Abstract
Antiretroviral drug therapy and cytotoxic T lymphocytes (CTL) both exert selective pressures on human immunodeficiency virus type 1, which influence viral evolution. Compared to chronically infected, antiretroviral-untreated patients, most chronically infected, treated patients with detectable viremia lack a cellular immune response against the Gag 77-85(SL9) epitope but show a new immunodominant response against an epitope in protease PR 76-84. Hence, mutations induced by antiretroviral therapy likely alter the profile of epitopes presented to T cells and thus the direction of the response. The consequences of dual pressures from treatment and CTL need to be considered in monitoring of drug therapy.
The course of human immunodeficiency virus type 1 (HIV-1) infection is characterized by the development of an intricate CD8+ cytotoxic T-lymphocyte (CTL) response directed against an evolving viral genome (24). CTL pressure can lead to early escape from a dominant epitope, as shown in the simian immunodeficiency virus model, where pressure on Tat leads to rapid escape and a change in dominance to recognition of the Gag CM9 epitope (3). A different pattern of changing epitopic usage over time is illustrated by responses to the immunodominant HLA-A2-restricted Gag 77-85 (77SLYNTVATL85 [SL9]) epitope in p17 Gag. SL9 is recognized by the majority of chronically infected individuals but not by most acutely infected individuals (4, 10, 16, 18, 21, 24, 37). This temporal relationship for CTL recognition of virus proteins/epitopes is also supported by observations that the HIV-1-Nef protein is recognized before other proteins (2, 5, 9, 28) and, in mouse models, by constantly evolving CTL immunodominance (7, 42-44).
In addition to CTL, therapy with antiretroviral drugs also exerts significant pressures on the viral genome. This treatment-associated pressure is largely direct and can lead to the rapid accumulation of drug-resistance associated mutations. Among treated patients with drug-resistant HIV, the steady-state viral load is often lower than the pretreated levels. This partial viral suppression is due in part to residual activity of antiretroviral drugs and reduced replicative capacity of the drug-resistant variants (6, 13). We and others, however, have argued that drug treatment may exert pressure indirectly via the immune system (1, 12, 23, 36, 38). This interaction can occur via a number of non-mutually exclusive mechanisms, including treatment-mediated selection of new mutations that act as novel epitopes (23, 31-33, 39-41) and/or treatment-mediated decreases in replicative capacity (“fitness”), which can lead to a reduction in the ability of HIV-1 to destroy antigen-specific T cells (6, 11, 13, 20).
Although several studies suggest that treatment can modify viral evolution via its effect on T-cell immunity, no study has comprehensively studied the impact of treatment on the hierarchy of immunodominant responses. To address this issue, we conducted a detailed study of the interactions between viral sequence changes and the specificity of the cellular immune responses. We focused our analysis on well-described HLA-A2-restricted epitopes, including SL9, and studied HLA-A2-positive treated and untreated subjects during primary and chronic HIV-1 infection.
A total of 38 HLA-A2-positive subjects were identified from the UCSF cohorts of primary infection (OPTIONS) (19, 24) and from the SCOPE cohort of chronically infected subjects (14) and were divided into three groups. The three groups were designated as follows. Group 1 contained subjects with acute infection and who were antiretroviral untreated (n = 8; median viral load of 4.4 logs, median CD4 cell count of 541 cells/mm3 and median estimated duration of infection 8 weeks), group 2 contained subjects with chronic infection and who were antiretroviral untreated (n = 10; median viral load of 4.4 logs, median CD4 cell count of 322 cells/mm3), and group 3 contained subjects with chronic infection and who were protease inhibitor treated with detectable viremia (n = 20; median viral load of 3.9 logs and median CD4 cell count of 287 cells/mm3). The protease inhibitor-treated subjects all had developed a number of resistance-associated mutations against both reverse transcriptase and protease inhibitors (23). Viral sequence data were generated using population-based sequencing from extracted viral RNA in plasma [Dynabeads Oligo(dT)25; Invitrogen Corporation, Dynal Biotech, Oslo, Norway] and viral DNA from peripheral blood mononuclear cells (QIAamp DNA blood kit; QIAGEN, Valencia, CA), as indicated in Table 1. Sequences obtained from the same individual originating from viral DNA and RNA always aligned together and showed high sequence identity with each other. When available, sequences obtained from viral RNA in plasma were used in the sequence analysis. The study was approved by the UCSF Institutional Review Board, and all subjects provided written informed consent.
TABLE 1.
Patient characteristics and treatment history of chronically untreated and treatment-experienced HIV-1-infected subjects
| Patient | Viral load (copies/ml) | CD4 count (cells/mm3) | Treatmenta | Origin of viral sequences
|
||
|---|---|---|---|---|---|---|
| Gag | Pol | Nef | ||||
| Chronically infected, untreated | ||||||
| 1028 | 8,626 | 411 | Last meds in 1992; no PIs | RNA/DNA | RNA | RNA |
| 1029 | 2,062 | 373 | Never | RNA | RNA | RNA |
| 1030 | 201,304 | 258 | Never | RNA/DNA | RNA | RNA |
| 1034 | 34,004 | 365 | Never | RNA | RNA | RNA |
| 1038 | 27,846 | 178 | Never | RNA/DNA | RNA | RNA |
| 1051 | 65,276 | 238 | Last meds in 1998; no PIs | RNA | RNA | RNA |
| 1057 | 8,638 | 216 | last meds in 1999; no PIs | RNA | RNA | RNA |
| 1058 | 63,932 | 585 | Never | RNA/DNA | RNA | RNA |
| 1074 | 9,946 | 688 | Never | RNA | RNA | RNA |
| 1079 | 25,655 | 438 | Never | RNA | RNA | RNA |
| Chronically infected, treated (viremic) | ||||||
| 3002 | 6,655 | 195 | 16 yr; PIs for 5 yr | RNA | RNA | RNA |
| 3007 | 8,712 | 288 | 14.5 yr; PIs for 5.5 yr | RNA/DNA | RNA | RNA |
| 3011 | 7,585 | 125 | 6.75 yr; PIs for 4 yr | RNA/DNA | RNA | RNA/DNA |
| 3040 | 21,487 | 237 | 10 yr; PIs for 10 yr | RNA/DNA | RNA | DNA |
| 3042 | 278 | 160 | 3.75 yr; PIs for 3 yr | RNA/DNA | RNA | RNA/DNA |
| 3057 | 20,760 | 514 | 10 yr; PIs for 6 yr | RNA/DNA | RNA | RNA/DNA |
| 3109 | 13,731 | 296 | 13 yr; PIs for 6 yr | DNA | RNA/DNA | RNA/DNA |
| 3151 | 28,610 | 184 | 4.5 yr; PIs for 2 yr | RNA/DNA | RNA/DNA | RNA/DNA |
| 3153 | 5,630 | 454b | 13 yr; PIs for 4 yr | RNA/DNA | RNA/DNA | RNA/DNA |
| 3156 | 175,000 | 891 | 1.5 yr; PIs for 1 yr | RNA/DNA | RNA/DNA | RNA/DNA |
meds, medications; PIs, protease inhibitors.
Estimated.
We first analyzed CTL responses targeting SL9 in a group of 20 chronically HIV-1-infected, antiretroviral-treated subjects with detectable viremia. Surprisingly, we found that these individuals generally lacked a response against this epitope. Using the intracellular cytokine flow cytometry (ICS) assay (23, 24), only 3 of 20 patients had a detectable CTL response measured by the production of interferon gamma (IFN-γ) against the SL9 epitope. Prior studies have repeatedly shown responses to the SL9 epitope in the majority of chronically infected, antiretroviral-untreated subjects (4, 18, 21, 22, 37), although these responses were not always immunodominant (8).
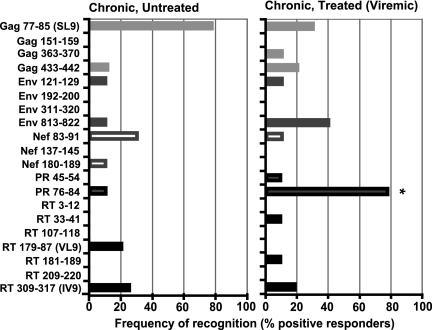
To evaluate if the lack of T-cell responses to SL9 were influenced by treatment exposure and sequence changes, we performed additional studies assessing Gag-, Env-, Nef-, and Pol-specific responses from 10 antiretroviral-treated and 10 antiretroviral-untreated subjects with chronic infection (Table 1). Using the sequence information obtained by population-based sequences of the Gag p17, protease, reverse transcriptase (partial), and Nef regions, we manufactured peptides corresponding to a panel of 20 epitopes and measured HIV-1-specific IFN-γ and tumor necrosis factor alpha (TNF-α) CTL responses using the ICS assay corresponding to autologous and consensus B sequences (Fig. 1) (24). As has been observed by others (4, 18, 21, 22, 37), the majority of chronically infected, untreated subjects had measurable CTL responses targeting the autologous variant of the SL9 epitope (seven of nine subjects tested) (Table 2). This is in contrast to the chronically infected, treated individuals, of whom only 2 of 6 had a measurable CTL response against the autologous SL9 epitope (P = 0.14; Fisher's exact test for chronically infected, treated versus untreated) and 2 of 10 had a response against the wild-type SL9 epitope (P = 0.02). Although the treated individuals lacked strong SL9-specific responses, they exhibited much stronger responses directed against an epitope in protease spanning two amino acid positions associated with resistance to protease inhibitor treatment (PR76-84) (Table 3). Seven out of nine antiretroviral-treated, chronically infected patients tested had a response targeting their autologous variant of the PR76-84 epitope compared to only 1 of 10 chronically infected, untreated subjects (treated versus untreated, P < 0.01; Fisher's exact test) (Fig. 1).
FIG. 1.
Antiviral treatment alters the distribution of epitope recognition. Most chronically infected, antiretroviral-treated patients have gained a response targeting an epitope, which is under strong protease inhibitor-mediated drug pressure (PR 77-85). In contrast to the untreated chronically infected subjects, the treated individuals generally lack responses against the SL9 epitope. A sample was considered positive when the responses were at least two times the experimental background and above 0.05% IFN-γ- and TNF-α-positive CD8+ CD4− CD3+ T cells. *, P < 0.01 (Fisher's exact test).
TABLE 2.
Treatment alters viral evolution and influence recognition of the SL9 epitopea
| Patient no. and sequence type | SL9 sequence | Frequency (%) | Affinity to HLA-A2 (nM)b | CD8+ T-cell response (% IFN-γ−producing cells)
|
|
|---|---|---|---|---|---|
| Wild type | Variantc | ||||
| Reference sequence | 77SLYNTVATL85 | ||||
| Chronically infected, untreated | |||||
| 1028 | --F--I-V- | 100 | 102 | −d | − |
| 1029 | --F------ | 70 | 144 | − | 0.286 |
| --F--I---e | 30 | 88 | − | 0.451 | |
| 1030 | -----I-V- | 90 | 102 | 0.184 | 0.074 |
| -------V-e | 10 | 172 | 0.184 | 0.104 | |
| 1034 | -----I--- | 100 | 99 | − | − |
| 1038 | -----I-V- | 80 | 102 | − | NAf |
| -------V-e | 20 | 172 | − | NA | |
| 1051 | --F-AI-V- | 100 | 33 | − | 0.336 |
| 1057 | -----I-V- | 100 | 102 | 0.087 | 0.078 |
| 1058 | -I--LI--- | 100 | 151 | − | 0.106 |
| 1074 | --F--I-V- | 100 | 102 | 0.170 | 0.265 |
| 1079 | -----I--- | 100 | 99 | 0.156 | 0.123 |
| Chronically infected, treated (viremic) | |||||
| 3002 | -------V- | 100 | 172 | − | NA |
| 3007 | --------- | 100 | 162 | 1.560 | NVTg |
| 3011 | -------V- | 100 | 172 | − | − |
| 3040 | -----I-V- | 100 | 102 | − | − |
| 3042 | --------- | 100 | 162 | 0.356 | NVT |
| 3057 | -------V- | 100 | 172 | − | − |
| 3109 | -----L--- | 100 | 140 | − | NA |
| 3151 | -------V- | 100 | 172 | − | NA |
| 3153 | --------- | 100 | 162 | − | NVT |
| 3156 | -----I-V- | 100 | 102 | − | NA |
Positions 77 to 85 in HXB2 protease.
Affinity to HLA-A2 as predicted by NetMHC 3.0 (http://www.cbs.dtu.dk/services/NetMHC/).
Autologous viral variant of the SL9 epitope tested.
−, negative.
Minor variant.
NA, not analyzed (CTL response against autologous variant epitope not analyzed due to limitation in cell number).
NVT, no autologous variant to test.
TABLE 3.
Antiretroviral treatment alters viral evolution within the HIV-1 protease region that may influence processing and recognition of the PR 76-84 epitope
| Patient no. and sequence type | Sequence of PR 76-84a | Affinity to HLA-A2 (nM)b | CD8+ T-cell responses (% IFN-γ-producing cells)
|
|
|---|---|---|---|---|
| Wild type | Variantc | |||
| Reference sequence | 64IEICGHKAIGTVLVGPTPVNIIGRNLLTQI93 | |||
| Chronically infected, untreated | ||||
| 1028 | ------------------------------ | 4,762 | −d | NVTe |
| 1029 | Z-------Z--------------------- | 4,762 | − | NVT |
| 1030 | -------V---------------------L | 4,762 | − | NVT |
| 1034 | ------------------------------ | 4,762 | − | NVT |
| 1038 | -----------------------------L | 4,762 | − | NVT |
| 1051 | ------------------------------ | 4,762 | 0.116 | NVT |
| 1057 | ------------------------------ | 4,762 | − | NVT |
| 1058 | --------E----I---------------- | 2,101 | − | − |
| 1074 | --------Q--------------------- | 4,762 | − | NVT |
| 1079 | ------------------------------ | 4,762 | − | NVT |
| Chronically infected, treated (viremic) | ||||
| 3002 | -------I----------N-------M--- | 16,091 | − | − |
| 3007 | --F----VVZ--------S---------K- | 12,353 | − | NAf |
| 3011 | V------VLS----------------M--- | 4,762 | 0.505 | NVT |
| 3040 | -------LTS--------A-------M--L | 5,931 | 0.306 | 0.619 |
| 3042 | V----Y-------I---------------- | 2,101 | − | − |
| 3057 | -------TZ---------------S-Z--L | 4,762 | 0.055 | NVT |
| 3109 | -----Z-T--S--I----A-------MZ-L | 2,778 | 0.427 | 0.467 |
| 3151 | -------ITZ----------V----VM-K- | 2,247 | 0.247 | 0.232 |
| 3153 | ---------T-----Z----V-----M--L | 2,247 | 1.972 | 0.559 |
| 3156 | --Z----Z--------------------ZL | 4,762 | 0.308 | NVT |
The sequence from positions 64 to 93 in HXB2 protease is shown. Predicted protease cleavage sites are underlined in the reference sequence. The portion of each sequence corresponding to the PR 76-84 epitope (76LVGPTPVNI84) is shown in boldface. The letter Z represents viral polymorphism at the nucleic acid level giving rise to two different amino acid variants, including the wild-type variant.
Affinity to HLA-A2 as predicted by NetMHC 3.0.
Autologous viral variant of the PR76-84 epitope tested.
−, negative.
NVT, no autologous variant to test.
NA, not analyzed.
As expected, an extensive number of HIV-1 Pol mutations were present in and around the protease epitope in the chronically infected, treated individuals (all subjects were receiving a protease inhibitor-based regimen [Table 3]). Most of these mutations were found in the flanking regions, and six of seven patients who had developed the resistance-associated mutation L90M showed reactivity against the PR 76-84 epitope. Since the L90M mutation was predicted to introduce an additional protease cleavage site at position L89 in Pol (NetChop 3.0), it may have influenced processing and presentation of the epitope.
We next considered what impact this apparent lack of HLA-A2-restricted Gag-specific (SL9) responses in favor of Pol-specific (PR 76-84) responses in treated subjects was having on viral evolution. It should be emphasized that mutations within the targeted epitope previously have been shown to lead to a decay of CTL against the wild-type epitope sequence in chronic infection (22). If, as suggested by our data, protease inhibitor treatment leads to a shift in immunodominant CTL responses from established epitopes (e.g., SL9) to new epitopes (e.g., PR 76-84), then presumably this lack of CTL response may facilitate reversion of CTL-induced escape mutations associated with a fitness cost (15, 17, 27). This would be particularly true for SL9 given evidence that mutations in the Gag region apparently reduce viral fitness (15, 21, 24, 27, 30, 35). In support of this hypothesis, as shown in Table 2, protease inhibitor-treated patients exhibited less sequence variation from the consensus in the SL9 epitope, as compared to untreated, chronically infected individuals. Also, compared to the untreated subjects, the treated subjects had a lower prevalence of the phenylalanine (79F) mutation in position 3 of the SL9 epitope (P = 0.08; Fisher's exact test) and a lower prevalence of isoleucine (82I) or leucine (82L) in position 6 (P = 0.02; Fisher's exact test). This loss of sequence diversity may reflect fitness-associated reversion back to wild type in the absence of any residual CTL activity. It is also possible that treatment-mediated changes in protease may have led to reduced Gag polypeptide processing (and reduced fitness) and ultimately to the emergence of compensatory changes in Gag (29, 34). These results suggest that treatment-induced sequence variations can alter immunodominance. The cross-sectional nature of our study, however, prohibits us from excluding the possibility that the lack of SL9-specific responses in our treated patients may have existed prior to treatment (and indeed may have reflected a poor immunologic response leading to the need to start therapy). A longitudinal study in which patients are studied before treatment and after long-term virologic failure is needed to more fully address these issues; however, such a study may not be feasible given that a large number of treatment-naïve patients would be needed to be studied as they failed several sequential regimens over a period of several years.
To further evaluate how the viral sequence may be changing at different stages of the infection and under the influence of antiviral therapy, we used viral epidemiology signature pattern analysis (25) to compare HIV-1 sequences obtained from our chronically infected subjects with those observed in eight HLA-A2-positive untreated subjects at primary infection (OP177, OP428, OP474, OP488, OP506, OP539, OP581, and OP583, as described in reference 24). Compared to sequences from untreated individuals with primary infections, the chronically infected, untreated individuals showed differences at four amino acid positions within Gag (K30R, V82I, T84V, and E102D). Positions 82 and 84 fall within the SL9 epitope, and position 102 is flanking a highly immunogenic region. In contrast, compared to sequences from primary infection, sequences from the chronically infected, treated subjects showed differences at only two positions (84 and 102). We next used the Shannon entropy score (26) to quantify variations in the protein sequence alignments between the different groups. Once again, the numbers of sites in the sequences showing a significant difference were greater between the chronically infected, untreated and treated subjects (nine positions) than between the chronically infected, treated subjects and untreated subjects with primary infections (three positions) (data not shown). Our sequence data therefore indicate that HIV-1 Gag sequences from chronically infected, treated individuals are more similar to untreated subjects with primary infection than chronically infected, untreated subjects. Although we have not followed the subjects longitudinally, it is possible that the potential reversions to a more consensus B-like viral sequence may be facilitated by the lack of Gag-specific CTL responses against the wild-type sequence.
In summary, we found an altered distribution of HIV-1 specific CTL epitope recognition in antiretroviral drug-treated subjects. Compared to chronically infected, untreated patients, most chronically infected, treated patients had lost a cellular immune response against the SL9 epitope but gained a new immunodominant response against an epitope in PR 76-84. We thus show that mutations induced by antiretroviral therapy alter the profile of epitopes presented to T cells and thus the direction of the response. The consequences of dual pressures from drugs and CTL need to be considered in monitoring of drug therapy, and as drug therapy becomes more widespread, transmission of dual drug and immune-shaped virus populations will become more prevalent.
The results also open the possibility of manipulating drug-resistant HIV-1 by targeted CTL boosting or using antiretroviral drugs to drive CTL escape reversion. Considering the important role of the cellular immune responses in durably controlling drug-resistant HIV-1 (14), we suggest that novel therapeutic approaches, including epitope-specific vaccinations, could be used to either prohibit the development of specific resistance-associated mutations or mobilize a response against the mutant virus.
Nucleotide sequence accession number.
The sequences obtained in this study have been submitted to GenBank and were given accession no. EU011832 to EU011921.
Acknowledgments
This work was supported in part by grants from the NIAID (AI052745, AI055273, and AI44595), the National Institutes of Health UCSF/Gladstone Institute of Virology & Immunology Center for AIDS Research (P30 MH59037 and P30 AI27763), the Center for AIDS Prevention Studies (P30 MH62246), the General Clinical Research Center at San Francisco General Hospital (5-MO1-RR00083-37), the AIDS Biology Program of the UCSF ARI, Fogarty grant D43 TW00003, and the Swedish Agency for International Development Cooperation-Sida (2005-001756 and 2006-018).
Footnotes
Published ahead of print on 1 August 2007.
REFERENCES
- 1.Alatrakchi, N., C. Duvivier, D. Costagliola, A. Samri, A. G. Marcelin, G. Kamkamidze, M. Astriti, R. Agher, V. Calvez, B. Autran, and C. Katlama. 2005. Persistent low viral load on antiretroviral therapy is associated with T cell-mediated control of HIV replication. AIDS 19:25-33. [DOI] [PubMed] [Google Scholar]
- 2.Ali, A., R. Lubong, H. Ng, D. G. Brooks, J. A. Zack, and O. O. Yang. 2004. Impacts of epitope expression kinetics and class I downregulation on the antiviral activity of human immunodeficiency virus type 1-specific cytotoxic T lymphocytes. J. Virol. 78:561-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen, T. M., D. H. O'Connor, P. Jing, J. L. Dzuris, B. R. Mothe, T. U. Vogel, E. Dunphy, M. E. Liebl, C. Emerson, N. Wilson, K. J. Kunstman, X. Wang, D. B. Allison, A. L. Hughes, R. C. Desrosiers, J. D. Altman, S. M. Wolinsky, A. Sette, and D. I. Watkins. 2000. Tat-specific cytotoxic T lymphocytes select for SIV escape variants during resolution of primary viraemia. Nature 407:386-390. [DOI] [PubMed] [Google Scholar]
- 4.Altfeld, M., T. M. Allen, E. T. Kalife, N. Frahm, M. M. Addo, B. R. Mothe, A. Rathod, L. L. Reyor, J. Harlow, X. G. Yu, B. Perkins, L. K. Robinson, J. Sidney, G. Alter, M. Lichterfeld, A. Sette, E. S. Rosenberg, P. J. R. Goulder, C. Brander, and B. D. Walker. 2005. The majority of currently circulating human immunodeficiency virus type 1 clade B viruses fail to prime cytotoxic T-lymphocyte responses against an otherwise immunodominant HLA-A2-restricted epitope: implications for vaccine design. J. Virol. 79:5000-5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bansal, A., E. Gough, S. Sabbaj, D. Ritter, K. Yusim, G. Sfakianos, G. Aldrovandi, R. A. Kaslow, C. M. Wilson, M. J. Mulligan, J. M. Kilby, and P. A. Goepfert. 2005. CD8 T-cell responses in early HIV-1 infection are skewed towards high entropy peptides. AIDS 19:241-250. [PubMed] [Google Scholar]
- 6.Barbour, J. D., T. Wrin, R. M. Grant, J. N. Martin, M. R. Segal, C. J. Petropoulos, and S. G. Deeks. 2002. Evolution of phenotypic drug susceptibility and viral replication capacity during long-term virologic failure of protease inhibitor therapy in human immunodeficiency virus-infected adults. J. Virol. 76:11104-11112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergmann, C. C., J. D. Altman, D. Hinton, and S. A. Stohlman. 1999. Inverted immunodominance and impaired cytolytic function of CD8+ T cells during viral persistence in the central nervous system. J. Immunol. 163:3379-3387. [PubMed] [Google Scholar]
- 8.Betts, M. R., J. P. Casazza, B. A. Patterson, S. Waldrop, W. Trigona, T.-M. Fu, F. Kern, L. J. Picker, and R. A. Koup. 2000. Putative immunodominant human immunodeficiency virus-specific CD8+ T-cell responses cannot be predicted by major histocompatibility complex class I haplotype. J. Virol. 74:9144-9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao, J., J. McNevin, S. Holte, L. Fink, L. Corey, and M. J. McElrath. 2003. Comprehensive analysis of human immunodeficiency virus type 1 (HIV-1)-specific gamma interferon-secreting CD8+ T cells in primary HIV-1 infection. J. Virol. 77:6867-6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dalod, M., M. Dupuis, J. C. Deschemin, C. Goujard, C. Deveau, L. Meyer, N. Ngo, C. Rouzioux, J. G. Guillet, J. F. Delfraissy, M. Sinet, and A. Venet. 1999. Weak anti-HIV CD8(+) T-cell effector activity in HIV primary infection. J. Clin. Investig. 104:1431-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deeks, S. G., R. Hoh, T. B. Neilands, T. Liegler, F. Aweeka, C. J. Petropoulos, R. M. Grant, and J. N. Martin. 2005. Interruption of treatment with individual therapeutic drug classes in adults with multidrug-resistant HIV-1 infection. J. Infect. Dis. 192:1537-1544. [DOI] [PubMed] [Google Scholar]
- 12.Deeks, S. G., J. N. Martin, E. Sinclair, J. Harris, T. B. Neilands, H. T. Maecker, E. Hagos, T. Wrin, C. J. Petropoulos, B. Bredt, and J. M. McCune. 2004. Strong cell-mediated immune responses are associated with the maintenance of low-level viremia in antiretroviral-treated individuals with drug-resistant human immunodeficiency virus type 1. J. Infect. Dis. 189:312-321. [DOI] [PubMed] [Google Scholar]
- 13.Deeks, S. G., T. Wrin, T. Liegler, R. Hoh, M. Hayden, J. D. Barbour, N. S. Hellmann, C. J. Petropoulos, J. M. McCune, M. K. Hellerstein, and R. M. Grant. 2001. Virologic and immunologic consequences of discontinuing combination antiretroviral-drug therapy in HIV-infected patients with detectable viremia. N. Engl. J. Med. 344:472-480. [DOI] [PubMed] [Google Scholar]
- 14.Emu, B., E. Sinclair, D. Favre, W. J. Moretto, P. Hsue, R. Hoh, J. N. Martin, D. F. Nixon, J. M. McCune, and S. G. Deeks. 2005. Phenotypic, functional, and kinetic parameters associated with apparent T-cell control of human immunodeficiency virus replication in individuals with and without antiretroviral treatment. J. Virol. 79:14169-14178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernandez, C. S., I. Stratov, R. De Rose, K. Walsh, C. J. Dale, M. Z. Smith, M. B. Agy, S.-L. Hu, K. Krebs, D. I. Watkins, D. H. O'Connor, M. P. Davenport, and S. J. Kent. 2005. Rapid viral escape at an immunodominant simian-human immunodeficiency virus cytotoxic T-lymphocyte epitope exacts a dramatic fitness cost. J. Virol. 79:5721-5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrari, G., W. Neal, J. Ottinger, A. M. Jones, B. H. Edwards, P. Goepfert, M. R. Betts, R. A. Koup, S. Buchbinder, M. J. McElrath, J. Tartaglia, and K. J. Weinhold. 2004. Absence of immunodominant anti-Gag p17 (SL9) responses among Gag CTL-positive, HIV-uninfected vaccine recipients expressing the HLA-A*0201 allele. J. Immunol. 173:2126-2133. [DOI] [PubMed] [Google Scholar]
- 17.Friedrich, T. C., E. J. Dodds, L. J. Yant, L. Vojnov, R. Rudersdorf, C. Cullen, D. T. Evans, R. C. Desrosiers, B. R. Mothe, J. Sidney, A. Sette, K. Kunstman, S. Wolinsky, M. Piatak, J. Lifson, A. L. Hughes, N. Wilson, D. H. O'Connor, and D. I. Watkins. 2004. Reversion of CTL escape-variant immunodeficiency viruses in vivo. Nat. Med. 10:275-281. [DOI] [PubMed] [Google Scholar]
- 18.Goulder, P. J., M. A. Altfeld, E. S. Rosenberg, T. Nguyen, Y. Tang, R. L. Eldridge, M. M. Addo, S. He, J. S. Mukherjee, M. N. Phillips, M. Bunce, S. A. Kalams, R. P. Sekaly, B. D. Walker, and C. Brander. 2001. Substantial differences in specificity of HIV-specific cytotoxic T cells in acute and chronic HIV infection. J. Exp. Med. 193:181-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hecht, F. M., M. P. Busch, B. Rawal, M. Webb, E. Rosenberg, M. Swanson, M. Chesney, J. Anderson, J. Levy, and J. O. Kahn. 2002. Use of laboratory tests and clinical symptoms for identification of primary HIV infection. AIDS 16:1119-1129. [DOI] [PubMed] [Google Scholar]
- 20.Iversen, A. K. N., R. W. Shafer, K. Wehrly, M. A. Winters, J. I. Mullins, B. Chesebro, and T. C. Merigan. 1996. Multidrug-resistant human immunodeficiency virus type 1 strains resulting from combination antiretroviral therapy. J. Virol. 70:1086-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iversen, A. K. N., G. Stewart-Jones, G. H. Learn, N. Christie, C. Sylvester-Hviid, A. E. Armitage, R. Kaul, T. Beattie, J. K. Lee, Y. Li, P. Chotiyarnwong, T. Dong, X. Xu, M. A. Luscher, K. Macdonald, H. Ullum, B. Klarlund-Pedersen, P. Skinhoj, L. Fugger, S. Buus, J. I. Mullins, E. Y. Jones, P. A. van der Merwe, and A. J. McMichael. 2006. Conflicting selective forces affect T cell receptor contacts in an immunodominant human immunodeficiency virus epitope. Nat. Immunol. 7:179-189. [DOI] [PubMed] [Google Scholar]
- 22.Jamieson, B. D., O. O. Yang, L. Hultin, M. A. Hausner, P. Hultin, J. Matud, K. Kunstman, S. Killian, J. Altman, K. Kommander, B. Korber, J. Giorgi, and S. Wolinsky. 2003. Epitope escape mutation and decay of human immunodeficiency virus type 1-specific CTL responses. J. Immunol. 171:5372-5379. [DOI] [PubMed] [Google Scholar]
- 23.Karlsson, A. C., S. G. Deeks, J. D. Barbour, B. D. Heiken, S. R. Younger, R. Hoh, M. Lane, M. Sällberg, G. M. Ortiz, J. F. Demarest, T. Liegler, R. M. Grant, J. N. Martin, and D. F. Nixon. 2003. Dual pressure from antiretroviral therapy and cell-mediated immune response on the human immunodeficiency virus type 1 protease gene. J. Virol. 77:6743-6752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karlsson, A. C., A. K. Iversen, J. M. Chapman, T. de Oliviera, G. Spotts, A. J. McMichael, M. P. Davenport, F. M. Hecht, and D. F. Nixon. 2007. Sequential broadening of CTL responses in early HIV-1 infection is associated with viral escape. PLoS ONE 2:e225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korber, B., and G. Myers. 1992. Signature pattern analysis: a method for assessing viral sequence relatedness. AIDS Res. Hum. Retrovir. 8:1549-1560. [DOI] [PubMed] [Google Scholar]
- 26.Korber, B. T. M., K. J. Kunstman, B. K. Patterson, M. Furtado, M. M. McEvilly, R. Levy, and S. M. Wolinsky. 1994. Genetic differences between blood- and brain-derived viral sequences from human immunodeficiency virus type 1-infected patients: evidence of conserved elements in the V3 region of the envelope protein of brain-derived sequences. J. Virol. 68:7467-7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leslie, A. J., K. J. Pfafferott, P. Chetty, R. Draenert, M. M. Addo, M. Feeney, Y. Tang, E. C. Holmes, T. Allen, J. G. Prado, M. Altfeld, C. Brander, C. Dixon, D. Ramduth, P. Jeena, S. A. Thomas, A. S. John, T. A. Roach, B. Kupfer, G. Luzzi, A. Edwards, G. Taylor, H. Lyall, G. Tudor-Williams, V. Novelli, J. Martinez-Picado, P. Kiepiela, B. D. Walker, and P. J. Goulder. 2004. HIV evolution: CTL escape mutation and reversion after transmission. Nat. Med. 10:282-289. [DOI] [PubMed] [Google Scholar]
- 28.Lichterfeld, M., X. G. Yu, D. Cohen, M. M. Addo, J. Malenfant, B. Perkins, E. Pae, M. N. Johnston, D. Strick, T. M. Allen, E. S. Rosenberg, B. Korber, B. D. Walker, and M. Altfeld. 2004. HIV-1 Nef is preferentially recognized by CD8 T cells in primary HIV-1 infection despite a relatively high degree of genetic diversity. AIDS 18:1383-1392. [DOI] [PubMed] [Google Scholar]
- 29.Mammano, F., C. Petit, and F. Clavel. 1998. Resistance-associated loss of viral fitness in human immunodeficiency virus type 1: phenotypic analysis of protease and gag coevolution in protease inhibitor-treated patients. J. Virol. 72:7632-7637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martinez-Picado, J., J. G. Prado, E. E. Fry, K. Pfafferott, A. Leslie, S. Chetty, C. Thobakgale, I. Honeyborne, H. Crawford, P. Matthews, T. Pillay, C. Rousseau, J. I. Mullins, C. Brander, B. D. Walker, D. I. Stuart, P. Kiepiela, and P. Goulder. 2006. Fitness cost of escape mutations in p24 Gag in association with control of human immunodeficiency virus type 1. J. Virol. 80:3617-3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mason, R. D., M. I. Bowmer, C. M. Howley, M. Gallant, J. C. Myers, and M. D. Grant. 2004. Antiretroviral drug resistance mutations sustain or enhance CTL recognition of common HIV-1 Pol epitopes. J. Immunol. 172:7212-7219. [DOI] [PubMed] [Google Scholar]
- 32.Mason, R. D., and M. D. Grant. 2005. A therapy-related point mutation changes the HLA restriction of an HIV-1 Pol epitope from A2 to B57 and enhances its recognition. AIDS 19:981-984. [DOI] [PubMed] [Google Scholar]
- 33.Mueller, S. M., B. Schaetz, K. Eismann, S. Bergmann, M. Bauerle, M. Schmitt-Haendle, H. Walter, B. Schmidt, K. Korn, H. Sticht, B. Spriewald, E. G. Harrer, and T. Harrer. 2007. Dual selection pressure by drugs and HLA class I-restricted immune responses on human immunodeficiency virus type 1 protease. J. Virol. 81:2887-2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Myint, L., M. Matsuda, Z. Matsuda, Y. Yokomaku, T. Chiba, A. Okano, K. Yamada, and W. Sugiura. 2004. Gag non-cleavage site mutations contribute to full recovery of viral fitness in protease inhibitor-resistant human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 48:444-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nietfield, W., M. Bauer, M. Fevrier, R. Maier, B. Holzwarth, R. Frank, B. Maier, Y. Riviere, and A. Meyerhans. 1995. Sequence constraints and recognition by CTL of an HLA-B27-restricted HIV-1 gag epitope. J. Immunol. 154:2189-2197. [PubMed] [Google Scholar]
- 36.Nixon, D. F., S. G. Deeks, B. L. Shacklett, and A. C. Karlsson. 2005. Multidrug-resistant, dual-tropic HIV-1 and rapid progression. Lancet 365:1924-1925. (Letter.) [DOI] [PubMed] [Google Scholar]
- 37.Ogg, G. S., X. Jin, S. Bonhoeffer, P. R. Dunbar, M. A. Nowak, S. Monard, J. P. Segal, Y. Cao, S. L. Rowland-Jones, V. Cerundolo, A. Hurley, M. Markowitz, D. D. Ho, D. F. Nixon, and A. J. McMichael. 1998. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science 279:2103-2106. [DOI] [PubMed] [Google Scholar]
- 38.Price, D. A., G. Scullard, A. Oxenius, R. Braganza, S. A. Beddows, S. Kazmi, J. R. Clarke, G. E. Johnson, J. N. Weber, and R. E. Phillips. 2003. Discordant outcomes following failure of antiretroviral therapy are associated with substantial differences in human immunodeficiency virus-specific cellular immunity. J. Virol. 77:6041-6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Samri, A., G. Haas, J. Duntze, J.-M. Bouley, V. Calvez, C. Katlama, and B. Autran. 2000. Immunogenicity of mutations induced by nucleoside reverse transcriptase inhibitors for human immunodeficiency virus type 1-specific cytotoxic T cells. J. Virol. 74:9306-9312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmitt, M., E. Harrer, A. Goldwich, M. Bauerle, I. Graedner, J. R. Kalden, and T. Harrer. 2000. Specific recognition of lamivudine-resistant HIV-1 by cytotoxic T lymphocytes. AIDS 14:653-658. [DOI] [PubMed] [Google Scholar]
- 41.Stratov, I., C. J. Dale, S. Chea, J. McCluskey, and S. J. Kent. 2005. Induction of T-cell immunity to antiretroviral drug-resistant human immunodeficiency virus type 1. J. Virol. 79:7728-7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van der Most, R. G., K. Murali-Krishna, J. G. Lanier, E. J. Wherry, M. T. Puglielli, J. N. Blattman, A. Sette, and R. Ahmed. 2003. Changing immunodominance patterns in antiviral CD8 T-cell responses after loss of epitope presentation or chronic antigenic stimulation. Virology 315:93-102. [DOI] [PubMed] [Google Scholar]
- 43.van der Most, R. G., A. Sette, C. Oseroff, J. Alexander, K. Murali-Krishna, L. L. Lau, S. Southwood, J. Sidney, R. W. Chesnut, M. Matloubian, and R. Ahmed. 1996. Analysis of cytotoxic T cell responses to dominant and subdominant epitopes during acute and chronic lymphocytic choriomeningitis virus infection. J. Immunol. 157:5543-5554. [PubMed] [Google Scholar]
- 44.Wherry, E. J., J. N. Blattman, K. Murali-Krishna, R. van der Most, and R. Ahmed. 2003. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 77:4911-4927. [DOI] [PMC free article] [PubMed] [Google Scholar]