Abstract
Cajal–Retzius (CR) cells are principal cells of layer I in the developing neocortex. They are able to generate action potentials, make synaptic contacts in layer I and receive excitatory GABAergic inputs before birth. Although CR cells participate in neuronal network activity in layer I, the properties of their synaptic inputs are not yet characterized. We recorded miniature (mIPSCs) and evoked (eIPSCs) postsynaptic currents using the whole-cell patch-clamp technique. Most of CR cells displayed two types of mIPSCs, namely those with fast (mIPSCF) and slow (mIPSCS) rise kinetics. The mIPSCF mean amplitude was significantly larger than that of mIPSCS, while their decay rates were not different. Peak-scaled non-stationary noise analysis revealed that mIPSCS and mIPSCF differed in their weighted single-channel conductance. In addition, zolpidem (100 nm), a modulator of α1 subunit-containing GABAA receptors, selectively affected mIPSCS suggesting that different postsynaptic GABAA receptors mediate mIPSCF and mIPSCS. eIPSCs also split into two populations with different rise kinetics. Fast eIPSCs (eIPSCF) displayed higher paired-pulse ratio (PPR) and lower GABA release probability than slowly rising eIPSCs (eIPSCS). As CGP55845, a GABAB receptor antagonist, eliminated the observed difference in PPR, the lower release probability at IPSCF connections probably reflects a stronger tonic GABAB receptor-mediated inhibition of IPSCF synapses. At low (0.1 Hz) stimulation frequency both inputs can effectively convert presynaptic action potentials into postsynaptic ones; however, only IPSCF connections reliably transfer the presynaptic activity patterns at higher stimulation rates. Thus, CR cells receive two GABAergic inputs, which differ in the quantal amplitude, the probability of GABA release and the frequency dependence of signal transfer.
During the development of the mammalian cerebral cortex, the earliest postmitotic neuroblasts form the primordial plexiform layer or preplate. Later generated neurons settle in the cortical plate, which splits the preplate into the subplate and the marginal zone (future layer I, for review see Super et al. 1998). Cajal–Retzius (CR) cells are the principal neurons in the marginal zone/layer I of the developing neocortex. These early born neurons have large pericarya with long horizontal dendrites and axonal arbors that are restricted to layer I. CR cells represent a transient cell population and disappear approximately by the end of the second postnatal week in rodents (for review see Marin-Padilla, 1998; Soriano & del Rio, 2005). However, because of their strategic location in layer I and the coincidence of their life span with the period of cortical migration, CR cells have been proposed to play a key role in the structural organization of the neocortex. Recent experimental findings have shown that CR cells synthesize and secrete reelin, an extracellular matrix protein necessary for cortical lamination (for review see Frotscher, 1998; Rice & Curran, 2001; Tissir & Goffinet, 2003). In reelin-deficient mice the preplate partition is disrupted and the undivided preplate forms the ‘superplate’ on the top of the inverted cortical plate (Caviness, 1982). CR cell degeneration in newborn animals results in a dramatic decrease in the number of radial glia cells and interrupts cortical layer formation (Super et al. 2000). Despite this wealth of information suggesting a crucial importance of CR cells for corticogenesis, their function is still the subject of discussion (for review see Marin-Padilla, 1998; Frotscher, 1998; Soriano & del Rio, 2005; Yoshida et al. 2006).
In addition to reelin production, CR cells are capable of generating action potentials early in development (Zhou & Hablitz, 1996; Albrieux et al. 2004) and express a variety of channels and neurotransmitter receptors including GABAA receptors (Schwartz et al. 1998; Cheng et al. 2006). CR cells have been shown to receive excitatory GABAergic inputs (Kilb & Luhmann, 2001; Radnikow et al. 2002). The axonal collaterals of CR cells form a dense horizontally orientated plexus in layer I, projecting over millimetres of cortical surface and making synaptic contacts with excitatory neurons (Radnikow et al. 2002). Taken together, morphological and electrophysiological data suggest that CR cells may be a part of an early cortical network. Indeed, they have been shown to be involved in the synchronized network activity in layer I in both wild-type and reelin-deficient mice (Aguilo et al. 1999). This correlated network activity can be blocked by GABAA receptor antagonists indicating that excitatory GABAergic projections play an essential role in this form of activity. A dense, transient GABAergic fibre plexus confined to layer I has been observed in the developing rodent cortex (Lauder et al. 1986). This GABAergic fibre plexus is proposed to be composed of axons of GABAergic cortical neurons (Marin-Padilla, 1998) and extrinsic projections from the zona incerta of the ventral thalamus (Lin et al. 1990). In addition, the axonal processes of subplate neurons terminate in the marginal zone (Friauf et al. 1990) and these inputs may be GABAergic (Voigt et al. 2001). Therefore, CR cells are likely to be innervated by functionally distinct GABAergic fibres. Unfortunately, detailed knowledge of the input and output structures of CR cells is still lacking (Kilb & Luhmann, 2001; Radnikow et al. 2002; Soda et al. 2003; Kirmse & Kirischuk, 2006a).
In this study, we report that neocortical CR cells receive two types of GABAergic inputs. Based on the rise kinetics, miniature and evoked IPSCs can be divided into two groups, namely slowly (IPSCS) and fast (IPSCF) rising IPSCs. IPSCF connections have larger quantal amplitudes and lower GABA release probability. Both projections can effectively elicit action potentials in CR cells at low levels of incoming activity (about 0.1 Hz). However, already at moderate (about 1 Hz) frequencies only IPSCF connections can reliably convert presynaptic activity patterns into postsynaptic action potentials. Our data suggests that neocortical CR cells receive distinct GABAergic inputs with different signal transfer capability and this may be of importance for the function and/or developmental adjustment of an immature cortical network.
Methods
Brain slices preparation
All experiments were carried out according to the guidelines laid down by the Office of Health Protection and Technical Safety of the regional government Berlin (Landesamt für Arbeitsschutz, Gesundheitsschutz und technische Sicherheit Berlin, T0406/03).
All experiments were conducted with pigmented C57BL/6J mice pups of postnatal days 5–7 (the day of birth was designated as P0). Animals were decapitated under deep ether anaesthesia. The brain was removed quickly and transferred into ice-cold saline that contained (mm): 125 NaCl, 4 KCl, 10 glucose, 1.25 NaH2PO4, 25 NaHCO3, 0.5 CaCl2 and 2.5 MgCl2, constantly aerated with a 5% CO2–95% O2 mixture (pH 7.3). The brain was separated into two hemispheres. Sagittal slices of both hemispheres were cut on a vibrating microtome (Integraslice 7550PSDS, Campden Instruments Ltd, Loughborough, UK). After preparation, slices (200 μm thick) were stored for at least 1 h at room temperature in artificial cerebrospinal fluid (ACSF) that contained (mm): 125 NaCl, 4 KCl, 10 glucose, 1.25 NaH2PO4, 25 NaHCO3, 2 CaCl2 and 1 MgCl2. pH was buffered to 7.3 by continuous bubbling with a 5% CO2–95% O2 mixture. The osmolarity was 330 mosmol l−1.
Electrophysiological recordings in acute slices
For recordings, slices were placed into a recording chamber (∼0.4 ml volume) on the microscope stage (Axioscope FS, Zeiss, Oberkochen, Germany) equipped with phase contrast optics. Slices were submerged with a constant flow of oxygenated ACSF. Flow rate was set to 1 ml min−1 using a gravity-driven manually operated superfusion system. A 40× water immersion objective (Zeiss, Oberkochen, Germany) was used in all experiments. Inhibitory postsynaptic currents (IPSCs) were recorded using the whole-cell configuration of the patch-clamp technique. Intra-pipette solution contained (mm): 100 potassium gluconate, 50 KCl, 5 NaCl, 0.5 CaCl2, 5 EGTA, 25 Hepes, 2 MgATP and 0.3 GTP, with pH set to 7.2 with KOH. The osmolarity was 320 mosmol l−1. Pipette resistance was 3–5 MΩ, when filled with the above saline. Electrophysiological signals were acquired using an EPC-7 amplifier (List, Darmstadt, Germany), a 16-bit AD/DA board (ITC-16, HEKA Elektronik, Lambrecht, Germany), and TIDA 4.11 software (HEKA Elektronik). The signals were filtered at 3 kHz and sampled at a rate of 10 kHz. Liquid junction potentials (about 5 mV) were not corrected, unless otherwise stated. The chloride reversal potential was about −20 mV, which is close to its physiological value (Mienville, 1998). In the voltage-clamp mode, the holding potential was set to −70 mV.
Access resistance was controlled by applying hyperpolarizing pulses of 10 mV. Cell capacitance and access resistance values were obtained by fitting a monoexponential function to the capacitance artifacts. Only recordings with a series resistance below 40 MΩ were accepted. Series resistance compensation was not applied. Cells exhibiting more than 20% changes in the access resistance during an experiment were discarded.
Cajal–Retzius cell identification
The identification of CR cells in the mouse cortex was described elsewhere (Hestrin & Armstrong, 1996; Radnikow et al. 2002). Briefly, CR cells were visually selected according to morphological criteria: (1) location in layer I, (2) horizontal orientation, (3) large ovoid soma, and (4) one thick tapered dendrite typically extending in parallel to the pial surface. Electrophysiologically, CR cells have been shown to exhibit (1) a relatively depolarized resting potential (Mienville & Pesold, 1999), and (2) a hyperpolarization-activated inward Ih current (Kilb & Luhmann, 2000).
Strychnine (30 μm), a glycine receptor antagonist, did not affected synaptic responses, while both spontaneous and evoked synaptic currents were completely and reversibly blocked by bicuculline methiodide (10 μm) or gabazine (20 μm) revealing their GABAergic nature (data not shown; Kirmse & Kirischuk, 2006a). In the present study, GABAA receptor-mediated postsynaptic currents will be referred to as ‘inhibitory’ postsynaptic currents (IPSCs) even though the action of GABA is depolarizing in CR cells (Mienville, 1998). It is worth mentioning that strychnine, GABAA and GABAB receptor blockers and the GAT-2/3 inhibitor SNAP-5114 did not affect either resting membrane potential or membrane resistance of CR cells suggesting that at least under these experimental conditions CR cells do not exhibit GABAA receptor-mediated tonic conductance.
Peak-scaled non-stationary noise analysis
For peak-scaled non-stationary analysis fast and slowly rising mIPSCs were divided into two groups and analysed separately using in-house-written software (C. Henneberger, Institute of Neurophysiology, Berlin). mIPSCs distorted by superimposed preceding or subsequent events were excluded from analysis. Within these groups, rise times and decay time constants were independent of the amplitude (Spearman rank test, P > 0.10 for all evaluated recordings). Analysis was then carried out as described (Traynelis et al. 1993). Briefly, a 250 ms long average mIPSC waveform was calculated from at least 40 mIPSCs, amplitude-scaled to each individual mIPSC and subtracted. The decay phase was divided into 25 bins on the basis of equal fractional reductions of the amplitude. For each bin, the average mIPSC amplitude (Ii) and variance (σ2i) with respect to the average waveform were determined. Ii and σ2i were averaged over all IPSCs. The initial part of this correlation was fitted by the linear equation σ2i=isIi+σ2b where is denotes the weighted mean single-channel current. Baseline noise (σ2b) was determined independently from a region devoid of events.
Electrical stimulation
Evoked postsynaptic currents were elicited by focal electrical stimulation through a glass pipette filled with ACSF (about 10 MΩ). In this case, N-(2,6-dimethy-lphenylcarbamoylmethyl)-triethylammonium bromide (QX 314, 2 mm) was added to the intracellular solution to prevent generation of action potentials in the tested neurons (Connors & Prince, 1982). An isolated stimulation unit was used to generate rectangular electrical pulses. Pulse duration was set to 0.5 ms. Pulse intensity was adjusted to activate a unitary synaptic input (minimal stimulation). Stimulation was accepted as minimal if the following criteria were satisfied: (1) eIPSC latency remained stable (< 20% fluctuations); (2) lowering stimulus intensity by 20% resulted in a complete failure of eIPSCs; and (3) an increase in stimulus intensity by 20% changed neither mean eIPSC amplitude nor eIPSC shape (see online supplemental material, Supplemental Fig. 1A and B). Typical pulse intensity required for minimal stimulation was between 1 and 2 μA.
Figure 1. Fast and slowly rising mIPSCs in a CR cell.
A, sample trace displays a 1 min recording of mIPSCs. The inset shows two consecutive mIPSCs displaying fast and slow rise kinetics. B, examples of fast (left) and slowly (right) rising mIPSCs. C and D, individual slowly (grey) and fast (black line) rising mIPSCs were amplitude scaled to illustrate that they have different rise kinetics (C), but similar decay kinetics (D).
Reversal potentials of eIPSCs were obtained using the standard (see above) and low chloride-containing intrapipette solutions. The latter contained (mm): 135 potassium gluconate, 15 KCl, 5 NaCl, 0.5 CaCl2, 5 EGTA, 25 Hepes, 2 MgATP and 0.3 GTP, with pH set to 7.2 with KOH. In this set of experiments, potentials were corrected for liquid junction potentials (5 mV for 50 mm intrapipette [Cl−] and 9 mV for 15 mm intapipette [Cl−]). Local application of GABA in the presence of TTX revealed that intracellular QX-314 led to about 6 mV shift of the reversal potential of GABAAR-mediated currents to more positive values. This effect of QX-314 was not corrected.
To estimate the size of the readily releasable pool (RRP), we used high frequency stimulation (Schneggenburger et al. 1999; Lu & Trussell, 2000; Kirischuk & Grantyn, 2003). Repetitive stimulation leads to a decrease in the eIPSC amplitudes. Assuming that the eIPSC depression is largely caused by a transient decrease in the number of readily releasable quanta, it is possible to estimate the RRP size on the basis of the cumulative eIPSC amplitude plot (Schneggenburger et al. 1999; Lu & Trussell, 2000; Kirischuk & Grantyn, 2003). Namely, stimulation trains of 40 pulses delivered at 20 Hz were applied (Kirmse & Kirischuk, 2006a) and cumulative eIPSC amplitudes were plotted versus stimulus number. Because repetitive stimulation causes a compound postsynaptic response (synchronous and asynchronous), IPSCs that peaked within a 3 ms interval following the end of a stimulus pulse were selected as stimulus-locked eIPSCs (Kirischuk & Grantyn, 2003). After 10–20 pulses, the cumulative eIPSCs reached a steady state, as indicated by the linear slope dependence of the cumulative eIPSC amplitude on the pulse number (Fig. 6B). Assuming that (1) the number of release sites remains constant throughout the experiment and (2) the linear component reflects vesicle recycling, the cumulative IPSC amplitude in the absence of pool replenishment can be estimated by back-extrapolation to the start of the train. Note that RRP estimations were performed without preceding or following mIPSC measurements. Therefore, RRP size in this case has a dimension of picoamperes and represents the product of the number of release sites and the quantal amplitude. Release probability (Pr) was calculated using the binomial model approximation, namely, Pr= mean eIPSC/RRP.
Figure 6. eIPSCS connections have higher release probability.
A, sample trace shows a compound IPSC elicited by a 20 Hz train of 40 pulses. The inset demonstrates late eIPSCs induced after RRP depletion. The mean amplitude of late eIPSCs has been taken as an estimate for the quantal amplitude. B, cumulative eIPSC amplitude plot. To obtain the RRP size, the last 20 points (from the 21st to 40th pulses) were fitted by linear regression (line), and back-extrapolated to time 0 (see Methods). C, mean late eIPSC amplitudes at different connections. D, the mean amplitude of late eIPSCF was significantly larger than mean late eIPSCS amplitude. E, RRP estimates obtained at different connections. F, RRP size did not depend on the type of connection. G, calculated release probability (Pr) at different synapses. H, release probability at eIPSCF connections was significantly smaller than at eIPSCS ones. *P < 0.05, **P < 0.01.
To obtain the number of vesicles in the RRP, an estimate of the number of release sites, the obtained RRP values were normalized to the mean amplitude of late eIPSCs. The latter was defined as the mean eIPSC amplitude in response to the last 20 stimuli in 20 Hz trains excluding failures (Fig. 6A). Here, we assumed that the RRP is depleted after about 20 pulses, and the rate of new vesicle recruitment determines the rate of late eIPSC occurrence. As a result, the latter should approach the quantal amplitude. Indeed, during 20 Hz trains the mean late eIPSC amplitude has been shown to be close to the median mIPSC amplitude in this preparation (Kirmse & Kirischuk, 2006a). It should be mentioned that only late eIPSCs but not asynchronous IPSCs (Lu & Trussell, 2000; Kirischuk et al. 2005) were selected to obtain an estimate of the quantal amplitude for the projection of interest. We did it because GABA is an excitatory neurotransmitter in the neocortex at this age, and asynchronous events occurring during high-frequency trains can reflect a GABA-driven recurrent activity of neocortical neuronal network and originate at synapses which do not belong to the stimulated axon.
Inhibitory postsynaptic potentials (IPSPs) and action potentials in CR cells were recorded in the current-clamp mode. Current injection was used to hold CR cells at about −60 mV, which is close to their resting potentials at this (P5–7) age (Zhou & Hablitz, 1996; Kilb & Luhmann, 2001). When action potentials were investigated, the intrapipette solution did not contain QX-314.
Solutions and chemicals
All experiments were performed at room temperature (22–25°C), unless otherwise stated (Figs 4 and 5). During experiments, 10 μm 6,7-dinitroquinoxaline-2,3-dione (DNQX, an AMPA/kainate receptor antagonist) and 50 μm dl-2-amino-5-phosphonopentanoic acid (APV, an NMDA receptor blocker) were added to the ACSF to block glutamatergic currents. In experiments using a lower (1 mm) Ca2+ solution, Ca2+ was replaced with an equimolar concentration of Mg2+ to keep the total concentration of divalent ions constant. Miniature IPSCs (mIPSCs) were recorded in the presence of tetrodotoxin (TTX, 1 μm). TTX was obtained from Alomone Laboratories (Jerusalem, Israel). 2S-3-[[(1S)-1-(3,4-Dichlorophenyl)ethyl]amino-2-hydroxypropyl] (phenylmethyl)phosphinic acid (CGP55845) was from Tocris (Bristol, UK). All other chemicals were obtained from Sigma-Aldrich (Munich, Germany).
Figure 4. Two types of eIPSCs.
A, original recordings showing individual eIPSCF (left) and eIPSCS induced by paired-pulse (ISI = 50 ms) stimulation in layer I. B, averaged eIPSCF (left) and eIPSCS (right) responses. Traces are an average of 40 trials. Data obtained from the same two CR cells as in A. C, amplitude scaled eIPSCF and eIPSCS responses are shown to illustrate the difference in the rise kinetics. The same traces as in B. D, rise time distributions of eIPSCF (open bars, n = 130) and eIPSCS (shaded bars, n = 179). Data obtained from the same two CR cells as in A. E, the mean eIPSC amplitudes are plotted versus their rise times. Data obtained from 75 CR cells. F, statistical data showing that the mean amplitudes of eIPSCF and eIPSCS were not significantly different both at room and at near physiological temperatures.
Figure 5. eIPSCF and eIPSCS display different paired-pulse plasticity.
A, eIPSCs recorded at different holding potentials. Traces are an average of 10 trials. B, reversal potentials of eIPSCF (filled symbols) and eIPSCS (open symbols) do not differ. Experiments were performed using two intracellular solutions containing 50 and 15 mm Cl−. C, PPR as a function of eIPSC rise times. Data obtained from 75 CR cells. D, eIPSCF and eIPSCS differ in paired-pulse ratio both at room and near physiological temperatures. **P < 0.01.
Data evaluation and statistics
Data were evaluated off-line using TIDA 5.0 (HEKA Elektronik). mIPSCs were analysed using PeakCount v3.2 software (C. Henneberger, Institute of Neurophysiology, Berlin). The program employs a derivative threshold-crossing algorithm to detect individual mIPSCs. Each automatically detected event is displayed for visual inspection. mIPSC rise times and decay time constants (a single exponential fit) can be also obtained. All results are presented as means ± s.e.m. The error bars in all figures indicate s.e.m. Differences between means were tested for significance using paired Student's t test, unless otherwise stated.
Results
Two populations of mIPSCs in CR cells
Spontaneous postsynaptic currents in neocortical CR cells have been observed in mice (Soda et al. 2003; Kirmse & Kirischuk, 2006a) and rats (Kilb & Luhmann, 2001; Radnikow et al. 2002). The synaptic currents were not sensitive to DNQX and APV, specific antagonists of AMPA and NMDA receptors, but they were completely and reversibly blocked by bicuculline methiodide (10 μm) or gabazine (20 μm), selective GABAA receptor blockers, revealing their GABAergic nature. Interestingly, we have observed fast and slowly rising mIPSCs (Fig. 1A–C), while both types of mIPSCs displayed no apparent difference in their decay rates (Fig. 1D). To corroborate the observation, we have first characterized the basic properties of mIPSCs in neocortical CR cells.
The frequency of mIPSCs was relatively low (0.07 ± 0.01 Hz, n = 72) in CR cells. Small number of synaptic events can radically modify the results of mIPSC analysis. To overcome the problem, we used the two following approaches. Firstly, we selected 11 cells from which more than 100 mIPSCs were collected. Secondly, neocortical slices were preincubated for 10 min with 10 μm of N-ethylmaleimide (NEM, n = 6). NEM, a sylfhydryl alkylating agent, uncouples pertussis toxin-sensitive Gi/o-type G proteins from receptors (Jakobs et al. 1982). In CR cells, NEM drastically increases mIPSC frequency, not affecting, however, mIPSC amplitudes and kinetics (Kirmse & Kirischuk, 2006b). Therefore, the NEM treatment shortens the time required to record a reasonable number of mIPSCs (> 100) to a couple of minutes. Because similar results were obtained using both approaches, the data obtained were pooled.
Miniature IPSCs had variable amplitudes, resulting in a skewed amplitude distribution (Fig. 2A and B). Fast and slowly rising mIPSCs were observed in all tested CR cells. Consequently, the distribution of 10–90% rise times showed a bimodal distribution (Fig. 2C and D). This result suggests the presence of at least two populations of mIPSCs. Indeed, the distribution of mIPSC rise times was better fitted with the sum of two Gaussians than with a single Gaussian function (Fig. 2D, P < 0.01, F test). In all CR cells tested, the centre of one Gaussian was observed at times shorter than 1 ms, while the other peaked at times longer than 1 ms. Mean rise times obtained by fitting two Gaussians were 0.52 ± 0.04 and 1.22 ± 0.05 ms, respectively (n = 17, P < 0.001). Therefore, to separate fast and slowly rising mIPSCs, we applied the following rule. Synaptic events that had a rise time of less than 1 ms were defined as fast rising mIPSCs (mIPSCF), while those with rise times longer than 1 ms were defined as slowly rising mIPSCs (mIPSCS). mIPSCF showed highly variable amplitudes, ranging from tens to hundreds of picoamperes, while mIPSCS amplitudes were distributed over a more narrow range (usually < 50 pA, Fig. 2G). Smaller amplitudes of mIPSCS could easily be explained by dendritic filtering of some synaptic currents originating distally on the dendritic tree. If this were the case, mIPSCS should also display slower decay kinetics. However, decay time constants (a monoexponential fit) showed a single Gaussian distribution (Fig. 2E and F). Moreover, there was no significant correlation between mIPSC rise times and their decay time constants (Fig. 2H). In general, only in 2 out of 17 CR cells mIPSCS and mIPSCF decay time constants were significantly different. It is worth mentioning that in some cases (7 out of 17 CR cells) a sum of two exponential functions described the decay of mIPSCF better than a monoexponential fit. Therefore, we also compared half-decay (T50) times of mIPSCS and mIPSCF. Both decay time constants (19.9 ± 1.3 and 19.7 ± 1.8 ms, P = 0.8) and T50 (9.9 ± 0.5 and 10.3 ± 0.6 ms for mIPSCF and mIPSCS, respectively, P = 0.24, n = 17, Fig. 2J) did not differ significantly. Although these results support the hypothesis of two different mIPSC populations, one cannot exclude the possibility that fast rise kinetics is more sensitive to dendritic filtering than a much slower process – mIPSC decay.
Figure 2. Properties of mIPSCs recorded in a CR cell.
A, mIPSC amplitudes showed large variability, but did not show an apparent drift throughout the experiment. B, mIPSC amplitude distribution was skewed to the right. C, mIPSC rise times (10–90%) displayed large variability, but did not show any temporal drift (> 12 min). D, mIPSC rise time distribution could be better described by the sum of two Gaussian functions (0.5 ± 0.13 and 1.05 ± 0.91 ms, mean ± s.d., n = 320). E, mIPSC decay time constants (a single exponential fit) were variable, but remained constant during the recordings. F, decay time constant distribution was close to a Gaussian distribution (16.5 ± 4.7 ms, mean ± s.d., n = 280). G, mIPSC rise times did not correlate with mIPSC amplitude. H, plot of mIPSC rise time against mIPSC decay time constant. Note lack of correlation between these two parameters. Panels A–H show data obtained from the same CR cell. I and J, statistical data obtained from 17 CR cells. mIPSCF amplitudes were larger than mIPSCS amplitudes (I), but both types of mIPSCs showed similar half-decay (T50) times and decay time constants (monoexponential fit, J). ***P < 0.001.
Different postsynaptic GABAA receptors mediate mIPSCF and mIPSCS
The median amplitude of mIPSCS (24.2 ± 1.6 pA) was significantly smaller than the median amplitude of mIPSCF (55.1 ± 5.2, n = 17, P < 0.001, Fig. 2I). Smaller amplitudes of mIPSCS may result from a variety of reasons, including dendritic filtering, smaller number of postsynaptic GABAA receptors and/or smaller single-channel conductance of GABAA receptors mediating mIPSCs. If mIPSC decay is not significantly modified by dendritic filtering, unitary conductance of GABAAR channels can be estimated by the peak-scaled non-stationary noise analysis of mIPSCs (Traynelis et al. 1993). We applied the following rules to separate mIPSCS and mIPSCF. mIPSCs were selected as mIPSCF if: (1) the rise time was smaller than 1 ms and (2) mIPSC amplitude was larger than 100 pA. mIPSCS were defined as having the rise time between 1 and 1.5 ms. Figure 3A and B summarizes the procedure applied to determine the variance from two groups of mIPSCs and shows the relationships between mean current and peak-scaled variance obtained for representative mIPSCF and mIPSCS populations (see Methods). Assuming a calculated Cl− driving force of 50 mV, we obtained a value for the mean weighted single-channel conductance of 34.4 ± 3.9 pS for mIPSCF and 18.4 ± 3.8 pS for mIPSCS (P < 0.001, n = 9, Fig. 3C). The observed difference in the single-channel conductance suggests that different GABAA receptors mediate mIPSCS and mIPSCF.
Figure 3. Different GABAA receptors mediate mIPSCF and mIPSCS.
A, individual mIPSCF and mIPSCS superimposed to the amplitude-scaled waveforms (continuous lines) of mIPSCF (top) and mIPSCS (bottom). B, representative relationships between mean current and peak-scaled variance obtained for mIPSCF and mIPSCS, approximated by the equation  (continuous lines, see Method). C, weighted mean single-channel conductance of GABAA receptors mediating mIPSCF was significantly larger as compared to those generating mIPSCS. The holding potential was −70 mV; the Nernst equilibrium potential for Cl− was −20 mV. D, sample traces show mIPSCs recorded in control and in the presence of zolpidem (100 nm). The insets show mIPSCF and mIPSCS. E and F, statistical data obtained from 18 CR cells. Zolpidem (Zol.) did not affect the median amplitudes of either mIPSCF or mIPSCS (E), but significantly increased only mIPSCS decay time constant (F). ***P < 0.001.
(continuous lines, see Method). C, weighted mean single-channel conductance of GABAA receptors mediating mIPSCF was significantly larger as compared to those generating mIPSCS. The holding potential was −70 mV; the Nernst equilibrium potential for Cl− was −20 mV. D, sample traces show mIPSCs recorded in control and in the presence of zolpidem (100 nm). The insets show mIPSCF and mIPSCS. E and F, statistical data obtained from 18 CR cells. Zolpidem (Zol.) did not affect the median amplitudes of either mIPSCF or mIPSCS (E), but significantly increased only mIPSCS decay time constant (F). ***P < 0.001.
To corroborate this observation, we examined the effects of a low concentration (100 nm) of zolpidem, a benzodiazepine agonist, on mIPSCF and mIPSCS. At this concentration zolpidem specifically modulates GABAA receptors containing α1 and γ2 subunits (Pritchett & Seeburg, 1990). In this set of experiments, brain slices were pretreated with 10 μm NEM for 10 min to elevate mIPSC frequency (Kirmse & Kirischuk, 2006b). The median amplitudes of both mIPSCF and mIPSCS were not significantly affected by zolpidem (Fig. 3D–F). However, the decay of mIPSCS (Fig. 3F), but not mIPSCF (Fig. 3E), was significantly prolonged by 100 nm zolpidem. The decay time constant of mIPSCS increased from 20.2 ± 1.3 in control to 29.3 ± 2.5 ms in the presence of zolpidem (P < 0.001, n = 18). At the same time, mIPSCS rise time was not significantly changed by zolpidem (1.28 ± 0.07 and 1.26 ± 0.07 ms in control and in the presence of zolpidem, respectively, P > 0.85, n = 18, data not shown). These results confirm that distinct GABAA receptors mediate mIPSCS and mIPSCF and show that α1γ2 subunit-containing GABAA receptors contribute to mIPSCS but not to mIPSCF.
eIPSCF and eIPSCS differ in paired-pulse plasticity
mIPSCs represent postsynaptic responses generated by the content of a single vesicle. The observation that there are two populations of mIPSCs raises the question whether both types of postsynaptic responses can be generated by a unitary connection. To address this question, we recorded evoked IPSCs (eIPSCs). The latter were elicited using a local electrical stimulation in layer I, and stimulation intensity was adjusted to activate only one axon, i.e. minimal stimulation (see Methods and Supplemental Fig. 1). When seeking a synaptic input on CR cells, both fast and slowly rising evoked IPSCs were observed in most of the tested cells. Unfortunately, low stimulation rate (0.1 Hz) has to be used to prevent a run-down of synaptic responses. As a consequence, it was not possible to acquire a reasonable (for a quantitative analysis) number of different types of synaptic responses from one CR cell. Therefore, only one type of eIPSCs has been recorded from each CR cell. Similar to mIPSCs, eIPSCs displayed different rise kinetics (Fig. 4A–D). Fast rising (< 1 ms) eIPSCs (eIPSCF) had the mean rise time of 0.58 ± 0.02 ms (n = 57), while the mean rise time of slowly rising eIPSCs (eIPSCS) was 1.24 ± 0.04 ms (n = 18). Decay time constants were not significantly different (21 ± 3 and 23 ± 2 ms for eIPSCF and eIPSCS, respectively). eIPSCS were observed in about 1/4 cases (18 out of 75 blindly selected connections). Although the mean amplitude of eIPSCS was slightly smaller than the mean amplitude of eIPSCF, this difference was not statistically significant (96 ± 11 and 84 ± 10 pA for eIPSCF (n = 57) and eIPSCS (n = 18), respectively, P = 0.53, unpaired Student's t test, Fig. 4E and F). Similar results were obtained at near physiological temperature. Corresponding values were 121.5 ± 27.5 and 87.9 ± 25.8 pA for the mean amplitude of eIPSCF (n = 12) and eIPSCS (n = 5), respectively (P = 0.35, unpaired Student's t test, Fig. 4F).
Smaller amplitude and slower rise kinetics of eIPSCS may mean a more distal position of corresponding synapses. The extended geometry of CR cells may result in an imperfect space clamp, which can alter the apparent time course of synaptic currents at distant locations. To examine the possibility, we determined reversal potentials of eIPSCF and eIPSCS (Fig. 5A and B). The obtained values were not significantly different (−11.2 ± 1.5 and −11.8 ± 0.9 mV for eIPSCF (n = 6) and eIPSCS (n = 5), respectively, P > 0.9, unpaired Student's t test). In addition, we performed a set of experiments using low Cl– containing (15 mm) intracellular solution. High [Cl−] concentration in CR cells is maintained by the sodium-dependent Cl− pump NKCC1 (Achilles et al. 2007). Therefore, one can expect a larger shift in reversal potential for synaptic inputs close to the cell body, where [Cl−] is almost clamped, than at distal sites, where the activity of membrane chloride pumps can maintain high internal chloride concentration. However, in this case eIPSCF and eIPSCS reversal potentials were also not significantly different (−26 ± 2 and −28 ± 2 mV for eIPSCF (n = 5) and eIPSCS (n = 4), respectively, P > 0.6, unpaired Student's t test, Fig. 5B) arguing against a more distal location of IPSCS synapses.
Next, we applied 100 nm zolpidem to examine whether different postsynaptic GABAA receptors mediate eIPSCF and eIPSCS. Zolpidem, similar to its effect on mIPSCs, did not influence mean amplitudes of either eIPSCF or eIPSCS. However, eIPSCS decay time constant increased from 21 ± 2 ms in control to 26 ± 2 ms in the presence of zolpidem (n = 5, P < 0.001), while eIPSCF decay rate was not affected (19 ± 2 and 20 ± 2 ms in control and in the presence of zolpidem, respectively, n = 6, P > 0.2, data not shown). These data show that distinct GABAA receptors contribute to eIPSCF and eIPSCS generation.
In addition, paired-pulse ratio (PPR) was significantly higher in the case of eIPSCF (1.49 ± 0.08 and 1.01 ± 0.05 for eIPSCF (n = 57) and eIPSCS (n = 18), respectively, P < 0.01, unpaired Student's t test, Fig. 5C and D). Similar results were obtained at near physiological temperature (1.5 ± 0.1 and 0.97 ± 0.03 for eIPSCF (n = 12) and eIPSCS (n = 5), respectively, P < 0.01, unpaired Student's t test, Fig. 5D). Because the mean amplitude of mIPSCS was significantly smaller than that of mIPSCF (Fig. 2I), comparable mean eIPSC amplitudes suggest that an action potential releases a larger number of synaptic vesicles at IPSCS connections. This may mean that IPSCS axons make more synaptic contacts and/or that synapses generating IPSCS have a higher release probability. The above observation that PPR was smaller at IPSCS connections supports the latter possibility. Nevertheless, we inspected both possibilities separately.
First, we estimated the size of the readily releasable pool (RRP) using a high frequency stimulation and cumulative eIPSC amplitude plot (see Methods, Fig. 6A and B). In our previous study (Kirmse & Kirischuk, 2006a), we have shown that 20 Hz trains of 40 pulses can be used for the RRP estimation. However, the RRP size obtained in this way is a product of the number of release sites and the quantal amplitude. To quantify the latter parameter, we have used the mean amplitude of late eIPSCs, i.e. eIPSCs elicited by the last 20 pulses in trains (Fig. 6A, inset). Similar to mIPSCs, the mean amplitudes of late eIPSCs at IPSCF connections were significantly larger than those at IPSCS synapses (54 ± 6 and 32 ± 3 pA for IPSCF (n = 22) and IPSCS (n = 10), respectively, P < 0.05, unpaired Student's t test, Fig. 6C and D). RRP values were not significantly different at IPSCF and IPSCS connections (12.1 ± 1.5 and 14.4 ± 2.5 for IPSCF (n = 22) and IPSCS (n = 10), P = 0.36, unpaired Student's t test, Fig. 6E and F). However, the calculated release probability (Pr) was significantly lower at IPSCF synapses (0.12 ± 0.01 and 0.19 ± 0.01 for IPSCF (n = 22) and IPSCS (n = 10), respectively, P < 0.01, unpaired Student's t test, Fig. 6G and H). We conclude that IPSCF and IPSCS connections contain a comparable number of readily releasable vesicles, but IPSCS connections have a higher release probability.
However, calculated release probability values are strongly dependent on the precision of the RRP estimates. RRP overestimation, for example due to desensitization of postsynaptic GABAA receptors, could result in a higher Pr value. In the frame of the binomial model, obtained RRP and Pr values allow calculation of the coefficient of variation (CV) of eIPSC amplitudes as CV =[(1 −Pr)/(RRP ×Pr)]1/2. If the estimates were correct, the predicted CVs should strongly correlate with the CV (CV =s.d./mean) of recorded eIPSCs. This was indeed the case (Supplemental Fig. 2A). Secondly, release probability was shown to affect various forms of short-term plasticity including PPR (Zucker & Regehr, 2002). In general, high release probability is usually associated with paired-pulse depression, while low Pr is associated with paired-pulse facilitation. If the obtained Pr values were correct, PPR and calculated Pr should exhibit a negative correlation. A strong correlation between PPR and Pr was observed (Supplemental Fig. 2B). These tests confirm the reliability of calculated RRP and Pr parameters.
IPSCS synapses are less inhibited by tonically activated presynaptic GABAB receptors
We have recently reported that GABAergic transmission on CR cells is inhibited via tonically activated presynaptic GABAB receptors (GABABRs) (Kirmse & Kirischuk, 2006a). Therefore, we asked whether the strength of GABABR-mediated inhibition is similar at IPSCF and IPSCS synapses. GABABRs were blocked by 1 μm of CGP55845, a specific GABABR antagonist. CGP55845 increased the mean eIPSC amplitude and decreased PPR at both types of connections (Fig. 7A). At eIPSCF synapses, PPR decreased from 1.68 ± 0.17 in control to 0.89 ± 0.11 in the presence of CGP55845 (P < 0.001, n = 16, Fig. 7B and C). At eIPSCS connections, PPR declined from 1.05 ± 0.05 in control to 0.87 ± 0.08 in the presence of CGP55845 (P < 0.05, n = 7, Fig. 7B and C). Interestingly, eIPSCF and eIPSCS synapses demonstrated similar PPR in the presence of CGP55845 (P > 0.8, unpaired Student's t test). This result suggests that the higher GABA release probability at IPSCS synapses may result from a weaker GABAB receptor-mediated inhibition at these connections. To investigate the suggestion, we applied 40 μm of SNAP-5114, a specific blocker of GABA transporter 2/3 (GAT-2/3). In our previous study, we have shown that GAT-2/3 releases GABA in neocortical layer I and this leads to presynaptic GABABR activation (Kirmse & Kirischuk, 2006a). If the above suggestion is correct, we should observe no difference in PPR of IPSCF and IPSCS connections in the presence of SNAP-5114. Indeed, SNAP-5114 reduced PPR both of IPSCF synapses (from 1.39 ± 0.27 in control to 0.81 ± 0.16, P < 0.01, n = 8) and of IPSCS connections (from 0.93 ± 0.07 in control to 0.78 ± 0.08, P < 0.05, n = 4, Fig. 7D and E). In the presence of SNAP-5114, eIPSCF and eIPSCS synapses showed comparable PPR (P > 0.8, unpaired Student's t test). These data support the suggestion that the different release probabilities observed at IPSCF and IPSCS connections may be determined by the strength of GABABR-mediated inhibition at these two types of synapses.
Figure 7. IPSCS synapses show weaker tonic GABABR-mediated inhibition.
A, sample traces show paired-pulse stimulation-induced eIPSCF and eIPSCS in control and in the presence of CGP55845 (CGP, 1 μm). Traces represent an average of 40 sequential responses. B, PPR plotted versus eIPSC rise times in control (open symbols) and in the presence of CGP55845 (filled symbols). C, statistical data shows CGP55845 effects on PPR at IPSCF and IPSCS connections. D, PPR plotted versus eIPSC rise times in control (open symbols) and in the presence of 40 μm of SNAP-5114 (SNAP, filled symbols). E, statistical data show SNAP effects on PPR at IPSCF and IPSCS connections. *P < 0.05, **P < 0.01, ***P < 0.001, ns – not significant.
The weaker tonic GABABR-mediated inhibition of mIPSCS synapses is not due to lack of presynaptic GABAB receptors
The lower sensitivity of IPSCS connections to GABABR blockers could result from a lower density of presynaptic GABABRs at IPSCS contacts. To inspect this possibility, we applied the GABABR agonist baclofen (10 μm). In all cases baclofen strongly suppressed the mean amplitude of eIPSCs and increased PPR (Fig. 8A). At IPSCF synapses, baclofen increased PPR to 2.17 ± 0.26 from 1.44 ± 0.16 in control (n = 8, P < 0.001, Fig. 8B and C). At IPSCS connections, PPR increased from 1.13 ± 0.09 in control to 1.99 ± 0.16 in the presence of baclofen (P < 0.01, n = 4, Fig. 8B and C). In the presence of baclofen, there was no significant difference in PPR between IPSCF and IPSCS synapses (P = 0.65, unpaired Student's t test). These results suggest that the density of presynaptic GABABRs at synapses is comparable at IPSCS and IPSCF synaptic contacts.
Figure 8. GABAB receptor activation inhibits both IPSCF and IPSCS synapses.
A, sample traces show paired-pulse stimulation-induced eIPSCF and eIPSCS in control and in the presence of baclofen (Bac., 10 μm). Traces represent an average of 40 sequential responses. B, PPR plotted versus eIPSC rise times in control (open symbols) and in the presence of baclofen (filled symbols). C, statistical data showing that baclofen influences both IPSCF and IPSCS connections. D, PPR plotted versus eIPSC rise times in control (open symbols) and in the presence of CGP55845 (CGP, filled symbols). Recordings were performed in ACSF containing 1 mm Ca2+. E, statistical data showing CGP55845 effects on PPR at IPSCF and IPSCS connections. **P < 0.01, ***P < 0.001, ns – not significant.
However, a stronger saturation of postsynaptic GABAA receptors at IPSCS synapses may underlie the observed weaker CGP55845 sensitivity. If GABAARs at IPSCS synapses are close to saturation in control, the CGP55845-induced potentiation of GABA release will result only in a small increase in the mean amplitude of eIPSCs and will not report the real strength of presynaptic GABABR-mediated inhibition. To examine this possibility, we performed experiments using low (1 mm) Ca2+ containing ACSF. Lower extracellular Ca2+ concentration decreases the release probability and therefore alleviates the saturation of postsynaptic GABAA receptors. Thus, in low extracellular Ca2+ ACSF, the tonic GABABR-mediated inhibition of eIPSCS, if present, is expected to show up. However, also in 1 mm Ca2+-containing ACSF, CGP55845 affected IPSCF connections much more strongly than IPSCS synapses. At IPSCF synapses CGP55845 decreased PPR to 1.37 ± 0.14 from 2.39 ± 0.25 in control (n = 6, P < 0.001, Fig. 8D and E). At IPSCS connections PPR decreased from 1.47 ± 0.14 in control to 1.29 ± 0.17 in the presence of CGP55845 (P = 0.15, n = 4, Fig. 8D and E). In the presence of CGP55845 there was no significant difference in PPR between IPSCF and IPSCS synapses (P = 0.7, unpaired Student's t test). We therefore conclude that weaker activation of presynaptic GABABRs by extracellular GABA underlies the observed difference in tonic GABABR-mediated inhibition at IPSCF and IPSCS synapses.
Basic properties of eIPSPF and eIPSPS
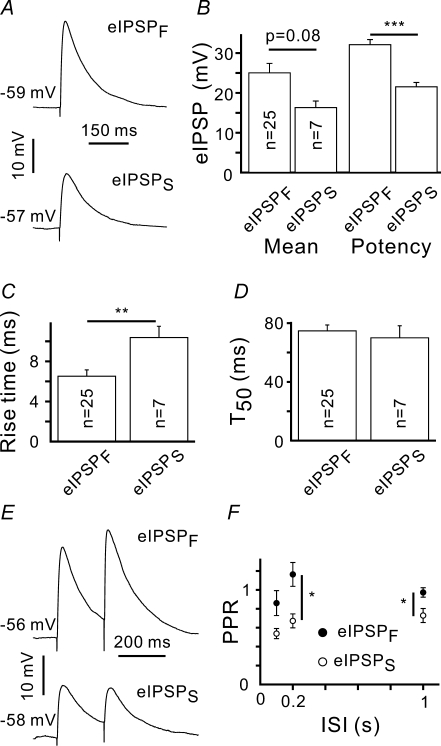
Because CR cells have high input resistance (Zhou & Hablitz, 1996; Kilb & Luhmann, 2001), even relatively small depolarizing synaptic currents can theoretically result in postsynaptic action potentials. Therefore, we examined evoked inhibitory postsynaptic potentials (eIPSP) mediated by fast (eIPSPF) and slow (eIPSPS) connections. The following experimental protocol was used. First, 20 eIPSCs were recorded in voltage clamp mode, to determine whether the input was IPSCF or IPSCS. Next, CR cells were held at −60 mV in current clamp mode, and eIPSPs were recorded (Fig. 9A). Like in the case of eIPSCs, mean eIPSPF amplitude (25 ± 2 mV, n = 25) was larger than the mean eIPSPS one (16 ± 2 mV, n = 7), but this difference was not statistically significant (Fig. 9B). On the other hand, eIPSPF and eIPSPS potencies (mean eIPSP amplitude excluding failures) differed significantly (32 ± 1 and 22 ± 1 mV for eIPSPF and eIPSPS, respectively, P < 0.001, Fig. 9B). As compared to eIPSPF, eIPSPS showed longer rise time (6.5 ± 0.6 and 10.4 ± 1.2 ms, P < 0.01, Fig. 9C), but similar half-decay time (74 ± 4 and 70 ± 8 ms for eIPSPF (n = 25) and eIPSPS (n = 7), respectively, P > 0.5, unpaired Student's t test). eIPSPF and eIPSPS showed different paired-pulse plasticity. eIPSPF displayed paired-pulse facilitation at ISI of 200 ms, while eIPSPS exhibited always paired-pulse depression (Fig. 9E and F). Even at reasonably long (1 s) ISI, eIPSPS displayed depression (0.73 ± 0.07, n = 6), while PPR at eIPSPF was close to 1 (0.97 ± 0.05, n = 13, P < 0.05, unpaired Student's t test).
Figure 9. Properties of eIPSPs elicited by stimulation of fast (eIPSPF) and slow (eIPSPS) projections.
A, averaged eIPSPF and eIPSPS responses. Traces are an average of 20 trials. B, statistical data showing that the mean amplitudes of eIPSPF and eIPSPS were not significantly different, while their potencies (mean amplitude excluding failures) differed. C and D, eIPSPS and eIPSCF differed in their rise times (C), but had similar half-decay times (D). E, averaged eIPSPF and eIPSPS responses induced by paired-pulse stimulation. Traces are an average of 20 trials. F, PPR as a function of ISI. *P < 0.05, **P < 0.01, ***P < 0.001.
Frequency-dependence of signal transfer by IPSCF and IPSCS connections
To investigate whether different properties of IPSPF and IPSPS influence the coupling between synaptic currents and CR cell firing, we inspected the efficacy of synaptic inputs. Efficacy was defined as the percentage of release events (eIPSPs) resulting in postsynaptic action potentials. Because the resting membrane potential of CR cells is about −60 mV and the action potential threshold is −45 mV, both eIPSPF- and eIPSPS-induced depolarization appears to be sufficient to elicit an action potential. Indeed, both types of inputs were highly efficient at low (0.1 Hz) stimulation frequency (76 ± 3 and 72 ± 4% for eIPSPF (n = 9) and eIPSPS (n = 6), respectively, P > 0.9, unpaired Student's t test, Fig. 10A and B). However, with increasing stimulation frequency, the efficacy of IPSPS inputs declined significantly more strongly than IPSPF synapses. Figure 10C shows representative firing responses of CR cells elicited by 40 stimuli delivered at 1 and 5 Hz to IPSCF and IPSCS connections. When stimulated by 1 Hz trains, IPSPF and IPSPS synapses induced postsynaptic action potentials in 65 ± 6% (n = 9) and 31 ± 5% (n = 6) of cases, respectively (P < 0.001, unpaired Student's t test, Fig. 10D). At 5 Hz the difference in efficacy between IPSPF (51 ± 5%, n = 9) and IPSPS inputs (7 ± 2%, n = 6) was even more drastic (P < 0.001, unpaired Student's t test, Fig. 10E). We conclude that both IPSPF and IPSPS inputs are equally effective at signalling the arrival of single presynaptic action potentials to a CR cell, but only IPSPF connections are capable of reliable signal transfer at higher frequencies.
Figure 10. IPSCF and IPSCS connection efficacies show different frequency dependence.
A, an action potential in a CR cell was elicited by stimulation of unitary GABAergic input. B, both IPSCF and IPSCS inputs were equally efficient when activated by 0.1 Hz trains. C, firing patterns of CR cells in response to 1 and 5 Hz stimulations (40 pulses) of IPSCF (upper) and eIPSCS (bottom traces) inputs. D and E, IPSCS synapses were less efficient than IPSCF connections when stimulated both by 1 (D) and 5 Hz (E) trains. ***P < 0.001.
Discussion
In the present study, we have shown that IPSCs recorded from neocortical CR cells fall into two distinct categories: slowly and fast rising responses. The mean amplitude of mIPSCF is considerably larger compared to mIPSCS. Postsynaptically, GABAA receptors mediating mIPSCF and mIPSCS differ in single-channel conductance and display different sensitivity to zolpidem. Presynaptically, IPSCF synapses have a lower resting release probability, as a consequence of tonic GABABR-mediated inhibition of GABA release. Despite the smaller quantal amplitudes, the higher release probability makes IPSCS synapses as effective as mIPSCF connections in eliciting a postsynaptic action potential in response to a single stimulus. However, the efficacy of IPSCS synapses declines dramatically as the presynaptic activity level increases.
Two types of IPSCs recorded from CR cells
Analysis of mIPSC rise time distributions allowed identification of two kinetically distinct populations of IPSCs recorded from CR cells. However, the extended morphology of CR cells leads to an imperfect space clamp, which can result in loss of voltage control and alteration of IPSC time course. The observation that the median amplitude of mIPSCS is about twofold smaller than that of mIPSCF gives further support to this possibility. However, several lines of evidence suggest that slow and fast mIPSCs represent two distinct GABA-gated currents. First, decay rates of mIPSCF and mIPSCS were not significantly different. Second, there was no obvious correlation between mIPSC amplitudes and rise times. Third, peak-scaled non-stationary noise analysis of mIPSCs revealed that GABAA receptors mediating IPSCF and IPSCS have different single-channel conductance. Fourth, the selective action of zolpidem on mIPSCS cannot be explained by space-clamp artifact. Two populations of mIPSCs that differed in their rise kinetics were also identified in granule cells of the olfactory bulb; and different postsynaptic GABAA receptors segregated to distinct synapses were suggested to underlie different kinetics of mIPSCs (Nusser et al. 1999). Because murine CR cells express various GABAA receptor subunits, namely α1, α2, α4, α5, β1, β2, β3, γ1, γ2 and γ3 (Cheng et al. 2006), segregation of different GABAA receptors to distinct synaptic sites seems to be a plausible explanation of our results.
In addition to mIPSCs, eIPSCs elicited by minimal stimulation in layer I also displayed slow and fast rise kinetics. In contrast to mIPSCs, eIPSC rise time distribution could be well fitted by a single Gaussian function (Fig. 4D) suggesting that a single projection predominantly generates IPSCs only of one kind. Selective effect of zolpidem on eIPSCS gives further support to the idea that similar GABAA receptors mediate m- and eIPSCS and m- and eIPSCF, respectively. However, also in this case, the slower onset of eIPSCS may be caused by dendritic filtering of identical responses generated at different locations. Several observations support the suggestion that two eIPSCs are indeed distinct. First, their decay rates did not significantly differ. Second, reversal potentials of eIPSCF and eIPSCS were the same both at close to physiological and at decreased intrapipette [Cl−]. Third, eIPSCF and eIPSCS displayed different paired-pulse plasticity, which should not be primarily dependent on the dendritic location of synaptic contacts. Fourth, slow and fast IPSCs differed in their sensitivity to both CGP55845 and SNAP-5114. The last two results evidence that eIPSCF and eIPSCS connections differ not only post- but also presynaptically.
Interestingly, similar results have been obtained in the adult hippocampus. Two kinetically distinct types of GABAA receptor-mediated IPSCs were observed in CA1 pyramidal neurons (Pearce, 1993). Fast and slow IPSCs were mediated by different GABAA receptors (Banks et al. 1998) and differed in paired-pulse plasticity (Pearce et al. 1995). Moreover, the observed difference in paired-pulse behaviour was in part dependent on the expression/activation of presynaptic GABAB receptors. In contrast to our results, however, in the hippocampus fast and large IPSCs were less sensitive to GABAB receptor modulators than slow and small IPSCs (Pearce et al. 1995). The authors suggested that relative insensitivity of fast IPSCs to GABAB receptors serves to prevent uncontrolled bursts of action potentials during repetitive activity. However, in contrast to the adult hippocampus, where GABA is an inhibitory neurotransmitter, GABA action is depolarizing in the developing cortex (Luhmann & Prince, 1991; Mienville, 1998; Owens et al. 1999). Because CR cells receive only GABAergic inputs (Kilb & Luhmann, 2001; Soda et al. 2003; Kirmse & Kirischuk, 2006a), GABAergic postsynaptic currents determine the firing pattern of CR cells. Consequently, stronger GABAB receptor-mediated inhibition of large IPSCF may protect CR cells and, in turn, the immature neuronal network of the neocortex from over-excitation.
Information transfer at IPSCS and IPSCF synapses
On the other hand, strong tonic inhibition of IPSCF may economize information transfer at fast synapses. Because mIPSCF are larger than mIPSCS, a smaller number of vesicles has to be simultaneously released at IPSCF synapses to initiate an action potential in a CR cell. Using the binomial model of synaptic transmission, we characterized basic presynaptic properties of two GABAergic inputs. Our data show that release probability at IPSCS synapses was almost twofold higher than at IPSCF terminals (Fig. 6). As a consequence, the mean amplitudes of eIPSCF and eIPSCS, as well as eIPSPF and eIPSPS, did not significantly differ. Thus, postsynaptic weakness of IPSCS synapses (the smaller quantal size) seems to be almost compensated by higher presynaptic release probability. As a consequence, both inputs can comparably effectively transfer information at low frequencies (Fig. 10B). However, similar efficacy of IPSCF and IPSCS synapses in response to a single presynaptic action potential has an important consequence. On average, an action potential releases 1.4 vesicles at IPSCF synapses and 2.9 vesicles at IPSCS ones. Because both types of synapses have similar RRP size, higher levels of presynaptic activity will result in a faster RRP depletion at IPSCS synapses unless the RRP exhaustion is compensated by the higher rate of vesicle supply/recycling. The latter was not observed. The efficacy of IPSCS connections drastically declined already at stimulation frequency of 1 Hz. In contrast to IPSCS projections, the lower release probability at IPSCF synapses makes them reliable over a wider range of stimulation frequencies.
Possible physiological role of two GABAergic inputs
An important question is whether there is a necessity of high-frequency information transfer in layer I of the perinatal neocortex. Although detailed knowledge of the input structures to CR cells is not yet available, a transient GABAergic fibre plexus confined to layer I has been identified in the prenatal rodent cortex (Lauder et al. 1986). Three types of fibres have been proposed to contribute to this plexus: axons of intrinsic GABAergic cortical neurons in layer I (Marin-Padilla, 1998), axons of GABAergic neurons located in the subplate (Voigt et al. 2001), and extrinsic projections from the zona incerta of the ventral thalamus (Lin et al. 1990). Definitely, two types of IPSCs may be generated by two distinct populations of GABAergic neurons in layer I (Xiang et al. 2002). However, pair-cell recordings performed in layer I failed to uncover a GABAergic input to CR cells suggesting that synaptic inputs of CR cells are most likely derived from underlying layers of the neocortex or subcortical structures (Soda et al. 2003). The zona incerta has high metabolic activity at early developmental stages (Nicolelis et al. 1995) and neurons projecting to neocortical layer I exhibit high frequencies of spontaneous action potentials and occasional burst firing (Dammerman et al. 2000). Incertocortical projections synapse on the distal dendrites of pyramidal neurons and, moreover, the postsynaptic depolarization induced by a single eIPSC was sufficient to evoke an action potential (Dammerman et al. 2000). Although it is not known yet if incertocortical axons synapse on CR cells, we suggest that IPSCF synapses would be capable of transferring the high activity level of projecting neurons in the zona incerta. Unfortunately, incertocortical projections cannot be preserved even in a 500 μm acute slice (Dammerman et al. 2000). The origin of IPSCS input is not identified either. However, giant GABAergic neurons located in the subplate have been proposed to project to layer I and, moreover, they fire at very low frequencies (Voigt et al. 2001). Our preliminary data show that electrical stimulation in the subplate induced only slowly rising eIPSCs in CR cells and, moreover, very low stimulation frequencies (< 0.1 Hz) had to be used to prevent a rapid run-down of responses. We therefore suggest that IPSCS input to CR cells could be derived from the subplate. Taken together, CR cells could receive and integrate functionally distinct inputs, presumably from the thalamus and the subplate (see Supplemental Fig. 3). As CR cells make synapses on apical dendrites of pyramidal neurons, their activity pattern may contribute to synaptogenesis in the developing cortical plate (see Supplemental Fig. 3).
Acknowledgments
The technical assistance of Mrs Kerstin Rückwardt is highly appreciated. This study was supported by German Research Council (Deutsche Forschungsgemeinschaft, KI1093/1-1 to S.K.). K.K. has received a scholarship from DFG as a member of the Graduate School (Graduiertenkolleg 238).
Supplemental material
Online supplemental material for this paper can be accessed at: http://jp.physoc.org/cgi/content/full/jphysiol.2007.145003/DC1 and http://www.blackwell-synergy.com/doi/suppl/10.1113/jphysiol.2007.145003
References
- Achilles K, Okabe A, Ikeda M, Shimizu-Okabe C, Yamada J, Fukuda A, Luhmann HJ, Kilb W. Kinetic properties of Cl− uptake mediated by Na+-dependent K+-2Cl− cotransport in immature rat neocortical neurons. J Neurosci. 2007;27:8616–8627. doi: 10.1523/JNEUROSCI.5041-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilo A, Schwartz TH, Kumar VS, Peterlin ZA, Tsiola A, Soriano E, Yuste R. Involvement of Cajal-Retzius neurons in spontaneous correlated activity of embryonic and postnatal layer 1 from wild-type and reeler mice. J Neurosci. 1999;19:10856–10868. doi: 10.1523/JNEUROSCI.19-24-10856.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrieux M, Platel JC, Dupuis A, Villaz M, Moody WJ. Early expression of sodium channel transcripts and sodium current by Cajal-Retzius cells in the preplate of the embryonic mouse neocortex. J Neurosci. 2004;24:1719–1725. doi: 10.1523/JNEUROSCI.3548-02.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks MI, Li TB, Pearce RA. The synaptic basis of GABAA,slow. J Neurosci. 1998;18:1305–1317. doi: 10.1523/JNEUROSCI.18-04-01305.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviness VS., Jr Neocortical histogenesis in normal and reeler mice: a developmental study based upon [3H]thymidine autoradiography. Brain Res. 1982;256:293–302. doi: 10.1016/0165-3806(82)90141-9. [DOI] [PubMed] [Google Scholar]
- Cheng Q, Yeh PW, Yeh HH. Cajal-Retzius cells switch from expressing γ-less to γ-containing GABA receptors during corticogenesis. Eur J Neurosci. 2006;24:2145–2151. doi: 10.1111/j.1460-9568.2006.05122.x. [DOI] [PubMed] [Google Scholar]
- Connors BW, Prince DA. Effects of local anesthetic QX-314 on the membrane properties of hippocampal pyramidal neurons. J Pharmacol Exp Ther. 1982;220:476–481. [PubMed] [Google Scholar]
- Dammerman RS, Flint AC, Noctor S, Kriegstein AR. An excitatory GABAergic plexus in developing neocortical layer 1. J Neurophysiol. 2000;84:428–434. doi: 10.1152/jn.2000.84.1.428. [DOI] [PubMed] [Google Scholar]
- Friauf E, McConnell SK, Shatz CJ. Functional synaptic circuits in the subplate during fetal and early postnatal development of cat visual cortex. J Neurosci. 1990;10:2601–2613. doi: 10.1523/JNEUROSCI.10-08-02601.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frotscher M. Cajal-Retzius cells, Reelin, and the formation of layers. Curr Opin Neurobiol. 1998;8:570–575. doi: 10.1016/s0959-4388(98)80082-2. [DOI] [PubMed] [Google Scholar]
- Hestrin S, Armstrong WE. Morphology and physiology of cortical neurons in layer I. J Neurosci. 1996;16:5290–5300. doi: 10.1523/JNEUROSCI.16-17-05290.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobs KH, Lasch P, Minuth M, Aktories K, Schultz G. Uncoupling of α-adrenoceptor-mediated inhibition of human platelet adenylate cyclase by N-ethylmaleimide. J Biol Chem. 1982;257:2829–2833. [PubMed] [Google Scholar]
- Kilb W, Luhmann HJ. Characterization of a hyperpolarization-activated inward current in Cajal-Retzius cells in rat neonatal neocortex. J Neurophysiol. 2000;84:1681–1691. doi: 10.1152/jn.2000.84.3.1681. [DOI] [PubMed] [Google Scholar]
- Kilb W, Luhmann HJ. Spontaneous GABAergic postsynaptic currents in Cajal-Retzius cells in neonatal rat cerebral cortex. Eur J Neurosci. 2001;13:1387–1390. doi: 10.1046/j.0953-816x.2001.01514.x. [DOI] [PubMed] [Google Scholar]
- Kirischuk S, Grantyn R. Intraterminal Ca2+ concentration and asynchronous release at single GABAergic boutons in rat collicular cultures. J Physiol. 2003;548:753–764. doi: 10.1113/jphysiol.2002.037036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirischuk S, Juttner R, Grantyn R. Time-matched pre- and postsynaptic changes of GABAergic synaptic transmission in the developing mouse superior colliculus. J Physiol. 2005;563:795–807. doi: 10.1113/jphysiol.2004.081216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirmse K, Kirischuk S. Ambient GABA constrains the strength of GABAergic synapses at Cajal-Retzius cells in the developing visual cortex. J Neurosci. 2006a;26:4216–4227. doi: 10.1523/JNEUROSCI.0589-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirmse K, Kirischuk S. N-ethylmaleimide increases release probability at GABAergic synapses in layer I of the mouse visual cortex. Eur J Neurosci. 2006b;24:2741–2748. doi: 10.1111/j.1460-9568.2006.05179.x. [DOI] [PubMed] [Google Scholar]
- Lauder JM, Han VK, Henderson P, Verdoorn T, Towle AC. Prenatal ontogeny of the GABAergic system in the rat brain: an immunocytochemical study. Neuroscience. 1986;19:465–493. doi: 10.1016/0306-4522(86)90275-7. [DOI] [PubMed] [Google Scholar]
- Lin CS, Nicolelis MA, Schneider JS, Chapin JK. A major direct GABAergic pathway from zona incerta to neocortex. Science. 1990;248:1553–1556. doi: 10.1126/science.2360049. [DOI] [PubMed] [Google Scholar]
- Lu T, Trussell LO. Inhibitory transmission mediated by asynchronous transmitter release. Neuron. 2000;26:683–694. doi: 10.1016/s0896-6273(00)81204-0. [DOI] [PubMed] [Google Scholar]
- Luhmann HJ, Prince DA. Postnatal maturation of the GABAergic system in rat neocortex. J Neurophysiol. 1991;65:247–263. doi: 10.1152/jn.1991.65.2.247. [DOI] [PubMed] [Google Scholar]
- Marin-Padilla M. Cajal-Retzius cells and the development of the neocortex. Trends Neurosci. 1998;21:64–71. doi: 10.1016/s0166-2236(97)01164-8. [DOI] [PubMed] [Google Scholar]
- Mienville JM. Persistent depolarizing action of GABA in rat Cajal-Retzius cells. J Physiol. 1998;512:809–817. doi: 10.1111/j.1469-7793.1998.809bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mienville JM, Pesold C. Low resting potential and postnatal upregulation of NMDA receptors may cause Cajal-Retzius cell death. J Neurosci. 1999;19:1636–1646. doi: 10.1523/JNEUROSCI.19-05-01636.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolelis MA, Chapin JK, Lin RC. Development of direct GABAergic projections from the zona incerta to the somatosensory cortex of the rat. Neuroscience. 1995;65:609–631. doi: 10.1016/0306-4522(94)00493-o. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Mody I. Differential regulation of synaptic GABAA receptors by cAMP-dependent protein kinase in mouse cerebellar and olfactory bulb neurones. J Physiol. 1999;521:421–435. doi: 10.1111/j.1469-7793.1999.00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens DF, Liu X, Kriegstein AR. Changing properties of GABAA receptor-mediated signaling during early neocortical development. J Neurophysiol. 1999;82:570–583. doi: 10.1152/jn.1999.82.2.570. [DOI] [PubMed] [Google Scholar]
- Pearce RA. Physiological evidence for two distinct GABAA responses in rat hippocampus. Neuron. 1993;10:189–200. doi: 10.1016/0896-6273(93)90310-n. [DOI] [PubMed] [Google Scholar]
- Pearce RA, Grunder SD, Faucher LD. Different mechanisms for use-dependent depression of two GABAA-mediated IPSCs in rat hippocampus. J Physiol. 1995;484:425–435. doi: 10.1113/jphysiol.1995.sp020675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchett DB, Seeburg PH. γ-Aminobutyric acidA receptor α5-subunit creates novel type II benzodiazepine receptor pharmacology. J Neurochem. 1990;54:1802–1804. doi: 10.1111/j.1471-4159.1990.tb01237.x. [DOI] [PubMed] [Google Scholar]
- Radnikow G, Feldmeyer D, Lubke J. Axonal projection, input and output synapses, and synaptic physiology of Cajal-Retzius cells in the developing rat neocortex. J Neurosci. 2002;22:6908–6919. doi: 10.1523/JNEUROSCI.22-16-06908.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice DS, Curran T. Role of the reelin signaling pathway in central nervous system development. Annu Rev Neurosci. 2001;24:1005–1039. doi: 10.1146/annurev.neuro.24.1.1005. [DOI] [PubMed] [Google Scholar]
- Schneggenburger R, Meyer AC, Neher E. Released fraction and total size of a pool of immediately available transmitter quanta at a calyx synapse. Neuron. 1999;23:399–409. doi: 10.1016/s0896-6273(00)80789-8. [DOI] [PubMed] [Google Scholar]
- Schwartz TH, Rabinowitz D, Unni V, Kumar VS, Smetters DK, Tsiola A, Yuste R. Networks of coactive neurons in developing layer 1. Neuron. 1998;20:541–552. doi: 10.1016/s0896-6273(00)80993-9. [DOI] [PubMed] [Google Scholar]
- Soda T, Nakashima R, Watanabe D, Nakajima K, Pastan I, Nakanishi S. Segregation and coactivation of developing neocortical layer 1 neurons. J Neurosci. 2003;23:6272–6279. doi: 10.1523/JNEUROSCI.23-15-06272.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano E, del Rio JA. The cells of Cajal-Retzius: still a mystery one century after. Neuron. 2005;46:389–394. doi: 10.1016/j.neuron.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Super H, del Rio JA, Martinez A, Perez-Sust P, Soriano E. Disruption of neuronal migration and radial glia in the developing cerebral cortex following ablation of Cajal-Retzius cells. Cereb Cortex. 2000;10:602–613. doi: 10.1093/cercor/10.6.602. [DOI] [PubMed] [Google Scholar]
- Super H, Soriano E, Uylings HBM. The functions of the preplate in development and evolution of the neocortex and hippocampus. Brain Res Rev. 1998;27:40–64. doi: 10.1016/s0165-0173(98)00005-8. [DOI] [PubMed] [Google Scholar]
- Tissir F, Goffinet AM. Reelin and brain development. Nat Rev Neurosci. 2003;4:496–505. doi: 10.1038/nrn1113. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Silver RA, Cull-Candy SG. Estimated conductance of glutamate receptor channels activated during EPSCs at the cerebellar mossy fiber-granule cell synapse. Neuron. 1993;11:279–289. doi: 10.1016/0896-6273(93)90184-s. [DOI] [PubMed] [Google Scholar]
- Voigt T, Opitz T, de Lima AD. Synchronous oscillatory activity in immature cortical network is driven by GABAergic preplate neurons. J Neurosci. 2001;21:8895–8905. doi: 10.1523/JNEUROSCI.21-22-08895.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Z, Huguenard JR, Prince DA. Synaptic inhibition of pyramidal cells evoked by different interneuronal subtypes in layer V of rat visual cortex. J Neurophysiol. 2002;88:740–750. doi: 10.1152/jn.2002.88.2.740. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Assimacopoulos S, Jones KR, Grove EA. Massive loss of Cajal-Retzius cells does not disrupt neocortical layer order. Development. 2006;133:537–545. doi: 10.1242/dev.02209. [DOI] [PubMed] [Google Scholar]
- Zhou FM, Hablitz JJ. Postnatal development of membrane properties of layer I neurons in rat neocortex. J Neurosci. 1996;16:1131–1139. doi: 10.1523/JNEUROSCI.16-03-01131.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.