Abstract
The Eph receptor tyrosine kinases and their membrane-anchored ligands, ephrins, are signaling proteins that act as axon guidance molecules during chick auditory brainstem development. We recently showed that Eph proteins also affect patterns of neural activation in the mammalian brainstem. However, functional deficits in the brainstems of mutant mice have not been assessed physiologically. The present study characterizes neural activation in Eph protein deficient mice in the auditory brainstem response (ABR). We recorded the ABR of EphA4 and ephrin-B2 mutant mice, aged postnatal day 18–20, and compared them to wild type controls. The peripheral hearing threshold of EphA4−/− mice was 75% higher than that of controls. Waveform amplitudes of peak 1 (P1) were 54% lower in EphA4−/− mice than in controls. The peripheral hearing thresholds in ephrin-B2lacZ/+ mice were also elevated, with a mean value 20% higher than that of controls. These ephrin-B2lacZ/+ mice showed a 38% smaller P1 amplitude. Significant differences in latency to waveform peaks were also observed. These elevated thresholds and reduced peak amplitudes provide evidence for hearing deficits in both of these mutant mouse lines, and further emphasize an important role for Eph family proteins in the formation of functional auditory circuitry.
Keywords: Brainstem, ABR, Eph receptor, ephrin, mouse
Introduction
Eph receptor tyrosine kinases and their membrane-anchored ligands, ephrins, constitute a large family of molecules that mediate intercellular signaling, with broad functions during development. In the nervous system, these proteins regulate cell migration (Krull et al., 1997; Mellitzer et al., 2000), axon outgrowth (Kullander and Klein, 2002; Murai and Pasquale, 2003; Wilkinson, 2001), synapse formation and stability (Dalva et al., 2007), and synaptic plasticity (Contractor et al., 2002; Dalva et al., 2007; Henderson et al., 2001). These functions for Eph proteins contribute to the orderly patterning and connectivity seen in the auditory system. In the ear, Eph proteins act as axon guidance molecules in developing spiral ganglion neurons (Bianchi and Gray, 2002; Brors et al., 2003), and mutations in EphB receptors result in altered distortion product otoacoustic emissions (Howard et al., 2003). Eph proteins are expressed in central auditory structures and have a role in auditory brainstem development (Cramer, 2005). Misexpression of Eph receptors in chick embryos results in targeting errors in the chick brainstem (Cramer et al., 2006; Cramer et al., 2004; Huffman and Cramer, 2007). Moreover, studies of mice with mutations in Eph genes show that these molecules regulate patterning in the mammalian brainstem after deafferentation (Hsieh et al., 2007).
Studies of Eph/ephrin protein functions must take into account the complexity of binding interactions and signaling mechanisms, which occur bidirectionally into both the Eph-expressing and ephrin-expressing cells upon contact. Eph receptors and ephrins are subdivided into A and B subclasses (Eph Nomenclature Committee, 1997). In general, ephrin-A ligands (1–6 in vertebrates) bind EphA receptors (1–10) and ephrin-B ligands (1–3) bind EphB receptors (1–6). The two exceptions to this rule are that EphA4 binds to ephrin-A ligands as well as ephrin-B2 and ephrin-B3 (Gale et al., 1996), and EphB2 binds to ephrin-B ligands and ephrin-A5 (Himanen et al., 2004). Binding between ephrins and Eph receptors occurs with high affinity and may mediate either attraction or repulsion (Pasquale, 2005). Because the Eph family is large and shows promiscuous binding between ligands and receptors, the effects of mutations in a single Eph gene are often subtle. An important question is whether mutations that cause anatomical abnormalities also result in a corresponding alteration of auditory function. Most of the studies on Eph proteins in the auditory system have focused on the auditory nerve and brainstem. We recently found that mice with mutations in EphA4 or in ephrin-B2 show significantly altered levels and patterns of activation in the auditory brainstem following pure tone stimulation (Miko et al., in press). In order to evaluate whether these mutations have a significant effect on hearing function, we performed auditory brainstem response (ABR) measurements on mice with mutations in EphA4 or ephrin-B2, and compared these measurements to wild type littermate controls. We found that both mutations result in elevated ABR thresholds, and that latencies of some peaks were significantly altered. These results suggest that EphA4 and ephrin-B2 are required for normal hearing.
Materials and Methods
Animals
The mice used in this study were deficient in either EphA4, a receptor tyrosine kinase, or ephrin-B2, a transmembrane ligand for EphA4. We used two strains of mice, which had mutations in either ephrin-B2 or EphA4, and mutations were linked to β-galactosidase expression. The ephrin-B2 mutant mice (Dravis et al., 2004) were bred in our colony and maintained on a CD-1/129 background. In this strain, the mutant allele encodes a membrane-bound ephrin-B2-β-galactosidase fusion protein in which the cytoplasmic domain of ephrin-B2 has been deleted. Within this strain, ABR measurements were performed on ephrin-B2+/+ or ephrin-B2lacZ/+ mice, as the ephrin-B2lacZ/lacZ mice are not viable postnatally. To study the effects of mutations in EphA4, we used EphA4 gene trap mice (Leighton et al., 2001) provided to us by Marc Tessier-Lavigne. These animals were maintained in our colony on a C57/Bl6 background. The mutant allele in this strain has a null mutation in EphA4 and expresses cytoplasmic β-galactosidase, which is inserted downstream of the EphA4 promoter region. ABR measurements were performed on wild type (EphA4+/+), heterozygous (EphA4+/−), and homozygous (EphA4−/−) mice. Mice were postnatal day (P) 18–20 at the time ABR recordings were made. At this age, mice are mature enough to produce an ABR waveform that includes all peaks present in the adult waveform (Song et al., 2006), yet young enough to minimize the impact of age-related hearing loss, which can occur quite early in the C57/Bl6 strain (Parham, 1997). All procedures were approved by the University of California Animal Care and Use Committee (IACUC).
ABR recordings
Prior to experiments, tympanic sound pressure level (SPL, expressed in dB re 20 Pa) was calibrated from 100 Hz to 30 kHz in 100-Hz steps under computer control using a 0.5-in condenser microphone model #4134 (Bruel & Kjaer). In addition, we ascertained that the mice were responsive to acoustic startle stimuli. Mice were anaesthetized with ketamine (75–85 mg/kg) and xylazine (0.1 – 0.5 mg/kg) until insensitive to toe pinch, and placed in a double-walled sound-attenuating chamber (Industrial Acoustics Corp.). Recordings were acquired as differentially recorded scalp potentials. Subcutaneous steel-tipped electrodes were placed at the vertex (positive), and the parallel to the mastoid (negative) ipsilateral to the sound presentation. A ground electrode was attached to the tail. At the left ear, a speaker was placed inside the pinna, against the entrance of the ear canal. Stimulus delivery was controlled from outside the acoustic chamber, and clicks were generated by a MALab system (Kaiser Instruments, Irvine, CA) that prompted a 100 microsecond square wave pulse ten times/second for each decibel level. The click stimulus was delivered in 5 dB steps from 20 dB to 60 dB, and thereafter in 10 dB steps until 100 dB. ABR signals were band pass filtered below 10 Hz and above 1000 Hz with a Grass P511K amplifier, acquired with MALab at a sampling rate of 1/0.02 ms, averaged online over 500 trials for each dB, and monitored in a 10 ms display window. The filtered signals were also sent to a digital oscilliscope that was triggered to the clicks, and EKG activity was monitored as an indicator of health during recordings.
Data Analysis
Each ABR averaged trace was saved as a vector number string containing 500 units, representing 10 ms of time for each dB SPL. For each mouse, all vectors representing the dB SPL stimulus set were exported to Excel for analysis. The first 2 ms portion of each trace was the pre-stimulus period. All traces were set to the baseline by obtaining the average voltage of the pre-stimulus period from each trace, and subtracting this average from each point in the stimulus-evoked voltage in the remaining 8 ms. This correction also reduced the influence of background noise and DC offset on the recorded trace. Threshold was defined as the first dB SPL in which peak structures emerged above baseline. These peaks were identified by their temporal correlation to peaks at the higher sound levels, where the waves are typically much larger in amplitude. Cases were excluded from threshold analysis if the background noise was large enough to mask the recognition of waves at low sound levels. For measurements of wave amplitude and latency, the 100 dB trace was plotted individually and values were read directly from this plot. For measurements of amplitude, the wave peak was defined as the first value of maximum voltage within a wave. Peak amplitude was then measured as the difference between the baseline value at the bottom of the positive-going wave, and the maximum value in the peak (Walsh et al., 1986). Latency was defined relative to the onset of the stimulus, and peak latency was the time coordinate of the maximum wave amplitude value. All measurements were made blind to genotype. These threshold, amplitude and latency values were then analyzed in JMP v4.0 for statistical significance. Data plots and figures were composed with JMP v4.0, Freehand v11.0, Adobe Illustrator v11.0, and Igor Pro v4.08.
PCR and Genotyping
We used PCR to determine the genotype of the mice used in this study. Tail clips were obtained after termination of the ABR recording. The procedures used are those reported previously (Dravis et al., 2004; Hsieh et al., 2007). Briefly, tail samples were digested overnight and DNA was extracted with 100% isopropanol and resuspended in distilled water at a concentration of 0.3 mg/ml. The primers for EphA4 genotyping were: GTTTCCGCTCTGAGCTTATACTGC, ACAGTGAGTGGACAAAGAGACAGG, and CGCTCTTACCAAAGGGCAAAC. The PCR amplification yielded a 639 bp band for the wild type allele and an 800 bp band for mutant allele. The primers for the ephrin-B2 mice were: AGGCGATTAAGTTGGGTAACG, TCTGTCAAGTTCGCTCTGAGG, and CTTGTAGTAAATGTTGGCAGGACT, and the reaction yielded a 500 bp band for the wild type allele and a 400 bp band for the mutant allele.
Results
We recorded and characterized the ABR of EphA4 and ephrin-B2 mutant mice, aged postnatal day 18–20, and compared them to wild type controls. An example ABR is shown in Figure 1A. Recordings were 10 ms long and included a 2 ms pre-stimulus period. There were typically 4 to 5 waves in each 10 ms trace, as reported previously by Song et al. (2006). The first three waves were termed P1, P2 and P3.
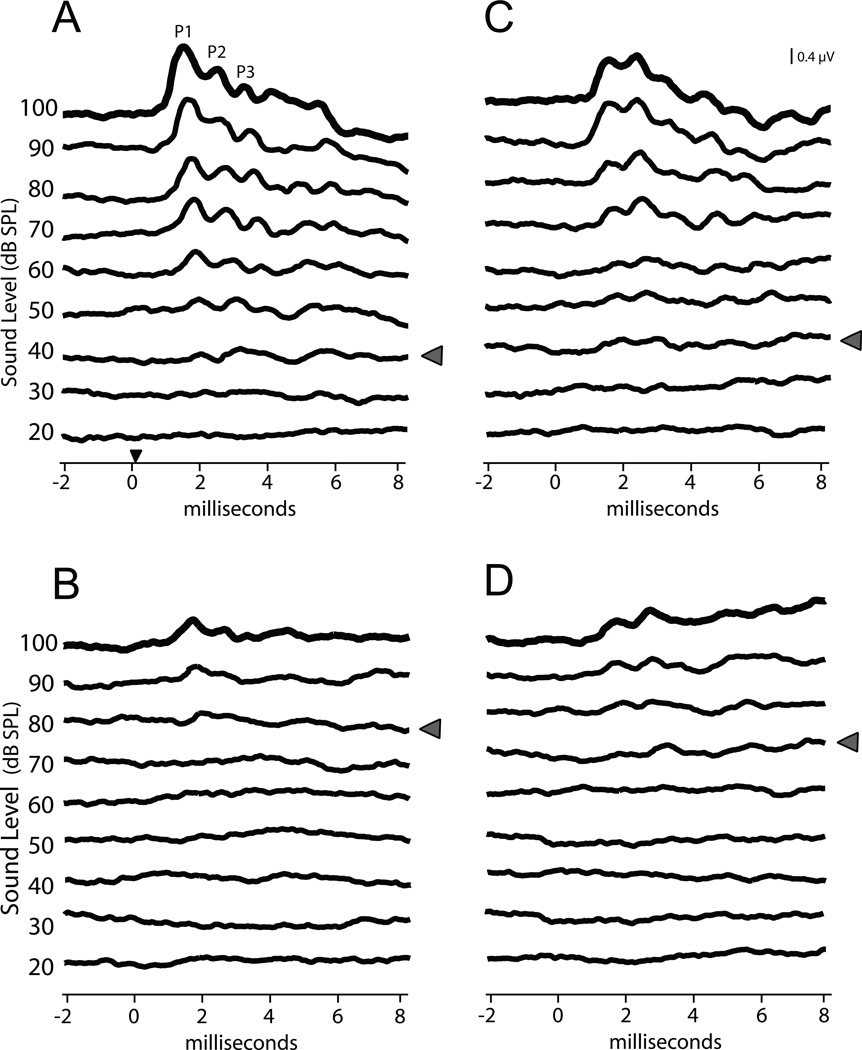
Figure 1.
ABR threshold. Averaged traces from each level of click presentation are shown in the same voltage scale, stacked in increasing dB SPL order. Grey arrowheads indicate ABR threshold. (A) A representative sample of traces from an EphA4+/+ mouse shows an ABR with threshold near 40dB. The small black arrowhead on the time axis indicates the approximate arrival of sound at the tympanic membrane (applies to all panels). (B) The EphA4−/− mouse shows a higher threshold, in this case close to 80dB. In addition, the peak amplitudes appear smaller in the EphA4−/− (compare 100dB traces in A and B). (C) Representative trace from an ephrin-B2+/+ mouse. (D) Similar to the EphA4 mutant, both an elevated threshold and reduced peak amplitude is seen in ephrin-B2lacZ/+ mice as compared to wild type (compare 100dB traces in C and D).
ABR thresholds were higher in both EphA4 and ephrin-B2 mutant mice than in wild type mice. Representative examples of ABR traces for genotypes used in the study are shown in Figure 1A–D. Data were collected every 5 dB between 20 and 60 dB to make a precise determination of threshold. Both the EphA4 heterozygote (EphA4+/−) and homozygote (EphA4−/−) mice required higher dB SPL to elicit a recognizable response (EphA4+/+: 31.5 ± 1.26 dB SPL, n = 17; EphA4+/−: 41.6 ± 3.89 dB SPL, n = 15; EphA4−/−: 55.0 ± 2.98 dB SPL, n = 10; ANOVA, p < 0.05; Fig 2A). Thus, the threshold value for EphA4−/− mice was 75% higher than wild type. Thresholds for ephrin-B2lacZ/+ mice were also significantly elevated, with a mean value 20% higher than wild type controls (ephrin-B2lacZ/+: 59.6 ± 2.36 dB SPL, n = 13 vs. ephrin-B2 +/+: 49.6 ± 1.74 dB SPL, n = 13; t-test, p < 0.05; Fig 2B).
Figure 2.
Measurement of ABR threshold in all genotypes. Both EphA4+/− and EphA4−/− mice show a significantly increased ABR threshold as compared to wild type (A), and the mutant value is 75% higher than control. The ephrin-B2lacZ/+ mice also shows a 20% elevated ABR threshold as compared to wild type (B).
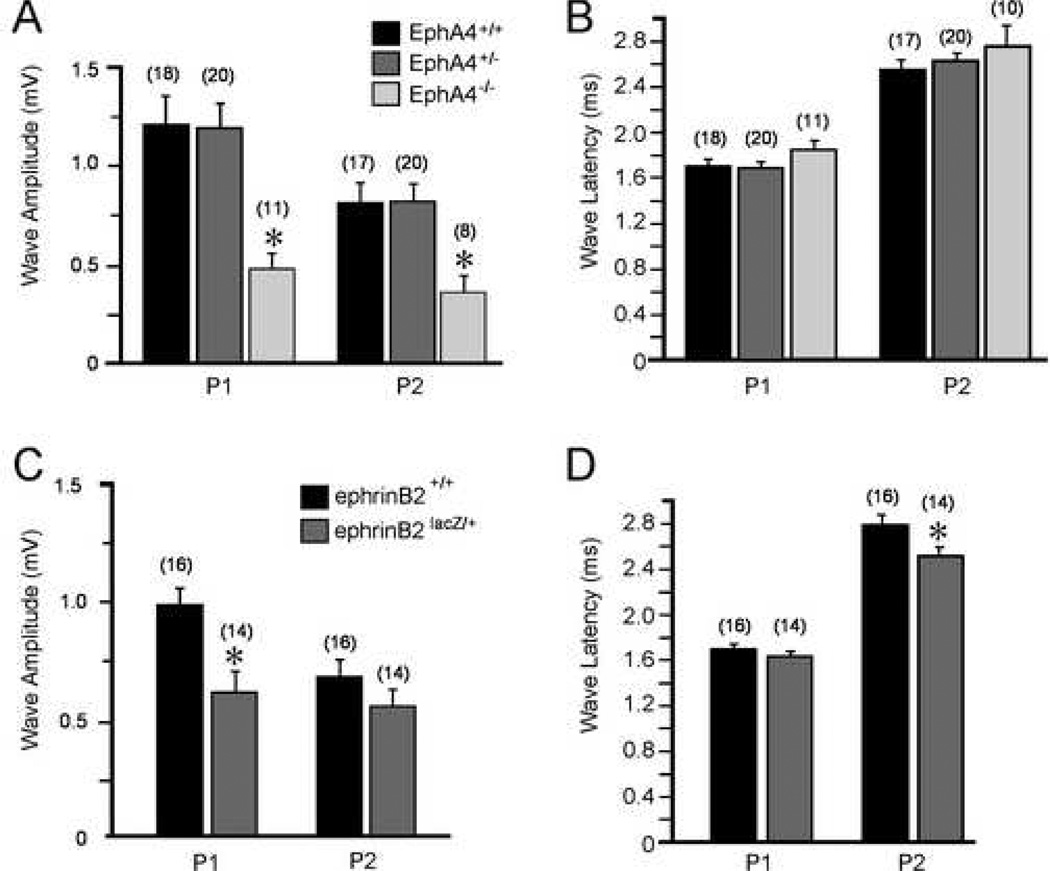
Further analysis of ABR traces revealed that individual waves were also different in some genotype groups (Fig. 3). Measures of amplitude show that P1 was 54% smaller in EphA4−/− mice (0.564 ± 0.09 µV) when compared to wild type controls (1.228 ± 0.14 µV), and P2 amplitude was 56% smaller (0.363 ± 0.04 µV vs. wild type = 0.817 ± 0.11 µV, ANOVA, p < 0.05; Fig. 3A). While P1 amplitude was 38% smaller in ephrin-B2lacZ/+ (0.620 ± 0.09 µV) as compared to wild types (0.994 ± 0.08 µV, t-test, p<0.05), P2 amplitude was not significantly different (ephrin-B2lacZ/+, 0.575 ± 0.08 µV vs. wild type, 0.702 ± 0.07 µV, t-test, p > 0.05; Fig 3C).
Figure 3.
A comparison of amplitude and latency measures in all genotypes. EphA4 genotype key applies to A and B and ephrin-B2 genotype key applies to C and D. P1 and P2 showed decreased amplitude in EphA4−/− mice (A), whereas there was no difference in latency for either peak (B). ephrin-B2lacZ/+ mice show a decreased amplitude in P1 (C ) and a decreased latency for P2 (D).
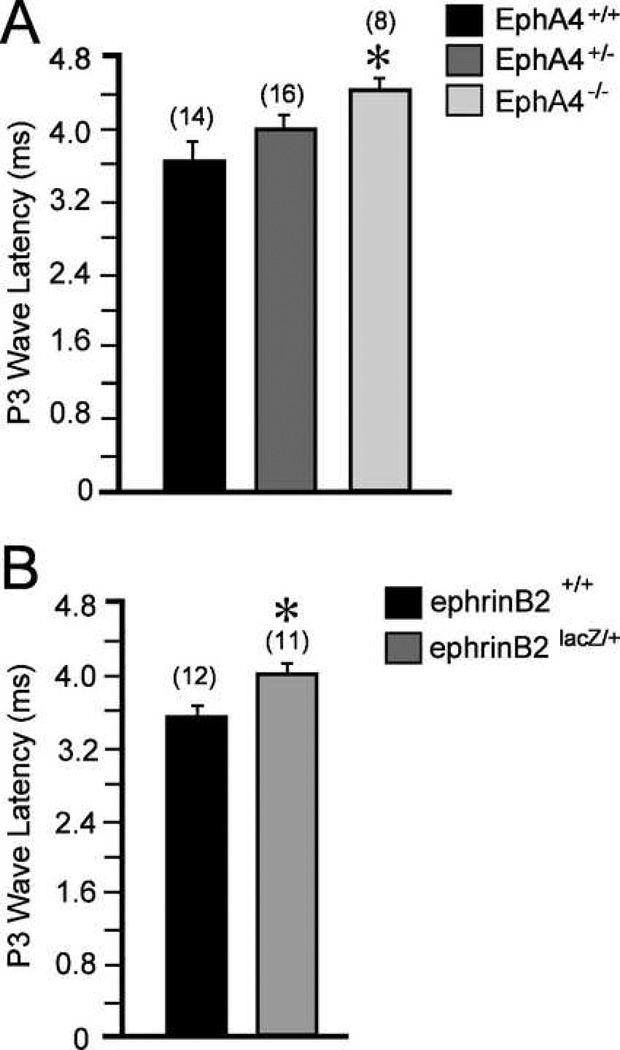
Measures of latency to waveform peak revealed that P1 and P2 were not different from wild type (1.72 ± 0.0448 ms), for either EphA4+/− (1.72 ± 0.0398 ms) or EphA4−/− (1.85 ± 0.079 ms) mice (ANOVA, p< 0.05; Fig 3B). Interestingly, ephrin-B2lacZ/+ mice showed a shorter P2 latency (2.53 ± 0.058 ms vs. wild type, 2.79 ± 0.080 ms; Fig 3D). However, P3 latency was significantly longer in both EphA4−/− (4.42 ± 0.178 ms vs. wild type, 3.71 ± 0.172 ms, ANOVA, p < 0.05; Fig 4A) and ephrin-B2lacZ/+ mice (4.07 ± 0.164 ms vs. wild type, 3.57 ± 0.11 ms, t-test, p < 0.05; Fig 4B). While the peak maxima were identifiable for these relative measures of latency, measures of P3 amplitude were not recorded as the waves were frequently merged with adjacent waveforms.
Figure 4.
The latency to P3 peak was longer in EphA4−/− (A) and ephrin-B2lacZ/+ (B) mice, as compared to matched wild types.
Discussion
EphA4 and ephrin-B2 mutant mice display an altered ABR in comparison to wild type controls, in measures of magnitude and timing. Hearing thresholds were significantly elevated for both homozygous and heterozygous mice, and a concurrent decrease in P1 amplitude was found in both mutants. P2 amplitude was also significantly decreased in EphA4 mutants, while ephrin-B2 mutants showed a shorter latency for P2 without any change in amplitude. Both mutants also displayed a longer latency of the P3 wave.
Several functions of Eph proteins might account for the changes we observed in the ABR in mutant mice. Our measurements of reduced amplitude of P1 and P2 waves in EphA4 mice and reduced P1 wave amplitude in ephrin-B2lacZ/+ mice suggest an auditory nerve and/or cochlear nucleus defect. The expression of EphA4 in the cochlea (van Heumen et al., 2000) suggests that the change in the ABR may originate peripherally, at least in part. Mutations in some EphB genes alter cochlear function as measured by DPOAEs (Howard et al., 2003), but the specific effect of EphA4 or ephrin-B2 mutation has not been reported. Both neural and non-neural regions of the cochlea express Eph proteins (Pickles, 2003), suggesting that the functions of these proteins may also include structural development (Pickles et al., 2002) and maintenance of the cochlea. In addition, Eph proteins may regulate ion concentrations essential to function in the ear (Dravis et al., 2007). The diversity of functions for Eph proteins, combined with the distinct expression patterns of Eph family members (Bianchi and Gale, 1998; Pickles, 2003), highlight the fact that these proteins may have multiple, complex interactions that ultimately support normal hearing.
Decreases in P1 and P2 wave amplitudes may be directly related to the observed increase in ABR threshold. These first two waves show the largest amplitudes in the mouse ABR trace, and therefore are the first to define the emergence of the peaks from baseline, the appearance at threshold. Previous work has suggested that P1 and P2 reflect activation of the auditory nerve and cochlear nucleus, respectively (Møller and Jannetta, 1985). Our findings of decreased amplitude in both peaks in the EphA4−/− mouse may reflect a significant role for this receptor tyrosine kinase in the auditory nerve-cochlear nucleus region. EphA4 is expressed in both the dorsal and ventral cochlear nucleus, and measures of tone-evoked activation of the immediate early gene c-fos have shown that the magnitude of activation is significantly decreased in the EphA4−/− dorsal cochlear nucleus (DCN) as well as medial nucleus of the trapezoid body (MNTB) (Miko et al., in press). Together with the ABR result presented here, there is strong evidence that EphA4 protein has a pronounced affect on the magnitude of evoked activation in auditory brainstem.
The 20% elevated threshold and 38% reduction of P1 amplitude in ephrin-B2 mutants may also reflect a poorly developed auditory nerve-cochlear nucleus junction. Developing mouse spiral ganglion cells express ephrin-B2 during development of cochlear innervation (Bianchi and Gray, 2002; Pickles, 2003), and cochlear nucleus neurons also express ephrin-B2 during early postnatal development (Miko et al., in press). Studies of adult cochlear nucleus show that ephrin-B2 is also abundant in both dorsal and ventral cochlear nucleus, as well as the superior olive and emanating lemniscal pathways. Our previous observation that ephrin-B2 deficiency increases the spread of tone-evoked activation in the dorsal cochlear nucleus without decreasing magnitude of activation (number of cells) (Miko et al., in press) may corroborate the decreases in P1 amplitude presented here. Thus, the voltage could be attenuated as a result of the same current spread over a larger volume of tissue. However, the use of higher impedance electrodes than those used in the present study would be necessary to explore the relationship between current magnitude and spread of activation over a smaller volume of tissue the size of cochlear nucleus.
The significantly delayed P3 latency in both EphA4 and ephrin-B2 mutants may reflect abnormality in the superior olivary complex. Previous work in the guinea pig brainstem has shown that a lesion at the ventral midline that resects the olivary nuclei causes a complete loss of P3, and suggests that the superior olivary complex contributes to this wave of the ABR (Wada and Starr, 1989). Our findings of increased P3 latency in the mutant animals may reflect a processing defect in this part of the brainstem. This observation is consistent with our finding that EphA4 and ephrin-B2 are expressed in the olivary nuclei and that tone-evoked activation of c-fos in the MNTB is reduced in both mutants (Miko et al., in press). A slower neural conduction time through the auditory pathway may be an indication of the integrity of myelin, as increases in ABR peak latencies have been observed in humans with multiple sclerosis (Keith et al., 1987). Interestingly, Eph proteins are known to interact with myelin through neuroglial interactions (Benson et al., 2005; Goldshmit et al., 2006; Liu et al., 2006). Furthermore, the ephrin-B2 ABR shows an intriguing shorter P2 latency coinciding with a longer P3 latency, which suggests that the weight of this impairment may indeed be in the processing between cochlear nucleus and the superior olivary complex.
Although the P18–20 mouse shows an adult-like ABR waveform (Song et al., 2006), there is evidence that protein expression and synaptic transmission are not fully mature in brainstem auditory structures until after P25 (Blaesse et al., 2006; Friauf et al., 1997; Happe and Morley, 2004). However, we focused on P18–20 because at this age responses to click stimuli are adult-like in the cochlear nerve (Sanes and Constantine-Paton, 1985), and the ABR has matured to include all peaks and latencies present in the adult waveform (Song et al., 2006), yet this age is young enough to minimize the effect of age-related hearing loss, which can occur quite early in the C57/Bl6 strain. The present findings involving Eph receptor impact on function may eventually be compensated for by later ages, but this would require ABR testing at multiple time points beyond the one tested here. Above all, deficits seen at P18–20 can have a long-term impact, as they would be present at weaning age, when the mouse uses its hearing to survive independent of the breeding group.
It is important to note that differences in ABR threshold were also apparent between the wild type of each mouse line, which shows that the impact of background strain can be a confounding influence. The definition of normal ABR thresholds were always made within background strain, and the results shown here emphasize the importance of comparing mutant mice with wild type littermates, as strain differences may impact results achieved with knockout mice.
ABR measurements have proven to be a robust method for analyzing the effects of genetic mutations on hearing in mice, both in mutagenesis screens (Kermany et al., 2006) and in the evaluation of candidate genes (Abraira et al., 2007; Blauwkamp et al., 2007; Polley et al., 2006). In these studies, the candidate genes are transcription factors or morphogens with identified roles in nervous system development. Similarly, the ABR data presented here reveal the significant impact of EphA4 and ephrin-B2 mutations on the effectiveness of auditory processing. Together with their previously established role as axon guidance molecules, these Eph proteins impact both anatomical and functional aspects of the auditory neural circuit. These abnormalities underscore the importance of Eph family proteins in the formation of normal auditory function.
Acknowledgments
The authors would like to thank Leonard Kitzes and Arnold Starr for helpful discussions, and Shazia Siddiqui and Shan Kuang for technical assistance. This research was supported by NIH DC005771 and T32 NS045540.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraira VE, Hyun N, Tucker AF, Coling DE, Brown MC, Lu C, Hoffman GR, Goodrich LV. Changes in Sef levels influence auditory brainstem development and function. J Neurosci. 2007;27:4273–4282. doi: 10.1523/JNEUROSCI.3477-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson MD, Romero MI, Lush ME, Lu QR, Henkemeyer M, Parada LF. Ephrin-B3 is a myelin-based inhibitor of neurite outgrowth. Proc Natl Acad Sci U S A. 2005;102:10694–10699. doi: 10.1073/pnas.0504021102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi LM, Gale NW. Distribution of Eph-related molecules in the developing and mature cochlea. Hear Res. 1998;117:161–172. doi: 10.1016/s0378-5955(98)00010-0. [DOI] [PubMed] [Google Scholar]
- Bianchi LM, Gray NA. EphB receptors influence growth of ephrin-B1-positive statoacoustic nerve fibers. Eur J Neurosci. 2002;16:1499–1506. doi: 10.1046/j.1460-9568.2002.02248.x. [DOI] [PubMed] [Google Scholar]
- Blaesse P, Guillemin I, Schindler J, Schweizer M, Delpire E, Khiroug L, Friauf E, Nothwang HG. Oligomerization of KCC2 correlates with development of inhibitory neurotransmission. J Neurosci. 2006;26:10407–10419. doi: 10.1523/JNEUROSCI.3257-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blauwkamp MN, Beyer LA, Kabara L, Takemura K, Buck T, King WM, Dolan DF, Barald KF, Raphael Y, Koenig RJ. The role of bone morphogenetic protein 4 in inner ear development and function. Hear Res. 2007;225:71–79. doi: 10.1016/j.heares.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brors D, Bodmer D, Pak K, Aletsee C, Schafers M, Dazert S, Ryan AF. EphA4 provides repulsive signals to developing cochlear ganglion neurites mediated through ephrin-B2 and -B3. J Comp Neurol. 2003;462:90–100. doi: 10.1002/cne.10707. [DOI] [PubMed] [Google Scholar]
- Contractor A, Rogers C, Maron C, Henkemeyer M, Swanson GT, Heinemann SF. Trans-synaptic Eph receptor-ephrin signaling in hippocampal mossy fiber LTP. Science. 2002;296:1864–1869. doi: 10.1126/science.1069081. [DOI] [PubMed] [Google Scholar]
- Cramer KS. Eph proteins and the assembly of auditory circuits. Hear Res. 2005;206:42–51. doi: 10.1016/j.heares.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Cramer KS, Cerretti DP, Siddiqui SA. EphB2 regulates axonal growth at the midline in the developing auditory brainstem. Dev Biol. 2006;295:76–89. doi: 10.1016/j.ydbio.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Cramer KS, Bermingham-McDonogh O, Krull CE, Rubel EW. EphA4 signaling promotes axon segregation in the developing auditory system. Dev Biol. 2004;269:26–35. doi: 10.1016/j.ydbio.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Dalva MB, McClelland AC, Kayser MS. Cell adhesion molecules: signalling functions at the synapse. Nat Rev Neurosci. 2007;8:206–220. doi: 10.1038/nrn2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dravis C, Yokoyama N, Chumley MJ, Cowan CA, Silvany RE, Shay J, Baker LA, Henkemeyer M. Bidirectional signaling mediated by ephrin-B2 and EphB2 controls urorectal development. Dev Biol. 2004;271:272–290. doi: 10.1016/j.ydbio.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Dravis C, Wu T, Chumley MJ, Yokoyama N, Wei S, Wu DK, Marcus DC, Henkemeyer M. EphB2 and ephrin-B2 regulate the ionic homeostasis of vestibular endolymph. Hear Res. 2007;223:93–104. doi: 10.1016/j.heares.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Eph Nomenclature Committee. Unified nomenclature for Eph family receptors and their ligands, the ephrins. Cell. 1997;90:403–404. doi: 10.1016/s0092-8674(00)80500-0. [DOI] [PubMed] [Google Scholar]
- Friauf E, Hammerschmidt B, Kirsch J. Development of adult-type inhibitory glycine receptors in the central auditory system of rats. J Comp Neurol. 1997;385:117–134. doi: 10.1002/(sici)1096-9861(19970818)385:1<117::aid-cne7>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Gale NW, Holland SJ, Valenzuela DM, Flenniken A, Pan L, Ryan TE, Henkemeyer M, Strebhardt K, Hirai H, Wilkinson DG, Pawson T, Davis S, Yancopoulos GD. Eph receptors and ligands comprise two major specificity subclasses and are reciprocally compartmentalized during embryogenesis. Neuron. 1996;17:9–19. doi: 10.1016/s0896-6273(00)80276-7. [DOI] [PubMed] [Google Scholar]
- Goldshmit Y, McLenachan S, Turnley A. Roles of Eph receptors and ephrins in the normal and damaged adult CNS. Brain Res Rev. 2006;52:327–345. doi: 10.1016/j.brainresrev.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Happe HK, Morley BJ. Distribution and postnatal development of alpha 7 nicotinic acetylcholine receptors in the rodent lower auditory brainstem. Brain Res Dev Brain Res. 2004;153:29–37. doi: 10.1016/j.devbrainres.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Henderson JT, Georgiou J, Jia Z, Robertson J, Elowe S, Roder JC, Pawson T. The receptor tyrosine kinase EphB2 regulates NMDA-dependent synaptic function. Neuron. 2001;32:1041–1056. doi: 10.1016/s0896-6273(01)00553-0. [DOI] [PubMed] [Google Scholar]
- Himanen JP, Chumley MJ, Lackmann M, Li C, Barton WA, Jeffrey PD, Vearing C, Geleick D, Feldheim DA, Boyd AW, Henkemeyer M, Nikolov DB. Repelling class discrimination: ephrin-A5 binds to and activates EphB2 receptor signaling. Nat Neurosci. 2004;7:501–509. doi: 10.1038/nn1237. [DOI] [PubMed] [Google Scholar]
- Howard MA, Rodenas-Ruano A, Henkemeyer M, Martin GK, Lonsbury-Martin BL, Liebl DJ. Eph receptor deficiencies lead to altered cochlear function. Hear Res. 2003;178:118–130. doi: 10.1016/s0378-5955(03)00068-6. [DOI] [PubMed] [Google Scholar]
- Hsieh CY, Hong CT, Cramer KS. Deletion of EphA4 enhances deafferentation-induced ipsilateral sprouting in auditory brainstem projections. J Comp Neurol. 2007;504:508–518. doi: 10.1002/cne.21465. [DOI] [PubMed] [Google Scholar]
- Huffman KJ, Cramer KS. EphA4 misexpression alters tonotopic projections in the auditory brainstem. Dev Neurobiol. 2007;67:1655–1668. doi: 10.1002/dneu.20535. [DOI] [PubMed] [Google Scholar]
- Keith RW, Garza-Holquin Y, Smolak L, Pensak ML. Acoustic reflex dynamics and auditory brain stem responses in multiple sclerosis. Am J Otol. 1987;8:406–413. [PubMed] [Google Scholar]
- Kermany MH, Parker LL, Guo YK, Miller D, Swanson DJ, Yoo TJ, Goldowitz D, Zuo J. Identification of 17 hearing impaired mouse strains in the TMGC ENU-mutagenesis screen. Hear Res. 2006;220:76–86. doi: 10.1016/j.heares.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Krull CE, Lansford R, Gale NW, Collazo A, Marcelle C, Yancopoulos GD, Fraser SF, Bronner-Fraser M. Interactions of Eph-related receptors and ligands confer rostrocaudal pattern to trunk neural crest migration. Curr. Biol. 1997;7:571–580. doi: 10.1016/s0960-9822(06)00256-9. [DOI] [PubMed] [Google Scholar]
- Kullander K, Klein R. Mechanisms and functions of Eph and ephrin signalling. Nat Rev Mol Cell Biol. 2002;3:475–486. doi: 10.1038/nrm856. [DOI] [PubMed] [Google Scholar]
- Leighton PA, Mitchell KJ, Goodrich LV, Lu X, Pinson K, Scherz P, Skarnes WC, Tessier-Lavigne M. Defining brain wiring patterns and mechanisms through gene trapping in mice. Nature. 2001;410:174–179. doi: 10.1038/35065539. [DOI] [PubMed] [Google Scholar]
- Liu BP, Cafferty WB, Budel SO, Strittmatter SM. Extracellular regulators of axonal growth in the adult central nervous system. Philos Trans R Soc Lond B Biol Sci. 2006;361:1593–1610. doi: 10.1098/rstb.2006.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellitzer G, Xu Q, Wilkinson DG. Control of cell behaviour by signalling through Eph receptors and ephrins. Curr Opin Neurobiol. 2000;10:400–408. doi: 10.1016/s0959-4388(00)00095-7. [DOI] [PubMed] [Google Scholar]
- Miko IJ, Nakamura PA, Henkemeyer M, Cramer KS. Auditory brainstem neural activation patterns are altered in EphA4 and ephrin-B2 deficient mice. doi: 10.1002/cne.21530. in press. [DOI] [PubMed] [Google Scholar]
- Møller AR, Jannetta PJ. Neural generators of the auditory brainstem response. In: Jacobson JT, editor. The Auditory Brainstem Response. San Diego: College Hill Press; 1985. [Google Scholar]
- Murai KK, Pasquale EB. 'Eph'ective signaling: forward, reverse and crosstalk. J Cell Sci. 2003;116:2823–2832. doi: 10.1242/jcs.00625. [DOI] [PubMed] [Google Scholar]
- Parham K. Distortion product otoacoustic emissions in the C57BL/6J mouse model of age-related hearing loss. Hear Res. 1997;112:216–234. doi: 10.1016/s0378-5955(97)00124-x. [DOI] [PubMed] [Google Scholar]
- Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol. 2005;6:462–475. doi: 10.1038/nrm1662. [DOI] [PubMed] [Google Scholar]
- Pickles JO. Expression of Ephs and ephrins in developing mouse inner ear. Hear Res. 2003;178:44–51. doi: 10.1016/s0378-5955(03)00029-7. [DOI] [PubMed] [Google Scholar]
- Pickles JO, Claxton C, Van Heumen WR. Complementary and layered expression of Ephs and ephrins in developing mouse inner ear. J Comp Neurol. 2002;449:207–216. doi: 10.1002/cne.10231. [DOI] [PubMed] [Google Scholar]
- Polley DB, Cobos I, Merzenich MM, Rubenstein JL. Severe hearing loss in Dlxl mutant mice. Hear Res. 2006;214:84–88. doi: 10.1016/j.heares.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Sanes DH, Constantine-Paton M. The development of stimulus following in the cochlear nerve and inferior colliculus of the mouse. Brain Res. 1985;354:255–267. doi: 10.1016/0165-3806(85)90177-4. [DOI] [PubMed] [Google Scholar]
- Song L, McGee JA, Walsh EJ. Consequences of combined maternal, fetal and persistent postnatal hypothyroidism on the development of auditory function in Tshrhyt mutant mice. Brain Res. 2006;1101:59–72. doi: 10.1016/j.brainres.2006.05.027. [DOI] [PubMed] [Google Scholar]
- van Heumen WR, Claxton C, Pickles JO. Expression of EphA4 in developing inner ears of the mouse and guinea pig. Hear Res. 2000;139:42–50. doi: 10.1016/s0378-5955(99)00158-6. [DOI] [PubMed] [Google Scholar]
- Wada S, Starr A. Anatomical bases of binaural interaction in auditory brain-stem responses from guinea pig. Electroencephalogr Clin Neurophysiol. 1989;72:535–544. doi: 10.1016/0013-4694(89)90231-9. [DOI] [PubMed] [Google Scholar]
- Walsh EJ, McGee J, Javel E. Development of auditory-evoked potentials in the cat. III. Wave amplitudes. J Acoust Soc Am. 1986;79:745–754. doi: 10.1121/1.393463. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG. Multiple roles of EPH receptors and ephrins in neural development. Nat Rev Neurosci. 2001;2:155–164. doi: 10.1038/35058515. [DOI] [PubMed] [Google Scholar]