Abstract
A commercial air ejector was coupled to an electrospray ionization linear ion trap mass spectrometer (LTQ) to transport remotely generated ions from both electrospray (ESI) and desorption electrospray ionization (DESI) sources. We demonstrate the remote analysis of a series of analyte ions that range from small molecules and polymers to polypeptides using the AE-LTQ interface. The details of the ESI-AE-LTQ and DESI-AE-LTQ experimental configurations are described and preliminary mass spectrometric data is presented.
Introduction
The introduction, development, and refinement of novel ionization sources, specifically ambient “direct analysis” methods continue to expand the analytical utility of mass spectrometry. They include atmospheric-pressure solids analysis probe1 (ASAP), direct analysis in real time2 (DART), desorption electrospray ionization3 (DESI), laser desorption atmospheric pressure chemical ionization (LD-APCI)4, electrospray assisted laser desorption electrospray ionization5, 6 (ELDI), and matrix assisted laser desorption electrospray ionization7, 8 (MALDESI). The sample to MS inlet distances typically range from 2–10 cm depending on the ionization source and sample (e.g., MALDI plate, tissue, drug tablet). This close proximity limits the size, location, and geometry of the sample to be analyzed. Enabling facile remote coupling for “direct analysis” ionization sources to a MS detector would be highly useful for a variety of applications including paints and coatings, forensics, and off-site environmental analysis. This has been partially addressed using portable mass spectrometers9–11 and recently Cooks and coworkers reported coupling DESI to a miniature mass spectrometer for a variety of field studies.12 However, the ionization source was still confined to the immediate entrance of the mass spectrometer limiting the analysis to small, well-defined sample substrates.
We report the development of a generally applicable remote sampling method that utilizes a commercial air ejector to transport ions from the point of ion formation to the MS inlet via a flexible polyethylene tube. This ion transfer interface is demonstrated using both ESI and DESI sources remotely coupled to a linear ion trap mass spectrometer. Unlike the recently introduced method by Cooks et al. that uses a rigid stainless steel tube for non proximate detection13 of explosives and chemical warfare stimulants by DESI14, this approach utilizes a flexible, non-conductive polyethylene tube that is robust and amendable to modifications (e.g., movable sampling wand) and optimization for the targeted analyte (vide infra). Furthermore, it does not require extensive modifications to the MS inlet. The commercial air ejector interface described herein facilitated the remote DESI analysis of surface-bound analytes ranging from small organic molecules to polypeptides.
Experimental
Materials
Rhodamine 6G, melittin, polypropylene glycol (average molecular weight = 1000 Da (PPG-1000), and formic acid were obtained from Sigma Aldrich (St. Louis, MO) and used without further purification. HPLC grade acetonitrile and water were purchased from Burdick & Jackson (Muskegon, MI). 0.5 mm thick polytetrafluoroethylene (PTFE) sheets (P/N 8711K82, McMaster Carr, Atlanta, GA) were used as the DESI substrates. Nitrogen (99.98%) and LTQ helium bath gas (99.999%) were obtained from MWSC High Purity Gases (Raleigh, NC).
Methods
Electrospray solutions were prepared by mixing acetonitrile:water (1:1 v/v) with 0.1% formic acid and then diluting Rhodamine 6G, melittin, and PPG-1000 to a concentration of 10 µM. DESI substrates for both melittin and PPG-1000 were prepared by spotting 10 µL of 1 mg/mL stock standard solutions of each (in 1:1 v/v acetonitrile:water with 0.1% formic acid) onto a PTFE surface and dried under ambient conditions immediately prior to analysis. DESI substrates for rhodamine 6G were prepared by spotting 10 µL of 20 µM solution (in 1:1 v/v acetonitrile:water with 0.1% formic acid) onto a PTFE surface.
Mass Spectrometer
All mass spectra were acquired on a LTQ mass spectrometer (Thermo Electron, San Jose CA) in the positive-ion mode. The maximum injection time was set to 300 ms for all experiments and three microscans were taken per mass spectrum. The AGC limit was set to 1 × 106 but never reached this target value allowing the trap to collect ions for the full 300 ms. The capillary temperature was kept at a constant 200°C. The LTQ-MS sample inlet was modified with a 0.5 mm i.d. × 154 mm stainless steel heated metal capillary that extended 55 mm from the threaded capillary ferrule.
ESI and DESI Sources
The ESI and DESI experiments were performed with the same ionization source which is based on the prototype DESI design from the Cooks laboratory.13, 15 Briefly, the source emitter is constructed of a single stainless steel 1/16″ Swagelok® Tee (P/N SS-100-3, Raleigh Valve and Fitting, Raleigh, NC) that houses two fused silica capillaries (Polymicro Technologies, Inc., Phoenix Arizona): the inner capillary (50 µm i.d., 150 µm o.d., P/N 2000015) transports the ESI solvent at 2 µL/min and fits inside the outer capillary (250 um i.d., 350 um o.d., P/N 2000026) that transports the nitrogen nebulizing gas. The nebulizing gas pressures for both ESI and DESI experiments were measured at the high pressure regulator (P/N 2053021-01-000, Controls Corp. of America, Virginia Beach, VA) at 40 and 110 psi respectively. A 4000 V voltage was applied to the ESI and DESI source for all experiments.
Air Ejector Interface
A Series EIX Air Ejector (Figure 1 picture inset) was purchased from Bosch Rexroth AG (P/N 0821305187; Charlotte, NC) and used without further modification. Figure 1 shows the experimental configuration of the air ejector relative to the LTQ-MS entrance and the ESI and DESI source. The operation of the air ejector is illustrated in Figure 1 where high pressure gas (nitrogen) is introduced at the “Inlet Pressure” and directed into the air ejector “Exhaust” via a small orifice in the center of the device. The flow of high pressure gas through the small orifice creates a vacuum via the Venturi effect. The induced vacuum is labeled “Sample Intake” at the terminus of the 3 foot section of 1/4” polyethylene tubing (P/N 14176121, Thermo Fisher Scientific, Pittsburgh, PA) at the point of ion formation by either ESI (Figure 1A) or DESI (Figure 1B). The 1/4” polyethylene tubing was connected to the air ejector using two brass Swagelok® fittings (P/N B-400-1-4). The terminus of the 1/4” polyethylene tubing (“Sample Intake”) was fitted with a stainless steel 1/4” Swagelok® nut (P/N SS-400-NFSET) coupled to a brass 1/4” Swagelok®-to-1/8” NPT converter (P/N B-400-1-2). Voltages were applied at the air ejector (50 V) and the brass fitting at the terminus of the 3 foot 1/4” polyethylene tubing (100 V). The “Exhaust” component of the air ejector was positioned 1 mm from the MS inlet capillary which was maintained at 37 V.
Figure 1.
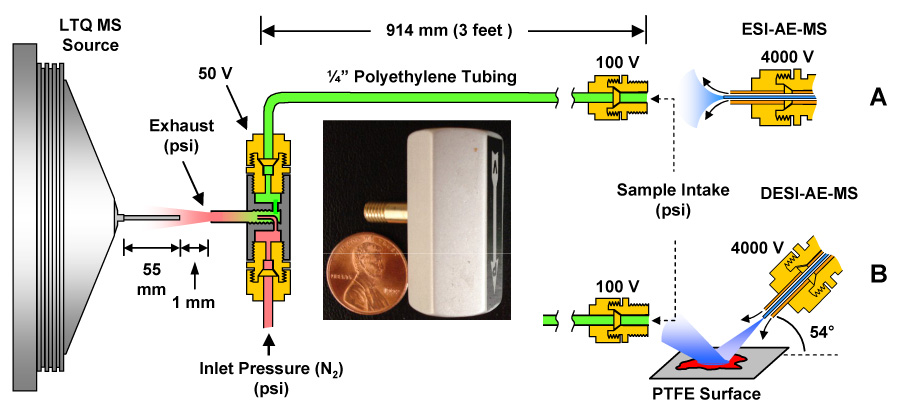
Schematic of the commercial air ejector (AE) interfaced to an LTQ-MS with (A) ESI and (B) DESI ion generation. ESI and DESI were achieved 3 feet from the inlet of the mass spectrometer. High pressure nitrogen was introduced at the “Inlet Pressure” and directed through a small orifice and then out of the “Exhaust” outlet directed at the LTQ heated metal capillary inlet. The vacuum is produced via the Venturi effect that occurs when the high pressure gas is forced through the orifice in the middle of the AE. The convergence of the high pressure and vacuum flows entrains the ions generated at the “Sample Intake” by either ESI (A) or DESI (B).
Air Ejector Pressure Measurements
Pressure profiling of the air ejector was carried out using a Mannix Handheld Digital Manometer (P/N DM8200, Hauppauge, NY). The “Inlet Pressure” of nitrogen was metered into the air ejector via a high pressure regulator and all reported “Inlet Pressures” were from the high pressure regulator gauge. Both the “Exhaust” and “Sampling Inlet” pressures were measured separately using the same Mannix Handheld Digital Manometer. This was done to most closely simulate the performance characteristics of the air ejector as it was used in these studies. The “Exhaust” pressure from the air ejector was measured by placing the Mannix sampling tubing directly over the outside of the brass “Exhaust” tubing with no observable leaking. The “Sampling Inlet” pressure was measured by coupling the Mannix tube directly to the brass 1/4” Swagelok® union (P/N B-400-6) at the terminus of the 3 foot length of 1/4” polyethylene tubing.
Results and Discussion
The air ejector interface illustrated in Figure 1 transports remotely generated ions via the high flow vacuum (“Sample Intake”) into the front of the LTQ mass spectrometer inlet. Ion generation using the ESI source was initially investigated to test the transport of ions via the AE interface due to the continuous nature of the ionization source. This allowed us to determine the pressure conditions for achieving optimum ion abundances which could not be readily measured using the DESI source (vide infra).
Figure 2 displays the results from the ESI-AE-LTQ interface (Figure 1A) for electrosprayed 10 µM rhodamine 6G (R6G), PPG-1000, and melittin solutions. Figure 2 shows the total ion chromatogram (left y-axis = Total Ion Abundance) of rhodamine 6G, PPG-1000, and melittin as a function of the ”Inlet Pressure” (Figure 1). The “Inlet Pressure” was adjusted over 5 psi intervals from 0–20 psi and then 10 psi from 20–70 psi with the pressure intervals representing 30 seconds each of LTQ analysis time (top x-axis). The “Exhaust” and “Sample Intake” pressures (right y-axis) were also plotted as a function of the “Inlet Pressure” to show the relationship of each pressure to the observed ion abundances. An approximate total ion abundance maximum for R6G was observed at an “Inlet Pressure” of ~5 psi whereas the maximum for PPG-1000 and melittin was observed at ~10 psi. Importantly, the data shows that it is possible to achieve approximately half the total ion abundance of rhodamine 6G using the ESI-AE-LTQ without any “Inlet Pressure” whereas no appreciable signal was observed under the same conditions for PPG-1000 and melittin. Furthermore, the rhodamine 6G total ion abundance shows a faster rate of decay after the 5 psi maximum relative to the PPG-1000 and melittin which appear more stable from 5 – 20 psi.
Figure 2.

Total ion abundance (left y-axis) of rhodamine 6G (R6G, dashed), melittin (dotted), and PPG-1000 (solid) plotted as a function of “Inlet Pressure” (bottom x-axis) and LTQ acquisition time (top x-axis). “Exhaust” (square) and “Sample Intake” (diamond) pressure (right y-axis) plotted as a function of “Inlet Pressure” (bottom x-axis) and LTQ acquisition time (top x-axis). Representative mass spectra of rhodamine 6G (A), PPG-1000 (B), and Melittin (C) are shown at an “Inlet Pressure” of 10 psi.
Representative LTQ mass spectra are shown for rhodamine 6G (Figure 2A), PPG-1000 (Figure 2B), and melittin (Figure 2C) at the “Inlet Pressure” of 10 psi. All spectra were collected for 300 ms and never reached the AGC limit of 1 × 106. Sodium-adducted PPG-1000 was observed to dominate the MS signal in Figure 2B.
The DESI-AE-MS (Figure 1B) spectra observed for all three surface-bound analytes rhodamine 6G, melittin, and PPG-1000 (not shown due to space constraints) were virtually identical to the directly infused ESI-AE-MS data shown in Figure 2A, 2B, and 2C. The “Inlet Pressure” that provided the best ion abundance for DESI-AE-MS was slightly higher (20 psi) than that of the ESI-AE-MS (5–10 psi). It is important to note that the nature of DESI does not readily allow for optimization experiments where the population of ions must be constant over an extended period of time. In other words, surface-bound samples are constantly being consumed. However, these data clearly indicate the potential for using the air ejector in DESI applications that require remote sampling via a flexible probe.
Limits of detection (LOD) for each of the surface-bound analytes were determined based on the amount of material deposited, analyte spot size, DESI plume interaction area on the surface, and extrapolation of signal intensity to a 3:1 S/N ratio. This ratio has previously been used by Cooks and coworkers to report experimental limits of detection.14 The deposition of 10 µL of analyte (1:1 H20:ACN) onto a PTFE surface resulted in a sample spot area of 11 mm². The DESI spray plume area was determined previously by fluorescence imaging to be 0.5 mm².16 Thus, the amount of analyte sampled by a single DESI spray plume corresponds to 4.5% (0.5 mm²/11 mm² × 100%) of the total analyte on the surface. This assumes a uniform coverage within the 11 mm% surface area and the “complete” removal of analyte within the DESI spray plume area. These calculations resulted in LOD’s for rhodamine 6G, melittin, and PPG to be 0.4 ng, 3.0 ng, and 91 ng respectively. However, it is important to note that this is a conservative calculation as multiple spectra could be obtained from a single interaction area in our experiments suggesting that the DESI spray plume does not remove all of the analyte within the interaction area.
The potential for using the AE as a remote sampling device is significant considering the variety of samples (e.g., large or geometrically complex substrates that do not readily fit into the standard ion source configurations on the front of most mass spectrometers) and ionization sources (e.g., ASAP1, DART2, LD-APCI4, ELDI5, 6, and MALDESI7, 8) that could benefit from a flexible sampling probe. Additional experiments were attempted with a 10′ coil of ¼” polyethylene tubing, under the same AGC and experimental conditions as the 3’ section of tubing, however appreciable signal was not attained. We are currently investigating the affect of experimental parameters such as the curvature of the tubing (which would significantly reduce sample/ion transport), inner diameter of the transport tubing (conductance limiting factor), tubing material, ejector design (materials, inlet/outlet diameters, geometry), applied voltage potentials, temperature, and inlet pressure in an effort to extend the working distance of the air ejector. Furthermore, we are working to couple the air ejector to an existing MALDESI source7, 8 and incorporate a voltage-assisted air amplifier17–19 to improve ion transmission.
Conclusions
The integration of a commercial air ejector with ESI and DESI demonstrates the remote analysis of non-standard sample substrates that are not amenable to standard ionization source configurations. The AE is inexpensive (<$100), robust, and easily mounted to the front of most mass spectrometers. Furthermore, the AE should be readily adaptable to other direct ionization techniques such as ASAP, DART, LD-APCI, ELDI, and MALDESI.
Acknowledgements
The authors gratefully acknowledge financial support received from the National Cancer Institute, National Institutes of Health (R33 CA105295), the W.M. Keck Foundation, and North Carolina State University. The authors also thank Professor Michael MacCoss and coworkers (University of Washington, Seattle, WA) for help with modifying the heated metal capillary.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McEwen CN, McKay RG, Larsen BS. Analysis of solids, liquids, and biological tissues using solids probe introduction at atmospheric pressure on commercial LC/MS instruments. Anal. Chem. 2005;77(23):7826–7831. doi: 10.1021/ac051470k. [DOI] [PubMed] [Google Scholar]
- 2.Cody RB, Laramee JA, Durst HD. Versatile new ion source for the analysis of materials in open air under ambient conditions. Anal. Chem. 2005;77(8):2297–2302. doi: 10.1021/ac050162j. [DOI] [PubMed] [Google Scholar]
- 3.Takats Z, Wiseman JM, Gologan B, Cooks RG. Mass spectrometry sampling under ambient conditions with desorption electrospray ionization. Science. 2004;306(5695):471–473. doi: 10.1126/science.1104404. [DOI] [PubMed] [Google Scholar]
- 4.Coon JJ, Harrison WW. Laser desorption-atmospheric pressure chemical ionization mass spectrometry for the analysis of peptides from aqueous solutions. Anal. Chem. 2002;74(21):5600–5605. doi: 10.1021/ac020402k. [DOI] [PubMed] [Google Scholar]
- 5.Shiea J, Huang MZ, HSu HJ, Lee CY, Yuan CH, Beech I, Sunner J. Electrospray-assisted laser desorption/ionization mass spectrometry for direct ambient analysis of solids. Rapid Commun. Mass Spectrom. 2005;19(24):3701–3704. doi: 10.1002/rcm.2243. [DOI] [PubMed] [Google Scholar]
- 6.Huang MZ, Hsu HJ, Lee LY, Jeng JY, Shiea LT. Direct protein detection from biological media through electrospray-assisted laser desorption ionization/mass spectrometry. J. Proteome Res. 2006;5(5):1107–1116. doi: 10.1021/pr050442f. [DOI] [PubMed] [Google Scholar]
- 7.Sampson JS, Hawkridge AM, Muddiman DC. Generation and detection of multiply-charged peptides and proteins by matrix-assisted laser desorption electrospray ionization (MALDESI) Fourier transform ion cyclotron resonance mass spectrometry. J. Am. Soc. Mass Spectrom. 2006;17(12):1712–1716. doi: 10.1016/j.jasms.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Sampson JS, Hawkridge AM, Muddiman DC. Direct characterization of intact polypeptides by matrix assisted laser desorption electrospray ionization (MALDESI) quadrupole fourier transfrom ion cyclotron resonance mass spectrometry. Rapid Commun. Mass Spectrom. 2007;21(7):1150–1154. doi: 10.1002/rcm.2947. [DOI] [PubMed] [Google Scholar]
- 9.Badman ER, Cooks RG. Special feature: Perspective - Miniature mass analyzers. Journal of Mass Spectrometry. 2000;35(6):659–671. doi: 10.1002/1096-9888(200006)35:6<659::AID-JMS5>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 10.Gao L, Song QY, Patterson GE, Cooks RG, Ouyang Z. Handheld rectilinear ion trap mass spectrometer. Anal. Chem. 2006;78(17):5994–6002. doi: 10.1021/ac061144k. [DOI] [PubMed] [Google Scholar]
- 11.Chaudhary A, van Amerom FHW, Short RT, Bhansali S. Fabrication and testing of a miniature cylindrical ion trap mass spectrometer constructed from low temperature co-fired ceramics. International Journal of Mass Spectrometry. 2006;251(1):32–39. [Google Scholar]
- 12.Mulligan CC, Talaty N, Cooks RG. Desorption electrospray ionization with a portable mass spectrometer: in situ analysis of ambient surfaces. Chemical Communications. 2006;(16):1709–1711. doi: 10.1039/b517357d. [DOI] [PubMed] [Google Scholar]
- 13.Takats Z, Wiseman JM, Cooks RG. Ambient mass spectrometry using desorption electrospray ionization (DESI): instrumentation, mechanisms and applications in forensics, chemistry, and biology. J. Mass Spectrom. 2005;40(10):1261–1275. doi: 10.1002/jms.922. [DOI] [PubMed] [Google Scholar]
- 14.Cotte-Rodriguez I, Cooks RG. Non-proximate detection of explosives and chemical warfare agent simulants by desorption electrospray ionization mass spectrometry. Chemical Communications. 2006;(28):2968–2970. doi: 10.1039/b606020j. [DOI] [PubMed] [Google Scholar]
- 15.Takats Z, Nanita SC, Cooks RG, Schlosser G, Vekey K. Amino acid clusters formed by sonic spray ionization. Anal. Chem. 2003;75(6):1514–1523. doi: 10.1021/ac0260793. [DOI] [PubMed] [Google Scholar]
- 16.Bereman MS, Muddiman DC. Detection of Attomole Amounts of Analyte by Desorption Electrospray Ionization Mass Spectrometry (DESI-MS) Determined Using Fluorescence Spectroscopy. J. Am. Soc. Mass Spectrom. 2007;18(6):1093–1096. doi: 10.1016/j.jasms.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Zhou L, Yue BF, Dearden DV, Lee ED, Rockwood AL, Lee ML. Incorporation of a venturi device in electrospray ionization. Anal. Chem. 2003;75(21):5978–5983. doi: 10.1021/ac020786e. [DOI] [PubMed] [Google Scholar]
- 18.Hawkridge AM, Zhou L, Lee ML, Muddiman DC. Analytical performance of a venturi device integrated into an electrospray ionization Fourier transform ion cyclotron resonance mass spectrometer for analysis of nucleic acids. Anal. Chem. 2004;76(14):4118–4122. doi: 10.1021/ac049677l. [DOI] [PubMed] [Google Scholar]
- 19.Yang PX, Cooks RG, Ouyang Z, Hawkridge AM, Muddiman DC. Gentle protein ionization assisted by high-velocity gas flow. Anal. Chem. 2005;77(19):6174–6183. doi: 10.1021/ac050711l. [DOI] [PubMed] [Google Scholar]


