Abstract
Neuregulin-1 (NRG-1) is a growth factor with potent neuroprotective capacity in ischemic stroke. We recently showed that NRG-1 reduced neuronal death following transient middle cerebral artery occlusion (tMCAO) by up to 90% with an extended therapeutic window. Here, we examined the neuroprotective potential of NRG-1 using a permanent MCAO ischemia (pMCAO) rat model. NRG-1 reduced infarction in pMCAO by 50% when administered prior to ischemia. We previously demonstrated using gene expression profiling that pMCAO was associated with an exaggerated excitotoxicity response compared to tMCAO. Therefore, we examined whether co-treatment with an inhibitor of excitotoxicity would augment the effect of NRG-1 following pMCAO. Both NRG-1 and the N-methyl-D-aspartate (NMDA) antagonist MK-801 similarly reduced infarct size following pMCAO. However, combination treatment with both NRG-1 and MK-801 resulted in greater neuroprotection that either compound alone, including a 75% reduction in cortical infarction compared to control. Consistent with these findings, NRG-1 reduced neuronal death using an in vitro ischemia model and this effect was augmented by MK-801. These results demonstrate the efficacy of NRG-1 in pMCAO rat focal ischemia model. Our findings further indicate the potential clinically relevance of NRG-1 alone or as a combination strategy for treating ischemic stroke.
Keywords: apoptosis; acetylcholine receptor inducing activity (ARIA); erbB, excitotoxicity; glial growth factor (GGF); heregulin; inflammation; ischemia; neu differentiation factor (NDF); stroke
1. Introduction
The neuregulins are a family of multipotent growth factors that includes acetylcholine receptor inducing activities (ARIAs), glial growth factors (GGFs), heregulins and neu differentiation factors (NDFs) [11,19,20,25,33]. A number of recent reports from our laboratory and others have shown that administration of NRG-1 reduces delayed ischemic cortical damage following transient middle cerebral artery occlusion (tMCAO) when administered before the onset of ischemia in rats [16,30,37] or after tMCAO with an extended therapeutic window [35]. The neuroprotective effects of the single administration of NRG-1 were seen up to 2 weeks following treatment. NRG-1 was neuroprotective if administered either before or 13.5 hours after transient MCAO and resulted in a significant improvement of functional neurological outcome. NRG-1 also prevented glial activation, apoptosis and pro-inflammatory gene expression, further suggesting a role for NRG-1 in preventing delayed neuronal death following ischemia.
Many stroke investigators consider permanent MCAO (pMCAO) more ideal than tMCAO as a model for human stroke [31]. Therefore, in this study we investigated the therapeutic potential of NRG-1 in the pMCAO model. Our findings demonstrated that NRG-1 is a potent neuroprotectant in pMCAO. We also showed that simultaneous NRG-1 administration and inhibition of glutamate excitotoxicity provided enhanced neuroprotection compared to either agent alone. These findings may result in the development of novel therapeutic strategies for the treatment of stroke.
2. Results
NRG-1 reduced neuronal damage and improves neurological outcome following MCAO
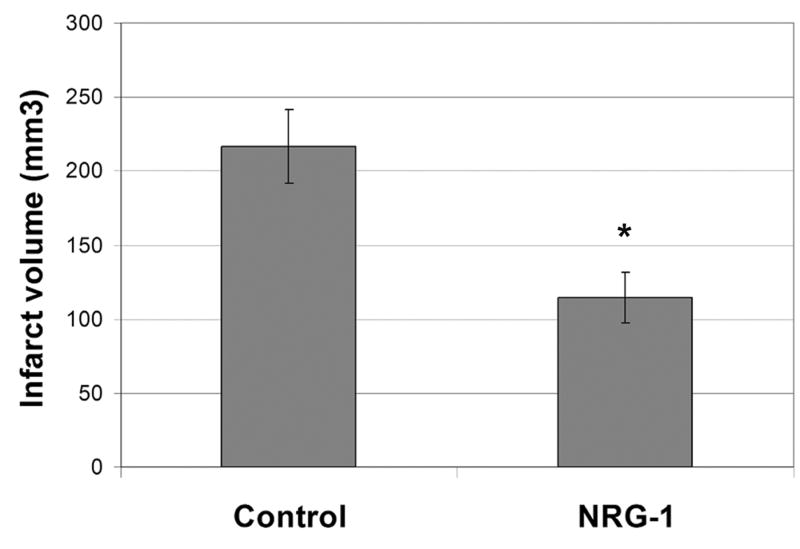
Rats were treated with NRG-1 immediately before pMCAO and sacrificed after 24 hours. Figure 1 illustrates a typical TTC staining of brain sections treated with vehicle or NRG-1 prior to pMCAO. Compared to control (Fig. 1a), pre-treatment with NRG-1 drastically reduced infarct volume after pMCAO (Fig. 1b). Infarct volume in the vehicle treated animals was 216.8 ± 25.0 mm3. NRG-1 reduced the total infarct volume by 47.2% (Fig. 2).
Figure 1.
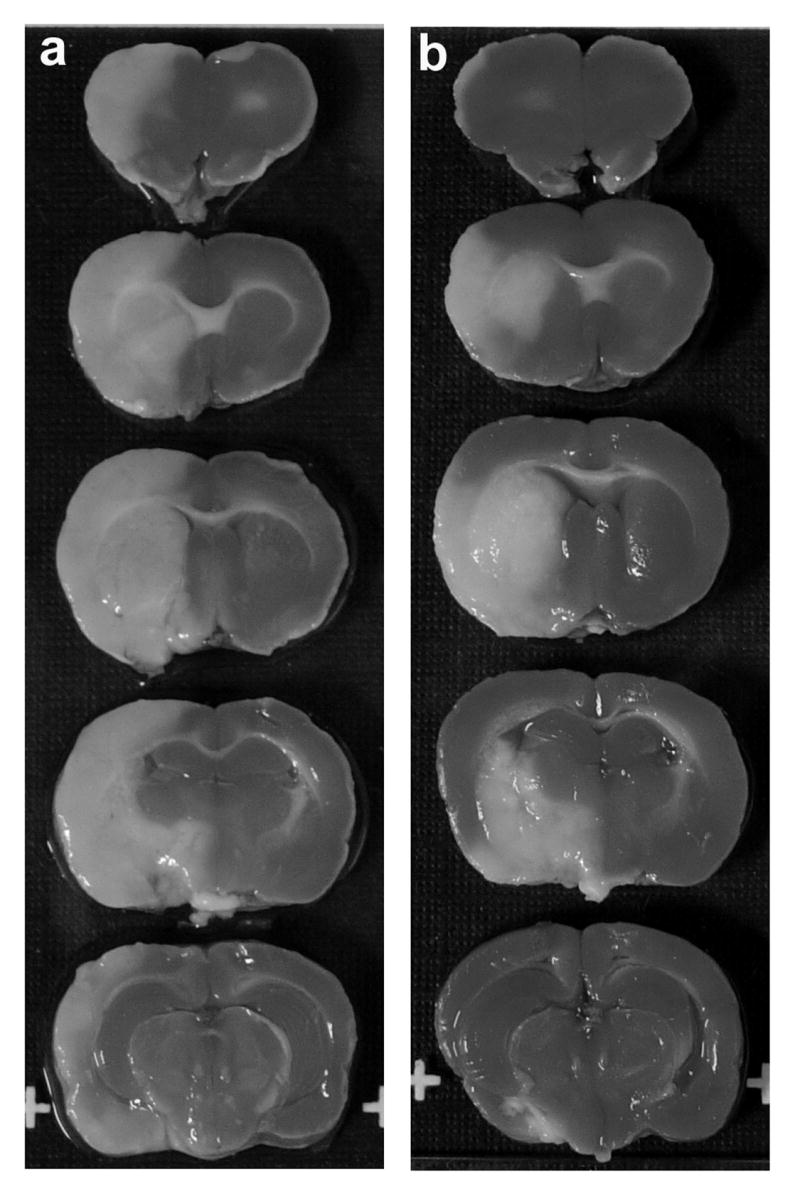
NRG-1 treatment reduces pMCAO-induced brain infarction. Representative TTC stained brain sections are shown where rats were injected with vehicle (a; n = 5), NRG-1 (b; n = 5) before pMCAO. Animals were killed 24 hours later and the brains were sliced into 2 mm sections and stained with 2,3,5-triphenyltetrazolium chloride (TTC). Infarct volumes in brains from vehicle and NRG-1 treated animals are shown in the graph (c). Values are presented as mean ± SD; * denotes significantly different from respective vehicle treated animals. (P< 0.01).
Figure 2.
NRG-1 treatment reduces pMCAO-induced brain infarction. Infarct volumes in brains from vehicle and NRG-1 treated animals are shown in the graph. Values are presented as mean ± SD; * denotes significantly different from respective vehicle treated animals. (P< 0.01).
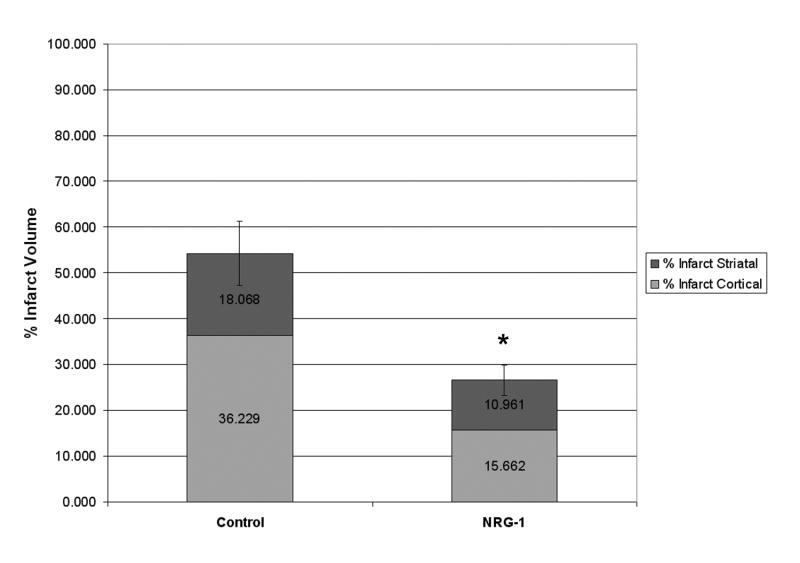
The relative reduction in cortical and subcortical neuronal death was calculated. The infarct volume of control animals represented 54.3% of the total size of the ipsilateral hemisphere. The majority of the injury was localized to cortical brain regions. Treatment with NRG-1 resulted in an infarct that was reduced to represent only 26% of the total hemisphere; a 51% reduction in infarct size. NRG-1 treatment reduced the percentage of infarction in the cerebral cortex by 57% and by 41% in subcortical regions (Fig. 3).
Figure 3.
The percentage of cortex and stratum with infarction after treatment with NRG-1 or vehicle are shown in the graph. Values are presented as mean ± SD; * denotes significantly different from respective vehicle treated animals. (P< 0.01).
Combination treatment with NRG-1 and MK-801 prevents infarction following pMCAO
We previously demonstrated that administration of NRG-1 prior to tMCAO prevented neuronal death by up to 90% following ischemia and reperfusion [34,37]. However, no further protection was conferred in the pMCAO model even when a twofold higher dose of NRG-1 was administered (data not shown). It is plausible that NRG-1 was not equally effective in the pMCAO model due to additional mechanisms that may be involved in pMCAO that are not available to NRG-1 treatment. Previous results from our laboratory using EASE software [12] showed that tMCAO/reperfusion was associated with apoptotic cell death and inflammation, which have been shown to be blocked by NRG-1 [16,36,37]. EASE identified distinct pathways upregulated in the pMCAO model that were related to excitotoxicity, including neurotransmission and ion channel function. Previous studies have shown that NRG-1 is ineffective against excitotoxic injury in neurons [21]. These results raised the possibility that co-treatment with NRG-1 and an inhibitor of excitotoxicity could have synergistic neuroprotective effects in the pMCAO model.
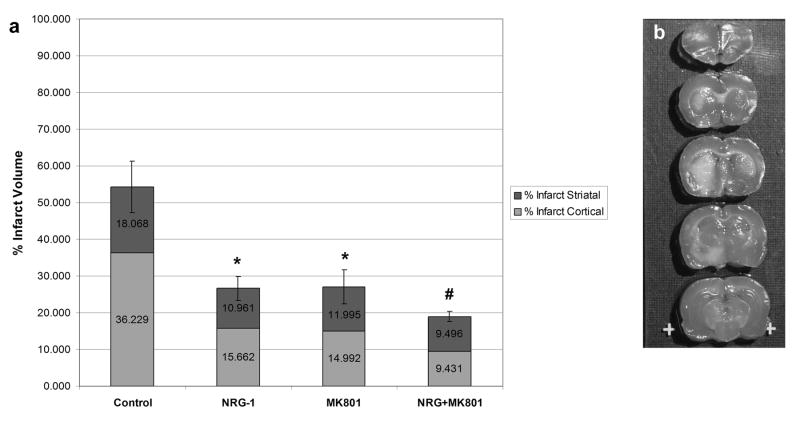
MK-801 is a potent, noncompetitive NMDA receptor antagonist that blocks the excitotoxic events of ischemia [6,26,38]. MK-801 reduces infarct size and improves neurological outcome in vitro and in animal models of cerebral ischemia. Compared to controls, MK-801 reduced neuronal death when administered before pMCAO to similar levels seen with NRG-1 treatment (Fig. 4a). NRG-1 and MK-801 both reduced the infarct size by 50%. However, combination treatment with both NRG-1 and MK-801 resulted in greater neuroprotection that either compound alone. The co-treatment of NRG-1 and MK-801 reduced the percentage of infarction to 35%, representing a 30% improvement over the response to either compound alone. Both NRG-1 and MK-801 similarly decreased cortical and subcortical infarction. Interestingly, combination treatment with NRG-1 and MK-801 resulted in an enhancement of cortical neuroprotection but no significant improvement in subcortical neuronal survival. Figure 4b is a representative TTC labeled brain showing how co-treatment with NRG-1 and MK-801 dramatically reduced cortical infarction following pMCAO.
Figure 4.
Combination treatment with NRG-1 and MK-801 results in enhanced neuroprotection following pMCAO. The percentage of cortex and stratum with infarction are shown in the graph (a) for control animals (n = 5), and animals treated with NRG-1 (n = 5), MK-801 (n = 5) or NRG + MK-801 (n = 5). Values are presented as mean ± SD; * denotes significantly different from respective vehicle treated animals. (P< 0.01); # denotes significantly treatment with NRG-1 or MK-801 alone. (P< 0.05). Representative TTC stained brain sections are shown where rats were injected with NRG-1 + MK-801 (b) before MCAO.
NRG-1 and MK-801 protect neurons from in vitro ischemia
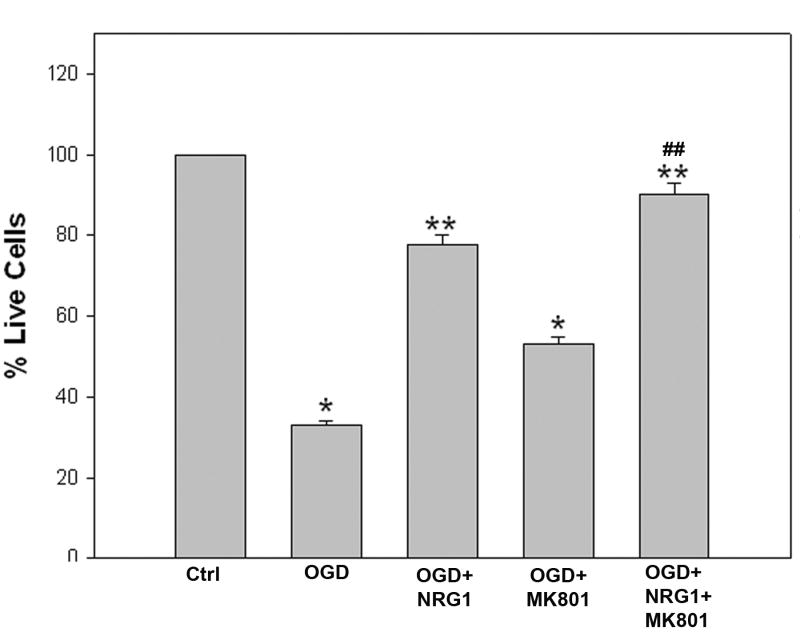
We also examined the neuroprotective effect of NRG-1 in combination with the NMDA receptor antagonist MK-801 in vitro. Rat B35 neuronal cultures were used to determine whether NRG-1 and MK-801 protected the cells from in vitro ischemia (OGD). Exposure of neurons to OGD resulted in a significant reduction in cell viability (Figure 5). Pre-treatment of cells NRG-1 resulted in a 2-3 fold increase in the number surviving cells following OGD. Treatment of neurons with MK-801 resulted in neuroprotection but was only half as effective as NRG-1. Neuroprotection by NRG-1 was enhanced by MK-801 in OGD-induced neuronal death, similar to in vivo findings. Neurons treated with both NRG-1 and MK-801 survived to near the levels seen in normoxic cultures.
Figure 5.
NRG-1 increases survival after ischemia in vitro. B-35 cells were subjected to 60 minutes of OGD 23 hours of reoxygenation. Cultures were treated with NRG-1, MK-801, NRG-1 + MK-801 or the vehicle control (CTRL) immediately before reoxygenation. Viability was measured using the live/dead assay. The graph illustrates the quantitative analysis of cell survival following the treatments. Each experiment was performed three or more times in triplicate. Values are represented as means ± SD; * denotes significantly different from control, ** denotes significantly different from OGD, ## denotes significantly different from OGD+NRG1, (p<0.05).
3. Discussion
Neuroprotective compounds have largely failed in clinical trials, so the development of novel strategies is crucial to combat the devastating effects and costs associated with stroke. The only approved therapy for acute stroke, thrombolysis, has been proven effective, but only in a limited patient population. The present study demonstrates that NRG-1 is capable of inhibiting neuronal damage in a rat pMCAO stroke model. We also show that combination treatment with NRG-1 and MK-801 provides enhanced neuroprotection from ischemia in the pMCAO model.
Previous findings from our laboratory and others demonstrated that NRG-1 reduced ischemic brain injury in a tMCAO model by up to 90% [16,30,35,37]. Several clinicians and scientists consider permanent MCAO (pMCAO) more ideal than tMCAO as a model for human stroke [31]. Therefore, in this study we examine the neuroprotective potential of NRG-1 using the pMCAO model. Our findings demonstrated that NRG-1 is a potent neuroprotectant in pMCAO as well as tMCAO. While NRG-1 is also neuroprotective in the pMCAO model, the level of protection was not as great as that seen in the tMCAO model even when higher concentrations of NRG-1 were administered. We therefore investigated whether that differences in the effectiveness of NRG-1 in the two models was due to distinct mechanisms that may be involved in pMCAO that could not be regulated by NRG-1. Previous results from our laboratory using microarray and EASE analysis software [12], identified distinct pathways upregulated in the pMCAO model that were related to excitotoxicity. Reperfusion following MCAO was associated with apoptotic cell death and inflammation. We and others have shown that neuroprotection by NRG-1 involves the inhibition of apoptotic and pro-inflammatory responses [16,30,36,37]. NRG-1 prevented mononuclear infiltration, astrocyte activation and cytokine production, which are known to be associated with delayed neuronal death following ischemia [36,37]. Since NRG-1 has been shown be ineffective against excitotoxic injury in neurons [21], we examined whether co-treatment with NRG-1 and an inhibitor of excitotoxicity could have synergistic neuroprotective effects in the pMCAO model. Indeed, the combination of NRG-1 and MK-801 provided enhanced neuroprotective compared to either compound alone as observed in both in vivo and in vitro studies. Blocking NMDA receptors in OGD has been shown to prevent glutamate-mediated excitotoxic, necrotic neuronal death, resulting in a shift from necrotic death to an apoptotic form of cell death in neuronal cultures [18]. This may therefore increase the effectiveness of NRG-1 by switching neurons to an apoptotic fate.
In additional to blocking distinct cellular mechanisms, it is plausible that there may be crosstalk between NRG-1 and NMDA receptor mediated pathways. In fact, NRG-1 was shown to specifically increase the expression of mRNA for the NR2C NMDA receptor subunit by more than 100-fold [27]. ErbB receptors, particularly erbB4, co-localizes with PSD-95 and NMDA receptors at postsynaptic densities and has been implicated in activity-dependent plasticity and synaptogenesis [13,28]. Overexpression of erbB4 enhanced AMPA currents and increased dendritic spine size, while reduced NRG1/erbB4 signaling resulted in glutamatergic hypofunction with a loss of NMDA currents and dendritic spines [23]. Direct effects of NRG-1 on NMDA receptor activity has been demonstrated where bath application of NRG-1 to acutely isolated and cultured pyramidal neurons resulted in a significant reduction of NMDA receptor currents [15].
Because there are multiple mechanisms involved in neuronal injury after ischemia, it is appropriate to consider using pharmacological agents that affect multiple mechanisms simultaneously or sequentially (for reviews, [4,9,22,24]). Therapeutic strategies have been designed typically to affect only one of several mechanisms in the ischemic cascade, but combinatorial therapies are likely to be more effective than monotherapy, as in the treatment of HIV/AIDS [10,14]. This has sometimes been referred to as the “cocktail” approach [1,39]. With the use of multiple neuroprotective therapies, each agent could be given either simultaneously or in rapid succession, allowing each agent to work on a different ischemic injury mechanism [2,3,5,7,8,17,26,29,32]. Therefore we suggest that a “combotemporal stroke therapy”, such as the combined use of a neuroprotective or anti-inflammatory agent and an excitatory amino acid antagonist simultaneously or in sequence, may provide greater neuroprotection than either agent alone. In addition, the effectiveness of mono- or combination therapy in a pMCAO models could provide treatments that circumvent the need for t-PA or extend the therapeutic window for thrombolytic treatment.
4. Experimental Procedures
Middle cerebral artery occlusion
All surgical procedures were performed by sterile/aseptic techniques in accordance with institutional guidelines. Adult male Sprague-Dawley rats weighing 250-300 g were used for this study. Animals were subjected to left MCA occlusion. Rats were anesthetized with a ketamine/xylazine solution (10mg/kg, IP). MCA occlusion was induced by the intraluminal suture MCAO method as previously described [12,35]. Briefly, the left common carotid artery (CCA) was exposed through a midline incision and was carefully dissected free from surrounding nerves and fascia. The occipital artery branches of the external carotid artery (ECA) were then isolated, and the occipital artery and superior thyroid artery branches of the ECA were coagulated. The ECA was dissected further distally. The internal carotid artery (ICA) was isolated and carefully separated from the adjacent vagus nerve, and the pterygopalatine artery was ligated close to its origin with a 6-0 silk suture. Then, a 40 mm 3-0 surgical monofilament nylon suture (Harvard Apparatus, Holliston, Massachusetts) was coated with poly-L-lysine with its tip rounded by heating near a flame or with rubber silicone. The filament was inserted from the external carotid artery (ECA) into the internal carotid artery (ICA) and then into the circle of Willis to occlude the origin of the left middle cerebral artery. The suture was inserted 18 to 20 mm from the bifurcation of the CCA to occlude the MCA. In pMCAO, the suture was left in place for 24 hours prior to sacrificing the animal. In tMCAO model, the nylon suture was withdrawn 1.5 hours following ischemia and the brain tissues were reperfused for 22.5 hours before sacrificing. To determine the effects of NRG-1 on ischemic stroke, rats were injected intra-arterially with a single bolus 10 μl dose of vehicle (% BSA in PBS) or NRG-1β ( nmol/L NRG-1 (EGF-like domain, R&D Systems, Minneapolis, Minnesota) in 1% BSA in PBS) through a Hamilton syringe as previously described [37]. This resulted in the administration of 2-3 µg of NRG-1/kg body weight. NRG-1 (n = 5) or vehicle (PBS; n = 5) was administered by bolus injection into the ICA through ECA immediately before MCAO. MK-801 (0.5 mg/kg; n = 5) was administered IA alone or simultaneously with NRG-1 (n = 5). All NRG-1 and vehicle treatment studies were performed in a blinded manner. Core body temperature was monitored with a rectal probe and maintained at 37 °C with a Homeothermic Blanket Control Unit (Harvard Apparatus) during anesthesia.
Measurement of infarct formation
Twenty four hours after MCAO, the animals were killed and the brain tissue was removed and sliced into 2.0 mm-thick sections. Brain slices were incubated in a 2% triphenyltetrazolium chloride (TTC) solution for 10 minutes at 37°C and then transferred into a 4% formaldehyde solution for fixation. TTC, a colorless salt, is reduced to form an insoluble red formazan product in the presence of a functioning mitochondrial electron transport chain. Thus, the infarcted region lacks staining and appears white, whereas the normal non-infarcted tissue appears red. Infarct area of four slices of 2 mm coronal sections of each brain was calculated in a blinded manner by capturing the images with a digital camera. Infarct volumes were analyzed by ANOVA; P<0.05 was regarded as significant.
Neuronal cultures and oxygen-glucose deprivation
B35 rat neuroblastoma cells (ATCC, Manassas, VA) were cultured in growth medium composed of Dulbecco’s modification of eagle’s medium, 2 mM L-glutamine, 10% fetal bovine serum (FBS) and 50U penicillin and maintained at 37° C in an atmosphere of 5% CO2. Cells were differentiated by removal of serum from growth medium 2 days prior to treatment. To create in vitro ischemic conditions, cultures were washed twice in phosphate buffered saline, then incubated in a balanced salt solution (BSS: 116 mM NaCl, 5.4 mM KCl, 0.8 mM MgSO4, 1.0 mM NaH2PO4, 26.2 Mm NaHCO3, 1.8 mM CaCl2, 0.01 mM glycine, and 10 mg/L phenol red) lacking glucose and placed in a hypoxic atmosphere (1% O2/5% CO2). Cultures were subjected to OGD for 90 minutes then returned to normoxic conditions. NRG-1 and MK-801 were prepared as 1000x stock solutions in PBS. After OGD, cultures were treated with 10nM NRG-1 ± 10μM MK-801 or vehicle (PBS) and returned to normoxic conditions (reoxygenation) for 22.5 hours. Control samples were placed in a balanced salt solution with 20mM D-glucose. Each experiment was performed three or more times in triplicate. Viability assays were performed using a LIVE/DEAD Viability/Cytotoxicity Kit (Invitrogen, Carlsbad, California) according to the manufacturers’ protocols.
Acknowledgments
This work was supported by NIH grants NS34194 and NS056446, an NSF Center for Behavioral Neuroscience Cooperative Agreement (#IBN-9876754) and the W.M. Keck Foundation. The investigation was conducted in a facility constructed with support from Research Facilities Improvement Grant #C06 RR-07571 from the National Center for Research Resources, NIH
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Andrews RJ. Neuroprotection for the new millennium. Matchmaking pharmacology and technology. Ann N Y Acad Sci. 2001;939:114–25. doi: 10.1111/j.1749-6632.2001.tb03618.x. [DOI] [PubMed] [Google Scholar]
- 2.Aronowski J, Strong R, Shirzadi A, Grotta JC. Ethanol plus caffeine (caffeinol) for treatment of ischemic stroke: preclinical experience. Stroke. 2003;34:1246–51. doi: 10.1161/01.STR.0000068170.80517.B3. [DOI] [PubMed] [Google Scholar]
- 3.Aspey BS, Alp MS, Patel Y, Harrison MJ. Effects of combined glutamate and platelet-activating factor inhibition on the outcome of focal cerebral ischaemia - an initial screening study. Metab Brain Dis. 1997;12:237–49. [PubMed] [Google Scholar]
- 4.Barone FC, Feuerstein GZ. Inflammatory mediators and stroke: new opportunities for novel therapeutics. J Cereb Blood Flow Metab. 1999;19:819–34. doi: 10.1097/00004647-199908000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Belayev L, Khoutorova L, Zhang Y, Belayev A, Zhao W, Busto R, Ginsberg MD. Caffeinol confers cortical but not subcortical neuroprotection after transient focal cerebral ischemia in rats. Brain Res. 2004;1008:278–83. doi: 10.1016/j.brainres.2004.02.039. [DOI] [PubMed] [Google Scholar]
- 6.Bertorelli R, Adami M, Di Santo E, Ghezzi P. MK 801 and dexamethasone reduce both tumor necrosis factor levels and infarct volume after focal cerebral ischemia in the rat brain. Neurosci Lett. 1998;246:41–4. doi: 10.1016/s0304-3940(98)00221-3. [DOI] [PubMed] [Google Scholar]
- 7.Culmsee C, Junker V, Kremers W, Thal S, Plesnila N, Krieglstein J. Combination therapy in ischemic stroke: synergistic neuroprotective effects of memantine and clenbuterol. Stroke. 2004;35:1197–202. doi: 10.1161/01.STR.0000125855.17686.6d. [DOI] [PubMed] [Google Scholar]
- 8.Ding G, Jiang Q, Zhang L, Zhang ZG, Li L, Knight RA, Ewing JR, Wang Y, Chopp M. Analysis of combined treatment of embolic stroke in rat with r-tPA and a GPIIb/IIIa inhibitor. J Cereb Blood Flow Metab. 2005;25:87–97. doi: 10.1038/sj.jcbfm.9600010. [DOI] [PubMed] [Google Scholar]
- 9.Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–7. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 10.Dybul M, Fauci AS, Bartlett JG, Kaplan JE, Pau AK. Guidelines for using antiretroviral agents among HIV-infected adults and adolescents. Ann Intern Med. 2002;137:381–433. doi: 10.7326/0003-4819-137-5_part_2-200209031-00001. [DOI] [PubMed] [Google Scholar]
- 11.Falls DL, Rosen KM, Corfas G, Lane WS, Fischbach GD. ARIA, a protein that stimulates acetylcholine receptor synthesis, is a member of the neu ligand family. Cell. 1993;72:801–15. doi: 10.1016/0092-8674(93)90407-h. [DOI] [PubMed] [Google Scholar]
- 12.Ford G, Xu Z, Gates A, Jiang J, Ford BD. Expression Analysis Systematic Explorer (EASE) analysis reveals differential gene expression in permanent and transient focal stroke rat models. Brain Res. 2006;1071:226–36. doi: 10.1016/j.brainres.2005.11.090. [DOI] [PubMed] [Google Scholar]
- 13.Garcia RA, Vasudevan K, Buonanno A. The neuregulin receptor ErbB-4 interacts with PDZ-containing proteins at neuronal synapses. Proc Natl Acad Sci U S A. 2000;97:3596–601. doi: 10.1073/pnas.070042497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gladstone DJ, Black SE, Hakim AM. Toward wisdom from failure: lessons from neuroprotective stroke trials and new therapeutic directions. Stroke. 2002;33:2123–36. doi: 10.1161/01.str.0000025518.34157.51. [DOI] [PubMed] [Google Scholar]
- 15.Gu Z, Jiang Q, Fu AK, Ip NY, Yan Z. Regulation of NMDA receptors by neuregulin signaling in prefrontal cortex. J Neurosci. 2005;25:4974–84. doi: 10.1523/JNEUROSCI.1086-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo WP, Wang J, Li RX, Peng YW. Neuroprotective effects of neuregulin-1 in rat models of focal cerebral ischemia. Brain Res. 2006;1087:180–5. doi: 10.1016/j.brainres.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 17.Gupta S, Kaul CL, Sharma SS. Neuroprotective effect of combination of poly (ADP-ribose) polymerase inhibitor and antioxidant in middle cerebral artery occlusion induced focal ischemia in rats. Neurol Res. 2004;26:103–7. doi: 10.1179/016164104773026624. [DOI] [PubMed] [Google Scholar]
- 18.Gwag BJ, Lobner D, Koh JY, Wie MB, Choi DW. Blockade of glutamate receptors unmasks neuronal apoptosis after oxygen-glucose deprivation in vitro. Neuroscience. 1995;68:615–9. doi: 10.1016/0306-4522(95)00232-8. [DOI] [PubMed] [Google Scholar]
- 19.Ho WH, Armanini MP, Nuijens A, Phillips HS, Osheroff PL. Sensory and motor neuron-derived factor. A novel heregulin variant highly expressed in sensory and motor neurons. J Biol Chem. 1995;270:26722. [PubMed] [Google Scholar]
- 20.Holmes WE, Sliwkowski MX, Akita RW, Henzel WJ, Lee J, Park JW, Yansura D, Abadi N, Raab H, Lewis GD, et al. Identification of heregulin, a specific activator of p185erbB2. Science. 1992;256:1205–10. doi: 10.1126/science.256.5060.1205. [DOI] [PubMed] [Google Scholar]
- 21.Krainock R, Murphy S. Heregulin upregulates the expression of nitric oxide synthase (NOS)-1 in rat cerebellar granule neurons via the ErbB4 receptor. J Neurochem. 2001;76:312–5. doi: 10.1046/j.1471-4159.2001.00089.x. [DOI] [PubMed] [Google Scholar]
- 22.Lee JM, Grabb MC, Zipfel GJ, Choi DW. Brain tissue responses to ischemia. J Clin Invest. 2000;106:723–31. doi: 10.1172/JCI11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li B, Woo RS, Mei L, Malinow R. The neuregulin-1 receptor erbB4 controls glutamatergic synapse maturation and plasticity. Neuron. 2007;54:583–97. doi: 10.1016/j.neuron.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- 25.Marchionni MA, Goodearl AD, Chen MS, Bermingham-McDonogh O, Kirk C, Hendricks M, Danehy F, Misumi D, Sudhalter J, Kobayashi K, et al. Glial growth factors are alternatively spliced erbB2 ligands expressed in the nervous system. Nature. 1993;362:312–18. doi: 10.1038/362312a0. [DOI] [PubMed] [Google Scholar]
- 26.Onal MZ, Li F, Tatlisumak T, Locke KW, Sandage BW, Jr, Fisher M. Synergistic effects of citicoline and MK-801 in temporary experimental focal ischemia in rats. Stroke. 1997;28:1060–5. doi: 10.1161/01.str.28.5.1060. [DOI] [PubMed] [Google Scholar]
- 27.Ozaki M, Sasner M, Yano R, Lu HS, Buonanno A. Neuregulin-beta induces expression of an NMDA-receptor subunit. Nature. 1997;390:691–4. doi: 10.1038/37795. [DOI] [PubMed] [Google Scholar]
- 28.Ozaki M, Tohyama K, Kishida H, Buonanno A, Yano R, Hashikawa T. Roles of neuregulin in synaptogenesis between mossy fibers and cerebellar granule cells. J Neurosci Res. 2000;59:612–23. doi: 10.1002/(SICI)1097-4547(20000301)59:5<612::AID-JNR4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 29.Scholler K, Zausinger S, Baethmann A, Schmid-Elsaesser R. Neuroprotection in ischemic stroke--combination drug therapy and mild hypothermia in a rat model of permanent focal cerebral ischemia. Brain Res. 2004;1023:272–8. doi: 10.1016/j.brainres.2004.01.094. [DOI] [PubMed] [Google Scholar]
- 30.Shyu WC, Lin SZ, Chiang MF, Yang HI, Thajeb P, Li H. Neuregulin-1 reduces ischemia-induced brain damage in rats. Neurobiol Aging. 2004;25:935–44. doi: 10.1016/j.neurobiolaging.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 31.STAIR. Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke. 1999;30:2752–8. doi: 10.1161/01.str.30.12.2752. [DOI] [PubMed] [Google Scholar]
- 32.Toung TJ, Chen TY, Littleton-Kearney MT, Hurn PD, Murphy SJ. Effects of combined estrogen and progesterone on brain infarction in reproductively senescent female rats. J Cereb Blood Flow Metab. 2004;24:1160–6. doi: 10.1097/01.WCB.0000135594.13576.D2. [DOI] [PubMed] [Google Scholar]
- 33.Wen D, Peles E, Cupples R, Suggs SV, Bacus SS, Luo Y, Trail G, Hu S, Silbiger SM, Levy RB, et al. Neu differentiation factor: a transmembrane glycoprotein containing an EGF domain and an immunoglobulin homology unit. Cell. 1992;69:559–72. doi: 10.1016/0092-8674(92)90456-m. [DOI] [PubMed] [Google Scholar]
- 34.Xu Z, Croslan DR, Harris AE, Ford GD, Ford BD. Extended therapeutic window and functional recovery after intraarterial administration of neuregulin-1 after focal ischemic stroke. J Cereb Blood Flow Metab. 2005 August 31; doi: 10.1038/sj.jcbfm.9600212. advance online publication. [DOI] [PubMed] [Google Scholar]
- 35.Xu Z, Croslan DR, Harris AE, Ford GD, Ford BD. Extended therapeutic window and functional recovery after intraarterial administration of neuregulin-1 after focal ischemic stroke. J Cereb Blood Flow Metab. 2006;26:527–35. doi: 10.1038/sj.jcbfm.9600212. [DOI] [PubMed] [Google Scholar]
- 36.Xu Z, Ford GD, Croslan DR, Jiang J, Gates A, Allen R, Ford BD. Neuroprotection by neuregulin-1 following focal stroke is associated with the attenuation of ischemia-induced pro-inflammatory and stress gene expression. Neurobiol Dis. 2005;19:461–70. doi: 10.1016/j.nbd.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 37.Xu Z, Jiang J, Ford G, Ford BD. Neuregulin-1 is neuroprotective and attenuates inflammatory responses induced by ischemic stroke. Biochem Biophys Res Commun. 2004;322:440–6. doi: 10.1016/j.bbrc.2004.07.149. [DOI] [PubMed] [Google Scholar]
- 38.Yang G, Chan PH, Chen SF, Babuna OA, Simon RP, Weinstein PR. Reduction of vasogenic edema and infarction by MK-801 in rats after temporary focal cerebral ischemia. Neurosurgery. 1994;34:339–45. doi: 10.1227/00006123-199402000-00018. discussion 345. [DOI] [PubMed] [Google Scholar]
- 39.Zhou H, Tong Z, McLeod JF. “Cocktail” approaches and strategies in drug development: valuable tool or flawed science? J Clin Pharmacol. 2004;44:120–34. doi: 10.1177/0091270003261333. [DOI] [PubMed] [Google Scholar]