Abstract
Sequential transformations enable the facile synthesis of complex target molecules from simple building blocks in a single preparative step. Their value is amplified if they also create multiple stereogenic centers. In the ongoing search for new domino processes, emphasis is usually placed on sequential reactions which occur cleanly and without forming by-products. As a prerequisite for an ideally proceeding one-pot sequential transformation, the reactivity pattern of all participating components has to be such that each building block gets involved in a reaction only when it is supposed to do so. The development of sequences that combine transformations of fundamentally different mechanisms broadens the scope of such procedures in synthetic chemistry. This mini review contains a representative sampling from the last 15 years on the kinds of reactions that have been sequenced into cascades to produce heterocyclic molecules.
Keywords: Cascade, domino, cycloaddition, rearrangement, electrocyclization, sigmatropic, metathesis
1. Introduction
Molecules containing heterocyclic substructures continue to be attractive targets for synthesis since they often exhibit diverse and important biological properties.1 Accordingly, novel strategies for the stereoselective synthesis of hetero-polycyclic ring systems continue to receive considerable attention in the field of synthetic organic chemistry.2,3,4,5,6,7,8 The efficiency with which heterocycles can be constructed is important not only because it affects the production costs for the desired material, but also the environmental impact associated with waste disposal, conservation of source materials like petroleum stocks, and energy consumption. The rate of increase in molecular intricacy as one progresses from simple starting materials to the final product can serve as a measure of efficiency.9 On one end of the continuum, a single synthetic step could convert an inexpensive material into a highly complex heterocyclic product. On the other end lies a linear series of transformations, wherein a single atom or group is added in each step to build complexity. As a prerequisite for an ideally proceeding one-pot sequential transformation, the reactivity pattern of all participating components has to be such, that each building block gets involved in a reaction only when it is supposed to do so. The reality of chemical synthesis is somewhere between these extremes, with the one-step process held as the ideal.
Domino reactions (reactions in which several bonds are formed in one sequence without the isolation of intermediates, the changing of reaction conditions, or the addition of reagents),10 multi-component reactions, and the so-called “telescoping” of reactions (the sequencing of multiple transformations in a single reaction vessel through the changing of conditions and/or adding of reagents at appropriate times) allow for a rapid increase in molecular complexity in a single chemical operation. The terms “tandem” and “cascade” have been applied to all three of these reaction types and are thus used as general descriptors in this work.11,12,13,14,15,16 Because of the rate at which they increase molecular intricacy, cascade reactions have received considerable attention from synthetic organic community. The development of sequences that combine transformations of differing fundamental mechanism broadens the scope of such procedures in synthetic chemistry.
This review contains a representative sampling from the last 15 years of the kinds of reactions that have been sequenced into cascades to produce heterocyclic molecules. The fact that multiple reactions give rise to a cascade sequence makes the categorization of these processes difficult. The structure we have imposed, therefore, is somewhat arbitrary but is loosely based upon what, in our judgment, is the key reaction in the cascade sequence. This mini-review is not intended to be a critical or comprehensive coverage, but rather provides an overview of the field and thus some cascade processes are covered in more detail than others.
2. [4π+2π]-Cycloadditions
2.1. [1,3]-Dipolar Cycloadditions
2.1.1 Metallo-carbenoid Initiated Cascades
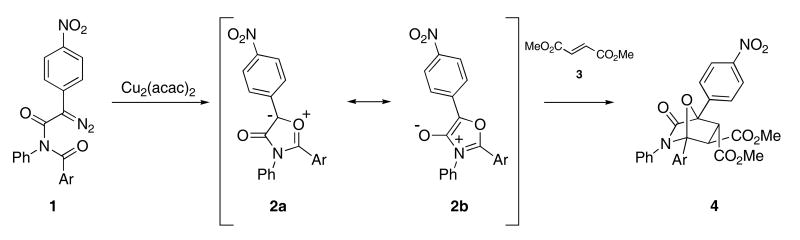
Many different examples of cascade processes that employ 1,3-dipoles as reactive intermediates have been described in the literature. The transition metal-catalyzed decomposition of diazoimides results in the formation of isomünchnone dipoles, a class of mesoionic betaines17 that are known to undergo ready 1,3-dipolar cycloaddition chemistry. Ibata and Hamaguchi were the first to report that diazoimide 1 formed isomünchnone 2 upon heating in the presence of Cu2(acac)2,18 and that this reactive dipole could be trapped with various dipolarophiles such as 3 to give the oxabicyclic product 4 (Scheme 1).19
Scheme 1.
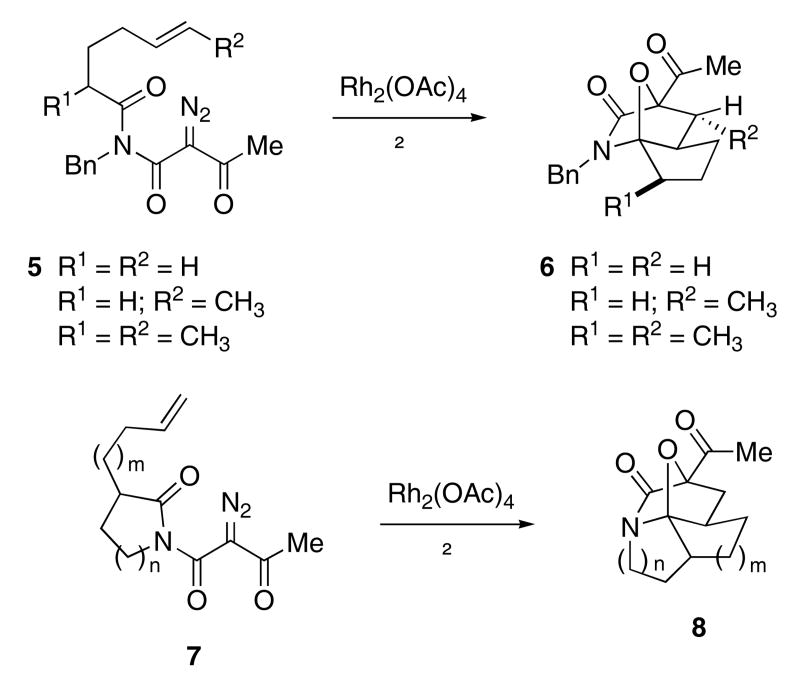
Rhodium(II) catalysts initiate a similar reaction.20 By including the dipolarophile moiety within the diazoimide, complex polycyclic compounds can be formed in a single step.21 Thus, heating compounds such as 5 with catalytic Rh2(OAc)4 produced cycloadduct 6 as a single diastereomer in 73 - 91% yield (Scheme 2). Over a period of years, Padwa and coworkers demonstrated that this cascade sequence is quite general. Diazoimides with the general structure 7 (n = 1, 2, 3; m = 1, 2) were readily converted to the corresponding polycyclic system 8.22
Scheme 2.
Compounds of type 6 and 8 contain an N,O-acetal functional group and have been used as precursors to N-acyliminium ions. This method was exploited for the synthesis of B-ring homologues of the erythrinane family of alkaloids.23 In these studies, diazoimides of type 9 were exposed to catalytic amounts of rhodium(II) and oxabicycles 10 are formed in 90 - 98% yield (Scheme 3). Treating 10 with BF3•OEt2 provided the ring opened products 11 in 85 - 95% yield as single diastereomers.
Scheme 3.
Isomünchnone dipoles generated by the cyclization of rhodium carbenoid intermediates with adjacent amido groups undergo cycloaddition with both electron-rich and certain heteroaromatic π-bonds. For example, the catalytic decomposition of diazoimide 12 provided dipole 13 which subsequently added across the indole π-bond to give a cycloadduct possessing the aspidosperma skeleton (Scheme 4).24
Scheme 4.
This sequence was recently used for the synthesis of the alkaloid (±)-aspidophytine (19). The key sequence of reactions began with the treatment of diazo ketoester 15 with Rh2(OAc)4 to generate a transient metallocarbene that reacted with the proximal imido carbonyl group to form dipole 16.25 A subsequent 1,3-dipolar cycloaddition across the tethered indole π-bond gave cycloadduct 17 in 97% yield. Oxabicycle 17 was then converted into 18 by the action of BF3•OEt2 in 70% yield and this compound was eventually converted into (±)-aspidophytine (19).
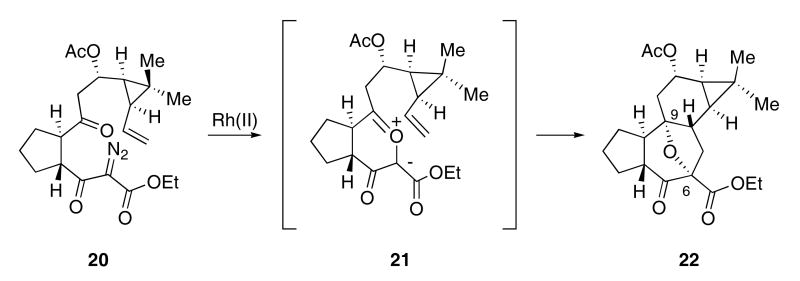
Dauben used a related cyclization-cycloaddition approach for the synthesis of tigliane 22. Carbonyl ylide 21, derived from the diazo ketoester 20, underwent intramolecular cycloaddition to form 22, a molecule which contains the C(6), C(9)-oxido bridge of the tigliane ring system (Scheme 5).26
Scheme 5.
2.1.2. Pummerer Initiated Cascade
A Pummerer-initiated cascade reaction was also used as a method for generating isomünchnones for further use in cycloaddition chemistry. For example, treatment of sulfoxide 23 with acetic anhydride first resulted in the formation of a reactive thionium ion that reacted with the distal amide carbonyl group to produce isomünchnone 24 (Scheme 6).27 Further exposure of 24 to a dipolarophile, such as N-phenylmaleimide, resulted in 1,3-dipolar cycloaddition to give 25 as a single diastereomer in 85% yield.
Scheme 6.
The specific conditions required to successfully effect this transformation were important and warrant comment. The initial attempts to form the isomünchnone intermediate, employing TFAA to promote the cyclization and Et3N to deprotonate the oxonium ion intermediate, failed to produce the isomünchnone dipole. Rather, cyclic ketene acetal 26 was obtained. After considerable experimentation, it was found that the slow addition of 23 to a mixture of acetic anhydride, a catalytic amount of p-TsOH, and the appropriate dipolarophile at 120 °C in toluene gave consistently good yields of the 1,3-cycloadduct. A variety of dipolarophiles were found to participate in these cycloadditions. When 24 was allowed to react with DMAD, the initially formed oxabicycle underwent a rapid fragmentation reaction to produce furan 27 (41% yield) and methyl isocyanate. The reaction of isomünchnone 24 with 1,4-naphthoquinone afforded cycloadduct 28 in 73% yield. Other suitable dipolarophiles include vinyl sulfones, maleic anhydride, and acrylate derivatives. Unactivated olefins also participated in the cycloaddition reaction when they were tethered to the isomünchnone dipole. For example, when sulfoxide 29 was treated with acetic anhydride, azapolycycle 30 was isolated as a single diastereomer in 73% yield.
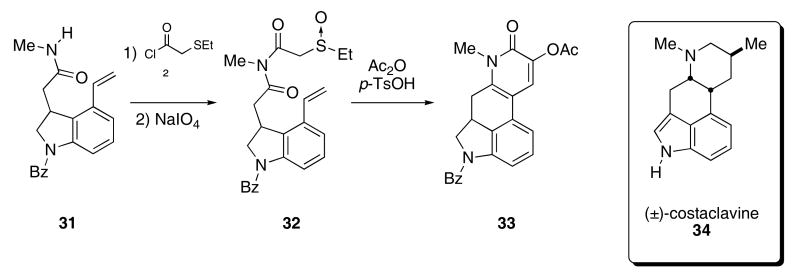
The synthesis of the ergot alkaloid (±)-costaclavine 34 demonstrates the utility of this methodology for the synthesis of natural products (Scheme 7).28 Construction of the ergot skeleton began by acylation of the methyl amide functionality of 31 with (ethylsulfanyl)acetyl chloride and this was followed by a subsequent oxidation of the sulfide with NaIO4 to provide sulfoxide 32. A tandem Pummerer cyclization/cycloaddition cascade, initiated by exposing 32 to acetic anhydride and catalytic quantities of p-TsOH, gave tetracycle 33 in 64% yield. Several functional group interconversions of 33 then delivered (±)-costaclavine 34. The synthesis of several other alkaloids, including onychine, dielsquinone, (±)-lupinine, and pumiliotoxin C, was also accomplished using this methodology.15
Scheme 7.
2.1.3. Nitrones
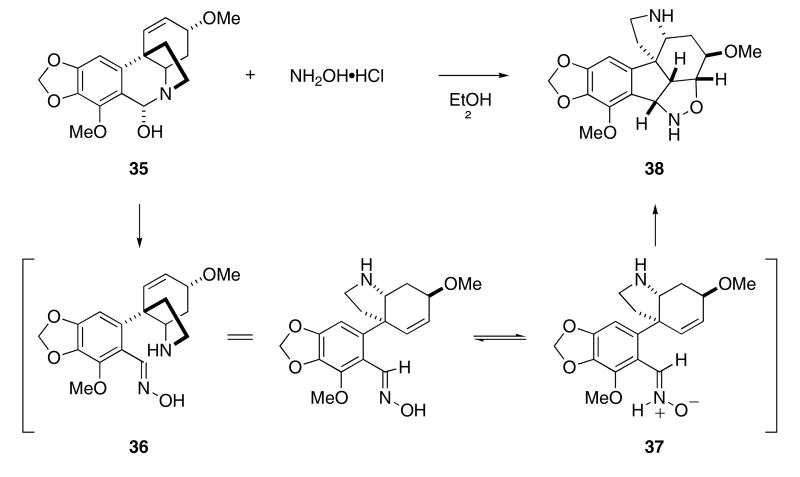
The intramolecular 1,3-dipolar cycloaddition of nitrones is a well precedented reaction for the formation of cyclic isooxazolidines. An interesting method that has been used for the generation of N-H nitrones from readily available starting materials is through the 1,2-prototropic shift of oximes. Although it is unusual to observe cycloadditions using these N-H nitrones, a few examples have been reported. For example, while studying the synthesis of a series of Amaryllicaceae alkaloids, Wildman observed that the reaction of 6-hydroxybuphandidrine (35) with hydroxylamine produced cycloadduct 38 in good yield (Scheme 8).29 This reaction presumably occurs by formation of the intermediate oxime 36 that undergoes a subsequent 1,2-prototropic shift to give nitrone 37. Cycloaddition of the nitrone dipole across the adjacent alkene moiety furnished cycloadduct 38.
Scheme 8.
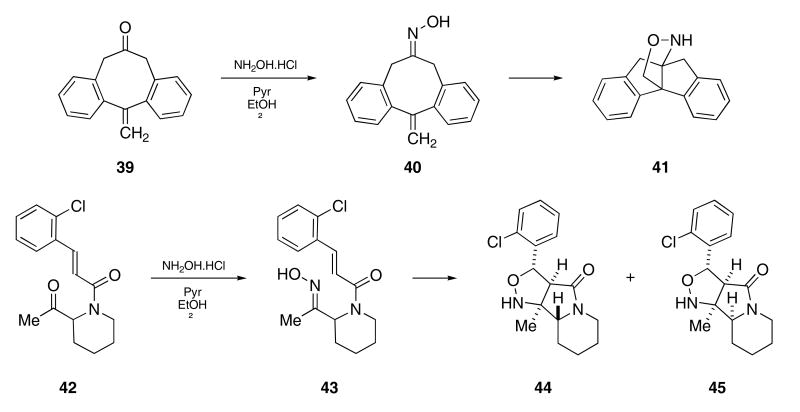
Christy and coworkers observed a similar cyclization upon condensing ketone 39 with hydroxylamine in hot ethanol which provided compound 41 (Scheme 9).30 Oxime 40 was prepared under milder conditions, and was found to undergo a 1,2-prototropic shift followed by intramolecular cycloaddition to provide 41 upon warming to 75 °C in toluene.
Scheme 9.
In a related study, Heathcock reported that ketone 42 reacted with hydroxylamine hydrochloride under similar conditions to produce 44.31 The intermediate oxime 43 that was first formed could be isolated under milder conditions. Whereas heating oxime 43 at reflux in acetonitrile for 30 h gave 44 in 92% yield, heating 43 in DMF solvent produced a 1:1-mixture of the diastereomeric cycloadducts 44 and 45.
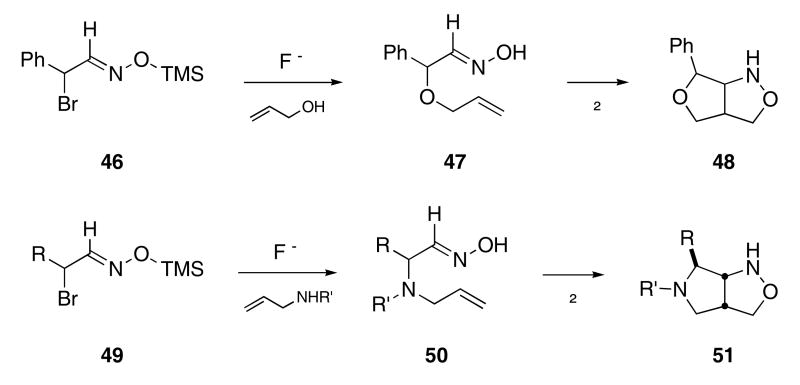
While exploring the chemistry of α-brominated aldoxime derivatives, Padwa and Hassner observed the cycloaddition of oximes that contained pendant olefins. For example, the reaction of 46 with fluoride ion in the presence of allyl alcohol produced oxime 47 (Scheme 10).32 Heating a benzene solution of 47 at 80 °C led to the formation of 48 as a single diastereomer, though only in 25% yield. The Hassner group later expanded the method to include the fluoride-mediated reaction of aldoxime 49 with various allyl amines to generate oximes 50 in 70 -80% yield.33 Heating toluene solutions of these oximes at reflux temperatures led to the formation of pyrrolidines 51.
Scheme 10.
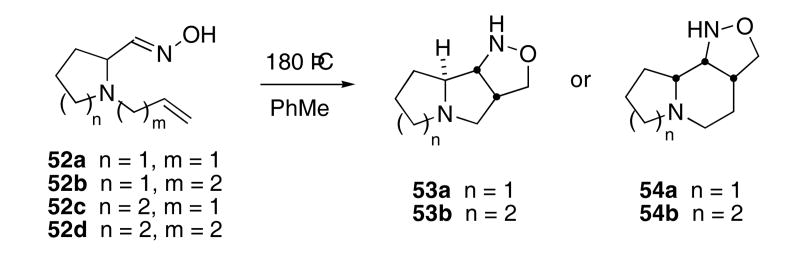
The cascade sequence was also used to synthesize indolizidine, pyrrolizidine, and quinolizidine structures. Thus, heating oximes 52 at 180 °C in a sealed tube provided cycloadducts 53 or 54 in 60 - 76% yields (Scheme 11).34 Each of the products were isolated as single diastereomers. When five-membered rings were obtained from the cycloaddition, cis-anti isomers (i.e. 53a,b) were formed, whereas formation of a six-membered ring led only to the cis-syn isomer (i.e. 54a,b).
Scheme 11.
The Grigg group also studied the tautomerization of oximes to N-H nitrones followed by a dipolar cycloaddition reaction. The well-known H-bonding dimeric association of oximes, in both solution and the solid state, allows for a concerted proton switch to occur and provides nitrone 56 (Scheme 12).35 Another possible pathway involves tautomerization of the oxime to an ene-hydroxylamine (i.e. 57) followed by a 1,4-hydride shift to give nitrone 58. To probe the ene-hydroxylamine mechanism, deuterated oxime 59 was prepared and heated at 140 °C in xylene. The physical characteristics of the isolated product, however, were consistent with compound 60, suggesting that the 1,2-prototropic reaction does not proceed via the ene-hydroxylamine. Grigg postulated that while the tautomerization between an oxime and a N-H nitrone is facile, dipolar cycloadditions involving these types of nitrones are relatively rare because (a) unactivated or electron rich dipolarophiles have too large a HOMO/LUMO gap, although intramolecular cycloadditions to form five-membered rings can overcome this gap, and (b) electron deficient dipolarophiles preferentially undergo Michael-type reactions with oximes.
Scheme 12.
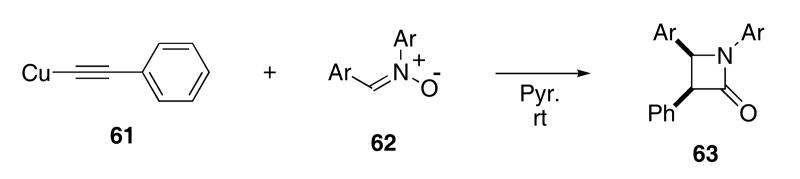
Another interesting cascade involving nitrones is the copper catalyzed reaction with alkynes to produce β-lactams that was originally reported by Kinugasa.36 Stoichiometric amounts of copper(I) phenylacetylide (61) react with various aryl nitrones 62 in pyridine solvent and gave β-lactams 63 in 50 - 60% yield (Scheme 13). In each case, only the cis-lactams were isolated.
Scheme 13.
Miura and coworkers showed that the reaction could also be carried out using catalytic amounts of CuI in the presence of pyridine.37 Asymmetric reactions were reported to occur with chiral bisoxazoline ligands producing β-lactams with moderate (40 - 68%) enantiomeric excess. Use of an oxazolidinone with a chiral auxiliary attached to the alkyne did provide enantiomerically pure products.38 In all of these latter reports, mixtures of cis and trans lactam isomers were obtained in which the trans-product predominates. It was also shown that the cis-isomer could easily be converted to the trans-product when exposed to base.
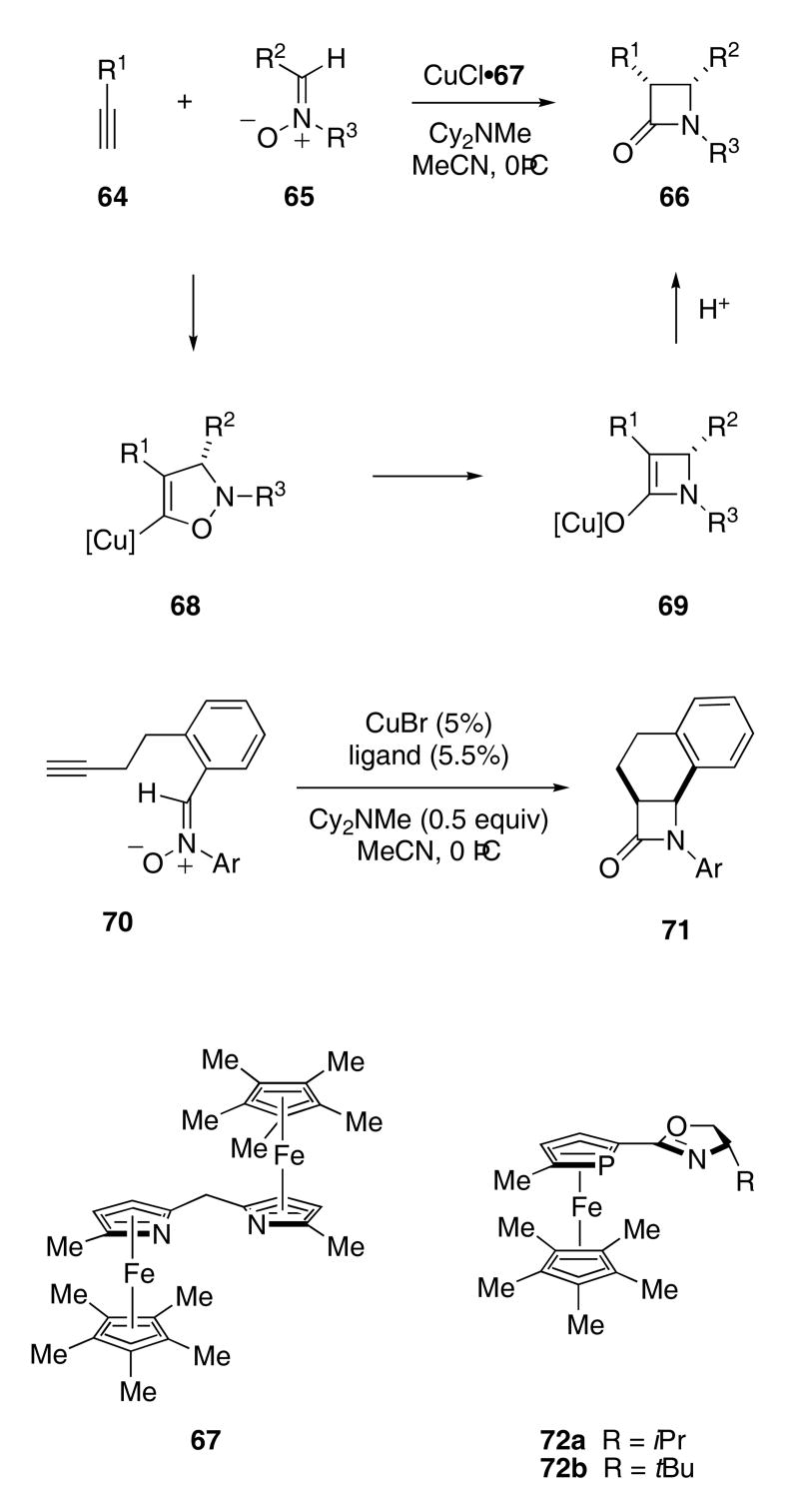
The Fu group recently reported the use of C2-symmetric planar-chiral bis(azaferrocene) ligands for the catalytic enantioselective Kinugasa reaction. A variety of terminal alkynes 64 (R1 = Ar, Bn, 1-cyclohexenyl) were allowed to react with nitrones 65 (R2 = Ar, Cy, PhCO; R3 = Ar) in the presence of catalytic amounts of the CuCl•67 complex to give diastereomeric mixtures (>90 : 10) predominating in cis-substituted β-lactams 66 in moderate to good yields (45 - 90%) and with good enantiomeric excess (67 - 92%; Scheme 14).39 With regard to the R3 group on nitrone 65, electron-rich aromatic groups increased the enantioselectivity, though the yields were somewhat lower.
Scheme 14.
An intramolecular variant of this catalytic enantioselective process was recently reported. Nitrone 70 was converted to azetidinone 71 in the presence of the CuBr•72a complex in 74% yield and with 88% ee.40 Ligand 72b was also quite effective, providing 71 with 90% ee, though the yield was only 47%.
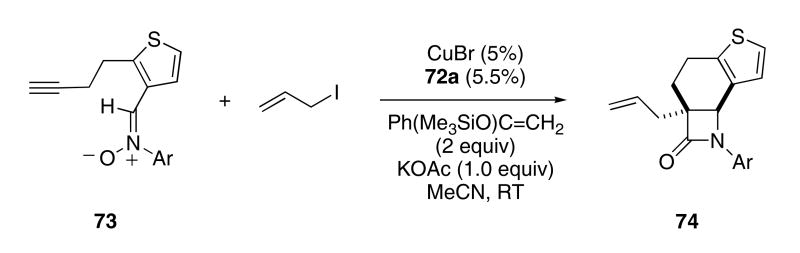
The mechanism for the Kinusaga reaction is thought to involve a [3+2]-cycloaddition of the nitrone with the copper-acetylide to give isoxazolidine 68. Rearrangement of 68 then provides the copper enolate of the corresponding β-lactam (i.e. 69), which is subsequently protonated to provide the observed product. The proton source for this last step is most likely the conjugate acid of the base used to generate the copper-acetylide. Through considerable experimentation, the Fu group developed conditions that allowed for the reaction of the enolate with added electrophiles. Thus, exposing 73 to CuBr•72a in the presence of KOAc, allyl iodide, and the silyl enol ether of acetophenone gave rise to β-lactam 74 in 70% yield and with 90% ee (Scheme 15).27
Scheme 15.
Brandi and coworkers developed an interesting tandem cycloaddition/thermal rearrangement cascade involving nitrones and methylenecyclopropane derivatives to produce 4-pyridones. For example, heating nitrone 75 with 76 at 110 °C for 7 days afforded pyridone 78 in 63% yield (Scheme 16).41 The intermediate isoxazolidine 77 was suggested to undergo homolytic cleavage of the weak N-O bond to give diradical 79 that underwent fragmentation to provide diradical 80.42 Cyclization of 80 then gave rise to 4-pyridone 78.
Scheme 16.
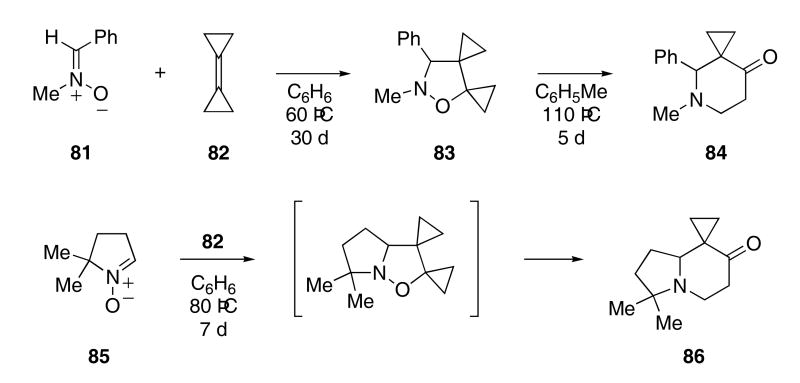
Nitrone 81 underwent a dipolar cycloaddition reaction with bicyclopropylidene (82) at 60 °C over a 30 day period to give isoxazolidine 83 in 93% yield (Scheme 17).43 Further heating of 83 in toluene at reflux for 5 days produced pyridone 84 in 63% yield. When a toluene solution of 81 and 82 was heated at reflux for several days, pyridone 84 was isolated in 61% yield. Nitrone 85 produced indolizidine 86 when heated with 82 in benzene at reflux temperatures for 7 days.
Scheme 17.
2.1.4. Nitrile Oxides
Brandi also examined the subsequent thermal behavior of the dipolar cycloadducts that arise from the reaction of nitrile oxides with methylenecyclopropanes. In general, the isoxazoline intermediates require much higher temperatures than their isoxazolidine counterparts for the rearrangement to occur, and the yields obtained from single pot cascades are somewhat low. In part, this is because some nitrile oxides rearrange to the corresponding isocycanates at elevated temperatures. Another complication is that the intermediate isoxazolines can act as dipolarophiles in [3 + 2]-cycloadditions with the starting nitrile oxides. For example, nitrile oxide 87a undergoes the cycloaddition/rearrangement cascade with 82 at 170 °C over 5 days to provide dihydrofuropyridine 88a in only 7% yield (Scheme 18).30 Similarly, nitrile oxide 87b reacted with 82 under the same conditions to give 88b in 21% yield.
Scheme 18.
Other cascade sequences have also been observed to occur from the thermolysis of isoxazolines, thereby increasing the utility of the nitrile oxide cycloaddition reaction. For example, in the context of synthesizing testosterone derivatives, Guarna and coworkers reported that the reaction of a nitrile oxide derived from oxime 89 with 76 gave isoxazoline 90 (Scheme 19).44 Hydrolysis of the ketal moiety provided cycloadduct 91, which was heated at reflux in DMF to furnish 92 in 30% yield.
Scheme 19.
2.1.5. Azides
The Pearson group studied a synthetically useful cascade in which azides undergo dipolar-cycloaddition with dienes followed by a thermal rearrangement to produce pyrrolidine containing products. Thus, heating azido diene 92 to 100 °C in CHCl3 for 15 h afforded the pyrrolizidine derivative 93 in 90% yield (Scheme 20).45 The phenylsulfanyl substituent was critical both in terms of the products isolated and the rates of the reaction. The mechanism for this cascade was suggested to involve an initial dipolar cycloaddition to provide an intermediate triazole 94 in the rate-determining step. Triazole 94 then fragments by loss of nitrogen to either produce a diradical or a zwitterionic intermediate 95. The resulting intermediate could cyclize either to 93 directly or alternatively give vinylaziridine 96. The formation of 96 would likely be reversible, and some products isolated with other substrates suggests its involvement as an intermediate. Formally, this process can be considered as a [4 + 1]-cycloaddition of a nitrene with a 1,3-diene.
Scheme 20.
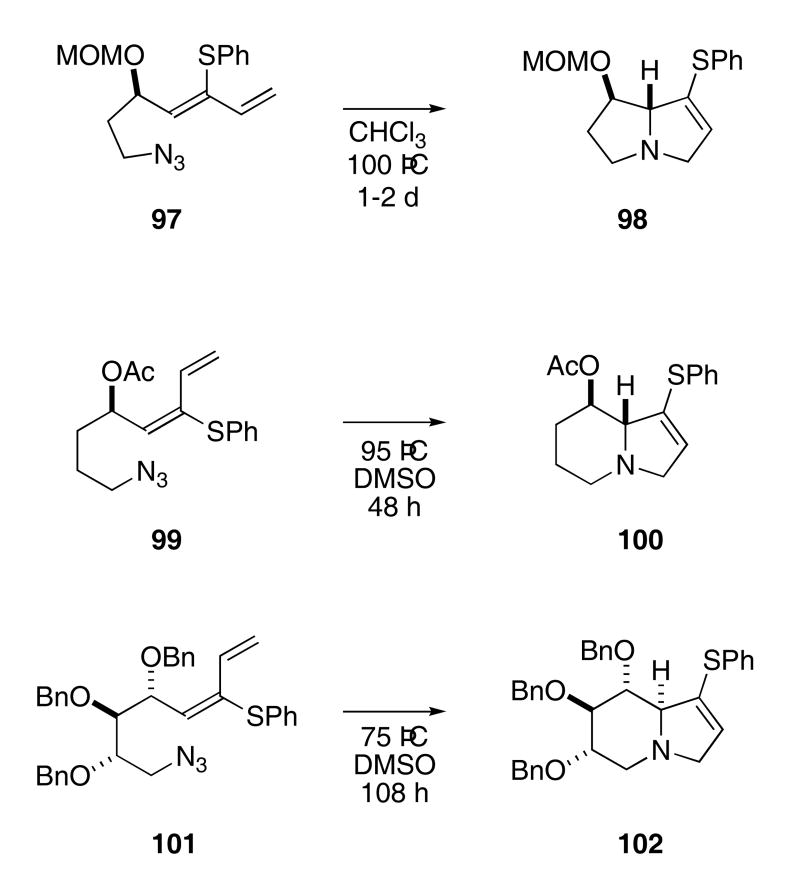
The stereoselectivity of the azide-diene cascade was examined using substrates bearing chiral centers. Choosing azido-dienes that could lead to natural product precursors, Pearson's group prepared azides 97, 99, and 101 (Scheme 21).32 Thermolysis of 97 at 100 °C in CHCl3 for 2 days provided 98 in 74% yield. Diene 99 produced 100 in 62% yield on heating at 95 °C in DMSO for 48 h, and the polyoxygenated azide 101 furnished 102 in 55% yield on heating at 75 °C in DMSO.
Scheme 21.
An unusual tandem Wittig/[3 + 2]-cycloaddition sequence of an azide was used for the synthesis of azasugars. Thus, acetal 103 was allowed to react with Ph3P=CHCO2Et to provide diazoamine 106 (Scheme 22).46 The Wittig reagent presumably underwent reaction with the ring-opened form of ketal 103 to provide the intermediate enoate 104. Dipolar cycloaddition of the pendant azide across the π-bond then produces triazole 105 that undergoes a subsequent dipolar cycloreversion reaction to give diazoamine 106.
Scheme 22.
2.2. Diels-Alder and Related Processes
2.2.1 Diels Alder
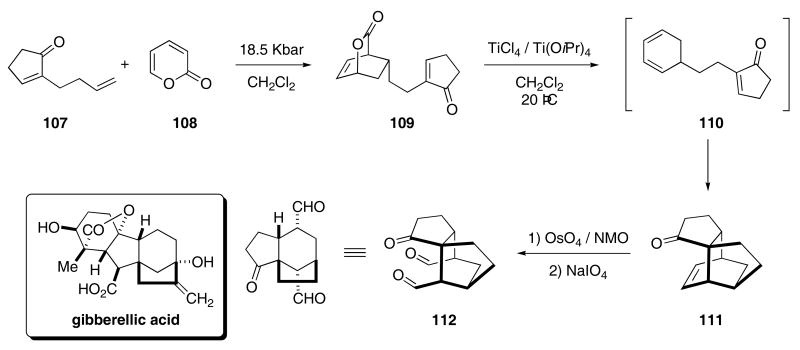
Diels-Alder cycloaddition chemistry has also been extensively exploited for many cascade reactions. A tandem Diels Alder approach47 toward tricyclic molecules was used by Markó's group as an approach toward gibbarellic acid. Enone 107 was reacted with 2-pyrone (108) under high pressure to provide the Diels-Alder bicycle 109 in 33% yield (Scheme 23).48 Exposure of 109 to TiCl4 effected the extrusion of CO2 to give the intermediate cyclohexadiene 110 that underwent cycloaddition to afford 111 in 69% yield. Dihydroxylation followed by a periodate mediated ring cleavage produced 112, which contains the core of gibberellic acid, in 80% yield.
Scheme 23.
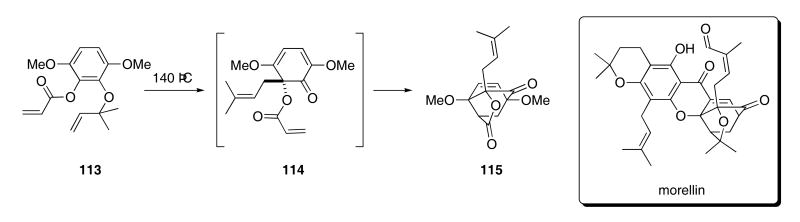
A tandem Claisen/Diels-Alder sequence was recently used to construct the tricyclic structure found in a series of Garcinia natural products, represented by morellin. Upon heating at 140 °C, acrylate ester 113 underwent an initial [3,3]-sigmatropic rearrangement to provide intermediate 114 (Scheme 24).49 A subsequent intramolecular Diels-Alder cycloaddition then produced 115 in 92% yield.
Scheme 24.
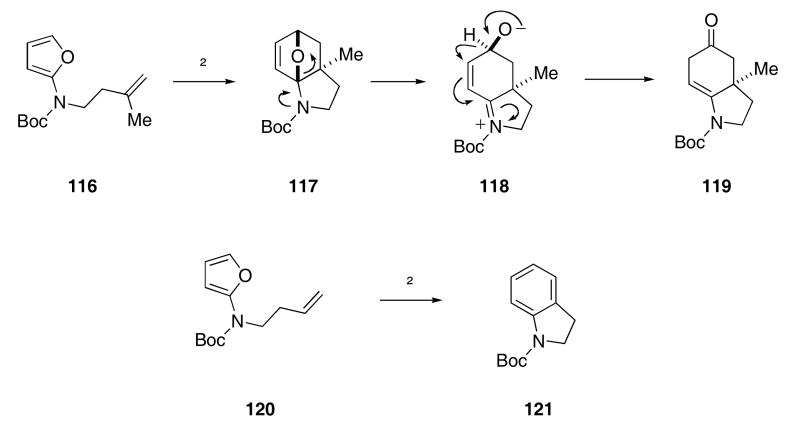
Several reaction cascades where amidofurans act as 4π components in Diels-Alder chemistry have recently been examined as a strategy for alkaloid synthesis.50 For example, the thermolysis of amidofuran 116 led to the formation of 119 in 71% yield (Scheme 25).51 In this reaction, an intramolecular [4+2]-cycloaddition of 116 first provides oxabicycle 117. Nitrogen-assisted opening of the oxygen bridge then leads to zwitterionic 118. A 1,2-hydride shift of 118, driven by the formation of a strong C=O double bond, results in the formation of 119. Interestingly, if the 2π reaction partner was not geminally substituted (as in 120), a deprotonation/dehydration cascade proceeds at a faster rate than the 1,2-hydride shift. This reaction sequence constitutes a de novo synthesis of the carbocyclic ring of an indole.
Scheme 25.
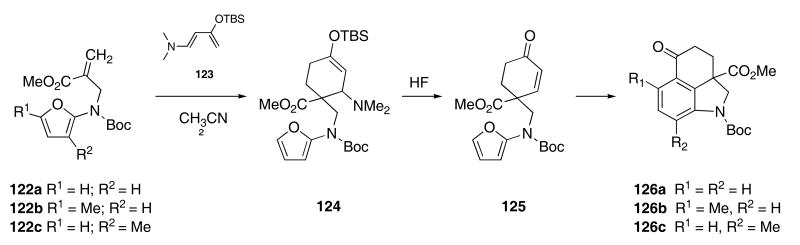
Coupling of this indole methodology with Rawal's azadiene work52 led to a synthesis of Kornfeld ketone analogues with substitution patterns that are difficult to otherwise obtain. Heating a mixture of 122a and Rawal's diene (123) in CH3CN at reflux for 2 h furnished a 2:1-mixture of diastereomeric amines 124 that was immediately treated with HF at room temperature to unmask the enone 125 (Scheme 26).53 The crude reaction mixture was then heated at reflux in toluene for 30 min to effect an IMDAF cycloaddition. Ring opening of the resulting Diels-Alder cycloadduct followed by dehydration provides the tricyclic ketone 126a in 60% yield from 122a. In a similar manner, amidofurans 122b,c were converted to dihydroindoles 126b,c.
Scheme 26.
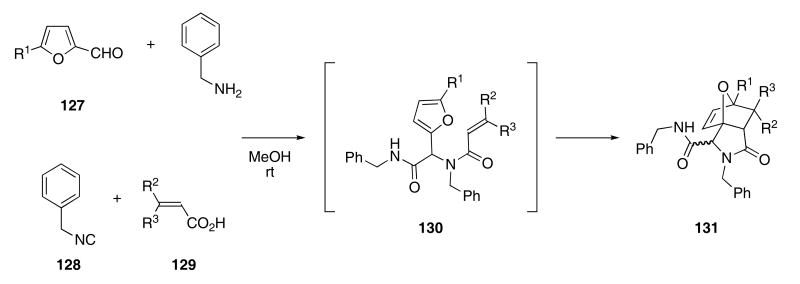
Furans are also useful 4π components for tandem Ugi condensation/intra-molecular Diels-Alder cascade reactions. For example, stirring a methanolic mixture of compounds 127-129 and benzylamine at room temperature provided the Ugi condensation product 130 that underwent a subsequent intramolecular Diels-Alder cycloaddition to furnish 131 in 70 - 90% yield (Scheme 27).54 This methodology also allowed for a solid phase synthesis by using an ArgoGel-Rink resin as the amine component, providing cycloadducts 131 (after cleavage from the resin) in ca 90-95% yields.
Scheme 27.
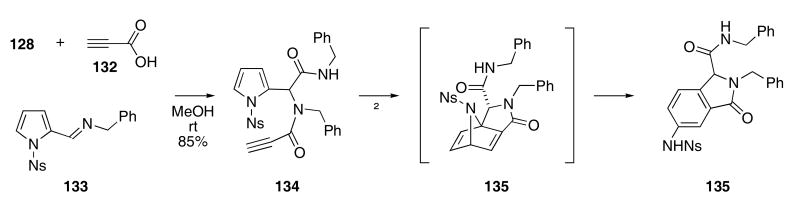
In a related sequence, pyrrole was found to act as a 4π reaction partner leading to the formation of aza-bridged derivatives. Propionic acid (132) was used in the Ugi condensation with 128 and 133 to provide alkyne 134 (Scheme 28).55 Heating 134 at reflux temperature in toluene promoted a somewhat rare intramolecular Diels-Alder reaction of a pyrrole, giving rise to the formation of intermediate 135. Ring opening of the nitrogen bridge in 135 produced isoindolone 136 in 65% yield.
Scheme 28.
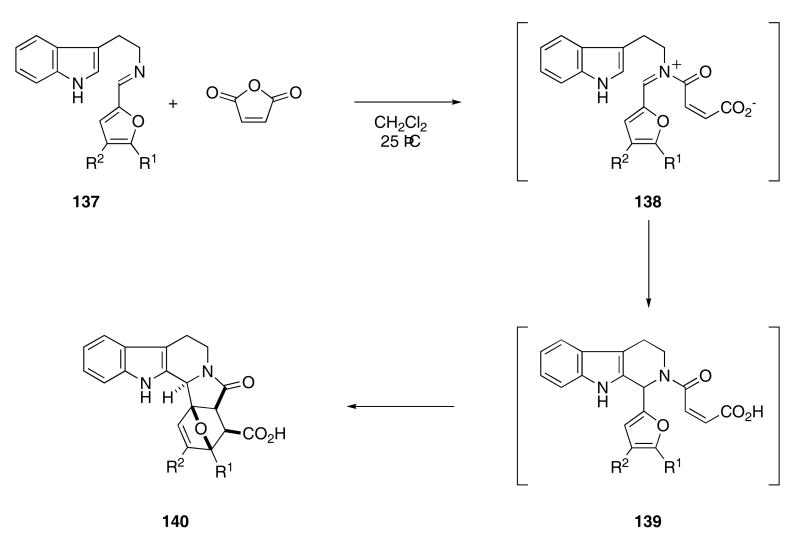
A novel tandem Pictet-Spengler/intramolecular Diels-Alder sequence has been used to prepare carboline derivatives. Reaction of imine 137 with maleic anhydride in CH2Cl2 provided cycloadduct 140 in 60 - 80% yields (Scheme 29).56 The reaction proceeds by acylation of the imine with the available anhydride to first produce iminium ion 138 that then cyclizes with the indole ring to give 139. An intramolecular Diels-Alder reaction of the furan with the proximal π-bond ultimately provides 140.
Scheme 29.
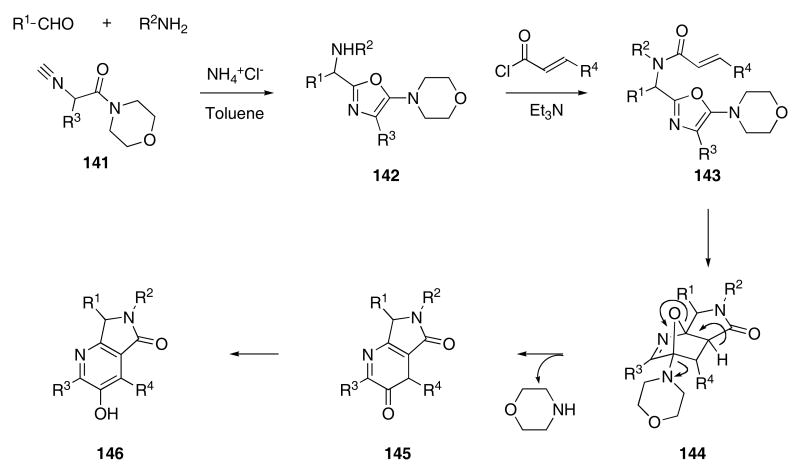
In another example of multi-component reactions involving Diels-Alder cycloadducts, Zhu and coworkers found that a mixture of an amine, an aldehyde, and isonitrile 141 led to oxazole 142 when the reaction was carried out in the presence of a mild acid catalyst (Scheme 30).57 The further reaction of 142 with a variety of α,β-unsaturated acid chlorides produced Diels-Alder substrates 143 that underwent cyclization to give bridged ethers 144. Ring opening with concomitant loss of morpholine afforded 145 that rapidly tautomerized to give 146 in 32 - 75% yield.
Scheme 30.
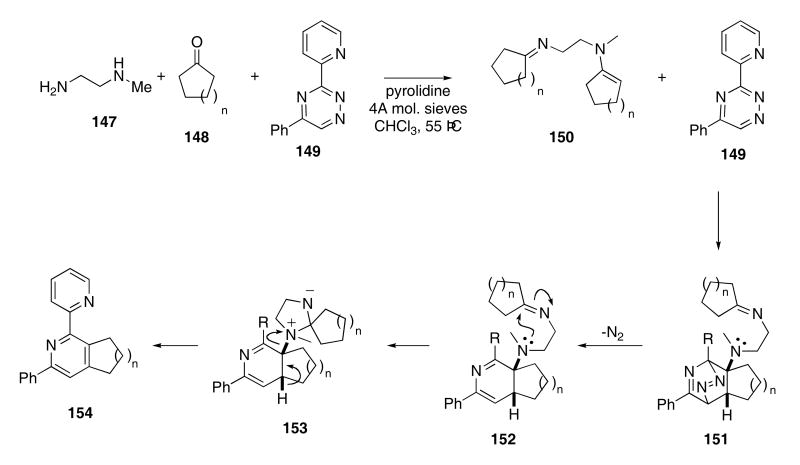
Taylor and Raw recently designed a tethered imine-enamine cascade sequence that converts 1,2,4-triazenes into substituted pyridines. In the presence of molecular sieves, N-methylethylenediamine (147) underwent condensation with excess cyclic ketone 148 (n = 1 - 4) to give imine-enamine 150 (Scheme 31).58 The enamine portion of the molecule then participated in an inverse-demand Diels-Alder cycloaddition reaction with 149 to provide intermediate 151. Cycloreversion of 151 with loss of N2 then gave 152 in which the tertiary resulting amino group added to the adjacent imine functionality to afford zwiterionic 153. Finally, an intramolecular Cope elimination produced 154 in 74 - 100% yield. Several other triazines were also shown to participate in this novel cascade.
Scheme 31.
2.2.2. Hetero Diels-Alder
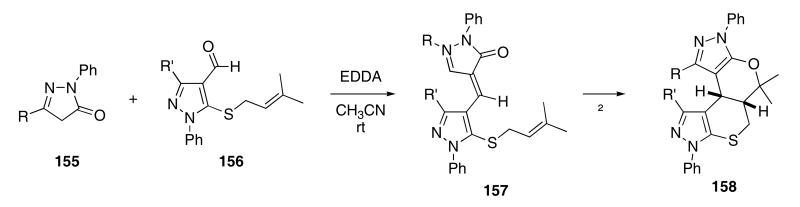
A domino Knoevenagel/hetero-Diels-Alder cycloaddition cascade was developed by Tietze3 and has continued to attract considerable attention. For example, variously substituted pyrazolones 155 and thio substituted heterocycles of type 156 were condensed to furnish novel heterocyclic structures (Scheme 32).59 The reaction of 155 and 156 in the presence of EDDA (ethylene diammonium diacetate) at room temperature in CH3CN gave 157. Upon heating the reaction mixture at reflux, hetero-Diels-Alder cycloadducts such as 158 could be isolated in good yields (81 - 87%).
Scheme 32.
A related domino process was used for the synthesis of coumarin derivatives that contain sugar-fused moieties. In the presence of NaOAc and HOAc, the reaction of coumarin 159 with prenylated sugar aldehyde 160 produced 161 in 82% yield (Scheme 33).60 A variety of 1,3-dicarbonyl compounds also participated in the reaction and provided tandem condensation/cycloaddition products in good yields (70 - 80%).
Scheme 33.
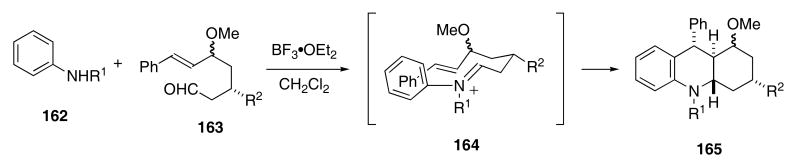
An interesting example of a formal [4+2]-cycloaddition has been found to occur upon condensing N-substituted anilines with ω-unsaturated aldehydes in the presence of Lewis Acids. In this study, N-phenylamines 162 underwent condensation with 163 to provide acridine products 165 in ca 60 - 75% yields (Scheme 34). The intermediate iminium ions 164 that are first formed either participate in a concerted [4+2]-cycloaddition (followed by proton transfer) or else undergo polar addition to the pendant alkene by addition of the resultant benzylic carbocation onto the aniline ring.
Scheme 34.
2.2.3. Nitroalkene [4+2]/[3+2]-Cycloadditions
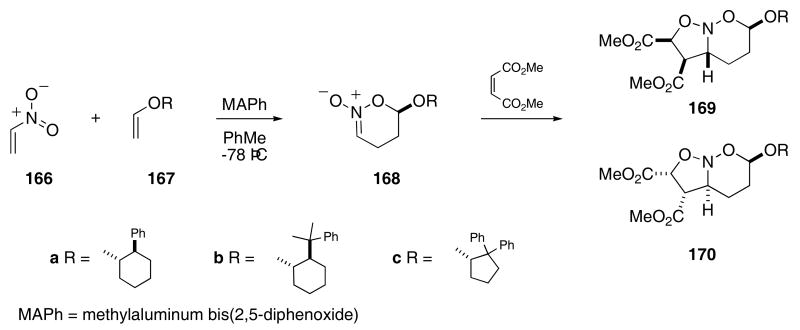
The Denmark laboratory has developed an elegant tandem [4+2]/[3+2]-cycloaddition strategy for the synthesis of a variety of alkaloid natural products.61 Nitroethylene (166) undergoes a ready Lewis acid promoted cycloaddition with vinyl ethers that contain a chiral auxiliary group to give nitronates 168 with good stereoselectivity (Scheme 35).62 For example, vinyl ether 167a provided 168a with a 20:11diastereoselectivity, whereas 167c afforded 168c with >50:1 selectivity. The initially formed nitronates 168 were unstable to silica gel chromatography, but the crude products underwent a ready [3+2]-cycloaddition reaction with electron deficient dipolarophiles. In these reactions, dimethyl maleate reacted with 168 to provide 6:1-mixtures of 169 and 170 in 84 - 89% yield.
Scheme 35.
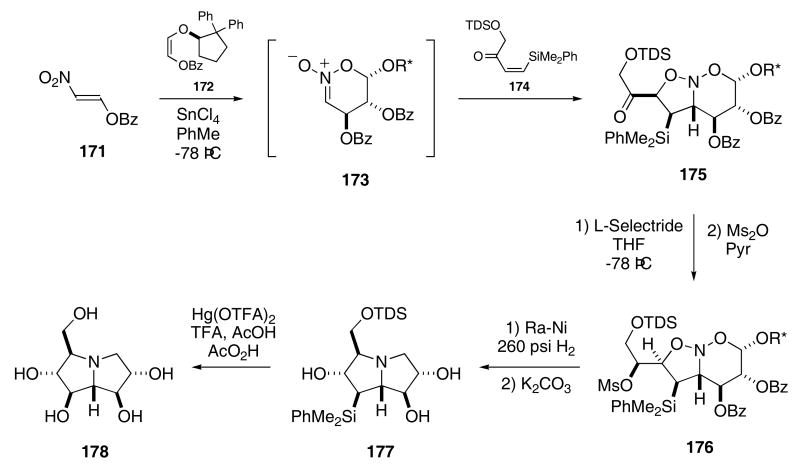
This tandem intermolecular [4+2]/intermolecular [3+2]-cycloaddition strategy was successfully applied to the synthesis of (+)-casuarine. In this synthesis, nitroalkene 171 was allowed to react with enol ether 172 in the presence of SnCl4 at −78 °C to give intermediate nitronate 173 (Scheme 36).63 A dipolar cycloaddition of 173 with 174 provided 175 in 76% yield as a mixture predominating in the stereoisomer shown in Scheme 31. Stereoselective reduction of the ketone moiety in 175, followed by conversion to the corresponding mesylate, gave 176 in 84% yield. Exposure of 176 to Raney nickel under high pressure afforded pyrrolizidine 177 in 64% yield and with 98% ee. Oxidative removal of the silyl group produced (+)-casuarine (178) in 84% yield.
Scheme 36.
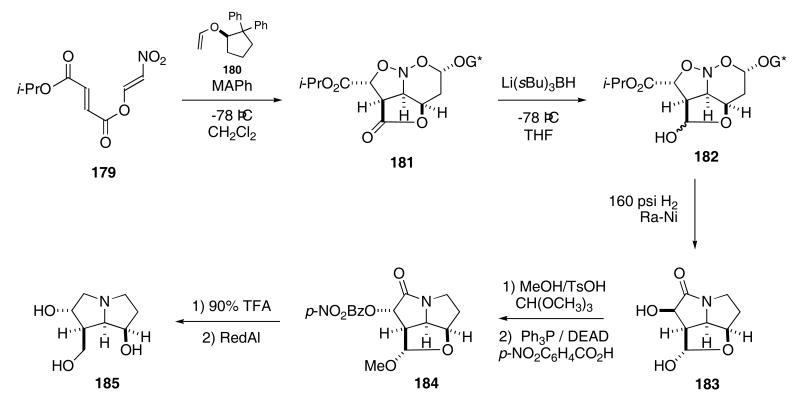
The Denmark group has developed several interesting variants of this sequence. For example, the intermolecular [4+2]/intramolecular [3+2]-cycloaddition cascade64 was used to construct several natural products, such as (−)-rosmarinecine.65 For this particular natural product, the Lewis acid promoted reaction of nitroalkene 179 with chiral enol ether 180 produced nitrosoacetal 181 in 94% yield and with excellent stereoselectivity (25:1- exo : endo) as shown in Scheme 37. Reduction of the lactone moiety afforded lactol 182 in 91% yield. Exposing 182 to Raney nickel under H2 (160 psi) gave the bicyclic lactam 183 in 64% yield. The chiral auxiliary could be recovered in 98% yield. Protection of the lactol followed by reaction with p-NO2-benzoic acid, Ph3P, and DEAD provided benzoate ester 184 in 69% yield from 183. Finally, deprotection of the lactol in compound 184 followed by exposure to RedAl produced rosmarinecine (185) in 57% yield for the two-step procedure.
Scheme 37.
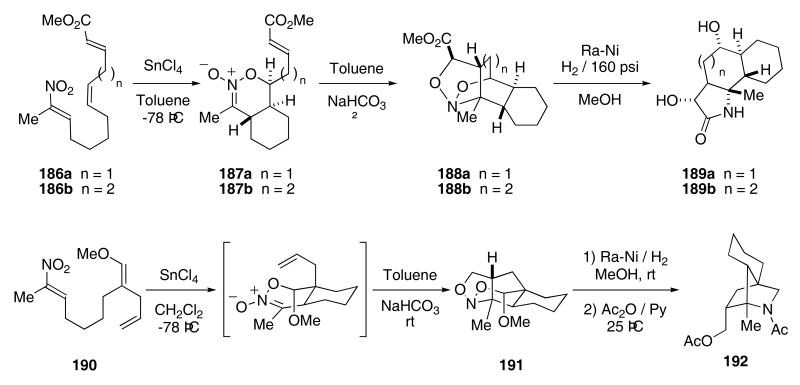
More recently, the Denmark group reported on the tandem intramolecular [4+2]/intramolecular [3+2]-cycloaddition of nitroalkenes. Exposure of nitrone 186a,b to SnCl4 produced nitronate 187a,b (Scheme 38).66 Warming the crude reaction mixture containing 187a in toluene at 80 °C for 90 min afforded 188a as a single diastereomer in 82% overall yield. Nitronate 187b required heating at 100 °C in toluene for 3 days in order to give 188b as a single diastereomer, though in only 44% yield (along with 40% of 187b). Reduction of 188a,b with Raney nickel under a hydrogen atmosphere (160 psi) provided the fused tricycles 189a,b in 71 and 78% yield, respectively. The selectivity of this tandem sequence is remarkable in that compounds 189a,b each contain six contiguous stereogenic centers. Similarly, nitroalkene 190 produced 191 in 87% yield when exposed to a Lewis acid. The reaction of 191 with Raney nickel in the presence of hydrogen provided the bridged tricycle 192 in 81% yield.
Scheme 38.
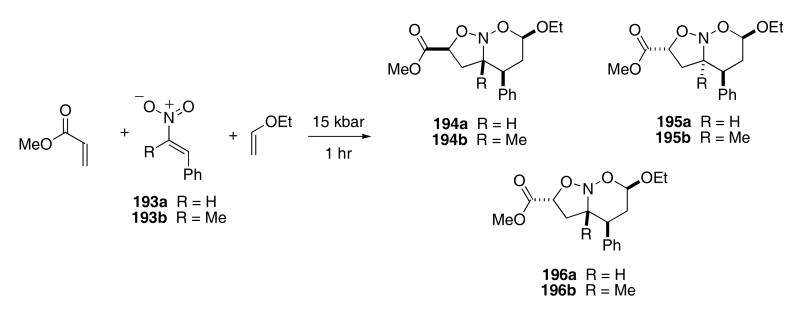
In these cited examples, Denmark employed a Lewis acid (often in 2-3 fold excess) to effect the tandem cycloaddition reaction. In an alternate approach, Scheeren promoted the tandem [4+2]/[3+2]-cycloadditions by using high pressure. For example, nitroalkene 193a reacted with methyl acrylate and ethyl vinyl ether under 15 kbar pressure to produce the bicyclic nitroso acetals 195a and 196a in 17 and 45% yield, respectively, after heating for 1 h (Scheme 39).67 Nitrone 193b reacted under similar conditions to produce 194b, 195b, and 196b in 29, 18, and 29% yields, respectively.
Scheme 39.
Heteroaromatic substituted nitroalkenes also participate in this high-pressure reaction sequence. For example, the reaction of 198a-c with 197 and methyl acrylate afforded diastereomeric mixtures of 199a-c in 53 - 74% yields (Scheme 40).68 In contrast, 198a reacted with 197 and N-phenyl maleimide to provide 200 as a single diastereomer.
Scheme 40.
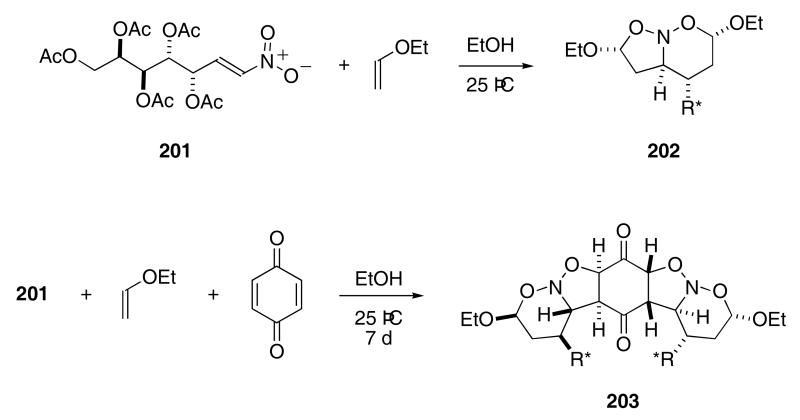
A tandem cycloaddition sequence involving nitroalkenes derived from carbohydrates was recently investigated. In this study, nitroalkene 201 reacted with ethyl vinyl ether in EtOH at 25 °C to produce 202 as a single diastereomer in 89% yield (Scheme 41).69 A subsequent reaction of 201 with ethyl vinyl ether and 1,4-benzoquinone gave rise to a single diastereomer, whose structure was tentatively assigned as 203, in 41% yield.70
Scheme 41.
2.2.4. [4+3]-Cycloadditions
Based upon the Harmata group's earlier work using alkoxyallyl sulfone71 and vinyl sulfoxide72 substrates, Bai and coworkers applied a Pummerer rearrangement/intra-molecular [4+3]-cycloaddition cascade toward the synthesis of pseudolaric acid A (Scheme 42).73 In their studies, sulfoxide 204 was allowed to react with TFAA in the presence of 2,6-lutidine to give cycloadduct 205 in 50% yield and with a remarkably high diastereoselectivity (>95% de). Hydrolysis of the trifluoroacetyl group delivered an advanced intermediate (206) that was used for the synthesis of pseudolaric acid A.
Scheme 42.
3. Rearrangements and Electrocyclizations
3.1 [2,3]-Sigmatropic Shifts
The [2,3]-sigmatropic rearrangement of ammonium ylides can lead to interesting heterocycles. Although it has been known for some time that the Simmons-Smith reagent (ClCH2)2Zn reacts with tertiary amines to provide quaternary ammonium salts, the chemistry of the intermediate ammonium ylide had received little attention. More recently, Aggarwal reported that the reaction of (ICH2)2Zn with allyl amine 207a produced the unreactive ylide 208.74 Treatment of 208 with BuLi, however, generated an activated zincate complex 209 that rearranged to give homoallyl amine 210a in 70% yield (Scheme 43). That a [2,3]-sigmatropic rearrangement occurs, as opposed to a Stevens rearrangement, was established by treating 207b with (ICH2)2Zn followed by n-BuLi to produce 210b in 76% yield. This reaction was also applied to oxazolidine 211, furnishing the eight-membered ring 212 in 72% yield and with a >98% diastereoselectivity.
Scheme 43.
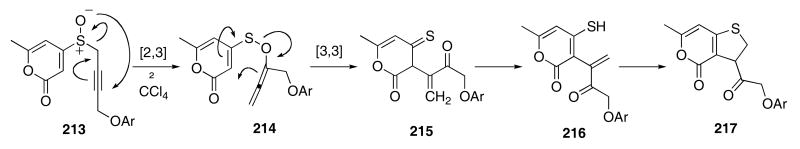
A novel cascade sequence was encountered during a study of the thermolysis of propargylic sulfoxide 213 which gave the rearranged structure 217 in 60 - 70% yield (Scheme 44).75 The cascade was initiated by a [2,3]-rearrangement of the sulfoxide which first produced the allene intermediate 214. A subsequent [3,3]-rearrangement of the transient allene then gave enone 215. Tautomerization of the thione functionality afforded 216 and this was followed by intramolecular Michael addition to give the observed product 217.
Scheme 44.
3.2 [3,3]-Sigmatropic Rearrangements
Of the various heterocycle-forming cascade reactions involving [3,3]-rearrangements, Overman's use of the Aza-Cope rearrangement /Mannich cyclization sequence certainly represents the best known example of this methodology.76 Condensation of a secondary homoallylic amine containing an allylic alcohol or ether such as 218 with aldehydes produces the intermediate iminium ion 219 (Scheme 45).77 A Cope rearrangement then provides a new iminium ion (220) that contains a transient enol which attacks the cationic center in a Mannich fashion to deliver pyrrolidines of type 221.78
Scheme 45.
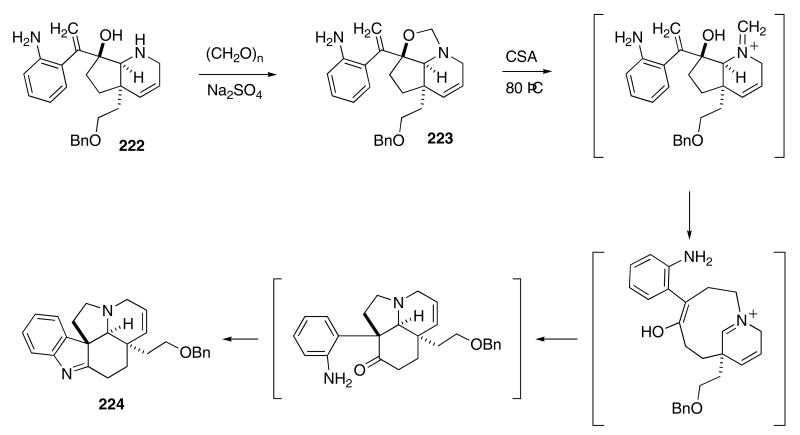
This methodology has been the strategic core of several clever synthetic endeavors carried out by the Overman group. For example, amine 222 was converted into the pentacyclic core of the aspidosperma alkaloid family (Scheme 46).79 Condensation of 222 with paraformaldehyde produced oxazoline 223. Heating 223 with excess camphorsulfonic acid (CSA) effected the Aza-Cope—Mannich cyclization cascade to furnish 224 in nearly quantitative yield.
Scheme 46.
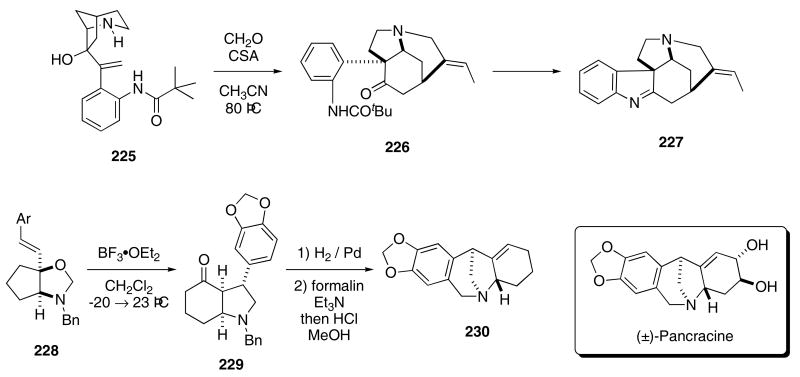
An efficient synthesis of the strychnos alkaloid skeleton was also achieved using this novel cascade process. The key transformation in this sequence occurs by heating bicyclic amine 225 with formaldehyde and CSA in CH3CN to give 226 as a single diastereomer in 88% yield (Scheme 47).80 Hydrolysis of the amide and subsequent condensation of the ketone with the aniline derivative provided dehydrotubifoline (227).
Scheme 47.
Overman also used his aza-Cope/Mannich cascade for a total synthesis of (±)-pancracine. In this particular synthesis, N,O-acetal 228 was allowed to react with BF3•OEt2 which resulted in the eventual formation of amine 229 in 97% yield.81 Hydrogenolysis removed the N-benzyl group, and the resulting amine was then heated with formaline in the presence of catalytic amounts of CSA to effect a Pictet-Spengler reaction, the product of which (230) contains the pancracine skeleton.
More recently, Overman designed a variant of this process for the construction of angularly substituted bicyclic amines. Heating ketal 231 with TFA and dimedone (232) resulted in condensation with the pendant amine group to give iminium ion 233 (Scheme 48).82 The [3,3]-rearrangement resulted in the formation of a second iminium ion 234 that was intercepted by enol 232 to give the Mannich adduct 235. Finally, elimination of the α-methylene 1,3-dione afforded amine 236. For ease of isolation, the crude reaction mixtures were subjected to the action of benzyl chloroformate. Several examples demonstrated the versatility of this sequence in that the original ring size could be varied (m = 1 - 3) as well as the annulated ring size (n = 1, 2) to produce predominantly cis-fused bicycles 237 in ca 65 -95% yields.
Scheme 48.
A novel [3,3]-sigmatropic process that involves an additive-Pummerer reaction that produces γ-butyrolactones by the reaction of dichloroketene with vinyl sulfoxides was developed by the Marino group.83 The oxygen atom of vinyl sulfoxide 238 first attacks dichloroketene to produce an internal salt 239 (Scheme 49). The resulting enolate present in 239 then undergoes a [3,3]-sigmatropic rearrangement to provide thionium ion intermediate 240. Finally, the resulting carboxylate adds to the neighboring thionium ion to furnish butyrolactone 241 whose stereochemistry depends upon the geometry of the starting olefin. The use of chiral sulfoxides 238 led to the enantiospecific formation of butyrolactones 241.84
Scheme 49.
This novel strategy was applied to a synthesis of (+)-aspidospermidine. In this approach, enantiomerically pure sulfoxide 242 was treated with trichloroacetyl chloride in the presence of zinc-copper couple (Zn-Cu) to give lactone 243 in 78% yield (Scheme 50).85 Removal of the chloro substituents followed by the deprotection of the ketal afforded 244 in 96% yield. Reaction of 244 with pyrrolidine effected an O- to N-transacylation with a subsequent elimination of thiolate to furnish the amido aldehyde 245 in 86% yield. Further exposure of 245 to pyrrolidine in the presence of 33% aqueous AcOH and 2-propanol promoted an intramolecular aldol reaction and simultaneously hydrolyzed the amide group to furnish an intermediate carboxylic acid. Conversion of the carboxylic acid to a mixed anhydride followed by the addition of 3-chloropropylamine gave 246 in 64% yield from 245. Exposure of 246 to NaH initiated a tandem intramolecular conjugate addition/alkylation to provide 247 in 86% yield. Subjection of the silyl enol ether of 247 to modified Segusa oxidation conditions delivered 248 (85%), which was subsequently carried on to (+)-aspidospermidine.
Scheme 50.
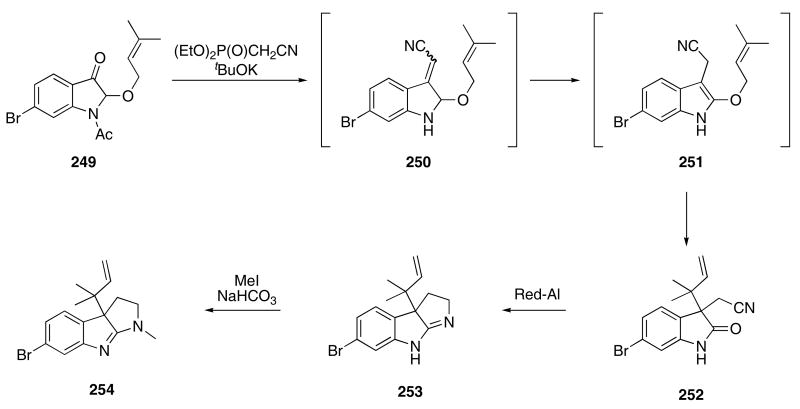
Kawasaki and Sakamoto developed a [3,3]-sigmatropic rearrangement cascade to introduce angular substituents found in several indole alkaloids. In one of the cases studied, the Claisen rearrangement was first preceded by a Horner-Emmons olefination of indolinone 249 to give 250 (Scheme 51).86 Isomerization of 250 provided indole 251 that then underwent a [3,3]-rearrangement to furnish 252 in 73% yield. Reduction of the nitrile by the action of Red-Al gave 253 in 89% yield. Methylation of the imine nitrogen in the presence of NaHCO3 then afforded flustramine C (254) in 38% yield. A more complete study on the scope of this cascade sequence has been reported, 87 and the application of domino Wittig-pericyclic reactions to bioactive heterocycles has recently been reviewed.88
Scheme 51.
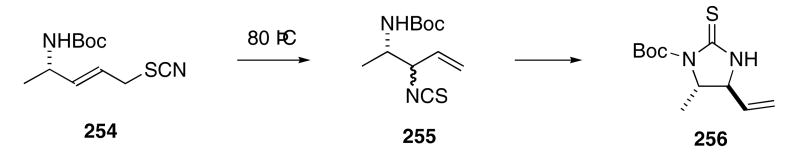
The stereoselective formation of imidazolidine thiones via the rearrangement of chiral thiocyanates have recently been reported. Heating allylic thiocyanates such as 254 at 80 °C for 3 h produced 1:1-mixtures of diastereomeric isothiocyanates 255 in 92% yield (Scheme 52).89 Prolonged heating, however, led to the isolation of the cyclic thiourea 256 as a single stereoisomer in 89 % yield. Several other examples, differing in the nature of the alkyl substituent, were also reported.
Scheme 52.
3.3. Other Rearrangements
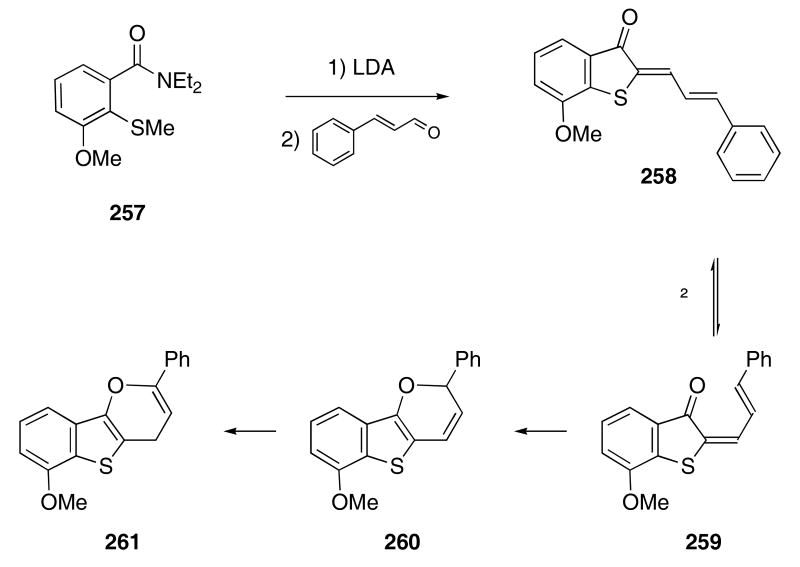
In the context of developing rapid access to thioaurone structures, De and coworkers observed an interesting 6π-electrocyclization/isomerization cascade. The reaction of sulfanyl amide 257 with an excess of LDA and cinnamaldehyde produced thioaruone 258 in 83% yield (Scheme 53).90 Upon heating at 210 °C, compound 258 isomerized to give 259 which underwent a subsequent electrocyclization reaction to produce 260. A formal [1,3]-hydride shift then furnished the observed product 261.
Scheme 53.
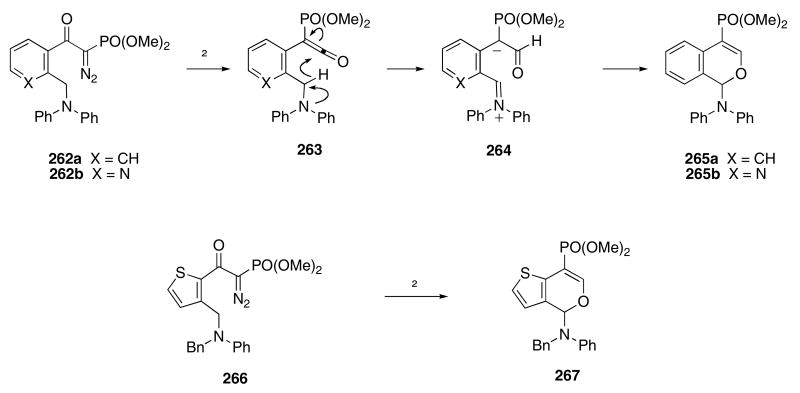
A tandem Wolff rearrangement / cyclization process has been used to synthesize benzopyran derivatives. In this sequence, α-diazo ketones 262a,b were heated to effect a Wolff rearrangement, giving rise to ketenes 263 (Scheme 54).91 The authors propose that a [1,5]-hydride shift then provided 264, and a subsequent cyclization gave 265a,b in 88 and 75% yields, respectively. Thiophene derivative 266 was also found to rearrange to 267 in 75% yield. However, the reaction of a related furan derivative led to extensive decomposition.
Scheme 54.
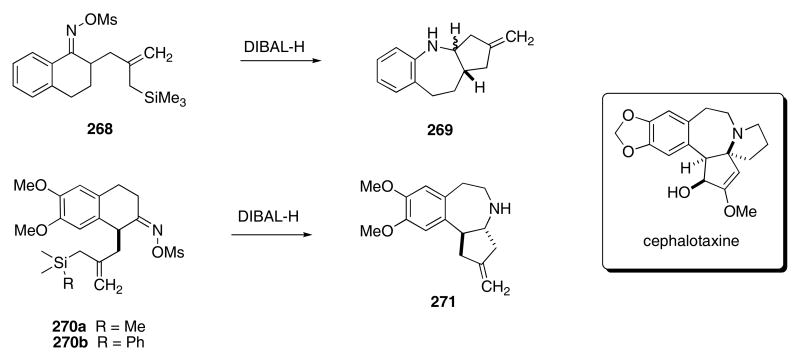
A clever synthetic approach toward the synthesis of cephalotaxine relies on an asymmetric Beckman rearrangement/allyl silane-terminated cation-cyclization cascade. In these studies, Schinzer and coworkers found that the reaction of racemic 268 with DIBAL-H produced 269 in 36% yield (Scheme 55).92 The racemic oxime ether 270a was converted into 271 in 23% yield under similar conditions. By changing the size of the silicon group (i.e., 270b), the yield was increased to 41%. Non-racemic (S)-270b was synthesized using a chiral chromium-arene complex and afforded (S,R)-271 in 55% yield and with 81% ee upon exposure to excess DIBAL-H.93
Scheme 55.
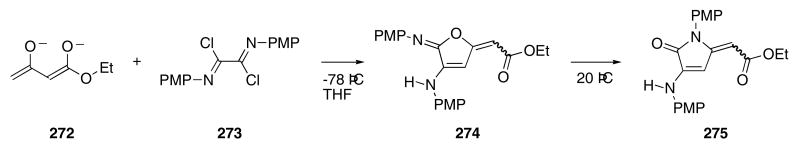
A tandem anionic cyclization/Dimroth rearrangement was employed for the preparation of γ-lactams containing alkylidene substituents.94 In this cascade sequence, the dianion of ethyl acetoacetate (272) reacted with 273 to provide furan derivative 274 (Scheme 56) which underwent a subsequent rearrangement to give 275 in 56% yield. 95
Scheme 56.
The rearrangement of bis-allenyl disulfides provides an interesting route to prepare fused thieno[3,4-c]thiophenes. Thus, Braverman reported that 276 reacted with lithium methoxide to give 280 in 70% yield (Scheme 57).96 Presumably, allene 276 first dimerized under the reaction conditions to generate disulfide 277. Cyclization of 277 would then produce a diradical 278 that fragments into 279a. A further cyclization of the E-isomer 279b nicely accounts for the formation of 280. The analogous diselenide underwent a related reaction to give the corresponding selenophene derivative.
Scheme 57.
4. Cation-Promoted Cyclization Cascades
4.1. Nitrogen Stabilized Carbocations
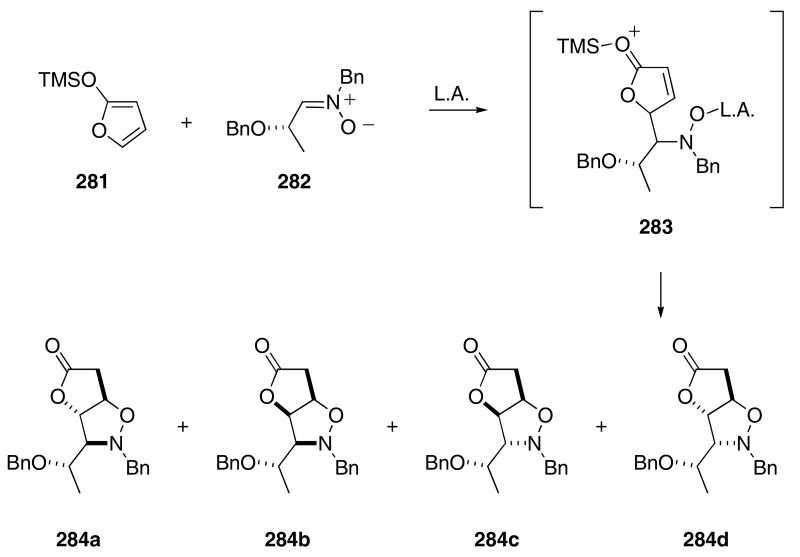
The Mannich reaction is a very common process that occurs in many tandem reaction sequences. For example, the Overman aza-Cope cascade sequence is terminated by a Mannich reaction (cf Scheme 45). Several groups have used variants of the Mannich reaction to initiate cascades that lead to the formation of heterocyclic molecules. Thus, the Lewis acid catalyzed intermolecular vinylogous Mannich reaction97 of silyloxy furan 281 with nitrone 282 produced a diastereomeric mixture (49:3:42:6) of azabicycles 284a-d in 97% combined yield (Scheme 58).98 These products arose from an intramolecular Michael addition of the initially formed oxonium ion 283.
Scheme 58.
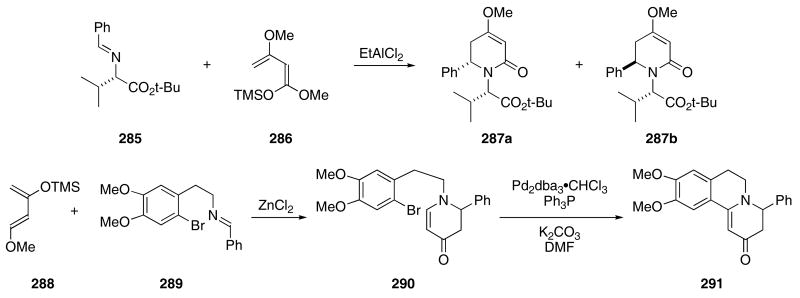
A Mannich / Michael reaction sequence was used by Waldman for the formation of several piperidone derivatives. The reaction of 285 with 286 in the presence of a variety of Lewis acids produced mixtures of 287a,b in 84% yield (Scheme 59).99
Scheme 59.
Using diene 288 and imine 289, the tandem Mannich / Michael reaction sequence afforded the vinylogous amide 290 in 66% yield.100 Imines derived from other aldehydes were also studied, providing derivatives of 290 in moderate yields (ca 40 - 65%). The palladium catalyzed cyclization of 290 furnished tricyclic benzoquinolizine 291 in 76% yield.
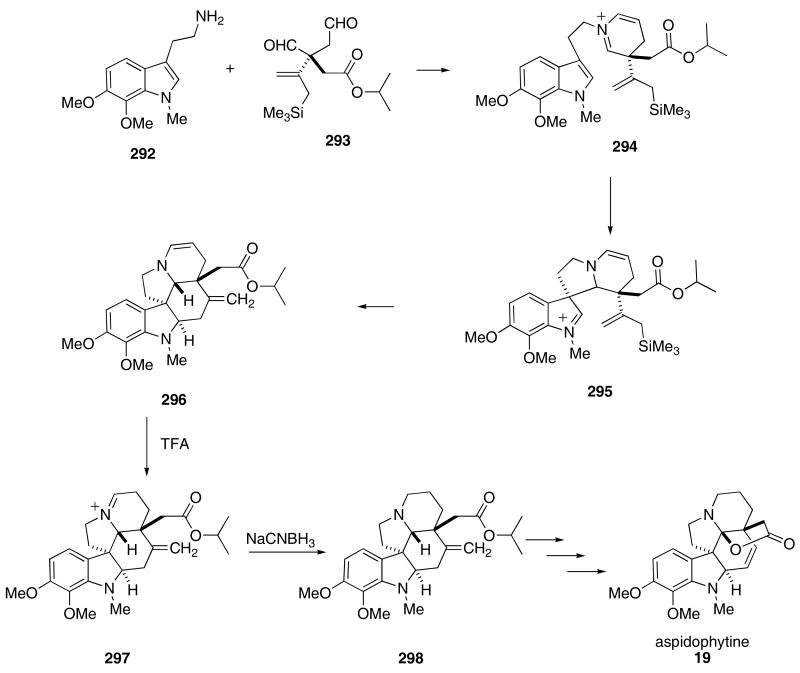
In addition to their use in Mannich (and variant) reactions, iminium ions are useful for other cationic type cyclizations. Corey employed a novel tandem iminium-ion cyclization as part of an elegant cascade used for the synthesis of aspidophytine. The reaction of the tryptamine 292 and dialdehyde 293 in CH3CN at ambient temperature afforded the pentacyclic skeleton of the alkaloid (296; Scheme 60).101 Condensation of the free amino functionality of 292 with the dialdehyde produced a dihydropyidinium intermediate 294 that then cyclized onto the indole π-bond to give 295. The iminium ion so produced underwent a second cyclization with the tethered allylsilane moiety to give 296. Protonation of the enamine in 296 provided still another iminium ion (297) that was then reduced with NaCNBH3 to furnish 298 in 66% yield. All of the above reactions could be made to occur in a single pot.
Scheme 60.
4.2. Pummerer Cascade Reactions
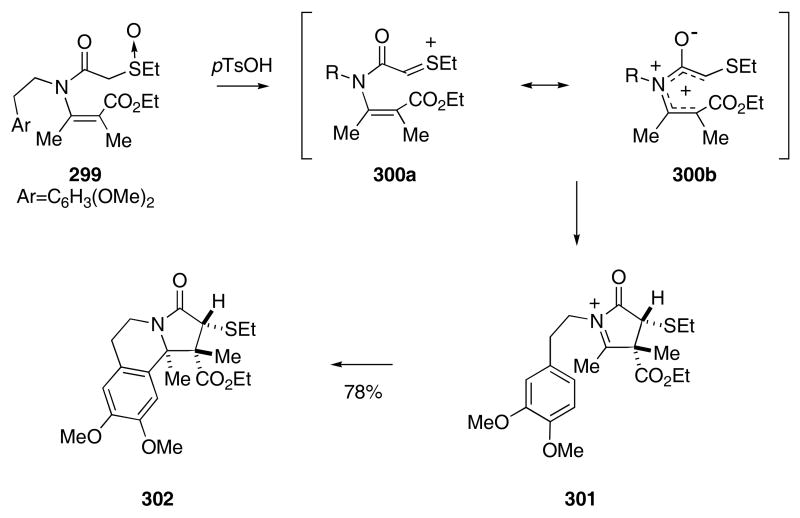
The combination of a Pummerer-based reaction102 followed by an N-acyliminium ion cyclization in tandem to form pyrrolidine-containing ring systems represents a unique method to synthesize heterocycles. In a typical example from the Padwa laboratory, enamide 299 was treated with p-TsOH in boiling benzene to produce thionium ion 300. A subsequent Nazarov-like ring closure of 300 furnished iminium ion 301. Finally, an intramolecular Pictet-Spengler reaction with the pendant aromatic ring of 301 provided 302 as a single diastereomer in 78% yield (Scheme 61).103 The stereochemistry of 302 was established by X-ray crystallographic analysis and is compatible with a conrotatory ring closure.
Scheme 61.
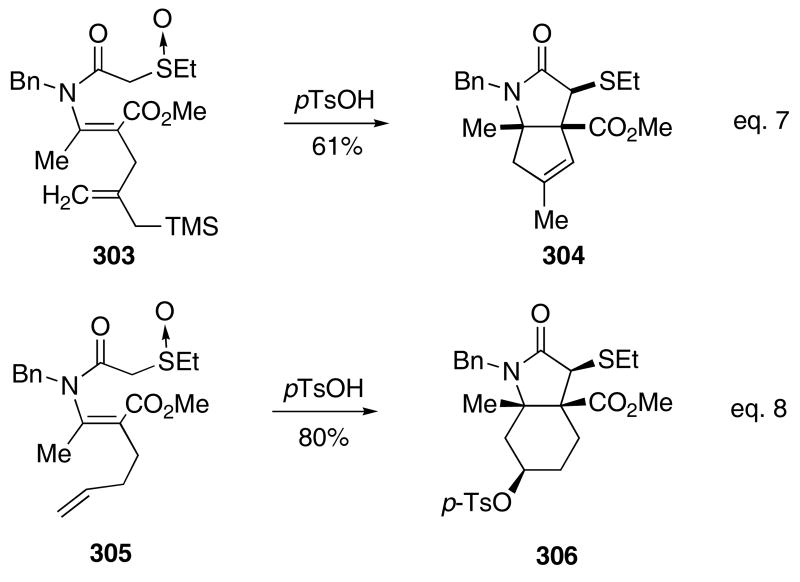
Other π-bonds were also found to efficiently participate in the Pummerer/Mannich ion cascade. For example, allylsilane 303 gave bicycle 304 in 61% yield when heated with p-TsOH (Scheme 62). The terminal alkene present in 305 cyclized to give 306, wherein the resultant secondary carbocation was captured by the sulfonate anion in 80% yield. In each case, only one diastereomer was isolated, suggesting that a concerted 4π-electrocyclization reaction occurs from the intermediate thionium ion.
Scheme 62.
This methodology was employed for the synthesis of the reported structure of the alkaloid jamtine.104 The key sulfoxide intermediate 307 was heated with CSA to produce several tricyclic products (98% yield) as a mixture (5:2:1:1) of diastereomers in which 308 predominated (Scheme 63). The stereochemistry of 308 was secured by X-ray crystallographic analysis, and is consistent with a Nazarov-type conrotatory 4π-electrocyclization followed by attack of the nucleophilically disposed aromatic ring from the least hindered side of the intermediate iminium ion. Reaction of α-ethylthio amide 308 with NaH effected an intramolecular alkylation to provide tetracycle 309.
Scheme 63.
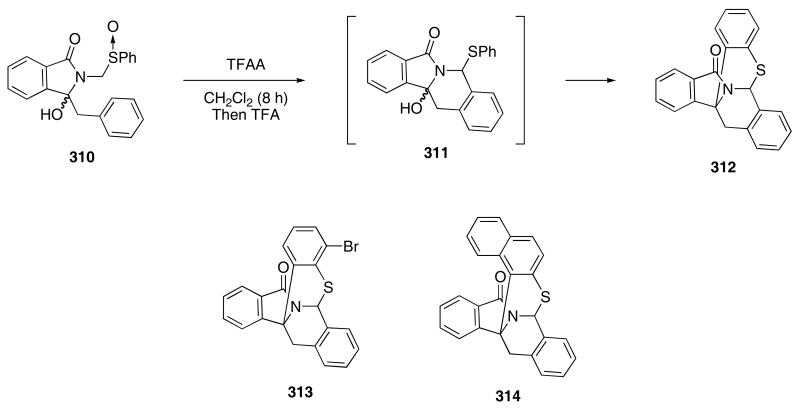
As part of their investigations dealing with N,S-fused polycyclic ring systems, Daich and coworkers reported the use of a tandem Pummerer/N-acyliminium ion cyclization to construct interesting isoquinolinone structures. Thus, treatment of sulfoxide 310 with TFAA in CH2Cl2 at rt for 8 h followed by the addition of TFA produced 312 in 42% yield through the intermediacy of 311 (Scheme 64).105 By conducting the reaction under buffered conditions (TFAA and pyridine), compound 311 could be isolated in 56% yield. An N-acyliminium ion intermediate was then generated by treating 311 with neat TFA and a subsequent cyclization of the resulting cationic intermediate gave 312 in 58% yield. Other arylthio groups were also studied, with compounds 313 and 314 being obtained from the TFAA/TFA conditions in 62 and 41% yield, respectively.
Scheme 64.
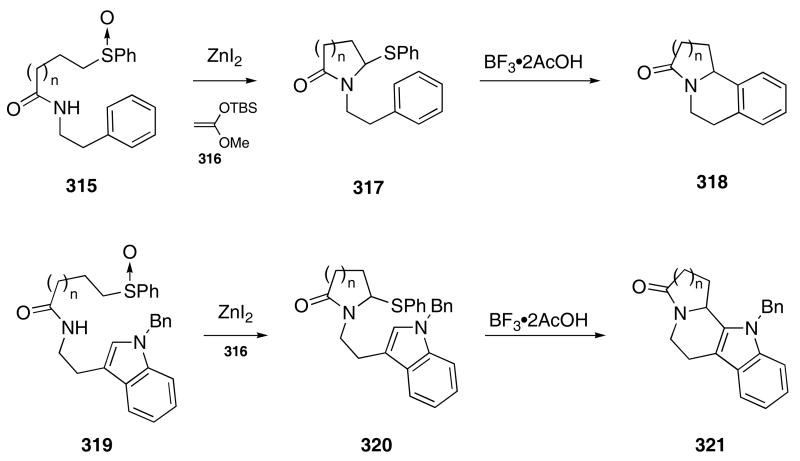
α-Thiophenylamides were also employed as precursors for the formation of N-acyliminium ions, which were then used as intermediates for subsequent cyclization chemistry. For example, treatment of amido sulfoxide 315 with silylketene acetal 316 in the presence of ZnI2 gave lactam 317 in excellent yield (>90%, Scheme 65).106 The action of BF3•2AcOH on 317 led to further ionization of the phenylthio group and cyclization of the resultant iminium ion onto the aromatic ring furnished 318 in 98% (n = 1) and 79% (n = 2) yield, respectively. The indole-substituted amido sulfoxide 319 gave compound 321 via the intermediacy of 320 in good overall yield, when subjected to these reaction conditions.
Scheme 65.
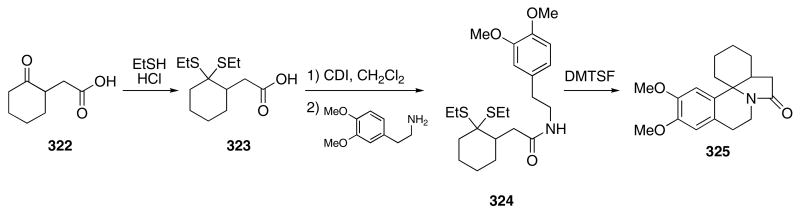
The above tandem Pummerer/Mannich cyclization cascade was modified to allow the use of dithioketals rather than sulfoxides as thionium ion precursors.107 This change in thionium ion precursor allowed the Pummerer cyclization to produce the requisite iminium ion in a single reaction vessel. An efficient synthesis of the erythrina alkaloid core demonstrated the utility of this cascade. Keto acid 322 was transformed into the thioketal 323 (Scheme 66). Coupling of 323 with 3,4-dimethoxyphenethylamine using carbonyl diimidazole (CDI) gave 324. Treatment of 324 with dimethyl(methylthio)-sulfonium tetrafluoroborate (DMTSF) in CH2Cl2 at reflux temperatures delivered the indolo-isoquinoline 325 in 71% yield.
Scheme 66.
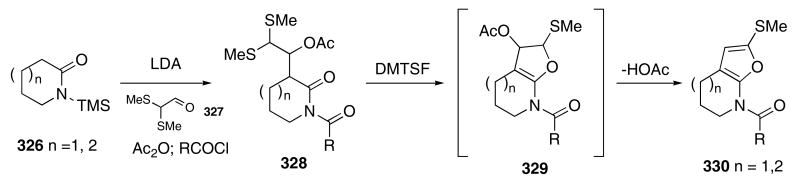
The Padwa group has also made extensive use of a Pummerer-based cyclization cascade for the formation of amidofurans.108 For example, the lithium enolate of cyclic amides such as 326 added cleanly to bis-(methylsulfanyl)acetaldehyde (327) to furnish aldol products of type 328 (Scheme 67).109 Reaction of 328 with DMTSF triggered a Pummerer cascade process by first inducing the loss of a methylthio group in 328 which provided a reactive thionium ion intermediate. This cation reacts with the proximal carbonyl group to give the dihydrofuran derivative 329. Elimination of acetic acid under the reaction conditions furnished amidofurans 330 in 70 - 80% isolated yields.
Scheme 67.
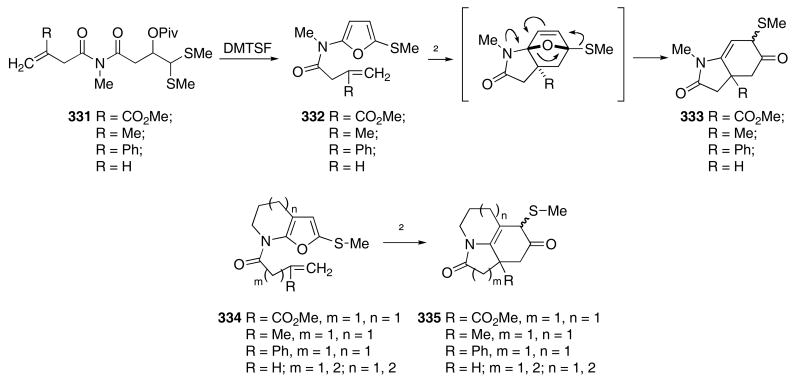
A variety of 2-methylthio-5-amidofuran systems containing a tethered π-bond on the amido nitrogen were prepared and utilized for a subsequent intramolecular Diels-Alder reaction.110 Thus, exposure of imides 331 to DMTSF resulted in the formation of furans 332 in 40 -70% yields (Scheme 68). Thermolysis of these furans in toluene at reflux initiated an intramolecular Diels-Alder reaction to first produce an intermediate oxabicyclo adduct. A subsequent fragmentation of the intermediate cycloadduct followed by a 1,2-thio shift provided the bicyclic amides 333 in good yields (ca 70%). In an analogous manner, the cycloaddition chemistry of amidofurans 334 provided the azatricyclic products 335. Apparently, the rate of the 1,2-thio shift of the initially formed cycloadduct is much faster than the deprotonation/dehydration pathway previously described in Scheme 25.
Scheme 68.
An interesting example of a Wagner-Merwein-type rearrangement that triggers a subsequent Pummerer cyclization has been recently been reported.111 Phenylsulfanyl-cyclopropane 336 was heated with p-TsOH in dry benzene at reflux. Ionization of the hydroxyl group occurred with concomitant ring expansion to give the transient cyclobutyl thionium 337 ion that was subsequently captured by the pendant aryl group to furnish 338 in 77% yield (Scheme 69). Other aryl groups, such as those containing a p-Me or a p-Cl substituent, also participated in this reaction, as did the unsubstituted analog (67-80% yield). Chromene 338 could be converted into the core structure of the radulanins by treatment with m-CPBA which gave sulfoxide 339 in 70% yield. Thermolysis of 339 in toluene resulted in the elimination of PhSOH producing 340 in 83% yield. Further exposure of 340 to m-CPBA induced a ring contraction reaction. This reaction presumably proceeds through the intermediacy of epoxide 341 and provides 342 whose carbon skeleton is found in the radulanin family of natural products.
Scheme 69.
4.3. Prins-Pinacol Cascades
The Overman group has made effective use of a pinacol-terminated Prins cyclization cascade for the synthesis of oxygen-containing heterocycles.112 His synthetic strategy for the synthesis of several Laurencia sesquiterpenes, such as kumausyne and kumausallene, focused on the acid mediated reaction of (1S,2R)-1-vinylcyclopentane-1,2-diol with 2-(benzyloxy)acetaldehyde. This reaction led to tetrahydrofuran 343 which contains the requisite stereochemistry for these natural products (Scheme 70). In this reaction, the p-TsOH mediated condensation first generated oxonium ion 344. A Prins cyclization then afforded carbocation 345 which underwent a pinacol rearrangement to furnish racemic 343 in 69% yield. Enantiomerically enriched starting (1S, 2R)-diol (84% ee) gave (−)-343 in 57% yield under similar conditions.
Scheme 70.
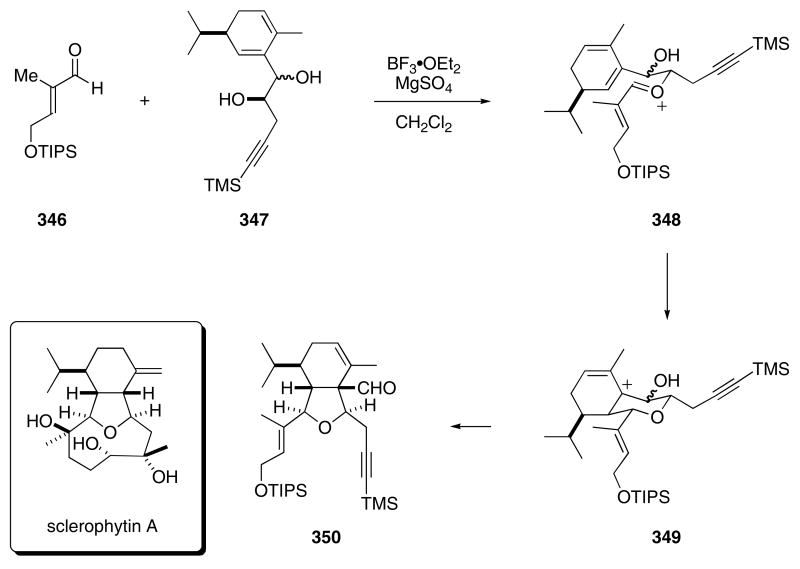
Application of the Prins-pinacol strategy also led to the synthesis of several cembranoid diterpenes. In these syntheses, BF3•OEt2 promoted the condensation of aldehyde 346 with diol 347 which generated oxonium ion 348 that underwent a subsequent Prins cyclization to provide 349 (Scheme 71). The Pinacol rearrangement of 349 then afforded tetrahydrofuran 350 in 79% yield. This compound was employed for the construction of several natural products, including sclerophytin A.
Scheme 71.
4.4. Other Cationic Cyclizations
A tandem Wagner-Merwein rearrangement/carbocation cyclization was used to synthesize several fenchone-derived systems.113 Heating a mixture of HCl and amide 351 at reflux temperature in aqueous ethanol for 24 h produced the indole derivative 352 in 60% yield (Scheme 72). Presumably, this reaction involves hydrolysis of amide 351 to initially produce compound 353. Solvolysis of 353 then provided carbocation 354 which undergoes a rearrangement to give 355. Carbocation capture by the adjacent nitrogen ultimately affords the ammonium salt of 352.
Scheme 72.
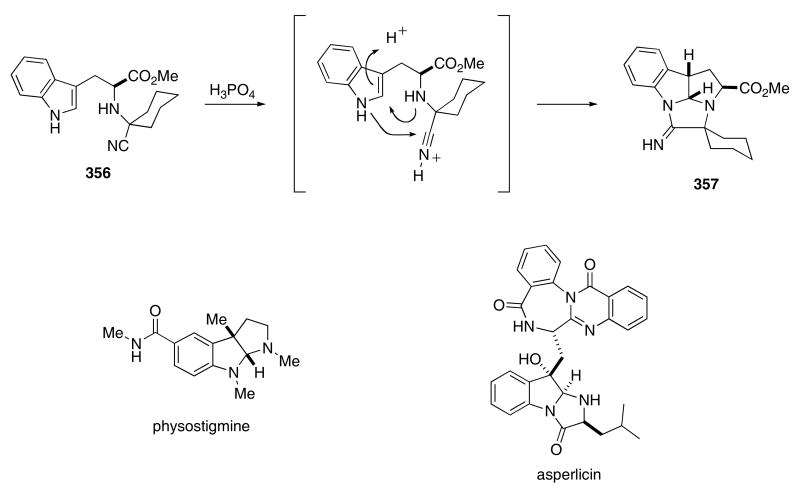
When stirred in 85% H3PO4, the tryptophan derived α-amino nitrile 356 underwent a stereospecific cyclization cascade to give 357 in nearly quantitative yield (Scheme 73).114 The formation of tetracyclic 357 is interesting because this compound incorporates both the tetrahydropyrrolo[2,3-b]indole structure, which is found in physostigmine and related alkaloids, and the tetrahydroimidazo[1,2-a]indole skeleton, which is present in asperlicin and related natural products.
Scheme 73.
5. Radical Cyclizations
5.1 Polycyclic Cascades
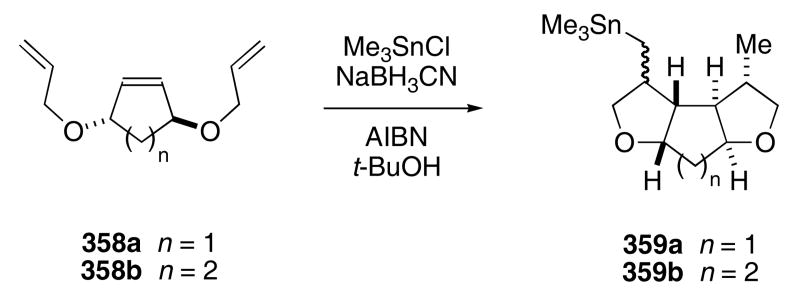
Polyethers are readily accessible by tandem radical cyclizations. For example, bis-allylether 358a,b reacts with a trimethyl tin radical and then undergoes a sequential radical cyclization to provide 359a,b in 86 and 85% yield, respectively (Scheme 74).115 A ceric ammonium nitrate oxidation of 359 was carried out in methanol and converted the stannyl moiety into the corresponding dimethylacetal.
Scheme 74.
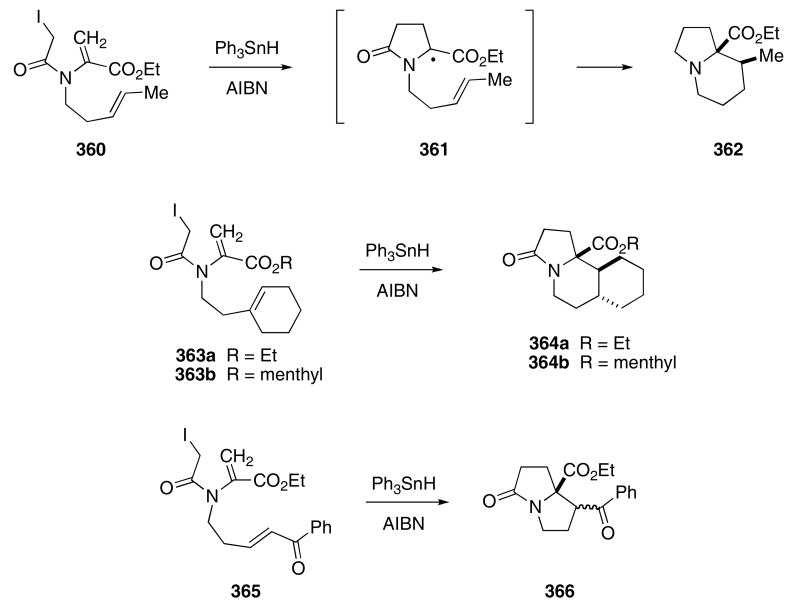
Several groups have reported the use of a radical cyclization cascade to form nitrogenous polycyclic structures. In one example, Parsons treated enamide 360 with Ph3SnH in the presence of AIBN to produce 362 in 40% yield (Scheme 75).116 In this case, cyclization of the intermediate α-amino ester radical proceeded through a 6-endo trig pathway rather than the typically more rapid 5-exo trig closure. The isolation of the 6-endo trig product most likely reflects the reversibility of the ring closure step, thereby allowing thermodynamic product stability to dictate the course of the reaction. When subjected to the same conditions, 363a produced 364a as a single diastereomer. Unfortunately, the incorporation of a menthol chiral auxiliary onto the ester group (i.e. 363b) led to 364b as mixture of six diastereomers in 38% yield, suggesting that this is not a suitable way to control stereoselectivity in these cyclization reactions.
Scheme 75.
The pyrrolizidinone ring can also be generated using this methodology if the intermediate α-amino ester radical undergoes cyclization onto an appropriately tethered electron-poor double bond. For example, enamide 365 reacted with Ph3SnH in the presence of AIBN to produce 366 in 52% yield as a 1.6 : 1-mixture of diastereomers, where the cis-isomer predominates.128b By incorporating a radical stabilizing group onto the π-bond, the reversibility of the 5-exo ring trig closure was reduced, thereby allowing isolation of the kinetically controlled product.
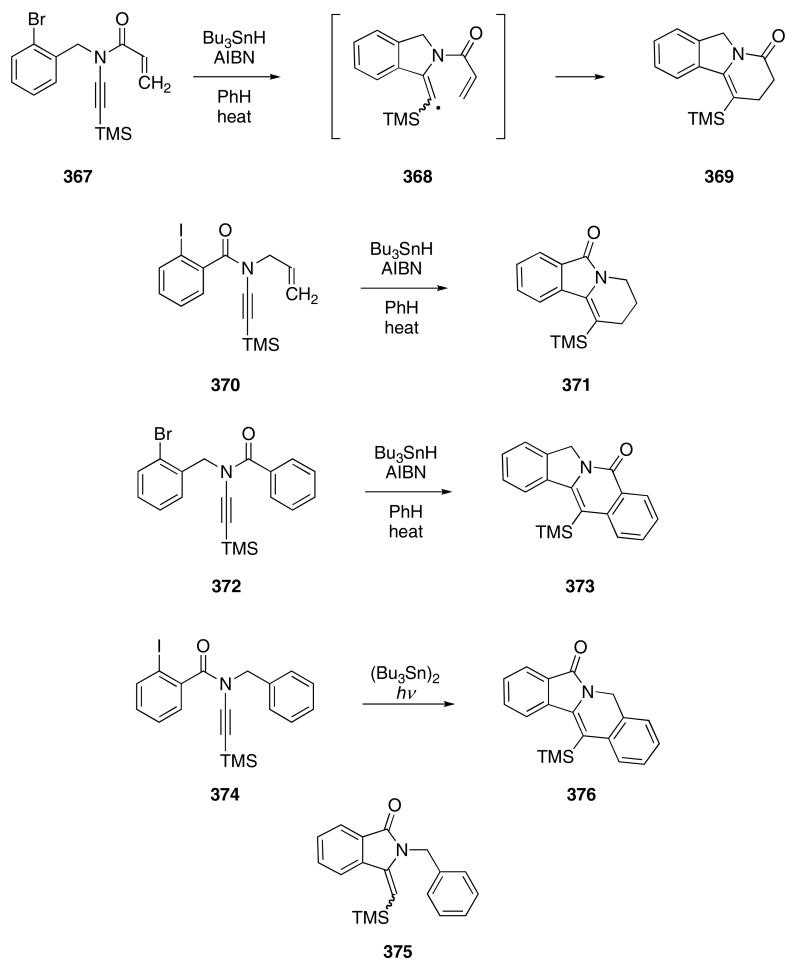
Ynamides also participate in radical cascade reactions. The Bu3SnH mediated cyclization of 367 afforded tricylic amide 369 in 70% yield via the intermediacy of radical 368 (Scheme 76).117 Similarly, subjection of ynamide 370, in which the carbonyl group is no longer part of the radical acceptor, to the same experimental conditions gave 371 in 90% yield. Ynamide 372, which contains a benzoyl radical acceptor, produced compound 373 in 67% yield, whereas 374 gave only pyrrolidinone 375 in 57% yield under identical conditions. Photolysis of (Bu3Sn)2, however, promoted the conversion of 374 into 376 in 46% yield.
Scheme 76.
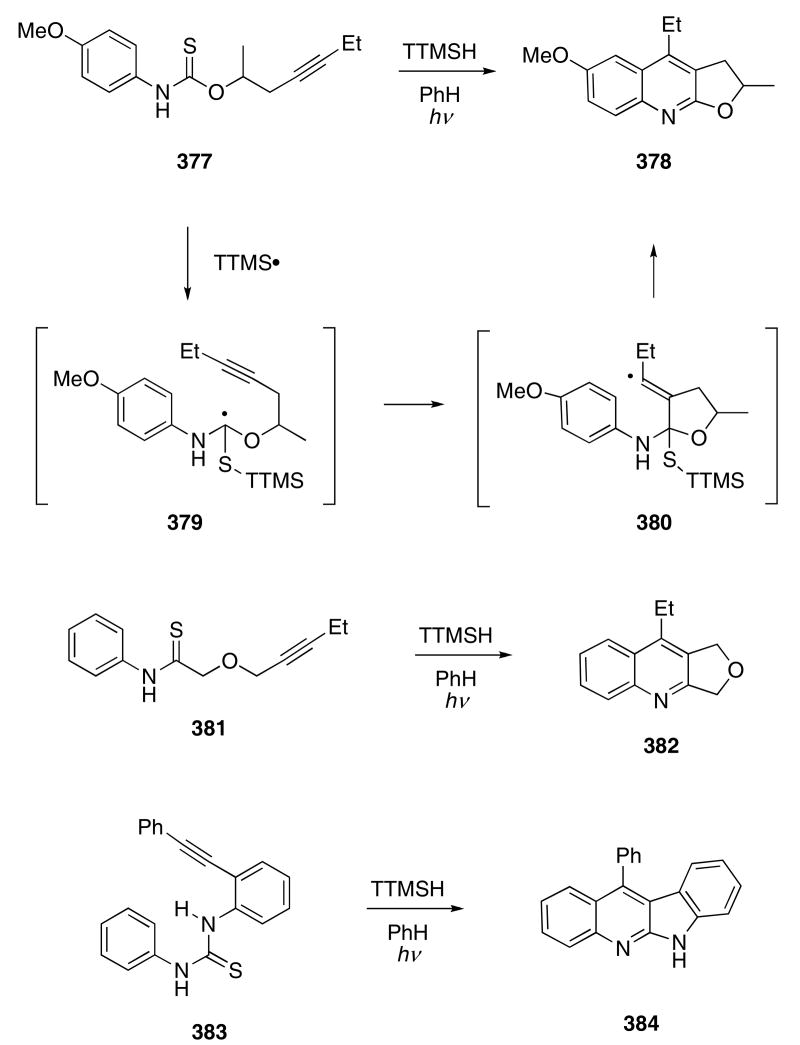
The Curran group has examined the use of thiocarbonyl derivatives for the radical cyclization cascade and employed this as a method to form quinoline dervatives. Thus, thiocarbamate 377 was allowed to react with tris-trimethylsilyl silane (TTMSH) under irradiation (UV) conditions and this resulted in the formation of 378 in 67% yield (Scheme 77).118 Tin reagents failed to mediate this reaction. Variously substituted analogs of 377 also participated in this cyclization cascade, affording quinoline derivatives (i.e. 378) in 44 - 88% yield. The mechanism of the cyclization is believed to involve addition of the radical derived from TTMSH onto the sulfur atom to generate an α-thioamino radical 379 which undergoes a subsequent cyclization onto the pendant alkyne to give vinyl radical 380. A second cyclization onto the aryl ring then provides 378 (Scheme 77). Substrates possessing substituents in the meta-position of the aryl ring afforded 1 : 1-mixtures of regioisomeric products.
Scheme 77.
Thioamides and thioureas also undergo the silyl mediated cascade reaction. For example, compound 381 gave 382 in 67% yield, while structural variants generally afforded related cyclized products in 50-87% yield. Thiourea 383 provided 384 in 64% yield, although a related substrate whose alkyne tether was conformationally more flexible failed to produce any cyclized product.
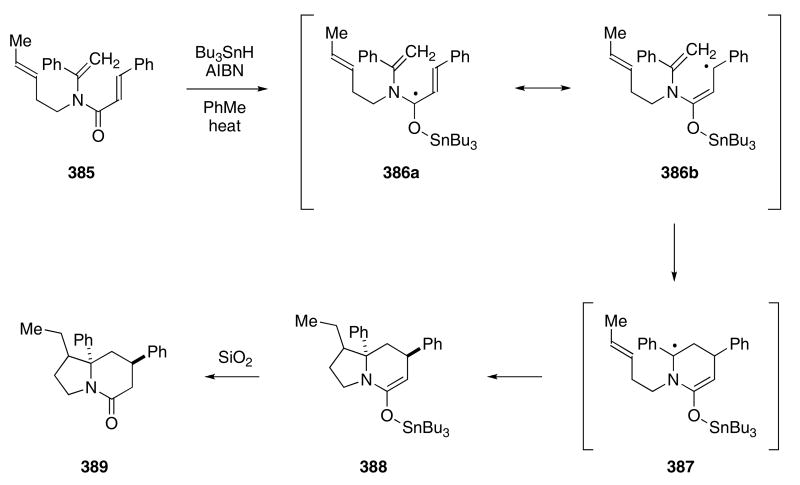
A novel application of the radical cascade for construction of the indolizidinone skeleton focused on the initial formation of O-stannyl ketyls. The tributyl tin radical was found to react with the carbonyl group of 385 to give ketyl 386 (Scheme 78).119 Consecutive 6-endo and 5-exo trig cyclizations then furnished stannyl enol ether 388. Eventual hydrolysis of the enol ether provided indolizidinone 389 in 36% yield as a 1 : 1-mixture of diastereomers. Again, the predominant isolation of the thermodynamic favored products derived from a 6-endo-trig cyclization can be attributed to the stability of 386b, suggesting that the cyclization to 387 is a reversible process. Without the stabilizing phenyl group, the conditions required to effect the first cyclization were much harsher, and a 5-exo-trig product was isolated.
Scheme 78.
Nitrogen centered radicals have received considerable attention in recent years. In particular, amidyl radicals have been shown to enter into cascade reactions to form pyrrolizidinone and indolizidinone derivatives. Thus, heating the O-benzoyl hydroxamic acid derivative 390 with Bu3SnH in the presence of AIBN produced 391 as a 3 : 2-mixture of diastereomers (Scheme 79).120 Separation of compound 391 from the tin residues was difficult, and the isolated yield (17%) was consequently low. When 392 was subjected to identical conditions, a 2 : 1-mixture of indolizidine 393 and pyrrolizidine 394 was isolated in 42% yield, along with the monocyclic product 395 in 5% yield. Attempts to induce addition of a radical intermediate onto an aromatic ring and thereby form molecules like 397 failed. However, by adding Cu+2 salts to the reaction mixture, this permitted the tandem radical cyclization to occur. It was suggested that the intermediate carbon centered radical was first oxidized to a carbocation and this was followed by a Friedel-Craft type reaction. Thus, under high dilution conditions in CH3CN, compound 396 was converted into 397 in 53% yield. Some reduced starting material (i.e., 398) was also produced in 40% yield.
Scheme 79.
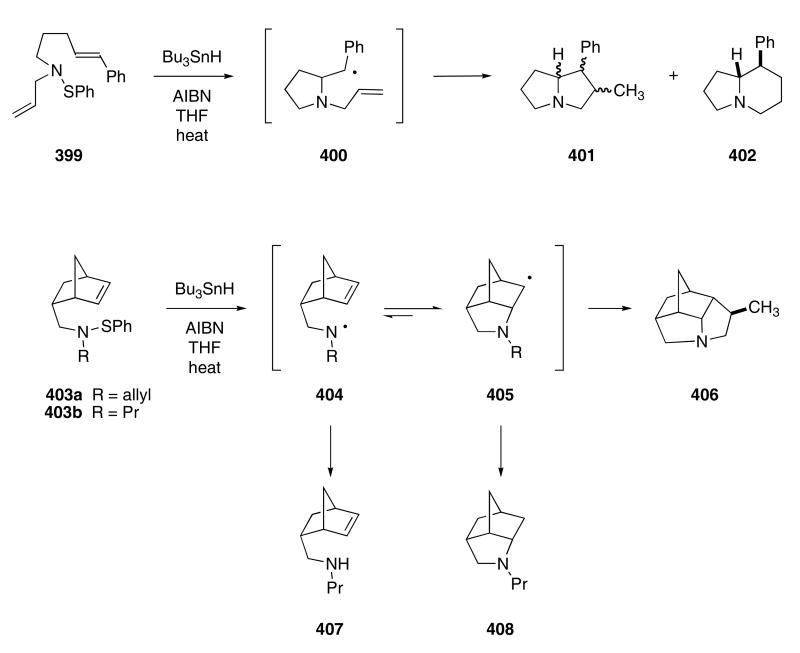
The Bowman group investigated different ways to use aminyl radicals for cyclization so as to produce azacycles. Aminyl radicals generally do not react well with alkenes. Bowman found, however, that these radicals will cyclize onto alkenes that are “activated.” For example, sulfenamide 399 when reacted with Bu3SnH and AIBN in THF at reflux temperatures delivered pyrrolizidine 401 as a mixture of three diastereomers in 49% yield, as well as indolizidine 402 and 14% yield (Scheme 80).121 Although the 5-exo trig cyclization pathway is kinetically favored, the 6-endo trig pathway does lead to the thermodynamically more stable radical. The formation of 402 suggests that cyclization of the intermediate radical 400 onto the tethered π-bond is a reversible process.
Scheme 80.
The difficulty associated with cyclization of the aminyl radical onto a π-bond is probably related to a competition between the rate of cyclization versus hydrogen abstraction from the tin hydride. Since the 5-exo-trig cyclization of the endo-2-(bicyclo[2.2.1]hept-2-en-5-yl)ethyl system is one of the fastest radical reactions known, sulfenamides 403a,b were constructed and then subjected to the cyclization conditions. The course of the reaction was found to depend upon the choice of the substituent group on nitrogen. When 403a was treated with Bu3SnH and AIBN, the N-allyl group acted as an internal radical trap and gave rise to 406 in 90% yield. Without the presence of the π-bond, products such as 407 were isolated in approximately 6% yield along with the cyclization product 408 (29% yield). The results were interpreted in terms of a fast but reversible cyclization of 404 to give 405. In the absence of the internal allyl group, hydrogen abstraction by radical 404 becomes competitive with hydrogen abstraction by radical 405.
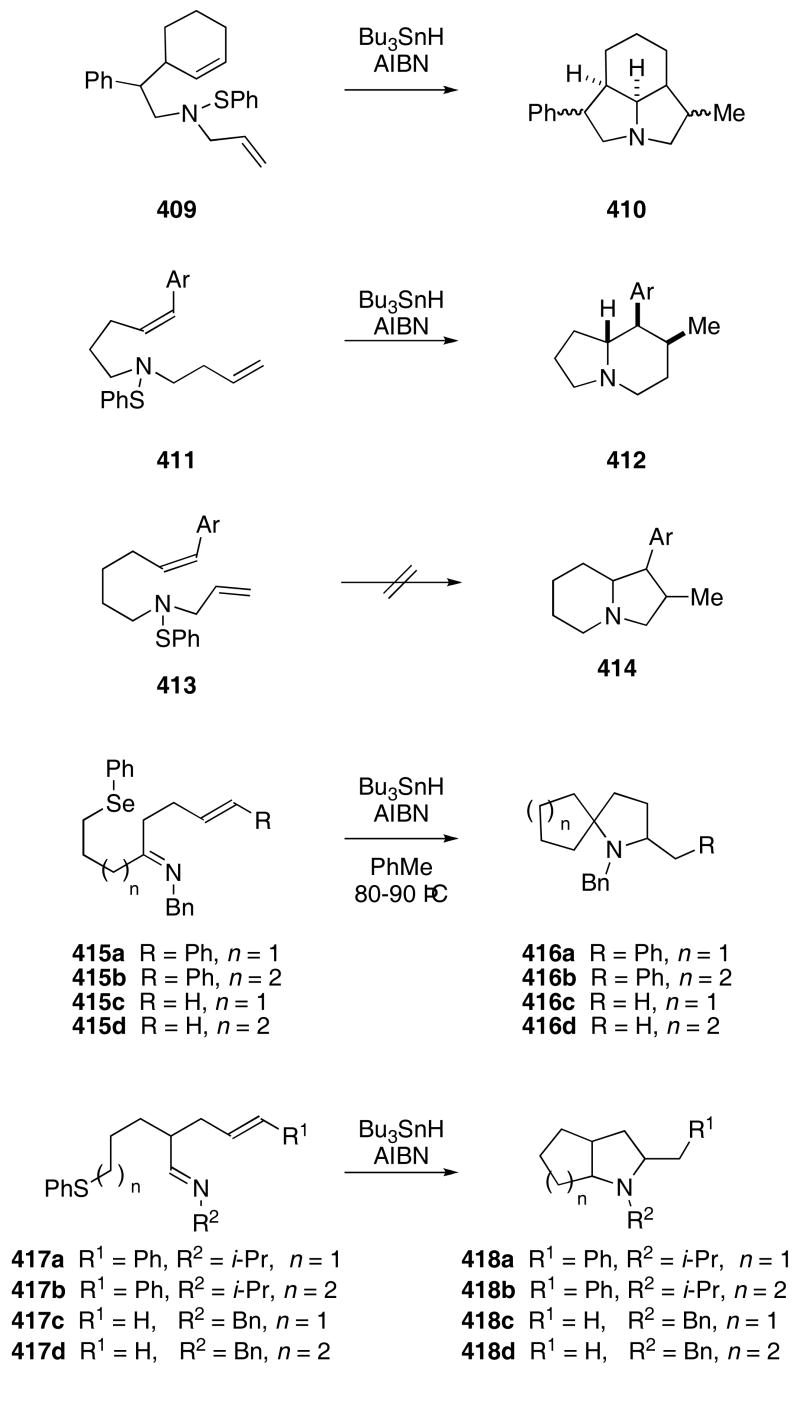
Having established that aminyl radicals can undergo cyclization, several different modes of reaction were explored. Allyl sulfenamide 409 was found to participate in a tandem radical cyclization reaction to produce a 2:2:1-mixture of hexahydroindolines 410 in 30% yield (Scheme 81).122 The cyclization failed when a related substrate lacking the allyl group was subjected to the same reaction conditions. Sulfenamide 411 underwent a tandem 5-exo/6-endo trig cyclization to give indolizidine 412 in 64% yield. Substrates that would require a 6-endo/5-exo cascade to form the indolizidine skeleton (i.e. 413) failed to cyclize under the radical conditions.
Scheme 81.
Spirocyclic amines can also be formed by using cyclization cascades that involve aminyl radicals. The AIBN promoted reaction of ketimines 415a,b with Bu3SnH in toluene provided 416a,b in 34 and 30% yields, respectively.123 Ketimines 415c,d failed to produce spirocycles under these conditions. However, in the presence of MgBr2•Et2O, compound 415c,d underwent the tandem cyclization reaction to give 416c,d in 24 and 33% yield, respectively. Aldimines 417a-d reacted similarly to afford bicyclic amines 418a-d in 40, 58, 27, and 33% yield. Again, substrates containing an aromatic group which provides stabilization for the intermediate radical produced from the cyclization, gave higher yields of bicyclic products. Adding MgBr2•Et2O to the reaction mixture increased the yield of 418c to 35% yield.
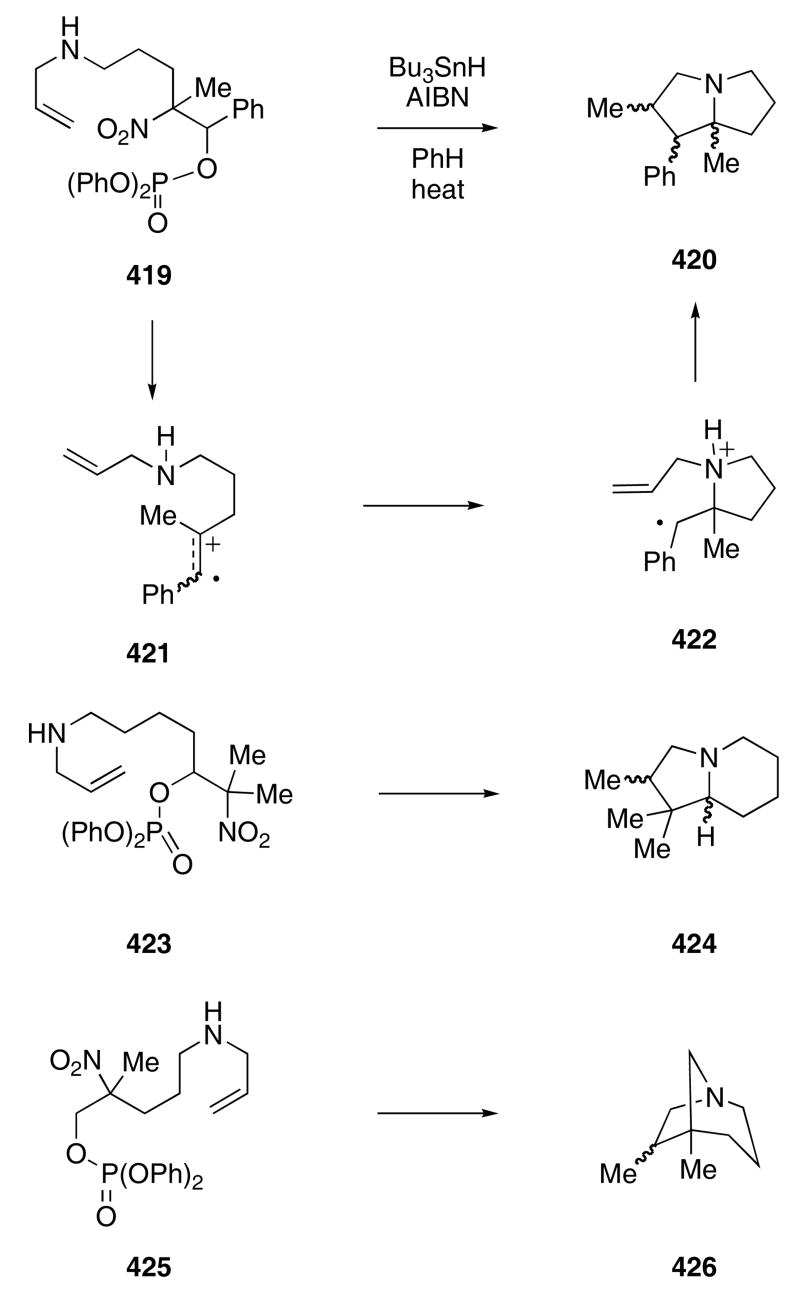
Crich and coworkers developed a novel radical-cation cascade for the construction of azapolycycles. Thermolysis of compound 419 with Bu3SnH and AIBN in benzene produced pyrrolizidine 420 as a mixture of diastereomers in 85% overall yield (Scheme 82).124 The reaction was thought to proceed via the formation of radical cation 421, which was generated by homolytic cleavage of the nitro group and ionization of the phosphate ester moiety. Intramolecular addition of the nitrogen onto the cationic center would then provide radical 422 that could cyclize to give the final product. Depending upon the substitution pattern of the starting nitro compound, a variety of fused and bridged bicyclic amines are possible. For example, allylamine 423 produced 424 as a 1 : 1-mixture of diastereomers in 78% yield, whereas 425 afforded 426 as a 2 : 1-mixture of diastereomers in 78% yield.
Scheme 82.
6. Metathesis
In recent years, the ring-closing olefin metathesis (RCM) reaction has become one of the more powerful tools for the synthesis of heterocycles,125 particularly medium-sized rings that are hard to form by other methods. Of the cascade reactions involving RCM, many are multiple sequential ring-closures or involve a tandem ring-opening/ring-closing reaction. Appropriately arrayed polyenes easily undergo multiple ring-closing metathesis reactions. For example, Harrity and coworkers demonstrated that the Grubb's first generation catalyst 428 catalyzed the conversion of tetraene 427 into the spirocyclic 429 in 90% yield (Scheme 83).126 None of the corresponding seven-membered ring product was isolated under these conditions. RCM reactions are known to be sensitive to conformational preferences within a molecule as well as substitution on the participating alkene. For example, diester 430 afforded 431 in 50% yield and macrocycle 432 in 19% yield, along with other dimeric material, when exposed to catalytic quantities of 428 at room temperature in CH2Cl2.127 Use of the second-generation Grubb's catalyst 428 increased the yield of 432 (up to 45 % yield), but no detectable amount of 433 was observed. Diether 435a underwent reaction with 428 to give mixtures that contained spirocycle 436, but only in 21% yield. Formation of five-membered rings continue to be the dominant pathway. To retard the formation of the five-membered ring products, diether 435b was synthesized and underwent reaction with 428 to produce spirocyclic 436 in 46% yield.
Scheme 83.
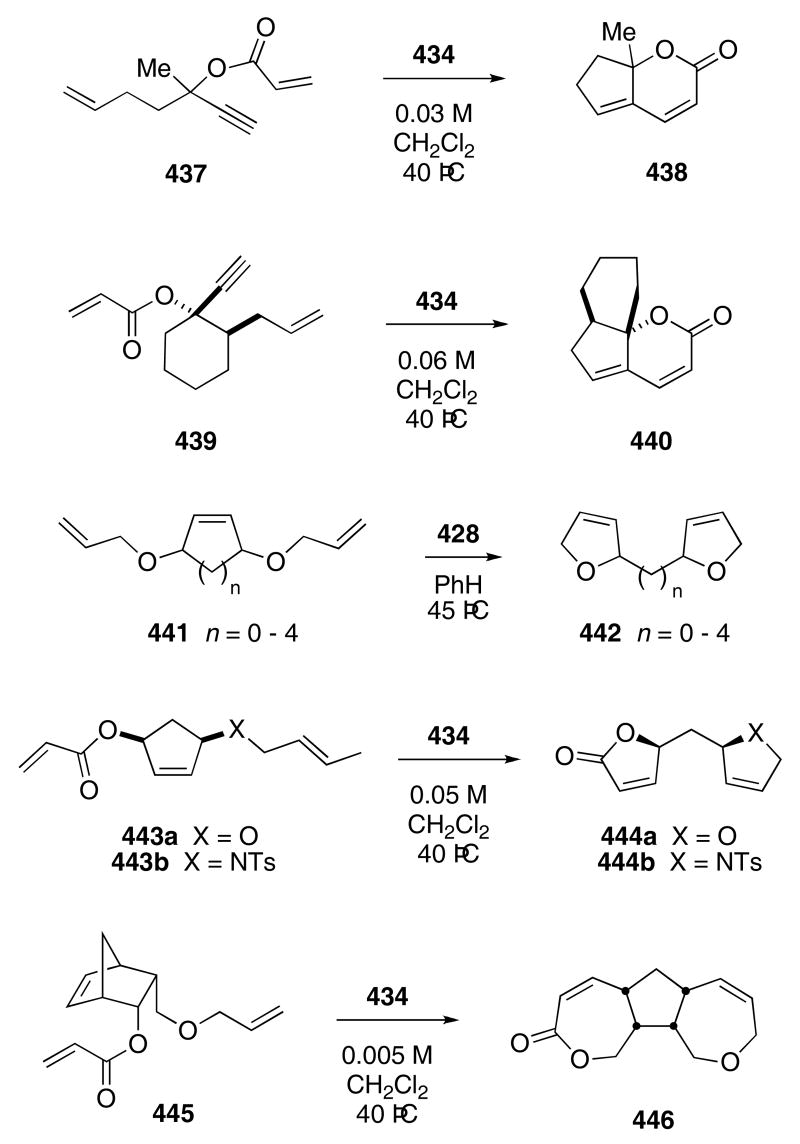
The Grubbs group has made extensive use of the tandem RCM cascade with tethered alkynes to produce heterocyclic compounds. For example, acyclic ester 437 reacted in the presence of 5 mol% of catalyst 434 in CH2Cl2 at 40 °C to furnish bicyclic lactone 438 in 95% yield (Scheme 84).128 The cyclic alkyne 439 reacted under the same conditions to give 440 in 74% yield.
Scheme 84.
Strained cycloalkenes that contain appropriately tethered olefins can also undergo the tandem ring-opening metathesis/RCM cascade. Using the first generation catalyst 428, Grubbs transformed a series of diallyloxy-cycloalkenes 441 into bis-furan derivatives 442 in moderate to good yields (57 - 90%).129 Likewise, cyclopentenes 443a,b reacted in the presence of catalyst 434 to afford 444a,b in 81 and 89% yield, respectively.128 Bicycloalkene 445 was converted into tricyclic 446 in 47% yield.
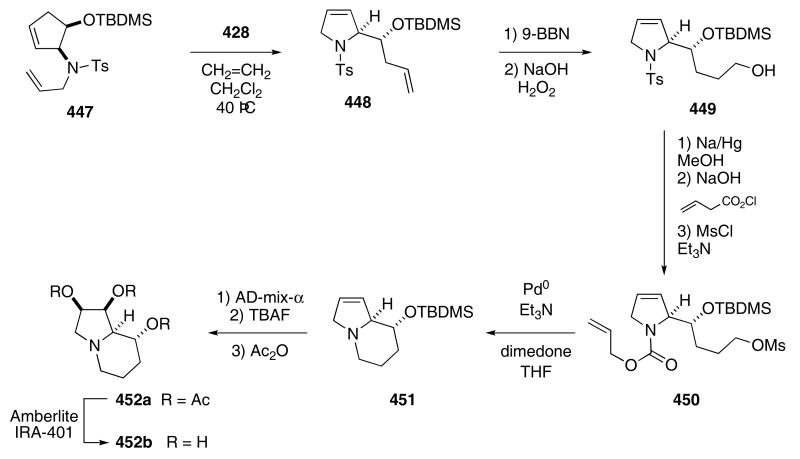
The Blechert group exploited the ring-opening/ring-closing cascade for the synthesis of several natural products. In a synthesis of (−)swainsonine (452b), sulfonamide 447 was heated at 40 °C with 5 mol% Grubbs catalyst 428 under an atmosphere of ethene to provide pyrroline derivative 448 in 98% yield.130 Hydroboration of the terminal alkene in 448 with 9-BBN followed by an oxidative workup under alkaline conditions gave alcohol 449 in 83% yield. The sulfonamide was cleaved by the action of Na/Hg. Acylation of the resulting free amino nitrogen with allyl chloroformate followed by mesylation afforded 450 in 87% yield from 449. Palladium mediated removal of the allyl carbamate group liberated a basic nitrogen that subsequently displaced the mesylate group to produce 451 in 95% yield. An asymmetric dihydroxylation of 451 produced an inseparable 20 : 1-mixture of diastereomeric diols. Separation of these diastereomers required removal of the silyl ether and conversion of the resulting triol into the triacetate occurred in 68% yield and with isomer 452a predominating. A base promoted hydrolysis then yielded the desired target 452b in 96% yield (Scheme 85).
Scheme 85.
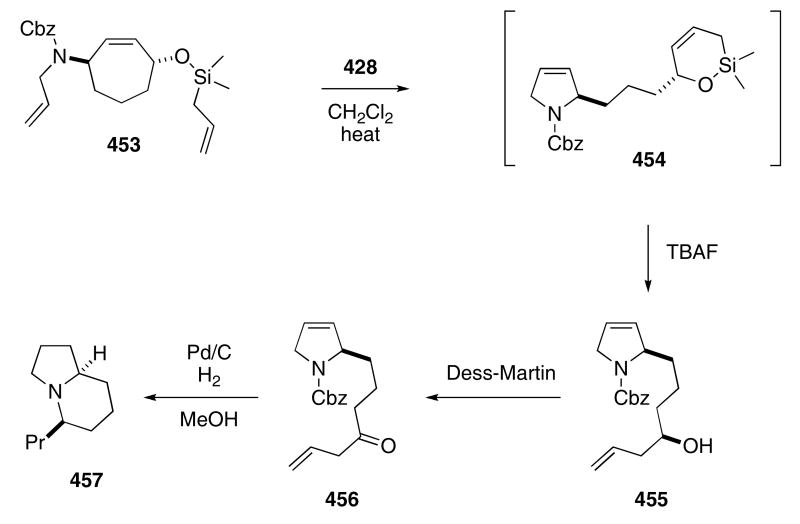
Extending this approach, Blechert's group reported a particularly efficient synthesis of indolizidine 167B (457)(Scheme 86). The cycloheptene derivative 453 was initially converted into silacycle 454 by the action of catalyst 428.131 A subsequent addition of TBAF to the reaction mixture promoted cleavage of the silicon group to give 455 in 92% yield from 453. Oxidation of the alcohol using the Dess-Martin periodane reagent afforded ketone 456 in 73% yield. A palladium-mediated hydrogenolysis of the benzyl carbamate was accompanied by intramolecular reductive amination of the ketone and this was followed by reduction of the alkene to provide 457 in 79% yield.
Scheme 86.
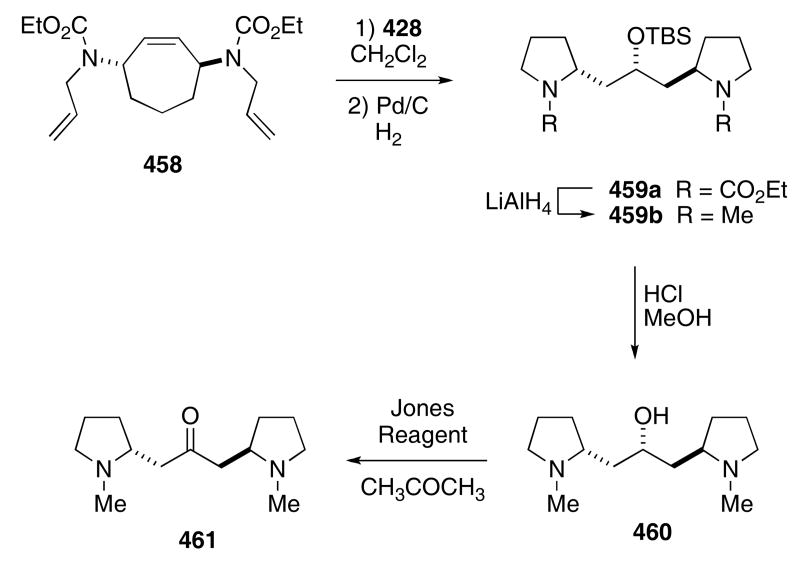
Synthesis of the alkaloid cuscohygrine (461) also came about from this methodology (Scheme 87). Exposing bis-carbamate 458 to catalyst 428 in hot CH2Cl2 produced a bis-pyrroline derivative whose double bonds proved to be unstable.132 Accordingly, the crude metathesis reaction mixture was treated with palladium in the presence of a hydrogen atmosphere to give 459a in 72% yield from 458. Reduction of the carbamate protecting group by the action of LiAlH4 afforded 459b in 92% yield. An acid mediated hydrolysis of the silyl ether provided (+)-dehydrocuscohygrine (460) in 89% yield. Further oxidation of the alcohol under Jones conditions produced the desired target 461 in 73% yield.
Scheme 87.
Recently, Hoveyda and Schrock have developed molybdenum-based catalysts, (i.e., 462a,b) for an asymmetric ring-opening metathesis (AROM) reaction (Scheme 88).133 Using a tandem AROM/RCM sequence, they examined the asymmetric synthesis of several heterocyclic compounds. For example, meso bis-ether 463 delivered 465 in 69% yield and with a 92% ee upon exposure to 5 mol% of 462a.134 In this case, ring opening by the catalyst was faster than the metathesis reaction with one of the pendant alkenes, and alkylidene carbene 464 was enantiospecifically produced as an intermediate. However, the less sterically hindered catalyst 462b mediated the transformation of 466 into 467 in 84% yield and with greater than 98% ee. Interestingly, 468 could be converted into 469 by the action of catalyst 462b in 60% yield and with 72% ee, whereas catalyst 462a did not promote the reaction. By adding diallyl ether to the reaction mixture, 462a did catalyze the reaction and provided 469 in 54% yield but with 92% ee.
Scheme 88.
Building on their earlier tandem ring-opening/cross-metathesis studies,135 Arjona and Plumet have applied the ring-opening/ring-closing/cross-metathesis cascade136 to the synthesis of various nitrogenous heterocycles. For example, bicyclic amides 470a,b reacted with ethene in the presence of Grubbs catalyst 428 to give 471a,b in 65 and 60% yields, respectively (Scheme 89).137 Bicyclic lactams 471 were the expected products of a ring-opening/ring-closing metathesis cascade without the initial cross-metathesis with ethylene. To demonstrate the actual cross-metathesis reaction, allyl acetate was used as the partner. In this case, compound 470a reacted with catalyst 428 and allyl acetate and gave 472 in 40% isolated yield along with 471a in 30% yield. By changing to catalyst 434, the yield of 472 was increased to 65% yield, though 471a was again produced in 30% yield.
Scheme 89.
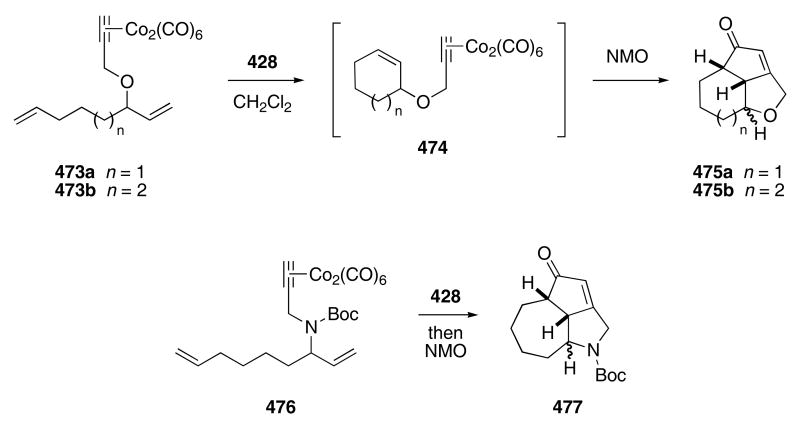
A one-pot sequential RCM/Pauson-Khand reaction sequence has been used to synthesize nitrogen- and oxygen-containing polycycles. The cobalt-alkyne complexes 473a,b reacted in the presence of Grubbs catalyst 428 to give 474 (Scheme 90).138 The addition of NMO to the crude reaction mixture promoted the Pauson-Khand reaction and provided 475a,b in 81 and 70% yield as mixtures of diastereomers. Similarly, 476 underwent the tandem RCM/Pauson-Khand to produce 477 in 67% yield.
Scheme 90.
7. Concluding Remarks
From the selective sampling of cascade reactions for the synthesis of heterocyclic molecules that has been outlined in this mini-review, it is clear that virtually any reaction can be incorporated into a tandem sequence. Some cascade sequences increase molecular complexity more than others, but each seems to provide complex heterocyclic structures in a more efficient manner than the corresponding chemistry wherein each intermediate is isolated. Indeed, many of these cascades rapidly construct hetero-polycyclic systems that are difficult to produce in other ways.
Several domino cascade sequences for heterocyclic synthesis have been well explored; Padwa's rhodium carbenoid-initiated dipolar cycloadditions, Denmark's nitroalkene [4+2]/[3+2]-cycloadditions, Overman's aza-Cope/Mannich cascade, and Grubb's ring closing metathesis chemistry have all matured into significant synthetic tools. Familiar multi-component reactions, such as the Ugi reaction, are being used in interesting ways. Others sequences not covered in this mini-review show tremendous promise; Fu's asymmetric Kinugasa reaction, indium initiated radical cascades, Buchwald's copper catalyzed N-arylation reactions, Trost's alkyne heterocyclization, and the Hoveyda and Schrock tandem AROM/RCM reactions all provide improvements in stereoselectivity and involve the use of environmentally benign reagents. Continued development of these domino cascade reactions will have a significant impact on the processes used to make heterocyclic compounds on an industrial scale.
Acknowledgments
AP wishes to acknowledge the research support of our cascade program in heterocyclic chemistry by the National Institutes of Health (GM 0539384) and the National Science Foundation (CHE-0450779).
Biographies
Albert Padwa*
Albert Padwa was born in New York City. He received both his B.A. and Ph.D. degrees from Columbia University. After a NSF postdoctoral position at the University of Wisconsin, he was appointed Assistant Professor of Chemistry at the Ohio State University in 1963. He moved to SUNY Buffalo in 1966 as Associate Professor and was promoted to Professor in 1969. Since 1979, he has been the William Patterson Timmie Professor of Chemistry at Emory University. He has held visiting positions at University Claude Bernard, France, University of California at Berkeley, the University of Wurzburg, Germany and Imperial College of Chemistry, UK. Professor Padwa has been the recipient of an Alfred P. Sloan Fellowship, John S. Guggenheim Fellowship, Alexander von Humboldt Senior Scientist Award, a Fulbright Hays Scholarship, Senior Award in Heterocyclic Chemistry from the International Society of Heterocyclic Chemists, ACS Arthur C. Cope Scholar Award and is the coauthor of more than 650 publications. He served as the Chairman of the Organic Division of the ACS and as President of the International Society of Heterocyclic Chemistry. He has also served as a member of the editorial boards of the Journal of the American Chemical Society, Journal of Organic Chemistry, Organic Letters, and has been the volume editor of Comprehensive Heterocyclic Chemistry, the Synthesis of Science (Vol 27) and is currently one of the Associate Editors of the Journal of Organic Chemistry. His research interests include heterocyclic chemistry, dipolar cycloadditions, alkaloid synthesis, tandem transformations, organometallic chemistry, and organic photochemistry. Aside from chemistry, his other passion is mountain climbing in various parts of the world.

Scott K. Bur received his B.S. from the University of Michigan in 1994, and worked as an extern with Parke-Davis pharmaceuticals in Ann Arbor for one year. He then earned his Ph.D. from the University of Texas at Austin in 2000 under the supervision of Professor Stephen F. Martin. After an NIH postdoctoral fellowship in the laboratories of Professor Albert Padwa at Emory University, he joined the faculty at Gustavus Adolphus College in 2003 as an Assistant Professor. His research interests include heterocyclic methodology, natural product synthesis, and the application of computational methods to the solution of synthetic organic problems.

Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Katritzky A, Rees CW, Scriven EF, editors. Comprehensive Heterocyclic Chemistry. Elsevier Science; Oxford, U.K.: 1996. [Google Scholar]
- 2.Kuehne ME, Matsko TH, Bohnert JC, Motyka L, Oliver-Smith D. J Org Chem. 1981;46:2002. [Google Scholar]; Kuehne ME, Earley WG. Tetrahedron. 1983;39:3707. [Google Scholar]; Kuehne ME, Brook CS, Xu F, Parsons R. Pure Appl Chem. 1994;66:2095. [Google Scholar]; Nkiliza J, Vercauteren J. Tetrahedron Lett. 1991;32:1787. [Google Scholar]; Rawal VH, Michoud C, Monestel RF. J Am Chem Soc. 1993;46:3030. [Google Scholar]
- 3.Overman LE, Sworin M, Burk RM. J Org Chem. 1983;48:2685. [Google Scholar]; Overman LE, Sugai S. Helv Chim Acta. 1985;68:745. [Google Scholar]; Overman LE, Robertson G, Robichaud AJ. J Org Chem. 1989;54:1236. [Google Scholar]
- 4.Gallagher T, Magnus P, Huffman JC. J Am Chem Soc. 1983;105:4750. [Google Scholar]; Magnus P, Gallagher T, Brown P, Pappalardo P. Acc Chem Res. 1984;17:35. [Google Scholar]
- 5.Rigby JH, Qabar MH. J Org Chem. 1993;58:4473. [Google Scholar]; Rigby JH, Qabar M, Ahmed G, Hughes RC. Tetrahedron. 1993;49:10219. [Google Scholar]; Rigby JH, Hughes RC, Heeg MJ. J Am Chem Soc. 1995;117:7834. [Google Scholar]; Rigby JH, Mateo ME. Tetrahedron. 1996;52:10569. [Google Scholar]; Rigby JH, Laurent S, Cavezza A, Heeg MJ. J Org Chem. 1998;63:5587. [Google Scholar]
- 6.Kraus GA, Thomas PJ, Bougie D, Chen L. J Org Chem. 1990;55:1624. [Google Scholar]; Sole D, Bonjoch J. Tetrahedron Lett. 1991;32:5183. [Google Scholar]; Bonjoch J, Sole D, Bosch J. J Am Chem Soc. 1993;115:2064. [Google Scholar]; Schultz AG, Holoboski MA, Smyth MS. J Am Chem Soc. 1993;115:7904. [Google Scholar]; Schultz AG, Guzzo PR, Nowak DM. J Org Chem. 1995;60:8044. [Google Scholar]; Schultz AG, Holoboski MA, Smyth MS. J Am Chem Soc. 1996;118:6210. [Google Scholar]
- 7.Pearson WH, Postich MJ. J Org Chem. 1994;59:5662. [Google Scholar]
- 8.Takano S, Inomata K, Ogasawara K. Chem Lett. 1992:443. [Google Scholar]; Uesaka N, Saitoh F, Mori M, Shibasaki M, Okamura K, Date T. J Org Chem. 1994;59:5633. [Google Scholar]; Mori M, Kuroda S, Zhang C, Sato Y. J Org Chem. 1997;62:3263. doi: 10.1021/jo9701187. [DOI] [PubMed] [Google Scholar]
- 9.Fuchs PL. Tetrahedron. 2001;57:6855–6875. [Google Scholar]
- 10.Tietze LF. Chem Rev. 1996;96:115–136. doi: 10.1021/cr950027e. [DOI] [PubMed] [Google Scholar]
- 11.Tietze LF, Beifuss U. Angew Chem Int Ed Engl. 1993;32:131. [Google Scholar]
- 12.Ho TL. Tandem Organic Reactions. Wiley; New York: 1992. [Google Scholar]; Bunce RA. Tetrahedron. 1995;51:13103. [Google Scholar]; Parsons PJ, Penkett CS, Shell AJ. Chem Rev. 1995;95:195. doi: 10.1021/cr950023+. [DOI] [PubMed] [Google Scholar]
- 13.Ziegler FE. In: Comprehensive Organic Synthesis, Combining C-C π-Bonds. Chapter 73 Paquette LA, editor. Vol. 5. Pergamon Press; Oxford: 1991. [Google Scholar]
- 14.Waldmann H. In: Domino Reaction in Organic Synthesis Highlight II. Waldmann H, editor. VCH; Weinheim: 1995. pp. 193–202. [Google Scholar]
- 15.Curran DP. In: Comprehensive Organic Synthesis. Trost BM, Fleming I, editors. Vol. 4. Pergamon; Oxford: 1991. p. 779. [Google Scholar]
- 16.Wender PA, editor. Frontiers in Organic Synthesis. Chem Rev. 1996;96:1–600. doi: 10.1021/cr9500803. [DOI] [PubMed] [Google Scholar]
- 17.Gribble GW. In: Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry toward Heterocycles and Natural Products. Padwa A, Pearson WH, editors. John Wiley & Sons, Inc; Hoboken, New Jersey: 2003. pp. 681–753. [Google Scholar]
- 18.Hamaguchi M, Ibata T. Tetrahedron Lett. 1974:4475–4476. [Google Scholar]
- 19.Hamaguchi M, Ibata T. Chem Lett. 1975:499. [Google Scholar]
- 20.Doyle MP, Pieters RJ, Tauton J, Pho HQ, Padwa A, Hertzog DL, Precedo L. J Org Chem. 1991;56:820. [Google Scholar]
- 21.Maier ME, Evertz K. Tetrahedron Lett. 1988;29:1677–1680. [Google Scholar]
- 22.Hertzog DL, Austin DJ, Nadler WR, Padwa A. Tetrahedron Lett. 1992;33:4731–4734. [Google Scholar]
- 23.Padwa A, Brodney MA, Marino JPJ, Osterhout MH, Price AT. J Org Chem. 1997;62:67–77. doi: 10.1021/jo9607267. [DOI] [PubMed] [Google Scholar]
- 24.Padwa A, Price AT. J Org Chem. 1995;60:6258–6259. [Google Scholar]
- 25.Mejia-Oneto J, Padwa A. Org Lett. 2006;8:3275–3278. doi: 10.1021/ol061137i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dauben WG, Dinges J, Smith TC. J Org Chem. 1993;58:7635–7637. [Google Scholar]
- 27.Padwa A, Heidelbaugh TM, Kuethe JT. J Org Chem. 1999;64:2038–2049. doi: 10.1021/jo982315r. [DOI] [PubMed] [Google Scholar]
- 28.Padwa A, Heidelbaugh TM, Kuehne ME. J Org Chem. 2000;65:2368–2378. doi: 10.1021/jo9915729. [DOI] [PubMed] [Google Scholar]
- 29.Slabaugh MR, Wildman WC. J Org Chem. 1971;36:3202–3207. [Google Scholar]
- 30.Christy ME, Anderson PS, Britcher SF, Colton CD, Evans BE, Remy DC, Engelhardt EL. J Org Chem. 1979;44:3117–3127. [Google Scholar]
- 31.Norman MH, Heathcock CH. J Org Chem. 1987;52:226–235. [Google Scholar]
- 32.Padwa A, Chiacchio U, Dean DC, Schoffstall AM, Hassner A, Murthy KSK. Tetrahedron Lett. 1988;29:4169–4172. [Google Scholar]
- 33.Hassner A, Maurya R, Mesko E. Tetrahedron Lett. 1988;29:5313–5316. [Google Scholar]
- 34.Hassner A, Maurya R. Tetrahedron Lett. 1989;30:2289–2292. [Google Scholar]
- 35.Grigg R, Heaney F, Markandu J, Surendrakumar S, Thorton-Pett M, Warnock WJ. Tetrahedron. 1991;47:4007–4030. [Google Scholar]
- 36.Kinugasa M, Hashimoto S. J Chem Soc Chem Commun. 1972:466–467. [Google Scholar]
- 37.Miura M, Enna M, Okura K, Nomura M. J Org Chem. 1995;60:4999–5004. [Google Scholar]
- 38.Basak A, Ghosh SC, Bhowmick T, Das AK, Bertolasi V. Tetrahedron Lett. 2002;43:5499–5501. [Google Scholar]
- 39.Lo MMC, Fu GC. J Am Chem Soc. 2002;124:4521–4573. [Google Scholar]
- 40.Shintani R, Fu GC. Angew Chem. 2003;42:4082–4085. doi: 10.1002/anie.200352103. [DOI] [PubMed] [Google Scholar]
- 41.Brandi A, Dürüst Y, Cordero FM, De Sarlo F. J Org Chem. 1992;57:5666–5670. [Google Scholar]
- 42.Brandi A, Cordero FM, De Sarlo F, Goti A, Guarna A. Synlett. 1993:1–8. [Google Scholar]
- 43.Goti A, Anichini B, Brandi A. J Org Chem. 1996;61:1665–1172. doi: 10.1021/jo951838l. [DOI] [PubMed] [Google Scholar]
- 44.Belle C, Cardelli A, Guarna A. Tetrahedron Lett. 1991;32:6395–6398. [Google Scholar]
- 45.Pearson WH, Bergmeier SC, Degan S, Lin KC, Poon YF, Schkeryantz JM, Williams JP. J Org Chem. 1990;55:5719–5738. [Google Scholar]
- 46.a) Herdeis C, Schiffer T. Tetrahedron. 1996;52:14745–14756. [Google Scholar]; b) Herdeis C, Tesler J. Eur J Org Chem. 1999:1407–1414. [Google Scholar]
- 47.a) Markó IE, Seres P, Swarbrick TM, Staton I, Adams H. Tetrahedron Lett. 1992;33:5649–5652. [Google Scholar]; b) Swarbrick TM, Markó IE, Kennard L. Tetrahedron Lett. 1991;32:2549–2552. [Google Scholar]
- 48.Markó IE, Seres P, Evans GR, Swarbrick TM. Tetrahedron Lett. 1993;34:7305–7308. [Google Scholar]
- 49.Tisdale EJ, Chowdhury C, Vong BG, Li H, Theodorakis EA. Org Lett. 2002;4:909–912. doi: 10.1021/ol017278w. [DOI] [PubMed] [Google Scholar]
- 50.a) Padwa A, Dimitroff M, Waterson AG, Wu T. J Org Chem. 1997;62:4088–4096. [Google Scholar]; b) Padwa A, Dimitroff M, Waterson AG, Wu T. J Org Chem. 1998;63:3986–3997. [Google Scholar]; c) Padwa A, Brodney MA, Satake K, Straub CS. J Org Chem. 1999;64:4617–4626. doi: 10.1021/jo982061+. [DOI] [PubMed] [Google Scholar]; d) Padwa 2002;#217] [Google Scholar]
- 51.Padwa A, Brodney MA, Dimitroff M. J Org Chem. 1998;63:5304–5305. doi: 10.1021/jo010020z. [DOI] [PubMed] [Google Scholar]
- 52.a) Kozmin SA, Janey JM, Rawal VH. J Org Chem. 1999;64:3039–3052. doi: 10.1021/jo981563k. [DOI] [PubMed] [Google Scholar]; b) Janey JM, Iwama T, Kozmin SA, Rawal VH. J Org Chem. 2000;65:9059–9068. doi: 10.1021/jo005619y. [DOI] [PubMed] [Google Scholar]
- 53.a) Bur SK, Padwa A. Org Lett. 2002;4:4135–4137. doi: 10.1021/ol0268992. [DOI] [PubMed] [Google Scholar]; b) Padwa A, Bur SK, Zahng HJ. J Org Chem. 2005;70:6833–6841. doi: 10.1021/jo0508797. [DOI] [PubMed] [Google Scholar]
- 54.Paulvannan K. Tetrahedron Lett. 1999;40:1851–1854. [Google Scholar]
- 55.Paulvannan K. J Org Chem. 2004;69:1207–1214. doi: 10.1021/jo030231z. [DOI] [PubMed] [Google Scholar]
- 56.Paulvannan K, Hale R, Mesis R, Chen T. Tetrahedron Lett. 2002;43:203–207. [Google Scholar]
- 57.Janvier P, Sun X, Bienaymé H, Zhu J. J Am Chem Soc. 2002;124:2560–2567. doi: 10.1021/ja017563a. [DOI] [PubMed] [Google Scholar]
- 58.Raw SA, Taylor RJK. Chem Commun. 2004:508–509. doi: 10.1039/b316107b. [DOI] [PubMed] [Google Scholar]
- 59.Ceulemans E, CVoets M, Emmers S, Uytterhoeven K, Van Meervelt L, Dehaen W. Tetrahedron. 2002;58:531–544. [Google Scholar]
- 60.Yadav JS, Reddy BVS, Narsimhaswamy D, Naga Lakshm P, Narsimulu K, Srinivasulu G, Kunwar AC. Tetrahedron Lett. 2004;45:3493–3497. [Google Scholar]
- 61.Denmark SE, Thorarensen A. Chem Rev. 1996;96:137–165. doi: 10.1021/cr940277f. [DOI] [PubMed] [Google Scholar]
- 62.Denmark SE, Hurd AR. J Org Chem. 1998;63:3045–3050. [Google Scholar]
- 63.Denmark SE, Hurd AR. J Org Chem. 2000;65:2875–2886. doi: 10.1021/jo991680v. [DOI] [PubMed] [Google Scholar]
- 64.a) Denmark SE, Middleton DS. J Org Chem. 1998;63:1604–1618. [Google Scholar]; b) Denmark SE, Thorarensen A. J Am Chem Soc. 1997;119:125–137. [Google Scholar]; c) Denmark SE, Dixon JA. J Org Chem. 1997;62:7086–7087. doi: 10.1021/jo9713215. [DOI] [PubMed] [Google Scholar]
- 65.Denmark SE, Thorarensen A, Middleton DS. J Am Chem Soc. 1996;118:8266–8277. [Google Scholar]
- 66.Denmark SE, Gomez L. Org Lett. 2001;3:2907–2910. doi: 10.1021/ol016385n. [DOI] [PubMed] [Google Scholar]
- 67.Uittenbogaard RM, Seerden JPG, Scheeren HW. Tetrahedron. 1997;53:11929–11936. [Google Scholar]
- 68.Kuster GJT, Steeghs RHJ, Scheeren HW. Eur J Org Chem. 2001:553–560. [Google Scholar]
- 69.Avalos M, Babiabo R, Cintas P, Higes FJ, Jiménez JL, Palacios JC, Silva MA. J Org Chem. 1996;61:1880–1882. doi: 10.1021/jo951728e. [DOI] [PubMed] [Google Scholar]
- 70.Avalos M, Babiabo R, Cintas P, Higes FJ, Jiménez JL, Palacios JC, Silva MA. J Org Chem. 1999;64:1494–1502. doi: 10.1021/jo981921j. [DOI] [PubMed] [Google Scholar]
- 71.a) Harmata M, Gamlath CB. J Org Chem. 1988;53:6154–6156. [Google Scholar]; b) Harmata M, Gamlath CB, Barnes CL. Tetrahedron Lett. 1990;31:5981–5984. [Google Scholar]
- 72.Harmata M, Fletcher VR, Claassen RJ., II J Am Chem Soc. 1991;113:9861–9862. [Google Scholar]
- 73.a) Hu Y, Ou L, Bai D. Tetrahedron Lett. 1999;40:545–548. [Google Scholar]; b) Ou L, Hu Y, Song G, Bai D. Tetrahedron. 1999;55:13999–14004. [Google Scholar]
- 74.Aggarwal VK, Fang Gy, Charmant JPH, Meek G. Org Lett. 2003;5:1757–1760. doi: 10.1021/ol034404i. [DOI] [PubMed] [Google Scholar]
- 75.Majumdar KC, Kundu UK, Ghosh S. Tetrahedron. 2002;58:10309–10313. [Google Scholar]
- 76.Jacobsen EJ, Levin J, Overman LE. J Am Chem Soc. 1988;110:4329–4336. [Google Scholar]
- 77.Overman LE, Kakimoto Ma. J Am Chem Soc. 1979;101:1310–1312. [Google Scholar]
- 78.For an early example involving an aza-Cope / vinylogous Mannich cascade in the synthesis of yohimbone, see Benson W, Winterfeldt E. Chem Ber. 1979;112:1913–1915.
- 79.Overman LE, Robertson GM, Robichaud AJ. J Am Chem Soc. 1991;113:2598–2610. [Google Scholar]
- 80.Fevig JM, Marquis RW, Overman LE. J Am Chem Soc. 1991;113:5085–5086. [Google Scholar]
- 81.Overman LE, Shim J. J Org Chem. 1991;56:5005–5007. [Google Scholar]
- 82.Aron ZD, Overman LE. Org Lett. 2005;7:913–916. doi: 10.1021/ol047330z. [DOI] [PubMed] [Google Scholar]
- 83.Marino JP, Neisser M. J Am Chem Soc. 1981;103:7687–7689. [Google Scholar]
- 84.a) Marino JP, de la Pradilla RF, Laborde E. Synthesis. 1987:1088–1091. [Google Scholar]; b) Marino JP, de la Pradilla RF. Tetrahedron Lett. 1985;26:5381–5384. [Google Scholar]; c) Ferreira JTB, Marques JA, Marino JP. Tetrahedron Asymmetry. 1994;5:641–648. [Google Scholar]
- 85.Marino JP, Rubio MB, Cao G, de Dios A. J Am Chem Soc. 2002;124:13398–13399. doi: 10.1021/ja026357f. [DOI] [PubMed] [Google Scholar]
- 86.Kawaskaki T, Terashima R, Sakaguchi Ke, Sekiguchi H, Sakamoto M. Tetrahedron Lett. 1996;37:7525–7528. [Google Scholar]
- 87.Kawaskaki T, Ogawa A, Terashima R, Saheki T, Ban N, Sekiguchi H, Sakaguchi Ke, Sakamoto M. J Org Chem. 2005;70:2957–2966. doi: 10.1021/jo040289t. [DOI] [PubMed] [Google Scholar]
- 88.Schobert R, Gordon GJ. Current Organic Chemistry. 2002;6:1181–1196. [Google Scholar]
- 89.a) Martinková M, Gonda J. Tetrahedron Lett. 1997;38:875–878. [Google Scholar]; b) Gonda J, Martinková M, Imrich J. Tetrahedron. 2002;58:1611–1616. [Google Scholar]
- 90.Kamila S, Kukherjee C, De A. Synlett. 2003:1479–1481. [Google Scholar]
- 91.Léost F, Chantegrel B, Deshayes C. Tetrahedron. 1998;54:6457–6474. [Google Scholar]
- 92.Schinzer D, Langkopf E. Synle. 1994:375–377. [Google Scholar]
- 93.Schinzer D, Abel U, Jones PG. Synlett. 1997:632–634. [Google Scholar]
- 94.Langer P, Döring M. Eur J Org Chem. 2002:221–234. [Google Scholar]
- 95.Anders JT, Görls H, Langer P. Eur J Org Chem. 2004:1897–1910. [Google Scholar]
- 96.Braverman S, Freund M. Tetrahedron. 1990;46:5759–5776. [Google Scholar]
- 97.Bur SK, Martin SF. Tetrahedron. 2001;57:3221–3242. [Google Scholar]
- 98.Castellari C, Lombardo M, Pietropaolo G, Trombini C. Tetrahedron: Asymmetry. 1996;7:1059–1068. [Google Scholar]
- 99.a) Waldmann H, Braun M, Dräger M. Tetrahedron: Asymmetry. 1991;2:1231–1246. [Google Scholar]; b) Lock R, Waldmann H. Chem Eur J. 1997;3:143–151. [Google Scholar]
- 100.Kirschbaum S, Waldmann H. Tetrahedron Lett. 1997;38:2829–2832. [Google Scholar]
- 101.He F, Bo Y, Altom JD, Corey EJ. J Am Chem Soc. 1999;121:6771–6772. [Google Scholar]
- 102.For a review of the Pummerer reaction in heterocyclic synthesis, see Bur SK, Padwa A. Chem Rev. 2004;104:2401–2432. doi: 10.1021/cr020090l.
- 103.a) Padwa A, Heidelbaugh TM, Kuethe JT, McClure MS. J Org Chem. 1998;63:6778–6779. doi: 10.1021/jo981349w. [DOI] [PubMed] [Google Scholar]; b) Padwa A, Heidelbaugh TM, Kuethe JT, McClure MS, Wang Q. J Org Chem. 2002;67:5928–5937. doi: 10.1021/jo020083x. [DOI] [PubMed] [Google Scholar]
- 104.a) Padwa A, Danca DM. Org Lett. 2002;4:715–717. doi: 10.1021/ol017193v. [DOI] [PubMed] [Google Scholar]; b) Padwa A, Danca DM, Hardcastle KI, McClure MS. J Org Chem. 2002;68:929–941. doi: 10.1021/jo026471g. [DOI] [PubMed] [Google Scholar]
- 105.Hucher N, Daich A, Decroix B. Org Lett. 2000;2:1201–1204. doi: 10.1021/ol005545c. [DOI] [PubMed] [Google Scholar]
- 106.a) Padwa A, Waterson AG. Tetrahedron. 2000;56:10159–10173. [Google Scholar]; b) Padwa A, Waterson AG. Tetrahedron Lett. 1998;39:8585–8588. [Google Scholar]
- 107.Padwa A, Waterson AG. J Org Chem. 2000;65:235–244. doi: 10.1021/jo991414h. [DOI] [PubMed] [Google Scholar]
- 108.a) Cochran JE, Padwa A. Tetrahedron Lett. 1995;36:3495–3498. [Google Scholar]; b) Cochran JE, Padwa A. J Org Chem. 1995;60:3938–3939. [Google Scholar]; c) Padwa A, Cochran JE, Kappe CO. J Org Chem. 1996;61:3706–3714. doi: 10.1021/jo960295s. [DOI] [PubMed] [Google Scholar]; d) Kappe CO, Padwa A. J Org Chem. 1996;61:6166–6174. doi: 10.1021/jo9607929. [DOI] [PubMed] [Google Scholar]
- 109.Ginn JD, Lynch SM, Padwa A. Tetrahedron Lett. 2000;41:9387–9391. [Google Scholar]
- 110.Padwa A, Ginn JD, Bur SK, Eidell CK, Lynch SM. J Org Chem. 2002;64:3412–3424. doi: 10.1021/jo0111816. [DOI] [PubMed] [Google Scholar]
- 111.Bernard AM, Cadoni E, Frongia A, Piras PP, Secci F. Org Lett. 2002;4:2565–2567. doi: 10.1021/ol026199x. [DOI] [PubMed] [Google Scholar]
- 112.Overman LE. Acc Chem Res. 1992;25:352–359. [Google Scholar]
- 113.Starling SM, Vonwiller SC. Tetrahedron Lett. 1997;38:2159–2162. [Google Scholar]
- 114.González-Vera JA, García-López MT, Herranz R. Org Lett. 2004;6:2641–2644. doi: 10.1021/ol049222i. [DOI] [PubMed] [Google Scholar]
- 115.Hanessian S, Léger R. J Am Chem Soc. 1992;114:3115–3117. [Google Scholar]
- 116.a) Baker SR, Parsons AF, Pons JF, Wilson M. Tetrahedron Lett. 1998;39:7197–7200. [Google Scholar]; b) Baker SR, Burton KI, Parsons AF, Pons JF, Wilson M. J Chem Soc, Perkin Trans I. 1999:427–436. [Google Scholar]
- 117.Marion F, Courillon C, Malacria M. Org Lett. 2003;5:5095–5097. doi: 10.1021/ol036177q. [DOI] [PubMed] [Google Scholar]
- 118.Du W, Curran DP. Org Lett. 2003;5:1765–1768. doi: 10.1021/ol0344319. [DOI] [PubMed] [Google Scholar]
- 119.Flisinska-Luczak J, Lesniak S, Nazarski RB. Tetrahedron. 2004;60:8181–8188. [Google Scholar]
- 120.Clark AJ, Filik RP, Peacock JL, Thomas GH. Synlett. 1999:441–443. [Google Scholar]
- 121.Bowman WR, Clark DN, Marmon RJ. Tetrahedron Lett. 1992;33:4993–4994. [Google Scholar]
- 122.Bowman WR, Clark DN, Marmon RJ. Tetrahedron. 1994;50:1295–1310. [Google Scholar]
- 123.Bowman WR, Stephenson PT, Young AR. Tetrahedron Lett. 1995;36:5623–5626. [Google Scholar]
- 124.Crich D, Ranganathan K, Neelamkavil S, Huang X. J Am Chem Soc. 2003;125:7942–7947. doi: 10.1021/ja035639s. [DOI] [PubMed] [Google Scholar]
- 125.a) Grubbs RH, Miller SJ, Fu GC. Acc Chem Res. 1995;28:446–452. [Google Scholar]; b) Deiters A, Martin SF. Chem Rev. 2004;104:2199–2238. doi: 10.1021/cr0200872. [DOI] [PubMed] [Google Scholar]
- 126.Bassindale MJ, Hamley P, Leitner A, Harrity JPA. Tetrahedron Lett. 1999;40:3247–3250. [Google Scholar]
- 127.Wybrow RAJ, Johnson LA, Auffray B, Moran WJ, Adams H, Harrity JPA. Tetrahedron Lett. 2002;43:7851–7854. [Google Scholar]
- 128.Choi TL, Grubbs RH. Chem Commun. 2001:2648–2649. [Google Scholar]
- 129.Zuercher WJ, Hashimoto M, Grubbs RH. J Am Chem Soc. 1996;118:6634–6640. [Google Scholar]
- 130.Bushmann N, Rückert A, Blechert S. J Org Chem. 2002;67:4325–4329. doi: 10.1021/jo025589u. [DOI] [PubMed] [Google Scholar]
- 131.Zaminer J, Stapper C, Blechert S. Tetrahedron Lett. 2002;43:6739–6741. [Google Scholar]
- 132.Stapper C, Blechert S. J Org Chem. 2002;67:6456–6460. doi: 10.1021/jo025666l. [DOI] [PubMed] [Google Scholar]
- 133.a) Alexander JB, La DS, Cefalo DR, Hoveyda AH, Schrock RR. J Am Chem Soc. 1998;120:4041–4042. [Google Scholar]; b) La DS, Alexander JB, Cefalo DR, Graf DD, Hoveyda AH, Schrock RR. J Am Chem Soc. 1998;120:9720–9721. [Google Scholar]; c) La DS, Ford JG, Sattely ES, Schrock RR, Hoveyda AH. J Am Chem Soc. 1999;121:11603–11604. doi: 10.1021/ja010684q. [DOI] [PubMed] [Google Scholar]
- 134.Weatherhead GS, Ford JG, Alexanian EJ, Schrock RR, Hoveyda AH. J Am Chem Soc. 2000;122:1828–1829. [Google Scholar]
- 135.Arjona O, Csákÿ AG, Plumet J. Eur J Med Chem. 2003:611–622. [Google Scholar]
- 136.Stragies R, Blechert S. Synlett. 1998:169–170. [Google Scholar]
- 137.Arjona O, Csákÿ AG, Medel R, Plumet J. J Org Chem. 2002;67:1380–1383. doi: 10.1021/jo016000e. [DOI] [PubMed] [Google Scholar]
- 138.Rosillo M, Casarrubios L, Domínguez G, Pérez-Castells J. Org Biomol Chem. 2003;1:1450–1451. doi: 10.1039/b302277c. [DOI] [PubMed] [Google Scholar]