Abstract
Study Objective
To evaluate the pharmacokinetic compatibility of a ritonavir-boosted indinavir-fosamprenavir combination among patients with human immunodeficiency virus (HIV).
Design
Single-center, nonrandomized, prospective, multiple-dose, two-phase pharmacokinetic study.
Setting
University research center.
Patients
Eight adult patients with HIV infection who had been receiving and tolerating indinavir 800 mg–ritonavir 100 mg twice/day for at least 2 weeks.
Intervention
After 12-hour pharmacokinetic sampling was performed on all patients (period A), fosamprenavir (a prodrug of amprenavir) 700 mg twice/day was coadministered for 5 days, with a repeat 12-hour pharmacokinetic sampling performed on the fifth day (period B).
Measurements and Main Results
Pharmacokinetic parameters were determined for indinavir, ritonavir, and amprenavir: area under the concentration-time curve from time 0 to 12 hours after dosing (AUC0–12), maximum plasma concentration (Cmax), and 12-hour plasma concentration (C12). For each parameter, the geometric mean, as well as the geometric mean ratio (GMR) comparing period B with period A, were calculated. Indinavir Cmax was lowered by 20% (GMR 0.80, 95% confidence interval [CI] 0.67–0.96), AUC0–12 was lowered by 6% (GMR 0.94, 95% CI 0.74–1.21), and C12 was increased by 28% (GMR 1.28, 95% CI 0.78–2.10). Ritonavir AUC0–12 was 20% lower (GMR 0.80, 95% CI 0.54–1.19), Cmax was 15% lower (GMR 0.85, 95% CI 0.55–1.32), and C12 was 7% lower (GMR 0.93, 95% CI 0.49–1.76). With the exception of indinavir Cmax, the changes in indinavir and ritonavir pharmacokinetic parameters observed after fosamprenavir coadministration were not statistically significant. The geometric means of amprenavir AUC0–12, Cmax, and C12 were 41,517 ng•hour/ml (95% CI 30,317–56,854 ng•hr/ml), 5572 ng/ml (95% CI 4330–7170 ng/ml), and 2421 ng/ml (95% CI 1578–3712 ng/ml), respectively.
Conclusion
The combination of indinavir 800 mg–ritonavir 100 mg–fosamprenavir 700 mg twice/day appears to be devoid of a clinically significant drug-drug interaction and should be evaluated as an alternative regimen in salvage HIV treatment. This combination may be suitable as part of a background regimen to optimize the therapeutic benefit of newer classes of antiretroviral agents such as the integrase and coreceptor inhibitors in the treatment of multidrug-resistant viruses.
Keywords: antiretroviral pharmacokinetics, ritonavir-boosted double–protease inhibitor combination, indinavir, fosamprenavir
Constructing an effective therapeutic regimen for antiretroviral-experienced patients with persistent viremia despite ongoing therapy is a nagging challenge in the management of human immunodeficiency virus (HIV) infection. Although several new classes of antiretroviral agents with activities against multidrug-resistant HIV-1 viral strains are in the developmental pipeline, the antiviral activities of these newer drugs are optimal in the presence of an active background antiretroviral therapy.1, 2 Therefore, the need exists to continue to identify combinations of agents among currently approved antiretroviral drugs that are pharmacokinetically compatible, safe, and potentially active against multidrug-resistant HIV strains.
Ritonavir-boosted dual–protease inhibitor regimens are used as a component of a salvage combination therapy.3-6 By inhibiting the cytochrome P450 (CYP) 3A4 isoenzyme, ritonavir enhances the pharmacokinetics of protease inhibitors to levels that could produce sustained virologic suppression for viral strains with moderate phenotypic resistance similar to ritonavir pharmacoenhancement in the naïve setting.7, 8 Furthermore, pharmacoenhancement of two or more active protease inhibitors with nonoverlapping resistance mutation patterns may result in a higher genetic barrier to resistance and possibly synergistic activity against HIV. For these reasons, a ritonavir-enhanced indinavir-amprenavir combination appears attractive for salvage therapy in HIV infection. Fosamprenavir, a prodrug of amprenavir, is rapidly hydrolyzed to its parent form by cellular phosphatases in the gut epithelium during absorption.9 Not only are the plasma concentrations of indinavir and amprenavir enhanced by ritonavir,10 the two agents have nonoverlapping primary resistance patterns.11, 12 However, our knowledge of limiting drug-drug interactions that occur with a number of boosted double–protease inhibitor combinations13-15 makes it essential to evaluate the pharmacokinetics of these regimens before their clinical use.
In this study, we evaluated the pharmacokinetic interaction of a ritonavir-boosted indinavir-fosamprenavir combination among HIV-infected patients. If pharmacokinetically compatible, a ritonavir-boosted indinavir-fosamprenavir regimen could be a suitable adjunct in salvage therapy of highly treatment-experienced HIV-infected patients with multidrug-resistant viruses.
Methods
Study Population
Patients with HIV who were 18 years or older and taking and tolerating an antiretroviral regimen that contained indinavir 800 mg plus ritonavir 100 mg twice/day for at least 2 weeks before enrollment were eligible. There were no restrictions on CD4+ T-cell count or HIV viral load. Patients were excluded if they had significant abnormalities in laboratory parameters, if they were using any CYP inhibitors or inducers other than ritonavir or indinavir, or if they were pregnant or breastfeeding. Patients were recruited from the Grady Health System Infectious Diseases Clinic, Atlanta, Georgia. All patients provided written informed consent before undergoing any study procedures. This study was designed according to the ethical guidelines for human studies and approved by the Emory University Institutional Review Board and Grady Health System Research Oversight Committee.
Intervention and Pharmacokinetic Design
This was a single-center, nonrandomized, prospective, multiple-dose, two-phase pharmacokinetic study. At enrollment, demographic and baseline clinical laboratory data were obtained. Adherence counseling was provided to each patient at study entry. In addition, pill counts for study drugs were conducted. Within 30 days of enrollment, patients underwent intensive blood sampling for baseline indinavir and ritonavir pharmacokinetic profiles at the General Clinical Research Center (GCRC). During that GCRC visit, observed doses of indinavir 800 mg–ritonavir 100 mg plus background nucleoside reverse transcriptase inhibitors (NRTIs) were administered orally with a standard breakfast. Serial blood samplings were obtained at time 0 (before dosing) and again at 1, 2, 3, 4, 6, 8, 10, and 12 hours after dosing.
After the pharmacokinetic sampling was performed, fosamprenavir 700 mg twice/day for 5 days was added to patients’ antiretroviral regimens, and adherence was reemphasized. The duration of fosamprenavir was limited to 5 days for a number of reasons. First, given fosamprenavir’s half-live of about 7.7 hours,9 plasma steady-state concentrations for fosamprenavir will be achieved within this exposure period. Second, patients were already treated with indinavir-ritonavir for at least 2 weeks before pharmacokinetic sampling; therefore, we presumed that the inhibitory effect of ritonavir on the CYP enzyme system would have attained steady state. Finally, fosamprenavir has previously been shown to significantly interact negatively with lopinavir-ritonavir, and vice versa,15 raising the concern that a similar interaction could occur with indinavir-ritonavir, compromising virologic suppression in this cohort of antiretroviral-treated patients.
On the fifth day of fosamprenavir, a second 12-hour GCRC visit was scheduled. An observed dose of fosamprenavir 700 mg with indinavir 800 mg–ritonavir 100 mg plus background NRTIs was administered orally with a standard breakfast. Serial blood samplings at time 0 and again at 1, 2, 3, 4, 6, 8, 10, and 12 hours after dosing were obtained.
Blood samples collected during both GCRC visits were kept on ice until processed (within 60 min of collection). Plasma was separated by centrifugation at 900 x g for 10 minutes, transferred to a polypropylene cryovial, and kept frozen at −70°C until analysis.
Analytic Methods
Bioanalysis of collected plasma samples was performed in the antiviral pharmacology laboratory at the University of Alabama at Birmingham. Plasma concentrations of indinavir, amprenavir, and ritonavir were quantitatively determined with sensitive and validated reverse-phase high-performance liquid chromatography (HPLC) and ultraviolet detection. Briefly, a rapid, sensitive, and specific HPLC assay was used for the simultaneous quantification of multiple antiretroviral agents in 200 μl of human plasma. Analytes included protease inhibitors (indinavir, amprenavir, saquinavir, atazanavir, ritonavir, lopinavir, and nelfinavir) and efavirenz. After liquid-liquid extraction in 2 ml of methyl tert-butyl ether at basic pH, samples were separated by means of reverse-phase liquid chromatography on a YMC C8 (4.6 × 100 mm, 3 μm) analytic column under isocratic conditions (55% 20 mmol/L sodium acetate, pH 4.88, 45% acetonitrile), with a total run time of 25 minutes. Ultraviolet detection at 212 nm provides adequate sensitivity with minimal interference from endogenous matrix components. The assay is linear over a concentration range of 25–20,000 ng/ml. Inter- and intraday accuracy and precision were less than 10%.
Pharmacokinetic Parameter Estimation
Pharmacokinetic parameters of indinavir, ritonavir, and amprenavir were determined by using noncompartmental methods (WinNonlin version 5.1; Pharsight Corp., Mountain View, CA). Calculated pharmacokinetic parameters were area under the concentration-time curve over the dosing interval (immediately before dosing [time 0] to 12 hours after dosing; AUC0–12), maximum plasma concentration (Cmax), time to Cmax (Tmax), 12-hour plasma concentration (C12), oral clearance (Cl/F, where F is bioavailability), terminal apparent distribution volume (Vz/F), and elimination half-life (t1/2). The AUC0–12 was determined by using the linear-log trapezoidal rule; Cmax, C12, and Tmax were taken directly from the observed concentrationtime data. The Cl/F was calculated as dose/AUC0–12. The Vz/F was calculated as dose divided by the product of the elimination rate constant (λz) and AUC0–12. The λz was determined by linear regression of the terminal elimination phase concentration-time points; t1/2 was calculated as ln(2)/λz. Measured samples below the assay limit of quantitation (25 ng/ml) were treated as 12.5 ng/ml. Regression analysis was used to estimate the 12-hour concentration if the measured value was below the assay limit of quantitation.
Statistical Analysis
Baseline demographic and clinical characteristics were summarized by descriptive statistics. Before statistical analysis, the values for the pharmacokinetic parameters (AUC0–12, Cmax, C12, and Cl/F) were logarithmically transformed (natural log). Pharmacokinetic parameters were summarized according to treatment periods: period A was indinavir-ritonavir only, and period B was indinavir-ritonavir plus fosamprenavir. The geometric mean for periods A and B, the geometric mean ratio (GMR) comparing the test period (period B) with the the reference period (period A), and their respective 95% confidence intervals (CIs) were calculated for each pharmacokinetic parameter. A paired t test was used to test the equality of the means between the two treatment periods for each pharmacokinetic parameter. By contrast, median with minimum and maximum were reported for two time variables (Tmax and t1/2), and Wilcoxon signed rank test was used to test the equality of median times between period A and period B. Repeated-measures analyses for log-transformed indinavir and ritonavir concentrations were performed with a means model using PROC MIXED software version 9 (SAS Institute, Cary, NC), providing separate estimates of the mean by sampling time (0, 1, 2, 3, 4, 6, 8, 10, and 12 hrs) and by period (A or B). A compound symmetry variance-covariance form in repeated measurements was assumed for each outcome. The estimated means and standard errors are reported based on back transformation of the log-transformed values to the usual arithmetic scale.
The sample size calculations for this study were based on the AUC0–24 outcome reported previously.16 The geometric mean (minimum and maximum) AUC0–24 at a dosage of indinavir 800 mg–ritonavir 100 mg every 12 hours was 142.5 mg•hour/L (112.8 and 239.4 mg•hr/L).16 Assuming AUC is normally distributed, the estimated standard deviation (SD) of the mean indinavir AUC0–24 is approximately 32 mg•hour/L. The outcome of interest in this study was the mean difference (period B minus period A) in indinavir AUC0–12, and we assumed a 30% difference in this parameter between the two periods to be clinically significant (i.e., 142.5 mg•hr/L in period A and 100 mg•hr/L in period B). A sample size of eight patients achieves greater than 90% statistical power to detect a mean difference in indinavir AUC (period B minus period A) of 42.5 mg•hour/L between the null hypothesis mean of 0.0 and the alternative hypothesis mean difference of 42.5 mg•hour/L with an estimated SD of 32 mg•hour/L and with a significance level (α) of 0.05, using a two-sided one-sample t test (PASS 2005 one-sample t test module software; NCSS, Kaysville, UT).
After data were collected on eight patients, additional power calculations were performed based on the following descriptive statistics: indinavir AUC0–12 (ng•hr/ml) period A (indinavir-ritonavir alone – mean = 48,171 ng•hr/ml, SD = 14,948 ng•hr/ml); period B (indinavir-ritonavir-fosamprenavir – mean = 45,369 ng•hr/ml, SD = 13,643 ng•hr/ml). The important statistic for sample size calculations is the SD for the mean difference, which was estimated from the data as approximately 12,000 ng•hour/ml. A sample size of eight patients achieves 81% statistical power to detect a mean difference of approximately 30% in AUC0–12 (period B minus period A) of 14,000 ng•hour/ml between the null hypothesis mean of 0.0 and the alternative hypothesis mean difference of 14,000 ng•hour/ml with an estimated SD of 12,000 ng•hour/ml and with a significance level (α) of 0.05 using a two-sided one-sample t test. Since the observed mean difference in indinavir AUC0–12 was much smaller than 30%, no additional patients were enrolled.
Results
Baseline demographic and clinical data are summarized in Table 1.
Table 1.
Baseline Demographic and Clinical Data for the Eight Study Patients
| Characteristic | Value |
|---|---|
| No. (%) of Patients | |
| Sex | |
| Male | 5 (62) |
| Female | 3 (38) |
| Race | |
| African-American | 7 (88) |
| Caucasian | 1 (12) |
| NRTI background | |
| Zidovudine | 6 (75) |
| Stavudine | 1 (12) |
| Lamivudine | 8 (100) |
| Tenofovir | 1 (12) |
|
| |
| Median (25th–75th percentile) | |
|
| |
| Age (yrs) | 43 (39.5–54.5) |
| Weight (kg) | 68.6 (62.7–87.1) |
| HIV-RNA (copies/ml) | 80 (50–1205) |
| CD4+ T-cell count (cells/mm3) |
214 (140–469) |
| Creatinine clearance (ml/min)a |
86.2 (69.7–104) |
| Alanine aminotransferase level (mg/dl) |
15 (13–21.5) |
| Aspartate aminotransferase level (mg/dl) |
25 (19.5–28.5) |
NRTI = nucleoside reverse transcriptase inhibitor; HIV = human immunodeficiency virus.
aCalculated by using the Cockcroft-Gault equation.
Pharmacokinetics
Results of pharmacokinetic analyses are summarized in Figures 1 and 2, and Tables 2 and 3. Comparison of the concentration-time plots for indinavir and ritonavir when indinavir-ritonavir was administered alone (period A) or in combination with fosamprenavir (period B) revealed that mean indinavir and ritonavir concentrations changed in significantly different ways across time (p<0.0001, test for interaction between period and sampling time; Figure 1). Mean indinavir concentrations were similar in both periods at baseline; however, at about the Tmax of indinavir, the mean concentration became significantly higher in period A (indinavir without fosamprenavir) at 2 hours (p<0.0001) and at 3 hours (p=0.05). No significant differences were seen in indinavir concentrations after 3 hours. Similarly, mean ritonavir concen-trations were similar in both periods at baseline but became significantly higher in period A (ritonavir without fosamprenavir) at 2 hours (p=0.005). No significant differences were seen in ritonavir concentrations after 2 hours.
Figure 1.

Effect of fosamprenavir on steady-state geometric mean (± standard error of the mean) indinavir and ritonavir concentrations in the eight study patients.
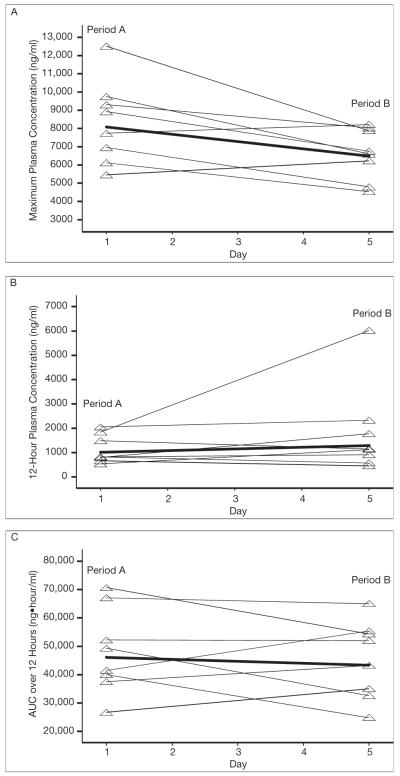
Figure 2.
Pharmacokinetic parameters for indinavir during periods A and B. Bold lines denote the geometric means; the other lines connect the period A and B measurements for the eight study patients. The p values for the comparison of the geometric mean change from period A to period B were as follows: p=0.02 for maximum plasma concentration (A), p=0.30 for 12-hour plasma concentration (B), and p=0.60 for area under the concentration-time curve over 12 hours (C).
Table 2.
Steady-State Indinavir Pharmacokinetic Parameters After Multiple Oral Doses of Indinavir-Ritonavir Alone and in Combination with Fosamprenavir in the Eight Study Patients
|
Parameter |
Period A (indinavir-ritonavir), Geometric Mean (95% CI) |
Period B (indinavir-ritonavir-fosamprenavir), Geometric Mean (95% CI) |
Period B vs Period A, Geometric Mean Ratio (95% CI) |
|---|---|---|---|
| AUC0–12 (ng•hr/ml) | 46,125 (35,333–60,213) | 43,425 (33,088–56,992) | 0.94 (0.74–1.21) |
| Cmax (ng/ml) | 8087 (6453–10,135) | 6501 (5380–7856) | 0.80a (0.67–0.96) |
| C12 (ng/ml) | 1011 (667–1533) | 1295 (653–2568) | 1.28 (0.78–2.10) |
| Cl/F (L/hr) | 15.6 (11.9–20.5) | 16.3 (11.8–22.4) | 1.04 (0.78–1.38) |
|
| |||
| Median (range) | Median (range) | ||
|
| |||
| Tmax (hrs) | 2.0 (1.0–3.0) | 3.0 (0.0–4.0) | |
| t½ (hrs) | 3.0 (2.3–4.5) | 2.3 (1.3–4.5) | |
CI = confidence interval; AUC0–12 = area under the concentration-time curve over the dosing interval of time 0–12 hours after dosing; Cmax = maximum plasma concentration; C12 = 12-hour plasma concentration; Cl/F = oral clearance, where F = bioavailability; Tmax = time to Cmax; t½ = half-life.
Statistically significant using the paired t test (p=0.02).
Table 3.
Steady-State Ritonavir and Amprenavir Pharmacokinetic Parameters After Multiple Oral Doses of Indinavir-Ritonavir Alone and in Combination with Fosamprenavir in the Eight Study Patients
| Parameter | Ritonavir, Period A (indinavir-ritonavir), Geometric Mean (95% CI) |
Ritonavir, Period B (indinavir-ritonavir-fosamprenavir), Geometric Mean (95% CI) |
Ritonavir, Period B vs Period A, Geometric Mean Ratio (95% CI) |
Amprenavir, Period B (indinavir-ritonavir-fosamprenavir), Geometric Mean (95% CI) |
|---|---|---|---|---|
| AUC0–12 (ng•hr/ml) | 13,162 (7404–23,397) | 10,582 (6282–17,826) | 0.80 (0.54–1.19) | 41,517 (30,317–56,854) |
| Cmax (ng/ml) | 1860 (1067–3241) | 1586 (977–2575) | 0.85 (0.55–1.32) | 5572 (4330–7170) |
| C12 (ng/ml) | 420 (233–756) | 392 (169–910) | 0.93 (0.49–1.76) | 2421 (1578–3712) |
| Cl/F (L/hr) | 6.4 (3.6–11.3) | 7.9 (4.4–14.3) | 1.24 (0.80–1.91) | 10.8 (7.6–15.5) |
|
| ||||
| Median (range) | Median (range) | Median (range) | ||
|
| ||||
| Tmax (hrs) | 3.5 (1.0–4.0) | 4.0 (0.0–12.0) | 1.5 (1.0–12.0) | |
| t1/2 (hrs) | 3.9 (3.0–5.9) | 3.1 (2.1–4.7) | 6.2 (3.4–13.2) | |
CI = confidence interval; AUC0–12 = area under the concentration-time curve over the dosing interval of time 0–12 hours after dosing; Cmax = maximum plasma concentration; C12 = 12-hour plasma concentration; Cl/F = oral clearance, where F = bioavailability; Tmax = time to Cmax; t1/2 = half-life.
Although indinavir AUC0–12 was reduced by 6% (GMR 0.94, 95% CI 0.74–1.21) and the C12 was increased by 28% (GMR 1.28, 95% CI 0.78–2.10) after fosamprenavir coadministration, these differences were not statistically significant (Table 2). However, a 20% reduction in indinavir Cmax with the 95% CI not including the significance boundary of 1 was observed (GMR 0.80, 95% CI 0.67–0.96), indicating that Cmax was significantly reduced by fosamprenavir coadministration (Table 2).
Ritonavir AUC0–12 was 20% lower (GMR 0.80, 95% CI 0.54–1.19), Cmax was 15% lower (GMR 0.85, 95% CI 0.55–1.32), and C12 was 7% lower (GMR 0.93, 95% CI 0.49–1.76) in the presence of fosamprenavir (Table 3). These differences observed in ritonavir pharmacokinetic parameters between the two periods were not statistically significant.
Indinavir and ritonavir Tmax, t1/2, and Cl/F were comparable between the two periods (Tables 2 and 3). At period B, the geometric means of amprenavir AUC0–12 , Cmax, and C12 were 41,517 ng•hour/ml, 5572 ng/ml, and 2421 ng/ml, respectively (Table 3).
Discussion
This pilot pharmacokinetic study to assess the interaction between indinavir-ritonavir and fosamprenavir demonstrated that these compounds can be administered together without concern for adverse pharmacokinetic interactions. Previous unpublished data (Acosta E, Parkin N, Monogram Biosciences, 2005) demonstrate that at high levels of lopinavir resistance (≥80-fold), both indinavir and amprenavir demonstrate considerably lower-fold resistance (e.g., 30–35-fold). This was the basis for conducting the current study. Fosamprenavir has been shown to significantly interact negatively with lopinavir-ritonavir, and vice versa.15 The goal in our study was to determine if this same type of interaction would occur with indinavir-ritonavir. Our results suggest that fosamprenavir coadministration resulted in a modest but significant reduction in indinavir Cmax, but did not significantly alter indinavir AUC0–12, C12, or Cl/F. None of the ritonavir pharmacokinetic parameters evaluated was affected by fosamprenavir.
Given these results, indinavir 800 mg–ritonavir 100 mg–fosamprenavir 700 mg twice/day could conceivably be studied in HIV-1–infected subjects who exhibit protease inhibitor resistance and who have not received previous indinavir or fosamprenavir therapy. The respective amprenavir and indinavir geometric mean C12 values of 2421 and 1011 ng/ml, respectively, observed in this study are 6- and 10-fold higher than the minimum target trough values recommended for the wild-type HIV-1 virus by the Department of Health and Human Services (DHHS) HIV treatment guideline.17 Although the target indinavir and amprenavir troughs in salvage situations would vary from subject to subject depending on the level of protease inhibitor resistance exhibited, based on our data, one could speculate that the plasma concentrations of indinavir and amprenavir (when used in a ritonavir-boosted double–protease inhibitor regimen) would exceed the IC50 (concentration required for 50% viral replication inhibition) for many strains of the HIV virus encountered in a treatment-experienced setting with multidrug-resistant mutations. Nonetheless, therapeutic drug monitoring is recommended by the DHHS HIV treatment guideline for the treatment of multidrug-resistant viruses because of the intersubject variability that could occur in plasma protease inhibitor concentrations.17
Furthermore, investigational drugs such as integrase inhibitors and coreceptor inhibitors have been shown to have superior virologic suppression in the presence of an active background therapy.1, 2 Therefore, the combination of indinavir-ritonavir-fosamprenavir could be a suitable adjunct when fashioning an optimized background therapy for these new classes of antiretroviral drugs.
According to the fosamprenavir package insert, the median Cmax and C12 observed in subjects treated with fosamprenavir 700 mg–ritonavir 100 mg twice/day orally were 6080 and 2120 ng/ml, respectively.9 In addition, among participants treated with identical doses of fosamprenavir-ritonavir in the AIDS Clinical Trial Group A5143 trial,15 the mean AUC0–12 and C12 were 42,7311 ng•hour/ml (range 33,101–55,112 ng•hr/ml) and 2340 ng/ml (range 1424–3223 ng/ml), respectively. Our results showed these same parameters (mean ± SD AUC0–12, Cmax, and C12) to be 43,913 ± 14,322 ng•hour/ml, 5782 ± 1562 ng/ml, and 2648 ± 965 ng/ml, respectively. The results of this study therefore suggest that amprenavir pharmacokinetic parameters are not significantly altered by the concomitant use of indinavir-ritonavir.
It is important to note, however, that the sample size of eight HIV-infected patients in this study is modest. In addition, by performing the pharmacokinetic sampling early after fosamprenavir exposure at day 5 rather than later, it is possible that the full effect of the fosamprenavir (inductive or inhibitory) on the activities of the CYP enzyme system in the setting of a ritonavir-boosted double–protease inhibitor regimen may not have been completely exerted. Taking into consideration these limitations, the pharmacokinetics of indinavir and ritonavir were statistically comparable in the absence and in the presence of fosamprenavir coadministration. The 20% reduction in indinavir Cmax observed in the presence of fosamprenavir, although statistically significant, was modest and unlikely to be clinically relevant. The AUC0–12, C12, and Cl/F parameters for indinavir and ritonavir were comparable in the presence or absence of fosamprenavir. The observed amprenavir pharmacokinetic parameters were comparable to historical data.
Conclusion
The combination of ritonavir-boosted indinavir plus fosamprenavir therefore appears to be pharmacokinetically compatible. Clinical trials using this regimen are warranted as it could be used as an alternative strategy for the treatment of antiretroviral-experienced patients with persistent viremia, particularly those failing a lopinavir-ritonavir–based therapy. This combination should also be evaluated as an option in the construction of a suitable background therapy to optimize the therapeutic benefit of newer classes of antiretroviral agents such as integrase or coreceptor inhibitors.
Acknowledgments
The authors thank all the patients who volunteered to participate in this study. We also acknowledge the contribution of the staff at the Grady Health System Infectious Diseases Program and the nurses and technicians at the Grady satellite of the Emory University General Clinical Research Center, Atlanta, Georgia.
Supported by the Emory Medical Care Foundation; Emory University General Clinical Research Center (National Institutes of Health [NIH] grant M01 RR00039); Emory University Center for AIDS Research, Clinical Research and Biostatistics cores (NIH grant P30 AI050409); and the AIDS Clinical Trials Group Minority Fellowship Training Award (NIH grant U01 A138858).
Footnotes
Presented in part at the International AIDS Society Conference, Sydney, Australia, July 22–25, 2007.
Contributor Information
Ighovwerha Ofotokun, Department of Medicine, Division of Infectious Diseases, School of Medicine, Emory University, Atlanta, Georgia
Edward P. Acosta, Division of Clinical Pharmacology, University of Alabama, Birmingham, Alabama
Jeffrey L. Lennox, Department of Medicine, Division of Infectious Diseases, School of Medicine, Emory University, Atlanta, Georgia
Yi Pan, Department of Biostatistics, Rollins School of Public Health, Emory University, Atlanta, Georgia
Kirk A. Easley, Department of Biostatistics, Rollins School of Public Health, Emory University, Atlanta, Georgia
References
- 1.Grinsztejn B, Nguyen B, Katlama C, et al. Safety and efficacy of the HIV-1 integrase inhibitor raltegravir (MK-0518) in treatment-experienced patients with multidrug-resistant virus: a phase II randomized controlled trial. Lancet. 2007;369:1261–9. doi: 10.1016/S0140-6736(07)60597-2. [DOI] [PubMed] [Google Scholar]
- 2.Zolopa AR, Mullen M, Berger D, et al. The HIV integrase inhibitor GS-9137 has potent antiretroviral activity in treatment-experienced patients; Presented at the 14th conference on retroviruses and opportunistic infections; Los Angeles, CA. February 25–28, 2007. [Google Scholar]
- 3.Staszewski S, Babacan E, Stephan C, Stürmer M, Dauer B. The LOPSAQ study: 48-week analysis of a boosted double protease inhibitor regimen containing lopinavir/ritonavir plus saquinavir without additional antiretroviral therapy; Presented at the 16th international AIDS conference; Toronto, Ontario, Canada. August 13–18, 2006; [DOI] [PubMed] [Google Scholar]
- 4.Ribera E, Azuaje C, Lopez R, et al. Atazanavir and lopinavir/ritonavir: pharmacokinetics, safety and efficacy of a promising double-boosted protease inhibitor regimen. AIDS. 2006;20:1131–9. doi: 10.1097/01.aids.0000226953.56976.ad. [DOI] [PubMed] [Google Scholar]
- 5.Luber AD. [Accessed June 14, 2007];Double-boosted protease inhibitor regimens: a pharmacologic and pharmacokinetic perspective. Clinical care options, HIV, management series, module 1. Available from http://www.clinicaloptions.com/HIV/Management%20Series/Double–Boosted%20PIs.aspx.
- 6.Hammer SM, Vaida F, Bennett KK, et al. Dual vs single protease inhibitor therapy following antiretroviral treatment failure: a randomized trial. JAMA. 2002;288:169–80. doi: 10.1001/jama.288.2.169. [DOI] [PubMed] [Google Scholar]
- 7.Lichterfeld M, Wöhrmann A, Schmeisser N, et al. Superior virological efficacy of ritonavir-boosted protease inhibitor regimens compared to single protease inhibitor therapy. Eur J Med Res. 2003;8:56–60. [PubMed] [Google Scholar]
- 8.Murphy RL, Brun S, Hicks C, et al. ABT-378/ritonavir plus stavudine and lamivudine for the treatment of antiretroviral-naive adults with HIV-1 infection: 48-week results. AIDS. 2001;15:F1–9. doi: 10.1097/00002030-200101050-00002. [DOI] [PubMed] [Google Scholar]
- 9.GlaxoSmithKline . Lexiva (fosamprenavir) prescribing information. Research Triangle Park, NC: 2004. [Google Scholar]
- 10.Moyle GJ, Back D. Principles and practice of HIV-protease inhibitor pharmaco-enhancement. HIV Med. 2001;2:105–13. doi: 10.1046/j.1468-1293.2001.00063.x. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt B, Korn K, Moschik B, Paatz C, Uberla K, Walter H. Low level of cross-resistance to amprenavir (141W94) in samples from patients pretreated with other protease inhibitors. Antimicrob Agents Chemother. 2000;44:3213–16. doi: 10.1128/aac.44.11.3213-3216.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D’Aquila RT, Schapiro JM, Brun-Vezinet F, et al. Drug resistance mutations in HIV-1. Top HIV Med. 2003;11:92–6. [PubMed] [Google Scholar]
- 13.Mauss S, Schmutz G, Kuschak D. Unfavourable interaction of amprenavir and lopinavir in combination with ritonavir? AIDS. 2002;16:296–7. doi: 10.1097/00002030-200201250-00023. [DOI] [PubMed] [Google Scholar]
- 14.Boffito M, Kurowski M, Kruse G, et al. Atazanavir enhances saquinavir hard-gel concentrations in a ritonavir-boosted once-daily regimen. AIDS. 2004;18:1291–7. doi: 10.1097/00002030-200406180-00007. [DOI] [PubMed] [Google Scholar]
- 15.Kashuba AD, Tierney C, Downey GF, et al. Combining fosamprenavir with lopinavir/ritonavir substantially reduces amprenavir and lopinavir exposure: ACTG protocol A5143 results. AIDS. 2005;19:145–52. doi: 10.1097/00002030-200501280-00006. [DOI] [PubMed] [Google Scholar]
- 16.Saah AJ, Winchell GA, Nessly ML, Seniuk MA, Rhodes RR, Deutsch PJ. Pharmacokinetic profile and tolerability of indinavir-ritonavir combination in healthy volunteers. Antimicrob Agents Chemother. 2001;45:2710–15. doi: 10.1128/AAC.45.10.2710-2715.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Institutes of Health, Department of Health and Human Services. the DHHS panel on antiretroviral guidelines for adults and adolescents—a working group of the Office of AIDS Research Advisory Council (OARAC) [Accessed June 14, 2007];Guidelines for the use of antiretroviral agents in HIV-1–infected adults and adolescents—October 10, 2006. Developed by. Available from http://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL.pdf.