active treatment: 5 weeks | |
| Participants | Inclusion criteria: male subjects, DSM III R major depression, HMD 18+ Age: 60+ Country: Italy Setting: outpatients | |
| Interventions | mianserin versus amitriptyline | |
| Outcomes | mean score at endpoint dropouts | |
| Notes | quality rating: 12 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Montgomery 1980.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: Feighner criteria of primary depressive illness Age: 42 average Country: UK Setting: inpatients | |
| Interventions | maprotiline versus amitriptyline | |
| Outcomes | responders mean score at endpoint dropouts | |
| Notes | quality rating: 18 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Muller‐Oerlingh 1979.
| Methods | Double blind RCT Active treatment: 3 weeks | |
| Participants | Inclusion criteria: depressive syndrome requiring antidepressants, HMD 16+ Age: 20‐65 Country: Germany Setting: inpatients | |
| Interventions | viloxazine versus amitriptyline | |
| Outcomes | dropouts | |
| Notes | quality rating: 19 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Murasaki 1990.
| Methods | Double blind RCT Active treatment: 5 weeks | |
| Participants | Inclusion criteria: patients with a primary diagnosis of depression Age: 18‐77 Country: Japan Setting: inpatients and outpatients | |
| Interventions | Trazodone versus amitriptyline | |
| Outcomes | responders dropouts patients with side‐effects | |
| Notes | quality rating: 21 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Murphy 1978.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: patients with a primary diagnosis of depression Age: 18‐70 Country: UK Setting: family practice | |
| Interventions | mianserin versus amitriptyline | |
| Outcomes | dropouts patients with side‐effects | |
| Notes | quality rating: 21 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Murphy 1980.
| Methods | Double blind RCT Active treatment: 8 weeks | |
| Participants | Inclusion criteria: primary diagnosis of depression Age: 18‐65 Country: UK Setting: family practice | |
| Interventions | trazodone versus amitriptyline | |
| Outcomes | dropouts | |
| Notes | quality rating: 13 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Naftulin 1972.
| Methods | Double blind RCT Active treatment: 5 weeks | |
| Participants | Inclusion criteria: mixed anxiety‐depression Age: 19‐51 Country: US Setting: outpatients | |
| Interventions | doxepin versus amitriptyline+perphenazine | |
| Outcomes | responders dropouts | |
| Notes | quality rating: 10 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Nelson 1982.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: RDC major depressive disorder Age: 19‐69 Country: US Setting: inpatients | |
| Interventions | imipramine versus amitriptyline | |
| Outcomes | dropouts | |
| Notes | quality rating: 11 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Nieto 1973.
| Methods | Double blind RCT, allocation concealment may be inadequate Active treatment: 4 weeks | |
| Participants | Inclusion criteria: depressive episode Age: 14‐58 Country: Mexico Setting: outpatients | |
| Interventions | maprotiline versus amitriptyline | |
| Outcomes | responders dropouts | |
| Notes | quality rating: 11 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Nugent 1979.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: female depressive patients requiring antidepressants Age: 60+ Setting: inpatients | |
| Interventions | viloxazine versus amitriptyline | |
| Outcomes | mean score at endpoint dropouts | |
| Notes | quality rating: 19 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Okasha 1976.
| Methods | Double blind RCT Active treatment: 3 weeks | |
| Participants | Inclusion criteria: patients suffering from depression Age: 10‐60 Country: Egypt Setting: outpatients | |
| Interventions | maprotiline versus amitriptyline | |
| Outcomes | responders dropouts patients with side‐effects | |
| Notes | quality rating: 17 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Peters 1990.
| Methods | Double blind RCT Active treatment: 5 weeks | |
| Participants | Inclusion criteria: ICD 9 endogenous depression, unipolar or bipolar, HMD 17+, Raskin 8+ and greater than Covi Age: 25‐63 Country: Germany Setting: outpatients | |
| Interventions | fluoxetine versus amitriptyline | |
| Outcomes | mean score at endpoint dropouts | |
| Notes | quality rating: 16 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Petrie 1982.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: ICD manic‐depression of the depressed type, involuntary melancholia, HMD 20+, Neurotic/Endogenous Rating Scale 6+ Age: 18‐60 Country: US Setting: outpatients | |
| Interventions | viloxazine versus amitriptyline | |
| Outcomes | responders dropouts | |
| Notes | quality rating: 19 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Preskorn 1991.
| Methods | Double blind RCT Active treatment: 6 week | |
| Participants | Inclusion criteria: DSM III major depressive disorder, HMD 20+ Age: 18+ Country: US Setting: outpatients | |
| Interventions | fluoxetine versus amitriptyline | |
| Outcomes | dropouts | |
| Notes | quality rating: 19 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Prusoff 1981.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: RDC major depression, Raskin 7+ Age: 18‐65 Country: US Setting: outpatients | |
| Interventions | amoxapine versus amitriptyline | |
| Outcomes | responders dropouts | |
| Notes | quality rating: 21 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Pugh 1982.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: depressive illness requiring antidepressants Age: 15‐75 Country: UK Setting: outpatients | |
| Interventions | lofepramine versus amitriptyline | |
| Outcomes | dropouts | |
| Notes | quality rating: 14 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Quadri 1980.
| Methods | Double blind RCT, allocation concealment may be inadequate Active treatment: 4 weeks | |
| Participants | Inclusion criteria: Feighner criteria of endogenous depression, HMD 20+ Age: 22‐52 Country: India Setting: inpatients | |
| Interventions | imipramine versus amitriptyline | |
| Outcomes | responders dropouts | |
| Notes | quality rating: 12 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Querol 1970.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: depressive syndrome Age: 15‐66 Country: Perù Setting: outpatients | |
| Interventions | doxepin versus amitriptyline | |
| Outcomes | responders dropouts patients with side‐effects | |
| Notes | quality rating: 15 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Rabkin 1984.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: RDC major depressive disorder, HMD 18+ Age: 18‐65 Country: US Setting: in and outpatients | |
| Interventions | mianserin versus amitriptyline | |
| Outcomes | responders mean score at endpoint | |
| Notes | quality rating: 19 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Rampello 1995.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: DSM III R major depression, unipolar or bipolar, HMD 20+ Age: 18‐62 Conutry: Italy Setting: outpatients | |
| Interventions | amineptine versus amitriptyline | |
| Outcomes | mean score at endpoint dropouts | |
| Notes | quality rating: 19 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Rees 1976.
| Methods | Single blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: mild to moderate depression Age: range not clear Country: UK Setting: outpatients | |
| Interventions | dothiepin versus amitriptyline | |
| Outcomes | responders dropouts patients with side‐effects | |
| Notes | quality rating: 17 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Rego 1974.
| Methods | Single blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: patients requiring antidepressants Age: 26‐67 Country: Spain Setting: in and outpatients | |
| Interventions | maprotiline versus amitriptyline | |
| Outcomes | responders | |
| Notes | quality rating: 12 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Reimherr 1990.
| Methods | Double blind RCT Active treatment: 8 weeks | |
| Participants | Inclusion criteria: DSM III major depression, HMD 18+, Raskin greater than Covi Age: 18‐65 Country: US, Canada Setting: outpatients | |
| Interventions | sertraline versus amitriptyline | |
| Outcomes | mean improvement in score dropouts | |
| Notes | quality rating: 25 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Remick 1994.
| Methods | Double blind RCT Active treatment: 7 weeks | |
| Participants | Inclusion criteria: DSM III R major depressive disorder, HMD 20+ Age: 18‐65 Country: Canada Setting: outpatients | |
| Interventions | fluvoxamine versus amitriptyline | |
| Outcomes | mean score at endpoint dropouts | |
| Notes | quality rating: 23 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Richmond 1964.
| Methods | Blind RCT (all assessment were made blindly, but not clear whether patients were blind) Active treatment: 3 weeks | |
| Participants | Inclusion criteria: patients with depression requiring antidepressants Age: not clear Country: UK Setting: outpatients | |
| Interventions | imipramine versus amitriptyline | |
| Outcomes | responders | |
| Notes | quality rating: 8 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Rickels 1970.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: neurotic depressed patients Age: 44 average Country: US Setting: family practice, outpatients | |
| Interventions | trimipramine versus amitriptyline | |
| Outcomes | dropouts | |
| Notes | quality rating: 18 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Rickels 1972.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: neurotic depression Age: 43 average Country: US Setting: family practice, outpatients | |
| Interventions | doxepin versus amitriptyline+perphenazine | |
| Outcomes | dropouts | |
| Notes | quality rating: 16 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Rickels 1974.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: non‐psychotic depression Age: 21‐65 Country: US Setting: family practice, outpatients | |
| Interventions | clomipramine versus amitriptyline | |
| Outcomes | dropouts | |
| Notes | quality rating: 19 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Rickels 1982.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: non‐psychotic depression, HMD 18+, Raskin 8+, Feighner 15+ Age: 41 average Country: US Setting: family parctice, outpatients | |
| Interventions | doxepine versus amitriptyline+perphenazine | |
| Outcomes | dropouts | |
| Notes | quality rating: 21 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Rickels 1982a.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: DSM III major depressive disorder Age: 40 average Country: US Setting: family practice, outpatients | |
| Interventions | trazodone versus amitriptyline | |
| Outcomes | responders dropouts | |
| Notes | quality rating: 18 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Rickels 1985.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: Feighner criteria for major depression, HMD 18+, Raskin 8+, Covi less or equal than Raskin Age: 39 average Country: US Setting: outpatients | |
| Interventions | doxepin versus amitriptyline | |
| Outcomes | dropouts | |
| Notes | quality rating: 23 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Rose 1965.
| Methods | RCT, not double blind Active treatment: 4 weeks | |
| Participants | Inclusion criteria: primary depressive disorder Age: less than 65 Country: UK Setting: in and outpatients | |
| Interventions | nortriptyline versus amitriptyline | |
| Outcomes | responders dropouts patients with side‐effects | |
| Notes | quality rating: 9 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Rush 1988.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: unipolar major non‐psychotic depression Age: 35 average Country: US Setting: inpatients | |
| Interventions | nortriptyline versus amitriptyline | |
| Outcomes | responders | |
| Notes | quality rating: 13 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Rush 1989.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: RDC criteria of nonpsychotic major depressive disorder Age: 18‐65 Country: US Setting: outpatients | |
| Interventions | desimipramine versus amitriptyline | |
| Outcomes | responders dropouts | |
| Notes | quality rating: 16 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Rybakowski 1991.
| Methods | RCT, not double‐blind Active treatment: 4 weeks | |
| Participants | Inclusion criteria: female patients with unipolar depressive disorder Age: 23‐66 Country: Poland | |
| Interventions | imipramine versus amitriptyline | |
| Outcomes | responders | |
| Notes | quality rating: 15 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Sacchetti 2002.
| Methods | Double blind RCT Active treatment:12 weeks | |
| Participants | Inclusion criteria: DSM‐III‐R patients with recurrent major depression, one or more episode of affective illness per year for the previous 3 years, or 3+ episodes of affective illness in the previous 2 years, or 2+ episodes of affective illness in the previous year HMD 18+ Age: 18‐70 Country: Italy Setting: outpatients | |
| Interventions | paroxetine versus amitriptyline | |
| Outcomes | responders dropouts patients with side‐effects | |
| Notes | quality rating: 30 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Sandifer 1965.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: female patients with depression, HMD 23+ Age: 40‐59 Country: US Setting: inpatients | |
| Interventions | imipramine versus amitriptyline | |
| Outcomes | responders dropouts | |
| Notes | quality rating: 21 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Sedman 1977.
| Methods | Double blind RCT Active treatment: 5 weeks | |
| Participants | Inclusion criteria: depressive illness requiring antidepressants Age: 52 average Country: UK Setting: inpatients | |
| Interventions | viloxazine versus amitriptyline (sustained release) | |
| Outcomes | responders dropouts | |
| Notes | quality rating: 22 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Sethi 1979.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: patients suffering from depression, HMD 25+, Beck 12+ Age: 43 average Country: India Setting: inpatients | |
| Interventions | amoxapine versus amitriptyline | |
| Outcomes | responders dropouts patients with side‐effects | |
| Notes | quality rating: 18 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Shaw 1986.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: DSM III major depressive illness, HMD 18+ Age: 18‐70 Country: UK Setting: in and outpatients | |
| Interventions | citalopram versus amitriptyline | |
| Outcomes | mean score at endpoint dropouts | |
| Notes | quality rating: 17 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Shipley 1985.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: RDC depression, HMD 30+ Age: 41 average Country: US Setting: inpatients | |
| Interventions | desipramine versus amitriptyline | |
| Outcomes | responders mean score at endpoint dropouts | |
| Notes | quality rating: 14 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Silverstone 1977.
| Methods | Double blind RCT Active treatment: 3 weeks | |
| Participants | Inclusion criteria: symptoms of depression of sufficient severity to be treated with antidepressants Age: 45 average Country: UK Setting: family practice | |
| Interventions | maprotiline versus amitriptyline | |
| Outcomes | dropouts patients with side‐effects | |
| Notes | quality rating: 16 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Sims 1980.
| Methods | Double blind RCT Active treatment: 5 weeks | |
| Participants | Inclusion criteria: depressive illness Age: 18‐65 Country: UK Setting: inpatients | |
| Interventions | maprotiline versus amitriptyline | |
| Outcomes | mean score at endpoint dropouts | |
| Notes | quality rating: 18 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Sinclair 1975.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: anxiety/depression syndrome requiring antidepressants Age: 65+ Country: UK Setting: family practice | |
| Interventions | nortriptyline+fluphenazine versus amitriptyline | |
| Outcomes | dropouts | |
| Notes | quality rating: 20 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Solis 1970.
| Methods | Double blind RCT Active treatment: 8 weeks | |
| Participants | Inclusion criteria: depression Age: not clear Country: Mexico Setting: in and outpatients | |
| Interventions | doxepin versus amitriptyline | |
| Outcomes | responders dropouts | |
| Notes | quality rating: 15 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Staner 1995.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: RDC major depression, HMD 18+ Age: 18‐65 Country: Belgium Setting: inpatients | |
| Interventions | paroxetine versus amitriptyline | |
| Outcomes | responders dropouts | |
| Notes | quality rating: 20 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Stier 1982.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: Feighner criteria of primary depressive disorder Age: 24‐60 Country: Israel Setting: in and outpatients | |
| Interventions | maprotiline versus amitriptyline | |
| Outcomes | responders mean score at endpoint dropouts | |
| Notes | quality rating: 16 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Stott 1993.
| Methods | Double blind RCT Active treatment: 8 weeks | |
| Participants | Inclusion criteria: patients with depression and anxiety, MADRS 16+, CAS 11+ Age: 18‐65 Country: UK Setting: family practice | |
| Interventions | paroxetine versus amitriptyline | |
| Outcomes | mean score at endpoint dropouts patients with side‐effects | |
| Notes | quality rating: 20 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Straker 1966.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: neurotic or psychotic depression Age: 27 average (males); 41 average (females) Country: Canada Setting: outpatients | |
| Interventions | imipramine versus protryptiline versus amitriptyline | |
| Outcomes | responders dropouts | |
| Notes | quality rating: 18 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Stuppaeck 1994.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: DSM III major depression, melancholic subtype, HMD 18+ Age: 18‐65 Country: Austria, Germany Setting: inpatients | |
| Interventions | paroxetine versus amitriptyline | |
| Outcomes | responders mean score at endpoint dropouts | |
| Notes | quality rating: 21 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Toru 1972.
| Methods | Double blind RCT Active treatment: 3 weeks | |
| Participants | Inclusion criteria: patients suffering from depression Age: 43 average Country: Japan Setting: in and outpatient | |
| Interventions | doxepin versus amitriptyline | |
| Outcomes | responders dropouts patients with side‐effects | |
| Notes | quality rating: 14 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Trappe 1973.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: patients with depression Age: 34 average Country: Finland Setting: outpatents | |
| Interventions | doxepin versus amitriptyline+chlordiazepoxide | |
| Outcomes | dropouts | |
| Notes | quality rating: 15 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Trick 1975.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: patients with depression Age: 18‐63 Country: UK Setting: not clear | |
| Interventions | maprotiline versus amitriptyline | |
| Outcomes | responders dropouts patients with side‐effects | |
| Notes | quality rating: 15 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Tsaras 1981.
| Methods | RCT, double blind (not clear) Active treatment: 4 weeks | |
| Participants | Inclusion criteria: anxious depression Age: 19‐74 Country: Switzerland Setting: outpatients | |
| Interventions | maprotiline versus amitriptyline+chlordiazepoxide | |
| Outcomes | patients with side‐effects | |
| Notes | quality rating: 14 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Upward 1988.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: patients with depressive illness Age: 24‐63 Country: UK Setting: outpatients | |
| Interventions | fluoxetine versus amitriptyline | |
| Outcomes | dropouts | |
| Notes | quality rating: 18 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Van Amerongen 1979.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: depressive state requiring antidepressants Age: 24‐79 Country: France Setting: outpatients | |
| Interventions | amineptine versus amitriptyline | |
| Outcomes | responders dropouts patients with side‐effects | |
| Notes | quality rating: 19 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Veith 1983.
| Methods | RCT, double‐blind (not clear) Active treatment: 3 weeks | |
| Participants | Inclusion criteria: Feighner criteria of primary unipolar affective disorder, Zung SDS 54+ Age: 19‐58 Country: US Setting: outpatients | |
| Interventions | desipramine versus amitriptyline | |
| Outcomes | responders mean score at endpoint dropouts | |
| Notes | quality rating: 17 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Versiani 1999.
| Methods | Double blind RCT Active treatment: 8 weeks | |
| Participants | Inclusion criteria: DSM‐IV criteria for major depression (except duration of illness of at least one month), HMD 18+, HMA 18+ Age: 18 or older Country: Brazil, Colombia, Mexico, Peru, Venezuela Setting: unclear | |
| Interventions | fluoxetine versus amitriptyline | |
| Outcomes | responders mean score at endpoint dropouts | |
| Notes | quality rating: 26 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Von Bauer 1969.
| Methods | Double blind RCT, allocation concealment may be inadequate Active treatment: 4 weeks | |
| Participants | Inclusion criteria: patients with depression Age: 53 average Country: Austria Setting: inpatients | |
| Interventions | doxepin versus amitriptyline | |
| Outcomes | responders dropouts | |
| Notes | quality rating: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Waite 1986.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: Feighner criteria for major depression, HMD 16+ Age: 65+ Country: UK Setting: inpatients | |
| Interventions | dothiepin versus mianserin versus amitriptyline | |
| Outcomes | responders dropouts | |
| Notes | quality rating: 25 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Watanabe 1978.
| Methods | Double blind RCT Active treatment: 5 weeks | |
| Participants | Inclusion criteria: involutional melancholia, reactive depression Age: 12‐70 Country: Japan Setting: in and outpatients | |
| Interventions | maprotiline versus amitriptyline | |
| Outcomes | responders dropouts | |
| Notes | quality rating: 16 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Weissman 1975.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: patients with depression, Raskin 7+ Age: 21‐65 Country: US Setting: outpatients | |
| Interventions | maprotiline versus amitriptyline | |
| Outcomes | dropouts | |
| Notes | quality rating: 20 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wheatley 1975.
| Methods | Double blind RCT Active treatment: 4 weeks | |
| Participants | Inclusion criteria: primary diagnosis of depression Age: 18‐65 Country:UK Setting: family practice | |
| Interventions | mianserin versus amitriptyline | |
| Outcomes | patients with side‐effects | |
| Notes | quality rating: 14 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wheatley 1992.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: DSM‐III‐R major depression, HMD 19+ Age: 47 mean Country: UK Setting: family practice | |
| Interventions | minaprine versus amitriptyline | |
| Outcomes | responders dropouts patients with side‐effects | |
| Notes | quality rating: 22 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wilcox 1994.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: DSM III major depression, HMD 18+ Age: 18+ Country: US Setting: outpatients | |
| Interventions | mianserin versus amitriptyline | |
| Outcomes | dropouts | |
| Notes | quality rating: 23 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wright 1976.
| Methods | Double blind RCT Active treatment: 8 weeks | |
| Participants | Inclusion criteria: patients with depression Age: 46 average Country: UK Setting: outpatients | |
| Interventions | lofepramine versus amitriptyline | |
| Outcomes | dropouts patients with side‐effects | |
| Notes | quality rating: 14 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Yamhure 1977.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: ICD‐8 and DSM II involutional melancholia, manic‐depressive illness ‐ depressive type, manic‐depressive illness ‐ cyrcular type depressed, psychotic reactive depression, depressive neurosis Age: 16+ Country: Colombia Setting: inpatients | |
| Interventions | amoxapine versus amitriptyline | |
| Outcomes | responders | |
| Notes | quality rating: 11 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Young 1987.
| Methods | Double blind RCT Active treatment: 6 weeks | |
| Participants | Inclusion criteria: RDC moderately to severe unipolar depression, HMD 18+ Age: 20‐65 Country: UK Setting: outpatients | |
| Interventions | fluoxetine versus amitriptyline | |
| Outcomes | mean score at endpoint dropouts | |
| Notes | quality rating: 19 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Ziegler 1977.
| Methods | RCT, not double‐blind Active treatment: 6 weeks | |
| Participants | Inclusion criteria: Feighner criteria of affective disorder, HMD 21+ Age: 18‐60 Country: US Setting: outpatients | |
| Interventions | nortriptyline versus amitriptyline | |
| Outcomes | responders dropouts | |
| Notes | quality rating: 16 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anonimous 1971 | imipramine versus propazepine versus dothiepin versus amitriptyline No efficacy/safety data available |
| Branconnier 1981 | mianserin versus amitriptyline no efficacy/safety data available |
| Claghorn 1984 | dothiepin versus amitriptyline no efficacy/safety study, hearth rate as outcome measure |
| Coppen 1976 | mianserin versus amitriptyline no efficacy/safety data available |
| Deuschle 2004 | data from open‐label mixed with data from double blind |
| Elwan 1976 | doxepin versus amitriptyline no efficacy/safety data available |
| Friedel 1979 | desipramine versus amitripyline no efficacy/safety data available |
| Hackett 1967 | doxepin versus amitriptyline no efficacy/safety data available |
| Hackett 1967a | doxepin versus amitriptyline no efficacy/safety data available |
| Hollister 1964 | imipramine versus amitriptyline no efficacy/safety data available |
| Hutchinson 1963 | imipramine versus amitriptyline no efficacy/safety data available |
| Kiebach 1982 | trazodone versus amitriptyline no efficacy/safety data available |
| Lambourn 1974 | dothiepin versus amitriptyline no efficacy/safety data available |
| Lloyd 1981 | amoxapine versus amitriptyline no efficacy/safety data available |
| Maier 1989 | imipramine versus amitriptyline no efficacy/safety data available |
| Marjerrison 1969 | imipramine versus nortriptyline versus amitriptyline no efficacy/safety data available |
| Montgomery 1978 | mianserin versus maprotiline versus amitriptyline no efficacy/safety data available |
| Moyes 1980 | clomipramine versus amitriptyline no efficacy/safety data available |
| Peet 1977 | mianserin versus amitriptyline no efficacy/safety data available |
| Renfordt 1976 | mianserin versus amitriptyline no efficacy/safety data available |
| Saletu 1969 | mianserin versus amitriptyline no efficacy/safety data available |
| Stratas 1984 | dothiepin versus amitriptyline no efficacy/safety data available |
| Taverna 1969 | |
| Van De Merwe 1984 | trazodone versus amitriptyline no efficacy/safety study |
| Vartanian 1984 | imipramine versus maprotiline versus amitriptyline no efficacy/safety data available |
| Vogel 1976 | mianserin versus amitriptyline no efficacy/safety data available |
Contributions of authors
CB and MH conceived the project and wrote the protocol. CB and GG extracted data from the articles. GG wrote a first draft of the review, that was supervised by CB
Declarations of interest
None.
Edited (no change to conclusions)
References
References to studies included in this review
Aberg 1977 {published data only}
- Aberg A, Holmberg G. Controlled trial of a new antidepressive, amoxapine, in comparison with amitriptyline. Current Therapeutic Research 1977;22:304‐15. [Google Scholar]
Altamura 1989 {published data only}
- Altamura AC, Mauri MC, Rudas N. Carpiniello B, Montanini R, Perini M, et al. Clinical activity and tolerability of trazodone, mianserin, and amitriptyline in elderly subjects with major depression: a controlled multicenter trial. Clinical Neuropharmacology 1989;12 Suppl 1:s25‐s33. [DOI] [PubMed] [Google Scholar]
Altamura 1989a {published data only}
- Altamura AC, Novellis F, Guercetti G, Invernizzi G, Percudani M, Montgomery SA. Fluoxetine compared with amitriptyline in elderly depression: a controlled clinical trial.. International Journal Clinical Pharmacology Research 1989;9:391‐6. [PubMed] [Google Scholar]
Amin 1973 {published data only}
- Amin MM, Brahm E, Bronheim LA, Klingner A, Ban TA, Lehmann HE. A double‐blind, comparative clinical trial with Ludiomil (CIBA 34,276‐Ba) and amitriptyline in newly admitted depressed patients. Current Therapeutic Research 1973;15:691‐9. [PubMed] [Google Scholar]
Amin 1978 {published data only}
- Amin MM, Cooper R, Khalid R, Lehman HE. A comparison of desipramine and amitriptyline plasma levels and therapeutic response. Psychopharmacology Bulletin 1978;14:45‐6. [PubMed] [Google Scholar]
Anderson 1972 {published data only}
- Anderson J, Lambert NG, Pigott PV. An evaluation of fluphenazine with nortriptyline in anxiety and depression. The Practitioner 1972;208:511‐7. [PubMed] [Google Scholar]
Anton 1990 {published data only}
- Anton R, Burch EA. Amoxapine versus amitriptyline combined with perphenazine in the treatment of psychotic depression. American Journal Psychiatry 1990;147:1203‐8. [DOI] [PubMed] [Google Scholar]
Ather 1985 {published data only}
- Ather SA, Ankier SI, Middleton RSW. A double‐blind evaluation of trazodone in the treatment of depression in the elderly. British Journal of Clinical Practice 1985;39:192‐9. [PubMed] [Google Scholar]
Balestrieri 1971 {published data only}
- Balestrieri A, Benassi P, Cassano GB, Castrogiovanni P, Catalano A, Colombi A, et al. Clinical comparative evaluation of maprotiline, a new antidepressant drug. International Pharmacopsychiatry 1971;6:236‐48. [DOI] [PubMed] [Google Scholar]
Bascara 1989 {published data only}
- Bascara B. A double blind study to compare the effectiveness and tolerability of paroxetine and amitriptyline in depressed patients. Acta Psychiatrica Scandinavica, Supplementum 1989;350:141‐2. [DOI] [PubMed] [Google Scholar]
Battegay 1985 {published data only}
- Battegay R, Hager M, Rauchfleisch U. Double‐blind comparitive study of paroxetine and amitriptyline in depressed patients of a university psychiatric outpatient clinic. Neuropsychobiology 1985;13:31‐7. [DOI] [PubMed] [Google Scholar]
Beaini 1980 {published data only}
- Beaini AY, Hindmarch I, Snaith RP. A re‐examination of the clinical effects of imipramine and amitriptyline in depressive illness. Journal of Affective Disorders 1980;2:89‐94. [DOI] [PubMed] [Google Scholar]
Beckmann 1975 {published data only}
- Beckmann H, Goodwin FK. Antidepressant response to tricyclics and urinary MHPG in unipolar patients. Archives General Psychiatry 1975;32:17‐21. [DOI] [PubMed] [Google Scholar]
Bennie 1976 {published data only}
- Bennie EH, Schiff AA. A comparison of amitriptyline and fluphenazine/nortriptyline preparation in anxiety‐depressive states. Scottish Medical Journal 1976;21:204‐9. [DOI] [PubMed] [Google Scholar]
Bersani 1994 {published data only}
- Bersani G, Rapisarda V, Ciani N, Bertolino A, Sorge G. A double‐blind comparative study of sertraline and amitriptyline in outpatients with major depressive episodes. Human Psychopharmacology 1994;9:63‐8. [Google Scholar]
Bianchi 1971 {published data only}
- Bianchi GN, Barr RF, Kiloh LG. A comparative trial of doxepin and amitriptyline in depressive illness. Medical Journal Australia. 1971;1:843‐6. [DOI] [PubMed] [Google Scholar]
Bignamini 1992 {published data only}
- Bignamini A, Rapisarda V. A Double‐blind multicentre study of paroxetine and amitriptyline in depressed outpatients. International Clinical Psychopharmacology 1992;6 Suppl 4:37‐41. [DOI] [PubMed] [Google Scholar]
Blacker 1988 {published data only}
- Blacker R, Shanks NJ, Chapman N, Davey A. The drug treatment of depression in general practice: a comparison of nocte administration of trazodone with mianserin, dothiepin and amitriptyline. Psychopharmacology 1988;95:s18‐s24. [DOI] [PubMed] [Google Scholar]
Botros 1989 {published data only}
- Botros WA, Ankier SI, Priest RG, McManus IC, Steinert J, Samir ZY. (1989) Clinical assessment and performance tasks in depression: a comparison of amitriptyline and trazodone. British Journal of Psychiatry 1989;155:479‐82. [DOI] [PubMed] [Google Scholar]
Browne 1969 {published data only}
- Browne MW. A comparison of two drug treatments in depressive illness. British Journal of Psychiatry 1969;115:693‐6. [DOI] [PubMed] [Google Scholar]
Burke 1967 {published data only}
- Burke BV, Sainsbury MJ, Mezo BA. A comparative trial of amitriptyline and trimipramine in the treatment of depression. Medical Journal of Australia 1967;1:1216‐8. [DOI] [PubMed] [Google Scholar]
Burrows 1980 {published data only}
- Burrows G, Norman TR, Davies BM. A comparative study of amoxapine and amitriptyline for depressive illness. Australian Family Physician 1980;9:762‐6. [PubMed] [Google Scholar]
Burt 1962 {published data only}
- Burt CG, Gordon WF, Holt NF, Horden A. Amitriptyline in depressive states: a controlled trial. Journal of Mental Science 1962;108:711‐29. [DOI] [PubMed] [Google Scholar]
Byrne 1989 {published data only}
- Byrne MM. Meta‐analysis of early phase II studies with paroxetine in hospitalized depressed patients. Acta Psychiatrica Scandinavica, Supplementum 1989;350:138‐9. [DOI] [PubMed] [Google Scholar]
Carman 1991 {published data only}
- Carman JS, Ahdieh H, Wyatt‐Knowles E, Warga E, Panagides J. A controlled study of mianserin in moderately to severely depressed outpatients. Psychopharmacology Bulletin 1991;27:135‐9. [PubMed] [Google Scholar]
Carney 1984 {published data only}
- Carney PA, Healy D, Leonard BE. A double‐blind study to compare trazodone with amitriptyline in depressed patients. Psychopathology 1994;17 Suppl 2:37‐8. [DOI] [PubMed] [Google Scholar]
Chouinard 1985 {published data only}
- Chouinard G. A double‐blind controlled trial of fluoxetine and amitriptyline in the treatment of outpatients with major depression. Journal Clinical Psychiatry 1985;46:32‐7. [PubMed] [Google Scholar]
Christiansen 1996 {published data only}
- Christiansen PE, Behnke K, Black CH, Ohrstrom JK, Bork‐Rasmussen H, Nilsson J. Paroxetine and amitriptyline in the treatment of depression in general practice. Acta Psychiatrica Scandinavica 1996;93:158‐63. [DOI] [PubMed] [Google Scholar]
Click 1982 {published data only}
- Click MA, Zisook S. Amoxapine and amitriptyline: serum levels and clinical response in patients with primary unipolar depression. Journal of Clinical Psychiatry 1982;43:369‐71. [PubMed] [Google Scholar]
Cohn 1990 {published data only}
- Cohn CK, Shrivastava R, Mendels J, Cohn JB, Fabre LF, Claghorn JL, et al. Double‐blind, mulitcenter comparison of sertraline and amitriptyline in elderly depressed patients. Journal of Clinical Psychiatry 1990;51 Suppl B:28‐33. [PubMed] [Google Scholar]
Cournoyer 1987 {published data only}
- Cournoyer G, Montigny C, Quellette J, Langlois R, Elie R, Caille G, et al. A comparative double‐blind controlled study of trimipramine and amitriptyline in major depression: lack of correlation with 5‐hydroxytryptamine reuptake blockade. Journal of Clinical Psychopharmacology 1987;7:385‐93. [PubMed] [Google Scholar]
Dahl 1981 {published data only}
- Dahl LE, Dencker SJ, Lundin L. A double‐blind study of dothiepin hydrochloride (Prothiaden) and amitriptyline in outpatients with masked depression. Journal of International Medical Research 1981;9:103‐7. [DOI] [PubMed] [Google Scholar]
Daly 1979 {published data only}
- Daly RJ, Browne PJ. Mianserin in the treatment of depressive illness: a comparison with amitriptyline. Irish Journal of Medical Science 1979;148:145‐8. [PubMed] [Google Scholar]
De La Barquera 1998 {published data only}
- Barquera J, Brandi F, Brunner E. Double‐blind study of fluoxetine vs amitriptiline in depressive and anxiety symtoms and life quality in adults with major depression [Estudio doble‐ciego sobre fluoxetina vs amitriptilina en los sintomas depresivos y de ansiedad, y calidad de vida de los adultos con depresion mayor]. Salud Mental 1998;21:58‐62. [Google Scholar]
De Ronchi 1998 {published data only}
- Ronchi D, Rucci P, Lodi M, Ravaglia G, Forti P, Volterra V. Fluoxetine and amitriptyline in elderly depressed patients. A 10‐week, double‐blind study on course of neurocognitive adverse events and depressive symptoms. Archives ofGerontology Geriatrics 1998;6 Suppl:125‐40. [Google Scholar]
Deering 1974 {published data only}
- Deering RB, Vallè‐Jones JC. A general practitioner double‐blind study of dothiepin hydrochloridre ('Prothiaden') and amitriptyline in depression. Current Medical Research Opinion 1974;2:471‐3. [DOI] [PubMed] [Google Scholar]
Del Zompo 1990 {published data only}
- Zompo M, Bernardi F, Burrai C, Bocchetta A. A double‐blind study of minaprine versus amitriptyline in major depression. Neuropsychobiology 1990;24:79‐83. [DOI] [PubMed] [Google Scholar]
Delaunay 1978 {published data only}
- Delaunay J, Meynard J. Clinical and comparative trial of dosulepin and amitriptyline [Essai clinique et comparatif de la Dosulepine et de l'Amitriptyline]. Societe Medico‐Psychologique 1978;136:1201‐7. [PubMed] [Google Scholar]
Dell 1977 {published data only}
- Dell AJ. A comparison of maprotiline and amitriptyline. Journal of International Medical Research 1977;5 Suppl 4:22‐4. [PubMed] [Google Scholar]
Demyttenaere 1998 {published data only}
- Demyttenaere K, Ganse E, Gregoire J, Gaens E, Mesters P. Compliance in depressed patients treated with fluoxetine or amitriptyline. International Clinical Psychopharmacology 1998;13:11‐7. [DOI] [PubMed] [Google Scholar]
Donlon 1981 {published data only}
- Donlon P, Biertuemphel H, Willenbring M. Amoxapine and amitriptyline in the outpatient treatment of endogenous depression. Journal of Clinical Psychiatry 1981;42:11‐5. [PubMed] [Google Scholar]
Doongaji 1993 {published data only}
- Doongaji DR, Bal S, Rajkumar S. Multicentre double‐blind comparison of lofepramine and amitriptyline in the treatment of major depressive disorders in Indian patients. British Journal of Clinical Research 1993;4:45‐53. [Google Scholar]
Dorman 1980 {published data only}
- Dorman T. Clinical trial comparison of a sustained release form of amitriptyline with dothiepin. Journal International Medical Research 1980;8:286‐92. [DOI] [PubMed] [Google Scholar]
Dorn 1980 {published data only}
- Dorn M. Psychotropic drugs in medical practice. Double‐blind studies of lofepramine against amitriptyline [Psychopharmaka in der praxis. Doppelblindprufung lofepramin gegen amitriptylin]. Zeitschrift fur Allgemeinmedizin 1980;56:133‐9. [PubMed] [Google Scholar]
Edwards 1996 {published data only}
- Edwards JG, Dinan TG, Waller DG, Greentree SG. Double‐blind comparative study of the antidepressant, unwanted and cardiac effects of minaprine and amitriptyline. British Journal Clinical Pharmacology 1996;42:491‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fawcett 1989 {published data only}
- Fawcett J, Zajecka J, Kravitz H. Fluoxetine vs amitriptyline in adult inpatients with major depression. Current Therapeutic Research 1989;45:821‐32. [Google Scholar]
Feighner 1983 {published data only}
- Feighner JP, Jacobs RS, Jackson RE, Hendrickson G, Merideth CH, & O'Meara PD. A double‐blind comparative trial with mianserin and amitriptyline in outpatients with major depressive disorders. British Journal of Clinical Pharmacology 1983;15:227s‐237s. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferrari 1987 {published data only}
- Ferrari G, Berardi D, Berlinzani L, Bellini M, Cervino G, Innamorati A. A double‐blind comparative trial with viloxazine and amitriptyline in inpatients with major non‐psychotic depressive disorders. Current Therapeutic Research 1987;42:1088‐95. [Google Scholar]
Forrest 1964 {published data only}
- Forrest AD, Affleck JW, McGibb IA, Priest RG. Comparative trial of nortriptyline and amitriptyline. Scottish Medical Journal 1964;9:341‐4. [DOI] [PubMed] [Google Scholar]
Forrest 1975 {published data only}
- Forrest AW. A comparison between daily and nightly dose regimen of amitriptyline and maprotiline (Ludiomil) in the treatment of reactive depression in general practice. Journal International Medical Research 1975;3 Suppl 2:120‐5. [Google Scholar]
Freed 1999 {published data only}
- Freed E, Goldney R, Lambert T, Tiller J, Johnston R. A double‐blind, multicentre study to assess the tolerability and efficacy of paroxetine compared with amitriptyline in the treatment of depressed patients in Australian general practice. Australian and New Zealand Journal of Psychiatry 1999;33:416‐21. [DOI] [PubMed] [Google Scholar]
Fruensgaard 1979 {published data only}
- Fruensgaard K, Hansen CE, Korsgaard S, Nymgaard K, Vaag UH. Amoxapine versus amitriptyline in endogenous depression. A double‐blind study. Acta Psychiatrica Scandinavica 1979;59:502‐8. [DOI] [PubMed] [Google Scholar]
Gasperini 1992 {published data only}
- Gasperini M, Gatti F, Bellini L, Anniverno R, Smeraldi E. Perspectives in clinical psychopharmacology of amitriptyline and fluvoxamine. Neuropsychobiology 1992;26:186‐92. [DOI] [PubMed] [Google Scholar]
Geretsegger 1995 {published data only}
- Geretsegger C, Stuppaeck CH, Mair M, Platz T, Fartacek R, Heim M. Multicentre double‐blind study of paroxetine and amitriptyline in elderly depressed inpatients. Psychopharmacology 1995;119:277‐81. [DOI] [PubMed] [Google Scholar]
Goldberg 1977 {published data only}
- Goldberg HL, Finnerty RJ. Which tricyclic for depressed outpatients, imipramine pamoate or amitriptyline?. Diseases of the Nervous System 1977;38:785‐9. [PubMed] [Google Scholar]
Goldberg 1980 {published data only}
- Goldberg HL, Finnerty RJ. Trazodone in the treatment of neurotic depression. Journal of Clinical Psychiatry 1980;41:430‐4. [PubMed] [Google Scholar]
Goldstein 1969 {published data only}
- Goldstein BJ, Pinosky DG. Clinical evaluation of doxepin in anxious depressed outpatients. Current Therapeutic Research 1969;11:169‐77. [PubMed] [Google Scholar]
Gomez‐Martinez 1968 {published data only}
- Gomez‐Martinez I. Preliminary double‐blind clinical trial wit a new antidepressive doxepin. Current Therapeutic Research 1968;10:116‐8. [PubMed] [Google Scholar]
Gravem 1987 {published data only}
- Gravem A, Amthor KF, Astrup C, Elgen K, Gjessing LR, Gunby B, et al. A double‐blind comparison of citalopram (Lu 10‐171) and amitriptyline in depressed patients. Acta Psychiatrica Scandinavica 1987;75:78‐86. [DOI] [PubMed] [Google Scholar]
Grof 1974 {published data only}
- Grof P, Saxena B, Cantor R, Daigle L, Hetherington D, Haines T. Doxepin versus amitriptyline in depression: a sequential double‐blind study. Current Therapeutic Research 1974;16:470‐6. [PubMed] [Google Scholar]
Grof 1977 {published data only}
- Grof P, Saxena B, Daigle L, Mahutte G. Dopaminergic agonist nomifensine compared with amitriptyline: a double‐blind clinical trial in acute primary depressions. British Journal Clinical Pharmacology 1977;4:221s‐225s. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guelfi 1989 {published data only}
- Guelfi JD, Pichot P, Dreyfus JF. Efficacy of tianeptine in anxious‐depressed patients: results of a controlled multicenter trial versus amitriptyline. Neuropsychobiology 1989;22:41‐8. [DOI] [PubMed] [Google Scholar]
Guy 1983 {published data only}
- Guy W, McEvoy JM, Ban TA, Wilson WH, Pate K. A double‐blind clinical trial of mianserin versus amitriptyline: differentiation by adverse symptomatology. Pharmacotherapy 1983;3:45‐51. [DOI] [PubMed] [Google Scholar]
Harding 1973 {published data only}
- Harding T. A comparative clinical trial of oral clomipramine (Anafranil) against amitriptyline. Journal of International Medical Research 1973;1:343‐6. [Google Scholar]
Harris 1991 {published data only}
- Harris B, Szujelecka TK, Anstee JA. Fluvoxamine versus amitriptyline om depressed hospital out‐patients: a mulitcentre double blind comparative trial. British Journal of Clinical Research 1991;2:89‐99. [Google Scholar]
Hegerl 1997 {published data only}
Hekimian 1978 {published data only}
- Hekimian LJ, Friedhoff AJ, Deever E. A comparison of the onset of action and therapeutic efficacy of amoxapine and amitriptyline. Journal of Clinical Psychiatry 1978;39:633‐7. [PubMed] [Google Scholar]
Hosak 2000 {published data only}
- Hosak L, Tuma I, Hanus H, Straka L. Costs and outcomes of use of amitriptyline, citalopram and fluoxetine in major depression: exploratory study. Acta Medica (Hradec Kralove) 2002;43:133‐7. [PubMed] [Google Scholar]
Hutchinson 1992 {published data only}
- Hutchinson DR, Tong S, Moon CA, Vince M, Clarke A. Paroxetine in the treatment of elderly depressed patients in general practice: a double‐blind comparison with amitriptyline. International Clinical Psychopharmacolology 1992;6 Suppl 4:43‐51. [DOI] [PubMed] [Google Scholar]
Invernizzi 1994 {published data only}
- Invenizzi G, Aguglia E, Bertolino A, Casacchia M, Ciani N, Marchesi GF, et al. The efficacy and safety of tianeptine in the treatment of depressive disorder: results of a controlled double‐blind multicentre study vs. amitriptyline. Neuropsychobiology 1994;30:85‐93. [DOI] [PubMed] [Google Scholar]
James 1982 {published data only}
- James B. A double‐blind comparative clinical study of amoxapine and amitriptyline in depressed, hospitalised patients. New Zealand Medical Journal 1982;95:391‐3. [PubMed] [Google Scholar]
Jaskari 1977 {published data only}
- Jaskari MO, Ahlfors UG, Ginman L, Lydekcne K, Tienari P. Three double‐blind comparative trials of mianserine (ORG GB 94) and amitriptyline in the treatment of depressive illness. Pharmacopsychiatry 1977;10:101‐3. [DOI] [PubMed] [Google Scholar]
Jessel 1981 {published data only}
- Jessel HJ, Jessel I, Wegener G. Therapy of elderly depressive patients. Lofepramine and amitriptyline under double‐blind conditions [Therapie von alterendepressiven patienten. Lofepramin und amitriptylin unter doppelblindbedingungen]. Zeitschrift fur Allgemeinmedizin 1981;57:784‐8. [PubMed] [Google Scholar]
Judd 1993 {published data only}
- Judd FK, Moore K, Norman TR, Burrows GD, Gupta RK, Parker G. A multicentre double blind trial of fluoxetine versus amitriptyline in the treatment of depressive illness. Australian and New Zealand Journal of Psychiatry 1993;27:49‐55. [DOI] [PubMed] [Google Scholar]
Kamijima 1997 {published data only}
- Kamijima K, Koyama T, Mita T. Clinical evaluation of sertraline hydrochloride, a selective serotonin reuptake inhibitor in the treatment of depression and depressive state: A double‐blind, group comparison study of sertraline by hydrochloride vs amitriptyline hydrochloride. Japanese Journal Neuropsychopharmacology 1997;19:529‐48. [Google Scholar]
Kampman 1978 {published data only}
- Kampman R, Nummikko‐Pelkonen A, Kuha S. Tricyclic antidepressants in the treatment of depression. Acta Psychiatrica Scandinavica 1978;58:142‐8. [DOI] [PubMed] [Google Scholar]
Kaumeier 1980 {published data only}
- Kaumier HS, Haase HJ. A double‐blind comparison between amoxapine and amitriptyline in depressed in‐patients. International Journal Clinical Pharmacology, Therapy and Toxicology 1980;18:177‐84. [PubMed] [Google Scholar]
Kay 1974 {published data only}
- Kay N, Davies B. A controlled trial of maprotiline (Ludiomil) and amitrptyline in general practice. Medical Journal Australia 1974;1:704‐5. [DOI] [PubMed] [Google Scholar]
Keegan 1991 {published data only}
- Keegan D, Bowen RC, Blackshaw S, Saleh S, Dayal N, Remillard F, et al. A comparison of fluoxetine and amitriptyline in the treatment of major depression. International Clinical Psychopharmacology 1991;6:117‐24. [DOI] [PubMed] [Google Scholar]
Kerr 1984 {published data only}
- Kerr TA, McClelland HA, Stephens DA, Ankier SI. Trazodone. A comparative clinical and predictive study. Acta Psychiatrica Scandinavica 1984;70:573‐7. [DOI] [PubMed] [Google Scholar]
Khan 1981 {published data only}
- Khan AU. A comparison of the therapeutic and cardiovascular effects of a single nightly dose of Prothiaden (dothiepin, dosulepin) and Lentizol (sustained‐release amitriptyline) in depressed elderly patients. Journal International Medical Research 1981;9:108‐12. [DOI] [PubMed] [Google Scholar]
Khan 1982 {published data only}
- Khan MC, Ancill RJ, Davey A. Treatment of severe depressive illness: a double‐blind comparison of mianserin and long‐acting amitriptyline. British Journal of Clinical Practice 1982;36:240‐2. [PubMed] [Google Scholar]
Kiloh 1979 {published data only}
- Kiloh LG, Bartrop RW, Franklin JA, Neilson MD. A double‐blind comparative trial of viloxazine and amitriptyline in patients suffering from endogenous depression. Australian New Zealand Journal of Psychiatry 1979;13:357‐60. [DOI] [PubMed] [Google Scholar]
Klieser 1988 {published data only}
- Klieser E, Lehmann E. Experimental comparison between the effect of standardised trazodone‐amitriptyline and placebo treatment in vitalised depressive patients. Psychopharmacology 1988;95:s3‐s5. [DOI] [PubMed] [Google Scholar]
Kline 1982 {published data only}
- Kline NS. A controlled comparison of trimipramine and amitriptyline. Journal of Clinical Psychiatry 1982;43:100‐4. [PubMed] [Google Scholar]
Kocsis 1986 {published data only}
- Kocsis JH, Hanin I, Bowden C, Brunswick D. Imipramine and amitriptyline plasma concentrations and clinical response in major depression. British Journal of Psychiatry 1988;148:52‐7. [DOI] [PubMed] [Google Scholar]
Kuhs 1989 {published data only}
- Kuhs H, Rudolf GA. A double‐blind study of the comparative antidepressant effect of paroxetine and amitriptyline. Acta Psychiatrica Scandinavica, Supplementum 1989;350:145‐6. [DOI] [PubMed] [Google Scholar]
Kyle 1998 {published data only}
- Kyle CJ, Petersen HE, Overo KF. Comparison of the tolerability and efficacy of citalopram and amitriptyline in elderly depressed patients treated in general practice. Depression and Anxiety 1998;8:147‐53. [PubMed] [Google Scholar]
Laakmann 1988 {published data only}
- Laakmann G, Blaschke D, Engel R, Schwarz A. Fluoxetine vs amitriptyline in the treatment of depressed out‐patients. British Journal of Psychiatry 1988;153 Suppl 3:64‐68. [PubMed] [Google Scholar]
Laakmann 1991 {published data only}
- Laakmann G. Selective re‐uptake‐hemmung und ihre bedeutung fur die depression. Springer Verlag, 1991. [Google Scholar]
Lapierre 1980 {published data only}
- Lapierre YD, Sussman P, Ghadirian A. Differential antidepressant properties of trazodone and amitriptyline in agitated and retarded depression. Current Therapeutic Research 1980;28:845‐54. [Google Scholar]
Lauritsen 1974 {published data only}
- Lauritsen BJ, Madsen H. A multinational, double‐blind trial with a new antidepressant maprotiline (Ludiomil) and amitriptyline. Acta Psychiatrica Scandinavica 1974;50:192‐201. [DOI] [PubMed] [Google Scholar]
Laursen 1985 {published data only}
- Laursen AL, Mikkelsen PL, Fievre Honore P. Paroxetine in the treatment of depression ‐ a randomised comparison with amitriptyline. Acta Psychiatrica Scandinavica 1985;71:249‐55. [DOI] [PubMed] [Google Scholar]
Leahy 1967 {published data only}
- Leahy MR, Martin ICA. Double‐blind comparison of nortriptyline and amitriptyline in depressive illness. British Journal of Psychiatry 1967;113:1433‐4. [DOI] [PubMed] [Google Scholar]
Lehmann 1982 {published data only}
- Lehmann LS, Bowden CL, Redmont FC, Stanton BC. Amitriptyline and nortriptyline response profiles in unipolar depressed patients. Psychopharmacology 1982;77:193‐7. [DOI] [PubMed] [Google Scholar]
Lennox 1978 {published data only}
- Lennox IG, Asbury JF, Couldrick WG, Beswick KB. Viloxazine and amitriptyline in depressive illness. A double‐blind controlled trial in general practice. The Practitioner 1978;220:153‐6. [PubMed] [Google Scholar]
Levin 1974 {published data only}
- Levin A. Maprotiline and amitriptyline in the treatment of depressive illness. A double‐blind comparison. South African Medical Journal 1974;48:47‐9. [PubMed] [Google Scholar]
Lipsedge 1971 {published data only}
- Lipsedge MS, Rees WL. A double‐blind comparison of dothiepin and amitriptyline for the treatment of depression with anxiety. Psychopharmacologia 1971;19:153‐62. [DOI] [PubMed] [Google Scholar]
Loga 1992 {published data only}
- Loga S, Milovanovic D, Eric LJ. A double‐blind, parallel‐group comparative study of dothiepin and amitriptyline in the treatment of depression in a yugoslavian population. Journal of Drug Development 1992;4:213‐8. [Google Scholar]
Loo 1988 {published data only}
- Loo H, Malka R, Defrance R, Benard JY, Niox‐Riviere H, Raab A, et al. Tianeptine and amitriptyline. Controlled double‐blind trial in depressed alcoholic patients. Neuropsychobiology 1998;19:79‐85. [DOI] [PubMed] [Google Scholar]
Lopez‐Ibor 1979 {published data only}
- Lopez‐Ibor Alino JJ, Ayuso Gutierrez JL, Montejo Iglesias ML, Ramos JL. Estudio clinico comparativo entre el nomifensin y la amitriptilina en el tratamiento de la depresion endogena. Actas Luso Espanolas de Neurologia, Psiquiatria y Ciencias Afines 1979;7:123‐32. [PubMed] [Google Scholar]
Lydiard 1997 {published data only}
- Lydiard RB, Stahl SM, Hertzman M, Harrison WM. A double‐blind, placebo‐controlled study comparing the effects of sertraline versus amitriptyline in the treatment of major depression. Journal of Clinical Psychiatry 1997;58:484‐91. [DOI] [PubMed] [Google Scholar]
Magnus 1977 {published data only}
- Magnus RV, Schiff AA. Once‐daily treatment for mixed anxiety/depressive states: a comparison of slow release amitriptyline and fluphenazine with notriptyline. Journal of International Medical Research 1977;5:109‐13. [DOI] [PubMed] [Google Scholar]
Marais 1974 {published data only}
- Marais GF. Clinical evaluation of the antidepressants maprotiline and amitriptyline. A double‐blind controlled trial. South African Medical Journal 1974;48:1530‐2. [PubMed] [Google Scholar]
Marchesi 1998 {published data only}
- Marchesi C, Ceccherininelli A, Rossi A, Maggini C. Is anxious‐agitated major depression responsive to fluoxetine? A double‐blind comparison with amitriptyline. Pharmacopsychiatry 1998;31:216‐21. [DOI] [PubMed] [Google Scholar]
Mariategui 1978 {published data only}
- Mariategui J, Chavez H, Olivares A. Lofepramina: estudio clinico comparativo con amitriptilina. Acta Physiologica Latinoamericana 1978;24:201‐9. [PubMed] [Google Scholar]
Marneros 1979 {published data only}
- Marneros A, Philipp M. A double‐blind trial with amitriptyline and lofepramine in the treatment of endogenous depression. International Pharmacopsychiatry 1979;14:300‐4. [DOI] [PubMed] [Google Scholar]
Masco 1985 {published data only}
- Masco HL, Sheetz MS. Double‐blind comparison of fluoxetine and amitryptyline in the treatment of major depressive illness. Advances in Therapy 1985;2:275‐84. [Google Scholar]
Mason 1990 {published data only}
- Mason BJ, Kocsis JH, Frances AJ, Mann JJ. Amoxapine versus amitriptyline for continuation therapy of depression. Journal of Clinical Psychopharmacology 1990;10:338‐43. [PubMed] [Google Scholar]
McCallum 1975 {published data only}
- McCallum P, Meares R. A controlled trial of maprotiline (Ludiomil) in depressed outpatients. Medical Journal Australia 1975;2:392‐4. [DOI] [PubMed] [Google Scholar]
McClelland 1979 {published data only}
- McClelland HA, Kerr TA, Stephens DA, Howell RW. The comparative antidepressant value of lofepramine and amitriptyline. Results of a controlled trial with comments on the scales used. Acta Psychiatrica Scandinavica 1979;60:190‐8. [DOI] [PubMed] [Google Scholar]
McConaghy 1965 {published data only}
- McConaghy N, Kingston WR, Stevenson HG, Atkinson I, Cole E, Fennessy LA. A controlled trial comparing amitriptyline and protriptyline in the treatment of out‐patient depressives. Medical Journal of Australia 1965;ii:403‐5. [DOI] [PubMed] [Google Scholar]
Melo de Paula 1977 {published data only}
- Melo de Paula AJ, Heckert U, Abizaid W, Sotto Maior M. Amoxapina e amitriptilina ‐ Um estudio duplo‐cego em pacientes deprimidos. Folha Médica 1977;75:165‐9. [Google Scholar]
Mendels 1968 {published data only}
- Mendels J. Comparative trial of nortriptyline and amitriptyline in 100 depressed patients. American Journal Psychiatry 1968;124:59‐62. [DOI] [PubMed] [Google Scholar]
Mendlewicz 1980 {published data only}
- Mendlewicz J, Linkowski P, Rees JA. A double‐blind comparison of dothiepin and amitriptyline in patients with primary affective disorder: serum levels and clinical response. British Journal Psychiatry 1980;136:154‐60. [DOI] [PubMed] [Google Scholar]
Mendlewicz 1982 {published data only}
- Mendlewicz J, Pinder RM, Stulemeijer SM, Dorth R. Monoamine metabolites in cerebrospinal fluid of depressed patients during treatment with mianserin or amitriptyline. Journal of Affective Disorders 1982;4:219‐26. [DOI] [PubMed] [Google Scholar]
Metha 1980 {published data only}
- Metha BM, Spear FG, Whittington JR. A double‐blind controlled trial of mianserin and amitriptyline in depression. Current Medical Research Opinion 1980;7:14‐22. [DOI] [PubMed] [Google Scholar]
Mindham 1977 {published data only}
- Mindham BA. A comparison of maprotiline (Ludiomil) and amitriptyline. Journal of International Medical Research 1977;5 Suppl 4:25‐33. [PubMed] [Google Scholar]
Moises 1981 {published data only}
- Moises HW, Kasper S, Beckmann H. Trazodone and amitriptyline in treatment of depressed inpatients. A double‐blind study. Pharmacopsychiatry 1981;14:167‐71. [DOI] [PubMed] [Google Scholar]
Moller 1993 {published data only}
- Moller HJ, Berzewski H, Eckmann F, Gonzalves N, Kissling W, Knorr W, et al. Double‐blind multicenter study of paroxetine and amitriptyline in depressed patients. Pharmacopsychiatry 1993;26:75‐8. [DOI] [PubMed] [Google Scholar]
Moller 1995 {published data only}
- Moller HJ, Kasper S, Muller H, Kissling W, Fuger J, Ruhrmann S. A controlled study of the efficacy and safety of mianserin and amitriptyline in depressive inpatients. Pharmacopsychiatry 1995;28:249‐52. [DOI] [PubMed] [Google Scholar]
Moller 1998 {published data only}
- Moller HJ, Gallinat J, Hegerl U, Arato M, Janka Z, Pflug B, et al. Double‐blind, multicenter comparative study of sertraline and amitriptyline in hospitalized patients with major depression. Pharmacopsychiatry 1998;31:170‐7. [DOI] [PubMed] [Google Scholar]
Moller 2000 {published data only}
- Moller HJ, Glaser K, Leverkus F, Gobel C. Double‐blind, multicenter comparative study of sertraline versus amitriptyline in outpatients with major depression. Pharmacopsychiatry 2000;33:206‐12. [DOI] [PubMed] [Google Scholar]
Molnar 1977 {published data only}
- Molnar G. Maprotiline ‐ a double‐blind study of a new tetracyclic antidepressant in severe depression. Canadian Psychiatric Association Journal 1977;22:19‐23. [DOI] [PubMed] [Google Scholar]
Montbrun 1976 {published data only}
- Montbrun F, Obermair W. Lofepramine versus amitriptyline. A double‐blind study [Doppelblindstudie lofepramin versus amitriptylin]. Therapiewoche 1976;26:8722‐6. [Google Scholar]
Monteleone 1994 {published data only}
- Monteleone P, Fabrazzo M. Blood levels of mianserin and amitriptyline and clinical response in aged depressed patients. Pharmacopsychiatry 1994;27:238‐41. [DOI] [PubMed] [Google Scholar]
Montgomery 1980 {published data only}
- Montgomery SA, McAuley R, Montgomery DB, Dawling S, Braithwaite RA. Pharmacokinetics and efficacy of maprotiline and amitriptyline in endogenous depression: a double‐blind controlled trial. Clinical Therapeutics 1980;3:292‐310. [PubMed] [Google Scholar]
Muller‐Oerlingh 1979 {published data only}
- Muller‐Oerlinghausen B, Ruther E, Adam HK. Clinical profile and serum concentration of viloxazine as compared to amitriptyline. Pharmakopsychiatry 1979;12:321‐37. [DOI] [PubMed] [Google Scholar]
Murasaki 1990 {published data only}
- Murasaki M, Kurihara M, Takahashi R, Mori A, Hasegawa K, Endo S, et al. Clinical Effects of Trazodone (KB‐831) on depression. Clinical Evaluation 1990;18:279‐313. [Google Scholar]
Murphy 1978 {published data only}
- Murphy JE, Bridgman KM. A comparative clinical trial of mianserin (Norval) and amitriptyline in the treatment of depression in general practice. Journal of International Medical Research 1978;6:199‐206. [DOI] [PubMed] [Google Scholar]
Murphy 1980 {published data only}
- Murphy JE, Ankier SI. An evaluation of trazodone in the treatment of depression. Neuropharmacology 1980;19:1217‐8. [DOI] [PubMed] [Google Scholar]
Naftulin 1972 {published data only}
- Naftulin DH, Ware JE. A behavioural and clinical evaluation of two psychotropic agents: doxepin‐hydrochloride & pherphenazine‐amitriptyline hydrochloride. Psychosomatics 1972;XIII:125‐30. [DOI] [PubMed] [Google Scholar]
Nelson 1982 {published data only}
- Nelson WH, Orr WW, Stevenson JM, Shane SR. Hypothalamic‐pituary‐adrenal axis activity and tricyclic response in major depression. Archives of General Psychiatry 1982;39:1033‐6. [DOI] [PubMed] [Google Scholar]
Nieto 1973 {published data only}
- Nieto D, Rincon HP. A new antidepressive agent: C 34,276‐Ba or maprotyline, 1st tetracyclic thymoanaleptic Clinical study [Un nuevo antidepresivo: el C 34,276‐Ba o maprotilina, primer timoanaleptico tetraciclico]. Prensa Medica Mexicana 1973;38(11):429‐34. [PubMed] [Google Scholar]
Nugent 1979 {published data only}
- Nugent D. A double‐blind study of viloxazine and amitriptyline in depressed geriatric patients. Clinical Trials Journal 1979;16:13‐7. [Google Scholar]
Okasha 1976 {published data only}
- Okasha A, Sadek A. A controlled, double‐blind clinical trial between maprotiline and imipramine in depressive illness. Journal of the Egyptian Medical Association 1976;59:557‐62. [PubMed] [Google Scholar]
Peters 1990 {published data only}
- Peters UH, Lenhard P, Metz M. Therapy of depression in the psychiatrist's office ‐ A double‐blind multicenter study [Ambulante therapie der depression mit fluoxetin ‐ eine multizentrische doppelblindstudie]. Nervenheihunde 1990;9:28‐31. [Google Scholar]
Petrie 1982 {published data only}
- Petrie WM, Ban TA, Wilson WH, Jamieson RC, Guy W. Viloxazine in the treatment of endogenous depression. A standard (amitriptyline) controlled clinical study. International Pharmacopsychiatry 1982;17:280‐6. [DOI] [PubMed] [Google Scholar]
Preskorn 1991 {published data only}
- Preskorn SH, Silkey B, Beber J, Dorey C. Antidepressant response and plasma concentrations of fluoxetine. Annals of Clinical Psychiatry 1991;3:147‐51. [Google Scholar]
Prusoff 1981 {published data only}
- Prusoff B, Weissman MM, Charney J, Dockerty J, Kleber H, Rounsavill BJ, et al. Speed of symptom reduction in depressed outpatients treated with amoxapine and amitriptyline. Current Therapeutic Research 1981;30:843‐55. [Google Scholar]
Pugh 1982 {published data only}
- Pugh R, Bell J, Cooper AJ, Dunstan R, Greedharry D, Pomeroy J, et al. Does lofepramine have fewer side effects than amitriptyline?. Journal of Affective Disorders 1982;4:355‐63. [DOI] [PubMed] [Google Scholar]
Quadri 1980 {published data only}
- Quadri AA, Shalini K, Channabasavanna SM. d‐Amphetamine as a predictor for response to imipramine and amitriptyline. Indian Journal of Psychiatry 1980;22:182‐4. [PMC free article] [PubMed] [Google Scholar]
Querol 1970 {published data only}
- Querol M. Double blind studies with antidepressive drugs [Estudio doble ciego con antidepresivos]. Revista de Neuro‐Psiquiatria 1980;XXXIII:251‐70. [PubMed] [Google Scholar]
Rabkin 1984 {published data only}
- Rabkin JG, McGrath PJ, Quitkin FM, Fyer A, Stewart JW, Liebowitz MR, et al. Mianserin versus amitriptyline for depression: a double‐blind 6‐week trial. Neuropsychobiology 1984;12:224‐8. [DOI] [PubMed] [Google Scholar]
Rampello 1995 {published data only}
- Rampello L, Nicoletti G, Raffaele R, Drago F. Comparative effects of amitriptyline and amineptine in patients affected by anxious depression. Pharmacopsychiatry 1995;31:130‐4. [DOI] [PubMed] [Google Scholar]
Rees 1976 {published data only}
- Rees JA, Cryer PC. A single‐blind comparative study of once daily dothiepin (Prothiaden) and divided daily doses of amitriptyline. Current Medical Research Opinion 1976;6:416‐21. [DOI] [PubMed] [Google Scholar]
Rego 1974 {published data only}
- Rego A, Sanchez De Vega J. Single‐blind comparative study between patients with ludiomil (Ciba 34,276‐Ba) and its evaluation with Hamilton's scale [Estudio comparativo simple‐ciego entre pacientes con Ludiomil (Ciba 34, 276‐Ba) y su valoraciòn con la escala de Hamilton]. Archivos Neurobiologica 1974;37:475‐84. [PubMed] [Google Scholar]
Reimherr 1990 {published data only}
- Reimherr FW, Chouinard G, Cohn CK, Cole JO, Itil T, Lapierre YD. Antidepressant efficacy of sertraline: a double blind, placebo‐ and amitriptyline‐controlled, multicenter comparison study in outpatients with major depression. Journal of Clinical Psychiatry 1990;51 Suppl B:18‐27. [PubMed] [Google Scholar]
Remick 1994 {published data only}
- Remick RA, Reesal R, Oakander M, Allen J, Claman J, Ramirez CE, et al. Comparison of fluvoxamine and amitriptyline in depressed outpatients. Current Therapeutic Research 1994;55:243‐50. [Google Scholar]
Richmond 1964 {published data only}
- Richmond PW, Roberts AH. A comparative trial of imipramine, amitriptyline, isocarboxazid and tranylcypromine in out‐patient depressive illness. British Journal of Psychiatry 1964;110:846‐50. [DOI] [PubMed] [Google Scholar]
Rickels 1970 {published data only}
- Rickels K, Gordon PE, Weise CC, Bazilian SE, Feldman HS, Wilson DA. (1970) Amitriptyline and trimipramine in neurotic depressed outpatients: A collaborative study. American Journal of Psychiatry 1970;127:126‐218. [DOI] [PubMed] [Google Scholar]
Rickels 1972 {published data only}
- Rickels K, Hutchinson JC, Weise CC, Csanalosi I, Chung HR, Case WG. Doxepin and amitriptyline‐perphenazine in mixed anxious‐depressed neurotic outpatients: a collaborative controlled study. Psychopharmacologia 1972;23:305‐18. [DOI] [PubMed] [Google Scholar]
Rickels 1974 {published data only}
- Rickels K, Weise CC, Csanalosi I, Chung HR, Feldman HS, Rosenfeld H, et al. Clomipramine and amitriptyline in depressed outpatients. A controlled study. Psychopharmacology 1974;34:361‐76. [DOI] [PubMed] [Google Scholar]
Rickels 1982 {published data only}
- Rickels K, Case WG. Trazodone in depressed outpatients. American Journal of Psychiatry 1982;139:803‐6. [DOI] [PubMed] [Google Scholar]
Rickels 1982a {published data only}
- Richels K, Csanalosi I, Werblowsky J, Weise CC, Weinstock R, Brown AS, et al. Amitriptyline‐perphenazine and doxepin in depressed outpatients: a controlled double‐blind study. Journal of Clinical Psychiatry 1982;43:419‐22. [PubMed] [Google Scholar]
Rickels 1985 {published data only}
- Richels K, Feighner JP, Smith WT. Alprazolam, amitriptyline, doxepin, and placebo in the treatment of depression. Archives of General Psychiatry 1985;42:134‐41. [DOI] [PubMed] [Google Scholar]
Rose 1965 {published data only}
- Rose JT, Leahy MR, Martin IC, Westhead TT. A comparison of nortriptyline and amitriptyline in depression. British Journal of Psychiatry 1985;III:1101‐3. [DOI] [PubMed] [Google Scholar]
Rush 1988 {published data only}
- Rush AJ, Weissenburger J, Vasavada N, Orsulak PJ, Fairchild CJ. Dexamethasone suppression test status does not predict differential response to nortriptyline versus amitriptyline. Journal of Clinical Psychopharmacology 1988;8:421‐5. [PubMed] [Google Scholar]
Rush 1989 {published data only}
- Rush AJ, Giles DE, Jarret RB, Feldman‐Koffler F, Debus JR, Weissenburger J, et al. Reduced REM latency predicts response to tricyclic medication in depressed outpatients. Biological Psychiatry 1989;26:61‐72. [DOI] [PubMed] [Google Scholar]
Rybakowski 1991 {published data only}
- Rybakowski J, Matkowski K, Linka M. Monitoring of the treatment of endogenous depression with imipramine and amitriptyline (preliminary report) [Monitorowane leczenie depresji endogennej imipramina i amitryptylina]. Psychiatria Polska 1991;25:111‐8. [PubMed] [Google Scholar]
Sacchetti 2002 {published data only}
- Sacchetti E, Casano GB, Penati G, Pavan L, Nardini M, Casacchia M, et al. Paroxetine versus amitriptyline in patients with recurrent major depression: a double‐blind trial. International Journal of Psychiatry in Clinical Practice 2002;6:23‐29. [DOI] [PubMed] [Google Scholar]
Sandifer 1965 {published data only}
- Sandifer MG, Wilson IC, Gambill JM. The influence of case selection and dosage in an antidepressant drug trial. British Journal of Psychiatry 1965;III:142‐8. [DOI] [PubMed] [Google Scholar]
Sedman 1977 {published data only}
- Sedman G. Double‐blind trial of sustained‐release amitriptyline compared with viloxazine in moderate to severe depressive illness. Current Medical Research and Opinion 1977;5:217‐25. [DOI] [PubMed] [Google Scholar]
Sethi 1979 {published data only}
- Sethi BB, Sharma I, Singh H, Metha UK. Amoxapine and amitriptyline: a double‐blind study in depressed patients. Current Therapeutic Research 1979;25:726‐37. [Google Scholar]
Shaw 1986 {published data only}
- Shaw DM, Thomas DR, Briscoe MH, Watkins SE, Crimmins R, Harris B, et al. A comparison of the antidepressant action of citalopram and amitriptyline. British Journal of Psychiatry 1986;149:515‐7. [DOI] [PubMed] [Google Scholar]
Shipley 1985 {published data only}
- Shipley JE, Kupfer DJ, Griffin SJ, Dealy RS, Coble PA, McEachran AB, et al. Comparison of effects of desipramine and amitriptyline on EEG sleep of depressed patients. Psychopharmacology 1985;85:14‐22. [DOI] [PubMed] [Google Scholar]
Silverstone 1977 {published data only}
- Silverstone JT. A comparison of maprotiline and amitriptyline in the treatment of depression in general practice. The Practitioner 1977;218:279‐82. [PubMed] [Google Scholar]
Sims 1980 {published data only}
- Sims AC. Comparison of the efficacy of sustained‐release amitriptyline with maprotiline in the treatment of depressive illness. Current Medical Research and Opinion 1980;6:534‐9. [DOI] [PubMed] [Google Scholar]
Sinclair 1975 {published data only}
- Sinclair JM, Walsh MR, Valle‐Jones JC, Schiff AA. Treatment of anxiety/depressive conditions in the elderly: a double‐blind comparative study of motival and amitriptyline. Age and Ageing 1975;4:226‐31. [DOI] [PubMed] [Google Scholar]
Solis 1970 {published data only}
- Solis HG, Molina GB, Pineyro A. Clinical evaluation of doxepin and amitriptyline in depressed patients. Current Therapeutic Research 1970;12:524‐7. [PubMed] [Google Scholar]
Staner 1995 {published data only}
- Staner L, Kerkhofs M, Detroux D, Leyman S, Linkowski P, Mendlewicz J. Acute, subchronic and withdrawal sleep EEG changes during treatment with paroxetine and amitriptyline: a double‐blind randomized trial in major depression. Sleep 1995;18:470‐7. [PubMed] [Google Scholar]
Stier 1982 {published data only}
- Stier CS, Neumann M, Elizur A. Comparative double‐blind study between maprotiline and amitriptyline in one fixed nightly dose in major depressive disorder. Current Therapeutic Research 1982;32:447‐56. [Google Scholar]
Stott 1993 {published data only}
- Stott PC, Blagden MD, Aitken CA. Depression and associated anxiety in primary care: a double‐blind comparison of paroxetine and amitriptyline. European Neuropsychopharmacology 1993;3:324‐5. [Google Scholar]
Straker 1966 {published data only}
- Straker M, Davanloo H, Moll A. A double‐blind comparison of a new antidepressant, protryptiline, with imipramine and amitriptyline. Canadian Medical Association Journal 1966;94:1220‐2. [PMC free article] [PubMed] [Google Scholar]
Stuppaeck 1994 {published data only}
- Stuppaeck CH, Geretsegger C, Whitworth AB, Schubert H, Platz T, Konig P, et al. A multicentre double‐blind trial of paroxetine versus amitriptyline in depressed inpatients. Journal Clinical Psychopharmacology 1994;14:241‐6. [PubMed] [Google Scholar]
Toru 1972 {published data only}
- Toru M, Takamizawa M, Kariya T, Kobayashi K, Takahashi R. A double‐blind sequential comparison of doxepin with amitriptyline in depressed patients. Psychosomatics 1972;XIII:241‐50. [DOI] [PubMed] [Google Scholar]
Trappe 1973 {published data only}
- Trappe B. Doxepin and amitriptyline‐chlordiazepoxide combination in neurotic states. Psychiatria Fennica 1973;4:269‐75. [Google Scholar]
Trick 1975 {published data only}
- Trick KL. Double‐blind comparison of maprotiline (Ludiomil) with amitriptyline in the treatment of depressive illness. Journal of International Medical Research 1975;3 Suppl 2:67‐70. [Google Scholar]
Tsaras 1981 {published data only}
- Tsaras G, Ambrus J. Comparative clinical trial of chlordiazepoxide plus amitriptyline against maprotiline in depression with a component of anxiety [Etude clinique comparative de l'association chlordiazepoxide‐amitriptyline et de la maprotiline chez des patients souffrant de dépression à composante anxieuse]. Revue Medicale de la Suisse Romande 1981;101:483‐6. [PubMed] [Google Scholar]
Upward 1988 {published data only}
- Upward JW, Edwards JG, Goldie A, Waller DG. Comparative effects of fluoxetine and amitriptyline on cardiac function. British Journal of Clinical Pharmacology 1988;26:399‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Amerongen 1979 {published data only}
- Amerongen P. Double‐blind clinical trial of the antidepressant action of amineptine. Current Medical Research and Opinion 1979;6:93‐100. [DOI] [PubMed] [Google Scholar]
Veith 1983 {published data only}
- Veith RC, Bielski RJ, Bloom V, Fawcett JA, Narasimhachari N, Friedel RO. Urinary MHPG excretion and treatment with desipramine or amitriptyline: prediction of response, effect of treatment, and methodological hazards. Journal of Clinical Psychopharmacology 1983;3:18‐27. [PubMed] [Google Scholar]
Versiani 1999 {published data only}
- Versiani M, Ontiveros A, Mazzotti G, Ospina J, Davila J, Mata S, et al. Fluoxetine versus amittriptyline in the treatment of major depression with associated anxiety (anxious depression): a double‐blind comparison. International Clinical Psychopharmacology 1999;14:321‐7. [DOI] [PubMed] [Google Scholar]
Von Bauer 1969 {published data only}
- Bauer G, Nowak H. Doxepin, a new antidepressant: comparison of effects with amitriptyline [Doxepin, ein neues antidepressivum: wirkungsvergleich mit amitriptylin]. Arzneimittezforschung 1969;19:1642‐6. [PubMed] [Google Scholar]
Waite 1986 {published data only}
- Waite J, Grundy E, Arie T. A controlled trial of antidepressant medication in elderly in‐patients. International Clinical Psychopharmacology 1986;1:113‐26. [DOI] [PubMed] [Google Scholar]
Watanabe 1978 {published data only}
- Watanabe S, Yokoyama S, Kubo S, Iwai H, Kuyama C. A double‐blind controlled study of clinical efficacy of maprotiline and amitriptyline in depression. Folia Psychiatrica et Neurologica Japonica 1978;32:1‐31. [DOI] [PubMed] [Google Scholar]
Weissman 1975 {published data only}
- Weissman MM, Lieb J, Prusoff B, Bothwell S. A double‐blind trial of maprotiline (Ludiomil) and amitriptyline in depressed outpatients. Acta Psychiatrica Scandinavica 1975;52:225‐36. [DOI] [PubMed] [Google Scholar]
Wheatley 1975 {published data only}
- Wheatley D. Controlled clinical trial of a new antidepressant (ORG.GB 94) of novel chemical formulation. Current Therapeutic Research 1975;18:849‐54. [PubMed] [Google Scholar]
Wheatley 1992 {published data only}
- Wheatley D. MInaprine in depression: a controlled trial with Amitriptlyline. British Journal of Psychiatry 1992;161:113‐5. [DOI] [PubMed] [Google Scholar]
Wilcox 1994 {published data only}
- Wilcox CS, Cohn JB, Katz BB, Mijares CP, Guarino JJ, Panagides J, et al. A double‐blind, placebo‐controlled study comparing mianserin and amitriptyline in moderately depressed outpatients. International Clinical Psychopharmacology 1994;9:271‐9. [DOI] [PubMed] [Google Scholar]
Wright 1976 {published data only}
- Wright S, Hermann L. Double‐blind comparative study on the effects of lofepramine and amitriptyline in depressive outpatients [Doppelblindversuch zum wirkungsvergleich von lofepramin und amitriptylin bei ambulant behandelten patienten mit depressiven zustandsbildern]. Arzneimittezforschung 1976;26:1167‐69. [PubMed] [Google Scholar]
Yamhure 1977 {published data only}
- Yamhure A, Villalobos A. Amoxapine: a double‐blind comparative clinical study of amoxapine and amitriptyline in depressed, hospitalised patients. Current Therapeutic Research 1977;21:502‐6. [Google Scholar]
Young 1987 {published data only}
- Young JPR, Coleman A, Lader MH. A controlled comparison of fluoxetine and amitriptyline in depressed outpatients. British Journal of Psychiatry 1987;151:337‐40. [DOI] [PubMed] [Google Scholar]
Ziegler 1977 {published data only}
- Ziegler VE, Clayton PJ, Biggs JT. A comparison study of amitriptyline and nortriptyline with plasma levels. Archives of General Psychiatry 1977;34:607‐12. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Anonimous 1971 {published data only}
- Anonymous. Multihospital controlled comparison of the therapeutic effects of four antidepressants. Activitas Nervosa Superiora 1971;13:166‐7. [PubMed] [Google Scholar]
Branconnier 1981 {published data only}
- Branconnier RJ, Cole JO, Ghazvinian S. The therapeutic profile of mianserin in mild elderly depressives. Psychopharmacology Bulletin 1981;17:129‐31. [PubMed] [Google Scholar]
Claghorn 1984 {published data only}
- Claghorn JL, Schroeder J, Goldstein BJ 2nd. Comparison of the electrocardiographic effect of dothiepin and amitriptyline. Journal of Clinical Psychiatry 1984;45:91‐3. [PubMed] [Google Scholar]
Coppen 1976 {published data only}
- Coppen A, Gupta R, Montgomery S, Ghose K, Burns B, Ridder JJ. Mianserin hydrochloride: a novel antidepressant. British Journal of Psychiatry 1976;129:342‐5. [DOI] [PubMed] [Google Scholar]
Deuschle 2004 {published data only}
- Deuschle M, Krumm B, Bindeballe N, et al. Open‐label non‐randomized versus double‐blind randomized antidepressive treatment: what are the advantages of clinical decision over randomization?. Pharmacopsychiatry 2004;37:299‐302. [DOI] [PubMed] [Google Scholar]
Elwan 1976 {published data only}
- Elwan O, Taher Y, Allam M, Hassan MA. A comparative study of antidepressants in the treatment of depressive states. Journal of International Medical Research 1976;4(3):202‐10. [DOI] [PubMed] [Google Scholar]
Friedel 1979 {published data only}
Hackett 1967 {published data only}
- Hackett E, Gold RL, Kline NS, Winick L. Controlled evaluation of psychotropic drugs in a private psychiatric practice. Doxepin vs. amitriptyline (Elavil). Psychosomatics 1967;8:162‐5. [DOI] [PubMed] [Google Scholar]
Hackett 1967a {published data only}
Hollister 1964 {published data only}
- Hollister LE, Overall JE, Johnson M, Pennington V, Katz G, Shelton J. Controlled comparison of amitriptyline, imipramine and placebo in hospitalised depressed patients. Journal of Nervous and Mental Disease 1964;139:370‐5. [DOI] [PubMed] [Google Scholar]
Hutchinson 1963 {published data only}
- Hutchinson JT, Smedberg D. Treatment of depression: a comparative study of ECT and six drugs. British Journal of Psychiatry 1963;109:536‐8. [DOI] [PubMed] [Google Scholar]
Kiebach 1982 {published data only}
- Kieback D. Comparison of the Hamilton Depression Rating Scale and the Self Rating Scale BS (v. Zerssen) within a double blind study on the antidepressant effects of trazodone verus amitriptyline [Vergleich der Hamilton Rating Scale for Depression (HRS) mit der v. Zerssen‐Befindlichkeitsskala (BS) am Beispiel einer klinischen Prufung von Trazodon versus Amitriptylin]. Pharmacopsychiatry 1982;15:97‐102. [DOI] [PubMed] [Google Scholar]
Lambourn 1974 {published data only}
- Lambourn J, Rees JA. A general practitioner study of dothiepin and amitriptyline. Journal of International Medical Research 1974;2:210‐3. [Google Scholar]
Lloyd 1981 {published data only}
- Lloyd C, Zisook S, Click M, Jaffe KE. Life events and response to antidepressants. Journal of Human Stress 1981;7:2‐15. [DOI] [PubMed] [Google Scholar]
Maier 1989 {published data only}
- Maier W, Philipp M, Schlegel S, Heuser I, Wiedemann K, Benkert O. Diagnostic determinants of response to treatment with tricyclic antidepressants: a polydiagnostic approach. Psychiatry Research 1989;30:83‐93. [DOI] [PubMed] [Google Scholar]
Marjerrison 1969 {published data only}
- Marjerrison G, Deza PC, Silzer JC. Verbal behaviour changes with depression treatment. Canadian Psychiatric Association Journal 1969;14:503‐8. [DOI] [PubMed] [Google Scholar]
Montgomery 1978 {published data only}
Moyes 1980 {published data only}
- Moyes IC, Ray RL, Moyes RB. Plasma levels and clinical improvement ‐ a comparative study of clomipramine and amitriptyline in depression. Postgraduate Medical Journal 1980;58 Suppl 1:127‐9. [PubMed] [Google Scholar]
Peet 1977 {published data only}
Renfordt 1976 {published data only}
- Renfordt E, Bush H. New diagnostic strategies in psychiatry by means of video‐technique I The use of time‐blind video analysis for the evaluation of antidepressant drug trials [Neue Strategien psychiatrischer Urteilsbildung durch Anwendung audiovisueller Techniken 1 Mitteilung: Verlaufsuntersuchung einer antidepressiven Therapie durch vergleichende und zeitblinde Auswertung von Fernsehaufnahmen]. Pharmakopsychiatrie Neuro‐Psychopharmakologie 1976;9:67‐75. [DOI] [PubMed] [Google Scholar]
Saletu 1969 {published data only}
Stratas 1984 {published data only}
- Stratas NE. A double‐blins study of the efficacy and safety of dothiepin hydrochloride in the treatment of major depressive disorder. Journal of Clinical Psychiatry 1984;45:466‐9. [PubMed] [Google Scholar]
Taverna 1969 {published data only}
- Taverna P, Ferrari G. Comparative evaluation of nortriptyline, amitriptyline and of a placebo in the treatment of anxiety and depressive disorders [Valutazione comparativa della nortriptilina dell'amitriptilina e del placebo nel trattamento delle forme ansiose e depressive]. Minerva Medica 1969;60:2417‐31. [PubMed] [Google Scholar]
Van De Merwe 1984 {published data only}
- Merwe TJ, Silverstone T, Ankie SI, Warrington SJ, Turner P. A double‐blind non‐crossover placebo‐controlled study between group comparison of trazodone and amitriptyline on cardiovascular function in major depressive disorder. Psychopathology 1984;17 Suppl 2:64‐76. [DOI] [PubMed] [Google Scholar]
Vartanian 1984 {published data only}
- Vartanyan FE. Effects of antidepressants in different populations [Effekty antidepressantov v razlichnykh populiatsiiakh]. Farmakologiia i Toksikologiia 1984;47:13‐7. [PubMed] [Google Scholar]
Vogel 1976 {published data only}
- Vogel HP, Bente D, Feder J, Helmchen H, Muller‐Oerlinghausen B, Bohacek N, et al. Mianserin versus amitriptyline. A double‐blind trial evaluated by the AMP system. International Pharmacopsychiatry 1976;11:25‐31. [PubMed] [Google Scholar]
Additional references
Altman 1996
- Altman DG, Bland JM. Detecting skewness from summary information. BMJ 1996;313:1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anderson 1995
- Anderson IM, Tomenson BM. Treatment discontinuation with selective serotonin reuptake inhibitors compared with tricyclic antidepressants: a meta‐analysis. BMJ 1995;310:1433‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anderson 1998
- Anderson IM. SSRIs versus tricyclic antidepressants in depressed inpatients: a meta‐analysis of efficacy and tolerability. Depression and Anxiety 1998;7 Suppl 1:11‐7. [PubMed] [Google Scholar]
Anderson 2000
- Anderson IM. Selective serotonin reuptake inhibitors versus tricyclic antidepressants: a meta‐analysis of efficacy and tolerability. Journal of Affective Disorders 2005;58:19‐36. [DOI] [PubMed] [Google Scholar]
Anon 1993 a
- Anon. Selective serotonin reuptake inhibitors for depression. Drug and Therapeutic Bulletin 1993;31(15):57‐8. [PubMed] [Google Scholar]
Anon 1993 b
- Anon. The treatment of depression in primary health care. Effective Health Care Bulletin 1993;1:5. [Google Scholar]
APA 1980
- American Psychiatric Association. Diagnostic and Statistical Manual of mental disorders. 3rd Edition. Washington DC: American Psychiatric Association, 1980. [Google Scholar]
APA 1994
- American Psychiatric Association. Diagnostic and Statistical Manual of mental disorders. 4th Edition. Washington DC: American Psychiatric Association, 1994. [Google Scholar]
Barbui 1999
- Barbui C, Campomori A, D'Avanzo B, Negri E, Garattini S. Antidepressant drug use in Italy since the introduction of SSRIs: national trends, regional differences and impact on suicide rates. Social Psychiatry Psychiatric Epidemiology 1999;34:152‐6. [DOI] [PubMed] [Google Scholar]
Barbui 2001a
- Barbui C, Hotopf M. Amitriptyline versus the rest: still the leading antidepressant after 40 years of randomised controlled trials. British Journal of Psychiatry 2001;178:129‐44. [DOI] [PubMed] [Google Scholar]
Barbui 2001b
- Barbui C, Hotopf M. Forty years of antidepressant drug trials. Acta Psychiatrica Scandinavica 2001;194(2):92‐5. [DOI] [PubMed] [Google Scholar]
CCOHTA 1997
- Canadian Coordinating Office for Health Technology Assessment. Selective serotonin reuptake inhibitors (SSRIs) for major depression. Part I. Evaluation of the clinical literature. Ottawa: Canadian Coordinating Office for Health Technology Assessment, 1997. [Google Scholar]
Der Simonian 1986
- Simonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
DTB 1993
- Drug, Therapeutic Bulletin. Selective serotonin reuptake inhibitors for depression. Drug and Therapeutic Bulletin 1993;31:57‐8.. [PubMed] [Google Scholar]
Effective H C 1993
- Effective Health Care. The treatment of depression in primary health care. Effective Health Care Bulletin 1993;5:No pagination. [Google Scholar]
Feighner 1972
- Feighner JP, Robins E, Guze B, Woodruff RA, Winokur G, Munoz R. Diagnostic criteria for use in psychiatric research. Archives of General Psychiatry 1972;26(1):57‐63. [DOI] [PubMed] [Google Scholar]
Garattini 1988
- Garattini S, Samanin R. Biochemical hypothesis on antidepressant drugs: a guide for clinicians or a toy for pharmacologists?. Psychological Medicine 1988;18:287‐304. [DOI] [PubMed] [Google Scholar]
Hamilton 1960
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry 1960;23:56‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hotopf 1997
- Hotopf MH, Hardy R, Lewis G. (1997) Discontinuation rates of SSRI and tricyclic antidepressants: a meta‐analysis and investigation of heterogeneity. British Journal of Psychiatry 1997;170:120‐7. [DOI] [PubMed] [Google Scholar]
Joffe 1996
- Joffe R, Sokolov S, Streiner D. Antidepressant treatment of depression. A meta‐analysis. Canadian Journal of Psychiatry 1996;41:613‐6. [DOI] [PubMed] [Google Scholar]
Moncrieff 2001
- Moncrieff J, Churchill R, Drummond C, McGuire H. Development of a quality assessment instrument for trials of treatments for depression and neurosis. International Journal of Methods in Psychiatric Research 2001;10(3):126‐33. [Google Scholar]
Montgomery 1979
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry 1979;134:382‐9. [DOI] [PubMed] [Google Scholar]
Perry 1996
- Perry PJ. Pharmacotherapy for major depression with melancholic features: relative efficacy of tricyclic versus selective serotonin reuptake inhibitor antidepressants. Journal of Affective Disorders 1996;39:1‐6. [DOI] [PubMed] [Google Scholar]
Spigset 1999
- Spigset O, Martensson B. Drug treatment of depression. BMJ 1999;318(7192):1188‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spitzer 1977
- Spitzer RL, Endicott J, Robins E. Research Diagnostic Criteria (RDC) for a selected group of functional disorders. 3rd Edition. New York, NY: Biometric Research, 1977. [Google Scholar]
WHO 1974
- World Health Organization. International Classification of Diseases. 8th Edition. Geneva: World Health Organisation, 1974. [Google Scholar]
WHO 1992
- World Health Organization. International Classification of Diseases. 10th Edition. Geneva: World Health Organisation, 1992. [Google Scholar]


