Abstract
Insertion of the lymphocytic choriomeningitis virus (LCMV) precursor glycoprotein C (GP-C) into the membrane of the endoplasmic reticulum is mediated by an unusual signal peptide (SPGP-C). It is comprised of 58 amino acid residues and contains an extended hydrophilic N-terminal region, two hydrophobic regions, and a short C-terminal region. After cleavage by signal peptidase, SPGP-C accumulates in cells and virus particles. In the present study, we identified the LCMV SPGP-C as being an essential component of the GP complex and show that the different regions of SPGP-C are required for distinct steps in GP maturation and virus infectivity. More specifically, we show that one hydrophobic region of SPGP-C is sufficient for the membrane insertion of GP-C, while both hydrophobic regions are required for the processing and cell surface expression of the GPs. The N-terminal region of SPGP-C, on the other hand, is essential for pseudoviral infection of target cells. Furthermore, we show that unmyristoylated SPGP-C exposes its N-terminal region to the exoplasmic side. This SPGP-C can promote GP-C maturation but is defective in pseudoviral infection. Myristoylation and topology of SPGP-C in the membrane may thus hold the key to an understanding of the role of SPGP-C in GP-C complex maturation and LCMV infectivity.
Most secretory and membrane proteins are synthesized as preproteins with an N-terminal signal sequence that is often cleaved off after insertion into the membrane of the endoplasmic reticulum (ER) (5, 35). Signal sequences are usually 15 to 25 amino acids (aa) in length and contain a central hydrophobic core of 7 to 10 residues (h region), a polar N-terminal region (n region), and a C-terminal region (c region) that contains the cleavage site for signal peptidase (SPase). Membrane insertion is thought to occur in a loop-like fashion such that the N terminus of the signal sequence is exposed on the cytoplasmic side of the ER membrane (36). After cotranslational cleavage of the signal sequence by SPase on the luminal side of the membrane (34), signal peptides (SPs) can be either directly degraded or processed by SP peptidase (37); a further alternative is that SPs are long-lived and accumulate in the membrane. This has been shown for the SP of the lymphocytic choriomeningitis virus (LCMV) glycoprotein C (GP-C) (precursor GP-C [pGP-C]) (15) and the GPs of two other arenaviruses, Lassa virus and Junín virus (14, 39).
The SP of LCMV pGP-C (SPGP-C) targets the GP to the ER membrane. After cotranslational cleavage of LCMV SPGP-C, the GP is further processed into GP-1 and GP-2 by the subtilase SKI-1/S1P (3). Together, the peripheral protein GP-1 and the membrane-anchored protein GP-2 build up viral GP spikes. The entry of LCMV into target cells is initiated by the binding of GP-1 to cell surface receptors (9). Docking is followed by endocytosis into smooth vesicles, where an acid-induced conformational change of the GP complex leads to membrane fusion (6).
Signal sequences of arenavirus pGP-Cs are longer than average SPs (58 aa), comprising an extended n region containing a myristoylation consensus site (25) and two hydrophobic regions separated by basic amino acid residues. Each of the hydrophobic domains of Lassa virus SPGP-C alone is able to insert pGP-C into the ER membrane, but both h regions were shown to be essential for the proteolytic processing of GP-C into its subunits (13). Lassa virus SPGP-C can perform this function even in trans and was therefore proposed to be a maturation factor for GP-C (12). Junín virus SPGP-C (SSP) was found to be myristoylated and an essential subunit of the mature GP complex required for the transport of the GP-C complex out of the ER (1) and for pH-dependent cell-cell fusion (39). LCMV SPGP-C was detected in viral particles and was therefore proposed to have further functions in the virus life cycle (15).
In this study, we have investigated signal sequence requirements for membrane insertion, processing, and cell surface expression of LCMV pGP-C and the role of SPGP-C in virus infection. Our results reveal that LCMV SPGP-C is part of the GP complex interacting with the GP-2 subunit and that each region of SPGP-C fulfills a specific and essential function in GP-C maturation and virus infection.
MATERIALS AND METHODS
LCMV GP-C expression plasmids and antibodies.
LCMV GP-Cs (wild type [wt] and mutants) were expressed by using either the pSV51l (17) or the pHCMV (38) expression vector. pGP-C was derived from the cDNA sequence of the recloned LCMV GP-C (WE-HPI) (GenBank accession number AJ297484) (2) using the BamHI restriction site. Mutant and deletion pGP-C-HA constructs were cloned by standard PCR and restriction digestion techniques. The generated C-terminal GP-C deletion mutant pGP-C/ΔC comprises aa 1 to 462. All constructs contain the 5′ untranslated region of LCMV GP-C (26) (bp 8 to 77). Sequences were confirmed by DNA sequencing.
The anti-SP7 antibody was obtained by immunization of rabbits with a synthetic peptide comprising aa 7 to 18 (MFEALPHIIDEV) of SPGP-C (15). KL25 is a mouse monoclonal antibody that is reactive with the LCMV GP subunit GP-1 (7). Anti-hemagglutinin (HA) antibody was purchased from Santa Cruz Biotechnology.
Transfection of cells for immunoprecipitation and Western blotting.
HeLa cells were obtained from the American Type Culture Collection and grown in Dulbecco's modified Eagle medium (DMEM; Gibco, Karlsruhe, Germany) supplemented with 10% fetal calf serum (FCS) and 2 mM glutamine under conditions recommended by the manufacturer. Expression vectors were transfected into HeLa cells by the calcium phosphate precipitation method for 20 to 24 h (19). Cells were grown for 48 h before being harvested.
Metabolic labeling and immunoprecipitation.
Plasmid-transfected HeLa cells were starved for 2 to 2.5 h at 37°C with DMEM lacking methionine and cysteine and labeled with 72 μCi/ml [35S]Met-Cys for 30 min or with 250 μCi/ml [3H]myristic acid for 7 h in the presence or absence of the myristoylation inhibitor 2-hydroxy myristic acid (2-HMA). Labeling medium was removed, and cells were washed with phosphate-buffered saline (PBS) and either directly analyzed or chased in DMEM medium for the indicated time periods in the presence or absence of 2-HMA, as indicated. The labeled cells were lysed in immunoprecipitation buffer containing 1% Triton X-100, and proteins were immunoprecipitated as described previously (15).
For coimmunoprecipitation experiments, cells were lysed for 20 min on ice in coimmunoprecipitation buffer containing 150 mM NaCl, 50 mM HEPES-NaOH (pH 7,5), 1.5 mM MgCl2, 2% digitonin, 10% glycerol, 1 mM EGTA (pH 8), and protease inhibitors (1 mM phenylmethylsulfonyl fluoride, complete protease inhibitor cocktail tablets [Roche, Mannheim, Germany]). Nonsoluble material was removed by centrifugation (16,000 × g for 5 min), and supernatants of cell lysates were incubated overnight with protein A-Sepharose and the indicated antibodies at 4°C.
Where indicated, the immunoprecipitated proteins were deglycosylated with peptide-N-glycosidase F (PNGase F) or endoglycosidase H (Endo H) (New England Biolabs, Schwalbach, Germany) according to instructions provided by the manufacturer.
Protein samples were boiled in sodium dodecyl sulfate (SDS) sample buffer (27) containing 200 mM dithiothreitol or 2% 2-mercaptoethanol and analyzed by SDS-polyacrylamide gel electrophoresis (PAGE) as described previously by Schägger and von Jagow (27) using a 10% to 16% polyacrylamide gradient gel and Tricine buffer followed by phosphorimaging using a BAS 1500 instrument (Fuji, Tokyo, Japan) for 35S-labeled proteins. [3H]myristic acid signals were first intensified using a NAMP100 amplifier (Amersham Biosciences, Little Chalfont, England) according to the supplier's instructions and subsequently detected by autoradiography using Kodak AR films at −80°C.
Western blot analysis.
HeLa cells were lysed 48 h posttransfection either in lysis buffer containing Triton X-100 (150 mM NaCl, 50 mM HEPES-NaOH [pH 7.5], 1.5 mM MgCl2, 1% Triton X-100, 10% glycerol, 1 mM EGTA [pH 8], protease inhibitors) or in SDS sample buffer. Where indicated, proteins were deglycosylated with PNGase F according to instructions provided by the manufacturer. Lysates were then separated by SDS-PAGE (27) and transferred onto nitrocellulose membranes. Membranes were blocked in 10% powdered milk in Tris-buffered saline-Tween and probed with anti-HA or anti-SP7 antibody. Proteins were visualized by standard Western blot analysis techniques (19) using anti-rabbit secondary antibody conjugated to horseradish peroxidase (Dianova, Hamburg, Germany) and detected by chemiluminescence (Roche, Mannheim, Germany).
Analysis of cell surface expression.
293T cells were obtained from the American Type Culture Collection and grown in DMEM with 4 mM glutamine, penicillin-streptomycin, and 10% FCS. To detect cell surface expression of LCMV GPs, 293T cells were transiently transfected with expression plasmids encoding LCMV pGP-C-HA (wt or mutants) or mock treated. Cells were grown in 24-well plates and transfected with 800 ng of plasmid DNA per well using Lipofectamine 2000 (Invitrogen, Karlsruhe, Germany). After 48 h, transfected cells were resuspended in PBS-3% FCS and incubated for 1 h at 4°C with 1 μg of the LCMV GP-1-specific monoclonal antibody KL25. After washing three times with PBS-3% FCS, cells were incubated with phycoerythrin-conjugated goat anti-mouse immunoglobulin G (Dianova, Hamburg, Germany) for 30 min at 4°C, washed intensively, and subsequently analyzed by flow cytometry on a FACSCalibur apparatus (Becton Dickinson, San Jose, CA).
Transient production and analysis of LCMV GP-C pseudotypes.
For the transient production of LCMV GP-C pseudotypes, one 10-cm dish of subconfluently grown 293T cells was transfected with 7.5 μg of pMP71-eGFP-pre, 12.5 μg of pSV-Mo-MLVgagpol, and 2 μg of LCMV pGP-C-HA expression plasmids (wt or mutant) using a calcium phosphate transfection kit (Sigma, Taufkirchen, Germany). Medium was replaced 6 to 8 h after transfection. Pseudotyped vectors containing supernatants were harvested after 39 and 48 h, filtered through a 0.45-μm Millex-HV filter (Millipore, Schwalbach, Germany), and used for transduction and Western blot analyses of GP-C.
For Western blot analysis, purified vector particles were obtained by ultracentrifugation of 10 ml of viral supernatants through 1.5 ml of a 20% sucrose cushion in an SW41 Beckman rotor (2 h, 25,000 rpm, 4°C). Viral pellets were resolved in SDS sample buffer (27) containing 100 mM dithiothreitol and analyzed by Western blotting. GP amounts were quantified using ImageJ software (National Institutes of Health).
For the infectivity assays, pseudotype titers were determined by fluorescence-activated cell sorter analysis of transduced cells. TE671 cells were seeded at a density of 5 × 104 cells in 24-well plates and incubated overnight. Dilutions of supernatants were added to the cells, and plates were centrifuged for 1 h at 1,000 × g. Twenty-four hours later, supernatants were removed, and the cells were cultivated in growth medium. The percentage of enhanced green fluorescent protein (eGFP)-positive cells was determined 72 h posttransduction by fluorescence-activated cell sorter analysis (4). Infectivity of pseudoviral particles was normalized to the amount of GP quantified from Western blotting of virus lysates.
RESULTS
Deletions in SPGP-C affect ER membrane insertion and processing of GP-C-HA into GP-1 and GP-2-HA.
Signal sequences have a typical tripartite structure with a polar N region that is usually positively charged, one central hydrophobic region, and a short C-terminal region containing the SPase cleavage site (34). The signal sequence of LCMV pGP-C, however, contains an overall negatively charged n region and two hydrophobic regions (h1 and h2) (Fig. 1A and B). In order to investigate possible functions of the two h regions, we deleted either of them and analyzed membrane insertion and intracellular transport of wt and mutant pGP-C-HA (Δh1 and Δh2). To identify a possible contribution of the SPGP-C n region to these processes, we deleted the n region, leaving the initiating methionine and either a negatively charged amino acid as in the wt (ΔnME) or a positively charged lysine residue (ΔnMK) in front of the h1 region.
FIG. 1.
Effects of SPGP-C deletions on membrane insertion of pGP-C-HA and its proteolytic processing into GP-1 and GP-2-HA. (A) Schematic representation of LCMV GP-C that is C-terminally HA tagged (pGP-C-HA). SPGP-C (aa 1 to 58) and subunits GP-1 (aa 59 to 265) and GP-2 (aa 266 to 498) containing the transmembrane region (TM) are indicated. Putative N-glycosylation sites (Y), the SPase, and SKI-1/S1P cleavage sites are shown. (B) SPGP-C wt sequence in one-letter amino acid code and schematic outline of SPGP-C regions and deletions. The N-terminal (n), hydrophobic (h1 and h2), and C-terminal (c) regions are shown. The region to which the SP antibody was raised (anti-SP7) is indicated. (C) Western blot analysis of SPGP-C deletion mutants. Cells transfected with vector control plasmid (V) or plasmids expressing wt pGP-C-HA or deletion mutants were solubilized, and where indicated, proteins were deglycosylated with PNGase F. Cell lysates were separated by SDS-PAGE, transferred onto a nitrocellulose membrane, and probed with anti-HA antibody. The N-glycosylated GP-C-HA and GP-2-HA and the deglycosylated proteins (*) are indicated. (D) Identification of cleaved wt and mutant SPGP-C after metabolic labeling. Transfected HeLa cells expressing wt pGP-C-HA or deletion mutants Δh1 or Δh2 were radioactively labeled and chased for the indicated time periods. After cell lysis, antigens were immunoprecipitated with anti-SP7 antibody and characterized by SDS-PAGE and autoradiography. The positions of SPGP-C and Δh2 SPGP-C are indicated.
HeLa cells were transiently transfected with plasmids expressing either wt pGP-C-HA or one of the SPGP-C deletion mutants (Δh1, Δh2, ΔnME, and ΔnMK). After lysis with 1% Triton, proteins were treated with PNGase F to remove N-linked oligosaccharide side chains or left untreated. Cell lysates were separated by SDS-PAGE, proteins were transferred onto nitrocellulose, and membranes were probed with anti-HA antibody (Fig. 1C). For the wt and all SPGP-C deletion mutants, the glycosylated GP-C-HA of about 70 kDa and, after PNGase F treatment, its unglycosylated lower-molecular-mass form of about 55 kDa were detected. As glycosylation requires the membrane translocation of GP-C-HA, our results show that only one SPGP-C h region is needed for the ER membrane insertion of pGP-C-HA. Expression levels of the mutants were comparable to those of the wt, with the exception of Δh2, which showed a reduced amount of GP-C-HA. The double banding pattern of unglycosylated GP-C-HA* might be due to inefficient deglycosylation. The processing of GP-C-HA into GP-1 and GP-2-HA, indicated by the appearance of glycosylated GP-2-HA (about 40 kDa), was detected only for the wt and the ΔnME mutant (Fig. 1C, lanes 2 to 5) but was not detected for the other deletion mutants (lanes 6 to 11). Thus, both h regions and a negatively charged amino acid in front of the h1 region are required for the processing of GP-C into GP-1 and GP-2. A positively charged amino acid in front of the h1 region prevents processing into GP-1 and GP-2.
To see whether the deletion of an h region affects the stability of SPGP-C, we metabolically labeled proteins with [35S]Met-Cys, chased for 0 and 3 h, and performed immunoprecipitation with an anti-SP7 antibody that was characterized previously (15). As the epitope that is recognized by the anti-SP7 antibody is deleted in the case of Δn SPGP-C, we were able to analyze only the Δh1 and Δh2 SPGP-C. Immunoprecipitated proteins were separated by SDS-PAGE, and labeled proteins were visualized by phosphorimaging (Fig. 1D). SPGP-C was detected for the wt (Fig. 1D, lanes 1 and 2), and the shorter Δh2 SPGP-C was detected for the Δh2 mutant (lanes 5 and 6). Δh1 SPGP-C was not detected (Fig. 1D, lanes 3 and 4). As no pGP-C was found for any of the mutants with the anti-SP7 antibody (data not shown), it is most likely that Δh1 SPGP-C is rapidly degraded after cleavage from the precursor protein.
Probing SPGP-C function and membrane topology by using point mutants.
To test the effect of single point mutants on the function and topology of SPGP-C, we introduced potential N-glycosylation sites throughout the SPGP-C. During ER membrane insertion of a preprotein, the SP usually spans the membrane, exposing the N terminus to the cytosol and the C terminus with the SPase cleavage site to the ER lumen. As SPGP-C has two hydrophobic regions, either one or both of them could span the membrane with the N terminus exposed on the cytosolic or the lumenal side of the ER membrane. Point mutants were chosen such that the overall properties of the n and h regions remained essentially unchanged (Fig. 2A). Glycosylation of the mutated SPGP-C would indicate that the particular consensus site is exposed on the lumenal side of the ER membrane. After expression in HeLa cells, the point mutants were analyzed by Western blotting using anti-HA antibody (Fig. 2B, top) or the anti-SP7 antibody (bottom). For each mutant, the glycosylated 70-kDa GP-C-HA was detected, indicating insertion into the ER membrane. However, the processing of GP-C-HA and the generation of GP-2-HA were seen only for the wt and I4N, V22T, and A39T mutants (Fig. 2B, lanes 2 to 4 and 6). No accumulation of GP-2-HA was seen for the I29N, L46N, and G54N mutants (Fig. 2B, lanes 5, 7, and 8). Probing the Western blot with anti-SP7 antibody revealed the accumulation of SPGP-C only for those proteins that were processed into GP-2-HA. Very small amounts of SPGP-C were seen for the I29N, L46N, and G54N mutants. In no case did we see a 3-kDa-higher-molecular-mass form of SPGP-C, indicative of glycosylation. In order to see whether SP cleavage from the preproteins had occurred for all mutants, we pulse labeled transfected cells and immunoprecipitated proteins with anti-SP7 antibody (Fig. 2C). The cleaved SPs could be detected for all mutants, although the amounts for the I29N, L46N, and G54N mutants were lower than those of wt SPGP-C. Also, in this analysis, no glycosylated form of SPGP-C could be detected. Taken together, these results show that even minor changes in the amino acid sequence of SPGP-C can result in drastic effects on GP processing and SP stability. As no glycosylated SPGP-C appeared, we cannot draw any conclusions about the topology of SPGP-C.
FIG. 2.
Effect of SPGP-C point mutants on GP-C-HA processing and assessment of SPGP-C membrane topology by using N-glycosylation consensus site mutations. (A) Schematic representation of SPGP-C point mutants. Positions of mutated amino acid residues that introduce potential N-glycosylation sites are indicated. (B) Western blot analysis of HeLa cells transfected with vector control plasmid (V) or plasmids expressing wt pGP-C-HA or point mutants. Proteins were separated by SDS-PAGE and identified using either anti-HA (top) or anti-SP7 (bottom) antibody. (C) Identification of mutant SPGP-Cs after metabolic labeling of transfected cells. The cleaved SPs were immunoprecipitated using anti-SP7 antibody.
Myristoylation of SPGP-C.
The conserved N-terminal amino acid residues of the LCMV pGP-C signal sequence match the myristoylation consensus sequence MGxxxT/S (Fig. 3A). This consensus site is conserved in the signal sequences of other arenavirus GP-Cs, and myristoylation has previously been shown for Junín virus (39). Myristoylation is a cotranslational event occurring after the initiating methionine has been removed. Myristate is then linked to Gly-2 via an amide bond (25). To investigate potential LCMV SPGP-C myristoylation, we mutated the Gly-2 residue to Ala (G2A) to abolish myristoylation. In order to investigate the effect of myristoylation on the orientation of the N-terminal region of SPGP-C, we generated a double mutant (G2A/I4N) in which myristoylation cannot occur, but the n region of SPGP-C can be N glycosylated if it is exposed to the ER lumen (Fig. 3A).
FIG. 3.
Myristoylation of SPGP-C and its effect on SPGP-C membrane topology. (A) Outline of SPGP-C and myristoylation consensus site. Point mutants to prevent SPGP-C myristoylation (G2A) and/or to allow N glycosylation (I4N) are shown. (B) Myristoylation of SPGP-C. HeLa cells expressing wt pGP-C-HA or the G2A or I4N mutant were metabolically labeled with [3H]myristic acid. An inhibitor of myristoylation (2-HMA) was added where indicated. Antigens were immunoprecipitated with anti-SP7 antibody. (C) Accumulation of mutant SPGP-C and processing of pGP-C-HA. Transfected cells were lysed in SDS-PAGE sample buffer, and proteins were separated by SDS-PAGE, followed by immunoblotting with anti-HA or anti-SP7 antibody. The positions of GP-C-HA, GP-2-HA, SPGP-C, and a 3-kDa-higher form of SPGP-C, marked by an arrow, are indicated. (D) Glycosylation of unmyristoylated I4N and G2A/I4N SPGP-C mutants. Transfected HeLa cells expressing the I4N or the G2A/I4N mutant were metabolically labeled with [35S]Met-Cys. Where indicated, the cells were grown in the presence of the myristoylation inhibitor. After cell lysis and immunoprecipitation with anti-SP7 antibody, aliquots of the immunoprecipitates were either left untreated (−) or treated with Endo H (E) or PNGase F (P) to remove selectively high mannose oligosaccharides or all N-linked carbohydrates, respectively. g-SPGP-C, glycosylated form of SPGP-C; V, vector control plasmid.
In order to characterize the myristoylation of SPGP-C, we labeled transfected HeLa cells with [3H]myristic acid. After immunoprecipitation with anti-SP7 antibody, one major protein corresponding to myristoylated SPGP-C was precipitated from cells expressing the wt and the I4N mutant (Fig. 3B, lanes 3 and 4). When myristoylation was inhibited by 2-HMA, the label of SPGP-C in the wt and I4N mutant was drastically reduced (Fig. 3B, lanes 5 and 6). For cells expressing the G2A mutant, no myristoylated form of SPGP-C was detected (Fig. 3B, lane 2). However, the overall amount of cleaved SPGP-C from the G2A mutant was comparable to the wt level, as shown by Western blotting (Fig. 3C, bottom). Taken together, these results show that SPGP-C is myristoylated and that the introduction of an N-glycosylation site (I4N) does not interfere with myristoylation.
To determine whether myristoylation interferes with the glycosylation of the I4N mutant, we labeled I4N-expressing cells with [35S]Met-Cys and treated them with the myristoylation inhibitor 2-HMA. In this experiment, an additional 3-kDa-larger form, consistent with the N glycosylation of SPGP-C, was immunoprecipitated with anti-SP7 antibody (Fig. 3D, lane 2). The larger form of SPGP-C disappeared at the expense of wt-sized SPGP-C after treatment with the deglycosylation enzyme Endo H or PNGase F (Fig. 3D, lanes 3 and 4). When myristoylation was prevented by a mutation of the Gly-2 residue to Ala (G2A/I4N), mainly the higher-molecular-mass form of SPGP-C was detected, which also disappeared after deglycosylation (Fig. 3C, lane 5, and D, lanes 5 to 7). Thus, the n region of I4N SPGP-C becomes N glycosylated and thus can translocate across the ER membrane when either myristoylation is inhibited or myristoylation is prevented by the G2A mutation.
We next asked whether the myristoylation of SPGP-C is a prerequisite for the intracellular transport and proteolytic processing of GP-C. Cells expressing the wt or the G2A, I4N, G2A/I4N mutant were lysed, and proteins were analyzed by Western blotting using the anti-HA antibody. Insertion and GP-C-HA processing into GP-1 and GP-2-HA could be observed in each case (Fig. 3C, top). In addition, the cleavage and accumulation of all SPGP-Cs, including the glycosylated form of the G2A/I4N mutant (Fig. 3C, lane 5, arrow), could be detected by using the anti-SP7 antibody (Fig. 3C, bottom). Thus, the inhibition of SPGP-C myristoylation and glycosylation at Asn in position 4 does not interfere with the processing of LCMV GP-C into GP-1 and GP-2.
Both SPGP-C h regions are needed for cell surface expression of GPs.
We next asked whether SPGP-C mutants can still promote the transport of the GPs to the cell surface. To test this, we used flow cytometry and the GP-1-specific antibody KL25 to monitor cell surface expression of the SPGP-C mutants. In Fig. 4, the relative amount of cell surface expression of the SPGP-C mutants compared to wt GP (set to 100%) is depicted. The wt and the ΔnME mutant were efficiently expressed on the cell surface, whereas very low or no surface expression was found for the other deletion mutants (ΔnMK, Δh1, and Δh2) (Fig. 4, left). The latter SPGP-C mutants also did not promote the processing of GP-C into GP-1 and GP-2 (Fig. 1). Thus, both hydrophobic regions of SPGP-C are required for the cell surface expression of the GP complex, while the SPGP-C n region is dispensable.
FIG. 4.
Cell surface expression of SPGP-C mutants. Transfected 293T cells expressing wt pGP-C-HA or SPGP-C mutants as well as untransfected control cells were analyzed by flow cytometry with the LCMV GP-1-directed monoclonal antibody KL25. Differently shaded bars represent two independent experiments. Data show percent cell surface expression of SPGP-C mutants relative to wt levels.
The cell surface expression of the SPGP-C point mutants was similarly investigated. The experiments show that only those mutants that promoted GP-C processing into GP-1 and GP-2 (G2A, I4N, G2A/I4N, V22T, and A39T) were expressed on the plasma membrane (Fig. 4, right). Note that the glycosylated SPGP-C expressed from the G2A/I4N mutant also efficiently promoted cell surface expression of the GPs.
Requirements for the interaction of SPGP-C with GP.
As SPGP-C is found in LCMV particles (15), it is likely that it assembles into particles in association with GP-1 and GP-2. To investigate a possible interaction, we tested if SPGP-C can be coimmunoprecipitated with the GPs. HeLa cells expressing wt pGP-C-HA were metabolically labeled with [35S]Met-Cys. After anti-HA immunoprecipitation and PNGase F treatment of solubilized proteins, GP-C-HA* and, in addition, SPGP-C were detected (Fig. 5B, lane 2). This coimmunoprecipitation of SPGP-C was still detectable after chase times of 3 and 6 h, when a large portion of GP-C-HA is processed into GP-1 and GP-2-HA (Fig. 5B, lanes 3 and 4). These results show that SPGP-C is interacting with the GPs and that the interaction persists during intracellular transport.
FIG. 5.
Coimmunoprecipitation of SPGP-C with GP-C-HA or deletions. (A) Outline of wt pGP-C-HA, GP-1-HA, or GP-2-HA fused to SPGP-C and a C-terminal deletion mutant of pGP-C. (B) Transfected HeLa cells were metabolically labeled and chased for the times indicated. After solubilization of the proteins with digitonin and immunoprecipitation with anti-HA antibody, the proteins were deglycosylated with PNGase F (*) to better resolve the GP-1 and GP-2-HA subunits. The positions of GP-C-HA*, the cleaved subunits, and the coimmunoprecipitated SPGP-C are indicated. (C) Coimmunoprecipitation of SPGP-C with GP-C-HA subunits and a pGP-C C-terminal deletion mutant. Radioactively labeled proteins were solubilized with digitonin and immunoprecipitated with KL25 antibody, anti-HA antibody, or an unspecific antibody as a control (c). The positions of the proteins are indicated. Nonglycosylated proteins are marked by an asterisk. V, vector control plasmid.
Next, we examined the subunit of GP-C that is interacting with SPGP-C. We expressed fusion constructs containing either GP-1-HA or GP-2-HA fused to SPGP-C (Fig. 5A). After radioactive labeling, proteins were immunoprecipitated using either the GP-1-specific KL25 antibody, anti-HA, or an unrelated control antibody (Fig. 5C). The KL25 antibody but not an unrelated antibody efficiently coimmunoprecipitated SPGP-C from cells expressing wt GP-C-HA (Fig. 5C, lanes 3 and 4). Essentially no coimmunoprecipitation of SPGP-C was detected with GP-1-HA (Fig. 5C, lanes 5 and 6). In contrast, coimmunoprecipitation of SPGP-C was detected with GP-2-HA, although this was not as efficient as that with the wt (Fig. 5C, lanes 7 and 8). Signal sequence cleavage could be detected for both pGP subunits (data not shown). To see whether the cytoplasmic portion of GP-C is required for the interaction with SPGP-C, we deleted the last 36 aa of pGP-C, generating pGP-C/ΔC (Fig. 5A). Figure 5C shows that upon immunoprecipitation with the KL25 antibody, SPGP-C is efficiently coimmunoprecipitation with GP-C/ΔC (lanes 9 and 10), indicating that the cytoplasmic part of GP-C is not needed for the interaction with SPGP-C. In no case was SPGP-C coimmunoprecipitated when an unrelated control antibody was used (Fig. 5C, lanes 2, 4, 6, 8, and 10).
SPGP-C requirements for interaction with GP-C.
To determine the requirements of SPGP-C for the interaction with GP-C, we analyzed the SPGP-C mutants for coimmunoprecipitation with GP-C-HA. As shown in Fig. 6A, the deletion of the SPGP-C n region still allows the specific coimmunoprecipitation of SP with GP-C-HA (lanes 4 to 7). However, the positively charged amino acid residue in front of the SPGP-C h1 region negatively affects coimmunoprecipitation with GP-C. In addition, this mutant failed to promote the processing of GP-C into GP-1 and GP-2, as shown in Fig. 1C. No specific coimmunoprecipitation was found for the Δh1 and Δh2 mutants (data not shown). This suggests that the two h regions of SPGP-C are sufficient and essential for the interaction of SPGP-C with GP-C. In addition, we analyzed the SPGP-C point mutants for coimmunoprecipitation of SPGP-C with GP-C-HA (Fig. 6B). All SPGP-Cs with point mutations could be coimmunoprecipitated with GP-C-HA. Note that even the glycosylated SPGP-C with the G2A/I4N mutation interacts with GP-C-HA (Fig. 6B, lane 5).
FIG. 6.
Coimmunoprecipitation of SPGP-C mutants with GP-C-HA. wt pGP-C-HA and SPGP-C mutants were expressed in HeLa cells and metabolically labeled with [35S]Met-Cys. Coimmunoprecipitation was performed, as depicted in Fig. 5, with either KL25 and an unspecific antibody as a control (c) (A) or anti-HA antibody (B). The positions of SPGP-C and Δn SPGP-C are indicated. A glycosylated form of SPGP-C is marked by an arrow. V, vector control plasmid.
SPGP-C requirements for pseudovirus infectivity.
LCMV particles assemble at the cell surface and bud to produce infectious virions (24). The LCMV GPs are an essential component of this assembly process and promote the entry of virions into target cells. The entry of LCMV particles is mediated by GP-1 binding to cell surface receptors (α-dystroglycan) (9), followed by endocytosis of the virions. The acidification of endocytosed vesicles leads to a conformational change of the GP complex and fusion with the vesicular membrane (6). To study the effect of SPGP-C mutations on viral infectivity, we analyzed LCMV pseudovirus formation and infection of target cells.
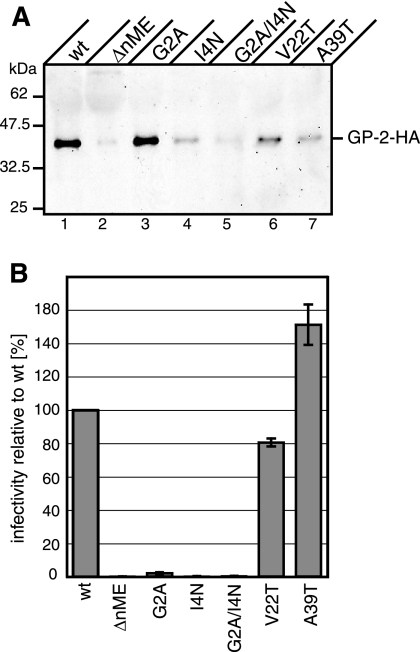
For the production of LCMV pseudoviral particles, 293T cells were transfected with plasmids encoding LCMV pGP-C-HA or SPGP-C mutants, murine leukemia virus gag pol, and a retroviral vector encoding eGFP (4). The incorporation of the GPs into retroviral particles was analyzed by Western blotting using anti-HA antibody. The infectivity of pseudoviral particles was determined after incubation of the cell supernatants with target cells (TE671) by measuring the green fluorescence of target cells expressing eGFP. Infectivity was normalized to the amount of GP-2-HA in pseudoviral particles calculated from Western blotting of concentrated supernatants (Fig. 7A).
FIG. 7.
Pseudovirus formation and infectivity of SPGP-C mutants. (A) Pseudovirus formation. Pseudoviruses were collected from the supernatant of transfected cells and pelleted by ultracentrifugation. Proteins were solubilized in SDS-PAGE sample buffer and separated by SDS-PAGE. After transfer onto nitrocellulose, the membrane was probed with anti-HA antibody. The position of GP-2-HA is indicated. GP amounts were quantified using ImageJ software. (B) Pseudovirus infectivity. TE671 cells were incubated with collected pseudoviruses, and infected eGFP-positive cells were quantified by flow cytometry. Data represent mean infectivities of pseudoviruses normalized to wt levels from three independent experiments (with the wt set to 100%) (± standard deviation).
Cleavage of the LCMV GP into GP-1 and GP-2 and cell surface expression are a prerequisite for the incorporation into retroviral particles and release from the cell surface (3). Therefore, only SPGP-C mutants that are cleaved and transported to the cell surface were investigated for pseudovirus formation and infectivity. A high amount of GP-2-HA incorporated into pseudoviral particles was detected for cells expressing wt GP-C-HA and the G2A mutant (Fig. 7A, lanes 1 and 3). The other mutants showed less efficient pseudoviral particle formation. Infection of target cells was seen only for pseudoviral particles assembled from wt GPs and V22T and A39T point mutants (Fig. 7B). Essentially no infectivity was detected for pseudoviral particles assembled from SPGP-C lacking the n region (ΔnME) or carrying a point mutation in the n region (G2A, I4N, and G2A/I4N). Taken together, the results show that the SPGP-C n region and, specifically, its myristoylation are essential for pseudovirus infectivity.
DISCUSSION
Signal sequences of arenavirus pGP-Cs are unusually long, containing an extended n region and two h regions. By using several deletion and point mutants of LCMV SPGP-C, we have investigated the requirements for GP-C membrane insertion, cleavage into GP-1 and GP-2, transport to the cell surface, and pseudoviral infectivity (summarized in Fig. 8).
FIG. 8.
(A) Summarized results for LCMV SPGP-C deletion and point mutants. Functions of SPGP-C and steps in GP-C maturation are indicated at the top of the figure. The different SPGP-C mutants are listed vertically. Results are given in comparison to wt amounts (indicated by +++): ++, less; +, drastically reduced; −, nothing; n.d., not determined. (B) Regions of SPGP-C sufficient or required for GP-C biosynthesis, transport to the cell surface, and viral infectivity. For ER insertion, only one hydrophobic region is needed, while both regions are necessary for the cleavage of the GP into GP-1 and GP-2, cell surface expression, and interaction with GP-C. For pseudovirus formation and infectivity, all regions of the SP are required, including myristoylation (myr) in the SPGP-C n region. (C) Postulated membrane topologies of the N-terminally-glycosylated SPGP-C. The hydrophobic segments (h1 and h2) are indicated by gray ovals. The positively charged lysine residue that separates the two h regions is indicated by a +. Proposed membrane topologies of the N-terminally-glycosylated SPGP-C during membrane insertion and SPase cleavage are indicated (a). When myristoylation does not occur, the N terminus is translocated across the membrane, with one or both h regions spanning the membrane (b or c), and can become glycosylated (*).
We found that SPGP-C associates with GP-C in the ER and remains part of the GP complex during transport to the cell surface. For the membrane insertion of pGP-C, we showed that one h region of SPGP-C is sufficient; however, both h regions, but not the n region, of SPGP-C are required for GP-C processing into GP-1 and GP-2 and cell surface expression. In addition, the n region of SPGP-C and its myristoylation were found to be essential for pseudovirus formation and viral infectivity. Most importantly, we showed that unmyristoylated SPGP-C exposes its N-terminal region to the exoplasmic side and still promotes GP-C maturation and transport to the cell surface. However, this SPGP-C is defective in pseudoviral infection. Our data imply that the myristoylation and membrane orientation of SPGP-C hold an important key to an understanding of the role of SPGP-C in LCMV infectivity.
Functional significance of the n and h regions of SPGP-C in the biogenesis and maturation of LCMV GP-C.
The n region of LCMV SPGP-C is, similar to Lassa virus SPGP-C, required neither for ER membrane insertion nor for the intracellular transport and processing of GP-C (13). However, there are certain requirements with regard to the charged amino acid residue following the initiating methionine. We find that placing a positively charged amino acid residue in front of the SPGP-C h1 region (ΔnMK) prevents the cleavage of GP-C into its subunits and negatively influences the interaction of SPGP-C with GP-C as well as the transport of the GP to the cell surface. N-terminal regions of signal sequences are usually positively charged (33), whereas the n region of SPGP-C has an overall negative-charge character. The unexpected requirement for a negatively charged amino acid residue in the n region might therefore be related to the fact that SPGP-C contains two hydrophobic regions, only one of which might function in ER targeting (20). The LCMV SPGP-Cs with either the h1 or h2 region deleted can mediate ER membrane insertion of pGP-C, although the h2 region is more efficient in mediating this process. The h2 region with the positively charged lysine residue at its N-terminal side and its proximity to the signal peptidase cleavage side at its C-terminal side show all characteristics of a minimal signal sequence for ER targeting (21). Therefore, the h2 region may function in ER targeting and, in this function, may span the membrane during membrane insertion and signal peptidase cleavage (22, 34) (Fig. 8C).
Mutant SPGP-Cs with only one h region mediate neither the processing of GP-C into GP-1 and GP-2 nor their cell surface expression. A similar result was found previously for Lassa virus SPGP-C (13). For LCMV SPGP-C, we find that even point mutations in either the h1 or the h2 region (I29N and L46N) prevent the processing of GP-C into GP-1 and GP-2 and cell surface expression, although they still mediate ER membrane insertion. As these mutant SPGP-Cs are produced but do not accumulate, we speculate that the destabilization of SPGP-C causes the block in GP-C transport. Taken together, it appears that the two h regions of SPGP-C are important structural elements for the intracellular transport of GP-C and its processing into GP-1 and GP-2.
Myristoylation and membrane topology of SPGP-C.
All SPs of arenavirus GP-Cs share a myristoylation consensus site at the N terminus (39). We found that LCMV SPGP-C is indeed myristoylated at its N-terminal Gly-2 and that myristoylation is not required for GP-C cleavage into GP-1 and GP-2 as it is true for SPGP-C (SSP) of Junín virus GP-C (39). When we tested the membrane topology of SPGP-C by making use of N-glycosylation sites at different positions along SPGP-C, we found that the n region of SPGP-C can be glycosylated when myristoylation is prevented. This clearly shows that the n region has an intrinsic property to translocate to the exoplasmic side of the ER membrane (Fig. 8C). Glycosylated SPGP-C with an N-exoplasmic/C-cytoplasmic (Nexo/Ccyt) topology can still interact with GP-C (Fig. 6B) and is able to promote the processing of GP-C into GP-1 and GP-2 and cell surface expression of the GP complex (Fig. 3C and 4). Thus, SPGP-C with an Nexo/Ccyt topology has all the functional properties required to promote the intracellular transport of GP-C. This topology, however, does not promote infection of target cells. One possible explanation is that myristoylation prevents the N-terminal membrane translocation of SPGP-C and that it is this topological change that prevents infectivity. An argument against this explanation is that unmyristoylated and glycosylated SPGP-C with an Nexo/Ccyt topology is fully functional in promoting GP-C maturation and cell surface expression. Thus, another reason why unmyristoylated SPGP-C is defective in viral infectivity might be that the myristoylated N-terminal region of SPGP-C has to be exposed on the exoplasmic side. In this case, myristoylation might then be directly required for the interaction of the GPs with cellular membranes. The fact that the glycosylation of the SPGP-C I4N mutant is seen only when myristoylation is inhibited does not necessarily mean that myristoylation prevents the translocation of the SPGP-C n region to the exoplasmic side. It is conceivable that myristoylation does not allow the access of oligosaccharyl transferase to the Asn-4 glycosylation site (23, 29). It should, however, be mentioned that there is a precedent for the glycosylation of an Asn-4 position when the Gly-2 position is myristoylated (31). Myristoylation does not principally prevent the N-terminal translocation of membrane proteins, as proteins that expose their myristoylated N-terminal regions on the exoplasmic side have been reported previously (10, 16, 18). Unfortunately, we were not able to directly determine the membrane topology of the myristoylated wt SPGP-C.
The fact that the n region of SPGP-C can translocate to the exoplasmic (lumenal) side of the membrane is certainly unusual, as signal sequences usually insert into the ER membrane in a loop-like fashion, exposing the N-terminal region on the cytoplasmic side and the C-terminal region on the exoplasmic side of the ER membrane. Major determinants for a Ncyt/Cexo topology are the positively charged amino acid residues usually found in n regions of simple signal sequences. SPGP-C can be considered to be a complex signal sequence that performs additional functions besides ER targeting and insertion. Consistent with N translocation is the overall negative charge of the SPGP-C n region and the finding that a negatively charged amino acid in front of the h1 region is required for postinsertion functions of SPGP-C. According to the so-called “positive-inside” rule, positively charged amino acids are indicators of a cytoplasmic localization of segments in membrane proteins (30). It is also conceivable that SPGP-C does not exclusively adopt one topology across the membrane. Examples of alternative topologies across membranes are well established, e.g., for the hepatitis B virus large envelope protein or the prion proteins (18, 32).
The membrane topology of Lassa virus SPGP-C has previously been analyzed by introducing epitopes of different lengths containing N-glycosylation acceptor sites (13). The cytoplasmic or exoplasmic localizations of these epitopes were determined either by immunofluorescence analysis of permeabilized cells or by glycosylation within the epitopes. For the modified Lassa virus SPGP-C, it was found that the N terminus is localized on the cytoplasmic side and that the h1 region spans the membrane. For several reasons, we think that this topological analysis cannot be extended to the authentic SPGP-C of Lassa virus or generally to SPGP-Cs of arenaviruses. The introduced epitopes are between 9 and 25 aa in length. Such insertions could drastically affect the topology of SPGP-C. Even more serious is the fact that only the mutated SPGP-C without its GP portion was investigated in the topological analysis (13). Therefore, not only were possible topological determinants added, but the entire pathway for ER membrane insertion might also be different from that of the authentic pGP-C.
SPGP-C as part of the GP complex.
We previously found that LCMV SPGP-C accumulates in viral particles (15). Using coimmunoprecipitation, we now show that SPGP-C is an integral part of the GP complex. SPGP-C was observed to interact with GP-C, GP-2 alone, and with GP-C lacking the cytoplasmic region. This suggests that LCMV SPGP-C interacts with the transmembrane region and/or exoplasmic parts of GP-2. For Junín virus, an interaction between Junín virus SPGP-C (SSP) and the cytoplasmic and transmembrane regions of GP-C was found (1). The cytoplasmic region of Junín virus GP-C was even found to be essential for SPGP-C (SSP) binding. We do not find that the cytoplasmic region of LCMV GP-C is required for the interaction with SPGP-C. Whether this reflects differences between LCMV and Junín virus GPs or different interactions at different stages of intracellular transport remains to be seen.
Requirements of SPGP-C for virus infection.
LCMV infection of target cells is initiated by the binding of the GP-1 subunit to α-dystroglycan on the target cell surface (9; reviewed in reference 8). After the internalization of bound virus into large, smooth-walled, endocytic vesicles, the viral nucleocapsid is delivered into the cell cytoplasm by pH-dependent membrane fusion (6, 11). At an acidic pH, GP-1 dissociates from GP-2, exposing the putative GP-2 fusion peptide (11). To analyze the effects of SPGP-C mutations on viral infectivity, we used LCMV pseudoviruses. The pseudovirus system allows the easy exchange of the GPs incorporated into the viral membrane by transfection of the appropriate constructs (4).
With the exception of the G2A mutant, all mutants tested (ΔnME, I4N, G2A/I4N, V22T, and A39T) showed a reduction in pseudovirus formation compared to the wt (Fig. 7). As the mutations are located in the n region as well as in the h regions of SPGP-C, this indicates that features of the entire SPGP-C contribute to the efficiency of pseudoviral particle formation. Analysis of pseudovirus infectivity revealed that pseudoviruses produced with the two h-region mutants, V22T and A39T, were still able to infect target cells, whereas mutations in the SPGP-C n region completely inhibit the infection (Fig. 7B). This strongly suggests that the n region of SPGP-C and, specifically, myristoylation are crucial for viral infection. The contribution of SPGP-C to viral infectivity could be indirect via modulating the conformation of the GP complex or a direct involvement of SPGP-C in viral entry or fusion. A role for SPGP-C in viral fusion is supported by the finding that the SPGP-C (SSP) of Junín virus GP-C is essential for pH-dependent membrane fusion, which was tested by syncytium formation (39). Participation of a small myristoylated peptide in membrane fusion is not without precedent. Considering size, topology, myristoylation, and sequence-specific effects on viral infectivity, the arenavirus SPGP-Cs share striking similarities with the fusion-associated small transmembrane (FAST) proteins. FAST proteins are small (10- to 15-kDa) membrane proteins that expose the N-terminally-myristoylated or palmitoylated region to the exoplasmic side of the plasma membrane. FAST proteins are proposed to mediate membrane fusion in cooperation with cellular proteins that may promote a close apposition of the membranes (28). In the case of arenavirus membrane fusion, SPGP-C may similarly cooperate with GP-2 in the fusion with cellular membranes. Clearly, further work is required to elucidate the functional contribution of SPGP-C to viral infection.
ADDENDUM
While the manuscript was under review, Saunders et al. (26a) reported, consistent with our observations, that LCMV SPGP-C (SSP) is required for the posttranslational maturation cleavage of GP-1 and GP-2, GP transport to the cell surface, and the formation of infectious virus particles. In addition, they showed that SPGP-C is required for acid pH-dependent GP-mediated cell fusion.
Agnihothram et al. (1a) reported the bitopic membrane topology of SPGP-C (SSP) of Junín virus GP-C with both the N and C termini of SPGP-C in the cytosol. As tagged versions of SPGP-C were used and signal peptidase cleavage was prevented, it is questionable whether such a topology also holds true for the authentic SPGP-C cleaved from GP-C.
Acknowledgments
We thank S. Bracharz and R. Seyd for excellent technical assistance.
This work was supported by funds from the Deutsche Forschungsgemeinschaft to B.D. (SFB 638).
Footnotes
Published ahead of print on 5 September 2007.
REFERENCES
- 1.Agnihothram, S. S., J. York, and J. H. Nunberg. 2006. Role of the stable signal peptide and cytoplasmic domain of G2 in regulating intracellular transport of the Junín virus envelope glycoprotein complex. J. Virol. 80:5189-5198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1a.Agnihothram, S. S., J. York, M. Trahey, and J. H. Nunberg. 2007. Bitopic membrane topology of the stable signal peptide in the tripartite Junín virus GP-C envelope glycoprotein complex. J. Virol. 81:4331-4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beyer, W. R., H. Miletic, W. Ostertag, and D. von Laer. 2001. Recombinant expression of lymphocytic choriomeningitis virus strain WE glycoproteins: a single amino acid makes the difference. J. Virol. 75:1061-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beyer, W. R., D. Popplau, W. Garten, D. von Laer, and O. Lenz. 2003. Endoproteolytic processing of the lymphocytic choriomeningitis virus glycoprotein by the subtilase SKI-1/S1P. J. Virol. 77:2866-2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beyer, W. R., M. Westphal, W. Ostertag, and D. von Laer. 2002. Oncoretrovirus and lentivirus vectors pseudotyped with lymphocytic choriomeningitis virus glycoprotein: generation, concentration, and broad host range. J. Virol. 76:1488-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blobel, G. 1980. Intracellular protein topogenesis. Proc. Natl. Acad. Sci. USA 77:1496-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borrow, P., and M. B. Oldstone. 1994. Mechanism of lymphocytic choriomeningitis virus entry into cells. Virology 198:1-9. [DOI] [PubMed] [Google Scholar]
- 7.Bruns, M., J. Cihak, G. Muller, and F. Lehmann-Grube. 1983. Lymphocytic choriomeningitis virus. VI. Isolation of a glycoprotein mediating neutralization. Virology 130:247-251. [DOI] [PubMed] [Google Scholar]
- 8.Buchmeier, M. J. 2002. Arenaviruses: protein structure and function. Curr. Top. Microbiol. Immunol. 262:159-173. [DOI] [PubMed] [Google Scholar]
- 9.Cao, W., M. D. Henry, P. Borrow, H. Yamada, J. H. Elder, E. V. Ravkov, S. T. Nichol, R. W. Compans, K. P. Campbell, and M. B. Oldstone. 1998. Identification of alpha-dystroglycan as a receptor for lymphocytic choriomeningitis virus and Lassa fever virus. Science 282:2079-2081. [DOI] [PubMed] [Google Scholar]
- 10.Corcoran, J. A., and R. Duncan. 2004. Reptilian reovirus utilizes a small type III protein with an external myristylated amino terminus to mediate cell-cell fusion. J. Virol. 78:4342-4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Simone, C., M. A. Zandonatti, and M. J. Buchmeier. 1994. Acidic pH triggers LCMV membrane fusion activity and conformational change in the glycoprotein spike. Virology 198:455-465. [DOI] [PubMed] [Google Scholar]
- 12.Eichler, R., O. Lenz, T. Strecker, M. Eickmann, H. D. Klenk, and W. Garten. 2003. Identification of Lassa virus glycoprotein signal peptide as a trans-acting maturation factor. EMBO Rep. 4:1084-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eichler, R., O. Lenz, T. Strecker, M. Eickmann, H. D. Klenk, and W. Garten. 2004. Lassa virus glycoprotein signal peptide displays a novel topology with an extended endoplasmic reticulum luminal region. J. Biol. Chem. 279:12293-12299. [DOI] [PubMed] [Google Scholar]
- 14.Eichler, R., O. Lenz, T. Strecker, and W. Garten. 2003. Signal peptide of Lassa virus glycoprotein GP-C exhibits an unusual length. FEBS Lett. 538:203-206. [DOI] [PubMed] [Google Scholar]
- 15.Froeschke, M., M. Basler, M. Groettrup, and B. Dobberstein. 2003. Long-lived signal peptide of lymphocytic choriomeningitis virus glycoprotein pGP-C. J. Biol. Chem. 278:41914-41920. [DOI] [PubMed] [Google Scholar]
- 16.Gripon, P., J. Le Seyec, S. Rumin, and C. Guguen-Guillouzo. 1995. Myristylation of the hepatitis B virus large surface protein is essential for viral infectivity. Virology 213:292-299. [DOI] [PubMed] [Google Scholar]
- 17.Huylebroeck, D., G. Maertens, M. Verhoeyen, C. Lopez, A. Raeymakers, W. M. Jou, and W. Fiers. 1988. High-level transient expression of influenza virus proteins from a series of SV40 late and early replacement vectors. Gene 66:163-181. [DOI] [PubMed] [Google Scholar]
- 18.Lambert, C., and R. Prange. 2001. Dual topology of the hepatitis B virus large envelope protein: determinants influencing post-translational pre-S translocation. J. Biol. Chem. 276:22265-22272. [DOI] [PubMed] [Google Scholar]
- 19.Maniatis, T., J. Sambrook, and E. F. Fritsch. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 20.Martoglio, B., and B. Dobberstein. 1995. Protein insertion into the membrane of the endoplasmic reticulum: the architecture of the translocation site. Cold Spring Harb. Symp. Quant. Biol. 60:41-45. [DOI] [PubMed] [Google Scholar]
- 21.Martoglio, B., and B. Dobberstein. 1998. Signal sequences: more than just greasy peptides. Trends Cell Biol. 8:410-415. [DOI] [PubMed] [Google Scholar]
- 22.Martoglio, B., and B. Dobberstein. 1996. Snapshots of membrane-translocating proteins. Trends Cell Biol. 6:142-147. [DOI] [PubMed] [Google Scholar]
- 23.Nilsson, I., and G. von Heijne. 2000. Glycosylation efficiency of Asn-Xaa-Thr sequons depends both on the distance from the C terminus and on the presence of a downstream transmembrane segment. J. Biol. Chem. 275:17338-17343. [DOI] [PubMed] [Google Scholar]
- 24.Perez, M., R. C. Craven, and J. C. de la Torre. 2003. The small RING finger protein Z drives arenavirus budding: implications for antiviral strategies. Proc. Natl. Acad. Sci. USA 100:12978-12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Resh, M. D. 1999. Fatty acylation of proteins: new insights into membrane targeting of myristoylated and palmitoylated proteins. Biochim. Biophys. Acta 1451:1-16. [DOI] [PubMed] [Google Scholar]
- 26.Romanowski, V., Y. Matsuura, and D. H. Bishop. 1985. Complete sequence of the S RNA of lymphocytic choriomeningitis virus (WE strain) compared to that of Pichinde arenavirus. Virus Res. 3:101-114. [DOI] [PubMed] [Google Scholar]
- 26a.Saunders, A. A., J. P. C. Ting, J. Meisner, B. W. Neuman, M. Perez, J. C. de la Torre, and M. J. Buchmeier. 2007. Mapping the landscape of the lymphocytic choriomeningitis virus stable signal peptide reveals novel functional domains. J. Virol. 81:5649-5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schägger, H., and G. von Jagow. 1987. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166:368-379. [DOI] [PubMed] [Google Scholar]
- 28.Shmulevitz, M., and R. Duncan. 2000. A new class of fusion-associated small transmembrane (FAST) proteins encoded by the non-enveloped fusogenic reoviruses. EMBO J. 19:902-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silberstein, S., and R. Gilmore. 1996. Biochemistry, molecular biology, and genetics of the oligosaccharyltransferase. FASEB J. 10:849-858. [PubMed] [Google Scholar]
- 30.Sipos, L., and G. von Heijne. 1993. Predicting the topology of eukaryotic membrane proteins. Eur. J. Biochem. 213:1333-1340. [DOI] [PubMed] [Google Scholar]
- 31.Utsumi, T., H. Ohta, Y. Kayano, N. Sakurai, and Y. Ozoe. 2005. The N-terminus of B96Bom, a Bombyx mori G-protein-coupled receptor, is N-myristoylated and translocated across the membrane. FEBS J. 272:472-481. [DOI] [PubMed] [Google Scholar]
- 32.Vishwanath, R., D. Lingappa, et al. 2002. Conformational control through translocational regulation: a new view of secretory and membrane protein folding. BioEssays 24:741-748. [DOI] [PubMed] [Google Scholar]
- 33.von Heijne, G. 1990. Protein targeting signals. Curr. Opin. Cell Biol. 2:604-608. [DOI] [PubMed] [Google Scholar]
- 34.von Heijne, G. 1985. Signal sequences. The limits of variation. J. Mol. Biol. 184:99-105. [DOI] [PubMed] [Google Scholar]
- 35.von Heijne, G. 1990. The signal peptide. J. Membr. Biol. 115:195-201. [DOI] [PubMed] [Google Scholar]
- 36.Walter, P., R. Gilmore, and G. Blobel. 1984. Protein translocation across the endoplasmic reticulum. Cell 38:5-8. [DOI] [PubMed] [Google Scholar]
- 37.Weihofen, A., K. Binns, M. K. Lemberg, K. Ashman, and B. Martoglio. 2002. Identification of signal peptide peptidase, a presenilin-type aspartic protease. Science 296:2215-2218. [DOI] [PubMed] [Google Scholar]
- 38.Yee, J. K., A. Miyanohara, P. LaPorte, K. Bouic, J. C. Burns, and T. Friedmann. 1994. A general method for the generation of high-titer, pantropic retroviral vectors: highly efficient infection of primary hepatocytes. Proc. Natl. Acad. Sci. USA 91:9564-9568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.York, J., V. Romanowski, M. Lu, and J. H. Nunberg. 2004. The signal peptide of the Junín arenavirus envelope glycoprotein is myristoylated and forms an essential subunit of the mature G1-G2 complex. J. Virol. 78:10783-10792. [DOI] [PMC free article] [PubMed] [Google Scholar]