Abstract
Poly-N-acetyl glucosamine (pGlcNAc) nanofiber-derived materials effectively achieve hemostasis during surgical procedures. Treatment of cutaneous wounds with pGlcNAc in a diabetic mouse animal model causes marked increases in cell proliferation and angiogenesis. We sought to understand the effect of the pGlcNAc fibers on primary endothelial cells (EC) in culture and found that pGlcNAc induces EC motility. Cell motility induced by pGlcNAc fibers is blocked by antibodies directed against αVβ3 and α5β1 integrins, both known to play important roles in the regulation of EC motility, in vitro and in vivo. pGlcNAc treatment activates mitogen-activated protein kinase and increases Ets1, vascular endothelial growth factor (VEGF) and interleukin 1 expression pGlcNAc activity is not secondary to its induction of VEGF; inhibition of the VEGF receptor does not inhibit the pGlcNAc-induced expression of Ets1 nor does pGlcNAc cause the activation of VEGF receptor. Both dominant negative and RNA interference inhibition of Ets1 blocks pGlcNAc-induced EC motility. Antibody blockade of integrin results in the inhibition of pGlcNAc-induced Ets1 expression. These findings support the hypothesis that pGlcNAc fibers induce integrin activation which results in the regulation of EC motility and thus in angiogenesis via a pathway dependent on the Ets1 transcription factor and demonstrate that Ets1 is a downstream mediator of integrin activation.
Keywords: Angiogenesis, Cell migration, Integrin, Ets1, Poly-N-acetyl glucosamine nanofiber
Introduction
Angiogenesis is the mechanism by which new blood vessels are formed from the pre-existing vascular network [1]. Among the cellular mechanisms that underlie angiogenesis, cell motility is the least understood. However, activation of the Ets1 transcription factor has emerged as an important downstream regulator of angiogenic cell movement. Endothelial cell (EC) growth factors, such as vascular endothelial growth factor (VEGF) and fibroblast growth factor, specifically upregulate the activity of Ets1 in primary EC [2, 3] resulting in the activation of downstream target genes, such as metalloproteinases and vimentin [4] that are important for matrix degradation and cell migration [5]. In addition, antisense Ets1 can block the proangiogenic function of VEGF in cultured EC [2, 6, 7]. Thus, Ets1 appears to be a major regulator of migratory potential in response to chemotactic growth factors, a function that is also evolutionarily conserved [2, 8]
Increased angiogenesis is a hallmark of cutaneous wound healing and is necessary to support new tissue formation [9]. VEGF production is strongly upregulated in wound healing, secreted by activated macrophage and keratinocytes working in consort to stimulate new capillary production within the wounded area. Impairment of new vessel formation results in decreased wound healing abilities [10–12]. Concentrated efforts have been made to increase vascularization for tissue regeneration and repair of chronic, nonhealing ischemic wounds [13]. New therapies using recombinant growth factors or vascular progenitor cells to foster the formation of new blood vessels have been proposed, some of which are presently in phase II/III trials [14, 15].
The isolation and formulation of highly pure and homogenous pGlcNAc (poly-N-acetyl glucosamine nanofibers) from a marine diatom are presently used as a hemostatic agent in the clinical arena [16, 17]. Although the mechanism of action is not completely defined, recent data show that pGlcNAc fiber-treated platelets are fully activated. The consequence of this activation is a marked increase in the formation of a fibrin matrix [18]. Importantly, platelet activation by pGlcNAc fibers is mediated by their association with integrin β3 and activation of integrin-mediated signaling. Indeed, pGlcNAc fibers have been shown to bind integrins specifically in pull-down assays [19]. Recent findings show that treatment of cutaneous wounds with pGlcNAc fiber-derived membranes results in increased kinetics of wound healing that can be attributed, in part, by a marked increase in angiogenesis [20].
We set out to test whether the increase in angiogenesis resulting from treatment of cutaneous wounds with pGlcNAc fibers was due to a direct effect on EC per se and whether the effect of pGlcNAc was integrin and/or VEGF dependent. We found that pGlcNAc treatment, in the absence of growth factor or serum, induces EC motility and increases in vitro angiogenesis as measured by cord formation in Matrigel assays. pGlcNAc-induced cell motility is found to be integrin mediated and results in the activation of mitogen-acivated protein kinase (MAPK) and the increased expression of Ets1, VEGF and interleukin (IL)-1. The effect of pGlcNAc is not a consequence of its induction of VEGF; blockade of VEGF receptor (VEGFR) did not block the pGlcNAc-induction of Ets1. Importantly, we show that Ets1 is required for pGlcNAc-induced cell motility. Indeed, antibody blockade of integrin activation results in a decreased induction of Ets1 by pGlcNAc. Taken together, these findings position Ets1 as a downstream effector of integrin-mediated cell signaling in primary EC.
Materials and Methods
Tissue Culture, Growth Factors and Transfection
Pooled, multiple-donor human umbilical cord vein EC (Cambrex) were maintained at 37°C with 5% CO2 in endothelial basal medium 2 (Cambrex) supplemented with EC growth medium 2 SingleQuots as described by Cambrex procedures. Serum starvation was performed at 80–90% confluency in RPMI-1640 supplemented with 0.1% fetal calf serum (Gibco BRL) for 24 h followed by stimulation with VEGF165 (20 ng/ml, R&D Systems) or with highly purified pGlcNAc nanofibers in sterile water (provided by Marine Polymer Technologies, Inc., Danvers, Mass., USA) with the amounts indicated in the text. For inhibition using the VEGFR inhibitor SU5416 (10 μm; R&D Systems), cells were pretreated for 15 min prior to stimulation with VEGF or pGlcNAc as described in the text.
Human umbilical cord vein EC were transfected using the Amaxa nucleofector system in procedures described by the manufacturer, obtaining transfection efficiencies up to 80%. All transfections were monitored by expression of green fluorescent protein (GFP) using a GFP expression vector pFP-C1 (Clontech) or a GFP-directed RNA interference (RNAi; Amaxa). Plasmid-based RNAi directed specifically against Ets1 was purchased from Pandomics, Inc., and the dominant-negative Ets (dn-Ets) construct contains the DNA-binding domain of Ets2 cloned into a pcDNA3 expression vector.
Antibodies and Western Blot Analyses
The antibodies used for Western blot analysis are as follows: anti-p85 subunit of PI3K (Upstate Biotechnology), anti-phospho-specific VEGFR2 (Cell Signaling), VEGFR2 (Santa Cruz), anti-phospho-specific p42/p44 (Promega), anti-phospho-specific focal adhesion kinase (FAK) and anti-phospho-specific VEGFR2 (BD Biosciences, Inc.), anti-p42/p44 Erk1/2, anti-VEGFR2 and anti-Ets1 (Santa Cruz).
Treated cells were washed once with phosphate-buffered saline (PBS) and lysed in 1 × RIPA lysis buffer (50 mm Tris-HCl, pH 7.5, 1% Triton X-100, 150 mm NaCl, 0.1% SDS, 1% sodium deoxycholate, 40 mm NaF), supplemented with complete protease inhibitors without EDTA (Roche) and 200 μm sodium orthovanadate. Protein concentrations were determined by a bicinchoninic acid protein assay (Pierce) resolved by SDS-PAGE and transferred onto Immobilon-P polyvinylidene fluoride membranes (Millipore). Western analyses followed standard procedures [21]. Proteins were visualized using Luminol reagent (Santa Cruz).
Cell Motility and Proliferation Assays
For ‘scratch’ wound closure assays, EC were grown to confluence on plastic tissue culture dishes and a single ‘wound’ was created using a pipette tip. Cells were then incubated in serum starvation media supplemented with or without VEGF (20 ng/ml) or pGlcNAc at the amounts indicated in the text for 16–18 h. Cells were washed once with PBS, fixed for 10 min in methanol, stained with 0.1% crystal violet for 10 min and rinsed thoroughly with water. Wounding assays were photographed at 10 × magnification using an Olympic light microscope equipped with digital imaging, and distance migrated was measured.
For modified transwell assays, transfected or untransfected EC were plated onto 8-μm pore size invasion chambers precoated with fibronectin at 20 μg/μl (Sigma), 5 × 104 cells per chamber in 500 μl of serum starvation media, and 500 μl of starvation media was added to the well. VEGF (20 ng/ml) or pGlcNAc (at the concentrations indicated in the text) was added to the upper chamber. Cells were incubated for 12 h at 37°C in the presence of 5% CO2 Cells that did not migrate were removed by wiping the top of each membrane with a cotton swab. The migrated cells were fixed in methanol for 10 min and stained with 0.1 μg/ml ethidium bromide in PBS. Migrated cells were counted using a Leica fluorescence microscope. Each assay was performed in triplicate at least 3 independent times, and at least 6 fields per transwell were counted.
For in vitro angiogenesis assays, EC were plated onto reduced growth factor Matrigel matrix (BD Laboratory) at 1.6 × 104 cells/50 μl per well of a 96-well plate in serum starvation media in the presence or absence of VEGF (20 ng/ml) or pGlcNAc at the concentrations indicated in the text. Cord formation was assessed for up to 8 h after plating. Cells were fixed and photographed when the VEGF- and pGlcNAc-treated cells began to form cords, while the controls retained a single cell layer. Assays were performed in duplicate and repeated 2 independent times.
For cellular proliferation/viability assessment, 2 different assays were used: trypan blue exclusion by direct cell counts using a hemacytometer and an MTT [3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide] assay in procedures described by the manufacturer (Promega).
Antibody Blockade
To block integrin-mediated cell motility and signaling, EC were preincubated for 15 min with blocking antibodies, at concentrations empirically determined (1 μg/ml), directed against αVβ3 or α5β1 (CD49e) purchased from Chemicon International or against the α5 subunit (Santa Cruz) prior to stimulation with pGlcNAc. Normal rabbit serum was used as a negative control. For inhibition of cell migration using the αVβ3 antibody, transwells were precoated with vitronectin (Sigma) rather than with fibronectin (20 μg/μl).
Reverse Transcription Polymerase Chain Reaction
For semiquantitative reverse transcription polymerase chain reaction (RT-PCR), cDNA was synthesized from total RNA (2–5 μg), isolated using RNA-STAT 60 (Tel-Test, Inc.) in procedures described by the manufacturer, with a Superscript First-Strand Synthesis Kit purchased from Gibco BRL using oligo(dT) following the manufacturer's instructions. PCR reactions contained equal amounts of cDNA and 1.25 μm of the appropriate primer pair (Proligo, Inc.). The primer sequences are as follows: Ets1, forward 5′-TTCTCAGAGCCCAGCTTCAT-3′, reverse 5′-AAAGTTTGAATTCCCAGCCAT-3′; metallothionein 2A, forward 5′-CAACCTGTCCCGACTCTAGC-3′, reverse 5′-AGGAGCAACTCCTGTCCTGA-3′; S26, forward 5′-CTCCGGTCCGTGCCTCCAAG-3′, reverse 5′-CAGAGAATAGCCTGTCTTCAG-3′; VEGF, forward 5′-CTACCTCCACCATGCCAAGT-3′, reverse 5′-TGGTGATGTTGGCTCCTCA-3′; IL-1, forward 5′-CTGCGCCAACACAGAAATTA-3′, reverse 5′-ATTGCATCTGGCAACCCTAC-3′; IL-8, forward 5′-TCGGATTTCACGATTTCTCC-3′, reverse 5′-GCTACAAGTGCGTCGTCAAA-3′. Cycling conditions were: 94°C for 5 min; 20–35 cycles of 94°C for 1 min, 50–65°C (based on primer Tm) for 1 min, 72°C for 1 min and 45 s + 2 s/cycle; 72°C for 7 min and cooled to 4°C. The cycle number was empirically determined to be within the linear range of the assay for each primer pair used. All semiquantitative RT-PCRs were performed in tandem with S26 primers as an internal control. Products were run on 1–1.5% agarose gels (based on product size) and visualized on a BioRad Molecular Imaging System.
Real-time PCR was performed using a Brilliant CYBR green quantitative PCR (QPCR) kit in combination with an Mx3000P real-time PCR system, both purchased from Stratagene. Real time was performed at least in triplicate at least 2 independent times. Internal control primers that detect the ribosomal protein subunit S26 were used.
Results
pGlcNAc Induces EC Motility
A recently published article demonstrates that treatment of full-thickness cutaneous wounds in a diabetic mouse model (db/db) with pGlcNAc fiber-containing membranes results in an increased wound closure rate that is correlated with a striking rise in angiogenesis [20]. To test if pGlcNAc fibers had a direct effect on EC, serum-starved EC cells were treated with VEGF or with different concentrations of pGlcNAc fibers. As shown in figure 1a, at 48 h after serum starvation, as compared with the total number of cells plated (control), there is a 2-fold reduction in the number of cells. This decrease in cell number is rescued by the addition of VEGF or by the addition of pGlcNAc fibers at either 5 or 10 μg/ml. These results indicate that like VEGF, pGlcNAc fiber treatment prevents cell death induced by serum deprivation. However, pGlcNAc does not result in a higher metabolic rate as measured by MTT assays (data not shown), suggesting that this polymeric material is not causing marked increases in cellular proliferation but is rescuing cell death by serum deprivation.
Fig. 1.

pGlcNAc treatment prevents cell death and stimulates EC motility. a EC were serum starved for 48 h (SS-48) in the presence or absence of pGlcNAc (NAG), at the concentrations indicated, or VEGF (20 ng/ml). ‘Control’ indicates the number of cells plated. Cell counts were performed using a hemacytometer. b ‘Scratch’ wounding assays were performed under conditions of serum starvation (SS) in the presence or absence of VEGF (20 ng/ml) or pGlcNAc (NAG) at 5 and 10 μg/ml. Photographs (×10) were taken, and the distance migrated into the wound was calculated using 6 different fields, 3 independent times. c Quantitation of transwell assays of EC migrating toward fibronectin under conditions of serum starvation (SS) in the presence or absence of VEGF (20 ng/ml) or pGlcNAc (NAG) at the concentrations indicated or with the addition of both (VEGF at 20 ng/ml and pGlcNAc at 5 and 10 μg/ml). Each assay was performed in triplicate, 3 independent times. d EC were plated onto growth factor-reduced Matrigel-coated plates under serum-starved conditions and treated with or without VEGF (20 ng/ml) or pGlcNAc (NAG, 10 μg/ml) and assessed for induction of cord formation within 6 h after plating. Matrigel assays were performed in duplicate, at least 3 independent times.
To test whether pGlcNAc fiber treatment of EC resulted in changes in cell motility, the ‘scratch’ wound closure assay was used. Migration of the cells into the wounded area was significantly increased in the presence of pGlcNAc at both 5 and 10 μg/ml. Wound closure was similar to that observed for VEGF treatment (fig. 1b). These results indicated that pGlcNAc treatment resulted in an increase in EC movement. To determine whether this increased cell motility correlated with an increased cellular invasion, EC motility was measured using transwell assays where membranes were precoated with the extracellular matrix protein, fibronectin. As shown in figure 1c, pGlcNAc treatment resulted in a 3-fold increase in migration toward fibronectin that was enhanced by the addition of VEGF (4-fold).
Stimulation of cell migration is a prerequisite for increased angiogenesis. To test, in vitro, whether pGlcNAc was proangiogenic, Matrigel assays were performed. EC were plated on growth factor-reduced Matrigel under conditions of serum starvation and assessed for cord formation in the presence or absence of VEGF or pGlcNAc fibers within 6 h. As shown in figure 1d, both VEGF and pGlcNAc treatment resulted in increased cord formation on Matrigel. These results suggest that pGlcNAc is proangiogenic.
pGlcNAc Activation of MAPK and Ets1 and Increased VEGF Expression
Our findings suggest that pGlcNAc stimulates EC motility, invasion and cord formation in a similar fashion to VEGF. To test whether pGlcNAc treatment resulted in the activation of pathways previously shown downstream of VEGFR signaling, EC were treated with pGlcNAc fibers for 15 min and assessed for the activation of Erk1/2 MAPK. As shown in figure 2a, pGlcNAc treatment results in a marked increase in the phosphorylation of Erk1/2. Additionally, pGlcNAc treatment also stimulates the expression of an important regulator of EC movement, the Ets1 transcription factor and a downstream target gene, metallothionein 2A (fig. 2b). Real-time PCR indicates that Ets1 is induced approximately 2-fold by pGlcNAc treatment (fig. 2c). This increase in message is accompanied by a higher protein expression as shown in the Western blot analysis in figure 2d. These results suggest that pGlcNAc stimulates pathways similar to those activated by VEGF and with similar kinetics.
Fig. 2.

pGlcNAc treatment stimulates MAPK and Ets1 expression. a Western blot analysis of total protein isolated from serum-starved EC (SS) treated with VEGF (V, 20 ng/ml) or pGlcNAc (NAG,10 μg/ml) for 15 min. Blots were probed with antibodies against phosphorylated Erk1 and Erk2 (P-Erk1/2). Blots were reprobed with antibodies against total Erk1 and Erk2. b Semiquantitative RT-PCR of total RNA isolated from serum-starved EC (SS) treated with or without VEGF (V) or pGlcNAc (NAG, 10 μg/ml) for 3 h. Primers were directed against Ets1, metallothionein 2A (MT) and S26 as an internal control. c QPCR of total RNA as described in b and assessed for Ets1 expression. d Western blot analysis of total protein isolated from serum-starved EC (SS) treated with pGlcNAc (NAG, 5 and 10 μg/ml) for 3 h and probed using antibodies directed against Ets1 and the p85 subunit of PI3 kinase as an internal control.
To test whether pGlcNAc treatment induces the expression of growth factors or cytokines known to be secreted by activated EC, serum-starved EC were treated with pGlcNAc for 12 h and assessed for changes in expression of VEGF, IL-1 and IL-8. As shown by RT-PCR and QPCR (fig. 3a, b), pGlcNAc treatment results in an increased expression of both VEGF and IL-1. These findings also suggest that the response of the EC to pGlcNAc is specific since there is no change in expression of another interleukin, IL-8.
Fig. 3.

pGlcNAc activity is independent of its induction of VEGF expression. a Semiquantitative RT-PCR of total RNA isolated from serum-starved EC (SS) treated with or without pGlcNAc (NAG, 5 or 10 μg/ml) for 12 h. Primers were directed against VEGF, IL-1, IL-8 and S26. The graph represents QPCR confirming the pGlcNAc-induction of VEGF and IL-1. b QPCR of total RNA isolated from serum-starved cells (SS) either stimulated with VEGF (V, 20 ng/ml) or pGlcNAc (NAG, 10 μg/ml) following a 15-min pretreatment with SU5416 (10 μm) using primers directed against Ets1 and S26 as an internal control. QPCR was performed at least in duplicate, 2 independent times. c Western blot analysis of total protein isolated from serum-starved EC (SS) treated with VEGF (20 ng/ml) or pGlcNAc (NAG,10 μg/ml) for the times indicated. Blots were probed with antibodies against phosphorylated VEGFR2 (P-VEGFR2) followed by antibodies against total VEGFR2 and p85 as an internal control.
To test the pGlcNAc-dependent induction of Ets1 expression, a transcription factor known to be regulated by VEGF [2, 3], secondary to the pGlcNAc effect on VEGF expression, activation of VEGFR was blocked using the pharmacological inhibitor, SU5416, prior to treatment with pGlcNAc. As shown by QPCR (fig. 3b), treatment of EC with this inhibitor blocked the induction of Ets1 by VEGF but had no effect on the induction of Ets1 by pGlcNAc. To confirm that pGlcNAc did not activate the VEGFR, a series of Western blots were performed using an antibody directed against the phosphorylated form of VEGFR2. As shown in figure 3c, VEGF treatment causes a rapid phosphorylation of VEGFR, accompanied by turnover of total VEGFR2 protein levels, whereas pGlcNAc had no effect either at these early time points shown, or up to 6 h after treatment (data not shown). These results confirm that pGlcNAc activity is independent of signaling through the VEGFR.
pGlcNAc-Induced Cell Motility Requires Integrin
Our findings thus far indicate that the early effects of pGlcNAc, i.e. activation of MAPK and Ets1, are mediated through a VEGF-independent mechanism. Recent findings have shown that pGlcNAc fibers activate platelets by a mechanism requiring integrin stimulation. Absorption experiments have shown that pGLcNAc tightly binds to a specific subset of platelet surface proteins including the β3 integrin subunit [19] To test whether the effects of pGlcNAc were integrin dependent, blocking antibodies were used to disrupt integrin-mediated signaling in EC. The effect of these antibodies on pGlcNAc-induced cell migration was assessed using transwell assays. Figure 4a shows the results when using an antibody directed against αVβ3 integrin in migration assays toward vitronectin (the αVβ3 receptor) and figure 4b shows a similar experiment using an antibody directed against α5β1 (CD49e) in transwells coated with fibronectin. Antibody blockade of either integrin subtype results in inhibition of pGlcNAc-induced cell motility on their cognate substrates. These results suggest that pGlcNAc stimulates cell motility via integrin activation.
Fig. 4.

pGlcNAc-induced cell motility is integrin dependent. a Quantitation of the number of cells migrated through transwells coated with fibronectin after pretreatment with antibodies (ab) directed against αVβ3 or α5β1 (CD49e) for 15 min prior to stimulation with pGlcNAc (NAG, 10 μg/ml). SS = Serum-starved EC. b As in a, except the transwells were coated with vitronectin. Each assay was performed in triplicate, 3 independent times. c Western blot analysis of total protein isolated from serum-starved EC (SS) treated with pGlcNAc (NAG, 10 μg/ml) for the times indicated. Blots were probed with antibodies (ab) against phosphorylated FAK (P-FAK) and the p85 subunit of PI3 kinase as a loading control.
FAK becomes phosphorylated in response to integrin clustering and activation. FAK is a key regulator of integrin and growth factor-mediated cell motility and invasion. To test integrin activation by pGlcNAc, EC were treated with pGlcNAc fibers for increasing amounts of time and assayed for changes in the level of FAK phosphorylation. As shown in figure 4c, pGlcNAc treatment results in the phosphorylation of FAK within 15 min of treatment. These results suggest that pGlcNAc-induced cell motility may involve the activation of FAK via integrin engagement.
Cell Motility Induced by pGlcNAc Is Ets1 Dependent
Our findings thus far suggest that pGlcNAc promotes cell movement by an integrin-dependent pathway. Interestingly, pGlcNAc also induces the expression of the proangiogenic transcription factor, Ets1, independently of VEGF. To test whether Ets1 was required for the motility induced by pGlcNAc, Ets1 was inhibited using both a dominant-negative approach as well as by RNAi. A dn-Ets construct expressing the conserved Ets DNA binding domain was transfected into EC. After 24 h to allow for expression of the dn-Ets, the cells were assessed for changes in cell migration toward fibronectin in transwell assays after treatment with pGlcNAc. As shown in figure 5a (left side), inhibition of Ets1 activity as well as of other family members expressed in EC results in a marked decrease in EC migration in response to pGLcNAc. As a control for the activity of the dn-Ets, figure 5b shows that transfection of increasing amounts of dn-Ets results in a decreased total Ets1 protein. Ets1 expression can be controlled not only by another family member [22], but can also be autoregulated [23]. Inhibition of Ets1 specifically by autoregulated RNAi also results in a decrease in cell motility induced by pGlcNAc on fibronectin (fig. 5a, right side). As a control for the RNAi experiment, figure 5 c shows the resultant expression levels of Ets1 in EC transfected with 2 amounts of plasmid-containing RNAi directed against Ets1. Expression of dn-Ets resulted in a more substantial reduction in cell migration than the Ets1 RNAi, probably due to its blockade of other family members expressed in EC. These findings support a role for Ets1 in the induction of cell motility by pGlcNAc.
Fig. 5.
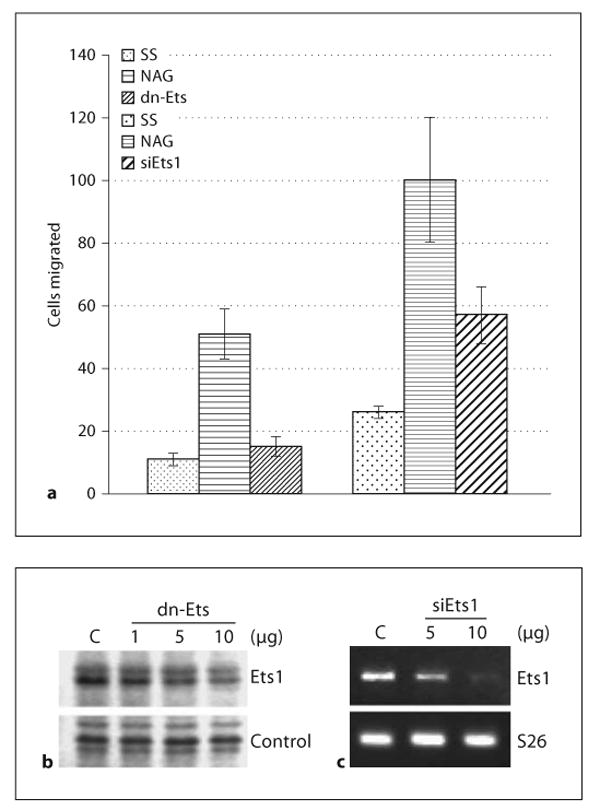
pGlcNAc-induced cell motility is Ets dependent. a Quantitation of the number of cells migrated through transwells coated with fibronectin after transfection with either dn-Ets or with Ets1 RNAi (siEts1), plated under serum-starved conditions (SS) and stimulated with pGlcNAc (NAG, 10 μg/ml). Each assay was performed in triplicate, 3 independent times. b Western blot analysis of Ets1 expression in cells transfected with increasing amounts of a dn-Ets expression plasmid. c Semiquantitative RT-PCR of total RNA isolated from cells transfected with 2 different amounts of Ets1 small interfering RNA (siEts1). S26 is used as a loading control. C = ■■■■.
A role for Ets1 in the transcriptional regulation of a number of integrin subunits has been described, positioning Ets1 upstream of integrins [24–27]. The finding that motility induced by pGlcNAc is dependent both on integrins and Ets1 implies that Ets1 may be regulated downstream of integrins. To confirm that integrin activation results in the regulation of Ets1 expression, blocking antibodies directed against α5β1 (the fibronectin receptor) or αVβ3 (the vitronectin receptor) were used to inhibit integrins by pGlcNAc. Figure 6a shows that antibody blockade of α5β1 integrin results in a reduction in pGlcNAc-induced Ets1 expression. This inhibition of Ets1 expression using a blockade of α5β1 integrin is recapitulated on the protein level (fig. 6b). However, although the αVβ3 integrin antibody blocked motility on vitronectin, it did not affect the pGlcNAc-induced expression of Ets1 (fig. 6), suggesting that pGlcNAc-induced cell motility on vitronectin may be Ets1 independent. Taken together, our results position Ets1 downstream of certain integrins in primary EC and suggest potential specificity in integrin signaling with respect to Ets1 expression in primary EC. These findings suggest that pGlcNAc can activate an integrin→Ets1 pathway leading to angiogenesis in a wound-healing model.
Fig. 6.

Ets1 expression is regulated by integrin activation. a Semiquantitative RT-PCR of total RNA isolated from serum-starved EC (SS) pretreated with or without antibodies directed against α5β1 or αVβ3 or with normal rabbit serum (NR) prior to stimulation with pGlcNAc (NAG, 10 μg/ml) and performed using primers directed against Ets1 or S26 as an internal control. b Western blot analysis of total protein from cells treated similarly to those in a and probed with an antibody directed against Ets1 and p85 as an internal control.
Discussion
Treatment of cutaneous wounds with a marine-derived nanofiber, pGlcNAc, results in increased wound closure that is correlated with a marked rise in angiogenesis. We set out to test whether this increased angiogenesis was due to a direct effect on EC per se. We found that in the absence of any exogenous growth factors, pGlcNAc induces EC motility and increases in vitro angiogenesis as measured by cord formation in Matrigel assays (fig. 1). This effect is mediated by early induction of an integrin-dependent pathway (fig. 3) that is independent of its ability to induce the expression of VEGF (fig. 3b, c). However, during prolonged treatment, pGlcNAc treatment causes an increased expression of both VEGF and IL-1 (fig. 3a) which likely contributes to the overall outcome of increased cell movement in vitro and increased wound healing in vivo. pGlcNAc treatment results in an upregulation of Ets1 expression and this induction is required for pGlcNAc-induced cell motility (fig. 5). Indeed, antibody blockade of integrin activation results in a decreased induction of Ets1 by pGlcNAc (fig. 6). Our findings support a model by which pGlcNAc stimulates EC motility through the activation of integrins and show that the proangiogenic transcription factor, Ets1, is downstream of α5β1 integrin (fibronectin receptor), suggesting a role for this pathway in wound-associated angiogenesis.
The increased population of aged individuals and the epidemic of diabetes have resulted in an intense interest in the development of new therapies for the treatment of ischemic or chronic wounds. One of the more fruitful methods of treatment has been to target angiogenesis, increasing blood vessel formation via delivery of certain growth factors or angiogenic precursors. Here, we describe the activity of a marine-derived polymeric material which effectively activates EC motility and has been efficacious in the treatment of cutaneous wounds in a model of delayed wound healing (db/db) [20]. This compound then provides a potential new avenue for treatment during wound healing.
Environmentally derived compounds have been used not only for their medicinal properties but also to uncover previously undefined cellular mechanisms. For example, use of fungal-derived antibiotics has lead to a greater understanding of the role of HSP90 in cell signaling and cancer [28–30]. Here, we show that pGlcNAc treatment stimulates integrins in the absence of exogenous growth factor and reduced serum. Integrin activation by this fiber material results in the induction of Ets1 expression. Integrin-dependent expression of Ets1 has not been described in EC, although a recent publication shows a link between integrins and Ets1 expression in myofibroblasts [31]. We have previously defined a pathway leading to Ets1 expression via VEGF and Akt activation in primary EC [2]. Although both VEGF and Ets1 are associated with changes in integrin expression and VEGFRs known to be modulated by integrin association, whether the VEGF→Akt pathway is impinged upon by integrin-mediated signaling remains to be determined.
This study suggests an important relationship between integrins and Ets1. The Ets family of transcription factors plays considerable roles in many processes and in many cell types. Ets1, in particular, controls the expression of a number of genes involved in angiogenesis [32], including cell survival [33] and cell motility [2, 34]. For example, Ets1 specifically regulates the expression of integrin genes [24, 35], matrix metalloproteinases required for matrix degradation [36, 37], as well as other endothelial specific genes such as vascular endothelial cadherin [38] and the Tie receptors [39, 40]. Importantly, Ets1 has been linked to the expression of VEGF [32] and IL-1 [41]. That some integrins can modulate the expression of this transcriptional regulator suggests a potentially more wide-ranging mechanism for the regulation of transcription factor circuitry in a broad range of cell types. Interestingly, our findings suggest that particular integrins, α5β1 in our studies, can activate the Ets1 pathway but that this phenomenon is not all-inclusive to the integrin family of proteins.
The mechanism by which pGlcNAc stimulates integrin-mediated motility and Ets1 expression remains to be determined. pGlcNAc could directly bind to the integrins, thus acting as a scaffold or mimicking appropriate extracellular matrix interactions. The pGlcNAc nanofibers consist of individual high molecular weight (approximately 3 million Da) polysaccharide polymers aligned in parallel to form a highly ordered and regular semicrystal-line structure. These fibers could act ‘extracellular matrix like’ to provide appropriate integrin/receptor-mediated interactions. In plasma- and platelet-derived extracts, pGlcNAc bind tightly to integrin proteins as well as to other, unidentified proteins [19]. Integrins can also be activated through associations with other cell surface molecules such as receptor tyrosine kinases [42–49] or possibly through adhesion proteins. pGlcNAc could be acting indirectly by receptor ‘clustering’, thus allowing activation of the integrin pathways. We have not ruled out the possibility that pGlcNAc may be stimulating the production of other growth factors or cytokines and may well be functioning also in this regard. However, we also show that pGlcNAc treatment does not activate the VEGFR, suggesting that the primary effect is probably on integrin activation and that any modulation of cytokine expression is probably a subsequent event.
We have shown that treatment of EC with a pure highly homogenous pGlcNAc nanofiber material shown to be efficacious in the treatment of wounds results in the stimulation of integrin-mediated cell motility and increases in vitro angiogenesis. These effects can be explained, at least in part, by the integrin-dependent regulation of the Ets1 transcription factor. Wound healing requires the coordinate interaction of numerous cell types and cytokines. That this material can stimulate wound healing and increased angiogenesis, at least in part by integrin engagement and by stimulating the expression of cytokines and growth factors, suggests its potential uses not only in a clinical arena but also as a tool to dissect the molecular mechanisms regarding cell-cell and cell-matrix interactions during the process of wound healing.
Acknowledgments
This work was supported by grants from the National Center for Research Resources (P20 RR16434, R01 HL84565) and from Marine Polymer Technologies to R.C.M. We wish to thank Vincent Dammai and Amy Bradshaw for their helpful suggestions.
References
- 1.Shiojima I, Walsh K. Role of Akt signaling in vascular homeostasis and angiogenesis. Circ Res. 2002;90:1243–1250. doi: 10.1161/01.res.0000022200.71892.9f. [DOI] [PubMed] [Google Scholar]
- 2.Lavenburg KR, Ivey J, Hsu T, Muise-Helmericks RC. Coordinated functions of Akt/PKB and ETS1 in tubule formation. FASEB J. 2003;17:2278–2280. doi: 10.1096/fj.03-0040fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Z, Fisher RJ, Riggs CW, Rhim JS, Lautenberger JA. Inhibition of vascular endothelial growth factor-induced endothelial cell migration by ETS1 antisense oligonucleotides. Cancer Res. 1997;57:2013–2019. [PubMed] [Google Scholar]
- 4.Sementchenko VI, Watson DK. Ets target genes: past, present and future. Oncogene. 2000;19:6533–6548. doi: 10.1038/sj.onc.1204034. [DOI] [PubMed] [Google Scholar]
- 5.Vu TH, Werb Z. Matrix metalloproteinases: effectors of development and normal physiology. Genes Dev. 2000;14:2123–2133. doi: 10.1101/gad.815400. [DOI] [PubMed] [Google Scholar]
- 6.Lelievre E, Mattot V, Huber P, Vandenbunder B, Soncin F. ETS1 lowers capillary endothelial cell density at confluence and induces the expression of VE-cadherin. Oncogene. 2000;19:2438–2446. doi: 10.1038/sj.onc.1203563. [DOI] [PubMed] [Google Scholar]
- 7.Mattot V, Vercamer C, Soncin F, Calmels T, Huguet C, Fafeur V, Vandenbunder B. Constitutive expression of the DNA-binding domain of Ets1 increases endothelial cell adhesion and stimulates their organization into capillary-like structures. Oncogene. 2000;19:762–772. doi: 10.1038/sj.onc.1203248. [DOI] [PubMed] [Google Scholar]
- 8.Hsu T, Schulz RA. Sequence and functional properties of Ets genes in the model organism Drosophila. Oncogene. 2000;19:6409–6416. doi: 10.1038/sj.onc.1204033. [DOI] [PubMed] [Google Scholar]
- 9.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 10.Galiano RD, Tepper OM, Pelo CR, Bhatt KA, Callaghan M, Bastidas N, Bunting S, Steinmetz HG, Gurtner GC. Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow-derived cells. Am J Pathol. 2004;164:1935–1947. doi: 10.1016/S0002-9440(10)63754-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong YK, Lange-Asschenfeldt B, Velasco P, Hirakawa S, Kunstfeld R, Brown LF, Bohlen P, Senger DR, Detmar M. VEGF-A promotes tissue repair-associated lymphatic vessel formation via VEGFR-2 and the alpha1beta1 and alpha2beta1 integrins. FASEB J. 2004;18:1111–1113. doi: 10.1096/fj.03-1179fje. [DOI] [PubMed] [Google Scholar]
- 12.Tonnesen MG, Feng X, Clark RA. Angiogenesis in wound healing. J Investig Dermatol Symp Proc. 2000;5:40–46. doi: 10.1046/j.1087-0024.2000.00014.x. [DOI] [PubMed] [Google Scholar]
- 13.Madeddu P. Therapeutic angiogenesis and vasculogenesis for tissue regeneration. Exp Physiol. 2005;90:315–326. doi: 10.1113/expphysiol.2004.028571. [DOI] [PubMed] [Google Scholar]
- 14.Yla-Herttuala S, Markkanen JE, Rissanen TT. Gene therapy for ischemic cardiovascular diseases: some lessons learned from the first clinical trials. Trends Cardiovasc Med. 2004;14:295–300. doi: 10.1016/j.tcm.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Katsube K, Bishop AT, Simari RD, Yla-Herttuala S, Friedrich PF. Vascular endothelial growth factor (VEGF) gene transfer enhances surgical revascularization of necrotic bone. J Orthop Res. 2005;23:469–474. doi: 10.1016/j.orthres.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 16.Hirsch JA, Reddy SA, Capasso WE, Linfante I. Non-invasive hemostatic closure devices: ‘patches and pads’. Tech Vasc Interv Radiol. 2003;6:92–95. doi: 10.1053/tvir.2003.36446. [DOI] [PubMed] [Google Scholar]
- 17.Palmer BL, Gantt DS, Lawrence ME, Rajab MH, Dehmer GJ. Effectiveness and safety of manual hemostasis facilitated by the Syvek-Patch with one hour of bedrest after coronary angiography using six-French catheters. Am J Cardiol. 2004;93:96–97. doi: 10.1016/j.amjcard.2003.08.077. [DOI] [PubMed] [Google Scholar]
- 18.Thatte HS, Zagarins S, Khuri SF, Fischer TH. Mechanisms of poly-N-acetyl glucosamine polymer-mediated hemostasis: platelet interactions. J Trauma. 2004;57:S13–S21. doi: 10.1097/01.ta.0000136743.12440.89. [DOI] [PubMed] [Google Scholar]
- 19.Fischer TH, Thatte HS, Nichols TC, Bender-Neal DE, Bellinger AD, Vournakis JN. Synergistic platelet integrin signaling and factor XII activation in poly-N-acetyl glucosamine fiber-mediated hemostasis. Biomaterials. 2005;26:5433–5443. doi: 10.1016/j.biomaterials.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 20.Pietramaggiori G, Yang HJ, Scherer SS, Kaipainene A, Chan RK, Alperovich M, Newalder J, Demcheva M, Vournakis JN, Valeri RC, Hechtman HB, Orgill DP. Effects of poly-N-acetyl glucosamine (pGlcNAc) patch on wound healing in db/db mice. J Trauma. doi: 10.1097/01.ta.0000244382.13937.a8. in press. [DOI] [PubMed] [Google Scholar]
- 21.Muise-Helmericks RC, Grimes HL, Bellacosa A, Malstrom SE, Tsichlis PN, Rosen N. Cyclin D expression is controlled post-transcriptionally via a phosphatdylinositol 3-kinase/Akt-dependent pathway. J Biol Chem. 1998;273:29864–29872. doi: 10.1074/jbc.273.45.29864. [DOI] [PubMed] [Google Scholar]
- 22.Sementchenko VI, Watson DK. Ets target genes: past, present and future. Oncogene. 2000;19:6533–6548. doi: 10.1038/sj.onc.1204034. [DOI] [PubMed] [Google Scholar]
- 23.Seth A, Papas TS. The c-ets-1 proto-oncogene has oncogenic activity and is positively autoregulated. Oncogene. 1990;5:1761–1767. [PubMed] [Google Scholar]
- 24.Oda N, Abe M, Sato Y. ETS-1 converts endothelial cells to the angiogenic phenotype by inducing the expression of matrix metalloproteinases and integrin beta3. J Cell Physiol. 1999;178:121–132. doi: 10.1002/(SICI)1097-4652(199902)178:2<121::AID-JCP1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 25.Tajima A, Miyamoto Y, Kadowaki H, Hayashi M. Mouse integrin alphaV promoter is regulated by transcriptional factors Ets and Sp1 in melanoma cells. Biochim Biophys Acta. 2000;1492:377–384. doi: 10.1016/s0167-4781(00)00121-4. [DOI] [PubMed] [Google Scholar]
- 26.Lu N, Heuchel R, Barczyk M, Zhang WM, Gullberg D. Tandem Sp1/Sp3 sites together with an Ets-1 site cooperate to mediate alpha11 integrin chain expression in mesenchymal cells. Matrix Biol. 2006;25:118–129. doi: 10.1016/j.matbio.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Rosen GD, Barks JL, Iademarco MF, Fisher RJ, Dean DC. An intricate arrangement of binding sites for the Ets family of transcription factors regulates activity of the alpha 4 integrin gene promoter. J Biol Chem. 1994;269:15652–15660. [PubMed] [Google Scholar]
- 28.Neckers L, Schulte TW, Mimnaugh E. Geldanamycin as a potential anti-cancer agent: its molecular target and biochemical activity. Invest New Drugs. 1999;17:361–373. doi: 10.1023/a:1006382320697. [DOI] [PubMed] [Google Scholar]
- 29.Richter K, Buchner J. Hsp90: chaperoning signal transduction. J Cell Physiol. 2001;188:281–290. doi: 10.1002/jcp.1131. [DOI] [PubMed] [Google Scholar]
- 30.Scheibel T, Buchner J. The Hsp90 complex – a super-chaperone machine as a novel drug target. Biochem Pharmacol. 1998;56:675–682. doi: 10.1016/s0006-2952(98)00120-8. [DOI] [PubMed] [Google Scholar]
- 31.Znoyko I, Trojanowska M, Reuben A. Collagen binding alpha2beta1 and alpha1beta1 integrins play contrasting roles in regulation of Ets-1 expression in human liver myofibroblasts. Mol Cell Biochem. 2006;282:89–99. doi: 10.1007/s11010-006-1400-0. [DOI] [PubMed] [Google Scholar]
- 32.Hashiya N, Jo N, Aoki M, Matsumoto K, Nakamura T, Sato Y, Ogata N, Ogihara T, Kaneda Y, Morishita R. In vivo evidence of angiogenesis induced by transcription factor Ets-1: Ets-1 is located upstream of angiogenesis cascade. Circulation. 2004;109:3035–3041. doi: 10.1161/01.CIR.0000130643.41587.DB. [DOI] [PubMed] [Google Scholar]
- 33.Zhang C, Kavurma MM, Lai A, Khachigian LM. Ets-1 protects vascular smooth muscle cells from undergoing apoptosis by activating p21WAF1/Cip1: ETS-1 regulates basal and inducible p21WAF1/Cip: ETS-1 regulates basal and inducible p21WAF1/Cip1 transcription via distinct cis-acting elements in the p21WAF/Cip1 promoter. J Biol Chem. 2003;278:27903–27909. doi: 10.1074/jbc.M304328200. [DOI] [PubMed] [Google Scholar]
- 34.Fafeur V, Tulasne D, Queva C, Vercamer C, Dimster V, Mattot V, Stehelin D, Desbiens X, Vandenbunder B. The ETS1 transcription factor is expressed during epithelial-mesenchymal transitions in the chick embryo and is activated in scatter factor-stimulated MDCK epithelial cells. Cell Growth Differ. 1997;8:655–665. [PubMed] [Google Scholar]
- 35.Davidson B, Goldberg I, Gotlieb WH, Kopolovic J, Risberg B, Ben-Baruch G, Reich R. Coordinated expression of integrin subunits, matrix metalloproteinases (MMP), angiogenic genes and Ets transcription factors in advanced-stage ovarian carcinoma: a possible activation pathway? Cancer Metastasis Rev. 2003;22:103–115. doi: 10.1023/a:1022272204045. [DOI] [PubMed] [Google Scholar]
- 36.Rutter JL, Mitchell TI, Buttice G, Meyers J, Gusella JF, Ozelius LJ, Brinckerhoff CE. A single nucleotide polymorphism in the matrix metalloproteinase-1 promoter creates an Ets binding site and augments transcription. Cancer Res. 1998;58:5321–5325. [PubMed] [Google Scholar]
- 37.Watabe T, Yoshida K, Shindoh M, Kaya M, Fujikawa K, Sato H, Seiki M, Ishii S, Fujinaga K. The Ets-1 and Ets-2 transcription factors activate the promoters for invasion-associated urokinase and collagenase genes in response to epidermal growth factor. Int J Cancer. 1998;77:128–137. doi: 10.1002/(sici)1097-0215(19980703)77:1<128::aid-ijc20>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 38.Lionneton F, Lelievre E, Baillat D, Stehelin D, Soncin F. Characterization and functional analysis of the p42Ets-1 variant of the mouse Ets-1 transcription factor. Oncogene. 2003;22:9156–9164. doi: 10.1038/sj.onc.1207241. [DOI] [PubMed] [Google Scholar]
- 39.Hegen A, Koidl S, Weindel K, Marme D, Augustin HG, Fiedler U. Expression of angiopoietin-2 in endothelial cells is controlled by positive and negative regulatory promoter elements. Arterioscler Thromb Vasc Biol. 2004;24:1803–1809. doi: 10.1161/01.ATV.0000140819.81839.0e. [DOI] [PubMed] [Google Scholar]
- 40.Iljin K, Dube A, Kontusaari S, Korhonen J, Lahtinen I, Oettgen P, Alitalo K. Role of Ets factors in the activity and endothelial cell specificity of the mouse Tie gene promoter. FASEB J. 1999;13:377–386. doi: 10.1096/fasebj.13.2.377. [DOI] [PubMed] [Google Scholar]
- 41.Redlich K, Kiener HP, Schett G, Tohidast-Akrad M, Selzer E, Radda I, Stummvoll GH, Steiner CW, Groger M, Bitzan P, Zenz P, Smolen JS, Steiner G. Overexpression of transcription factor Ets-1 in rheumatoid arthritis synovial membrane: regulation of expression and activation by interleukin-1 and tumor necrosis factor alpha. Arthritis Rheum. 2001;44:266–274. doi: 10.1002/1529-0131(200102)44:2<266::AID-ANR43>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 42.Cascone I, Napione L, Maniero F, Serini G, Bussolino F. Stable interaction between alpha5beta1 integrin and Tie2 tyrosine kinase receptor regulates endothelial cell response to Ang-1. J Cell Biol. 2005;170:993–1004. doi: 10.1083/jcb.200507082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Le Boeuf F, Houle F, Huot J. Regulation of vascular endothelial growth factor receptor 2-mediated phosphorylation of focal adhesion kinase by heat shock protein 90 and Src kinase activities. J Biol Chem. 2004;279:39175–39185. doi: 10.1074/jbc.M405493200. [DOI] [PubMed] [Google Scholar]
- 44.Masson-Gadais B, Houle F, Laferriere J, Huot J. Integrin alphavbeta3, requirement for VEGFR2-mediated activation of SAPK2/p38 and for Hsp90-dependent phosphorylation of focal adhesion kinase in endothelial cells activated by VEGF. Cell Stress Chaperones. 2003;8:37–52. doi: 10.1379/1466-1268(2003)8<37:ivrfva>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ling Y, Maile LA, Clemmons DR. Tyrosine phosphorylation of the beta3-subunit of the alphaVbeta3 integrin is required for membrane association of the tyrosine phosphatase SHP-2 and its further recruitment to the insulin-like growth factor I receptor. Mol Endocrinol. 2003;17:1824–1833. doi: 10.1210/me.2003-0143. [DOI] [PubMed] [Google Scholar]
- 46.Schneller M, Vuori K, Ruoslahti E. Alphav-beta3 integrin associates with activated insulin and PDGFbeta receptors and potentiates the biological activity of PDGF. EMBO J. 1997;16:5600–5607. doi: 10.1093/emboj/16.18.5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Falcioni R, Antonini A, Nistico P, Di Stefano S, Crescenzi M, Natali PG, Sacchi A. Alpha 6 beta 4 and alpha 6 beta 1 integrins associate with ErbB-2 in human carcinoma cell lines. Exp Cell Res. 1997;236:76–85. doi: 10.1006/excr.1997.3695. [DOI] [PubMed] [Google Scholar]
- 48.DeMali KA, Balciunaite E, Kazlauskas A. Integrins enhance platelet-derived growth factor (PDGF)-dependent responses by altering the signal relay enzymes that are recruited to the PDGF beta receptor. J Biol Chem. 1999;274:19551–19558. doi: 10.1074/jbc.274.28.19551. [DOI] [PubMed] [Google Scholar]
- 49.Wang Y, Miao H, Li S, Chen KD, Li YS, Yuan S, Shyy JY, Chien S. Interplay between integrins and FLK-1 in shear stress-induced signaling. Am J Physiol Cell Physiol. 2002;283:C1540–C1547. doi: 10.1152/ajpcell.00222.2002. [DOI] [PubMed] [Google Scholar]


