Abstract
To investigate the effects of carboxylic ester and acid moieties as the N-substituent of opioids, a short series of racemic N-substituted normetazocines was prepared. The introduction of both groups as the normetazocine N-substituent produced compounds which displayed low potency in vitro and in vivo, with the esters displaying the greater activity. The pharmacology of the compounds is discussed with implications resulting from potential in vivo metabolic hydrolysis.
Keywords: Opioid, Benzomorphan, Normetazocine
1. Introduction
Modification and replacement of the functional group attached to the basic nitrogen in opioids has been the subject of much exploration in opioid chemistry. The nature of the N-substituent determines, to a large extent, the pharmacological profile in a majority of classes of opioids, affecting affinity, potency, and efficacy at the three opioid receptor types mu (μ), kappa (κ), and delta (δ).1 The naturally occurring archetypal opiates morphine and codeine each have an N-methyl substituent and, in general, an N-methyl substituent produces a compound with opioid agonist activity. Changing from an N-methyl group (oxymorphone) to an N-allyl group (naloxone) converts a compound which exhibits μ opioid agonist activity to a compound which exerts μ opioid antagonism.1 Changing the N-substituent from an N-phenethyl to an N-benzyl, in the benzomorphan class of compounds transforms a compound with high agonist potency into a compound without opioid activity.2 While not the sole determinant the N-substituent is very important for opioid pharmacology, yet the molecular interactions responsible for such an important role are not clear.3
The lipophilicity and hydrophilicity of the N-substituent play a large role in the activity of opioids. Generally, lipophilic substituents are required for opioid activity and hydrophilic substituents are not well tolerated.1 The classic example of the effects of lipophilic and hydrophilic N-substituent replacement is the development of remifentanil (N-ester) from fentanyl (carfentanil) (both, N-phenethyl) in the 4-anilidopiperidine class of opioids.4 When remifentanil, an N-ester, is compared to the corresponding N-acid congener, opioid activity is greatly diminished. The change from lipophilic ester to hydrophilic acid results in changes in ED50 from 3.55 nM (N-ester, remifentanil) to 1.95 μM (N-acid, metabolite) in the guinea pig ileum (GPI) assay, and from 4.4 μg/kg to 1.6 mg/kg in the rat tail withdraw (RTW) assay.4 Outside of this series (remifentanil) of compounds, few opioid N-carboxylic ester or acid substituted compounds have been reported in the literature, fewer still reported with pharmacology. A literature report of an N-ester and acid pair (NME-1 and NMA-1) in the N-normetazocine (1) (benzomorphan) series presented limited data for the two compounds.5 The report was investigating the possible metabolites of pentazocine and included both the ester and acid. The data presented showed NME-1 inactive as an agonist but weakly antagonized the effects of meperidine with an AD50 of 66 (pentazocine AD50 3.9 from the same study) in the rat tail flick (RTF) assay. The corresponding N-acid (NMA-1) was devoid of both agonist and antagonist activity in this study.5 To further study the effects of N-esters and N-acids, and the effects of changing the lipophilicity/hydrophilicity of the N-substituent we synthesized a series of N-substituted-N-normetazocine analogs, with the aim of improving the understanding of molecular interactions between the N-substituent and the opioid receptors.
2. Results and Discussion
The racemic (±)-N-normetazocine-N-ester (NME-1–3) and -N-acid (NMA-1–3) analogs (Scheme 1) in this series displayed low potency in both the in vitro assays and in vivo antinociceptive assays (Table 1). Overall the compounds examined in this series required high (micromolar) concentrations to display significant effects. Since this series of compounds was tested as racemates the activity profiles may display decreased potency, as the greater opioid potency is known to reside in the (-)-isomers.1 Despite their racemic nature, the compounds in this series present an intriguing profile of N-substituent effects.
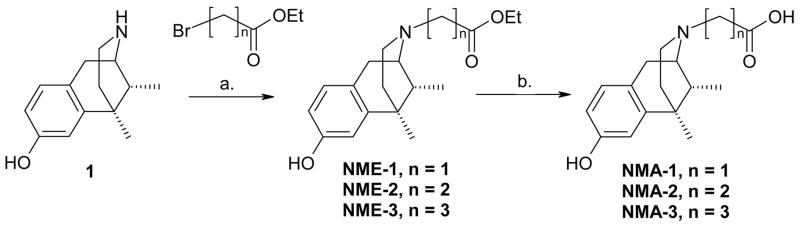
Scheme 1.
Reagents and conditions: a. DMF/THF, K2CO3, reflux 3–6 h; b. EtOH, H2O, KOH, reflux 1–2 h.
Table 1.
The Pharmacological Effects of N-ester and N-acid substituted-N-normetazocines
 |
In Vivo ED50 (mg/kg, sc) | In Vitro EC50 (M) | |||||
|---|---|---|---|---|---|---|---|
| Compound | R | HP | PPQ | TF | TFA | Binding | MVD |
| NME-1 | CH2COOEt | I | I | I | I, * | 2.08 μM | NE, ANTa |
| NME-2 | CH2CH2COOEt | I | I | I | I | NT | NT |
| NME-3 | CH2CH2CH2COOEt | I | 7.1 (2.4–21.4) | I | I | 1.02 μM | 17.9 μMb,c |
| NMA-1 | CH2COOH | I | I | I | I | >6 μMd | 9.79 μMb,c |
| NMA-2 | CH2CH2COOH | I | 11.4 (2.9–44.6) | I | I | >6 μMe | NEf |
| NMA-3 | CH2CH2CH2COOH | I | 1.4 (0.3–6.4) | I | I | >6 μMg | NEf |
| Morphine | --- | 0.98 | 0.23 | 5.8 | --- | 23.6 nM | 395 nM |
| (-) Metazocine | CH3 | 0.62 | 0.3 | 0.81 | I | ||
|
| |||||||
| Nalorphine | --- | 9.9 | 0.6 | I | 2.6 | 20.0 nMh | |
|
| |||||||
| Naloxone | --- | I | I | I | 0.03 | 6.26 nM | ANTi |
|
| |||||||
| Naltrexone | --- | I | I | I | 0.007 | 0.63 nM | ANTj |
Weak antagonist [30 μM] of sufentanil (μ), U50,488 (κ), and DSLET (δ);
Neither altered or reversed the effects of sufentanil;
Antagonized by 10−7 M naltrexone and 10−7 M β-fulnaltrexamine but not 10−7 M ICI-174864;
7.0% inhibition at 6 μM;
26% inhibition at 6 μM;
Did not block or reverse the effects of sufentanil (μ), U50,488 (κ), or DSLET (δ);
27% inhibition at 6 μM;
concentration of etorphine for displacement of this ligand was 3.0 nM (compare to codeine 34.7 μM, morphine 142nM, naloxone 9.1nM, and naltrexone 2.0nM when etorphine employed at 3.0nM);
pA2 values 7.99, 6.90, 7.35 for sufentanil, U50,488, and DSLET,
pA2 values 8.76, 7.74, 7.41 for sufentanil, U50,488, and DSLET.
I = inactive, did not reach 50% at highest dose (≥30 mg); NE = No Effect at any concentration tested (10−7 to 10−5 M); NT = Not Tested; ANT = Antagonist
MVD = Mouse Vas Deferens, Inhibition of electrically driven twitch, Inhibitory EC50 (M); Binding = Displacement of specific [3H]-etorphine binding (0.5 nM) from rat cerebrum homogenates, in presence of 150 mM NaCl; *previous report showed activity as an antagonist of meperidine in rats.4
The compounds in this series displayed activity in only one of the four in vivo assays for activity, the phenylquinone antiwrithing assay (PPQ) (Table 1.). Since the PPQ is an antinociceptive assay which is non-selective for narcotic analagesia (opioid) and can also detect anitinflamatory (NSAID type) analgesia, no conclusions can be made from this assay alone as to the type of analgesia detected.6 In both the N-ester series and the N-acid series there is a gain of antinociceptive activity in the PPQ with increasing chain length. This increased potency with increasing chain length is similar to the increase in potency reported for the remifentanil ester series of compounds.4 In the remifentanil series of esters potency increased from the methyl acetate 39 mg/kg ED50 to the methyl propionate 4.4 μg/kg (remifentanil), to the methyl butyrate 1.6 μg/kg in the RTW.4 The corresponding propionic acid analog of remifentanil had an ED50 of 1.6 mg/kg in the RTW, no data was presented on the acetic or butyric acid congeners.4 While interesting to note this similarity of in vivo activity with increasing spacing chain lengths, no direct comparisons can be made due to the differing assay types. Further, the activity of NMA-2 and NMA-3 is likely due to non-opioid effects, suggested by the lack of both binding and opioid agonist activity in the in vitro experiments.
Another result from the in vivo studies presented here which bears comment is the failure of the compound NME-1 to show antagonist activity in the mouse tail flick antagonism of morphine (TFA) assay. This compound was previously reported to be active though weak (AD50 of 66), as an antagonist.5,7 The differences in studies may have contributed to the lack of in vivo activity reported here. The use of rats instead of mice, freebase instead of oxalate salt, meperidine instead of morphine, and higher vs. lower tested dosage used in the original vs. experiments presented here could all factor into the lack of antagonist activity reported. However the in vitro experiments presented here argue for the opioid antagonistic effect of NME-1.
The ethyl acetate analog (NME-1) displayed micromolar binding affinity and weak opioid antagonist activity in the MVD assay. The opioid antagonist activity of NME-1 in the MVD was, though weaker, similar to that of naltrexone. NME-1 produced 12 fold, 11.6 fold, and 2.8 fold right shifts in the concentration-effect curves of sufentanil (μ), U50,488 (κ), and DSLET (δ) respectively, without altering the maximum response of any of the agonists (a similar profile as naltrexone in this assay). For a comparison of antagonist potency, if the 2.8 fold shift for NME-1 vs. DSLET is used as a basis for calculation, pA2 value of 4.52 was obtained.8 Naltrexone has a pA2 value of 7.41 for DSLET, and the comparison of values indicates that NME-1 is three orders of magnitude less potent than naltrexone.9 These results support the previously reported evidence of the compound’s weak in vivo antagonist effect on meperidine induced antinociception.5 The corresponding acetic acid analog (NMA-1), previously reported to be devoid of agonist opioid activity in vivo in the RTW or antagonism of meperidine’s effects in the RTW, here displayed opioid agonist activity in the in vitro MVD assay.
In the in vitro tests NMA-1 displayed μ opioid agonist activity in the MVD, but failed to show binding activity. In the MVD, NMA-1 exhibited a profile consistent with (mu) opioid agonists (inhibition of the twitch, and reversal of its effects by both naltrexone and β-fulnaltrexamine but not ICI 174864).10 At no concentration did NMA-1 inhibit the effects of sufentanil in the MVD. These results suggest NMA-1 has weak opioid agonist effects in the MVD. The lack of binding activity of NMA-1 should be noted, and argues against an opioid classification. The binding activity may have been revealed if higher concentrations were tested. Of the remaining analogs, only the ethyl butyrate analog (NME-3) exhibited significant binding and either agonist or antagonist activity at the doses tested. NME-3 displayed micromolar (μ) opioid agonist activity in the MVD (its effects were reversed by naltrexone, and β-fulnaltrexamine, but not ICI 174864), and micromolar binding affinity. Though both compounds (NMA-1 and NME-3) have very weak opioid activity in the MVD, and only NME-3 displayed significant opioid receptor binding, their profiles are consistent with that of opioid agonists.10
The compounds presented here can be examined for comparisons of the effects of esters and acids. One comparison of the compounds, examined as two series of different functional group types, N-esters and N-acids, indicates varying activity by chain length. In vitro, the Nester series ethyl acetate analog (NME-1) displayed an opioid antagonist profile and the ethyl butyrate analog (NME-3) displayed an opioid agonist profile. In vitro, the N-acetic acid analog (NMA-1), was the only N-acid compound which displayed any opioid efficacy or activity (opioid agonist activity) while the other two acids (NMA-2 and NMA-3) lost all opioid activity. Both N-esters and N-acids show a change in in vitro activity with increasing spacing chain length. In vivo both the N-ester series and the N-acid series gain antinociceptive activity with increasing chain length. While in vitro assays show the N-esters change from antagonist activity to agonist activity, and the N-acids loose activity with increasing chain length. This observation is consistent with other benzomorphans we have studied previously where in vitro and in vivo activity do not always correlate.2, 3 The broad picture gleaned from this in vitro based comparison is N-esters tend to retain opioid activity (though switching efficacy from antagonism to agonist activity) with increasing chain length, and N-acids lost opioid activity with increasing chain length. Non-specific antinociception (in vivo PPQ activity) was revealed in both the Nester and the N-acid series as chain length increased.
Another comparison examines the compounds in pairs with identical spacing chain lengths, representing the before and after effects of hydrolysis of the ester functional group. An examination of calculated log P (CLogP) values (a model for comparison of lipophilicity), shows a significant decrease in lipophilicity when N-esters are compared to their N-acid congeners (hydrolysis product) (Table 2).11 Though each analog pair shows a significant decrease in lipophilicity resulting from the modeled hydrolysis, no consistent effect other than change in activity is seen between the pairs. The ethyl acetate (NME-1) acetic acid (NMA-1) pair shows an opioid antagonist (NME-1) transformed into an opioid agonist (NMA-1) in the MVD. In this pair, in vitro functional activity is reversed when the before (ester, lipophilic) and after (acid, hydrophilic) components of hydrolysis are compared. The ethyl butyrate (NME-3) butyric acid (NMA-3) pair shows an opioid agonist (NME-3, ester, lipophilic) transformed into a compound devoid of opioid activity (NMA-3, acid, hydrophilic) in the MVD. Both pairs show a change in activity when hydrolysis is simulated in vitro. However, the in vivo data (non-opioid specific antinociception) show little change in activity when comparing the before (NME-3) and after (NMA-3) components of hydrolysis. These results are again similar to previous observations where in vitro and in vivo activity does not correlate.2, 3
Table 2.
Effects of N-substituents on lipophilicity
| Compound | N-substituent | Opioid class | ClogP |
|---|---|---|---|
| Fentanyl* | Phenyletheyl | Anilidopiperidine | 3.621 |
| Carfentanil* | Phenyletheyl | Anilidopiperidine | 3.686 |
| Remifentanil* | Ethylproprionate (Carbethoxyethyl) | Anilidopiperidine | 1.956 |
| Hydrolyzed Remifentanil* | Propionic acid (Carboxyethyl) | Anilidopiperidine | −0.777 |
| Metazocine | Methyl | Benzomorphan | 2.965 |
| NME-1 | Ethylacetate (Carbethoxymethyl) | Benzomorphan | 3.751 |
| NME-2 | Ethylpropionate (Carbethoxyethyl) | Benzomorphan | 3.992 |
| NME-3 | Ethylbutyrate (Carbethoxypropyl) | Benzomorphan | 4.229 |
| NMA-1 | Acetic Acid (Carboxymethyl) | Benzomorphan | 0.459 |
| NMA-2 | Propronic acid (Carboxyethyl) | Benzomorphan | 0.598 |
| NMA-3 | Butryic Acid (Carboxypropyl) | Benzomorphan | 0.852 |
Compound data from Feldman et al19; Calculated log P values (CLogP) were calculated using ChemDraw Ultra 10.0, CambridgeSoft;
The broad picture from comparing N-ester, N-acid congener pairs indicates change in in vitro activity. The NME-3, NMA-3 pair compares similarly to the change observed in the remifentanil series of compounds where an opioid agonist is reduced in potency when changed from an ester to an acid. The NME-1, NMA-1 pair also displays a change in activity but the change is in efficacy instead of potency. It is interesting to speculate that this change may be due to the physical properties of the short chain compounds interacting with receptor.
Overall the results indicate that a combination of both the functional group character (lipophilicity and electronic effects) and the spacing carbon chain length between the basic nitrogen and the carbonyl carbon appeared to determine the compound’s activity (or lack thereof), and not solely the functional group itself. The opioid antagonist activity of NME-1 reported here, supports the previous report of the antagonist activity of this compound. The confirmation of antagonist activity for an opioid N-ester, an efficacy profile opposite the profile of the N-ester remifentanil, suggests that the ester functional group’s effects on efficacy may be dependant upon opioid class. This observation is supported by the analysis of other opioid classes where antagonist activity is dependant upon binding mode, and not solely on the nature of the N-substituent.12, 13 The potential of this observation is interesting, however additional compounds and data are required to make generalizations.
3. Conclusions
The compounds presented here expand upon the current knowledge of functional group tolerance for opioid carboxylic ester and acid N-substituents. Although displaying significantly lower potency than alkyl substituents, esters appear to be tolerated in the benzomorphan class of opioids, and produced both opioid agonist and an opioid antagonist. Acids in the benzomorphan series appeared less well tolerated as they are extended out from the basic nitrogen leading to loss of opioid activity with extension beyond one methylene spacing unit. The data presented in this study show that an N-ester can produce opioid antagonism, which is the opposite efficacy of the remifentanil series of N-esters. This finding suggests both an expanded profile of N-ester substituent effects upon opioid efficacy, and also supports the observation that N-substituent efficacy effects are also dependant upon opioid class. These observations will be incorporated into future models of efficacy at opioid receptors underway in this laboratory.
4. Experimental
4.1 Chemistry
The N-ester-normetazocines (NME-1–3) were prepared from racemic normetazocine (1) and the appropriate halogenated esters, in a suspension of DMF/THF in the presence of sodium carbonate. The N-acid-normetazocine compounds (NMA-1–3) were prepared from the corresponding N-ester-normetazocine analogs by hydrolysis in potassium hydroxide in water, followed by neutralization with 10% hydrochloric acid (Scheme 1). All were compounds were converted into oxalate or HCl salts.14
Typical procedures
(±)-2-carbethoxymethyl-5,9-α-dimethyl-2’hydroxy-6,7-benzomorphan oxalate = N-Carboethoymethyl-α-N-normetazocine oxalate C18H25NO3.(COOH)2.(H2O)0.5 (NME-1)
(±)-N-normetazocine (1) (2.0 g), ethylbromoacetate (1.5 g), and K2CO3 (2.0 g) were added to a mixture of of tetrahydrofuran (6 mL) and dimethylformamide (2 mL) and the resulting mixture stirred under reflux for 5 hours. Solvents were evaporated in vacuo. The residue was diluted with water and extracted three times with diethyl ether. The resulting extracts were combined, washed with brine, dried with magnesium sulfate, and concentrated under vacuum which gave 2.8g of base. The acidification of the freebase with oxalic acid in acetone gave the hemihydrated oxalate salt. m.p. 116–118 °C.
α(±)-2-carboxymethyl-5,9-α-dimethyl-2’hydroxy-6,7-benzomorphan oxalate = N-Carboxymethyl-α-N-normetazocine oxalate C16H21NO3. (HCl)1.(H2O)0.25 (NMA-1)
NME-1 (1.0 g, freebase), ethanol (0.1g) and KOH (1.0 g) were refluxed in water (2.5 mL) for 1.5 hours. The ethanol was evaporated in vacuo and the residue neutralized to pH7 with 10% HCl. The resulting white solid was collected by filtration, washed with cold water yielding 0.65g of free acid (m.p. 195–200). The free acid was dissolved in a mixture of methanol and ethylacetate and precipitated as the hydrochloride salt by bubbling HCl gas through the solution. m.p. 232–233 °C.
4.2 pharmacology
Hot plate (HP), tail flick (TF), antagonism of morphine in the tail-flick (TFA), and phenylquinone antiwrithing (PPQ) assays were carried out in mice as previously described at does of 0.25, 1, 10, and 30 mg/Kg.15, 16 Binding was determined in rat cerebrum membrane preparations, and EC50 determinations by displacement of 0.5nM [3H]-etorphine from rat brain homogenates using the standard procedures of the Drug Evaluation Committee (DEC), College on Problems of Drug Dependence (CPDD).17, 18 This assay measures binding at all three opioid receptors, and does not differentiate between the opioid receptor types (μ, κ, δ). Functional activity in the Mouse Vas Deferens (MVD) was determined by suppression of electrically stimulated twitch using 25–30 g NIH Swiss mice, in the presence and absence of antagonists to determine the specific receptor responsible for the actions, again following standard procedures of DEC.17 Briefly, in the MVD assay, opioid agonists are characterized by the suppression of twitch height and inhibition by naltrexone. If the compound suppresses twitch height but is not inhibited by naltrexone the compound’s effect is non-opioid in nature. Compounds are evaluated for opioid antagonist activity in the MVD by their ability to reverse the effects of morphine’s suppression of twitch height. A compound, opioid agonist or antagonist, can be further evaluated for receptor specific effects with the use of receptor specific agonists and antagonists replacing naltrexone (for agonists) or morphine (for antagonists). Full details for these standard protocols and procedures are presented in the work of Smith.10 The in vitro experiments (binding and MVD assays) were carried out with concentrations ranging from 10−7 to 10−5 M. Results are listed in Table 1.
Acknowledgments
The authors would like to thank the College on Problems of Drug Dependence, the Drug Evaluation Committee, NIDA grant DA-13583, and DA-00254. MDM is the recipient of a Ruth L. Kirschstein NRSA fellowship DA-18025, and AC is the recipient of an Independent Scientist Award, DA-19634.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Casy AF, Parfitt RT. Opioid Analgesics. New York and London: Plenum Press; 1986. [Google Scholar]
- 2.May EL, Jacobson AE, Mattson MV, Coop A, Aceto MD, Bowman ER, John R, Traynor JR, Woods JH, Harris LS. Med Chem Res. 1998;8:311. [Google Scholar]
- 3.May EL, Coop A, Woods JH, Aceto MD, Bowman ER, Harris LS, Traynor JR. Bioorg Med Chem. 2003;11:31. doi: 10.1016/s0968-0896(02)00435-2. [DOI] [PubMed] [Google Scholar]
- 4.Feldman PL, James MK, Brackeen MF, Bilotta JM, Schuster SV, Lahey AP, Lutz MW, Johnson MR, Leighton HJ. J Med Chem. 1991;34:2202. doi: 10.1021/jm00111a041. [DOI] [PubMed] [Google Scholar]
- 5.Albertson NF, McKay FC. J Med Chem. 1977;20:602. doi: 10.1021/jm00214a036. [DOI] [PubMed] [Google Scholar]
- 6.Taber RI. Adv Biochem Psychopharmacol. 1973;8:191. [PubMed] [Google Scholar]
- 7.Defined by the authors of the study (ref 5) as the dose causing a 50% decrease in effect of an approximate ED80 dose of meperidine.
- 8.pA2 = −log[antagonist] which causes a 2 fold shift to the right in dose response curve of agonist.
- 9.Due to testing protocols employed; pA2 values for NME-1 for sufentanil and U50,488 were not calculated.
- 10.Smith CB. NIDA Research Monograph. 1986;76:288. [PubMed] [Google Scholar]
- 11.CLogP values calculated from structures using ChemDraw Ultra 10.0, CambridgeSoft.
- 12.Carroll FI, Melvin MS, Nuckols MC, Mascarella SW, Navarro HA, Thomas JB. J Med Chem. 2006;49:1781. doi: 10.1021/jm058264p. [DOI] [PubMed] [Google Scholar]
- 13.Carroll FI. J Med Chem. 2003;46:1775. doi: 10.1021/jm030092d. [DOI] [PubMed] [Google Scholar]
- 14.Spectra were consistent with the assigned structures; the salts gave satisfactory microanalyses (± 0.4%).
- 15.May EL, Jacobson AE, Mattson MV, Traynor JR, Woods JH, Harris LS, Bowman ER, Aceto MD. J Med Chem. 2000;43:5030. doi: 10.1021/jm000317+. [DOI] [PubMed] [Google Scholar]
- 16.Pearl J, Harris LS. J Pharmacol Exp Ther. 1966;154:319. [PubMed] [Google Scholar]
- 17.Woods JH, Traynor JR. Problems of Drug Dependence 2000, NIDA Research Monograph. 2001;181:141. [Google Scholar]
- 18.Negus SS. Drug Alcohol Depend. 2006;82:182. doi: 10.1016/j.drugalcdep.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Feldman PL, James MK, Brackeen MF, Bilotta JM, Schuster SV, Lahey AP, Lutz MW, Johnson MR, Leighton HJ. J Med Chem. 1991;34:2202. doi: 10.1021/jm00111a041. [DOI] [PubMed] [Google Scholar]