Abstract
An increase in bone resorption is one of the main symptoms of osteoporosis, a disease that affects more and more individuals every day. Bisphosphonates are known to inhibit bone resorption, and thus are being used as a treatment for osteoporosis. Aminobisphosphonates present a functionality that can be easily used for conjugation to other molecules, such as peptides, proteins, and ligands for protein recognition. In this study, an aminobisphosphonate conjugated with biotin was used as a model linker for protein attachment to bone. With this system, the interaction of biotinylated aminobisphosphonate with hydroxyapatite, a major mineral component of bone, was investigated. Quantification of the binding of aminobisphosphonate to hydroxyapatite was performed using a fluorescently labeled antibody for biotin. Additionally, the interaction of the biotinylated aminobisphosphonate with multiple treatments of cortical bone from the mid-shaft of a cow femur was studied. It was demonstrated that modified aminobisphosphonate reagents can bind hydroxyapatite and bone at high levels, while the biotin functionality is free to be recognized by the fluorescently labeled anti-biotin antibody, suggesting that modified aminobisphosphonates could be used to link other peptides or proteins to the bone surface.
Introduction
In 2000, over 10 million people in the United States had been diagnosed with osteoporosis (1), a disease characterized by a reduction in bone mass caused by an increase in bone resorption with minimal regeneration. Another 34 million were affected by low bone mineral density. Two types of bone cells, osteoblasts and osteoclasts, constantly work together to remodel bone. Osteoblasts, or bone-forming cells, regenerate bone tissue, while bone is resorbed by the osteoclast cells (2). In healthy individuals, the rate of bone resorption is similar to the rate of bone regeneration. In a patient diagnosed with osteoporosis, the balance of osteoblastic and osteoclastic activity is disrupted in such a way that the rate of bone resorption is higher than the rate of bone regeneration (3).
Popular treatments for osteoporosis include calcium and vitamin D supplementation, hormone replacement therapy, and use of bisphosphonates, among others (4). Calcium and vitamin D supplementation and hormone replacement therapy have been criticized recently over their inability to prevent fractures (5, 6) and the increased side effects with treatment (7–9), respectively. It has also been suggested that calcium and vitamin D supplements may result in only minimal increase in bone mineral density (10, 11). While this may be beneficial overall, it was found recently that supplementation has little to no effect at preventing fractures and can increase the occurrence of kidney stones (6). A high rate of fracture with minimal injury is one of the main symptoms of osteoporosis. Hormone replacement therapy, involving the administration of a combination of estrogen and progestin, had been widely prescribed as a treatment for osteoporosis in postmenopausal women (7) because of the effect that estrogen has at inhibiting the action of osteoclast cells (12, 13). Unfortunately, the long term effects of estrogen therapy were found to include an increased risk of breast cancer, heart attack, stroke, blood clots, and Alzheimer’s disease and other dementias (8, 9). Because of these risks, hormone replacement therapy is now only prescribed for postmenopausal women with extreme osteoporosis, where the benefits outweigh the risks associated with treatment.
A treatment for osteoporosis that is gaining popularity is bisphosphonate therapy. Bisphosphonates are antiresorptive medications that bind to the mineral phase of bone and inhibit the activity of osteoclasts (14). Bisphosphonates have a long history with many applications including use as detergent additives in hard water treatment, in toothpaste to prevent tartar build-up, as treatment for Paget’s disease, and as a diagnostic tool for bone tumor treatment (14, 15). In recent years, they have been mainly studied as an osteoporosis therapy because of their ability to inhibit bone resorption. The complex mechanism of action of bisphosphonates has only recently been understood (16–18), but in general, bisphosphonate binds to hydroxyapatite, the main mineral component of bone. When osteoclasts begin to resorb the bone, bisphosphonate is taken up by the cell, which then loses its resorptive function and undergoes apoptosis. The loss in the ability of the osteoclast to resorb bone following the administration of bisphosphonates causes an increase in bone mineral density.
While both osteoblasts and osteoclasts are essential to bone remodeling, a majority of osteoporosis research focuses on inhibiting the osteoclast (3, 4, 14, 16, 17, 19–24). The innate structure of bisphosphonates allows for numerous analogs to be produced that can have a wide scale of activities. Nitrogen-containing bisphosphonates, or aminobisphosphonates, have already been shown to have higher antiresorptive effects than bisphosphonates that do not contain a nitrogen atom (16). The conjugation of a peptide or protein that can stimulate bone formation to an aminobisphosphonate could produce an agent that would not only slow resorption, but would also help to increase bone mineral density.
Aminomethylene bisphosphonic acid (aminobisphosphonate) presents an amine functionality that can be easily attached to a biomolecule. Conjugation of aminobisphosphonate to proteins has previously been explored in the literature (25–29), demonstrating that various proteins conjugated with bisphosphonate have a high affinity for both hydroxyapatite and bone. In these studies, aminobisphosphonate was conjugated at multiple sites on the proteins leading to more than one bisphosphonate per protein. This results in various binding orientations of the protein on the bone surface. We propose to conjugate aminobisphosphonate with a protein-binding ligand that subsequently can be used to recruit proteins to the bone surface. The linked protein could be presented in a rationally oriented manner based on the attachment site of the ligand. Toward this goal, a novel aminobisphosphonate was synthesized that contains a conjugated biotin moiety. This molecule was explored as a model system for delivery of a linker for protein attachment to bone. The biotinylated aminobisphosphonate was incubated with both hydroxyapatite and bone. The attachment to the mineral surfaces was quantified using a fluorescently labeled anti-biotin antibody. We have demonstrated that a conjugated aminobisphosphonate could be bound to the bone surface and subsequently recognized by a biomolecule.
Experimental Procedures
Materials
Dibenzyl amine, triethyl orthoformate, silica gel, palladium on carbon, active carbon, dimethylformamide, reagent grade hydroxyapatite (HA) powder (catalog number 289396), d-biotin, bovine serum albumin (BSA), casein from bovine milk, and monoclonal anti-biotin-FITC antibody were purchased from Sigma-Aldrich (St. Louis, MO). Hexane, methanol, sodium hydroxide, and sodium phosphate dibasic heptahydrate were purchased from Mallinckrodt (Hazelwood, MO). Sodium chloride, sodium sulfate, hydrochloric acid, and triethylamine were obtained from EMD Chemicals (Gibbstown, NJ). Avidin from egg white was purchased from EMD Biosciences (San Diego, CA). Diethyl phosphite and cyclohexene were received from Fluka (Milwaukee, WI). Deuterium oxide NMR solvent was purchased from Cambridge Isotope Laboratories (Andover, MA). Argon gas was supplied through Scott-Gross Company (Lexington, KY). Ethyl acetate and ether were obtained from VWR (Westchester, PA). Fetal bovine serum (FBS) was obtained from Invitrogen (Carlsbad, CA). Tetraethyl(aminomethylene) bisphosphonate was purchased from Epsilon Chimie (Brest, France). 2-Anilinonaphthalene-6-sulfonic acid was obtained from Molecular Probes (Eugene, OR).
Apparatus
1H NMR spectra were obtained on a Varian INOVA 400 MHz Spectrometer (Palo Alto, CA). Electrospray ionization mass spectrometry was performed on a ThermoFinnigan LCQ Mass Spectrometer (Waltham, MA). Lyophilization was performed using a Christ Alpha 2–4 freeze dryer (Martin Christ, Osterode am Harz, Germany) equipped with a Pfeiffer Vacuum Pump (Asslar, Germany). Fluorescence readings were performed on a Cary Eclipse Fluorescence Spectrophotometer (Varian, Walnut Creek, CA). Deionized water was produced using a Milli-Q Water Purification System (Millipore, Bedford, MA).
Synthesis of-Aminomethylenediphosphonic Acid Triethylammonium Salt Biotinyl (AMB-Biotin)
Aminomethylene bisphosphonic acid (aminobisphosphonate) 3 was synthesized following the procedure published by Kantoci et al. (30) and outlined in Scheme 1. N,N- Dibenzylamine-bisphosphonate tetraethyl ester 1 was synthesized from dibenzylamine, diethyl phosphite, and triethyl orthoformate. The amine was deprotected using Pd/C and hexene to form tetraethyl(aminomethylene) bisphosphonate 2. Hydrogenation of the ester groups using aqueous hydrochloric acid produced 3. Biotin N-hydroxysuccinimide ester (biotin NHS ester) was conjugated with 3 in a one-step synthesis. Triethylamine (600 μL) was added to a solution of 3 (100 mg, 0.52 mmol) in deionized water (3 mL). A solution of biotin NHS ester (179 mg, 0.52 mmol) in dimethylformamide (DMF, 6 mL) was added drop-wise at 0 °C. The solution was stirred for 30 min at this temperature and then overnight at room temperature. The reaction mixture was evaporated to a minimal volume, and the final product was precipitated with diethyl ether. The solid was collected using a Buchner funnel and washed first with a few drops of DMF, then a few drops of ether, and finally with a few drops of methanol. Biotinyl-aminomethylenediphosphonic acid triethylammonium salt (AMB-Biotin) 4 was obtained as a white solid in 80% yield. Intermediate compound 2 was also purchased from Epsilon Chimie (Brest, France) and used in the synthesis of 4. 1H NMR (400 MHz, D2O): δ 1.55 (triplet, SCHCH of biotin ring, 1H), δ 1.63 (triplet, SCH2CH of biotin ring, 2H), δ 2.58 (triplet, 2H), δ 2.79 (singlet, 6H), δ 3.02 (singlet, PO(OH)2CHPO(OH)2 of bisphosphonate, 1H), δ 4.46, 4.63 (multiplets, NHCHCHNH of bridge of biotin ring, 2H). Mass spectral data were obtained at the University of Kentucky Mass Spectrometry Facility. m/z (ESI-MS) 416 ([M-H]−, 100%).
Scheme 1.
Synthesis of biotinyl-aminomethylenediphosphonic acid triethylammonium salt (AMB-Biotin).
Preparation of Bone Samples
Bone samples were taken from the mid-shaft of a cow femur. Cortical bone was cut into a rod approximately 7 mm × 7 mm square. The rod was then sliced into 500 μm thick pieces. Of the bone slices, half of the samples were left untreated, while the remaining samples were demineralized by soaking them in 0.6 M hydrochloric acid for 48 h. After 24 h, fresh acid solution was exchanged. After acid treatment, the bone was rinsed thoroughly with deionized water. Additional samples of untreated bone, approximately 7 mm × 7 mm × 1 cm in size, were ashed by heating the bone to 800 °C for 24 h. Subsequently, the ashed bone was crushed into a powder using a mortar and pestle.
Binding Studies of AMB-Biotin on Hydroxyapatite
A 3-mg sample of AMB-Biotin was dissolved in 1 mL of 0.5 M phosphate buffer, pH 7.4. HA, in the amount of 6 mg, was added to this solution, and the sample was incubated overnight at room temperature on a rotator (Glas-Col mini-rotator, Terre Haute, IN). The suspension was centrifuged for 15 min at 13000 rpm (Eppendorf Centrifuge 5417R, Brinkmann Instruments, Westbury, NY) to collect the insoluble HA, and the pellet was washed twice with buffer and three times with deionized water. The sample was then lyophilized overnight and resuspended in 1 mL of FITC-labeled anti- biotin antibody in phosphate buffer at a concentration of 29 μg/mL. The suspension was incubated on a rotator for 3 h at 4 °C. After incubation, the sample was centrifuged again to collect the solid, and the fluorescence of the supernatant was measured. As controls, a 3-mg sample of aminobisphosphonate (AMB) and a 1-mL sample of 29 μg/mL FITC-labeled anti-biotin antibody in phosphate buffer were subjected to the same conditions for incubation with HA as above. All samples were prepared in triplicate.
AMB-Biotin Binding on Hydroxyapatite in the Presence of Free Biotin
Three samples were prepared to study the interactions with HA in the presence of free biotin. These samples were examined to determine whether AMB-Biotin could interact with HA through the bisphosphonate or the biotin moieties. First, a 3-mg sample of AMB-Biotin was prepared in 1 mL phosphate buffer. The second sample contained 3 mg of free biotin in 1 mL phosphate buffer. Finally, a sample was prepared that contained 3 mg each of both AMB-Biotin and free biotin dissolved together in 1 mL phosphate buffer to determine what effect, if any, free biotin had on the interactions between AMB-Biotin and HA. An amount of 6 mg of HA was added to all three samples. Samples were prepared in triplicate and treated as described for AMB-Biotin on HA.
AMB-Biotin Binding on Hydroxyapatite in the presence of BSA or Serum
Because bisphosphonates are administered either orally or intravenously, the ability of the modified bisphosphonate to interact with HA in the presence of biological molecules was examined. For the BSA studies, a total of five samples were prepared to study the effect of BSA on AMB-Biotin binding to HA. The first sample was prepared by dissolving 3 mg of AMB-Biotin in 1 mL phosphate buffer. For the second sample, 0.3 mg of BSA was dissolved in 1 mL phosphate buffer. The third sample contained both 3 mg of AMB-Biotin and 0.3 mg BSA in 1 mL phosphate buffer to determine how the presence of BSA affects the interaction with HA. To each of these three samples, 6 mg of HA was added. These first three samples were treated as described previously for AMB-Biotin.
The final two samples were prepared differently. For these two samples, 3 mg of AMB-Biotin and 0.3 mg of BSA were each preincubated with 6 mg HA. These samples were washed and lyophilized as before. However, after lyophilization, the AMB-Biotin sample was resuspended in a 0.3-mg/mL BSA solution in phosphate buffer, and the BSA sample was resuspended in a 3-mg/mL solution of AMB-Biotin in phosphate buffer. These two samples were incubated for 3 h at room temperature on the rotator before centrifuging to remove the insoluble HA. The sample of (AMB-Biotin + HA) + BSA examined the strength of the interaction of the preincubated AMB-Biotin sample, while the (BSA + HA) + AMB-Biotin sample helped to determine the specificity of the AMB-Biotin interaction with the HA surface.
All five samples were then resuspended in 1 mL of 29 μg/mL FITC-labeled anti-biotin antibody in phosphate buffer and incubated on a rotator for 3 h at 4 °C. HA was collected by centrifugation, and the fluorescence of the supernatants was measured and compared to a standard of FITC-labeled anti-biotin antibody.
While the BSA studies looked at the effect of a low concentration of albumin (0.3 mg/mL) on AMB-Biotin binding to HA, additional studies were performed to study binding at higher concentrations of protein using fetal bovine serum (FBS). Three milligram samples of AMB-Biotin were prepared in 1-mL solutions of 25%, 50%, and 75% FBS in phosphate buffer along with solutions of 100% buffer and 100% FBS. Six milligrams of HA were added to each of the samples at the various levels of serum. The samples were treated following the procedure for AMB-Biotin.
Binding Studies of AMB-Biotin on Bone
A 3-mg sample of AMB-Biotin was dissolved in 1 mL of 0.5 M phosphate buffer, pH 7.4. To this solution, either a slab of untreated or demineralized bone or 6 mg of crushed ashed bone was added. The sample was incubated overnight at room temperature on a rotator. The sample was centrifuged for 15 min at 13000 rpm to collect the bone, which was washed twice with buffer and three times with deionized water. The sample was then lyophilized overnight and resuspended in 1 mL of FITC-labeled anti-biotin antibody in phosphate buffer at a concentration of 5.8 μg/mL. The suspension was incubated on a rotator for 3 h at room temperature. After the incubation, the sample was centrifuged again to collect the solid, and the fluorescence of the supernatant was measured. As controls, a 3-mg sample of free biotin and a 1-mL sample of FITC-labeled anti-biotin antibody in phosphate buffer were subjected to the same conditions as listed for the AMB-Biotin sample.
AMB-Biotin Binding on Ashed Bone in the Presence of Casein
Samples were again prepared to examine the binding of AMB-Biotin in the presence of a biological molecule, this time using casein. Four total samples were prepared to study the interactions of AMB-Biotin and casein with ashed bone. The first sample was prepared by dissolving 3 mg AMB-Biotin in 1 mL phosphate buffer. For the second sample, 5 mg of casein was dissolved in 1 mL of phosphate buffer. The third sample contained both 3 mg of AMB-Biotin and 5 mg of casein in 1 mL phosphate buffer. Ashed bone in an amount of 6 mg was added to all three of these samples, which were treated as previously described for the AMB-Biotin samples on HA.
The final sample was prepared by preincubating 3 mg of AMB-Biotin with 6 mg of ashed bone in 1 mL phosphate buffer. The washes and lyophilization were performed along with the other samples. However, after lyophilization, this sample was resuspended in a 1-mL solution of 5 mg/mL casein in phosphate buffer. The sample was incubated on a rotator at room temperature for 3 h. After incubation, the sample was centrifuged to collect the solid and the supernatant was removed.
All four of the samples were then resuspended in 1 mL each of 5.8 μg/mL FITC-labeled anti-biotin antibody, placed on a rotator and incubated for 3 h at room temperature. Subsequently, the samples were centrifuged to collect the bone, and the fluorescence of the supernatant was measured and compared to a standard of FITC-labeled anti-biotin antibody.
Kinetics of AMB-Biotin Binding to Ashed Bone
The kinetics of AMB-Biotin binding to bone were established by using a fluorescence assay based on avidin and 2-anilinonaphthalene-6-sulfonic acid (2,6-ANS), where 2,6-ANS occupies the biotin binding sites of avidin (31). 2,6-ANS has two different emission wavelengths, depending on whether it is in the bound or unbound state. In the presence of biotin, or in this case AMB-Biotin, the fluorescent probe is released from avidin. The amount of AMB-Biotin remaining unbound in solution can be determined using a calibration plot of increasing concentrations of AMB-Biotin in the presence of the avidin-2,6-ANS complex.
Three milligram samples of AMB-Biotin were incubated with 6 mg each of ashed bone for 0, 15, 30, 60, and 120 min in 1 mL of phosphate buffer. After the designated time period, the samples were centrifuged at 13000 rpm for 15 min. Aliquots of 12.5 μL of each of the supernatants were incubated with 800 μL of the avidin-2,6-ANS complex that was prepared at 8 μM avidin and 50 μM 2,6-ANS. Samples for the calibration plot were prepared using a solution of 3 mg/mL AMB-Biotin. AMB-Biotin was added to 800 μL of avidin-2,6-ANS in 12.5 μL increments. Ten total samples were prepared ranging from 0.09 mg/mL AMB-Biotin to 0.7 mg/mL. The excitation wavelength was set at 328 nm, while the fluorescence emission was measured from 400 nm to 500 nm. 2,6-ANS has an emission maximum at 438 nm when bound to avidin and 462 nm when unbound.
Results and Discussion
To synthesize AMB-Biotin, aminobisphosphonate was prepared first following a published procedure (30). This compound was subsequently functionalized with biotin. For this, biotin N-hydroxysuccinimide ester was used because of its reactivity toward aliphatic amines. AMB-Biotin was produced in high yield, and the NMR and MS data confirmed the production of a ligand-modified aminobisphosphonate.
AMB-Biotin binds to HA through its bisphosphonate portion. This binding can be followed by using a fluorescently labeled anti-biotin antibody that recognizes the biotin functionality on AMB-Biotin. Because of the difficulty in measuring fluorescence on the solid microparticles, the extent of binding was determined by measuring the fluorescence remaining in solution after the antibody reaction with the immobilized AMB-Biotin. Excitation of the fluorescent antibody was performed at 490 nm, while the emission was scanned from 500 to 600 nm. Figure 1A shows a representative set of spectra of AMB-Biotin binding on HA and the corresponding controls. The emission spectra have a maximum at 523 nm, which is characteristic of FITC-labeled proteins. The fluorescence intensity at 523 nm was used to calculate relative binding using the following equation:
where F0 is the fluorescence of the solution of anti-biotin antibody prior to addition of HA, FSample is the fluorescence remaining in the solution following the binding event, and FHighest is the fluorescence of the sample exhibiting the highest binding. Figure 1B shows data for the relative binding calculations for all samples of AMB-Biotin on HA and the controls.
Figure 1.
Binding of FITC-labeled monoclonal anti-biotin antibody to hydroxyapatite treated with AMB-Biotin and various controls. (A) Fluorescence signal of the supernatants (λex 490 nm) after removal of insoluble HA treated with AMB (•••) and AMB-Biotin (— • —), and untreated HA (— — —) compared to the spectrum of the FITC-labeled anti-biotin antibody solution used in the experiments (solid line). (B) Relative binding for untreated HA (n = 5), AMB treated HA (n = 6), and AMB-Biotin treated HA (n = 6). The * denotes statistically similar results. AMB-Biotin is statistically different from the AMB-treated and untreated HA samples at p > 0.01.
As can be seen in the spectra in Figure 1A, a considerable loss in the solution fluorescence is observed when hydroxyapatite was treated with AMB-Biotin. This suggests that AMB-Biotin is bound to the insoluble hydroxyapatite; anti-biotin antibody binds to the immobilized biotin and is removed from solution following centrifugation. When the relative binding is calculated, the AMB-Biotin treated hydroxyapatite exhibited the highest extent of binding (Figure 1B). This binding is more than twice the binding seen for the nonspecific interaction of the antibody with untreated HA and HA treated with an aminobisphosphonate lacking the biotin moiety. Analysis of variance (ANOVA) and post-hoc tests were performed to determine the statistical similarity or difference between the samples. In Figure 1B, the symbol * denotes that there is no statistical difference between the sample of HA treated with AMB and the untreated HA. However, both of these samples are statistically different from the AMB-Biotin + HA sample at a probability (p) of greater than 0.01.
Strong binding of AMB-Biotin is also observed in the presence of free biotin, as can be seen in Figure 2. In this figure, the symbols * and † denote statistically similar samples. There is no statistical difference between the Untreated HA, Free Biotin + HA, and AMB-Biotin + Free Biotin + HA samples, as denoted by the symbol *. There is also no statistical difference between the AMB-Biotin + HA sample and the AMB-Biotin + Free Biotin + HA sample, denoted by the symbol †. There is, however, a statistically significant difference (p > 0.01) between the Untreated HA and AMB-Biotin + HA samples. There is also a significant difference between the Free Biotin + HA and AMB-Biotin + HA samples (p > 0.01). The difference observed between the free biotin and AMB-Biotin samples demonstrates that the binding of the biotinylated bisphosphonate is indeed through the bisphosphonate moiety and not through the biotin functionality. Binding does not occur through the biotin group since no statistical difference was found between the Free Biotin + HA and the Untreated HA samples.
Figure 2.
Binding measurements in the presence of free biotin quantified using a fluorescently labeled anti-biotin antibody. Relative binding for untreated HA, free biotin treated HA, AMB-Biotin and free biotin incubated together with HA, and HA treated with AMB-Biotin. For all samples, n equals three. The symbols * and † denote statistically similar results. There is no statistical difference between the Untreated HA, Free Biotin, and AMB-Biotin + Free Biotin + HA samples, nor is there a difference between the AMB-Biotin + Free Biotin + HA and AMB-Biotin samples. However, there is a statistical difference (p > 0.01 for each sample) between the Untreated HA, Free Biotin + HA, and the AMB-Biotin +HA samples.
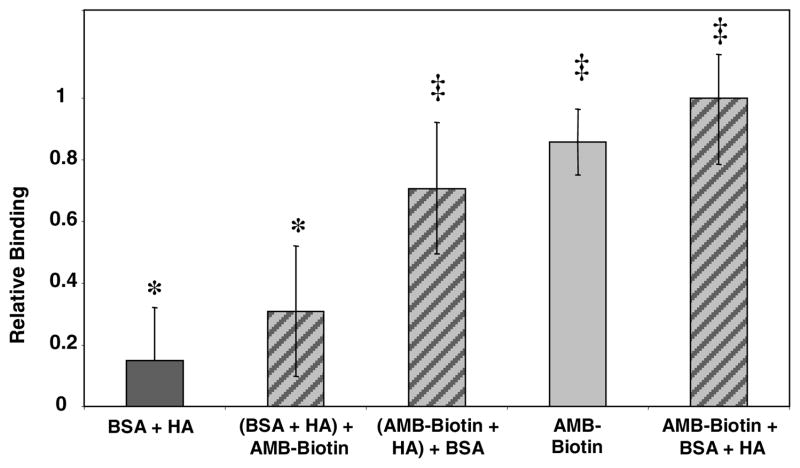
The results observed for the binding of AMB-Biotin in the presence of BSA are summarized in Figure 3. The highest relative binding observed was from the sample treated simultaneously with both AMB-Biotin and BSA. High relative binding was also observed for the AMB-Biotin + HA sample at almost 90%, and for the sample of HA pretreated with AMB-Biotin and subsequently incubated with BSA at over 70%. In the ANOVA and post-hoc calculations, these three samples were found to be statistically similar, as denoted by the symbol ‡. These three samples are statistically different (p > 0.05) from the two samples where HA was treated with either BSA alone or pretreated with BSA and then exposed to AMB-Biotin, which are statistically similar denoted by the symbol *. These results emphasize the specific binding ability of AMB-Biotin on HA, even when BSA is present in the sample.
Figure 3.
Binding measurements of AMB-Biotin in the presence of BSA based on the loss in fluorescence of the supernatants after incubation with a FITC-labeled anti-biotin antibody. Relative binding for BSA, BSA preincubated with HA, AMB-Biotin preincubated with HA, AMB-Biotin alone on HA, and AMB-Biotin and BSA incubated together with HA. All samples were prepared in triplicate. The symbols * and ‡ denote statistically similar samples. The statistically significant difference between the samples denoted with the symbol * and those with the symbol ‡ was at least p > 0.05.
While the BSA studies were performed at a low protein concentration (0.3 g/L), additional studies were carried out at higher levels of protein using FBS. Binding of AMB-Biotin to HA was performed in solutions of FBS in phosphate buffer at concentrations of 0%, 25%, 50%, 75%, and 100% (v/v) FBS. The highest binding was seen for 0% FBS. The sample showing the least amount of binding was the 75% serum sample, which showed binding of only 40% relative to the 0% FBS sample (data not shown). These levels of binding indicate that even at high concentrations of protein, AMB-Biotin still binds to HA. This leads one to believe that in the complexity of a living system, where there is a high level of protein and other biomolecules present, there will still be specific interaction of a conjugated aminobisphosphonate with the natural bone matrix.
The initial characterization of the biotinylated aminobisphosphonate was performed under conditions of a model system, specifically using hydroxyapatite to simulate bone and BSA as a general blocking protein. While this model system is beneficial initially, additional studies were performed to better understand how this bisphosphonate would behave with real bone. Three different treatments were used to prepare the bone samples. Samples of untreated, demineralized, and ashed bone were incubated with AMB-Biotin. Data from the bone samples can be seen in Figure 4. Figure 4A contains the data from the untreated bone samples, Figure 4B summarizes the data for the demineralized bone samples, and Figure 4C displays the results for the ashed bone samples. The symbol * denotes statistically similar binding between samples. Although a positive trend was observed for binding of AMB-Biotin to the untreated bone samples versus free biotin, there was not a statistical difference between the samples. Additionally, there is no statistical difference observed between the samples of ashed bone. The only bone treatment that showed a statistical difference between the free biotin sample and AMB-Biotin sample was the demineralized bone. This difference is observed at p > 0.025. It is unclear why there was no difference between biotin and AMB-biotin binding in the case of untreated and ashed bone. The data suggest that biotin is likely attached to the surface of these two samples through ion-exchange because of residual positive charges on the surface of the bone samples. Ashing the bone samples removes organic bone matrix that blocks access to the mineral phase (26). More binding sites on the mineral surface are exposed, thus increasing the probability of binding through electrostatic interactions.
Figure 4.
Binding measurements of AMB-Biotin and free biotin on multiple treatments of bone. The three treatments studied were untreated bone (A), demineralized bone (B), and ashed bone (C). Binding was quantified based on the binding of a fluorescently labeled anti-biotin antibody. For all samples, n equals three. The symbol * denotes statistically similar results. There is no statistical difference between the free biotin and AMB-Biotin samples on untreated and ashed bone. There is only a statistically significant (p > 0.025) difference between the AMB-Biotin and Free Biotin samples on Demineralized Bone.
The binding of AMB-Biotin to bone was further investigated in the presence of protein. In four different sets of experiments, ashed bone was treated with (a) casein, (b) AMB-Biotin, (c) AMB-Biotin followed by exposure to casein, and (d) simultaneous exposure to AMB-Biotin and casein. The results of this study can be seen in Figure 5. Casein proves to be a good blocking protein, allowing 15% of the binding of the anti-biotin antibody on the ashed bone relative to the ashed bone treated with AMB-Biotin. The extent of binding of AMB-Biotin on ashed bone was the highest for this sample set. Based on the ANOVA and post-hoc tests, it was found that there was no statistical difference between the Casein + AMB-Biotin + Ash, (AMB-Biotin + Ash) + Casein, and AMB-Biotin + Ash samples. This similarity is denoted in Figure 5 by the symbol *. All three of these samples were found to be statistically different from the Casein + Ash sample by a probability of at least greater than 0.05. Again, this suggests that the binding of AMB-Biotin has the greater impact on the system because there is still strong binding of the biotinylated aminobisphosphonate in the presence of protein.
Figure 5.
Binding measurements of AMB-Biotin in the presence of casein. Binding was measured using FITC-labeled anti-biotin antibody. Relative binding on ashed bone of casein, casein and AMB-Biotin, AMB-Biotin preincubated with bone, and AMB-Biotin. Samples were prepared in triplicate. The symbol * denotes statistically similar samples. There is no statistical difference between the Casein + AMB-Biotin + Ash, (AMB-Biotin + Ash) + Casein, and AMB-Biotin + Ash samples. All are statistically different from the Casein + Ash sample by at least p > 0.05.
It is generally accepted that conjugation of a therapeutic agent to bisphosphonate affects the properties of the bisphosphonate in terms of kinetics, affinity, and its ability to function as a drug (32, 33). Therefore, the kinetics of AMB-Biotin association to bone were determined. To evaluate the kinetics of the interaction between AMB-Biotin and ashed bone, a study was performed under pseudo first order conditions, where the ashed bone was present in excess over the AMB-Biotin. A calibration plot was used to determine the concentration of AMB-Biotin remaining in solution after incubation with ashed bone at various time intervals. The rate constant of the reaction was calculated to be 5.7 (± 1.7) x 10−4 min−1, where the error is the standard deviation of the data set. After two hours of incubation with ashed bone, the amount of AMB-Biotin remaining in solution was negligible. Since in all experiments described the bisphosphonate samples were incubated with either HA or bone overnight, there was sufficient time allowed to ensure binding of the bisphosphonate to the matrix material.
This work shows that a ligand-modified aminobisphosphonate can bind bone successfully. While this is a model system used to explore the conjugation of a protein linking molecule to an aminobisphosphonate, it may also help deliver peptides and proteins to the bone surface by linking them with a biotin-binding protein, such as an anti-biotin antibody. The availability of “humanized” antibodies overcomes the immunogenic challenges of avidin and streptavidin. A humanized antibody or antibody fragment could be conjugated to a therapeutic agent for delivery to the bone surface. Antibodies can also be used to introduce sites for the delivery of small molecules, such as drug carriers and anticancer, antibacterial, and antiresorptive agents (34).
Overall, the applications for bisphosphonates in the field of osteoporosis research are endless. The novel biotinylated aminobisphosphonate prepared herein retains its ability to bind to both hydroxyapatite and bone. The binding is specific enough that competing reagents, such as free biotin, or protein blocking agents, such as BSA, FBS, or casein, do not inhibit the affinity of the biotinylated bisphosphonate for bone. Aminomethylene bisphosphonic acid was chosen because it has a simple structure and fairly straightforward synthesis. The conjugation with biotin was performed using NHS ester chemistry. This technique could be performed on a variety of other bisphosphonates that are currently prescribed for osteoporosis treatment, such as pamidronate, alendronate, olpadronate, and ibandronate (34). Any of these molecules could be functionalized with biotin in the manner described and could therefore also be used to target proteins to the bone surface. While currently prescribed bisphosphonates function mainly to inhibit further bone resorption by the osteoclast, linking a molecule with osteogenic properties to a bisphosphonate would not only inhibit resorption but also stimulate regeneration.
Acknowledgments
The authors acknowledge financial support from the National Institutes of Health (AR048700). Also acknowledged are Dr. Bert Lynn and Dr. Jack Goodman of the University of Kentucky Mass Spectrometry Facility, Lexington, KY. Mr. Don Cho and Mr. Jivan Yewle (University of Kentucky, Lexington, KY) are thanked for assistance in acquiring NMR spectra.
References
- 1.Kiberstis P, Smith O, Norman C. Bone health in the balance. Science. 2000;289:1497. [Google Scholar]
- 2.Ducy P, Schinke T, Karsenty G. The osteoblast: A sophisticated fibroblast under central surveillance. Science. 2000;289:1501–1504. doi: 10.1126/science.289.5484.1501. [DOI] [PubMed] [Google Scholar]
- 3.Teitelbaum SL. Bone Resorption by Osteoclasts. Science. 2000;289:1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 4.Rodan GA, Martin TJ. Therapeutic approaches to bone diseases. Science. 2000;289:1508–1514. doi: 10.1126/science.289.5484.1508. [DOI] [PubMed] [Google Scholar]
- 5.Finkelstein JS. Calcium plus vitamin D for postmenopausal women -bone appetit? N Engl J Med. 2006;354:750–751. doi: 10.1056/NEJMe068007. [DOI] [PubMed] [Google Scholar]
- 6.Jackson RD, LaCroix AZ, Gass M, Wallace RB, Robbins J, Lewis CE, Bassford T, Beresford SAA, Black HR, Blanchette P, Bonds DE, Brunner RL, Brzyski RG, Caan B, Cauley JA, Chlebowski RT, Cummings SR, Granek I, Hays J, Heiss G, Hendrix SL, Howard BV, Hsia J, Hubbel FA, Johnson KC, Judd H, Kotchen JM, Kuller LH, Langer RD, Lasser NL, Limacher MC, Ludlam S, Mason JE, Margolis KL, McGowan J, Ockene JK, O'Sullivan MJ, Phillips L, Prentice RL, Sarto GE, Stefanick ML, Van Horn L, Wactawski-Wende J, Whitlock E, Anderson GL, Assaf AR, Barad D. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669–683. doi: 10.1056/NEJMoa055218. [DOI] [PubMed] [Google Scholar]
- 7.Fletcher SW, Colditz GA. Failure of estrogen plus progestin therapy for prevention. J Am Med Assoc. 2002;288:366–368. doi: 10.1001/jama.288.3.366. [DOI] [PubMed] [Google Scholar]
- 8.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SAA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. J Am Med Assoc. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 9.Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, III, BNJ, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women. J Am Med Assoc. 2003;289:2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 10.Cumming RG. Calcium intake and bone mass: A quantitative review of the evidence. Calcif Tissue Int. 1990;47:194–201. doi: 10.1007/BF02555919. [DOI] [PubMed] [Google Scholar]
- 11.Shea B, Wells G, Cranney A, Zytaruk N, Robinson V, Griffith L, Ortiz Z, Peterson J, Adachi J, Tugwell P, Guyatt G. Meta-analyses of therapies for postmenopausal osteoporosis. VII. Meta-analysis of calcium supplementation for the prevention of postmenopausal osteoporosis. Endocr Rev. 2002;23:552–559. doi: 10.1210/er.2001-7002. [DOI] [PubMed] [Google Scholar]
- 12.Horowitz MC. Cytokines and estrogen in bone: Anti-osteoporotic effects. Science. 1993;260:626–627. doi: 10.1126/science.8480174. [DOI] [PubMed] [Google Scholar]
- 13.Sunyer T, Lewis J, Collin-Osdoby P, Osdoby P. Estrogen's bone-protective effects may involve differential IL-1 receptor regulation in human osteoclast-like cells. J Clin Invest. 1999;103:1409–1418. doi: 10.1172/JCI4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin JH. Bisphosphonates: A review of their pharmacokinetic properties. Bone. 1996;18:75–85. doi: 10.1016/8756-3282(95)00445-9. [DOI] [PubMed] [Google Scholar]
- 15.Williams KR. The bisphosphonate story: From detergents to bone disease. J Chem Educ. 2004;81:1406–1407. [Google Scholar]
- 16.Reszka AA, Rodan GA. Mechanism of action of bisphosphonates. Curr Osteoporosis Rep. 2003;1:45–52. doi: 10.1007/s11914-003-0008-5. [DOI] [PubMed] [Google Scholar]
- 17.Rodan GA, Reszka AA. Bisphosphonate mechanism of action. Curr Mol Med. 2002;2:571–577. doi: 10.2174/1566524023362104. [DOI] [PubMed] [Google Scholar]
- 18.Reszka AA, Halasy-Nagy JM, Masarachia PJ, Rodan GA. Bisphosphonates act directly on the osteoclast to induce caspase cleavage of Mst1 kinase during apoptosis. J Biol Chem. 1999;274:34967–34973. doi: 10.1074/jbc.274.49.34967. [DOI] [PubMed] [Google Scholar]
- 19.Bagi CM. Targeting of therapeutic agents to bone to treat metastatic cancer. Adv Drug Delivery Rev. 2005;57:995–1010. doi: 10.1016/j.addr.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 20.Bansal G, Gittens SA, Uludağ H. A di(bisphosphonic acid) for protein coupling and targeting to bone. J Pharm Sci. 2004;93:2788–2799. doi: 10.1002/jps.20186. [DOI] [PubMed] [Google Scholar]
- 21.Frith JC, Monkkonen J, Blackburn GM, Russell RGG, Rogers MJ. Clodronate and liposome-encapsulated clodronate are metabolized to a toxic ATP analog, adenosine 5'-(β, γ-dichloromethylene) triphosphate, by mammalian cells in vitro. J Bone Miner Res. 1997;12:1358–1367. doi: 10.1359/jbmr.1997.12.9.1358. [DOI] [PubMed] [Google Scholar]
- 22.Green JR. Zoledronic acid: pharmacologic profile of a potent bisphosphonate. J Organomet Chem. 2004;690:2439–2448. [Google Scholar]
- 23.Halasy-Nagy JM, Rodan GA, Reszka AA. Inhibition of bone resorption by alendronate and risedronate does not require osteoclast apoptosis. Bone. 2001;29:553–559. doi: 10.1016/s8756-3282(01)00615-9. [DOI] [PubMed] [Google Scholar]
- 24.Lipton A. Toward new horizons: The future of bisphosphonate therapy. The Oncologist. 2004;9:38–47. doi: 10.1634/theoncologist.9-90004-38. [DOI] [PubMed] [Google Scholar]
- 25.Gittens SA, Matyas JR, Zernicke RF, Uludağ H. Imparting bone affinity to glycoproteins through the conjugation of bisphosphonates. Pharm Res. 2003;20:978–987. doi: 10.1023/a:1024445903306. [DOI] [PubMed] [Google Scholar]
- 26.Uludağ H, Kousinioris N, Gao T, Kantoci D. Bisphosphonate conjugation to proteins as a means to impart bone affinity. Biotechnol Progr. 2000;16:258–267. doi: 10.1021/bp990154m. [DOI] [PubMed] [Google Scholar]
- 27.Gittens SA, Bagnall K, Matyas JR, Löbenberg R, Uludağ H. Imparting bone mineral affinity to osteogenic proteins through heparin-bisphosphonate conjugates. J Control Release. 2004;98:255–268. doi: 10.1016/j.jconrel.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Uludağ H, Gao T, Wohl GR, Kantoci D, Zernicke RF. Bone affinity of a bisphosphonate-conjugated protein in vivo. Biotechnol Progr. 2000;16:1115–1118. doi: 10.1021/bp000066y. [DOI] [PubMed] [Google Scholar]
- 29.Uludağ H, Yang J. Targeting systemically administered proteins to bone by bisphosphonate conjugation. Biotechnol Progr. 2002;18:604–611. doi: 10.1021/bp0200447. [DOI] [PubMed] [Google Scholar]
- 30.Kantoci D, Denike JK, Wechter WJ. Synthesis of aminobisphosphonate. Synth Commun. 1996;26:2037–2043. [Google Scholar]
- 31.Przyjazny A, Bachas LG. Competitive-binding approach to liquid chromatographic postcolumn reactions with fluorimetric detection. Anal Chim Acta. 1991;246:103–112. [Google Scholar]
- 32.Nancollas GH, Tang R, Phipps RJ, Henneman Z, Gulde S, Wu W, Mangood A, Russell RGG, Ebetino FH. Novel insights into actions of bisphosphonates on bone: Differences in interactions with hydroxyapatite. Bone. 2006;38:617–627. doi: 10.1016/j.bone.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 33.Uludağ H. Bisphosphonates as a foundation of drug delivery to bone. Curr Pharm Des. 2002;8:1929–1944. doi: 10.2174/1381612023393585. [DOI] [PubMed] [Google Scholar]
- 34.Zhang S, Gangal G, Uludağ H. “Magic bullets” for bone diseases: Progress in rational design of bone-seeking medicinal agents. Chem Soc Rev. 2007;36:507–531. doi: 10.1039/b512310k. [DOI] [PubMed] [Google Scholar]