Abstract
Tumor protein D52 (TPD52) is involved in transformation and metastasis and has been shown to be over-expressed in tumor cells compared to normal cells and tissues. Murine TPD52 (mD52) shares 86% protein identity with the human TPD52 orthologue (hD52). To study TPD52 protein as a target for active vaccination recombinant, mD52 was administered as a protein-based vaccine. Naïve mice were immunized with either mD52 protein and CpG/ODN as a molecular adjuvant or CpG/ODN alone. Two weeks following the final immunization, mice were challenged s.c. with syngeneic tumor cells that over-express mD52. Two distinct murine tumor cell lines were used for challenge in this model, mKSA and 3T3.mD52. Half of the mice immunized with mD52 and CpG/ODN rejected or delayed onset of mKSA s.c. tumor cell growth, and 40% of mice challenged with 3T3.mD52 rejected s.c. tumor growth, as well as the formation of spontaneous lethal lung metastases. Mice immunized with mD52 and CpG/ODN generated detectable mD52-specific IgG antibody responses indicating that mD52 protein vaccination induced an adaptive immune response. In addition, mice that rejected tumor challenge generated tumor-specific cytotoxic T lymphocytes’ responses. Importantly, microscopic and gross evaluation of organs from mD52 immunized mice revealed no evidence of autoimmunity as assessed by absence of T cell infiltration and absence of microscopic pathology. Together, these data demonstrate that mD52 vaccination induces an immune response that is capable of rejecting tumors that over-express mD52 without the induction of harmful autoimmunity.
Keywords: Vaccine, Metastasis, mD52, TPD52, Murine, CpG
Introduction
The tumor protein D52 (TPD52) gene family [1] comprises three genes, D52 [2], D53 [1, 3], and D54 [4, 5]. The first human D52-like gene to be identified, human TPD52 (hD52), was found to be over-expressed in approximately 40% of breast carcinomas [2]. Subsequent reports have shown that hD52 is over-expressed in cancers of the lung [6], prostate [7], colon [8], and ovary [9]. Byrne and colleagues localized the hD52 gene to human chromosome 8q21 [2], a region frequently gained in breast and prostate carcinomas [10–14], and have since reported that hD52 represents a target for gene amplification in human breast cancer [15]. To our knowledge hTPD52 is the first and only chromosome 8q21 target gene to have been identified in any cancer type. Additional studies suggest that hTPD52 and hTPD53 genes encode regulators of cell proliferation [1]. Murine TPD52 naturally mirrors human TPD52 with respect to known function and over-expression in tumor cells, and shares 86% protein identity with the human orthologue. Our recent studies demonstrated that transfection and stable expression of murine TPD52 (mD52) cDNA in mouse 3T3 fibroblasts induced increased proliferation, anchorage independent cell growth, and the ability to form subcutaneous tumors and spontaneous lethal lung metastases in vivo when 3T3.mD52 cells were inoculated subcutaneously into naïve, syngeneic, immuno-competent mice [16]. Together these data strongly suggest that TPD52 is important for initiating and perhaps maintaining a tumorigenic and metastatic phenotype, and thus may be important for tumor cell survival making the TPD52 protein an excellent target for cancer vaccine development.
It has been convincingly demonstrated that unmethylated CpG dinucleotides largely found in bacterial DNA have the capacity to stimulate innate and adaptive immunity [17]. The mechanism of action lies within the ability of oligodeoxynucleotides (ODN), made up of unmethylated CpG dinucleotides, to bind Toll Like Receptor 9 (TLR9) on antigen presenting cells to include dendritic cells (DC) resulting in the enhancement of anti-cancer vaccination [17–19]. It has also been demonstrated that CpG/ODN are stronger than complete Freund’s adjuvant in eliciting TH1-type immune responses, which are believed to be important for the immunologic rejection of solid tumors, following protein antigen immunization [20, 21].
In the present study we sought to determine whether it was possible to induce an immune response to mD52 via active vaccination and to determine whether tumor immunity could be generated in the absence of autoimmunity. Recombinant mD52 protein and ODN 1826 (TCCATGACGTTCCTGACGTT) [20] were administered to naïve mice in the form of an active vaccination regimen. Immunized mice were challenged subcutaneously with a tumorigenic dose of syngeneic tumor cells naturally over-expressing mD52 protein. Immunization of mice resulted in tumor rejection, the generation mD52 antigen-specific, MHC-restricted cytotoxic T lymphocytes (CTLs) and the production of IgG antibodies against mD52 protein. Pathologic analysis of vital organs shown to express detectable levels of mD25 naturally, revealed no evidence of autoimmunity induction. Together these data indicate that active immunization with mD52 protein and CpG/ODN as an adjuvant is effective at generating an immune response capable of rejecting tumor cells that naturally over-express mD52 protein without the induction of potentially harmful autoimmunity.
Materials and methods
Mice and tumor cell lines
Female 6- to 8-week-old Balb/c mice were purchased from the NIH (Frederick, MD, USA). All animals were cared for and treated according to Institutional Animal Care and Use Committee guidelines at Texas Tech University Health Sciences Center (Lubbock, TX, USA). The tumorigenic Balb/c 3T3.mD52 cell line [16] and the tumorigenic SV40-transformed Balb/c murine kidney cell line designated mKSA were used for tumor challenge following immunization. The C57BL/6 autochthonous TRAMP-C1 and TRAMP-C2 tumor cells lines were used as MHC class-I mis-matched, mD52 positive controls. mKSA and 3T3.mD52 Tumor cell lines were cultured in RPMI 1640 (Fisher Scientific, Pittsburgh, PA, USA) supplemented with 10% heat-inactivated fetal bovine serum, 2 mmol/l l-glutamine, 250 ng/ml fungizone, 50 IU/ml penicillin, 50 μg/ml streptomycin, 50 μg/ml gentamicin sulfate, and 10 mmol/l HEPES. Autochthonous TRAMP cell lines were cultured as previously reported [22].
RT-PCR and real-time RT-PCR analysis
Total RNA was isolated from cell lines using TRIZOL reagent (Bio Whittaker, Walkersville, MD, USA), and 1 μg was reverse transcribed to obtain cDNA as previously described [16]. Murine normal tissue cDNA panels (multiple tissue cDNA panels I and III) were purchased from Clontech (Mountain View, CA, USA). Both mD52 and GAPDH (an internal reference control) amplification reactions were carried out as described previously [16] using 30 cycles for all PCR reactions and annealing temperatures of 62 and 60°C, respectively. All PCR reactions were performed in 50 μl volumes. The results were visualized by electrophoresis using 2% agarose gels containing ethidium bromide.
Real time RT-PCR was performed using cDNA samples generated as described above or using equivalent concentrations of murine normal tissue cDNA panels (Clontech) and the ABI Prism 7000 Sequence Detection System and ABI SYBR green PCR core reagents kit according to the manufacturer’s instructions (Applied Biosystems, Foster City, CA, USA). Real time RT-PCR conditions for 40 cycles were as follows: 50°C for 2 min, 95°C for 10 min, 95°C for 15 s, and an annealing temperature of 62°C for 1 min for mD52 primers (60°C annealing temperature was used for GAPDH control reactions). Additional controls were no template and no enzyme and were included in all real-time RT-PCR reactions as previously described [16].
Recombinant mD52 purification
The MicroSpin GST Purification Module (Amersham Biosciences, Piscataway, NJ, USA) was used to purify recombinant mD52–Glutathione S-transferase (GST) fusion protein using the manufacturer’s instructions and previously published methods [4]. Briefly, a 5 ml culture of recombinant Escherichia coli expressing mD52–GST fusion protein was grown to log phase and protein expression induced with IPTG. Cultured cells were harvested and lysed by repeated freeze thaw cycles, and the mD52–GST fusion protein was purified using a Glutathione Sepharose 4B MicroSpin column. Protein purity was assessed by SDS-PAGE and silver staining, as well as by western blot analysis using an anti-TPD52 polyclonal antibody (generated by immunizing rabbits with N-terminal, carrier conjugated peptide GC AYKKTSETLSQAGQKAS; italics represents a region of TPD52 protein that is conserved between human and mouse) (Bio Synthesis, Inc, Lewisville, TX, USA). Protein concentration was determined by measuring the absorbance at 280 nm according to the manufacturers’ guidelines (Amersham Biosciences). The GST affinity tag was approximated by 1 A280 ∼ 0.5 mg/ml, based on the extinction coefficient of the GST monomer using a Bradford protein assay. mD52 protein concentration was determined based on a 1:1 molar ratio of mD52 to GST in the fusion construct.
Western blot analysis
To determine if vaccination induced anti-mD52 antibodies specific for native denatured mD52 from tumor cells, Western blot analyses were performed using mKSA whole cell lysates, as previously reported by our laboratory [23]. mKSA cells were grown to confluency. Cells were harvested, counted, and whole cell protein lysates were prepared used Pierce Whole Cell Lysis Buffer (Pierce Biotechnology, Inc., Rockford, IL, USA) to obtain lysates of 1 × 108 cell equivalents/ml. Lysates were loaded on a standard reducing SDS-PAGE (4% stacking, 12% resolving) and electrophoresed at 200 V for 1 h, followed by electrolytic transfer to nitrocellulose for immunoblotting. The nitrocellulose membranes were blocked in PBS containing 1% normal goat serum, 1% milk, and 0.02% Tween for 2 h at room temperature. After blocking, membranes were cut into 5 mm strips and incubated overnight at 4°C with a 1:100 dilution of serum collected prior to, and following each immunization. Following incubation with serum, the strips were washed three times with PBS-0.02% Tween. Antibody binding was detected by the addition of goat-anti-mouse IgG horseradish peroxidase conjugated antibody (GAM-HRP) (SIGMA) at a 1:1,000 dilution for 2 h at room temperature. Strips were then washed three times with PBS-0.02% Tween and developed with 3.3 V diaminobenzidine reagent as substrate (Sigma, St. Louis, MO, USA) to visualize mD52-specific antibody reactivity. Strips probed with polyconal rabbit anti-TPD52 polyclonal antibody (generated by immunizing rabbits with N-terminal, carrier conjugated peptide GC AYKKTSETLSQAGQKAS; italics represents a region of TPD52 protein that is conserved between human and mouse) (Bio Synthesis, Inc) served as a positive control.
Immunization and tumor challenge
Individual mice were immunized via intramuscular (i.m.) injection every 14 days with 5–10 μg of recombinant mD52 protein admixed with 5–10 μg of CpG oligonucleotide (ODN 1826 TCCATGACGTTCCTGACGTT) [20] as an alum precipitate for a total of three injections. CpG/ODN in alum alone served as a control immunization. Mice in all groups were bled from the dorsal tail vein prior to immunization and 2 weeks following each immunization. Two weeks following the final immunization, mice in all groups were challenged with the following tumorigenic dose of tumor cells: 3T3.mD52 (1 × 106), or mKSA (5 × 105). Tumor cells were harvested, counted, and re-suspended in versene (PBS/EDTA, Fisher Scientific) to prevent aggregation and 100 μl of viable cell suspension was injected subcutaneously (s.c.) in the right flank of each mouse for tumor challenge. Tumor size was determined by taking perpendicular measurements with calipers every 2 to 3 days, and tumor volume (mm3) was calculated using the following formula: (a × b 2)/2, where b is the smaller of the two measurements.
Analysis of cytotoxic T lymphocyte (CTL)-mediated tumor cell lysis
T cells from spleens of immunized mice that survived tumor challenge were isolated and subjected to standard CTL-mediated tumor cell lysis analysis. CTLs were generated by culturing spleen cells in the presence of irradiated mKSA or 3T3.mD52 tumor cells (using the same tumor cell line as was used for the in vivo challenge) in the presence of IL-2 (10 ng/ml), IL-7 (5 ng/ml), and IL-12 (5 ng/ml) at 37°C for 5–7 days. Specificity was evaluated by mixing various numbers of CTLs with a constant number of target cells (5 × 103 cells per well) in 96 well round bottom plates. Specific lysis was determined using a Europium time-resolved fluorescence based 2 h method and measured using a Victor3™ plate reader (Perkin Elmer, Boston, MA, USA). Percent lysis was calculated as: % specific lysis = 1 − (E − S)/(M − S) × 100, where E represents Eu release in the presence of effector cells, S is spontaneous Eu release in medium alone and M represents maximum Eu released in the lysis buffer [24, 25]. To confirm MHC class-I restricted tumor recognition, CTL blocking assays were performed by incubating tumor cells with anti-H-2Kd (anti-Kd) or anti-H-2Kb (control Ig) mAb prior to incubation with CTLs. Briefly, 10 μl of mAb in PBS (a final concentration of 30 μg/ml) was added to individual wells of 96 well round bottom plates in triplicate. Next, 100 μl tumor cell targets were added to each well and incubated for 30 min at room temperature. Finally, 100 μl of effectors were added to the appropriate wells, and the plates were incubated for 24 h at 37°C. Assays were analyzed using the Victor3™ plate reader (Perkin/Elmer, Wallac).
Flow cytometry
Lymphocytes from spleens cultured as described earlier for CTL assay were stained with monoclonal antibodies specific for CD3, CD4, CD19, NK marker CD49b (detected with the DX5 antibody) and CD8, and MHC class-I expression was assessed on tumors cell lines, using antibodies purchased from BD-Bioscience (San Jose, CA, USA). Cells were fixed in 1% paraformaldehyde at 4°C for 1 h, and then analyzed by flow cytometry using a BD-FacsVantage™.
Assessment of vaccine induced autoimmunity
Kidneys from immune and control mice were blindly evaluated for T cell subset infiltrates using fluorescent immunohistochemistry (IHC) methods (Pathology, Texas Tech University Health Sciences Center). Kidneys from MRL/lpr mice (a murine Lupus model), a generous gift from Dr. Mark Mamula (Yale University), served as positive controls for T cell infiltration analysis by IHC.
Enumeration of 3T3.mD52 spontaneous lung metastases
Analysis of tumor metastasis to the lungs was performed by removing the lungs of animals following euthanization and injection of the lungs with India ink to visualize individual tumor nodules [24]. Briefly, an India ink solution was injected through the trachea and allowed to fill the lungs. The lungs were removed and placed in Fekete’s solution for de-staining. Tumor nodules do not absorb India ink, which results in the normal lung tissue staining black and the tumor nodules remaining white. Tumor nodules were counted blindly, and the size was noted by three individuals.
Statistical analysis
When necessary, tumor challenge data were analyzed with a Student’s t test to determine whether significant differences existed between mean tumor volume for mD52 immunized and that of control immunized groups of mice.
Results
Expression of mD52 in tumor cell lines and normal tissues
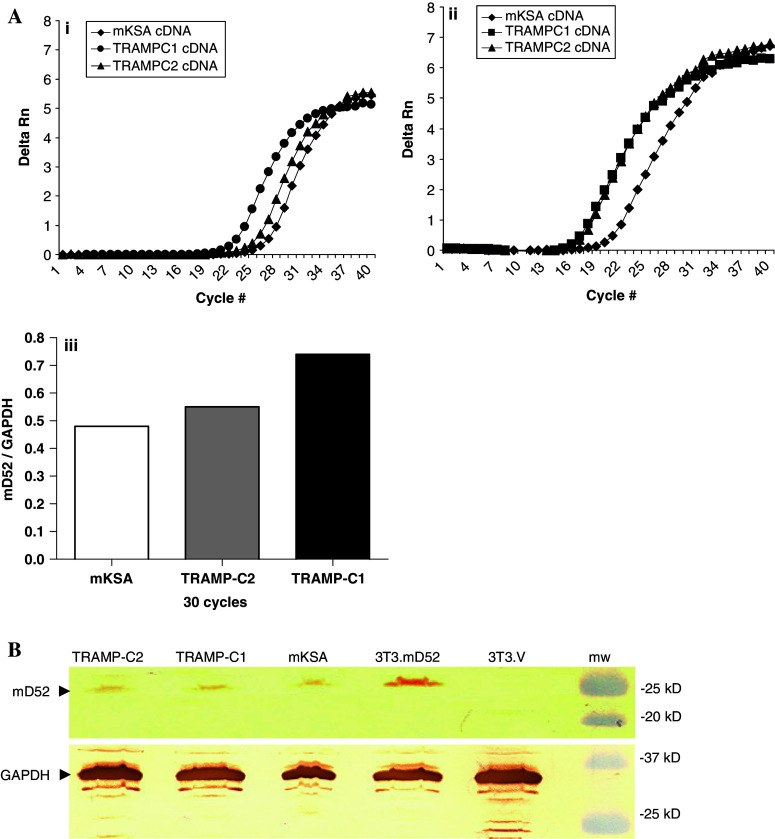
Prior to immunization with murine TPD52 (mD52) and evaluation of protection from tumor cell challenge, expression of mD52 was determined for tumor cell lines used in this study. The tumorigenic and metastatic cell line 3T3.mD52 was generated in our laboratory and shown to be positive for mD52 expression previously [16]. Expression of mD52 by the Balb/c kidney-derived tumor cell line, mKSA and the C57BL/6 tumor cell lines TRAMP-C1 and TRAMP-C2 was confirmed using a Syber green relative quantitation method for real-time RT-PCR (Fig. 1a). All three of the murine tumor cell lines expressed mD52 at detectable levels at 30 cycles of amplification. The delta Rn for mD52 expression at 30 cycles of amplification was similar for all three cell lines (Fig. 1, panel i). When comparing the relative expression of mD52 to the respective expression of GAPDH for the same cell line at 30 cycles of amplification, mKSA and TRAMP-C2 cells were similar, whereas TRAMP-C1 demonstrated greater expression of mD52 (Fig. 1a, panel iii). Expression of mD52 protein was confirmed for all tumor cell lines by Western blot analysis using an anti-mD52 antibody. A band of approximately 27 kDa was observed for the 3T3.mD52, mKSA, TRAMP-C1, and TRAMP-C2 cell lines (Fig. 1b). Examination of the 3T3.V empty vector transfected, negative control cell line revealed no detectable levels of mD52 protein expression by Western blot analysis (Fig. 1b). In addition, expression of MHC class-I molecules by the tumor cell lines was evaluated using specific monoclonal antibodies and flow cytometry. All four of the tumor cell lines expressed high levels of surface MHC class-I molecules (not shown). Taken together these data demonstrate that the tumor cell lines, mKSA, 3T3.mD52, TRAMP-C1, and TRAMP-C2 express mD52 protein and MHC class-I molecules.
Fig. 1.
Expression of Murine TPD52 (mD52) in tumor cell lines. a mRNA expression of mD52 in murine tumor cell lines. (i) Real-time RT-PCR showing mD52 expression in mKSA, TRAMP-C1 and TRAMP-C2 murine tumor cell lines. (ii) Real-time RT-PCR showing expression of GAPDH in mKSA, TRAMP-C1 and TRAMP-C2 murine tumor cell lines, as an internal reference control. (iii) Relative expression of mD52 over GAPDH in tumor cell lines at 30 cycles of amplication. b mD52 protein expression in murine tumor cell lines. Western blot analysis of murine tumor cell lysates generated from 1 × 108 cell equivalents demonstrating protein expression of mD52 using an antibody with specificity for mD52. GAPDH served as a control for protein loading. Shown are the representatives of three independent experiments
Others have reported the detection of human TPD52 expression in some normal tissues. TPD52 expression was shown to be pronounced in normal human kidney, prostate and breast with very little expression detected in the testes, thymus, and spleen [6]. TPD52 expression was also demonstrated in human B cells but not in T cells [26]. Since human and mouse TPD52 proteins are 86% identical and we demonstrated natural expression of mD52 in tumor cell lines (Fig. 1), it was of interest to assess the relative expression of mD52 in normal murine tissues. We were only interested in relative expression of mD52 in normal tissues compared to tumor cells to corroborate previous studies and to justify our evaluation of autoimmunity, given that expression of mD52 in normal tissues could result in tolerance or autoimmunity following active vaccination, and that absolute quantitation of mD52 was not necessary at this juncture for evaluation of autoimmunity and tolerance. Sixteen normal murine tissues were examined for relative mD52 expression using RT-PCR and real-time RT-PCR (Fig. 2). Pronounced expression of mD52 was observed for kidney, muscle, lung, brain prostate, lymph node, and eye. A much less expression of mD52 was observed for normal testes, liver, spleen, heart, uterus, stomach, thymus, and placenta (Fig. 2a, panels i and ii). Real-time RT-PCR analysis supported the mD52 expression findings observed for end point RT-PCR (Fig. 2b). Interestingly, expression of mD52 appeared to be the highest in normal kidney. Real-time RT-PCR produced a delta Rn of approximately 4.5 at 30 cycles of amplification for kidney with lung, spleen, and muscle, for example, producing delta Rn values less than 4.0 (Fig. 2b, panel i). These data indicate that the relative expression pattern of mD52 in normal murine tissues is similar to what has been reported for human TPD25 expression in normal tissues. Since mD52 expression in normal kidneys was the highest among the normal tissues tested, and the kidney is a vital organ, kidney pathology following vaccination with mD52 protein was monitored as an indication of vaccine induced autoimmunity.
Fig. 2.
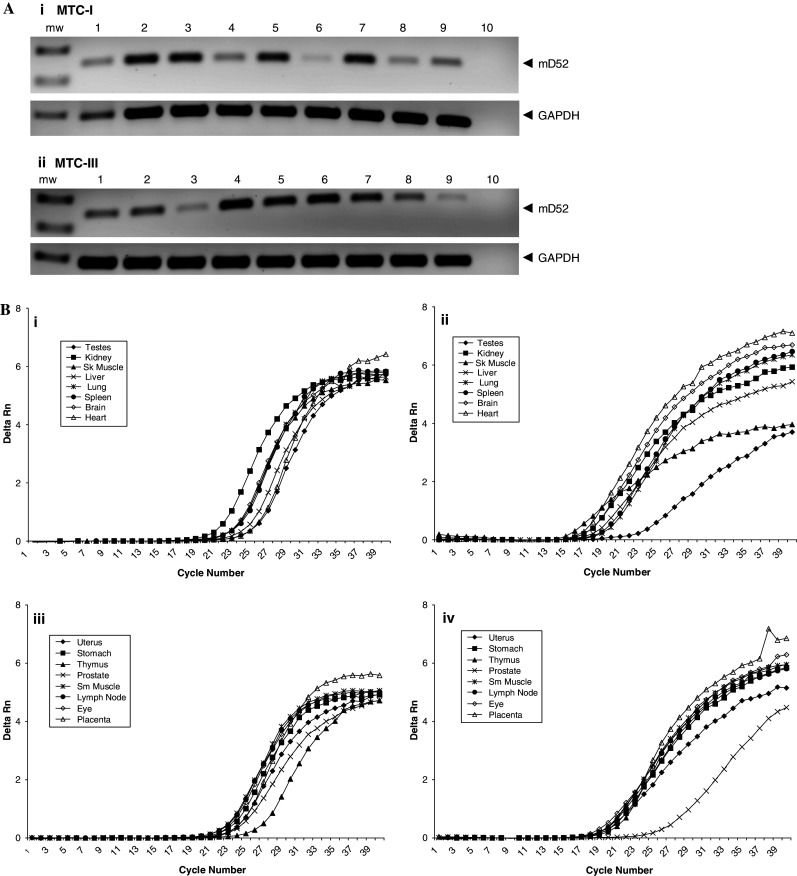
Expression of Murine TPD52 (mD52) in normal tissues. a RT-PCR showing mRNA expression of mD52 in normal tissues. (i) Multiple tissue cDNA panel-I (MTC-I) lane 1, testes; lane 2, kidney; lane 3, skeletal muscle; lane 4, liver; lane 5, lung; lane 6, spleen; lane 7, brain; lane 8, heart; lane 9, manufacturer’s control (pooled mouse liver cDNA); lane 10, H2O (no template control). (ii) Multiple tissue cDNA panel-III (MTC-III) lane 1, uterus; lane 2, stomach; lane 3, thymus; lane 4, prostate; lane 5, smooth muscle; lane 6, lymph node; lane 7, eye; lane 8, placenta; lane 9, manufacturer’s control (pooled mouse liver cDNA); lane 10, H2O (no template control). GAPDH expression served as a control. mw molecular weight standard. b Real-time RPT-PCR showing expression of mD52 in normal murine tissues. (i) Real-time RT-PCR showing expression of mD52 in normal tissue panel MTC-I. (ii) Real-time RT-PCR showing expression of GAPDH in normal tissue panel MTC-I. (iii) Real-time RT-PCR showing expression of mD52 in normal tissue panel MTC-III. (iv) Real-time RT-PCR showing expression of GAPDH in normal tissue panel MTC-III. Shown are the representatives of three independent experiments
Tumor protection following immunization with mD52 protein
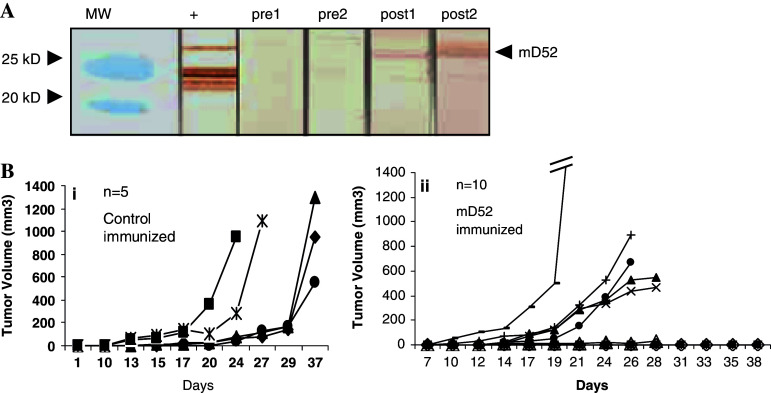
To determine whether it was possible to induce tumor protective immunity following vaccination with mD52 protein, groups of mice were immunized with purified recombinant mD52 protein (Fig. 3a) admixed with CpG/ODN, and rejection of subsequent tumor challenge was evaluated. Individual mice were immunized i.m. every 14 days, according to the schedule in Fig. 3b. Immunization groups included mD52 protein admixed with CpG/ODN as an alum precipitate or CpG/ODN in alum alone as control immunization. Since the vaccine was administered as protein in alum, the induction of immunity prior to tumor challenge was assessed by the detection of anti-mD52 IgG antibodies. Following each immunization, serum samples were collected and monitored for the presence of anti-mD52 antibodies using Western blot analysis. Mice immunized with mD52 protein and CpG/ODN generated detectable IgG antibodies to mD52 following three immunizations (Fig. 4a, post1 and post2), whereas mice immunized with CpG/ODN alone in alum generated no detectable IgG antibodies (not shown). Serum samples collected prior to immunization were also negative for IgG antibodies with specificity for mD52 protein as determined by Western blot analysis (Fig. 4a, pre1 and pre2). These data demonstrate that immunity to mD52 can be induced following immunization with recombinant protein admixed with CpG/ODN in alum. In addition, IgG antibodies with specificity for mD52 do not appear to exist in mice prior to immunization with mD52 and CpG/ODN. Interestingly, immunization with recombinant mD52 protein in alum in the absence of CpG/ODN failed to induce detectable IgG antibodies (not shown).
Fig. 3.
Purification of mD52 protein and mD52 immunization schedule. a Purification of recombinant mD52 protein. (i) Western blot analysis of purified mD52-GST fusion protein, lane 1, second elution of purified protein; lane 2, first elution of purified protein; lanes 3 and 4, subsequent washes; lanes 5 and 6, subsequent flow through samples prior to washing; lane 7, insoluble material from freeze thaw lysate; lane 8, freeze thaw lysate prior to loading on affinity column. mw molecular weight standard. (ii) Silver stained SDS-PAGE gel exact representation of the western blot shown in (i). mw molecular weight standard. b Schematic diagram of the schedule for immunization with purified recombinant mD52 protein (see Materials and methods for concentration of protein and CpG/ODN)
Fig. 4.
Immune response following immunization with mD52 protein and challenge with mKSA tumor cells. a Western Analysis of Serum from mD52 Immunized Mice. Preimmune serum from mouse 1 (pre1); and mouse 2 (pre2); serum following three immunizations with mD52 and rejection of mKSA tumor challenge mouse 1 (post1); and mouse 2 (post2). A TPD52 specific antibody that recognizes both human and murine TPD52 was used to detect mD52 protein in mKSA tumor cell lysates (+). MW molecular weight standards. Shown are sera from representative animals, and representative results from repeated experiments. b Effect of mD52 immunization on mKSA subcutaneous tumor growth in vivo. (i) Tumor growth for control immunized mice (CpG/ODN in alum). (ii) Tumor growth for mD52 immunized mice (mD52 protein + CpG/ODN in alum). Animals were immunized i.m. every 14 days with 5–10 μg of mD52 + 5–10 μg of CpG/ODN or 5–10 μg of CpG/ODN alone for a total of three injections followed on day 42 with a s.c. challenge with 5 × 105 live, syngeneic mKSA tumor cells. Shown are representative results for two independent experiments
To determine whether tumor immunity was induced following immunization with mD52 protein and CpG/ODN in alum, mice were challenged s.c. with a tumorigenic dose of syngeneic mKSA tumor. Tumor inoculation and growth was determined as described in the Materials and methods section. Fifty percent of mice immunized with mD52 protein admixed with CpG/ODN in alum rejected a tumorigenic challenge with mKSA tumor cells (Fig. 4b, panel ii), whereas none of the animals immunized with CpG/ODN in alum were capable of rejecting a tumorigenic challenge with mKSA (Fig. 4b, panel i). Similarly, mice immunized with recombinant mD52 in alum without CpG/ODN failed to reject mKSA tumor challenge (not shown). These data demonstrate that immunization with mD52 protein and CpG/ODN in alum prior to challenge with tumor cells that naturally express mD52 protein resulted in rejection of the tumor challenge compared to control immunized animals.
Induction of cytotoxic T lymphocytes following immunization with mD52 protein
Immunization with mD52 and CpG/ODN induced mD52-specific IgG antibodies (Fig. 4a) suggesting that specific T cells were induced to facilitate the switch to the IgG isotype. Since mD52 expressed by tumor cells is an intracellular protein it was of interest to determine if MHC class-I-restricted CTLs were also induced in mice following immunization with mD52 protein and CpG/ODN, and involved in the rejection of subsequent tumor challenge. Splenocytes were harvested and analyzed for tumor-specific killing as described in the Materials and methods section. The effector cells generated by 5–7 days mKSA mixed lymphocyte tumor culture (MLTC) were determined by flow cytometry to contain approximately 33% CD4+ T cells and 32% CD8+ T cells (not shown). Targets consisted of syngeneic MHC class-I matched 3T3 fibroblasts, 3T3.mD52 tumor cells, mKSA tumor cells, and allogeneic MHC class-I mis-matched TRAMP-C2 tumor cells. 3T3.mD52, mKSA, and TRAMP-C2 all over-express mD52, whereas 3T3 non-transformed fibroblasts do not (Fig. 1a, b) [16].
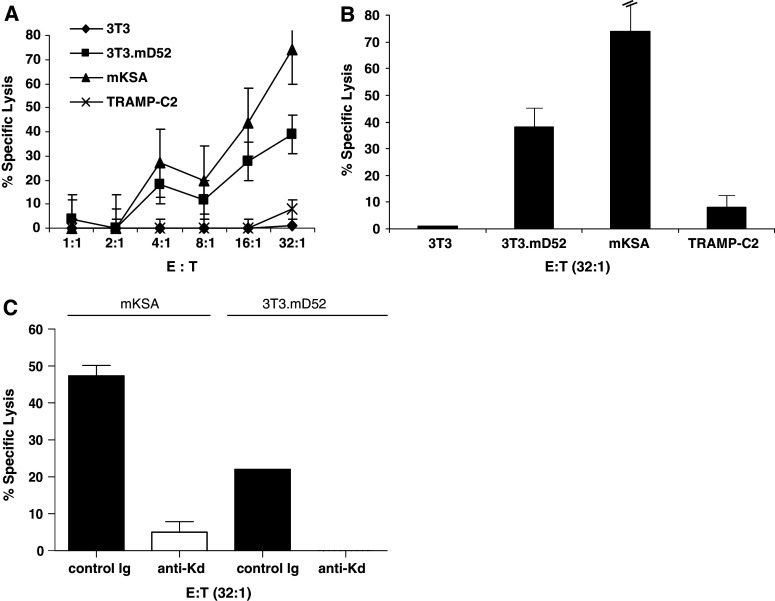
The CTLs generated from mice immunized with mD52 protein and CpG/ODN in alum and challenged with mKSA tumor cells demonstrated tumor-specific killing in Eu-release assays (Fig. 5a). Both mKSA (H-2Kd) the challenging tumor, and the MHC class-I matched (H-2Kd), mD52 positive cell line 3T3.mD52 were lysed by CTLs (>70 and >35% lysis, respectively, for an effector to target cell ratio of 32:1) (Fig. 5b), whereas the percent-specific lysis was <10% for both control targets 3T3 and TRAMP-C2 (Fig. 5a, b). The control targets either lack the correct MHC class-I molecule (TRAMP-C2; H-2Kb) or the targeted antigen mD52 (3T3; H-2Kd). Taken together, these data suggest that CTLs generated following immunizations with mD52 protein and CpG/ODN in alum are antigen-specific and MHC class-I-restricted. To confirm MHC class-I restricted tumor recognition, CTL blocking assays were performed by incubating tumor cell targets with anti-H-2Kd (anti-Kd) or anti-H-2Kb (control Ig) mAb prior to incubation with CTLs at an effector to target cell ration of 32:1. CTL mediated lysis of both mKSA and 3T3.mD52 was inhibited 90 to 100%, respectively, in the presence of anti-H-2Kd mAb. The control anti-H-2Kb (control Ig) mAb failed to inhibit CTL-specific lysis of either mKSA or 3T3.mD52 (Fig. 5c). These data further support the conclusions that the CTL generated following mD52 and CpG/ODN immunization were mD52 specific and MHC class-I restricted.
Fig. 5.
MHC-restriction and mD52-specificity of CTL from mD52 immunized tumor challenged mice. a Specific lysis of mD52 expressing tumor cells; b bar graph showing lysis at a single E:T ratio of 32:1. c Antibody blocking of CTL lysis of tumor cells. Control Ig, monoclonal antibody specific for mouse H-2Kb MHC class-I; anti-Kd, monoclonal antibody specific for mouse H-2Kd MHC class-I. For a and b TRAMP-C2 served as an MHC mismatched mD52 expressing negative control. 3T3 served as an MHC matched mD52 minus negative control. 3T3.mD52 and mKSA served as an MHC matched mD52 expressing positive control. Values shown are the mean ± SEM for triplicate determinations and are representative of two independent experiments
Immunization with mD52 does not induce autoimmunity
Since mD52 is a “self antigen”, i.e. it is expressed in some normal tissues as well as in tumors, it is possible that tolerance could prevent the induction of an immune response. Conversely, if tolerance is broken and an immune response is generated against mD52, there is the potential for the induction of harmful autoimmunity. As shown by the generation of IgG antibodies and MHC class-I-restricted antigen-specific CTLs to mD52, an immune response was indeed induced against mD52 following immunization, and the response was capable of rejecting mD52-expressing tumor challenge in vivo. Therefore, it was of interest to assess whether autoimmunity was also induced. To assess autoimmunity induction, kidneys of mice that were immunized with mD52 protein and CpG/ODN in alum and survived tumor challenge were harvested and evaluated for T cell infiltration and evidence of microscopic pathology. Individually immunized mice that survived tumor challenge showed no gross morbidity and appeared healthy throughout the study. Immunohistochemical analysis of kidneys showed no T cell infiltrates and no evidence of microscopic pathology compared to kidneys from naïve mice serving as normal controls (Fig. 6). No evidence of gross pathology was observed for livers, lungs or spleens. These data demonstrate that immunization with mD52 protein and CpG/ODN in alum does not result in the induction of autoimmunity.
Fig. 6.
Evaluation of autoimmune associated kidney pathology following mD52 immunization and tumor rejection. Shown are representative 5 μm, formalin fixed, paraffin embedded sections of kidneys from naïve mice or mice immunized with mD52 and subsequent tumor rejection (×100 magnification). The panels a through h are representative immuno-fluorescence micrographs staining for T cell infiltration. Panels a, b IgG-FITC control; panel c, d CD3-FITC naïve mouse kidneys; panel e, f CD3-FITC mouse kidneys following mD52 immunization and tumor rejection; panels g, h CD3-FITC kidneys from MRL/lpr autoimmune lupus mice as a positive control for T cell infiltration as depicted by the brighter fluorescent rings around kidney tubules (arrows). Panel i H&E stain for naïve mouse kidney; panel j H&E stain for kidney from mD52 immunized and tumor challenged animal. All assays were performed by an experienced pathologist and the determinations done blindly. Shown are representative micrographs from individual animals and two independent experiments
Prevention of spontaneous lung metastases following immunization with mD52
We reported previously that 3T3.mD52 tumor cells spontaneously metastasize to the lungs, forming lethal tumor burdens [16]. To determine if immunization with mD52 protein and CpG/ODN in alum was capable of preventing lethal lung metastases, animals were challenged s.c. with 3T3.mD52 tumor cells. Enumeration of tumor nodules was performed by harvesting lungs and staining with an India ink solution. Immunization with mD52 protein and CpG/ODN in alum resulted in the rejection of 40% of subcutaneous 3T3.mD52 tumors (Fig. 7a, panel ii), and the prevention of spontaneous lung metastases (Fig. 7b). In contrast, mice immunized with CpG/ODN in alum alone and challenged with 3T3.mD52 developed visible s.c. tumor nodules (Fig. 7a, panel i) and lung metastases (Fig. 7b). Of note, mice that rejected a primary 3T3.mD52 tumor challenge also rejected a second 3T3.mD52 tumor challenge (1× 106 cells) in the opposite flank, given 90 days after the initial tumor challenge (not shown). Taken together, these data indicate that immunization with mD52 protein and CpG/ODN in alum induces a memory immune response capable of preventing lethal lung metastasis by mD52 positive 3T3.mD52 tumor cells.
Fig. 7.

Immune response following immunization with mD52 protein and challenge with 3T3.mD52 tumor cells. a Effect of mD52 immunization on 3T3.mD52 subcutaneous tumor growth in vivo. (i) Tumor growth for control immunized mice (CpG/ODN in alum). (ii) Tumor growth for mD52 immunized mice (mD52 protein + CpG/ODN in alum). b Effect of mD52 immunization on 3T3.mD52 spontaneous lung metastasis in vivo. Control immunized mice, CpG/ODN in alum; mD52 immunized mice, mD52 protein + CpG/ODN in alum. For a and b, animals were immunized i.m. every 14 days with 5 μg of mD52 + 10 μg of CpG/ODN or 10 μg of CpG/ODN alone for a total of three injections followed on day 42 with a s.c. challenge with 1 × 106 live, syngeneic 3T3mD52 tumor cells. Shown are representative results for two independent experiments
Discussion
The use of vaccination against infectious disease in modern medicine has had a major impact on worldwide health. The power of the immune system has also been applied to fight cancer. Anti-cancer vaccines targeting self-proteins have been applied to treat or prevent multiple cancers in preclinical studies and clinical trials [27, 28]. Some examples include targeting MAGE in melanoma [29, 32], PAP [30, 33–36], PSMA [30, 31, 36], and PSA [30, 31, 36–38] in prostate cancer, and MUC1 [28] in breast cancer. Tumor protein D52 (TPD52) is a novel and potentially important tumor associated antigen (TAA) due to its over-expression in a number of fatal and common cancers to include prostate [39, 40], breast [2], ovary [9], and lung [6, 41] carcinomas. Scanlan and colleagues identified human TPD52 (hD52) as a candidate breast cancer TAA by using sera from breast cancer patients to screen a library of expressed genes from breast cancer tissue, demonstrating that hD52 is capable of inducing IgG antibodies [42]. This report suggests that TPD52 may be immunogenic and capable of inducing a cellular immune response, thus warranting study of TPD52 as an anti-cancer vaccine to induce cellular immunity. To address this, we hypothesized that vaccines targeting murine TPD52 (mD52) would induce cellular immune responses capable of rejecting tumor cells that over-express mD52 protein in murine models of cancer. Recombinant mD52 protein was administered along with CpG/oligodeoxynucleotide (ODN) in alum as an intramuscular vaccine, followed by challenge with a tumorigenic dose of syngeneic tumor cells that naturally over-express mD52 protein.
In the present study we immunized mice with recombinant mD52 protein admixed with CpG/ODN in alum which resulted in the induction of immune responses with specificity for mD52. As a test of tumor immunity we challenged mice with syngeneic tumor cells that over-express mD52, and tumor growth was monitored. All mice that received control immunizations developed lethal tumor burdens and had to be killed (Figs. 4b: panel i, 7a: panel i). In addition, mice immunized with CpG/ODN in alum alone and challenged with the spontaneously metastatic 3T3.mD52 tumor cells developed lung metastases (Fig. 7b). However, mice immunized with recombinant mD52 protein and CpG/ODN in alum resulted in the rejection of tumor challenge in 50% of mice challenged with mKSA tumor cells (Fig. 4b, panel ii) and 40% of mice that received 3T3.mD52 tumor cell challenge (Fig. 7b, panel ii). Mice immunized with mD52 protein and CpG/ODN followed by 3T3.mD52 tumor cell challenge also prevented the formation of spontaneous lethal lung metastases (Fig. 7b). Rejection of mKSA tumor challenge was associated with the induction of mD52-specific, MHC-restricted CTLs (Fig. 5). Importantly, there was no detectable evidence of autoimmunity following immunization with mD52 and CpG/ODN in alum (Fig. 6). Together, these data demonstrate that the “self” TAA mD52 is sufficiently immunogenic when administered as a protein-based vaccine admixed with CpG/ODN as a molecular adjuvant. Moreover, the generation of anti-mD52 CTL resulted in rejection of syngeneic tumor cells that naturally over-express mD52 protein without induction of detectable autoimmunity. The form of recombinant mD52 protein used was a fusion protein of mD52 and GST to facilitate purification (Fig. 3a). It is possible that GST functions as a carrier protein; however, experiments employing mD52-GST protein in the absence of CpG/ODN as a molecular adjuvant followed with mKSA tumor challenge (performed in conjunction with studies represented in Fig. 4) failed to induce protective tumor immunity (not shown). More importantly, the form of mD52 protein recognized by specific CTLs (Fig. 5) and IgG antibodies (Fig. 4) was a natural murine tumor cell derived mD52, not recombinant mD52-GST fusion protein.
It is known that TGF-β1 is involved in tumor-mediated immune suppression. We demonstrated previously that 3T3.mD52 tumor cells secrete significant quantities of TGF-β1 [16] which could account for decreased rejection of 3T3.mD52 tumors following immunization compared to mKSA tumors, which secrete nearly 50% less TGF-β1 than 3T3.mD52 tumor cells (not shown). Studies are underway to overcome immune suppression by TGF-β1 and increase protection in mice challenged with 3T3.mD52 or mKSA tumor cells by using TGF-β1 neutralizing mAb in vivo or siRNA to TGF-β1 to knock out or down TGF-β1 expression and secretion in vitro in tumor cells without interfering with in vivo tumorigenic or metastatic characteristics of tumor cells. More traditional adjuvants, such as IFA, different TLR agonists as molecular adjuvants, different routes and schedules of administration of mD52 to increase anti-tumor efficacy in vivo are also being explored. In addition, studies to assess mD52 vaccination as a treatment of pre-existing tumors are underway.
It is now widely accepted that bacterial derived, unmethylated CpG deoxynucleotides are potent molecular adjuvants when administered with active vaccinations regimens [17, 19, 43]. Nearly 10 years have passed since the first report on the potency of CpG DNA as a molecular adjuvant when administered with a protein-based vaccine [20]. In this study Davis and colleagues demonstrated that CpG DNA could overcome the ability of alum and protein vaccines to induce TH2 immunity in Balb/c mice and instead drive immunity to a TH1-type response. In addition, they proved that addition of CpG DNA could increase specific TH1-type IgG2a antibody responses to HBsAg fivefold above HBsAg without CpG DNA. This group subsequently went on to characterize three CpG/ODN classes with distinct immunostimulatory properties [44]. Of importance to our studies was the characterization of what they termed B class CpG/ODN. These CpG/ODN are potent activators of B cells and TH1-type cytokines capable of driving cellular immunity [44, 45]. The prototype B class CpG/ODN is represented by the ODN 1826 [20, 44, 45]. Since mD52 is an intracellular protein we were interested in the generation of TH1-type cellular immunity and CTLs which would be necessary for the rejection of mD52 expressing tumors. To this end, we chose the B class CpG/ODN 1826 as our molecular adjuvant. Others have demonstrated that CpG/ODN 1826 is a potent molecular adjuvant for vaccination against various cancers. Vaccine studies utilizing CpG/ODN 1826 admixed with TAA in various murine cancer models include; HPV-16 E7 protein [46], anti-idiotype vaccine that mimics carcinoembryonic antigen (CEA) [47, 48], and synthetic peptide from HER-2/neu oncoprotein in a model of spontaneous breast cancer [49]. Further support of the immunopotency of CpG/ODN 1826 in mice was the report by Shao and colleagues demonstrating that CpG/ODN 1826 could convert a weak autoantigen like uveitogenic rat interphotoreceptor retinoid-binding protein into a strong auto-antigen that induces uveitis [50]. This study demonstrated that molecular adjuvants based on CpG/ODN 1826 could enable tolerance to be broken to self-antigens delivered as active vaccination strategies.
In the present study we have shown for the first time that the self-TAA mD52 is immunogenic when administered as a recombinant protein-based vaccine admixed with CpG/ODN 1826 in alum. Further, the immune response generated is capable of rejecting tumor cells that naturally over-express mD52 protein without inducing harmful autoimmunity, suggesting the human TPD52 may be a potent vaccine antigen that could be administered to patients to treat or prevent cancers that over-express TPD52.
Acknowledgments
This work was supported in part by NIH Grant CA 77351, funds from the Southwest Cancer Treatment and Research Center and by a Howard Hughes Medical Institute grant through the Undergraduate Biological Sciences Education Program to Texas Tech University.
Abbreviations
- TPD52
Tumor protein D52
- mD52
Murine TPD52
- hD52
Human TPD52
- 3T3.mD52
mD52 Transformed 3T3 cells
- ODN
Oligodeoxynucleotide
- TAA
Tumor associated antigen
Footnotes
L.A. Payton and J.D. Lewis contributed equally to this study.
References
- 1.Byrne JA, Mattei MG, Basset P. Definition of the D52 gene/protein family through cloning of D52 homologues in human (hD53) and mouse (mD52) Genomics. 1997;35:523–532. doi: 10.1006/geno.1996.0393. [DOI] [PubMed] [Google Scholar]
- 2.Byrne JA, Tomasetto C, Garnier JM, Rouyer N, Mattei MG, Bellocq JP, Rio MC, Basset P. A screening method to identify genes commonly overexpressed in carcinomas and the identification of a novel complementary DNA sequence. Cancer Res. 1995;55:2896–2903. [PubMed] [Google Scholar]
- 3.Byrne JA, Mattei MG, Basset P, Gunning P. Identification and in situ hybridization mapping of a mouse Tpd52l1 (D53) orthologue to chromosome 10A4-B2. Cytogenet Cell Genet. 1998;81:199–201. doi: 10.1159/000015029. [DOI] [PubMed] [Google Scholar]
- 4.Byrne JA, Nourse CR, Basset P, Gunning P. Identification of homo- and heteromeric interactions between members of the breast carcinoma-associated D52 protein family using the yeast two-hybrid system. Oncogene. 1998;16:873–882. doi: 10.1038/sj.onc.1201604. [DOI] [PubMed] [Google Scholar]
- 5.Nourse CR, Mattei MG, Gunning P, Byrne JA. Cloning of a third member of the D52 gene family indicates alternative coding sequence usage in D52-like transcripts. Biochim Biophys Acta. 1998;1443:155–168. doi: 10.1016/s0167-4781(98)00211-5. [DOI] [PubMed] [Google Scholar]
- 6.Chen S-L, Maroulakou IG, Green JE, Romano-Spica V, Modi W, Lautenberger J, Bhat NK. Isolation and characterization of a novel gene expressed in multiple cancers. Oncogene. 1996;12:741–751. [PubMed] [Google Scholar]
- 7.Dhanasekaran SM, Barrette TR, Ghosh D, Shah R, Varambally S, Kurachi K, Pienta KJ, Rubin MA, Chinnaiyan AM. Delineation of prognostic biomarkers in prostate cancer. Nature. 2001;412:822–826. doi: 10.1038/35090585. [DOI] [PubMed] [Google Scholar]
- 8.Malek RL, Irby RB, Guo QM, Lee K, Wong S, He M, Tsai J, Frank B, Liu ET, Quackenbush J, Jove R, Yeatman TJ, Lee NH. Identification of Src transformation fingerprint in human colon cancer. Oncogene. 2002;21:7256–7265. doi: 10.1038/sj.onc.1205900. [DOI] [PubMed] [Google Scholar]
- 9.Byrne JA, Balleine RL, Schoenberg Fejzo M, Mercieca J, Chiew YE, Livnat Y, St Heaps L, Peters GB, Byth K, Karlan BY, Slamon DJ, Harnett P, Defazio A. Tumor protein D52 (TPD52) is overexpressed and a gene amplification target in ovarian cancer. Int J Cancer. 2005;117:1049–1054. doi: 10.1002/ijc.21250. [DOI] [PubMed] [Google Scholar]
- 10.Kallioniemi A, Kallioniemi OP, Piper J, Tanner M, Stokke T, Chen L, Smith HS, Pinkel D, Gray JW, Waldman FM. Detection and mapping of amplified DNA sequences in breast cancer by comparative genomic hybridization. Proc Natl Acad Sci USA. 1994;91:2156–2160. doi: 10.1073/pnas.91.6.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schoenberg Fejzo M, Ashar HR, Krauter KS, Powell WL, Rein MS, Weremowicz S, Yoon SJ, Kucherlapati RS, Chada K, Morton CC. Translocation breakpoints upstream of the HMGIC gene in uterine leiomyomata suggest dysregulation of this gene by a mechanism different from that of lipomas. Genes Chromosomes Cancer. 1996;17:1–6. doi: 10.1002/(SICI)1098-2264(199609)17:1<1::AID-GCC1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 12.Forozan F, Karhu R, Kononen J, Kallioniemi A, Kallioniemi OP. Genome screening by comparative genomic hybridization. Trends Genet. 1997;13:405–409. doi: 10.1016/S0168-9525(97)01244-4. [DOI] [PubMed] [Google Scholar]
- 13.Cher ML, Bova GS, Moore DH, Small EJ, Carroll PR, Pin SS, Epstein JI, Isaacs WB, Jensen RH. Genetic alterations in untreated metastases and androgen-independent prostate cancer detected by comparative genomic hybridization and allelotyping. Cancer Res. 1996;56:3091–3102. [PubMed] [Google Scholar]
- 14.Virgin JB, Hurley PM, Cher MP, Nahhas F, Bebchuk KG, Mohamed AN, Sakr WA, Bright RK, Cher ML. Isochromosome 8q is associated with 8p loss of heterozygosity in a prostate cancer cell line. Prostate. 1999;41:49–57. doi: 10.1002/(SICI)1097-0045(19990915)41:1<49::AID-PROS7>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 15.Balleine RL, Schoenberg Fejzo M, Sathasivam P, Basset P, Clarke CL, Byrne JA. The D52 (TPD52) gene is a candidate target gene for events resulting in increased 8q21 copy number in human breast carcinoma. Genes Chromosomes Cancer. 2000;29:48–57. doi: 10.1002/1098-2264(2000)9999:9999<::AID-GCC1005>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 16.Lewis JD, Payton LA, Whitford JG, Byrne JA, Smith DI, Yang L, Bright RK. Induction of tumorigenesis and metastasis by the murine orthologue of tumor protein D52. Mol Cancer Res. 2007;5:133–144. doi: 10.1158/1541-7786.MCR-06-0245. [DOI] [PubMed] [Google Scholar]
- 17.Krieg AM. CpG Motifs in bacterial DNA and their immune effects. Annu Rev Immunol. 2002;20:709–760. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 18.Krieg AM. CpG motifs: the active ingredient in bacterial extracts? Nat Med. 2003;9:831–835. doi: 10.1038/nm0703-831. [DOI] [PubMed] [Google Scholar]
- 19.Klinman DM. Immunotherapeutic uses of CpG oligodeoxynucleotides. Nat Rev Immunol. 2004;4:249–258. doi: 10.1038/nri1329. [DOI] [PubMed] [Google Scholar]
- 20.Davis HL, Weeranta R, Waldschmidt TJ, Tygrett L, Schorr J, Krieg AM. CpG DNA is a potent enhancer of specific immunity in mice immunized with recombinant Hepatitis B surface antigen. J Immunol. 1998;160:870–876. [PubMed] [Google Scholar]
- 21.Lipford GB, Sparwasser T, Zimmermann S, Heeg K, Wagner H. CpG-DNA-mediated transient lymphadenopathy is associated with a state of Th1 predisposition to antigen-driven responses. J Immunol. 2000;165:1228–1235. doi: 10.4049/jimmunol.165.3.1228. [DOI] [PubMed] [Google Scholar]
- 22.Foster BA, Gingrich JR, Kwan ED, Madias C, Greenberg NM. Characterization of prostatic epithelial cell lines derived from transgenic adenocarcinoma of the mouse prostate (TRAMP) model. Cancer Res. 1997;57:3325–3330. [PubMed] [Google Scholar]
- 23.Bright RK, Shearer MH, Kennedy RC. SV40 large tumor antigen associated synthetic peptides define native antigenic determinants and induce protective tumor immunity in mice. Mol Immunol. 1994;31:1077–1087. doi: 10.1016/0161-5890(94)90103-1. [DOI] [PubMed] [Google Scholar]
- 24.Lewis JD, Shearer MH, Kennedy RC, Bright RK. Surrogate Tumor antigen vaccination induces tumor-specific immunity and the rejection of spontaneous metastases. Cancer Res. 2005;65:2938–2946. doi: 10.1158/0008-5472.CAN-04-2874. [DOI] [PubMed] [Google Scholar]
- 25.Bright RK, Kimchi ET, Shearer MH, Kennedy RC, Pass HI. SV40 Tag-specific cytotoxic T lymphocytes generated from the peripheral blood of malignant pleural mesothelioma patients. Cancer Immunol Immunother. 2002;50:682–690. doi: 10.1007/s00262-001-0240-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tiacci E, Orvietani PL, Bigerna B. Tumor protein D52 (TPD52): a novel B-cell/plasma-cell molecule with unique expression pattern and Ca2+-dependent association with annexin VI. Blood. 2005;105:2812–2820. doi: 10.1182/blood-2004-07-2630. [DOI] [PubMed] [Google Scholar]
- 27.Gilboa E. The promise of cancer vaccines. Nat Rev Cancer. 2004;4:401–411. doi: 10.1038/nrc1359. [DOI] [PubMed] [Google Scholar]
- 28.Lewis JD, Reilly BD, Bright RK. Tumor associated antigens: from discovery to immunity. Int Rev Immunol. 2003;22:81–112. doi: 10.1080/08830180305221. [DOI] [PubMed] [Google Scholar]
- 29.Finn OJ. Cancer vaccines: between the idea and the reality. Nature. 2003;3:630–641. doi: 10.1038/nri1150. [DOI] [PubMed] [Google Scholar]
- 30.Shen L, Rock KL. Priming of T cells by exogenous antigen cross-presented on MHC class I molecules. Curr Opin Immunol. 2006;18:85–91. doi: 10.1016/j.coi.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Medzhitov R. CpG DNA: security code for host defense. Nat Immunol. 2001;2:15–16. doi: 10.1038/83121. [DOI] [PubMed] [Google Scholar]
- 32.Hunt DF, Henderson RA, Shabanowitz J, Sakaguchi K, Michel H, Sevilir N, Cox AL, Appella E, Engelhard VH. Characterization of peptides bound to the class I MHC molecule HLA-A2.1 by mass spectrometry. Science. 1992;255:1261–1263. doi: 10.1126/science.1546328. [DOI] [PubMed] [Google Scholar]
- 33.Waldman TA. Immunotherapy: past, present and future. Nat Med. 2003;9:269–277. doi: 10.1038/nm0303-269. [DOI] [PubMed] [Google Scholar]
- 34.Liang P, Pardee AB. Analysing differential gene expression in cancer. Nat Rev Cancer. 2003;3:869–876. doi: 10.1038/nrc1214. [DOI] [PubMed] [Google Scholar]
- 35.Liang P, Pardee AB. Differential display of eukaryotic mRNA by means of the polymerase chain reaction. Science. 1992;257:967–971. doi: 10.1126/science.1354393. [DOI] [PubMed] [Google Scholar]
- 36.Disis ML, Gooley TA, Rinn K. Generation of T-cell immunity to HER-2/neu protein after active immunization with HER-2/neu peptide-based vaccines. J Clin Oncol. 2002;20:2624–2632. doi: 10.1200/JCO.2002.06.171. [DOI] [PubMed] [Google Scholar]
- 37.Chomez P, DeBacker O, Bertrand M. An overview of the MAGE gene family with the identification of all human members of the family. Cancer Res. 2001;61:5544–5551. [PubMed] [Google Scholar]
- 38.Burch PA, Breen JK, Buckner JC. Priming tissue-specific cellular immunity in Phase I trial of autologous dendritic cells for prostate cancer. Clin Cancer Res. 2000;6:2175–2182. [PubMed] [Google Scholar]
- 39.Byrne JA, Mattei MG, Basset P. Definition of the tumor protein D52 (TPD52) gene family through cloning of D52 homologues in human (hD52) and mouse (mD52) Genomics. 1996;35:523–532. doi: 10.1006/geno.1996.0393. [DOI] [PubMed] [Google Scholar]
- 40.Rubin MA, Varambally S, Beroukhim R, Tomlins SA, Rhodes DR, Paris PL, Hofer MD, Storz-Schweizer M, Keufer R, Fletcher JA, His BL, Byrne JA, Pienta KJ, Collins C, Sellers WR, Chinnaiyan AM. Overexpression, amplification, and androgen regulation of TPD52 in prostate cancer. Cancer Res. 2004;64:3814–3822. doi: 10.1158/0008-5472.CAN-03-3881. [DOI] [PubMed] [Google Scholar]
- 41.Chen SL, Zhang XK, Halverson DO. Characterization of human N8 protein. Oncogene. 1997;15:2577–2588. doi: 10.1038/sj.onc.1201437. [DOI] [PubMed] [Google Scholar]
- 42.Scanlan MJ, Gout I, Gordon CM, Williamson B, Stockert E, Gure AO, Jager D, Chen YT, Mackay A, O’Hare MJ, Old LJ. Humoral immunity to human breast cancer: antigen definition and quantitative analysis of mRNA expression. Cancer Immun. 2001;1:4–20. [PubMed] [Google Scholar]
- 43.Krieg AM, Davis HL. Enhancing vaccines with immune stimulatory CpG DNA. Curr Opin Mol Ther. 2001;3:15–24. [PubMed] [Google Scholar]
- 44.Vollmer J, Weeranta R, Payette P, Jurk M, Schetter C, Laucht M, Wader T, Tluk S, Liu M, Davis HL, Krieg AM. Characterization of three CpG oligodeoxynucleotide classes with distinct immunostimulatory activities. Eur J Immunol. 2004;34:251–262. doi: 10.1002/eji.200324032. [DOI] [PubMed] [Google Scholar]
- 45.van Ojik HH, Bevaart L, Dahle CE, Bakker A, Jansen MJH, van Vugt MJ, van de Winkel JGJ, Weiner GJ. CpG-A and B olidodeoxynucleotides enhance the efficacy of antibody therapy by activating different effector cell populations. Cancer Res. 2003;63:5595–5600. [PubMed] [Google Scholar]
- 46.Kim T-Y, Myoung H-J, Kim J-H, Moon I-S, Kim T-G, Ahn W-S, Sin J-I. Both E7 and CpG-oligodeoxynucleotide are required for protective immunity against challenge with human papillomavirus 16 (E6/E7) immortalized tumor cells: involvement of CD4+ and CD8+ T cells in protection. Cancer Res. 2002;62:7234–7240. [PubMed] [Google Scholar]
- 47.Baral RN, Saha A, Chatterjee SK, Foon KA, Krieg AM, Weiner GJ, Bhattacharya-Chatterjee M. Immunostimulatory CpG oligonucleotides enhance the immune response of anti-idiotype vaccine that mimics carcinoembryonic antigen. Cancer Immunol Immunother. 2003;52:317–327. doi: 10.1007/s00262-002-0351-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saha A, Baral RN, Chatterjee SK, Mohanty K, Pal S, Foon KA, Primus FJ, Krieg AM, Weiner GJ, Bhattacharya-Chatterjee M. CpG oligonucleotides enhance the tumor antigen-specific immune response of an anti-idiotype antibody-based vaccine strategy in CEA transgenic mice. Cancer Immunol Immunother. 2006;55:515–527. doi: 10.1007/s00262-005-0009-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nava-Parada P, Forni G, Knutson KL, Pease LR, Celis E. Peptide vaccine given with toll-like receptor agonist is effective for the treatment and prevention of spontaneous breast tumors. Cancer Res. 2007;67:1326–1334. doi: 10.1158/0008-5472.CAN-06-3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shao H, Lei S, Sun SL, Xiang J, Kaplan HJ, Sun D. CpG-containing oligodeoxynucleotide 1826 converts the weak uveitogenic rat interphotoreceptor retinoid-binding protein peptide 1181–1191 into a strong uveitogen. J Immunol. 2003;171:4780–4785. doi: 10.4049/jimmunol.171.9.4780. [DOI] [PubMed] [Google Scholar]