Abstract
Injury to the cornea leads to formation of mediators that initiate and amplify inflammatory responses and neovascularization. Among these are lipid mediators generated by a cytochrome P450 (CYP) enzyme identified as CYP4B1. Increased corneal CYP4B1 expression increases limbal angiogenic activity through the production of 12-hydroxyeicosatrienoic acid (12-HETrE), a potent inflammatory and angiogenic eicosanoid. We used siRNA duplexes targeting CYP4B1 to substantiate the link between CYP4B1 expression, 12-HETrE production and angiogenesis in a model of suture-induced corneal neovascularization. Intrastromal sutures induced a time-dependent neovascular response which was significantly attenuated by CYP4B1-specific siRNAs but not by nonspecific siRNA. CYP4B1 mRNA was reduced by 60% and 12-HETrE’s levels were barely detected in corneal homogenates from eyes treated with the CYP4B1-specific siRNA. The decreased neovascular response in CYP4B1 siRNA-treated eyes was associated with a 75% reduction in corneal VEGF mRNA levels. Transfection of rabbit corneal epithelial cells with CYP4B1 cDNA induced VEGF expression. Conversely, treatment with CYP4B1 siRNA or addition of a CYP4B1 inhibitor significantly decreased VEGF mRNA levels; addition of 12-HETrE potently increased them. The results strongly implicate the corneal CYP4B1 as a component of the inflammatory and neovascular cascade initiated by injury and further suggest that CYP4B1-12-HETrE is a proximal regulator of VEGF expression.
INTRODUCTION
Neovascularization of the normally avascular cornea is seen in many pathological conditions which include infection, mechanical and chemical injury, chronic exposure to hypoxia and following corneal transplantation. Corneal neovascularization, whatever the cause, leads to decreased vision, recurrent corneal erosion and incompetent barrier function, thus presenting a serious clinical problem for which treatment is poor. The molecular mechanisms that control corneal neovascularization are not fully understood. Since inflammation frequently precedes corneal neovascularization, many studies have concentrated on the role of humoral and corneal-derived inflammatory mediators in the regulation of this process. Among these mediators are arachidonic acid-derived eicosanoids of the cytochrome P450 monooxygenase (CYP) pathways.
Previous studies have identified a CYP-dependent arachidonic acid metabolism in the corneal epithelium and established it as a primary corneal epithelial inflammatory pathway in experimental models of ocular surface inflammation.1 This corneal epithelial CYP metabolizes arachidonic acid to the potent inflammatory and angiogenic 12-hydroxyeicosatrienoic acid (12-HETrE). 12-HETrE is a critical corneal-derived mediator of ocular surface inflammation based on studies demonstrating that: 1) its synthesis in the corneal epithelium and primarily that of the bioactive R enantiomer increases following hypoxic or chemical injury in vitro and in vivo;2–4 2) its levels in the cornea positively correlate with the in situ inflammatory and neovascular response;5,6 and 3) its biological activities, in particular those of 12(R)-HETrE, in vitro and in vivo, are characteristic of potent inflammatory mediators (including vasodilation,7 neutrophil chemotaxis,8 and angiogenesis9). The importance of 12-HETrE to human pathophysiology is underscored by its presence in human tears, and more significantly, by the fact that its levels are much higher in tears from subjects with ocular inflammation.10
We have isolated a 1.82 kb CYP cDNA from hypoxic rabbit corneal epithelium with a 98% sequence homology to the lung CYP4B1.11,12 The expression of the CYP4B1 in rabbit corneal organ cultures was induced by hypoxia and its increased expression was associated with increased production of 12-HETrE.11 Further studies demonstrating that antibodies against CYP4B1 inhibited hypoxia-induced 12-HETrE synthesis11 provided substantial evidence that in the corneal epithelium CYP4B1 is involved in the hypoxia-induced 12-HETrE synthesis and ocular surface inflammation. Indeed, increased expression of CYP4B1 mRNA in the corneal epithelium during hypoxic injury in vivo corresponded well with the progression of the anterior surface inflammatory response, including corneal thickness and inflammatory score, as well as with the synthesis of 12-HETrE.13 In mice experiencing exaggerated and unresolved inflammation, corneal CYP4B1 expression is highly elevated and so are the corneal levels of 12-HETrE.14 In a recent study, we provided substantial evidence that the CYP4B1 is the enzymatic source for the production of 12-HETrE in the cornea. Moreover, transfection of CYP4B1 into the cornea yielded an inflammatory response in the ocular surface and stimulated angiogenic activity of limbal explants that was mediated by increased production of 12-HETrE.15
The current study was undertaken to further substantiate the cause and effect relationship between CYP4B1 expression, 12-HETrE production and neovascularization in the cornea. We used small interfering RNAs (siRNA) targeting CYP4B1 in a well-established model of inflammatory corneal neovascularization.16–19 Herein, we demonstrate that siRNA targeting the corneal CYP4B1 inhibits corneal neovascularization further supporting the role of CYP4B1 as an angiogenic pathway in the cornea.
MATERIALS AND METHODS
Design of gene targets and SiRNA
SiRNA duplexes were derived from the coding sequence of the rabbit CYP4B1 cDNA (GenBank AF176914).15 Synthesis of siRNAs was done by GeneLink (Hawthorne, NY). Sequences identified were BLASTed against the GenBank database. All siRNAs were 21-nucleotide long double stranded RNA oligos with a two-nucleotide (TT) overhang at the 3′ end. The target sequence (5′ to 3′) and the siRNA duplexes, designated as 4B1a, 4B1b and 4B1c, are shown in Table 1, with the position of the sequence in the rabbit CYP4B1 cDNA shown in parentheses; a nonspecific siRNA duplex was used as control. SiRNA solutions were made in RNAse free water at concentration of 200 μM.
TABLE 1.
Sequences of siRNA duplexes targeting CYP4B1
| SiRNA duplex | CYP4B1 sequence 5′-3′ [cDNA sequence] | SiRNA duplex sequence |
|---|---|---|
| 4B1a | gcatccacctctacttgaa [1499–1518] | GCAUCCACCUCUACUUGAATT
TTCGUAGGUGGAGAUGAACUU |
| 4B1b | gcacctacttgcttcagaa [1745–1757] | GCACCUACUUGCUUCAGAATT
TTCGUGGAUGAACGAAGUCUU |
| 4B1c | tgcacttgatcggctttat [1914–1932] | UGCACUUGAUCGGCUUUAUTT
TTACGUGAACUAGCCGAAAUA |
In vitro efficacy of siRNA
Immortalized rabbit corneal epithelial (RCE) cells were a generous gift from Dr. K. Araki, Department of Ophthalmology, University of Osaka, Japan.20 Cells were grown to 80% confluence in DMEM/F-12’s 1:1 Ham’s nutrient mixture (Hyclone, Logan, UT)) supplemented with 15% heat-inactivated fetal bovine serum and 1% antibiotics and antimycotic solution. Rabbit CYP4B1 cDNA (1.82Kb; GenBank #AF176914) was cloned into the multiple cloning site of pIRES-EGFP vector (Clontech, Mountain View, CA) at AccI and XmaI restriction sites15. The pIRES-EGFP plasmid was used as control. One day before transfection, the medium was substituted with serum-free medium. For the transfection, cells were incubated in DMEM (500 ml/well) containing 1μg of plasmid, either pIRES2-EGFP or pIRES2-EGFP-4B1, and 4 μl of the LipofectAMINETM (Invitrogen, Carlsbad, CA) for 6 h at 37°C. After incubation, the medium was replaced with serum-containing medium and incubation continued overnight. Cells were then placed in serum- and antibiotics-deprived DMEM (500 ml/well) containing 40nM siRNA and 6 μl of LipofectAMINETM for 6 hour at 37°C. The medium was replaced with DMEM/F12 supplemented with 15% (v/v) FBS and cells were left overnight at 37°C. Expression of pIRES-EGFP and pIRES-EGFP-CYP4B1 was monitored with a Nikon Eclipse TE2000-U immunofluorescent microscope and recorded with a CCD digital camera attached to the microscope. To assess CYP4B1 activity, cells were washed with PBS twice and processed for measurement of arachidonic acid metabolism as previously described.3 Briefly, cells were incubated with 14C-arachidonic acid (1 μCi) and NADPH (1 mM) for 60 min at 37oC. The incubation medium was extracted with ethyl acetate and radiolabeled metabolites were separated by HPLC and identified by co-elution with authentic standards. The levels of 12-HETrE were further quantified by negative chemical ionization-gas chromatography-mass spectrometry as previously described.21
Corneal neovascularization and in vivo delivery of siRNA
All animal studies adhered to the ARVO statement for the Use of Animals in Ophthalmic and Vision research. Male New Zealand rabbits (2.2–2.5 kg) were anesthetized with Ketamine (50 mg/kg) and Xylazine (20 mg/kg) intramuscularly. Following a local administration of 0.5% proparacaine hydrochloride to minimize discomfort, a single 7.0 silk suture was inserted at midstromal depth approximately 2 mm from the limbus. Immediately after suture placement, siRNA duplexes (20 μl) were administered by subconjunctival injection adjacent to the suture location using a 32-gauge Hamilton syringe. The left eye received a subconjunctival injection of 20μl solution of CYP4B1 siRNA (200 μM); the right eye received 20μl of nonspecific siRNA (200 μM). Injections of siRNA were repeated at day 2 and 4 after suture placement. In some experiments, eyes were treated with a daily drop (10 μl, 30 μM) of the selective CYP4B1 inhibitor 17-octadecynoic acid (17-ODYA)4,15. Corneal neovascularization was evaluated at days 2, 4 and 7 after suture placement by slit lamp microscopy attached to a video camera (Q Imaging, Canada). Neovascularization was quantified in digitalized images by comparing total vessel length. Vessel length was determined by tracing all and each vessel from the limbus to the suture. Total length is the sum of these vessels in mm and was determined using Image Pro-Express Software (Cyber Media) as previously described.15 Rabbits were sacrificed 7 days after suture insertion and corneal wedges containing the sutures were dissected and processed for measurements of CYP4B1 mRNA expression. Functional expression of CYP4B1 was further evaluated by measuring the levels of 12-HETrE, its arachidonic acid metabolite. Briefly, corneal wedges were homogenized and lipids were extracted with ethyl acetate as previously described. Extracts were constituted with methanol and subjected to reverse phase HPLC. 12-HETrE was detected by absorbance at 205 nm and identified by its co-elution with chemically synthesized 12-HETrE standard22. In addition, microsomes prepared from corneal wedges were incubated with arachidonic acid (7 nmol) and NADPH (1 mM) for 1 h at 37°C. 12-HETrE synthesis was measured by gas chromatography/mass spectrometry as previously described.10
Real time PCR
Corneas were dissected from eyes using sterile instruments and were cleaned in sterile PBS (4°C) under a dissecting microscope to remove all non-corneal tissue. Total RNA was isolated using TRiZol reagent (Invitrogen, Carlsbad, CA) and RNA integrity was verified by agarose gel electrophoresis and quantitated by spectrophotometry using the Nanodrop ND-1000 spectrophotometer (Nanodrop Technologies, Wilmington, Delaware). RT reaction of total RNA (5 μg) was performed using SuperscriptTM III First-Strand Synthesis System (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instruction. Quantitative Real-Time PCR was performed using Brilliant® SYBR Green QPCR Master Mix and the Mx3000p™ Real-Time PCR System (Stratagene, La Jolla, CA). Specific primers were designed based on published sequences (Genebank) and were as follows: CYP4B1 sense 5′-TCTCTGGGTT-GGACAGTTCATTG-3′ and antisense 5′-TGTCTCCTTTGCCAAACGTACAC-3′ (359 bp ) ; VEGF A sense 5′-GGAGTACCCTGATGAGATCGA-3′ and antisense 5′-CTTTGGTCTGCATTCACATTTGT-3′ (165 bp); 28S sense 5′-AAACTCTGGTGGAGGTCCGT-3′ and antisense 5′-CTTACCAAAAGTGGCCCACTA-3′ (305 bp). PCR efficiency for each primer pair was determined by quantitating amplification with increasing concentrations of template cDNA (15.6, 31.2, 62.5, 125.0, 250.0, and 500.0 ng) and specific amplification was verified by subsequent analysis of melt curve profiles for each amplification. A non-template control served as the negative control to exclude the formation of primer dimers or any other non-specific PCR products. RNA expression of target genes was calculated based on the real-time PCR efficiency (E) and the threshold crossing point (CP) and is expressed in comparison to the reference gene 28S RNA as described.23
VEGF mRNA expression in RCE cells
RCE cells were grown to 80% confluency in complete medium and then incubated for 24 h in serum-free medium before adding 12-HETrE (0.1–100 nM) or placing the cells under hypoxic condition using a modular tissue culture chamber (Billups-Rothenburg, DelMar, CA.) supplied continuously with 5%CO2/2% O2/93% N2 (hypoxia), bubbled through deionized H2O into the chamber within a 37°C incubator. Total RNA was extracted with TriZol, quantified by spectrometry and processed for real time PCR analysis as described above. VEGF mRNA levels were also measured in cells transfected with the plasmids pIRES2-EGFP or pIRES2-EGFP-4B1 and treated with and without CYP4B1 siRNA duplexes (40 nM) or 17-ODYA (20 μM).
RESULTS
In vitro efficacy of CYP4B1 siRNA
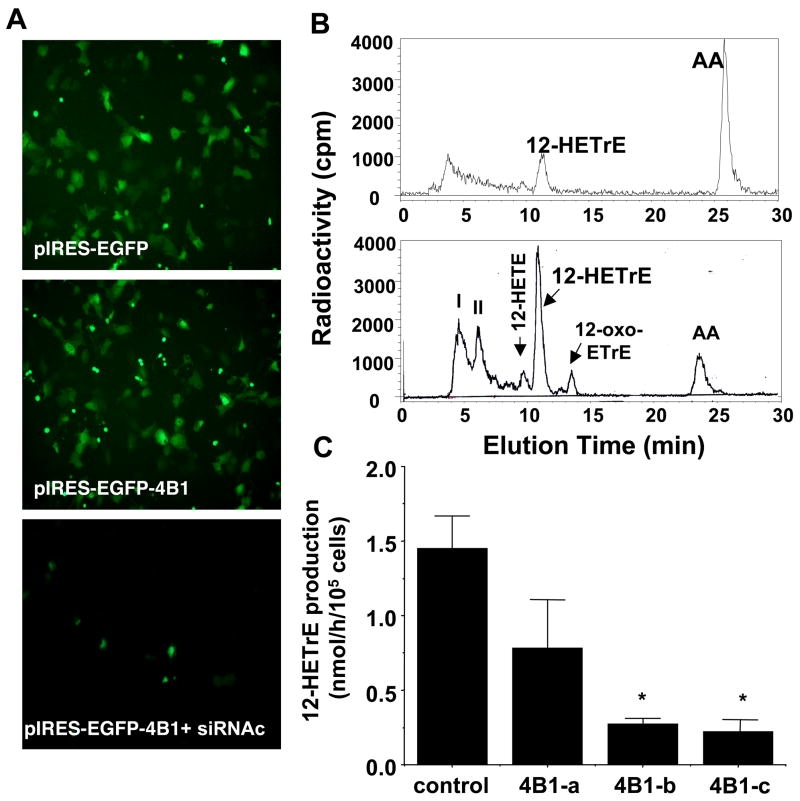
We have previously shown that RCE cells overexpressing the CYP4B1 readily converted arachidonic acid to 12-HETrE, whereas, native cells exhibited very low levels of CYP4B1 expression and showed little capacity of 12-HETrE production.24 Therefore, RCE cells overexpressing CYP4B1 were used to evaluate the efficacy of siRNA duplexes targeting CYP4B1. Figure 1A depicts GFP fluorescence in cells transfected with the bicistronic control plasmid pIRES-EGFP (upper panel) and the plasmid expressing the CYP4B1, pIRES-EGFP-CYP4B1 (middle panel); both exhibited similar levels of GFP fluorescence. However, they differ in their capacity to produce 12-HETrE the major arachidonic acid metabolite of CYP4B1. As seen in Figure 1B, HPLC profiles of arachidonic acid metabolites in cells transfected with the CYP4B1-containing plasmid clearly showed that 12-HETrE production is 3–4 fold higher than cells transfected with the control plasmid pIRES-EGFP. Treatment of pIRES-EGFP-CYP4B1-transfected cells with CYP4B1-specific siRNA attenuated GFP fluorescence (Figure 1A, lower panel), suggesting inhibition of CYP4B1 expression. The effect of siRNA on CYP4B1 functional expression was further determined by measuring the production of 12-HETrE. Quantitative analysis is given in Figure 1C. 12-HETrE production in CYP4B1-transfected cells treated with two CYP4B1-specific siRNAs (4B1-b and 4B1-c) was markedly inhibited (81 and 85%, respectively) as compared to untreated CYP4B1-transfected cells. 12-HETrE production was unaltered in cells treated with nonspecific siRNA and amounted to 1.4±0.3 nmol/h/106 cells (n=3). The CYP4B1 siRNA duplex 4B1-a was less effective in inhibiting 12-HETrE production in CYP4B1-transfected cells. The 4B1-c siRNA duplex was used in all subsequent experiments as the CYP4B1-specific siRNA.
Figure 1.
Inhibition of 12-HETrE production by CYP4B1-specific siRNA in RCE cells. A) EGFP fluorescence in RCE cells transfected with the pIRES2-EGFP (upper panel) and pIRES2-EGFP-4B1 without (middle panel) and with (lower panel) CYP4B1 specific siRNA (X40). B) Representative HPLC elution profiles of arachidonic acid metabolites in RCE cells transfected with the pIRES2-EGFP (upper panel) and pIRES2-EGFP-4B1 (lower panel). The major metabolite co-eluted with 12-HETrE authentic standatd; other radioactive peaks correspond to: I, unknown polar metabolites; II, 12,20-diHETE; 12-HETE; and 12-oxo-ETrE as previously reported42,43 C) Quantitative analysis of 12-HETrE production in RCE cells treated with siRNA duplexes targeting CYP4B1 (4B1-a, 4B1-b, 4B1-c) and non-specific siRNA (control) and incubated with arachidonic acid. Results are mean±SE, n=3, *p<0.05 from control siRNA.
CYP4B1 siRNA inhibits corneal neovascularization
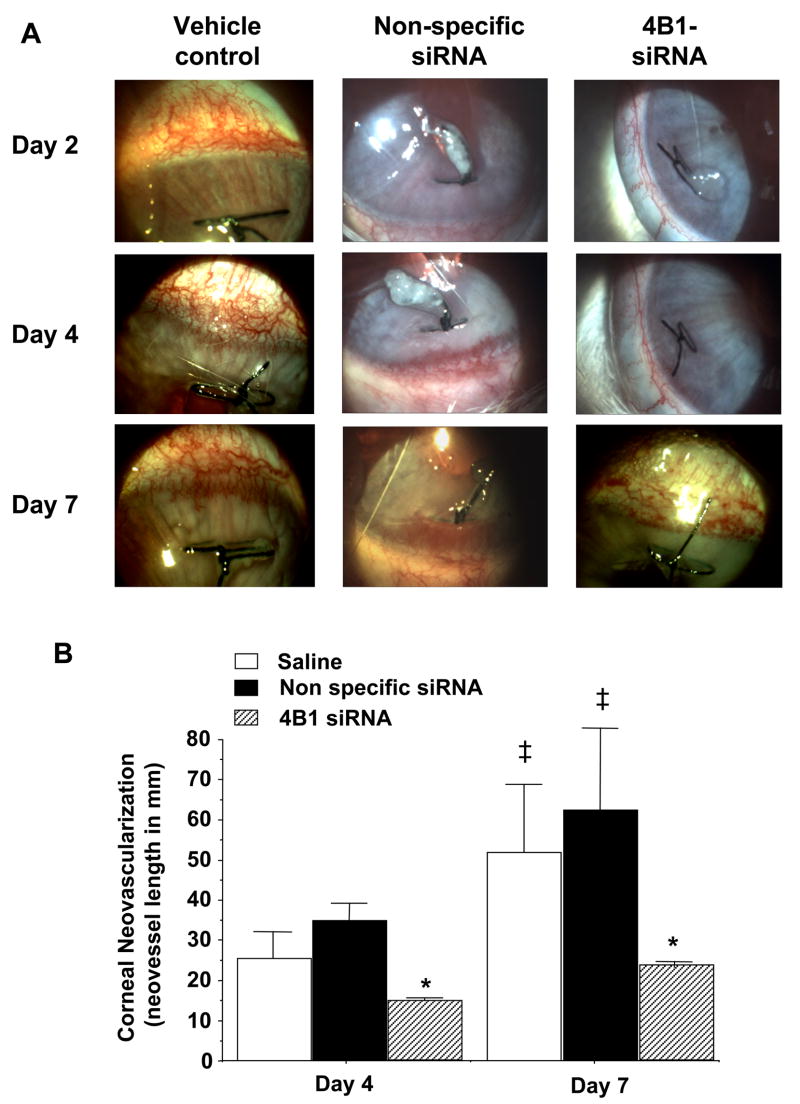
Corneal neovascularization was apparent in eyes with sutures as early as 2 days after its placement. In eyes treated with saline (vehicle control), the neovascular response increased with time encompassing larger area of the cornea by day 7 after suture placement (Figure 2A). Similar neovascular response was observed in eyes treated with control nonspecific siRNA duplexes (Figure 2A). In contrast, corneal neovascularization was markedly reduced in eyes receiving subconjunctival injections of CYP4B1-specific siRNAs as compared to control eyes or eyes receiving the control nonspecific siRNA (Figure 2A). Quantitative analysis showed that the neovascular response to suture placement in eyes receiving the CYP4B1-specific siRNA was inhibited by about 50 and 60% at day 4 and 7, respectively, when compared to either control eyes or eyes treated with the non-specific siRNA (Figure 2B). To further substantiate the notion that the neovascular response is mediated at least in part by CYP4B1 activity via production of 12-HETrE, experiments were conducted using daily topical treatment of 17-ODYA. Eyes treated with 17-ODYA exhibited a 50% reduction of the neovascular response at day 4 after suture placement as compared to eyes treated with the vehicle control, viz., from 14.95±1.21 to 8.29±1.26 mm neovessels (n=4, p<0.05). These results, combined with the results obtained with the CYP4B1-specific siRNA, lend support to the notion that the CYP4B1-derived 12-HETrE is a key pathway in the development of corneal neovascularization.
Figure 2.
Effect of siRNA treatment on corneal neovascularization. A) Representative pictures depicting corneal neovascularization in control eyes (treated with the vehicle saline) and in eyes receiving subconjunctival injections of CYP4B1-specific (4B1-siRNA) or nonspecific siRNAs at days 2, 4 and 7 after suture placement (X16). B) Quantitative analysis of corneal neovascularization 4 and 7 days after suture placement. Results are mean±SE; n=6; *p<0.05 from control siRNA-treated eyes; ‡p<0.05 from day 4.
CYP4B1 siRNA inhibits CYP4B1 expression and 12-HETrE Synthesis
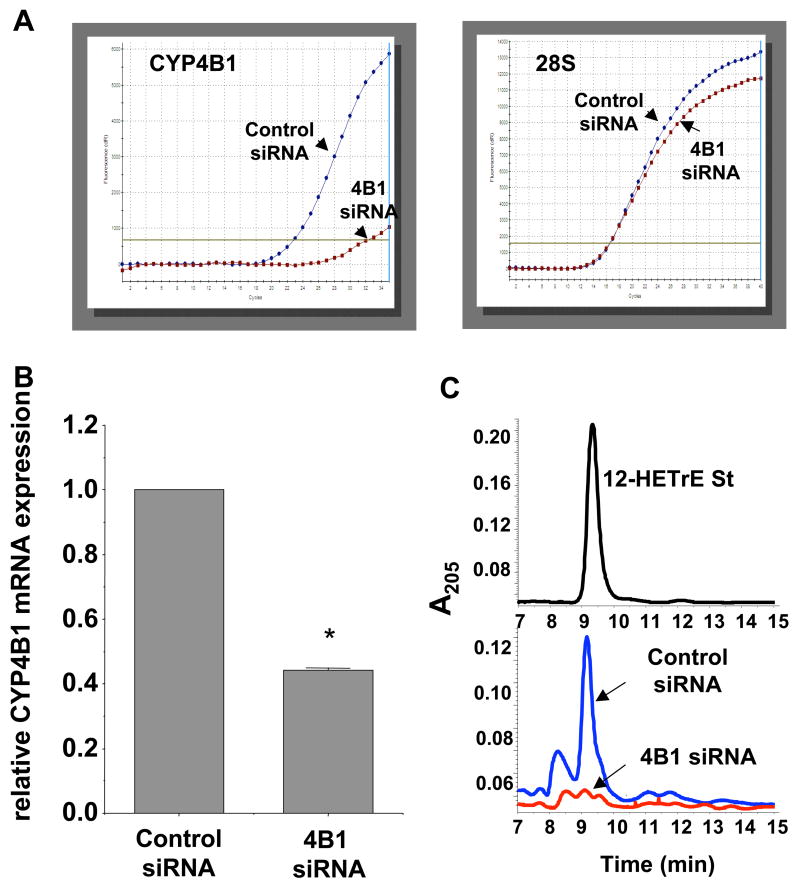
The effect of siRNA treatment on CYP4B1 mRNA levels was assessed by real time PCR analysis. As seen in Figure 3A, CYP4B1 mRNA levels of corneal limbal wedges indicated a significant inhibition of CYP4B1 mRNA expression in CYP4B1 siRNA-treated eyes. (Figure 3A). Since specific antibody against the rabbit CYP4B1 protein is unavailable, CYP4B1 functional expression was examined by measuring levels of 12-HETrE, the arachidonic acid metabolite of CYP4B1 enzymatic activity. Reverse phase HPLC analysis of homogenates of corneal wedges containing the sutures indicated the presence of 12-HETrE as detected by UV absorbance at 205 nm and co-eluted with synthetic 12-HETrE standard (Figure 3B). 12-HETrE was clearly present in homogenates from corneas treated with the control nonspecific siRNA. In contrast, 12-HETrE was barely detectable in homogenates from corneas treated with CYP4B1-specific siRNA (Figure 3B). Additional experiments were carried out to examine CYP4B1 enzymatic activity in corneal wedges from eyes treated with siRNA duplexes by measuring their capacity to metabolize arachidonic acid to 12-HETrE. Corneal wedges from sutured eyes treated with the CYP4B1 specific siRNA produced 12-HETrE in a significantly lower rate as compared to corneal wedges from nonspecific siRNA-treated sutured eyes, viz., 0.13±0.03 vs 0.18±0.04 pmol12-HETrE/mg protein/h, n=3, p<0.05.
Figure 3.
Effect of siRNA treatment on corneal CYP4B1 mRNA and 12-HETrE levels 7 days after intrastromal suture placement. A) Representative amplification plots for CYP4B1 (left) and 28S rRNA mRNA (right) by real time PCR analysis in RNA from corneas treated with control (blue line) and CYP4B1-specific (red line) siRNA. B) Quantitative analysis of mRNA levels. Results are presented as relative expression and are mean±SE, n=5, *p<0.05 from control siRNA. C) HPLC analysis of 12-HETrE levels in corneal homogenates from control and CYP4B1 (4B1) specific siRNA treated eyes; upper panel, elution profile of 12-HETrE standard (st); lower panel, representative analyses (n=3) of corneal homogenates from eyes treated with control (blue line) and CYP4B1-specific (red line) siRNAs.
CYP4B1 siRNA decreases corneal VEGF expression
We have previously shown a relationship between CYP4B1 and VEGF expression in the cornea following hypoxic injury,13 an injury that is associated with neovascularization. We also demonstrated that the angiogenic activity of 12-HETrE is mediated at least in part by VEGF; 12-HETrE induces VEGF expression and activity and inhibition of VEGF function diminishes 12-HETrE-induced angiogenesis.25 Therefore, we evaluated the effect of siRNA on VEGF expression and its relation to neovascularization. Real time PCR analysis clearly demonstrated that VEGF expression was markedly reduced by CYP4B1-specific siRNA but was not affected by treatment with nonspecific siRNA (Figure 4).
Figure 4.
Effect of siRNA treatment on corneal VEGF mRNA 7 days after intrastromal suture placement. A) Representative amplification plots for VEGF in corneas treated with control siRNA and CYP4B1 (4B1)-specific siRNA. B) Quantitative analysis of mRNA levels. Results are presented as relative expression and are mean±SE, n=5, *p<0.05 from control siRNA-treated eyes.
VEGF expression is regulated by the CYP4B1-12HETrE pathway in RCE cells
To further establish the link between CYP4B1 and VEGF expression, experiments were carried out using RCE cells. As seen in Figure 5A, VEGF mRNA levels were significantly higher in cells transfected with CYP4B1 cDNA than non-transfected cells or cells transfected with the control plasmid pIRES. More importantly, addition of CYP4B1-specific siRNA inhibited CYP4B1-stimulated VEGF induction by 46% (Figure 5A). There was no significant change in VEGF expression levels in CYP4B1-transfected cells treated with the control siRNA (data not shown). Likewise, neither control siRNA nor CYP4B1 siRNA had an effect on VEGF expression in control cells or cells transfected with the control plasmid pIRES. That the effect of CYP4B1 overexpression on VEGF mRNA levels is linked to CYP4B1 catalytic activity is documented in Figure 5B; addition of the CYP4B1 inhibitor 17-ODYA attenuated the effect of CYP4B1 on VEGF mRNA levels by 44%. Since RCE cells transfected with the CYP4B1 cDNA readily produce 12-HETrE from either exogenous or endogenous arachidonic acid,15 it is likely that 12-HETrE mediates, at least in part, the CYP4B1 effect on VEGF expression levels. To this end, addition of 12-HETrE to RCE cells induces VEGF expression in a concentration-dependent manner (Figure 5C); 12-HETrE at 1 nM induced VEGF to the same extent as hypoxia (2% O2) attesting to the potency of 12-HETrE as an angiogenic lipid mediator.
Figure 5.
Effect of CYP4B1 expression and 12-HETrE on VEGF mRNA levels in RCE cells. Levels of mRNA were measured by real time PCR. A) VEGF mRNA levels in non transfected (control) and cells transfected with the control plasmid pIRES and pIRES plasmid containing the CYP4B1 cDNA (4B1) in the absence and presence of CYP4B1-specific siRNA (4B1+siRNA). Results are expressed as relative expression normalized to 28S levels and are mean±SE; n=3; *p<0.05 from control or pIRES transfected cells; ‡p<0.05 from 4B1 transfected cells. B) VEGF mRNA levels in cells transfected with pIRES-GFP-CYP4B1 (4B1) and treated with the CYP4B1 inhibitor 17-ODYA (20 μM). Results are expressed as relative expression normalized to 28S levels and are mean±SE; n=3; *p<0.05 from pIRES transfected cells; ‡p<0.05 from 4B1 transfected cells. C) VEGF mRNA levels in RCE cells treated with 12-HETrE (0.1–100 nM) or subjected to hypoxia (2% O2). Results are expressed as relative expression normalized to 28S levels and are mean±SE; n=3; *p<0.05 from control.
DISCUSSION
Corneal neovascularization poses a real threat to vision; even modest numbers of new vessels will reduce visual acuity. In recent years, we have identified in the corneal epithelium the CYP4B1 enzymatic pathway which upon injury is activated and produces a potent angiogenic eicosanoid, namely, 12-HETrE. To date, the link between CYP4B1 expression, 12-HETrE production and neovascularization of the corneal surface was based primarily on studies showing correlation between the progression of the inflammatory and neovascular response to injury, increased CYP4B1 expression and increased production of 12-HETrE. The current study is the first to establish a direct cause and effect relationship between the CYP4B1 pathway and inflammatory neovascularization in the cornea. Moreover, the current study provides additional evidence for a link between the expression of CYP4B1 and VEGF and suggests that CYP4B1 may be a proximal regulator of VEGF expression and bioaction in the cornea.
Gene silencing by siRNA is a useful approach for suppressing gene expression with great specificity and potency and a powerful alternative to other genetic knockdown methods for the analysis of loss-of-function phenotypes; it can be employed locally and at any stage of the experiment. This approach has been successfully used to silence VEGF and VEGF receptor gene expression in several models of corneal neovascularization.26–29 Using this approach, we examined whether CYP4B1 gene silencing diminishes the corneal neovascular response in a model of inflammatory neovascularization. CYP4B1 expression is primarily localized to the corneal epithelium. In the intact cornea, CYP4B1 mRNA levels and CYP4B1 catalytic activity as an arachidonic acid metabolic pathway are relatively low; injury, however, rapidly and strongly induces CYP4B1 mRNA followed by an increase in its enzymatic activity, i.e., production of 12-HETrE. To specifically target the localized and induced expression of CYP4B1, siRNA duplexes were injected subconjunctively at the limbus at the time of suture instillation and every other day during the development of neovascularization. The results clearly indicated that, using this experimental approach and siRNA duplexes which shown to exert specificity and potency in vitro, CYP4B1 expression as measured by real time PCR was reduced by 60%. More importantly, treatment with CYP4B1-specific siRNA greatly attenuated the neovascular response suggesting that CYP4B1 is a key participant in the regulation of corneal neovascularization. The corneal CYP4B1 is involved in arachidonic acid metabolism and has been the primary enzymatic source of the angiogenic eicosanoid 12-HETrE.24 Accordingly, treatment with CYP4B1-specific siRNA greatly reduced the corneal levels of 12-HETrE. It is likely that the decrease in CYP4B1 activity and consequently 12-HETrE levels contributed to the reduced neovascular response brought about by the CYP4B1-specific siRNA. It is also likely that suture-induced corneal neovascularization is mediated, at least in part, via the production of 12-HETrE whose levels were barely detected in CYP4B1 siRNA-treated corneas but were predictably prominent in the untreated sutured corneas.
The demonstration that administration of siRNA that specifically targeted CYP4B1 greatly attenuated VEGF mRNA levels is of great significance. We have previously shown that CYP4B1 pattern of expression is parallel to that of VEGF and that a functional interaction links these two angiogenic pathways. This functional interaction is manifested by the demonstration that 12-HETrE’s angiogenic activity and, by extension, CYP4B1 angiogenic capacity is mediated, at least in part by VEGF.11,13,25 It is also supported in this study by the demonstrations that 1) suppression of CYP4B1 expression in vivo reduced VEGF mRNA levels; 2) overexpression of CYP4B1 in corneal epithelial cells or addition of 12-HETrE in vitro increased the levels of VEGF mRNA; and 3) inhibition of CYP4B1 enzymatic activity in vitro significantly decreased VEGF mRNA levels. Noteworthy, is a report by Chen at al30 which showed that an inhibitor of the CYP4 enzymes, including CYP4B1, inhibited VEGF-induced angiogenesis in human endothelial cells in vitro and corneal neovascularization elicited by VEGF in vivo.
VEGF has long been considered as a key angiogenic factor in the cornea;31,32 it is induced in response to injury and inhibition of its expression or blockade of its receptors inhibits corneal neovascularization in numerous experimental models.27,33,34 VEGF within the injured cornea originates from multiple cell types including resident and recruited inflammatory cells.35,36 While the cellular source of 12-HETrE within the injured cornea is primarily the corneal epithelium3, it is also likely that infiltrating cells such as neutrophils contribute to its increased levels as well.37 Notably, 12-HETrE bioactivity includes both chemotactic as well as angiogenic capacity.8 Hence, the CYP4B1-12-HETrE pathway may constitute an upstream regulator of VEGF expression and action and an amplification system of neovascularization. Accordingly, injury rapidly induces CYP4B1 activity to produce 12-HETrE; this lipid mediator acting as a chemoattractant, amplifies infiltration of inflammatory cells, a well-established source of VEGF, and activates resident cells including epithelial cells and the adjacent limbal endothelial cells to produce and use VEGF as the mediator of neovascularization.
In summary, this study is the first to demonstrate that inhibition of CYP4B1 expression attenuates corneal neovascularization. The results strongly implicate the corneal CYP4B1 as a component of the inflammatory and neovascular cascade initiated by injury to the ocular surface. Recent interest in inhibiting neovascularization has focused on VEGF.38 Our previous studies showing that VEGF mediates the angiogenic activity of 12-HETrE25 and that injury to the cornea is associated with a concerted induction of CYP4B1 and VEGF,13 together with the results presented here, raise the possibility of a new approach to the problem of preventing corneal neovascularization that targets the CYP4B1-12-HETrE system. To this end, we have shown the presence of the cytochrome P450 system in human corneas39 and documented the presence of 12-HETrE in human tears at concentrations that readily induce inflammatory and angiogenic response including VEGF induction.10 More importantly, ocular surface inflammation of diverse etiologies was associated with increased 12-HETrE tear levels. 12-HETrE’s levels were especially high in contact lens-related inflammation in which all patients had slept with their lenses in place and had an acute ocular surface inflammation on waking. Hypoxia, which is believed to be a major factor in contact lens-induced inflammation, has been shown to induce CYP4B1 expression and stimulate 12-HETrE synthesis.11,21 Herpes simplex keratitis and corneal foreign body, both injuries to the corneal epithelium, also had marked elevations of tear film 12-HETrE.10 The significant increase of 12-HETrE in the tear film of inflamed human eyes supports a positive correlation between 12-HETrE production and ocular surface inflammation that is consistent with the relationship previously established in rabbit models and with its hypothesized role as a paracrine mediator of inflammation. The fact that 12-HETrE is produced in other inflamed tissues40,41 further extends its clinical relevance.
Acknowledgments
This study was supported by the National Eye Institute Grant EY06513.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Laniado Schwartzman M, Green K, Edelhauser HF, et al. Cytochrome P450 and arachidonic acid metabolism in the corneal epithelium: Role in inflammation. New York: Plenum Press; 1997. [Google Scholar]
- 2.Conners MS, Stoltz RA, Webb SC, et al. A closed eye-contact lens model of corneal inflammation. I. Induction of cytochrome P450 arachidonic acid metabolism. Invest Ophthalmol Vis Sci. 1995;36:828–40. [PubMed] [Google Scholar]
- 3.Conners MS, Urbano F, Vafeas C, et al. Alkali burn-induced synthesis of inflammatory eicosanoids in rabbit corneal epithelium. InvestOphthalmolVis Sci. 1997;38:1963–71. [PubMed] [Google Scholar]
- 4.Vafeas C, Mieyal PA, Urbano F, et al. Hypoxia stimulates the synthesis of cytochrome P450-derived inflammatory eicosanoids in rabbit corneal epithelium. JPharmacolExp Ther. 1998;287:903–10. [PubMed] [Google Scholar]
- 5.Conners MS, Stoltz RA, Dunn MW, et al. A closed eye-contact lens model of corneal inflammation. II. Inhibition of cytochrome P450 arachidonic acid metabolism alleviates inflammatory sequelae. Invest Ophthalmol Vis Sci. 1995;36:841–50. [PubMed] [Google Scholar]
- 6.Laniado Schwartzman M, Abraham NG, Conners MS, et al. Heme oxygenase induction with attenuation of experimentally-induced corneal inflammation. Biochem Pharmacol. 1997;53:1069–75. doi: 10.1016/s0006-2952(97)00080-4. [DOI] [PubMed] [Google Scholar]
- 7.Murphy RC, Falck JR, Lumin S, et al. 12(R)-hydroxyeicosatetrienoic acid: a vasodilator cytochrome P450 dependent arachidonate metabolite from bovine corneal epithelium. JBiol Chem. 1988;263:17197–202. [PubMed] [Google Scholar]
- 8.Masferrer JL, Rimarachin JA, Gerritsen ME, et al. 12(R)-hydroxyeicosatrienoic acid, a potent chemotactic and angiogenic factor produced by the cornea. Exp Eye Res. 1991;52:417–24. doi: 10.1016/0014-4835(91)90037-f. [DOI] [PubMed] [Google Scholar]
- 9.Stoltz RA, Conners MS, Gerritsen ME, et al. Direct stimulation of limbal microvessel endothelial cell proliferation and capillary formation in vitro by a corneal-derived eicosanoid. AmJ Pathol. 1996;148:129–39. [PMC free article] [PubMed] [Google Scholar]
- 10.Mieyal PA, Dunn MW, Schwartzman ML. Detection of endogenous 12-hydroxyeicosatrienoic acid in human tear film. Invest Ophthalmol. 2001;42:328–32. [PubMed] [Google Scholar]
- 11.Mastyugin V, Aversa E, Vafeas C, et al. Hypoxia-Induced Production of 12-Hydroxyeicosanoids in the Corneal Epithelium: Involvement of a CYP4B1 Isoform. JPharmacolExp Ther. 1999;289:1611–9. [PubMed] [Google Scholar]
- 12.Mastyugin V, Mezentsev AV, Zhang WX, et al. Promoter Activity and Regulation of the Corneal CYP4B1 Gene by Hypoxia. J Cell Biochem. 2004;15:1218–38. doi: 10.1002/jcb.20018. [DOI] [PubMed] [Google Scholar]
- 13.Mastyugin V, Mosaed S, Bonazzi A, et al. Corneal epithelial VEGF and cytochrome P450 4B1 expression in a rabbit model of closed eye contact lens wear. Curr Eye Res. 2001;23:1–10. doi: 10.1076/ceyr.23.1.1.5422. [DOI] [PubMed] [Google Scholar]
- 14.Seta F, Bellner L, Rezzani R, et al. Heme oxygenase-2 is a critical determinant for execution of an acute inflammatory and reparative response. Am J Pathol. 2006;169(5):1612–23. doi: 10.2353/ajpath.2006.060555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mezentsev A, Mastyugin V, Seta F, et al. Transfection of Cytochrome P4504B1 into the Cornea Increases Angiogenic Activity of the Limbal Vessels. J Pharmacol Exp Ther. 2005;315(1):42–50. doi: 10.1124/jpet.105.088211. [DOI] [PubMed] [Google Scholar]
- 16.Streilein JW, Bradley D, Sano Y, Sonoda Y. Immunosuppressive properties of tissues obtained from eyes with experimentally manipulated corneas. Invest Ophthalmol Vis Sci. 1996;37(2):413–24. [PubMed] [Google Scholar]
- 17.Cursiefen C, Chen L, Borges LP, et al. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest. 2004;113(7):1040–50. doi: 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samolov B, Steen B, Seregard S, et al. Delayed inflammation-associated corneal neovascularization in MMP-2-deficient mice. Exp Eye Res. 2005;80(2):159–66. doi: 10.1016/j.exer.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 19.Holzer MP, Solomon KD, Vroman DT, et al. Photodynamic therapy with verteporfin in a rabbit model of corneal neovascularization. Invest Ophthalmol Vis Sci. 2003;44(7):2954–8. doi: 10.1167/iovs.02-0572. [DOI] [PubMed] [Google Scholar]
- 20.Araki K, Ohashi Y, Kinoshita S, et al. Immortalization of rabbit corneal epithelial cells by a recombinant SV40-adenovirus vector. InvestOphthalmolVis Sci. 1993;34:2665–71. [PubMed] [Google Scholar]
- 21.Mieyal PA, Bonazzi A, Jiang H, et al. The effect of hypoxia on endogenous corneal epithelial eicosanoids. Invest Ophthalmol Vis Sci. 2000;41:2170–6. [PubMed] [Google Scholar]
- 22.Shin DS, Yadagiri P, Falck JR, et al. Synthesis and structure of compound D, a pro-inflammatory arachidonate metabolite. Tetrahedron Lett. 1989;30:3923–6. [Google Scholar]
- 23.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):2002–7. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mezentsev A, Mastyugin V, Seta F, et al. Transfection Of Cyp4b1 Into The Cornea Increases Angiogenic Activity Of The Limbal Vessels. J Pharmacol Exp Ther. 2005 doi: 10.1124/jpet.105.088211. [DOI] [PubMed] [Google Scholar]
- 25.Mezentsev A, Seta F, Dunn MW, et al. Eicosanoid regulation of vascular endothelial growth factor expression and angiogenesis in microvessel endothelial cells. J Biol Chem. 2002;277(21):18670–6. doi: 10.1074/jbc.M201143200. [DOI] [PubMed] [Google Scholar]
- 26.Reich SJ, Fosnot J, Kuroki A, et al. Small interfering RNA (siRNA) targeting VEGF effectively inhibits ocular neovascularization in a mouse model. Mol Vis. 2003;9:210–6. [PubMed] [Google Scholar]
- 27.Kim B, Tang Q, Biswas PS, et al. Inhibition of ocular angiogenesis by siRNA targeting vascular endothelial growth factor pathway genes: therapeutic strategy for herpetic stromal keratitis. Am J Pathol. 2004;165(6):2177–85. doi: 10.1016/S0002-9440(10)63267-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen J, Samul R, Silva RL, et al. Suppression of ocular neovascularization with siRNA targeting VEGF receptor 1. Gene Ther. 2006;13(3):225–34. doi: 10.1038/sj.gt.3302641. [DOI] [PubMed] [Google Scholar]
- 29.Murata M, Takanami T, Shimizu S, et al. Inhibition of ocular angiogenesis by diced small interfering RNAs (siRNAs) specific to vascular endothelial growth factor (VEGF) Curr Eye Res. 2006;31(2):171–80. doi: 10.1080/02713680500514636. [DOI] [PubMed] [Google Scholar]
- 30.Chen P, Guo M, Wygle D, et al. Inhibitors of cytochrome P450 4A suppress angiogenic responses. Am J Pathol. 2005;166(2):615–24. doi: 10.1016/S0002-9440(10)62282-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amano S, Rohan R, Kuroki M, et al. Requirement for vascular endothelial growth factor in wound- and inflammation-related corneal neovascularization. Invest Ophthalmol Vis Sci. 1998;39:18–22. [PubMed] [Google Scholar]
- 32.Philipp W, Speicher L, Humpel C. Expression of vascular endothelial growth factor and its receptors in inflamed and vascularized human corneas. Invest Ophthalmol Vis Sci. 2000;41(9):2514–22. [In Process Citation] [PubMed] [Google Scholar]
- 33.Stechschulte SU, Joussen AM, von Recum HA, et al. Rapid ocular angiogenic control via naked DNA delivery to cornea. Invest Ophthalmol Vis Sci. 2001;42(9):1975–9. [PubMed] [Google Scholar]
- 34.Lai CM, Brankov M, Zaknich T, et al. Inhibition of angiogenesis by adenovirus-mediated sFlt-1 expression in a rat model of corneal neovascularization. Hum Gene Ther. 2001;12(10):1299–310. doi: 10.1089/104303401750270959. [DOI] [PubMed] [Google Scholar]
- 35.Moromizato Y, Stechschulte S, Miyamoto K, et al. CD18 and ICAM-1-dependent corneal neovascularization and inflammation after limbal injury. Am J Pathol. 2000;157(4):1277–81. doi: 10.1016/S0002-9440(10)64643-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Usui T, Ishida S, Yamashiro K, et al. VEGF164(165) as the pathological isoform: differential leukocyte and endothelial responses through VEGFR1 and VEGFR2. Invest Ophthalmol Vis Sci. 2004;45(2):368–74. doi: 10.1167/iovs.03-0106. [DOI] [PubMed] [Google Scholar]
- 37.Wainwright S, Falck JR, Yadagiri P, Powell WS. Metabolism of 12(S)-hydroxy-5,8,10,14-eicosatetraenoic acid and other hydroxylated fatty acids by the reductase pathway in porcine polymorphonuclear leukocytes. Biochem. 1990;29:10126–35. doi: 10.1021/bi00495a017. [DOI] [PubMed] [Google Scholar]
- 38.Witmer AN, Vrensen GF, Van Noorden CJ, Schlingemann RO. Vascular endothelial growth factors and angiogenesis in eye disease. Prog Retin Eye Res. 2003;22:1–29. doi: 10.1016/s1350-9462(02)00043-5. [DOI] [PubMed] [Google Scholar]
- 39.Abraham NG, Lin J, Dunn MW, Schwartzman ML. Presence of heme oxygensae and NADPH cytochrome P450 (c) reductase in human corneal epithelium. InvestOphthalmolVis Sci. 1987;28:1464–72. [PubMed] [Google Scholar]
- 40.Camp RDR, Cunningham FM, Fincham NJ, et al. Psoriatic skin lesions contain a novel lipid neutrophil chemokinetic compound which is distinct from known chemoattractant compounds. BrJ Pharmacol. 1988;94:1043–50. doi: 10.1111/j.1476-5381.1988.tb11620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Du L, Yermalitsky V, Hachey DL, et al. A Biosynthetic Pathway Generating 12-Hydroxy-5,8,14-eicosatrienoic Acid from Arachidonic Acid Is Active in Mouse Skin Microsomes. J Pharmacol Exp Ther. 2006;316(1):371–9. doi: 10.1124/jpet.105.093922. [DOI] [PubMed] [Google Scholar]
- 42.Nishimura M, Schwartzman ML, Falck JR, et al. Metabolism of 12(R)-hydroxy-5,8,10,14-eicosatetraenoic acid (12(R)-HETE) in corneal tissues: formation of novel metabolites. Arch Biochem Biophys. 1991;290:326–35. doi: 10.1016/0003-9861(91)90548-w. [DOI] [PubMed] [Google Scholar]
- 43.Yamamoto S, Nishimura M, Conners MS, et al. Oxidation and keto reduction of 12-hydroxy-5,8,10,14-eicosatetraenoic acids in bovine corneal epithelial microsomes. Biochim Biophys Acta. 1994;1210:217–25. doi: 10.1016/0005-2760(94)90124-4. [DOI] [PubMed] [Google Scholar]