Abstract
We used a propagation-defective, single-cycle, alphavirus replicon vector system to produce virus-like replicon particles (VRP) expressing the hemagglutinin (HA) and neuraminidase (NA) proteins from influenza A/Wyoming/03/2003 (H3N2). Efficient production methods were scaled to produce pilot lots of HA VRP and NA VRP and clinical lots of HA VRP. HA VRP-induced high-titered antibody responses in mice, rabbits and rhesus macaques, as measured by ELISA or hemagglutination inhibition (HI) assays, and robust cellular immune responses in mice and rhesus macaques, as measured by IFN-γ ELISPOT. NA VRP also induced cellular immune responses in mice. A toxicology study with HA VRP and NA VRP in rabbits showed no adverse effects in any parameter. These studies support clinical testing of alphavirus replicon vaccines for influenza.
Keywords: Influenza vaccine, Alphavirus replicon vaccine, Cellular immunity
1. Introduction
Influenza is an acute, usually self-limited febrile illness characterized by systemic and respiratory signs and symptoms and caused by infection with influenza virus type A or B. Continuous evolution of the hemagglutinin (HA) and neuraminidase (NA) genes encoding major virion surface antigens leads to minor antigenic differences from year-to-year (antigenic drift) and major antigenic differences that lead to global pandemics (antigenic shift). During inter-pandemic periods, influenza transmission occurs every year and antigenic drift contributes to epidemics of influenza A that occur every few years and are associated with excess mortality related to influenza-associated pneumonia in the very young and the elderly [1]. Existing influenza vaccines provide substantial protection when administered prior to exposure, and protective immunity is generally considered to be primarily dependent on neutralizing antibodies directed against HA [2,3], although there is evidence that antibodies against NA can also confer protection [4,5]. The only vaccines available for widespread use are inactivated vaccines prepared from influenza viruses grown in embryonated eggs, but their supply is limited, in large part by a paucity of specific pathogen-free eggs, and the need for new approaches to influenza vaccines is well recognized [6].
A large number of alternatives to inactivated influenza vaccines have been studied, including recombinant proteins [7,8], poxvirus vectors [9], DNA vaccines [10], immunostimulatory complexes [11], proteosome vaccines [12] and live attenuated vaccines [13,14]. In the present study, we describe the construction, process development and preclinical evaluation of a propagation-defective, single-cycle, alphavirus replicon vaccine for influenza that is suitable for clinical testing.
2. Materials and methods
2.1. Plasmid construction
Alphavirus replicon plasmids containing the HA or NA gene from the A/Wyoming/3/2003 (H3N2) strain of influenza virus (CDC# 2003714420), under control of an EV71 IRES, were constructed as described by Kamrud et al. [15]. The HA and NA genes were amplified by reverse transcriptase-polymerase chain reaction (RT-PCR) from purified viral RNA using gene-specific primers which incorporated XbaI restriction sites. The PCR products were separately subcloned into the pCDNA3.3/MS transfer plasmid. A region spanning the IRES and the HA gene was digested from the transfer plasmid using AscI enzyme and cloned into pERK spacer-replicon vectors containing spacers of different sizes. For both HA and NA, a replicon vector containing a spacer of 383 nucleotides in length upstream of an EV71 IRES was selected based on protein expression and replicon packaging titers. DNA sequencing showed no differences from the published sequences for HA and NA.
2.2. VRP production and characterization
VRP were produced using a modification of previously described methods [16,17]. Purified DNA plasmids were linearized by NotI endonuclease digestion and used as templates for in vitro RNA transcription using RNA Express T7 kits (Promega, Madison, WI). RNA was treated with DNase, purified by anion exchange chromatography followed by desalting, and stored at −80 °C until use. A Vero working cell bank, cryopreserved at passage 142, was thawed and cultured in Eagle’s minimum essential medium (EMEM) with 5% fetal bovine serum (FBS) in 175 cm2 flasks at 37 °C, 5% CO2. Culture medium was changed after 24 h and 72 h later cells were washed with phosphate buffered saline (PBS), detached by treatment with 0.05% trypsin (HyClone, Logan, UT) and transferred to 850 cm2 roller bottles. After 72 h cells were harvested, washed and re-suspended in PBS to a concentration of 1.5–2.0 × 108 cells/mL, mixed with RNA (30 µg each of replicon, capsid helper and glycoprotein helper), transferred to 0.4 cm gap cuvettes and electroporated using a Gene Pulser Xcell electroporation unit (BioRad Laboratories, Hercules, CA). Electroporated cells were resuspended in 100 mL OptiPRO SFM (Invitrogen, Carlsbad, CA) with 4 mM glutamine and cultured at 37 °C, 5% CO2 in 850 cm2 roller bottles. After 16–24 h the medium and cells were pooled and drawn into a Sartopore capsule filter (Sartorius, Edgewood, NY). Cells collected on the filter were washed with PBS and VRP recovered by washing with a high salt buffer. A portion of the salt wash material (a total of 3 × 108 infectious units) was tested in a cytopathic effect (CPE) assay to confirm the absence of detectable replication-competent virus as previously described [15]. In brief, VRP eluted by salt wash were added to Vero cell culture monolayers in T75 tissue culture flasks at a controlled multiplicity of infection (MOI) of <0.5 and incubated at 37 °C in a 5% CO2 atmosphere for 1 h. The inoculum was removed and the cells were incubated for 24 h. The cell culture supernatant from each Passage 1 flask was transferred to a fresh flask of Vero cells and incubated for 1 h, the inoculum removed and fresh culture medium added. CPE was assessed after incubation for 72 h.
The salt wash material was concentrated on a Hydrosart 100,000 molecular weight cutoff regenerated cellulose flat-sheet tangential flow filtration (TFF) membrane (Sartorius) and diafiltered against 2 M NaCl and then against PBS with 3 mM MgCl2. After treatment with Benzonase to degrade contaminating Vero DNA, VRP were diafiltered against 2 M NaCl and then 300 mM NaCl in 10 mM phosphate. The TFF pool was filtered through a 0.2 µm filter and loaded on a Cellufine Sulfate column that was sequentially washed with 250 mM NaCl and 500 mM NaCl in 10 mM phosphate. VRP were eluted with a step gradient to 800 mM NaCl in 10 mM phosphate. Purified VRP were sampled for quality control analysis and formulated as bulk vaccine in an excipient mix that included either human serum albumin (HSA), rabbit serum albumin (RSA) or normal mouse serum (NMS) to stabilize the VRP during storage at −80 °C.
VRP concentration, expressed as infectious units (IU) per mL, was determined by an immunofluorescence assay (IFA) in which serial dilutions of VRP were added to Vero cell monolayers in 48-well plates, cultured overnight, and reacted with goat antibody specific for HA or NA followed by fluorescein isothiocyanate-labeled anti-goat antibody to detect cells expressing the HA or NA protein. In some experiments, VRP were added to Vero cell monolayers in 48-well plates at a MOI of 10 IU/cell, cultured overnight, washed three times with cold PBS, lysed in 150 µL of extraction buffer (50 mM Tris–HCl, pH 7.5, 100 mM NaCl, 1 mM EDTA, 0.5% NP-40, 0.5% SDS, 1 × Complete® Protease Inhibitor (Roche, Indianapolis, IN)) and 1 µg of total protein per lane was resolved on 4–12% gradient SDS-PAGE (Invitrogen). HA and NA proteins were visualized by Western blot analysis after transfer onto PVDF membranes (BioRad), reaction with goat polyclonal antibodies specific for HA or NA followed by alkaline phosphatase (AP) conjugated anti-goat antibody, and color development using an AP conjugate substrate kit (BioRad).
2.3. Quality control testing of VRP
Various process pools were tested for residual protein, DNA and Benzonase concentrations, sodium dodecyl sulfate polyacrilamide gel electrophoresis (SDS-PAGE) and Western blot characterization, Southern blot estimation of residual Vero DNA size and quantitative polymerase chain reaction (qPCR) to determine genome equivalent concentration. Protein was measured by the bicinchoninic acid (BCA) method using a commercially available kit (Pierce Biotechnology, Rockford, IL) and bovine serum albumin (BSA) as the reference standard. DNA was measured by the picogreen method using a commercially available kit (Invitrogen). Benzonase was measured by enzyme-linked immunosorbent assay (ELISA) using a commercially available kit (EMD Chemicals, Gibbstown, NJ). SDS-PAGE was performed on NuPAGE 4–12% gradient Bis–tris gels (Invitrogen) under reducing conditions. Western blot analysis was performed as above using mouse antibodies against VEE virus envelope glycoproteins and capsid protein. For Southern blot analysis, samples were treated with proteinase K and DNA extracted on Minelute spin columns (Qiagen), separated by agarose gel electrophoresis, blotted to a nylon membrane and cross-linked. The DNA was hybridized to denatured, psoralen-biotin-labeled, AluI-digested Vero DNA, washed, reacted with streptavidin-labeled alkaline phosphatase (Ambion) and a chemiluminescent reagent, and the image captured on X-ray film. qPCR was performed on an Applied Biosystem 7500 Fast Real-Time PCR system. Ampliset primers and probes were constructed based on the nsP2 region of the replicon, and RNA was quantified during 40 cycles from a standard curve generated using standards containing from 1 × 103 to 1 × 106 copies of control RNA per 5 µL.
2.4. Immunization of mice
Groups of 6- to 7-week-old female BALB/c mice (n=6) housed at Integrated Laboratory Systems (ILS, Research Triangle Park, NC) were immunized twice with 5 × 105 IU of HA VRP or NA VRP, formulated with NMS, on study day (SD) 1 and 22, by bilateral footpad injection of 20 µL. Serum was collected on SD-1, 21 and 29 and splenic lymphocytes were collected on SD 29.
2.5. Immunization of rhesus macaques
Groups of rhesus macaques of Chinese origin were housed at Southern Research Institute (SRI, Fredrick, MD) and immunized three times, on SD 1, 29 and 162, by intramuscular (IM) injection of 0.5 mL containing 1 × 109 IU of HA VRP (n = 3) or an irrelevant VRP (n = 4) formulated with HSA. Blood for separation of serum and peripheral blood mononuclear cells (PBMC) was collected on SD 1, 15, 29, 43, 57, 71, 113, 162, 169, 176, 190 and 204.
2.6. Toxicology
A toxicology study conducted in compliance with Good Laboratory Practices (GLP) was conducted using five groups of 16 New Zealand white rabbits each (eight males and eight females) that were immunized on four occasions at 2-week intervals, on SD 1, 15, 29 and 43. Group 1 animals were treated with PBS via subcutaneous (SC) injection in the forelimb and IM injection in the hind limb. Groups 2 and 3 were treated with a mixture of HA VRP and NA VRP formulated with RSA via SC or IM injection, respectively. Groups 4 and 5 were treated with a mixture of HA VRP and NA VRP formulated with HSA via SC or IM injection, respectively. The target dosage level was a total of 1 × 109 IU per injection with equal numbers of HA VRP and NA VRP. On the day of each injection, aliquots of vaccine and placebo were shipped via overnight courier and tested to confirm that shipping and storage conditions did not result in any change in the potency or other characteristics of the products. Toxicity was evaluated by recording mortality/morbidity, body temperature, body weight, food consumption and ophthalmic examinations. Blood samples for clinical pathology (hematology, chemistry and coagulation parameters) were obtained before the first dose, on SD 3 and at termination. Serum for measurement of antibodies to influenza virus and HSA was obtained before each dose and at termination. Local reactogenicity was evaluated by examining the injection sites daily for 7 days after each injection and at termination, using a dermal Draize scoring system (none = 0, minimal = 1, mild = 2, moderate = 3, severe = 4).
Half of the animals were sacrificed 2 or 3 days after the last injection (SD 45/46) and the other half 2 weeks after the last injection (SD 57/58). A gross necropsy, which included examination of the external surface of the body, the injection/treatment sites, all orifices, the cranial, thoracic, and abdominal cavities and their contents, was conducted as soon as possible following euthanasia. The following organs (sex appropriate) were weighed as soon as possible after dissection: adrenal glands, brain, epididymides, heart, kidneys, liver, lungs (with mainstem bronchi), ovaries, spleen, testes, thymus and uterus (with cervix). All tissues recommended in the World Health Organization guidance document on non-clinical testing of vaccines [18] were collected and preserved in 10% neutral buffered formalin (NBF) with the exception of the eyes, testes, epididymides and optic nerves, which were fixed in Modified Davidson’s fixative. For all animals, the following tissues (sex appropriate) were histologically evaluated: injection site (skin and underlying muscle), draining lymph nodes (axillary lymph nodes for subcutaneous route and iliac lymph nodes for intramuscular route), mandibular and mesenteric lymph nodes, brain, heart, kidneys, liver, lung, ovaries, spleen, testes, thymus and gross lesions. For animals in Groups 1, 4 and 5, all other collected tissues were also histologically evaluated.
2.7. ELISA
ELISA was used to measure antibodies to HA. Briefly, 96-well plates were coated overnight with 50 ng of recombinant HA protein (A/Wyoming/03/2003, Protein Sciences, Meridien, CT) per well and blocked with 3% BSA in PBS. Serial two-fold dilutions of serum in PBS containing 1% BSA and 0.5% Tween 20 were added to wells and incubated at 30 °C for 1 h. Wells were washed with PBS, alkaline phosphatase-labeled goat anti-mouse, anti-rabbit or anti-human IgG was added and the plates were incubated at 30 °C for 1 h. Wells were washed and alkaline phosphatase substrate (p-nitrophenyl phosphate) added. Absorbance was measured in a VERSA max plate reader approximately every 5 min until the OD405 was ≥ the expected value in positive control wells while remaining <0.2 in negative control wells. Serum endpoint titer was defined as the reciprocal of the maximal dilution at which OD405 was ≥0.2 and the OD405 for the next highest dilution was <0.2.
2.8. HI assay
Antibodies to influenza virus were measured by hemagglutination inhibition (HI) assay. Briefly, sera were treated with receptor-destroying enzyme from Vibrio cholerae (Denka Seiken, Campbell, CA) overnight at 37 °C, heat inactivated for 60–90 min at 56 °C, adsorbed with 2% turkey erythrocytes (CBT Farms, Chestertown, MD) for 1 h at 2–8 °C and centrifuged at 1000 × g for 5 min. Supernatants were then serially diluted in V-shaped well microtiter plates in a final volume of 25 µL. An equal volume containing 4 agglutinating units of MDCK cell-grown influenza A/Wyoming/3/2003 virus (seed virus obtained from CDC, Atlanta, GA) was added, plates were incubated at room temperature for 45 min before adding 50 µL of 0.5% turkey erythrocytes in PBS, and HI titers were read after 30–60 min. Titer was defined as the reciprocal of the maximal dilution at which hemagglutination was inhibited.
2.9. Ouchterlony assay
Because data from previous toxicology studies indicated that VRP formulated in HSA sometimes induced an Arthus-type reaction, antibodies to HSA were measured by Ouchterlony assay. Five mL of 1% agarose (Fisher, Fairlawn, NJ) in PBS was added to 60 mm Petri dishes and allowed to solidify for 2 h at room temperature. Using a punch rig and vacuum tip punch, one center well and six surrounding wells were cut in the agarose. HSA (Buminate 25%, Baxter, West-lake, CA) was diluted 1:1000 with PBS and 20 µL added to the center well. Twenty µL of undiluted positive control antibody (rabbit anti-HSA IgG, Accurate Chemical and Scientific, Westbury CT) was added to one well and 20 µL of undiluted test serum was added to the remaining five wells on each dish. Dishes were incubated at 37 °C in a humidified incubator with 5% CO2 for 41 h and inspected for the presence of lines of precipitation between wells.
2.10. IFN-γ ELISPOT assay
T cell responses to HA and NA were measured by gamma interferon enzyme-linked immunospot (IFN-γ ELISPOT) assay using previously described methods [19]. In brief, splenic lymphocytes from mice or PBMC from rhesus macaques, isolated by density gradient centrifugation, were stimulated with pools of HA or NA peptides (15-mers overlapping by 11 amino acids) at a final concentration of 1 µg/mL for each peptide, or with no peptide, an irrelevant control peptide, or a mitogen. Cells were added to ELISPOT assay plates coated with anti-mouse or anti-primate (Mab Tech clone G2.4) IFN-γ antibody and left undisturbed at 37 °C in a humidified atmosphere with 5% CO2 for 16–20 h. Wells were washed with PBS containing 0.05% Tween 20, treated with a biotinylated anti-mouse or anti-primate (Mab Tech clone 7-B6-1) IFN-γ monoclonal antibody, washed with PBS, and incubated with Avidin-Peroxidase Complex for 1 h at room temperature. Wells were then washed, incubated with substrate (3-amino-9-ethylcarbazole) for 4 min at room temperature and spot development stopped by distilled water rinse. After drying overnight, plates were shipped to Zellnet Consulting (New York, NY) for spot enumeration by automated analysis with a Zeiss KS ELISPOT system. The mean number of spot-forming cells (SFC) from duplicate wells, after subtraction of counts from cells cultured with no peptide, was determined for each animal. A response was considered positive if this value was greater than 20 SFC per 106 splenic lymphocytes or PBMC.
An outline of the animal immunization studies is provided in Table 1.
Table 1.
Outline of animal immunization studies
| Mice | Rabbits | Rhesus Macaques | |
|---|---|---|---|
| VRP tested | HA VRP, NA VRP | HA VRP, NA | HA VRP |
| Routes of administration | SC (footpad) | SC, IM | IM |
| Protein in formulation buffer | NMS | HSA, RSA | HSA |
| Immunogenicity tests performed | ELISA, HI, IFN-γ ELISPOT | ELISA, HI | ELISA, HI, IFN-γ ELISPOT |
Abbreviations: VRP, virus-like replicon particles; HA, hemagglutinin; NA, neuraminidase; SC, subcutaneous, IM, intramuscular; NMS, normal mouse serum; HSA, human serum albumin; RSA, rabbit serum albumin; ELISA, enzyme-linked immunosorbent assay; HI, hemagglutination inhibition assay; IFN-γ ELISPOT, gamma interferon enzyme-linked immunospot assay.
2.11. Statistical analysis
In the toxicology study, body weights, body weight changes, food consumption, body temperature, organ weight, and clinical pathology parameters were analyzed using the Kolmogorov-Smirnov test for normality, the Levene Median test for equal variance, and by one-way analysis of variance (ANOVA). If the ANOVA indicated statistical significance among experimental groups then the Dunnett’s t-test was used to delineate which groups (if any) differed from the control. Statistical analysis was conducted using Sigma-Stat™ Statistical Software, Version 1 (Jandel Scientific, San Rafael, CA), with a two-tailed probability value of <0.05 as the critical level of significance for all tests.
3. Results
3.1. Characterization of HA and NA replicon constructs
Different concentrations of replicon, capsid and glycoprotein RNA were tested prior to pilot lot and clinical lot manufacture to determine the optimal ratio for VRP production. An RNA concentration of 30 µg for replicon, capsid helper and glycoprotein helper was found to be optimal for production of both HA VRP and NA VRP. Western blot analysis of proteins extracted from Vero cells infected with VRP for 18–22 h showed expression of proteins of the expected molecular weights that were reactive with antibodies specific for HA or NA (Fig. 1).
Fig. 1.

Characterization of replicon constructs by Western blot analysis. Proteins extracted from Vero cells that were left untreated (Ctrl) or infected with NA VRP or HA VRP were probed with antibodies specific for NA (left panel) or HA (right panel). The positions of molecular weight markers are indicated on the left and the positions of NA or HA proteins are indicated on the right.
3.2. Production of pilot lots and GMP lots of VRP
Pilot lots of HA VRP and NA VRP were produced from a total of 20 cuvette electroporations per lot and used to provide material for reference standards, stability studies and toxicology studies. Clinical lots of influenza HA VRP were produced from a total of 60 cuvette electroporations per lot using the same process as the pilot lots.
The production process was robust and reproducible for HA VRP during scale-up from pilot lot to clinical manufacturing. The overall process productivity, defined as infectious units produced per cell, and percent yields during purification of HA VRP and NA VRP are shown in Table 2. The yields for the harvest by high salt elution (first step in the purification process) were calculated based upon a mass balance of VRP in the high salt wash pool and VRP in the medium and low salt wash pools. The yield in the formulated bulk is the overall yield after all process sampling and a single freeze thaw on the bulk material. No significant change in titer was observed after the freeze thaw.
Table 2.
Process productivity and yields in pilot and clinical lots of influenza VRP vaccines
| HA VRP | NA VRP | ||
|---|---|---|---|
| Pilot lot | Clinical lot | Pilot lot | |
| Productivity (IU/cell) | 357 | 300 | 335 |
| % Recovery harvest | 98.8 | 97.7 | 98.9 |
| % Recovery formulated bulk | 33.8 | 50.2 | 33.5 |
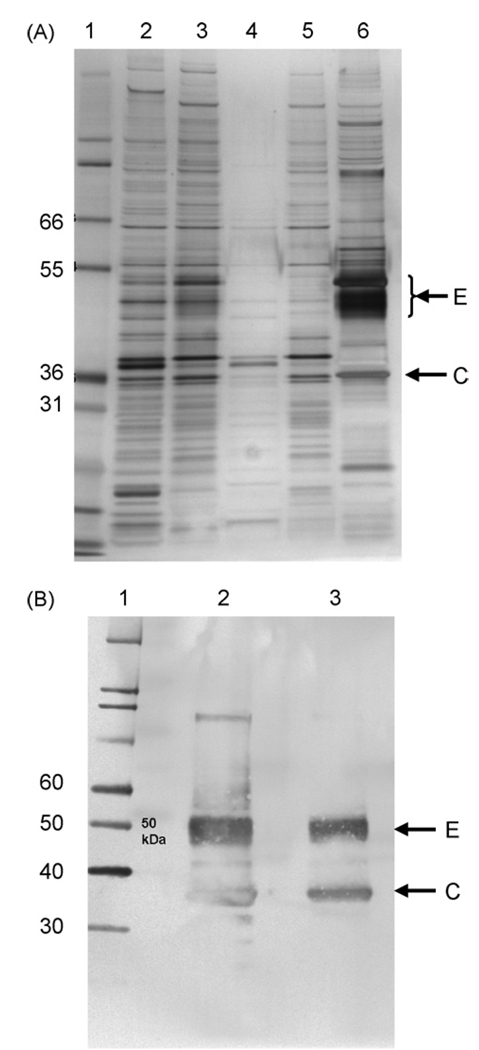
The purity of VRP with respect to protein and DNA in the product eluted from the Cellufine Sulfate column was assessed prior to addition of excipients to produce the formulated bulk and was generally consistent in process intermediate pools from pilot to clinical scale production operations, although the DNA concentration was lower in the clinical lot (Table 3). SDS-PAGE analysis of purified HA VRP (Fig. 2) and NA VRP (data not shown) demonstrated prominent protein bands that were confirmed by Western blot analysis to be VEE envelope and capsid proteins. Silver-stained bands representing the alphavirus envelope and capsid proteins could be visualized amongst the many protein species on the gel after purification by TFF but were more clearly seen when material was purified and concentrated during elution from the Cellufine Sulfate chromatography resin. As visualized by Southern blot analysis, the size of Vero DNA detected ranged from >10,000 base pairs in upstream process samples to very small fragments <300 base pairs at the end of TFF, and DNA was not detectable in VRP eluted from the Cellufine Sulfate chromatography resin (Fig. 3). The absence of DNA in the Cellufine Sulfate elution pool was not due to interfering substances, as 1 ng of digested Vero DNA spiked into process samples was clearly visible in the control lane.
Table 3.
Levels of VRP, protein, DNA and Benzonase in purified bulk vaccine prior to formulation
| HA VRP | NA VRP | ||
|---|---|---|---|
| Pilot lot | Clinical lot | Pilot lot | |
| Titer (IU/mL) | 6.3×1010 | 2.8×1010 | 5.5×1010 |
| Protein (µg/mL) (µg/108 IU) | 104 (0.17) | 126 (0.45) | 150 (0.27) |
| Vero DNA (ng/mL) (ng/108 IU) | 38.6 (0.06) | 4.0 (0.01) | 46.3 (0.07) |
| Benzonase (ng/mL) (pg/108 IU) | <0.125 (<0.2) | <0.125 (<0.4) | <0.125 (<0.2) |
Fig. 2.
(A) Silver-stained SDS-PAGE analysis of process pools from a pilot lot of HA VRP. Lane 1, molecular weight markers; lane 2, salt wash harvest pool; lane 3, pool of material at end of tangential flow filtration; lane 4, tangential flow filtration permeate; lane 5, Cellufine Sulfate unbound fraction; lane 6, VRP eluted from Cellufine Sulfate column. (B) Western blot of HA VRP probed with antibodies to VEE virus envelope and capsid proteins. Lane 1, molecular weight markers; lane 2, VRP eluted from Cellufine Sulfate column (undiluted); lane 3, VRP eluted from Cellufine Sulfate column (1 × 108 IU). The positions of molecular weight markers (kDa) are indicated on the left and the positions of the VEE virus envelope (E) and capsid (C) proteins are indicated on the right.
Fig. 3.

Southern blot analysis of process pools from a pilot lot of HA VRP. Lane 1, DNA size markers; lane 2, 1 ng of AluI-digested Vero DNA loaded on the gel as a control that underwent no sample processing; lane 3, salt wash harvest pool; lane 4, pool of VRP taken after Benzonase treatment; lane 5, pool of VRP taken after Benzonase treatment and spiked with 1 ng of AluI-digested Vero DNA; lane 6, pool of material at end of tangential flow filtration (TFF); lane 7, TFF pool spiked with 1 ng of AluI-digested Vero DNA; lane 8, Cellufine Sulfate elution pool; lane 9, Cellufine Sulfate elution pool spiked with 1 ng of AluI-digested Vero DNA. The locations of nucleotide size markers (kbp) are indicated on the left.
By qPCR analysis of formulated bulk vaccine, the genome equivalent to IU ratio was 18.3 and 15.7 for the pilot lot and clinical lot of HA VRP and 25.5 for the pilot lot of NA VRP.
3.3. Humoral immune responses in mice, rabbits and rhesus macaques
Mice, rabbits and rhesus macaques immunized with HA VRP developed anti-HA antibodies as measured by ELISA and HI assays (Table 4).
Table 4.
Antibody response to immunization of mice, rabbits and rhesus macaques with HA VRP
| Time point | Geometric mean (range) antibody response |
|||||
|---|---|---|---|---|---|---|
| Micea |
Rabbitsb |
Macaquesc |
||||
| ELISA | HI | ELISA | HI | ELISA | HI | |
| Baseline | <80 | <10 | <40 | <10 | <40 | 20 |
| Post-prime | 2560 (1280–5120) | 113 (40–320) | 538 (80–2560) | NT | 403 (160–640) | NT |
| Post-boost | 20,480 (10,240–40,960) | 320 (80–1280) | 4695 (1280–10,240) | 87 (40–320) | 25,803 (10,240–81,920) | 320 (160–1,280) |
ELISA, enzyme-linked immunosorbent assay; HI, hemagglutination inhibition assay; NT, not tested.
Mice were immunized at study day (SD) 1 and SD 22 and serum was obtained at SD 1, 21 (post-prime) and 29 (post-boost).
Rabbits were immunized at SD 1, 15, 29 and 43. Data shown are for serum obtained at SD 1, 15 (post-prime) and 42 (post-boost) from rabbits immunized by the subcutaneous route with VRP formulated in rabbit serum albumin. Data for additional time points, routes of administration and formulations are provided in Fig. 4 and in the text.
Rhesus macaques were immunized at SD 1, 29 and 162 and serum was obtained at SD 1, 29 (post-prime) and 57 (post-boost).
In the rabbit toxicology study, anti-HA antibodies developed in all rabbits immunized with VRP and none of the animals immunized with PBS. Antibody responses increased with repeated immunizations and were higher in animals immunized by the SC route than in animals immunized by the IM route (Fig. 4). The geometric mean (range) HI antibody titer at SD 42 was <10 in placebo recipients, 55 (20–80) and 55 (20–80) in animals immunized by the IM route with VRP formulated with RSA or HSA, respectively, and 87 (40–320) and 83 (40–640) in animals immunized by the SC route with VRP formulated with RSA or HSA, respectively. Anti-HSA antibodies, as measured by Ouchterlony assay, developed in 31 of 32 animals immunized with VRP formulated in HSA and in none of the animals immunized with PBS or with VRP formulated in RSA.
Fig. 4.

Anti-HA ELISA titers in rabbits immunized with placebo or with a mixture of HA VRP and NA VRP, formulated with 1% human serum albumin (HSA) or 1% rabbit serum albumin (RSA), by the subcutaneous (SC) or intramuscular (IM) route, on SD 1, 15, 29 and 43 (indicated by arrows). Each data point represents the geometric mean ± SEM for serum from 16 rabbits (or from eight rabbits at study days 45/46 and 57/58).
3.4. Cellular immune responses in mice and rhesus macaques
Spleen cells from mice immunized with HA VRP or NA VRP developed robust T cell responses as measured by IFN-γ ELISPOT assay. After immunization with 5 × 105 IU of VRP at Days 1 and 22, mean SFC per 106 splenic lymphocytes at Day 29 were 324 (range 186–592) for HA and 2183 (range 1558–2791) for NA.
PBMC from rhesus macaques immunized with HA VRP developed robust T cell responses as measured by IFN-γ ELISPOT assay (Fig. 5). After immunization with 1 × 109 IU of HA VRP at Days 1, 29 and 162, modest T cell responses were detected in two of three animals after the first dose of vaccine (24 and 51 SFC per 106 PBMC at Day 15) and strong T cell responses were detected in three of three animals after the second dose (698, 1155 and 1167 SFC per 106 PBMC at Day 43). T cell responses persisted at elevated but lower levels (81–388 SFC per 106 PBMC) at most later time points during the study, and these responses did not appear to be boosted after the third dose of vaccine at Day 162. Among four animals immunized with 1 × 109 IU of an irrelevant VRP, one had 25 SFC per 106 PBMC at SD 169 and one had 54 and 75 SFC per 106 PBMC at SD 57 and 169, respectively. The mean (±SD) T cell response for the other 41 post-immunization values in these four animals was 2.1 ± 3.9 (range 0–15).
Fig. 5.

IFN-γ ELISPOT responses in rhesus macaques immunized on SD 1, 29 and 163 (indicated by arrows) with HA VRP (top panel) or an irrelevant control VRP (bottom panel). Each data point represents the mean number of spot-forming cells (SFC) per 106 PBMC after stimulation with a pool of overlapping peptides spanning the HA protein.
3.5. Results from toxicology testing
Potency testing of samples obtained on the day of each injection demonstrated that animals in the active vaccine groups received an average of 2.4 × 108 IU of HA VRP and 4.1 × 108 IU of NA VRP per dose. Multiple inoculations had no adverse effects on clinical and cageside observations, body weights and body weight changes, food consumption, body temperature, or absolute and relative organ weights. Treatment with HA VRP and NA VRP had little effect on dermal Draize observations. Most animals were normal throughout the treatment period. Minimal edema or erythema was noted in all treated groups, affecting briefly one to three animals/sex/group. Mild edema or erythema was even more sporadic, affecting briefly one Group 4 male, one Group 3 female, and three Group 4 females. The mean maximum dermal Draize score at any time point in any group did not exceed 0.4 for edema and did not exceed 0.88 for erythema. In the animals that did develop edema or erythema, the injection sites were generally normal in appearance within 2–4 days after each immunization.
Clinical pathology evaluation revealed statistically significant increases in total protein and globulin concentration and decreased albumin to globulin ratio values in animals treated with VRP compared to the PBS control. These were considered to be most likely due to polyclonal immunoglobulin synthesis induced by test material administration. Statistically significant differences in coagulation parameters (activated partial thromboplastin time, fibrinogen concentration, and prothrombin time) were also considered to be a reflection of a host inflammatory response. None of these differences were considered to be biologically or toxicologically adverse.
Multiple inoculations with HA VRP and NA VRP had no adverse effect on gross pathology and no effect on organ weights, organ-to-body weight ratio or organ-to-brain weight ratio. Treatment-related gross pathology findings were limited to red discoloration, sometimes with gelatinous material, at the injection sites in a few animals treated with VRP formulated with HSA and necropsied on SD 45, and occasional enlargement or discoloration of draining lymph nodes in several animals in each of the five treatment groups.
Microscopic examination of tissues from SD 45/46 revealed test-article-related lesions at the intramuscular and subcutaneous injections sites, consistent with host inflammatory and immunologic reactions to injection of the test material. Lesions in the iliac and axillary draining lymph nodes and fascia adjacent to the sciatic nerves were consistent with drainage of the sites of inflammation. Lesions associated with VRP formulated with HSA were greater in severity grade and incidence than those associated with VRP formulated with RSA. Vasculitis was observed at the injection site only in animals that received VRP formulated with HSA. The incidence and severity of lesions had decreased at the recovery autopsies on SD 57/58.
4. Discussion
There is a compelling need for new approaches to influenza immunization. The limited duration of protective antibody responses after immunization with inactivated vaccines, and the continuous evolution of influenza viruses to produce variants that are not inhibited by antibodies to previous strains, contribute to the need for annual remanufacture and administration of influenza vaccines, and global production capacity is currently inadequate. The alphavirus replicon vector system offers a new approach to addressing these problems.
The VRP vaccines described in this paper had high-level expression of HA and NA that appeared to have authentic conformational structure. Both proteins were glycosylated, HA0 was processed to the expected HA1 and HA2 fragments, and immunization with HA VRP induced antibodies in mice, rabbits and macaques that were active in hemagglutination inhibition assays. In addition, preliminary experiments indicated that cells transfected with replicons expressing HA or NA had hemagglutinating or neuraminidase activity, respectively (data not shown), indicating that HA and NA expressed from replicons are able to form authentic, biologically active multimers.
The platform process used to scale up production of HA VRP and NA VRP was robust and gave products of yield and purity similar to that of VRP expressing other genes [17], including an HIV Gag VRP vaccine that demonstrated excellent safety and dose-dependent immunogenicity in a Phase 1 clinical trial [20]. The yields during product purification were excellent (33–50%) and the final product had low levels of protein and DNA contaminants.
When these studies were initiated, it was envisioned that an alphavirus replicon vaccine for influenza would include both HA VRP and NA VRP. After the GLP toxicology study was performed, analysis of efficacy data in ferrets immunized with different VRP vaccines indicated that a VRP vaccine expressing only the HA protein provided protective efficacy as good as that provided by a mixture of VRP vaccines expressing HA and NA proteins [21]. Therefore it was decided to proceed to clinical trials with a vaccine containing HA VRP only, and this product was also evaluated in nonhuman primates.
Protective immunity to influenza has been correlated with anti-influenza antibodies measured in the HI assay [2], and immunization with HA VRP induced high titers of HI antibodies in mice, rabbits and rhesus macaques. The role of cellular immune responses in protection against influenza is less clear. Although mice lacking functional class I major histocompatibility complex (MHC) glycoproteins and class I MHC-restricted, CD8+ effector T cells are able to clear influenza virus infections [22], CD8+ cyto-toxic T lymphocytes (CTL) can protect against influenza in the absence of antibodies to influenza [23,24], and CTL responses to influenza have been correlated with protection against influenza in humans [25]. Mice and rhesus macaques immunized with HA VRP developed T cell responses as measured by IFN-γ ELISPOT assay, and CD4+ and CD8+ T cells secreting IFN-γ and TNF-α have been identified by cytokine flow cytometry in PBMC from rhesus macaques immunized with HA VRP (E.A. Reap, unpublished data). Because the antigenic protein is produced within the VRP-infected cell, the antigen is proteolytically processed and presented by both Class I and Class II MHC proteins to stimulate both CD4+ and CD8+ T cell responses. In contrast, conventional influenza vaccines contain preformed proteins that are taken up by antigen-presenting cells and processed for presentation by Class II MHC proteins, inducing only CD4+ T cell responses. The ability of VRP to induce potentially protective CD8+ T cell responses may provide an additional benefit for VRP vaccines compared to conventional inactivated influenza vaccines.
VRP, like the alphaviruses from which that are derived, are enveloped particles, and purified preparations require the presence of protein in the formulation buffer to achieve stability. The stabilizing protein used for the vaccine to be tested in humans is HSA, but because mouse and rabbit albumin are sufficiently different from that in humans, repeated injections of VRP formulated in HSA can cause an immune response in these species, and VRP for studies in these species were formulated in NMS or RSA. However, GLP toxicology testing requires that the exact product to be used clinically must be the product tested in a toxicology study. Because previous toxicology studies in rabbits with other VRP vaccines formulated with HSA had sometimes shown increasing local reactogenicity with repeated dosing accompanied by histological evidence of inflammatory reactions, including necrotizing vasculitis, consistent with an Arthus-type reaction (J.D. Chulay, unpublished data), a GLP toxicology study was performed with HA VRP and NA VRP formulated with either HSA or RSA. Results of this study showed only mild local reactogenicity with no systemic toxicity. By histological examination, inflammatory responses were greater and evidence of vasculitis was seen only in animals immunized with VRP formulated with HSA. The Arthus-type reactions induced by VRP formulated with HSA may be related in part to the ability of VRP to provide adjuvant activity when co-administered with a foreign protein [26].
In summary, results of these studies, which describe efficient methods for production of VRP vaccines expressing influenza proteins that induce potent humoral and cellular immune responses in mice, rabbits and rhesus macaques with no adverse effects in GLP toxicology testing, support clinical testing of alphavirus replicon vaccines for influenza. Phase 1 testing of an HA VRP vaccine was initiated in April, 2007
Acknowledgements
We thank Randy Lamm, Kevin Williams, Tim Wagner, Deepa Patel, Renee Doggett and Holly Stone for performing analytical assays, ILS for immunizations and sample collection in mice, SRI for immunizations and sample collection in rhesus macaques, and Bridge GPS (formerly Gene Logic) for performing the GLP toxicology study in rabbits. Studies involving the use of animals complied with all relevant federal guidelines and institutional policies and were approved by an Institutional Animal Care and Use Committee. This research was supported in part by NIH grants UC1-AI62632 and UC1-AI062582.
Footnotes
This article was published in an Elsevier journal. The attached copy is furnished to the author for non-commercial research and education use, including for instruction at the author’s institution, sharing with colleagues and providing to institution administration.
Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited.
In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright
References
- 1.Treanor JJ. Influenza virus. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and practice of infectious diseases. fifth ed. Philadelphia: Churchill Livingstone; 2000. pp. 1823–1849. [Google Scholar]
- 2.Hobson D, Curry RL, Beare AS, Ward-Gardner A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg (Lond) 1972;70(Dec 4):767–777. doi: 10.1017/s0022172400022610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas DB, Patera AC, Graham CM, Smith CA. Antibody-mediated immunity. In: Nicholson KG, Webster RG, Hay AJ, editors. Textbook of Influenza. Oxford: Blackwell Science Ltd; 1998. pp. 267–277. [Google Scholar]
- 4.Beutner KR, Chow T, Rubi E, Strussenberg J, Clement J, Ogra PL. Evaluation of a neuraminidase-specific influenza A virus vaccine in children: antibody responses and effects on two successive outbreaks of natural infection. J Infect Dis. 1979;140(Dec 6):844–850. doi: 10.1093/infdis/140.6.844. [DOI] [PubMed] [Google Scholar]
- 5.Sandbulte MR, Jimenez GS, Boon AC, Smith LR, Treanor JJ, Webby RJ. Cross-Reactive Neuraminidase Antibodies Afford Partial Protection against H5N1 in Mice and Are Present in Unexposed Humans. PLoS Med. 2007;4(Feb 2):e59. doi: 10.1371/journal.pmed.0040059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Webby RJ, Webster RG. Are we ready for pandemic influenza? Science. 2003;302(Nov 5650):1519–1522. doi: 10.1126/science.1090350. [DOI] [PubMed] [Google Scholar]
- 7.Lakey DL, Treanor JJ, Betts RF, Smith GE, Thompson J, Sannella E, et al. Recombinant baculovirus influenza A hemagglutinin vaccines are well tolerated and immunogenic in healthy adults. J Infect Dis. 1996;174(Oct 4):838–841. doi: 10.1093/infdis/174.4.838. [DOI] [PubMed] [Google Scholar]
- 8.Treanor JJ, Betts RF, Smith GE, Anderson EL, Hackett CS, Wilkinson BE, et al. Evaluation of a recombinant hemagglutinin expressed in insect cells as an influenza vaccine in young and elderly adults. J Infect Dis. 1996;173(June 6):1467–1470. doi: 10.1093/infdis/173.6.1467. [DOI] [PubMed] [Google Scholar]
- 9.Bender BS, Rowe CA, Taylor SF, Wyatt LS, Moss B, Small PA., Jr Oral immunization with a replication-deficient recombinant vaccinia virus protects mice against influenza. J Virol. 1996;70(Sep 9):6418–6424. doi: 10.1128/jvi.70.9.6418-6424.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ulmer JB, Donnelly JJ, Parker SE, Rhodes GH, Felgner PL, Dwarki VJ, et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259(March 5102):1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 11.Coulter A, Harris R, Davis R, Drane D, Cox J, Ryan D, et al. Intranasal vaccination with ISCOMATRIX adjuvanted influenza vaccine. Vaccine. 2003;21(Feb 9–10):946–949. doi: 10.1016/s0264-410x(02)00545-5. [DOI] [PubMed] [Google Scholar]
- 12.Plante M, Jones T, Allard F, Torossian K, Gauthier J, St-Felix N, et al. Nasal immunization with subunit proteosome influenza vaccines induces serum HAI, mucosal IgA and protection against influenza challenge. Vaccine. 2001;20(Oct 1–2):218–225. doi: 10.1016/s0264-410x(01)00268-7. [DOI] [PubMed] [Google Scholar]
- 13.Treanor JJ, Kotloff K, Betts RF, Belshe R, Newman F, Iacuzio D, et al. Evaluation of trivalent, live, cold-adapted (CAIV-T) and inactivated (TIV) influenza vaccines in prevention of virus infection and illness following challenge of adults with wild-type influenza A (H1N1), A (H3N2), and B viruses. Vaccine. 2000;18(9–10):899–906. doi: 10.1016/s0264-410x(99)00334-5. [DOI] [PubMed] [Google Scholar]
- 14.Kroger AT, Atkinson WL, Marcuse EK, Pickering LK. General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2006;55(Dec RR15):1–48. [PubMed] [Google Scholar]
- 15.Kamrud KI, Custer M, Dudek JM, Owens G, Alterson KD, Lee JS, et al. Alphavirus replicon approach to promoterless analysis of IRES elements. Virology. 2007;360(April 2):376–387. doi: 10.1016/j.virol.2006.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pushko P, Parker M, Ludwig GV, Davis NL, Johnston RE, Smith JF. Replicon-helper systems from attenuated Venezuelan equine encephalitis virus: expression of heterologous genes in vitro and immunization against heterologous pathogens in vivo. Virology. 1997;239(2):389–401. doi: 10.1006/viro.1997.8878. [DOI] [PubMed] [Google Scholar]
- 17.Talarico T, Maughan M, Pancorbo B, Ruiz J, Graham A. Development and manufacture of alphavaccines. Bioprocessing. 2006 Fall;:8–14. [Google Scholar]
- 18.Griffiths E, Gruber M, Masset D, Verdier F, Wood D, Knezevic I. WHO guidelines on nonclinical evaluation of vaccines. http://wwwwhoint/biologicals/publications/nonclinical_evaluation_vaccines_nov_2003pdf.
- 19.Reap EA, Dryga SA, Morris J, Rivers B, Norberg PK, Olmsted RA, et al. Cellular and humoral immune responses to alphavirus replicon vaccines expressing cytomegalovirus pp65, IE1 and gB proteins. Clin Vaccine Immunol. 2007;14(6):748–755. doi: 10.1128/CVI.00037-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chulay J, Burke D, Karim SSA, Russell N, Wecker M, Allen M, et al. AIDS Vaccine 06; 29 Aug–1 Sep 2006. Amsterdam: Safety and immunogenicity of an alphavirus replicon HIV Gag vaccine (AVX101) in healthy HIV-uninfected adults. p. 11-09. [Google Scholar]
- 21.Hubby B, Boyers A, Ellis W, Copp L, Mann A, Lambkin R, et al. Immunogenicity and efficacy in ferrets of alphavirus replicon vaccines for influenza. Second European Influenza Conference; 2005; 11–14 Sep 2005; Malta. 2005. [Google Scholar]
- 22.Eichelberger M, Allan W, Zijlstra M, Jaenisch R, Doherty PC. Clearance of influenza virus respiratory infection in mice lacking class I major histocompatibility complex-restricted CD8+ T cells. J Exp Med. 1991 Oct 1;174(Oct 4):875–880. doi: 10.1084/jem.174.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yap KL, Ada GL, McKenzie IF. Transfer of specific cytotoxic T lymphocytes protects mice inoculated with influenza virus. Nature. 1978;273(May 5659):238–239. doi: 10.1038/273238a0. [DOI] [PubMed] [Google Scholar]
- 24.Taylor PM, Askonas BA. Influenza nucleoprotein-specific cytotoxic T-cell clones are protective in vivo. Immunology. 1986;58(July 3):417–420. [PMC free article] [PubMed] [Google Scholar]
- 25.McMichael AJ, Gotch FM, Noble GR, Beare PA. Cytotoxic T-cell immunity to influenza. N Engl J Med. 1983;309(July 1):13–17. doi: 10.1056/NEJM198307073090103. [DOI] [PubMed] [Google Scholar]
- 26.Thompson JM, Whitmore AC, Konopka JL, Collier ML, Richmond EM, Davis NL, et al. Mucosal and systemic adjuvant activity of alphavirus replicon particles. Proc Natl Acad Sci USA. 2006;103(March 10):3722–3727. doi: 10.1073/pnas.0600287103. [DOI] [PMC free article] [PubMed] [Google Scholar]