Abstract
Many factors affect the sensitivity and reliability of tumor vasculature assessment at the small doses of contrast agent necessary for imaging mice. In this study we investigate the dose–response relationship of ultrasound contrast agent for a minimal exposure power Doppler technique (minexPD) in a murine melanoma model. K1735 murine melanomas grown in 25 C3H/HeN mice were imaged by power Doppler ultrasound using different doses of contrast agents, Optison® and Definity®. Six mice were treated with an antivascular agent, combretastatin A4-phosphate (CA4P), and imaged before and after treatment. The color-weighted fractional area (CWFA) of the peak-enhanced image was measured to assess tumor perfusion on a relative scale of 0 to 100. CWFA increased logarithmically with dose (R2=0.97). Treatment with CA4P resulted in pronounced reduction in tumor perfusion 2 h after contrast injection, but perfusion recovered in the tumor periphery after 2 days. CWFA was significantly different between pre- and post-treatment for all doses at 2 h and 2 days (p < 0.05, respectively). There was no significant difference detectable between the two contrast agents, Optison® and Definity® (p = 0.46). In vivo tumor enhancement in mice increases as logarithmic function with dose. Although the extent of enhancement is dose dependent, the difference between pre- and post-therapy enhancement is relatively unchanged and uniform at varying doses. The two contrast agents tested in this study performed equally well. These results suggest that quantitative contrast-enhanced power Doppler imaging is an effective method for monitoring therapy response of tumors in mice.
Keywords: Contrast-enhanced ultrasound, dose, antivascular, cancer, mouse, melanoma
Introduction
Formation of new blood vessels (angiogenesis) is essential for tumor growth beyond 1–2 mm31,2. Convenient intravenous application and the possibility of affecting a large number of tumor cells by destroying one single vessel makes tumor vessels a promising target for cancer treatment. Various antivascular agents that damage the existing tumor vasculature are currently under investigation in rodent models3,4. Combretastatin A4-phosphate (CA4P) is an example of a vascular targeting agent that rapidly shuts down blood supply to the tumor5. To effectively monitor early response to antivascular therapy, it is important to non-invasively monitor tumor perfusion. Because ultrasound imaging is non-invasive, relatively inexpensive and widely available6–8, there is a growing interest in developing contrast-enhanced imaging in mice6,9–14. Mice are common animal models for human cancer research and are widely used for evaluating the effectiveness of various vascular disrupting agents6,8,15. However, scaling down the imaging methods developed originally for imaging humans for use in mouse models presents several challenges.
Conventional Doppler ultrasound methods are not very sensitive for the slow blood flow in tumor vessels7. Contrast-specific imaging modes such as contrast harmonic imaging or contrast-enhanced power Doppler imaging, however, visualize tissue vascularity with high sensitivity7. However, application of contrast-enhanced power Doppler ultrasound to imaging tumor perfusion is limited because the microbubbles, a component of contrast agent, are destroyed by the ultrasound imaging pulses. The slow flow in tumor vessels16 means that the microbubbles take longer to transit through the image plane. During the longer transit time, the bubbles are exposed to a greater number of ultrasound pulses and are increasingly destroyed which dampens image enhancement. To overcome the problem of bubble destruction in a slow flow environment, we proposed a power Doppler imaging technique that minimizes ultrasound exposure (minexPD) by gating the scanner at 0.5 Hz10,11,17 during bolus injection of contrast agent. With minexPD the microbubbles traveling through the image field of view are exposed to imaging ultrasound pulses once every 2 s. The reduced ultrasound exposure minimizes bubble destruction and enables visualization tumor perfusion10,11,17. Among the various factors that can influence the reliability and sensitivity of minexPD, the dose of contrast agent used for tumor visualization plays an important role. A large dose of contrast agent saturates the image, while delivery of a low dose is difficult on a consistent basis. Also, at low doses, the sensitivity of the method is considerably reduced due to insufficient image enhancement caused by significant destruction of the microbubbles in the tumor vasculature, which is known to have slow and inconsistent blood flow16.
This study investigates the dose–response relationship of ultrasound contrast agents for minexPD in a murine melanoma model. The ability to detect perfusion changes after therapy was tested by imaging mice pre- and post-treatment with an antivascular agent at different contrast doses. Contrast enhancement of two different contrast agents was compared and the influence of contrast agent dilution (prior to bolus injection) was evaluated.
Materials and methods
The trial was sponsored by OxiGene Inc. Watertown, MA. The sponsor provided the antivascular agent, combretastatin A4-phosphate (CA4P). The authors of this article had sole control of the data generated by this trial.
Animal models
Animal experiments were approved by the University of Pennsylvania Animal Care and Use Committee. Twenty-five female mice (6–8 weeks of age; C3H/HeN strain; Charles River Laboratories, Wilmington, MA), were kept in microisolator cages under sterile conditions. 2 × 106 K1735 murine melanoma cells (syngeneic with C3H/HeN mice) were injected subcutaneously into the right hind limb of mice anesthetized using 140 mg/kg ketamine hydrochloride (Abbott Laboratories, N. Chicago, IL) and 1.3 mg/kg xylazine hydrochloride (Phoenix Pharmaceutical Inc, St Joseph, MO) administered intraperitoneally. After the first ultrasonographic examination of the untreated tumor, antivascular treatment was performed in 6 of the 25 mice by intraperitoneal administration of 100 mg/kg combretastatin A4-phosphate (CA4P) (OxiGene Inc. Watertown, MA).
Ultrasound imaging
When tumor size reached 5 mm maximal diameter, catheters (26 gauge Abbocath, Abbot Ireland, Sligo, Ireland) were placed in the tail vein and the mice were anesthetized with 1.25% isoflurane in air (Isosol, Halocarbon Laboratories, River Edge, NJ). The mice were placed under a heat lamp, hair overlying the tumor was removed using a depilatory cream and the tumors were imaged by power Doppler ultrasound.
Imaging was performed using an HDI 5000 with spatial compound imaging (SonoCT, Philips Ultrasound, Bothell, WA, USA) with a 7–15 MHz linear probe. The scanner was gated externally with a frequency of 0.5 Hz, using a mechanical index (MI) of 0.9, a pulse repetition frequency (PRF) of 700 Hz was used. Tumors were scanned in a dorsal plane, using the image plane with the maximum length of the tumor. Once a good image of the tumor was obtained, the probe was fixed in position and not moved during the procedure. Color rejection threshold settings and Doppler gain settings were optimized to reduce blooming artifact at the beginning and were kept constant throughout the study. A single focal zone was placed at the level of the proximal third of the tumor. Four different doses (10, 20, 50 and 100 μl) of microbubble contrast agent (Optison®, GE Healthcare, Princeton, NJ, USA) were injected into the tail vein catheter. The injections were performed by injecting through a stylet inserted into the catheter to reduce dead space and assure proper mixing of the contrast agent. Flush after contrast injection was therefore not used. A time interval of 4–5 min between injections allowed clearance of the previously injected dose based on subjective lack of contrast enhancement of the image as well as guidelines from the manufacturer.
To compare two different contrast agents, two different doses (20, 50 μl) of Definity® (Bristol-Myers Squibb Medical Imaging Inc., NY, USA) were injected in addition to Optison® in 6 of the mice (3 with untreated, 3 with treated tumors). Since application of very small doses such as 10 μl can be difficult, in 5 different mice with untreated tumors, 10 μl of Optison® were injected undiluted and in a dilution of 1:5 in phosphate buffer solution (PBS) to assess the use of diluted contrast. Treated tumors were imaged immediately before, 2 h and 2 days after intraperitoneal treatment with CA4P.
Image analysis
Images were recorded on videotape (S-VHS format) for a total duration of 3 min immediately after contrast injection. Images were then digitized frame by frame (30 frames/s; 24 bit; Adobe Premiere 6.5, Adobe Systems Inc, San Jose, CA) using a digitizer (MediaConvertor, DVMC-DA2, Sony, Tokyo, Japan) connected to a standard personal computer (2.8 MHz Intel Pentium 4 CPU, Micron PC, Dell Inc, Austin, TX), and stored in an uncompressed format (QuickTimePlayer, 6.5.1, Apple Computer Inc, Cupertino, CA). Images at peak enhancement once the contrast was well distributed in the blood were used. On the digitized image, color level was analyzed within a region of interest (ROI) superimposed on the entire tumor using custom-made computer software (University of Pennsylvania). The color palette was used for calibration and remained constant throughout the study. Color-weighted fractional area (CWFA) of the colored pixels within the region of interest was measured as described before18,19. The color of each pixel in the contrast enhanced power Doppler image measures the fractional volume of the contrast flowing through the pixel and the color-weighted fractional area (product of color level and fraction area covered by colored pixels) measures the contrast volume per unit area of tumor. Mean ± standard error (SEM) of the CWFA was calculated for each dose in the control and treatment groups and CWFA vs. contrast dose curves were fitted to a logarithmic model to determine dose–response relationships.
Statistical analysis
An unpaired t-test (MedCalc Software version 7.1 (Mariakerke, Belgium) was used to determine whether the decrease in vascularity following treatment was statistically significant (p < 0.05) at each dose. Enhancement resulting from 10 μl of Optison® diluted and undiluted as well as comparison between Optison® and Definity® was compared using a paired t-test (MedCalc Software version 7.1; Mariakerke, Belgium).
Histologic analysis
Animals were euthanized and the tumors were removed and frozen in liquid nitrogen. Sections were cut in a dorsal plane through the center of the tumor (the plane used for the ultrasound scan) using a cryostat and stained with hematoxylin and eosin to evaluate the presence of tissue necrosis. Specimens were viewed with a microscope (Nikon E600 Eclipse, Nikon, Melville, NY) and images were acquired (Photometrics Coolsnap CF CCD camera, Roper Scientific Inc, Trenton, NJ) using image acquisition software (IP Lab, Scanalytics Inc, Fairfax, VA).
Results
The smallest volume that can be injected using a 26-gauge catheter is 10 μl due to dead space in the catheter. Still, detachment of 10 μl of contrast from the catheter lumen was unreliable and in 20% of the cases did not result in enhancement of the tumor. This prompted us to dilute the contrast agent so that small amounts of the contrast agent could be injected more reliably with a larger volume. In 5 mice where both 10 μl of Optison® diluted as well as undiluted were successfully injected, no statistical difference in tumor enhancement was observed between the two methods (p = 0.65).
Untreated tumors
Untreated K1735 tumors were examined in 25 mice. Contrast enhancement in untreated K1735 tumors was consistently strong and evenly distributed throughout the tumor as previously described10. Higher doses of contrast medium provided stronger enhancement (Fig. 1). This is reflected in the CWFA showing that measured relative perfusion increased with dose. The relationship was best described by a logarithmic model (Fig. 2). The equations were y=13.5ln(x)+8.74, R2=0.97.
Figure 1.
Dose-dependency of Optison® enhancement of K1735 tumor perfusion. Long axis view of the same K1735 tumor after injection of 10 (top left), 20 (top right), 50 (lower left) and 100 μl (lower right) of Optison®; the transducer is not moved between the injections. Increasing contrast dose results in stronger enhancement with considerable blooming. Note the very uniform vascularity of this tumor model.
Figure 2.
Optison® dose relationship to enhancement of tumor perfusion. Dose–response relationship of Optison® in untreated K1735 tumors (n = 25). Color-weighted fractional area (CWFA) parameters (mean ± standard error) plotted over dose show a logarithmic increase of the CWFA with increasing dose as shown by the equation included in the figure.
Treated tumors
Treatment with CA4P was performed in 6 mice and resulted in profound loss of perfusion 2 h after injection (Fig. 3). In half of the cases, there was a persistent rim of peripheral enhancement present 2 h after treatment. In all cases, tumor perfusion had recovered to a certain degree 2 days after treatment in the periphery of the tumor (Fig. 4). The dose–response relationship at both time points could be described by a logarithmic function with the equation y = 6.03ln(x)−13.6, R2=0.92 for 2 h post-treatment and y = 15.69ln(x)−22.68, R2=0.99 for 2 days after treatment (Fig. 4). A significant difference was detected between untreated and treated tumors after 2 h and 2 days at all doses (p < 0.05).
Figure 3.
Effect of treatment with combretastatin A4-phosphate on tumor perfusion. Long axis view of a K1735 tumor before, 2 h after and 2 days after treatment with combretastatin A4-phosphate (CA4P) (from left to right). At 2 h there is profound loss of perfusion, which recovers primarily in the periphery after 2 days.
Figure 4.
Dose–response relationship of Optison® enhancement of perfusion in tumors treated and untreated with combretastatin A4-phosphate. Dose–response relationship of Optison® in untreated K1735 tumors (n = 25), and K1735 tumors 2 h and 2 days after treatment with combretastatin A4-phosphate (n = 6). The vascularity is significantly decreased 2 h after treatment and recovers slightly 2 days later. The difference between treated and untreated tumors is evident at all contrast doses.
Comparison between Optison® and Definity®
Subjectively, application of Definity® resulted in a clearer demarcation of the vessels with less blush of the adjacent tissues (Fig. 5). Optison® cleared much more quickly from the tissues than Definity®; typically the power Doppler signal had returned to baseline after 2–3 min after injection of Optison®, whereas the enhancement persisted for 5–10 min after injection of Definity®. There were no statistically significant differences in CWFA measurements between Optison® and Definity® using 20 μl (p = 0.46) and 50 μl (p = 0.8). Both contrast agents performed equally well, differentiating between treated and untreated tumors and showed statistically significant differences at the examined doses of 20 μl and 50 μl (p <0 05).
Figure 5.
Contrast enhancement of tumor perfusion by Optison® versus Definity®. K1735 tumor scanned after injection of 20 and 50 μl of Optison® (top row, left to right) and 20 and 50 μl of Definity® (bottom row, left to right). Note the very similar enhancement pattern with subjectively better definition of the vessels using Definity®.
Histology
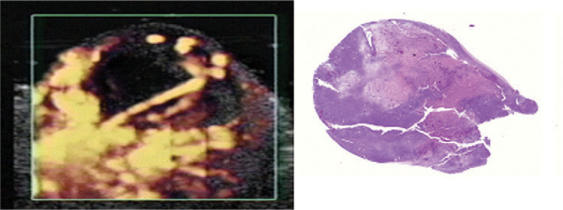
Untreated tumors were characterized by tumor cell proliferation with minimal necrosis or hemorrhage, confirming the uniform vascularization of this tumor model seen ultrasonographically. Histological examination of the tumors treated with the antivascular agent confirmed large areas of necrosis in the center corresponding to the perfusion defects seen with contrast-enhanced power Doppler ultrasound (Fig. 6). A small rim of viable tumor tissue with normal vessels could be seen in the periphery of the necrotic areas.
Figure 6.
Comparison between contrast-enhanced ultrasound imaging of tumor perfusion and tumor histopathology. K1735 tumor scanned 2 days after treatment with combretastatin A4-phosphate with corresponding hematoxylin and eosin staining of the tumor. Two large areas of necrosis divided by a vascularized zone are seen as perfusion defects on the power Doppler image.
Discussion
There is considerable interest in measuring angiogenesis and tumor perfusion using imaging techniques. Contrast-enhanced ultrasound imaging is an attractive choice because of its ease of use and low cost. Although several ultrasound techniques have been developed6,8,13,15,17,20,21, the effect of contrast agent dose on minexPD for the assessment of tumor vasculature has not been studied in vivo.
Mouse imaging, widely used in cancer research22,23, poses unique problems. The small body size of the mouse prevents the use of the same dose per weight as used in human patients. While it is desirable to use the smallest possible contrast dose resulting in adequate image quality, to avoid problems such as volume overload and contrast agent toxicity24,25, injection of small doses is limited by the dead space within the catheter lumen and inconsistencies in measuring very small contrast doses. The problem of a large dead space within the catheter sleeve was partially solved in this study by injecting contrast through a stylet. With this method, small amounts (equivalent to a small drop) of contrast can be delivered directly into the tail vein from the tip of the catheter. Ten microliters was the lowest possible injectable dose with the catheter size used in this study. However, deposition of 10 μl of contrast was unreliable, as not all injections resulted in enhancement of any tissue included in the scan field. This most likely resulted from failure of detachment of the small volume from the tip of the catheter stylet. This could be improved by diluting the contrast agent to increase the volume. While dilution is not recommended for administering contrast agents, it may be a valuable alternative because it will increase the volume while keeping the amount of contrast agent low. CWFA at peak enhancement using diluted and non-diluted contrast at a low dose of 10 μl was not significantly different in this study.
Using much higher doses, on the other hand, can lead to saturation and blooming artifacts. Blooming artifacts occur early after injection when the concentration of contrast is highest26–28. The color rejection threshold is considered to be the main cause of blooming artifacts, but adjustment of the color rejection settings alone does not eliminate the artifact completely26,29. Adjustment of Doppler gain setting and reducing the concentration of contrast also helps diminish the artifact30. Saturation of imaging fields with higher doses of contrast impaired detection of small coronary stenosis and led to the recommendation to reduce the contrast dose for myocardial contrast echography31.
As both too high or too low doses potentially decrease the sensitivity of the system, it is important to know in which part of the dose–response relationship curve one must operate for clinical application of ultrasound contrast in rodent tumors and ultimately also in humans. Since often the main purpose of performing contrast-enhanced Doppler ultrasound in tumors is to detect changes in vascularity after antiangiogenic or antivascular treatment, we chose to compare untreated and treated tumors at different doses to determine which dose is the most sensitive for detecting changes in tumor perfusion. The results of these experiments show that treatment with combretastatin produced such a significant reduction in tumor perfusion that the logarithmic curve leveled at a much lower CWFA value than the untreated group (Fig. 4). Two days after treatment, perfusion improved (Fig. 4) but was still significantly lower than the original pre-treatment curve. Because of the near-parallel nature of the curves, the difference between the pre- and post-treatment curves at different contrast concentrations was small, suggesting that all the doses used in these experiments were equally sensitive in detecting difference in perfusion resulting from this form of treatment. However, it should be noted that these results are specific to the experimental conditions described here and to the treatment that produced significant reduction in tissue perfusion. In the K1735 tumor model, tumor perfusion is very uniform even in relatively large tumors10,32,33 and is known to be sensitive to treatments. In tumor models with heterogeneous tissue perfusion, the treated and untreated curves may not be distinguished as well (Fig. 4), in which case there may be greater dependence on contrast dose for detecting perfusion changes. In other words, if the perfusion defects are smaller, it may be advantageous to reduce the contrast dose to operate in the steepest portion of the dose–response relationship curve. At higher doses, blooming artifacts might obscure smaller perfusion defects30.
The reason for the subjectively observed difference between the two contrast agents is not known. Many different characteristics of microbubble contrast media such as compressibility, bubble size, properties of the bubble shell and gas density could result in slightly different imaging characteristics30. More extensive studies of the physical characteristics of the two agents will be necessary to determine the cause of our observations.
An interesting feature of the treatment of K1735 tumors with CA4P is that it leads to profound perfusion defects after 2 h and some reperfusion, especially in the periphery, after 2 days. It appears that unlike the slower and smaller effects of antiangiogenic agents, an antivascular agent such as CA4P cause an acute collapse of the tumor blood vessels within minutes after injection. Alterations in the endothelial cytoskeleton and increase in permeability of the tumor vasculature with leakage of plasma proteins and increased interstitial fluid pressure are the most likely mechanisms leading to this rapid and selective shutdown of tumor vessels34. The peripheral sparing or viable rim has been described before34–36 and was observed in this group of mice as well, in some cases immediately after treatment and in all cases 2 days after treatment. It is a poorly understood feature of this antivascular treatment and leads to rapid regrowth if a single treatment is applied. Higher interstitial pressure in the center of the tumor leading to a larger amount of necrosis is a possible explanation for this phenomenon34.
There is only limited information available about the effect of contrast agent dose on contrast enhancement parameters and detection of tumor perfusion17,37,38. Ultrasound contrast enhancement of tumor vasculature is dose-dependent. Theoretical modeling suggests that contrast enhancement of Doppler images should increase as a log function of the concentration of the contrast agent17. This is because ultrasound scanners use log compression of the echo signal to display images. Although the log compression of the signal for displaying images is well recognized in the scientific community, it has not been shown experimentally if the logarithmic nature of the curve persists even after multiple layers of image processing. The dose–response relationship of Optison® contrast medium in this study followed a logarithmic curve which is consistent with the expectations based on theory and instrument design. A linear relationship between image enhancement and contrast medium concentration is often assumed in dye-dilution theory. The results of this study show that it is important to consider a non-linear relationship between dose and enhancement when considering dose dependence of ultrasound contrast agents in the evaluation of tumor vasculature.
The limitations of this study include the low number of mice in the treatment groups and the comparison groups between the two contrast media and the diluted vs. undiluted contrast. While previous studies have found a sample size of 6 to be sufficient10,17, increasing the number of mice would have facilitated detection of small differences between groups.
In addition, only four different doses were administered, and no intra-subject reproducibility was assessed. The number of injections per subject was restricted in order to reduce the risk of creating volume overload in the mouse. The limitation of conventional contrast-enhanced power Doppler with a high mechanical index leading to massive microbubble destruction was overcome in this study by using minexPD.
Conclusion
The results of this study show that contrast enhancement of tumor vasculature is dose dependent, and increases logarithmically with contrast dose. Treatment with vascular disrupting agents like CA4P leads to profound vessel shutdown which was easily measured by contrast enhanced power Doppler imaging. The choice of contrast agent did not influence the perfusion measurements. Although the enhancement was dose dependent, it did not significantly affect the ability of the contrast agent to monitor tumor response to antivascular therapy. While these results are encouraging, suggesting different doses can be used effectively to monitor tumor response to therapy, it is conceivable that the dose–response relationship may change if a spontaneous tumor model or a drug with less profound effects on the vasculature are used. In such situations dose–response studies similar to that presented in this study must be repeated to identify the optimum dose.
Acknowledgements
This work was supported by NIH grants RO1 EB001713 (C.M.S.) and U54 CA105008 (W.M.F.L.). The authors thank OxiGene Inc for providing combretastatin A4-phosphate.
References
- 1.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 2.Nyberg P, Xie L, Kalluri R.2 Endogenous inhibitors of angiogenesis. Cancer Res. 2005;65:3967–79. doi: 10.1158/0008-5472.CAN-04-2427. [DOI] [PubMed] [Google Scholar]
- 3.Cao Y. Antiangiogenic cancer therapy. Semin Cancer Biol. 2004;14:139–45. doi: 10.1016/j.semcancer.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 4.Tozer GM. Measuring tumour vascular response to antivascular and antiangiogenic drugs. Br J Radiol. 2003;76:S23–35. doi: 10.1259/bjr/30165281. [DOI] [PubMed] [Google Scholar]
- 5.Rehman S, Jayson GC. Molecular imaging of antiangiogenic agents. The Oncologist. 2005;10:92–103. doi: 10.1634/theoncologist.10-2-92. [DOI] [PubMed] [Google Scholar]
- 6.Krix M, Kiessling F, Vosseler S, et al. Sensitive noninvasive monitoring of tumor perfusion during antiangiogenic therapy by intermittent bolus-contrast power Doppler sonography. Cancer Res. 2003;63:8264–70. [PubMed] [Google Scholar]
- 7.Ferrara KW, Merritt CR, Burns PN, Foster FS, Mattrey RF, Wickline SA. Evaluation of tumor angiogenesis with US: imaging, Doppler and contrast agents. Acad Radiol. 2000;7:824–39. doi: 10.1016/s1076-6332(00)80631-5. [DOI] [PubMed] [Google Scholar]
- 8.Iordanescu I, Becker C, Zetter B, Dunning P, Taylor GA. Tumor vascularity: evaluation in a murine model with contrast-enhanced color Doppler US – effect of angiogenesis inhibitors. Radiology. 2002;222:460–7. doi: 10.1148/radiol.2222010660. [DOI] [PubMed] [Google Scholar]
- 9.Bunte RM, Ansaloni S, Sehgal CM, Lee WM, Wood AK. Histopathological observations on the antivascular effects of physiotherapy ultrasound on a murine neoplasm. Ultrasound Med Biol. 2006;32:453–61. doi: 10.1016/j.ultrasmedbio.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Ziemer LS, Lee WMF, Vinogradov SA, Sehgal CM, Wilson DF. Oxygen distribution in murine tumors: characterization using oxygen-dependent quenching of phosphorescence. J Appl Physiol. 2005;98:1503–10. doi: 10.1152/japplphysiol.01140.2004. [DOI] [PubMed] [Google Scholar]
- 11.Wood AK, Ansaloni S, Ziemer LS, Lee WM, Feldman MD, Sehgal CM. The antivascular action of physiotherapy ultrasound on murine tumors. Ultrasound Med Biol. 2006;32:453–61. doi: 10.1016/j.ultrasmedbio.2005.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yankeelov TE, Niermann KJ, Huamani J, et al. Correlation between estimates of tumor perfusion from microbubble contrast-enhanced sonography and dynamic contrast-enhanced magnetic resonance imaging. J Ultrasound Med. 2006;25:487–97. doi: 10.7863/jum.2006.25.4.487. [DOI] [PubMed] [Google Scholar]
- 13.Lucidarme O, Kono Y, Corbeil J, et al. Angiogenesis: noninvasive quantitative assessment with contrast-enhanced functional US in murine model. Radiology. 2006;239:730–9. doi: 10.1148/radiol.2392040986. [DOI] [PubMed] [Google Scholar]
- 14.Forsberg F, Ro RJ, Potoczek M, et al. Assessment of angiogenesis: implications for ultrasound imaging. Ultrasonics. 2004;42:325–30. doi: 10.1016/j.ultras.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 15.Korpanty G, Carbon JG, Grayburn PA, Fleming JB, Brekken RA. Monitoring response to anticancer therapy by targeting microbubbles to tumor vasculature. Clin Cancer Res. 2007;13:323–30. doi: 10.1158/1078-0432.CCR-06-1313. [DOI] [PubMed] [Google Scholar]
- 16.Cuenod CA, Fournier L, Balvay D, Guinebretiere JM. Tumor angiogenesis: pathophysiology and implications for contrast-enhanced MRI and CT assessment. Abdom Imaging. 2006;31:188–93. doi: 10.1007/s00261-005-0386-5. [DOI] [PubMed] [Google Scholar]
- 17.Kamotani Y, Lee WM, Arger PH, Cary TW, Sehgal CM. Multigated contrast-enhanced power Doppler to measure blood flow in mice tumors. Ultrasound Med Biol. 2003;29:977–84. doi: 10.1016/s0301-5629(03)00054-1. [DOI] [PubMed] [Google Scholar]
- 18.Sehgal CM, Arger PH, Silver AC, et al. Renal blood flow changes induced with endothelin-1 and fenoldopam mesylate at quantitative Doppler US: initial results in a canine study. Radiology. 2001;219:419–426. doi: 10.1148/radiology.219.2.r01ma13419. [DOI] [PubMed] [Google Scholar]
- 19.Weinstein SP, Conant EF, Sehgal CM, Woo IP, Patton JA. Hormonal variations in the vascularity of breast tissue. J Ultrasound Med. 2005;24:67–72. doi: 10.7863/jum.2005.24.1.67. [DOI] [PubMed] [Google Scholar]
- 20.Chomas JE, Pollard RE, Sadlowski AR, Griffey SM, Wisner ER, Ferrara KW. Contrast-enhanced US of microcirculation of superficially implanted tumors in rats. Radiology. 2003;229:439–46. doi: 10.1148/radiol.2292020536. [DOI] [PubMed] [Google Scholar]
- 21.Fleischer AC, Donnelly EF, Grippo RJ, Black AS, Hallahan D. Quantification of tumor vascularity with contrast-enhanced Sonography. J Ultrasound Med. 2004;23:37–41. doi: 10.7863/jum.2004.23.1.37. [DOI] [PubMed] [Google Scholar]
- 22.Carver BS, Pandolfi PP. Mouse modelling in oncologic preclinical and translational research. Clin Cancer Res. 2006;12:5305–11. doi: 10.1158/1078-0432.CCR-06-0482. [DOI] [PubMed] [Google Scholar]
- 23.Cespedes MV, Casanova I, Parreno M, Mangues R. Mouse models in oncogenesis and cancer therapy. Clin Transl Oncol. 2006;8:318–29. doi: 10.1007/s12094-006-0177-7. [DOI] [PubMed] [Google Scholar]
- 24.Li P, Armstrong WF, Miller DL. Impact of myocardial contrast echocardiography on vascular permeability: comparison of three different contrast agents. Ultrasound Med Biol. 2004;30:83–91. doi: 10.1016/j.ultrasmedbio.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Miller DL, Quddus J. Diagnostic ultrasound activation of contrast agent gas bodies induces capillary rupture in mice. Proc Natl Acad Sci U S A. 2000;97:10179–84. doi: 10.1073/pnas.180294397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu CY, Lo MT, Tsao J, Chu A, Chou YH, Tiu CM. Factor analysis in both spatial and temporal domains of color blooming artifacts in ultrasound investigations utilizing contrast agents. Comput Med Imaging Graphics. 2004;28:129–40. doi: 10.1016/j.compmedimag.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Forsberg F, Liu JB, Merton DA, Rawool NM, Goldberg BB. Parenchymal enhancement and tumor visualization using a new sonographic contrast agent. J Ultrasound Med. 1995;14:949–57. doi: 10.7863/jum.1995.14.12.949. [DOI] [PubMed] [Google Scholar]
- 28.Pugh CR, Arger PH, Sehgal CM. Power, spectral and color flow Doppler enhancement by a new ultrasonographic contrast agent. J Ultrasound Med. 1996;15:843–52. doi: 10.7863/jum.1996.15.12.843. [DOI] [PubMed] [Google Scholar]
- 29.Forsberg F, Liu JB, Burns PN. Artifacts in ultrasonic contrast agent studies. J Ultrasound Med. 1994;13:357–65. doi: 10.7863/jum.1994.13.5.357. [DOI] [PubMed] [Google Scholar]
- 30.McCulloch M, Gresser C, Moos S, et al. Ultrasound contrast physics: a series on contrast echocardiography, article 3. J Am Soc Echocardiogr. 2000;13:959–67. doi: 10.1067/mje.2000.107004. [DOI] [PubMed] [Google Scholar]
- 31.Masugata H, DeMaria AN, Peters B, et al. Difference of optimal dose of contrast agent between grey-scale and power Doppler imaging in assessing graded coronary stenosis by myocardial contrast echocardiography. Invest Radiol. 2003;38:550–8. doi: 10.1097/01.RLI.0000073641.93901.b9. [DOI] [PubMed] [Google Scholar]
- 32.Gee MS, Koch CJ, Evans SM, et al. Hypoxia-mediated apoptosis from angiogenesis inhibition underlies tumor control by recombinant interleukin 12. Cancer Res. 1999;59:4882–9. [PubMed] [Google Scholar]
- 33.Gee MS, Saunders HM, Lee JC, et al. Doppler ultrasound imaging detects changes in tumor perfusion during antivascular therapy associated with vascular anatomic alterations. Cancer Res. 2001;61:2974–82. [PubMed] [Google Scholar]
- 34.Tozer GM, Kanthou C, Baguley BC. Disrupting tumor blood vessels. Nature Reviews. 2005;5:423–35. doi: 10.1038/nrc1628. [DOI] [PubMed] [Google Scholar]
- 35.Tozer GM. Measuring tumour vascular response to antivascular and antiangiogenic drugs. Br J Radiol. 2003;76:S23–35. doi: 10.1259/bjr/30165281. spec no 1. [DOI] [PubMed] [Google Scholar]
- 36.Beauregard DA, Thelwall DE, Chaplin DJ, Hill SA, Adams GE, Brindle KM. Magnetic resonance imaging and spectroscopy of combretastatin A4 prodrug-induced disruption of tumour perfusion and energetic status. Br J Cancer. 1998;77:1761–7. doi: 10.1038/bjc.1998.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sehgal CM, Arger PH. Mathematical modelling of the dilution curves for ultrasonographic contrast agents. J Ultrasound Med. 1997;16:471–9. doi: 10.7863/jum.1997.16.7.471. [DOI] [PubMed] [Google Scholar]
- 38.Li J, Dong B, Yu X, Wang X, Li C. Grey-scale contrast enhancement in rabbit liver with Sonovue™ at different doses. Ultrasound Med Biol. 2005;31:185–90. doi: 10.1016/j.ultrasmedbio.2004.10.009. [DOI] [PubMed] [Google Scholar]