Abstract
Pulpitis pain may be triggered by a cold stimulus, yet the cellular mechanisms responsible for this phenomenon are largely unknown. One possible mechanism involves the direct activation of cold-responsive thermoreceptors. The purpose of this study was to evaluate the possible role of the TRPM8 thermoreceptor in cold-mediated noxious pulpal pain mechanisms by comparing expression patterns in pulpal nerves from healthy control molars to cold-sensitive painful molars with irreversible pulpitis. Samples were identically processed with the indirect immunofluorescence method and images obtained with confocal microscopy. The immunofluorescence intensity and area occupied by TRPM8 within N52/PGP9.5 identified nerve fibers were quantified. Results showed that relative to normal samples, TRPM8 nerve area expression was significantly less in the cold-sensitive painful samples (34.9% vs. 8%, p<0.03), but with no significant difference in immunofluorescence intensity between the two groups. These results suggest that TRPM8 is most likely not involved in cold-mediated noxious pulpal pain mechanisms.
Keywords: human, pulp, pain, cold, transient receptor potential
Introduction
Hot and/or especially cold thermal stimuli may differentially induce pain in teeth with irreversible pulpitis (1). Although the transduction of thermal stimuli and subsequent activation of pulpal nerve endings is usually attributed to the Brannstrom hydrodynamic theory (2, 3), the recent identification of thermoresponsive transient receptor potential (TRP) ion channels provides an additional potential mechanism where thermal stimuli may directly activate nerve endings (4). This direct activation of nerves by thermoreceptors may act independent or in concert with nerve activity produced by hydrodynamic theory-based mechanisms. Therefore, the exact molecular mechanism(s) leading to thermally-induced pulpal pain responses remain unknown.
The TRPV1 receptor represents the first identified thermoresponsive TRP channel (5) and additional studies have established a critical role for TRPV1 in the development of heat hyperalgesia following inflammatory insults (6, 7). This finding suggests a possible role for other TRPs in pain mechanisms. The recent identification of the TRPM8 (8, 9) and TRPA1 (10) as putative cold -responsive thermoreceptors offers a mechanism where cold thermal stimulation may directly initiate nerve activity. Although further studies have questioned the role of TRPA1 in cold sensation (11), TRPM8 expressing neurons are responsive to cold (12, 13), whereas their contribution to cold-induced hyperalgesia is unknown. The application of a cold stimulus to teeth with irreversible pulpitis may produce a long-lasting pain response and a change in the expression of cold thermoreceptors may be associated with this response. Additional information regarding the contribution of putative cold thermoreceptors to the production of a cold-induced hyperalgesic response may be provided by a comparison of thermoreceptor expressions in normal human dental pulp to those seen in cold-sensitive painful pulp. In this study we use quantitative techniques to test the hypothesis that there is a differential expression of the cold sensitive TRPM8 thermoreceptor within axons of normal and cold-sensitive painful human dental pulp. This is an important issue to address since increased TRPM8 expression patterns in cold-sensitive painful teeth would implicate the involvement of this ion channel in noxious cold transduction whereas decreased expressions would suggest otherwise.
Materials and Methods
Subjects
This study was approved by the Human Subjects Institutional Review Board at the University of Texas Health Science Center at San Antonio. The informed consent of all human subjects who participated in the experimental investigation reported or described in this manuscript was obtained after the nature of the procedure and possible discomforts and risks had been fully explained. Teeth were obtained from subjects having extraction of normal healthy wisdom teeth with fully formed apices or extraction of molar teeth diagnosed with irreversible pulpitis (n = 10 in each group). All wisdom teeth included in this study were erupted, lacked periodontal disease and carious lesions, responded briefly to cold stimuli and were not associated with spontaneous pain. Teeth with irreversible pulpitis were associated with severe to moderate pain as rated over the 24 hour period before extraction and showed an exaggerated and lingering painful response to cold. All painful teeth had the presence of a carious lesion that extended minimally into the pulpal tissues, while those with large necrotic pulpal lesions were excluded.
Tissue Processing
Extracted teeth were collected in 0.1M phosphate buffer (PB), later the same day the teeth were split longitudinally, pulpal tissue was removed and fixed in 4% paraformaldehyde in 0.1M PB (pH 7.4) for 30 minutes. The tissue was rinsed in 0.1M PB and placed in 0.1M PB with 30% sucrose overnight at 4°C. The next morning, the samples were stored in Neg-50 embedding medium (Richard-Allan Scientific, Kalamazoo, MI) and stored at −80°C until ready for sectioning. The pulpal samples were thawed and a normal sample was placed next to a painful sample and then this sample pair was embedded in Neg-50. Each sample pair was serially sectioned in a longitudinal plane at 30 μm with a cryostat. Tissue sections were placed onto Superfrost glass slides (Fisher Scientific, Pittsburgh, PA), and once dried were stored at −20°C. This method of matching pairs of normal and painful pulp sections removed any confound that might have occurred due to differences in any of the immunohistochemical steps or during the microscopic analysis.
Tissue Staining and Evaluation
A single slide was selected from each of the 10 different sample pairs and consisted of a section where the coronal and radicular pulpal regions were represented in both samples. All steps described below were performed at room temperature. All sample pairs used in this analysis were stained the same day with the same solutions. The specimens were rinsed in 0.1M phosphate buffered saline (PBS). Non-specific binding was decreased by incubation in 0.1M PBS with 0.3% Triton X-100 (Fisher Scientific), 2% bovine gamma-globulin (Sigma, St. Louis, MO), and 4% normal goat serum (Sigma) as a blocking solution for 90 minutes in a humidifier. The blocking solution was removed and the tissue was incubated in primary antibodies diluted in blocking solution and placed overnight in a humidifier. TRPM8 was identified with the cold menthol receptor antibody (CMR; Phoenix Pharmaceuticals, Catalog #H-050-50) at a 1:100 dilution. The specificity of this antibody in human dental pulp has been demonstrated previously (14). Nerve fibers were identified with the use of a protein gene product 9.5 antibody (PGP9.5; Chemicon, Catalog #AB5898) at a 1:300 dilution as a pan-neuronal nerve fiber marker, and anti-neurofilament 200 (N-52) antibody (Sigma, Catalog # N0142) at a 1:2000 dilution as a sensitive marker for myelinated axons. The next day the tissue was rinsed in 0.1M PBS followed by incubation in secondary antibodies at a 1:100 dilution in blocking solution for 90 minutes in a humidifier while protected from light. An Alexa Fluor 633-conjugated anti-guinea pig IgG secondary antibody (Molecular Probes, Eugene, OR) was used to visualize the PGP9.5-immunoreactivity (IR), an Alexa Fluor 568-conjugated anti-rabbit IgG secondary antibody (Molecular Probes) was used to visualize TRPM8-IR, while an AlexaFluor 488-conjugated anti-mouse IgG secondary antibody (Molecular Probes) was used to visualize the N-52-IR. PGP9.5 and N-52 are neuronal markers and serve to positively identify neuronal terminals in the sections. Tissues were rinsed in 0.1M PBS, then water, allowed to dry, coverslipped with Vectashield, (Vector Labs, Burlingame, CA) and stored at 4°C. Tissue specimens were evaluated with a Nikon C1si laser scanning confocal microscope equipped with three lasers (Nikon Instruments, Melville, NY). EZ-C1 v3.20 (Nikon) was used for acquisition of all images. Image processing for illustration purposes was done with Adobe Photoshop CS and CorelDRAW 12. Controls consisted of evaluation of tissues that were stained as described above but that lacked primary antibodies. These control sections lacked specific immunofluorescence.
Quantification of TRPM8 Staining
Longitudinal sections of dental pulp that had been triple-stained with TRPM8, N-52, and PGP9.5 antibodies as described above were examined with the confocal microscope. The tissues were minimally evaluated (to avoid photobleaching) to determine optimum laser gain settings that were associated with the maximum presence of 4–10 saturated pixels per acquired image, so as to allow a range of immunofluorescence intensities. A “z-series” of individual images were obtained at 5 μm increments from the middle 20 μm of each 30 μm thick section with a 40X oil immersion objective lens. Laser gain levels were the same for all acquired images. Z-series images were captured sequentially in channel series to prevent bleed-through. A z-series of images were obtained from similar regions in the upper radicular pulp in each normal and painful sample. Analysis was limited to this region since more coronal regions in painful samples generally lacked TRPM8 expression (see Results). Individual color channels were saved as separate 12-bit.ics/.ids files at a resolution of 1024×1024, with each square pixel being ~0.3 μm in length and representing an area of ~0.09 μm2. Representative images of TRPM8 staining patterns seen near coronal pulp horns in normal and painful samples were obtained for qualitative analysis. Further quantitative analysis (see below) was limited to those samples that showed N52/PGP9.5 staining within intact fibers, while excluding those with such staining in fragmented fibers, since fragmented fibers most likely represent those that are degenerating. All of the normal samples and 8/10 painful samples met this criteria.
Using NIH ImageJ software (available at http://rsb.info.nih.gov/ij/), the single channel files were opened using ICS Opener plugin for ImageJ (Nico Stuurman, UCSF; available at http://valelab.ucsf.edu/~nico/IJplugins/Ics_Opener.html). The corresponding PGP9.5 and N-52 z-stacks were combined using the “Max Operation” in the Image Calculator. The staining intensities in all images were filtered by applying a threshold value to remove low intensity pixels that represent nonspecific/background values. The threshold value was determined with mean and standard deviation pixel intensity values obtained from a histogram analysis of every TRPM8 and combined N52/PGP9.5 image slice from normal samples. Mean values for mean and standard deviation pixel intensities were then obtained independently for the TRPM8 and combined N52/PGP9.5 groups and then a threshold value (mean + 2 times the standard deviation) for each group was applied to remove (filter) background staining. This thresholding process was applied in a consistent manner to all images from the normal and painful sample groups and resulted in images where N52/PGP9.5 staining was limited to nerve fibers. The thresholded nerve fiber area was recorded by creating a selection in every slice and saved as regions of interests (ROIs) of the combined N52/PGP9.5 images. The nerve area ROI was transposed onto the corresponding thresholded TRPM8 image. The thresholded image was then redirected to the original TRPM8 image so that when it was analyzed, the actual TRPM8 immunofluorescence pixel intensities within the ROI were recorded. The particles were then analyzed, and the average intensity and percent area of nerve fiber occupied by TRPM8 were recorded. Statistical significance was analyzed with the use of an unpaired Student’s t-test and standard error of the mean (SEM).
Results
Qualitative Results
Evaluation of the TRPM8 staining within N52/PGP9.5 identified pulpal nerve fibers in normal samples showed that TRPM8 was widely expressed in a subset of the larger and medium-sized axons located within bundles throughout the radicular and coronal regions of the pulp and in the subodontoblastic plexus, yet was mostly lacking within fine axons that were located within and that traversed the odontoblastic layer (Figs. 1A, B and 2A, B). The TRPM8 staining of fibers within axon bundles was prominent both on the surface and within the axoplasm of the larger fibers contained within these bundles. The diameter of the largest fibers with TRPM8 staining was generally in the range of 2–3 μms and this finding is consistent with A-delta nociceptors. The TRPM8 staining on the surface of these axons appeared as a “halo” that surrounded the colocalization of TRPM8 with N52/PGP9.5 seen within the axoplasm (Figs. 2A, B). An examination of the TRPM8 expression in N52/PGP9.5 identified nerve fibers in the cold-sensitive painful teeth showed an overall downregulation within all regions of the pulp when compared to the expression seen in normal samples. This downregulation was striking within axons in the coronal regions located near carious lesions (Figs. 1C, D) and also apparent in axon bundles located in the upper radicular pulp (Figs. 2C, D).
Figure 1.

Confocal micrographs of combined TRPM8 (red), N52 (green) and PGP9.5 (blue) stainings (left side; A and C) and the corresponding TRPM8 image alone (right side; B and D) in the coronal pulp of a normal sample (A, B) and a painful sample (C, D). (A, B) Normal sample; TRPM8 expression is seen within a subset of larger and medium-sized N52/PGP9.5 identified nerve fibers (arrowheads), while lacking in the smaller axons, such as those that are seen in the odontoblastic layer (O; arrows). (C, D) Painful sample; TRPM8 (red) expression is generally lacking in the N52/PGP9.5 identified nerve fibers (arrows) located adjacent to inflammatory cells associated with a carious lesion, including the nerve fibers that traverse the odontoblastic layer (O). All scale bars = 100 μms.
Figure 2.
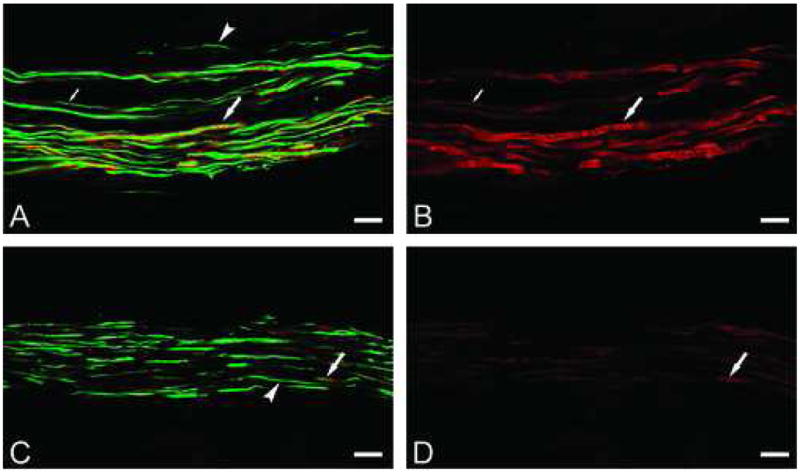
Confocal micrographs of combined TRPM8 (red), N52 (green) and PGP9.5 (blue) stainings (left side; A and C) and the corresponding TRPM8 image alone (right side; B and D) in axon bundles located in the upper radicular pulp of a normal sample (A, B) and a painful sample (C, D). (A, B) Normal sample; TRPM8 (red) expression is seen both on the surface and within the axoplasm of the larger N52/PGP9.5 identified nerve fibers (large arrows), while some of the smaller fibers express TRPM8 (small arrows) and others do not (arrowhead). The TRPM8 expression on the surface of the larger fibers that extends beyond the N52/PGP9.5 staining is seen as a “halo” and most likely represents the staining of myelin. (C, D) Painful sample; TRPM8 (red) is weakly expressed in some of the larger fibers (arrows), while the smaller fibers generally lack expression (arrowhead). All scale bars = 20 μms.
Quantitative Results
Quantitative analysis was limited to the TRPM8 expression within N52/PGP9.5 identified axon bundles located in the upper radicular pulp, since TRPM8 expression was still apparent in these axons in painful samples. This analysis was performed on the four optical image slices obtained from the middle of each normal sample (n=10) and the 8/10 painful samples that showed N52/PGP9.5 staining in intact fibers. Results showed that relative to normal samples, TRPM8 expression within N52/PGP9.5 identified nerve area was significantly less in cold-sensitive painful samples (34.9% +/−9.3% SEM vs. 8.0% +/−4.3% SEM; p<0.03). Even though the percent nerve area occupied by TRPM8 was less, the average immunofluorescence intensity was not significantly different among the normal and painful groups (normal- 1035 +/−108 SEM vs. painful- 847 +/− 46 SEM). The percent nerve area occupied by TRPM8 and the immunofluorescence intensity results for each sample are shown in Figure 3. Additional analysis showed that there was no significant difference in the average total nerve area (μm2/sample) that was evaluated between normal and painful samples (normal- 13,336 +/−1683 SEM vs. painful- 8066 +/−2429 SEM).
Figure 3.

Image analysis results obtained for each sample (N = normal and P = painful). (A) The percent of N52/PGP9.5 identified nerve fiber area occupied by TRPM8 within each sample. (B) The average immunofluorescence intensity of TRPM8 staining within each sample as based on 12 bit images with a 0–4095 range. Error bars represent standard error of the means.
Discussion
The results of our quantitative evaluation of TRPM8 expression in N52/PGP9.5 identified nerve fiber bundles showed a decreased expression within the total percent of nerve area in cold-sensitive painful molar teeth when compared to normal control samples. In contrast, the immunofluorescence intensity of TRPM8 staining was not significantly different among the two sample groups. Although we selected fibers with intact N52/PGP9.5 staining, the decreased nerve area expression seen in the painful samples may involve a reduction in the number of vital axons rather than a reduction in receptor density. Even though TRPM8 appeared to be widely expressed within pulpal axons of normal samples, the decreased nerve area expression noted in painful teeth where a cold stimulus triggered a prolonged painful response suggests that TRPM8 activation may not be involved in the transduction and activation of cold-induced pulpal pain responses.
Other human dental pulp studies have examined changes in protein expression between normal, and carious and/or painful samples within N52 (15) and PGP9.5 (16) identified nerve areas. An advantage of this analytical method over others, such as ELISA, is that expression changes can be selectively evaluated within neural components. Most of these studies were done with the fluorescent microscope in contrast to the laser-scanning confocal microscope used in our study. The use of the confocal microscope provides certain advantages, since it allows information regarding the immunofluorescence intensity of staining within individual optical sections. A difference in immunofluorescence intensity between sections from normal and painful samples that have been processed and stained the same allows an assessment of the biological condition on relative changes in protein expression (17). Even though the immunofluorescence intensity analysis performed in our study did not find a significant difference between normal and painful samples, this method of image analysis has broad applications and has been used by others in human dental pulp studies (18).
The TRP subtypes that have been implicated in thermal transduction include TRPV1, TRPM8 and TRPA1 and each is activated by distinct temperatures (4, 19). TRPV1 has been identified as a transducer for noxious heat (5), and is especially important in the development of heat hyperalgesia after inflammatory insults (6, 7). Recently, TRPV1 has been identified in painful and nonpainful human tooth pulp (20), and in human dental pulp cell cultures (21), where it may play a role in the perception of heat-induced pulpal pain. In contrast, the specific transducer(s) that underlie cold sensitivity in general and especially a cold-induced pain response are less well understood (22). Although most attention has been placed on TRPM8 and TRPA1, more recent evidence suggests that TRP-independent mechanisms may also be involved in cold sensation (23). Even though evidence does support a role for the TRPM8 receptor in cold detection (8, 9), a role in the transduction of noxious cold stimuli is generally lacking. Evidence to suggest a possible role for TRPM8 in noxious cold transduction includes its activation by the cooling compound menthol (24) and the ability of topically applied menthol to produce pain (25) and cold hyperalgesia (26).
Most studies that have examined TRPM8 localization in the peripheral nervous system have evaluated expressions in the cell bodies of sensory neurons within the dorsal root and trigeminal ganglia, and in general these studies have found TRPM8 expression in small to medium-sized cell bodies that are somewhat distinct from those that express TRPV1 and TRPA1 (8–10, 27, 28). Fewer studies have evaluated the peripheral nerve fiber expression of TRPM8 (14, 28, 29) and taken together these cell body and fiber findings suggest associations with peripheral c- and A-delta nociceptors. Our finding that identified TRPM8 in larger and medium-sized fibers, but while lacking in smaller fibers, suggests a more important role for TRPM8 in A-delta mediated pulpal pain mechanisms. Given the results of our study that show a prominent axonal expression in normal teeth and a downregulation in cold-sensitive painful teeth and the ability of cold to activate TRPM8 receptors as shown by others, the role of TRPM8 in pulpal pain mechanisms may be more likely related to the detection of innoxious cold stimuli rather than noxious cold (30). Confounding to a possible role in innoxious cold detection was the minimum expression of TRPM8 within the fine nerve endings in the odontoblastic layer where activation by a thermal stimulus seems most likely. This finding suggests that the hydrodynamic theory remains the best explanation for the painful response that follows the application of a cold stimulus to human dentin (31).
An unexpected finding was the TRPM8 expression on the surface of some of the larger fibers. This labeling pattern most likely corresponds to a myelin location and the significance of this finding is unknown. The TRPM8 localization on the myelin of peripheral nerves has been reported previously (14) and appears to be a consistent finding since it was seen with the use of two different antibodies, including the one used in this study. Although TRPM8 expression appeared on the myelin of these fibers, the TRPM8 was also present in the axoplasm of these same fibers where it was colocalized with the axoplasmic expression of PGP9.5 and especially N52. Since the quantitative techniques used in our analysis examined the expression of TRPM8 in the N52/PGP9.5 identified nerve area, this TRPM8 expression on myelin was minimized in our analysis. This aspect of the analysis would not have been possible without the use of the confocal microscope and its ability to produce optical sections that allow differentiation between the myelin sheath and the axoplasm. Although others have identified TRPM8 expression in the peripheral nerve endings in human tooth pulp (14) changes in its expression in inflammatory pulpal conditions have not previously been reported. In contrast to the downregulation seen in our study, an upregulation was reported in nerve fibers within the painful and overactive human bladder (14). This difference may be related to different inflammatory mechanisms associated with each condition. Interestingly, in contrast to a possible role in producing pain, TRPM8 has recently been implicated in the production of neuropathic pain analgesia by central mechanisms (32).
Although the recent identification of thermally-activated ion channels provides a possible mechanism where hot and/or cold stimuli can differentially contribute to the development of hyperalgesia in painful teeth with irreversible pulpitis, the results of our quantitative analysis suggest that TRPM8 may not be involved in this process. Even so, the TRP’s represent an important class of receptors involved in peripheral pain mechanisms. For example, an increased expression of TRPM2 was recently identified within nonneuronal cells in the painful human dental pulp (18). This finding suggests that the role of TRP’s in pain mechanisms may expand beyond their neuronal expressions. Additional studies are needed to further understand the contribution of the TRPs and other yet to be discovered thermally-sensitive ion channels to pulpal pain mechanisms, especially in the context of the hydrodynamic theory and the other attributes that uniquely characterize toothache pain.
Acknowledgments
This research was supported by the AAE Foundation and by R01 grants DE 015576 from the National Institute of Dental and Craniofacial Research (M.A.H.). The authors thank Gabriela Helesic for technical support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Petersson K, Soderstrom C, Kiani-Anaraki M, Levy G. Evaluation of the ability of thermal and electrical tests to register pulp vitality. Endod Dent Traumatol. 1999;15:127–31. doi: 10.1111/j.1600-9657.1999.tb00769.x. [DOI] [PubMed] [Google Scholar]
- 2.Brannstrom M. Dentin sensitivity and aspiration of odontoblasts. J Am Dent Assoc. 1963;66:366–70. doi: 10.14219/jada.archive.1963.0104. [DOI] [PubMed] [Google Scholar]
- 3.Brannstrom M. The hydrodynamic theory of dentinal pain: sensation in preparations, caries, and the dentinal crack syndrome. J Endod. 1986;12:453–7. doi: 10.1016/S0099-2399(86)80198-4. [DOI] [PubMed] [Google Scholar]
- 4.Dhaka A, Viswanath V, Patapoutian A. Trp ion channels and temperature sensation. Annu Rev Neurosci. 2006;29:135–61. doi: 10.1146/annurev.neuro.29.051605.112958. [DOI] [PubMed] [Google Scholar]
- 5.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–24. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 6.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–13. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 7.Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–7. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- 8.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–8. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 9.Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, et al. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–15. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 10.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–29. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 11.Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–82. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 12.de la Pena E, Malkia A, Cabedo H, Belmonte C, Viana F. The contribution of TRPM8 channels to cold sensing in mammalian neurones. J Physiol. 2005;567:415–26. doi: 10.1113/jphysiol.2005.086546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nealen ML, Gold MS, Thut PD, Caterina MJ. TRPM8 mRNA is expressed in a subset of cold-responsive trigeminal neurons from rat. J Neurophysiol. 2003;90:515–20. doi: 10.1152/jn.00843.2002. [DOI] [PubMed] [Google Scholar]
- 14.Mukerji G, Yiangou Y, Corcoran SL, Selmer IS, Smith GD, Benham CD, et al. Cool and menthol receptor TRPM8 in human urinary bladder disorders and clinical correlations. BMC Urol. 2006;6:6. doi: 10.1186/1471-2490-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Renton T, Yiangou Y, Plumpton C, Tate S, Bountra C, Anand P. Sodium channel Nav1.8 immunoreactivity in painful human dental pulp. BMC Oral Health. 2005;5:5. doi: 10.1186/1472-6831-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodd HD, Boissonade FM. Comparative immunohistochemical analysis of the peptidergic innervation of human primary and permanent tooth pulp. Arch Oral Biol. 2002;47:375–85. doi: 10.1016/s0003-9969(02)00012-2. [DOI] [PubMed] [Google Scholar]
- 17.Rastogi KS, Cooper RL, Shi ZQ, Vranic M. Quantitative measurement of islet glucagon response to hypoglycemia by confocal fluorescence imaging in diabetic rats: effects of phlorizin treatment. Endocrine. 1997;7:367–75. doi: 10.1007/BF02801332. [DOI] [PubMed] [Google Scholar]
- 18.Rowland KC, Kanive CB, Wells JE, Hatton JF. TRPM2 immunoreactivity is increased in fibroblasts, but not nerves, of symptomatic human dental pulp. J Endod. 2007;33:245–8. doi: 10.1016/j.joen.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 19.Caterina MJ. Transient receptor potential ion channels as participants in thermosensation and thermoregulation. Am J Physiol Regul Integr Comp Physiol. 2007;292:R64–76. doi: 10.1152/ajpregu.00446.2006. [DOI] [PubMed] [Google Scholar]
- 20.Renton T, Yiangou Y, Baecker PA, Ford AP, Anand P. Capsaicin receptor VR1 and ATP purinoceptor P2X3 in painful and nonpainful human tooth pulp. J Orofac Pain. 2003;17:245–50. [PubMed] [Google Scholar]
- 21.Miyamoto R, Tokuda M, Sakuta T, Nagaoka S, Torii M. Expression and characterization of vanilloid receptor subtype 1 in human dental pulp cell cultures. J Endod. 2005;31:652–8. doi: 10.1097/01.don.0000155259.22746.ae. [DOI] [PubMed] [Google Scholar]
- 22.McKemy DD. How cold is it? TRPM8 and TRPA1 in the molecular logic of cold sensation. Mol Pain. 2005;1:16. doi: 10.1186/1744-8069-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munns C, Alqatari M, Koltzenburg M. Many cold sensitive peripheral neurons of the mouse do not express TRPM8 or TRPA1. Cell Calcium. 2007;41:331–42. doi: 10.1016/j.ceca.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 24.Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–24. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 25.Wasner G, Schattschneider J, Binder A, Baron R. Topical menthol--a human model for cold pain by activation and sensitization of C nociceptors. Brain. 2004;127:1159–71. doi: 10.1093/brain/awh134. [DOI] [PubMed] [Google Scholar]
- 26.Namer B, Seifert F, Handwerker HO, Maihofner C. TRPA1 and TRPM8 activation in humans: effects of cinnamaldehyde and menthol. Neuroreport. 2005;16:955–9. doi: 10.1097/00001756-200506210-00015. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi K, Fukuoka T, Obata K, Yamanaka H, Dai Y, Tokunaga A, et al. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J Comp Neurol. 2005;493:596–606. doi: 10.1002/cne.20794. [DOI] [PubMed] [Google Scholar]
- 28.Abe J, Hosokawa H, Okazawa M, Kandachi M, Sawada Y, Yamanaka K, et al. TRPM8 protein localization in trigeminal ganglion and taste papillae. Brain Res Mol Brain Res. 2005;136:91–8. doi: 10.1016/j.molbrainres.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 29.Katsura H, Obata K, Mizushima T, Yamanaka H, Kobayashi K, Dai Y, et al. Antisense knock down of TRPA1, but not TRPM8, alleviates cold hyperalgesia after spinal nerve ligation in rats. Exp Neurol. 2006;200:112–23. doi: 10.1016/j.expneurol.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 30.Babes A, Zorzon D, Reid G. Two populations of cold-sensitive neurons in rat dorsal root ganglia and their modulation by nerve growth factor. Eur J Neurosci. 2004;20:2276–82. doi: 10.1111/j.1460-9568.2004.03695.x. [DOI] [PubMed] [Google Scholar]
- 31.Naylor MN. Studies on sensation to cold stimulation in human teeth. Br Dent J. 1964;117:482–6. [Google Scholar]
- 32.Proudfoot CJ, Garry EM, Cottrell DF, Rosie R, Anderson H, Robertson DC, et al. Analgesia mediated by the TRPM8 cold receptor in chronic neuropathic pain. Curr Biol. 2006;16:1591–1605. doi: 10.1016/j.cub.2006.07.061. [DOI] [PubMed] [Google Scholar]


