Abstract
The catalytic subunit of protein phosphatase type 1 (PP1) has an essential role in mitosis, acting in opposition to the Ipl1/Aurora B protein kinase to ensure proper kinetochore-microtubule interactions. However, the regulatory subunit(s) that completes the PP1 holoenzyme that functions in this capacity is not known. We show here that the budding yeast Ypi1 protein is a nuclear protein that functions with PP1 (Glc7) in this mitotic role. Depletion of cellular Ypi1 induces mitotic arrest due to activation of the spindle checkpoint. Ypi1 depletion is accompanied by a reduction of nuclear PP1 and by loss of nuclear Sds22, a Glc7 binding partner that is found in a ternary complex with Ypi1 and Glc7. Expression of a Ypi1 variant that binds weakly to PP1 also activates the spindle checkpoint and suppresses the temperature sensitivity of an ipl1-2 mutant. These results, together with genetic interactions among YPI1, GLC7, and SDS22 mutants, indicate that Ypi1 and Sds22 are positive regulators of the nuclear Glc7 activity that is required for mitosis.
INTRODUCTION
Bipolar attachment of spindle microtubules to chromosome kinetochores is a prerequisite for the equal segregation of chromosomes to daughter cells and is therefore a critical step in mitosis. The general mechanism leading to the bipolar attachment of chromosomes appears to be a two-step process (reviewed in Gadde and Heald, 2004; Hauf and Watanabe, 2004). Dynamic spindle microtubules first capture kinetochores in a relatively inexact process. Microtubule-kinetochore interactions are then regulated by a mechanism that strengthens appropriate or weakens inappropriate contacts. The spindle checkpoint prevents entry into anaphase until all chromosomes are bioriented (reviewed in Lew and Burke, 2003; Pinsky and Biggins, 2005; Tanaka et al., 2005).
The evolutionarily conserved Ipl1/Aurora B protein kinase plays a major role in promoting biorientation of kinetochores by promoting the turnover of kinetochore-microtubule contacts in response to tension (Tanaka et al., 2002; Hauf et al., 2003; Cimini et al., 2006; Pinsky et al., 2006b). Presumably, phosphorylation of one or more kinetochore proteins by Ipl1/Aurora B reduces the interaction between microtubules and kinetochores. Candidate substrates include components of inner kinetochore complexes (Westermann et al., 2003), the DAM/DASH complex (Kang et al., 2001; Cheeseman et al., 2002; Li et al., 2002; Shang et al., 2003; Pinsky et al., 2006b), and Ndc80/Hec1 (Cheeseman et al., 2006; DeLuca et al., 2006). Together, these results indicate that phosphorylation of kinetochore proteins by Ipl1/Aurora B serves to increase microtubule-kinetochore dynamics and allow for proper biorientation of kinetochore microtubules. The corollary of this hypothesis is that phosphorylated substrates should become dephosphorylated when kinetochores become bioriented. A large body of evidence suggests that type 1 protein phosphatase (PP1) carries out this dephosphorylation. The injection of anti-PP1 antibodies into mammalian cells induces metaphase arrest (Fernandez et al., 1992), and mammalian PP1α and PP1γ isoforms are located at kinetochores (Trinkle-Mulcahy et al., 2003, 2006). PP1 mutations in Drosophila, yeast, and fungi can induce mitotic arrest (Doonan and Morris, 1989; Ohkura et al., 1989; Axton et al., 1990; Hisamoto et al., 1994; Black et al., 1995; MacKelvie et al., 1995; Baker et al., 1997; Bloecher and Tatchell, 1999; Andrews and Stark, 2000). In mutants in the yeast PP1 catalytic subunit, glc7-129 and glc7-10, this arrest requires a functional spindle checkpoint (Bloecher and Tatchell, 1999; Sassoon et al., 1999). Extracts from glc7-10 mutants also have low levels of kinetochore-microtubule binding activity (Sassoon et al., 1999). Furthermore, GLC7 mutations can partially suppress the temperature sensitivity of ipl1 mutants (Francisco et al., 1994; Hsu et al., 2000; Pinsky et al., 2006a) and restore the normal phosphorylation levels for several Ipl1 substrates (Hsu et al., 2000; Pinsky et al., 2006a). Collectively, these results suggest that Glc7 either regulates Ipl1 activity or acts to dephosphorylate Ipl1 substrates. Although this question has not been definitively answered, Pinsky et al. (2006a) have found no evidence that Glc7 activity influences Ipl1 levels, localization, or activity, suggesting that Glc7 acts directly on Ipl1 substrates.
In contrast to the convincing biochemical evidence that Ipl1/Aurora B phosphorylates kinetochore components and modulates microtubule-kinetochore interactions, the evidence that PP1/Glc7 dephosphorylates these substrates is less convincing. Although mammalian PP1 colocalizes with kinetochores (Trinkle-Mulcahy et al., 2003, 2006), yeast Glc7, while abundant in the nucleus, has not been identified as a component of kinetochore complexes. Glc7 does colocalize with the kinetochore protein Nuf2 (Bloecher and Tatchell, 2000), but this protein is largely associated with spindle pole bodies when Glc7 and Nuf2 colocalize in anaphase (Janke et al., 2001). Another unknown is the nature of the Glc7 holoenzyme that would be acting in opposition to Ipl1. In general, substrate specificity of PP1 enzymes is regulated by a large collection of targeting and/or regulatory subunits that direct the phosphatase to specific substrates and may regulate activity differentially toward different substrates (reviewed in Cohen, 2002; Ceulemans and Bollen, 2004). Although targeting subunits are essential for many PP1/Glc7-dependent processes, the targeting subunit or subunits involved in its cell cycle activities are still subject to debate. One potential nuclear subunit is Sds22, a leucine-rich repeat protein that was first identified as a multicopy suppressor of the Schizosaccharomyces pombe dis2-11 PP1 mutant (Ohkura and Yanagida, 1991), and subsequently was implicated with regulating Glc7 in Saccharomyces cerevisiae (Hisamoto et al., 1995; MacKelvie et al., 1995). Temperature-sensitive sds22 mutants do not undergo cell cycle arrest at the nonpermissive temperature but exhibit high levels of chromosome loss and suppress the temperature sensitivity of the ipl1-2 mutant (Peggie et al., 2002). Recently, Yeast Phosphatase Inhibitor 1 (Ypi1), a Glc7-interacting protein (Ho et al., 2002; Garcia-Gimeno et al., 2003; Hazbun et al., 2003; Krogan et al., 2006), so named because it inhibits Glc7 catalytic activity in vitro (Garcia-Gimeno et al., 2003), was shown to associate with Glc7 and Sds22, forming a ternary complex that enhances the ability of Ypi1 to inhibit Glc7 activity in vitro (Pedelini et al., 2007). Increased expression of either Sds22 or Ypi1 was shown to suppress the temperature sensitivity of ipl1–132 mutants (Pedelini et al., 2007), consistent with the possibility that Ypi1 acts as an inhibitor in vivo. However, depletion of Ypi1 resulted in mitotic arrest, which is more consistent with Ypi1 acting as a positive regulator of Glc7. Here we report our analysis of the relationship between Ypi1 and Glc7. Our results indicate that Ypi1 acts with Sds22 as an important positive regulator of the nuclear Glc7 activity that opposes the Ipl1/Aurora B kinase.
MATERIALS AND METHODS
Yeast Strains, Media, and General Methods
The yeast strains used in this work are listed in Table 1 and are congenic to JC482 (Cannon and Tatchell, 1987). Yeast strains were grown at 30°C on YPD medium (2% Bacto peptone, 1% yeast extract, and 2% glucose) or synthetic complete (SC) medium unless stated otherwise. Strains were sporulated at 24°C on medium containing 2% Bacto peptone, 1% yeast extract, and 2% potassium acetate. SC medium and media lacking specific amino acids were made as described by Sherman et al. (1986). Benomyl (15 μg/ml) plates (used to score mad1Δ strains) were made as described in Straight et al. (1997). Yeast transformation, manipulation of Escherichia coli, and the preparation of bacterial growth media were performed as described previously (Maniatis et al., 1989; Gietz et al., 1992). The expression of YPI1 in PGAL1-3HA-YPI1 strains was induced by growing the strains in YPGal (2% Bacto peptone, 1% yeast extract, and 2% galactose) medium overnight at 30°C. Cultures were then diluted back into YPGal and grown at 30°C to midlog phase (density of ∼1–5 × 107 cells per ml). To repress expression of YPI1, these cells were then pelleted, washed, and resuspended in YPD medium to a final density of 1 × 104 cells per ml. Cell densities were determined using a Coulter Counter (Beckman Coulter, Miami, FL). Drop growth assays were done as described previously (Williams-Hart et al., 2002). Loss of cell viability was determined as described by Wang and Burke (1995).
Table 1.
Yeast strains
| Strain | Genotype | Source or Reference |
|---|---|---|
| KT1112 | MATaleu2 ura3-52 his3 | Stuart et al. (1994) |
| KT1113 | MATα leu2 ura3-52 his3 | Stuart et al. (1994) |
| KT1963 | MATaleu2 ura3-52 his3 ipl1-2 | This study |
| KT1968 | MATa leu2 ura3-52 his3 ipl1-2 glc7-127 | Hsu et al. (2000) |
| KT2422 | MATaleu2 ura3-52 his3 GLC7-mYFP::SpHIS5 | This study |
| KT2432 | MATaleu2 ura3-52 his3 GLC7-mYFP::SpHIS5 kanMX6:PGAL1-3HA-YPI1 | This study |
| KT2453 | MATaleu2 ura3-52 his3 GLC7-tdimer2::SpHIS5 | This study |
| KT2487 | MATaleu2 ura3-52 his3 GLC7-mCherry::SpHIS5 | This study |
| KT2572 | MATaleu2 ura3-52 his3 GLC7-mYFP::SpHIS5 YPI1-GFP:kanMX6 | This study |
| KT2668 | MATaleu2 ura3-52 his3::GFP-lacI:HIS3 trp1::(lacO)256:TRP1 kanMX6:P GAL1-3HA-YPI1 | This study |
| KT2729 | MATaleu2 ura3-52 his3 GLC7-tdimer2::SpHIS5 SDS22-mYFP:SpHIS5 | This study |
| KT2739 | MATaleu2 ura3-52 his3 GLC7-tdimer2::SpHIS5 kanMX6:PGAL1-3HA-YPI1 | |
| KT2767 | MATaleu2 ura3-52 his3 GLC7-mYFP::SpHIS5 POM34-mCherry:SpHIS5 kanMX6:PGAL1-3HA-YPI1 | This study |
| KT2771 | MATaleu2 ura3-52 his3 GLC7-mYFP::SpHIS5 POM34-mCherry:SpHIS5 | This study |
| KT2779 | MATaleu2 ura3-52 his3 kanMX6:PGAL1-3HA-YPI1 | This study |
| KT2780 | MATaleu2 ura3-52 his3 GLC7-mCherry::SpHIS5 kanMX6:PGAL1-3HA-YPI1 | This study |
| KT2732 | MATaleu2 ura3-52 his3 GLC7-mCherry::SpHIS5 SDS22-mYFP:SpHIS5 | This study |
| LKD3 | MATaleu2 his3 ura3::GFP-PPZ1:URA3 | This study |
| LKD4 | MATaleu2 his3 ura3::GFP-PPZ1:URA3 kanMX6:PGAL1-3HA-YPI1 | This study |
| JB256 | MATa/α leu2/leu2 ura3-52/ura3-52 his3/his3 ypi1::kanMX6/+ | This study |
| JB275-1A | MATaleu2 ura3-52 his3 ypi1::kanMX6 pJB27 | This study |
| JB276-8B | MATα leu2 ura3-52 his3 ypi1::kanMX6 pJB43 | This study |
| JB278-16B | MATa leu2 ura3-52 his3 ypi1::kanMX6 GLC7-mYFP::SpHIS5 pJB43 | This study |
| JB279-2C | MATα leu2 ura3-52 his3 ypi1::kanMX6 ipl1-2 pJB43 | This study |
| JB280-5B | MATaleu2 ura3-52 his3 ypi1::kanMX6 ipl1-2 pJB27 | This study |
| JB282-1B | MATα leu2 ura3-52 his3 YPI1-GFP:kanMX6 | This study |
| JB284-2C | MATaleu2 his3 trp1–1 ura3::GFP-TUB1:URA3 kanMX6:PGAL1-3HA-YPI1 | This study |
| JB289-1A | MATaleu2 his3 ura3::GFP-TUB1:URA3 | This study |
| JB289-1C | MATaleu2 his3 ura3::GFP-TUB1:URA3 mad1::HIS3MX6 | This study |
| JB289-4B | MATaleu2 his3 ura3::GFP-TUB1:URA3 mad1::HIS3MX6 kanMX6:PGAL1-3HA-YPI1 | This study |
| JB290-1B | MATaleu2 ura3-52 his3 glc7-127 | This study |
| JB290-1D | MATaleu2 ura3-52 his3 YPI1-GFP:kanMX6 glc7-127 | This study |
| JB295-1A | MATaleu2 ura3-52 his3 ipl1-2 YPI1-GFP:kanMX6 | This study |
| JB297-2A | MATaleu2 ura3-52 his3 glc7-109 | This study |
| JB297-1A | MATaleu2 ura3-52 his3 YPI1-GFP:kanMX6 glc7-109 | This study |
| JB298-5B | MATaleu2 ura3-52 his3 glc7-132 | This study |
| JB298-7A | MATaleu2 ura3-52 his3 YPI1-GFP:kanMX6 glc7-132 | This study |
| JB300-3A | MATα leu2 ura3-52 his3 ypi1::kanMX6 GLC7-mYFP::SpHIS5 pJB27 | This study |
| JB308-7A | MATaleu2 his3 ypi1::kanMX6 ura3::GFP-TUB1:URA3 pJB43 | This study |
| JB309-8B | MATaleu2 his3 ypi1::kanMX6 ura3::GFP-TUB1:URA3 pJB27 | This study |
| JB320-1B | MATα leu2 ura3-52 his3 SDS22-mYFP:SpHIS5 | This study |
| JB328-12C | MATaleu2 ura3-52 his3 SDS22-mYFP:SpHIS5 POM34-mCherry:SpHIS5 | This study |
| JB331-3A | MATaleu2 ura3-52 his3 SDS22-mYFP:SpHIS5 POM34-mCherry:SpHIS5 kanMX6:PGAL1-3HA-YPI1 | This study |
| JB338-3A | MATα leu2 ura3-52 his3::GFP-lacI:HIS3 trp1::(lacO)256:TRP1 | This study |
| JB338-13B | MATaleu2 ura3-52 his3::GFP-lacI:HIS3 trp1::(lacO)256:TRP1 mad1::HIS3MX6 kanMX6:PGAL1-3HA-YPI1 | This study |
| JB338-14D | MATα leu2 ura3-52 his3::GFP-lacI:HIS3 trp1::(lacO)256:TRP1 mad1::HIS3MX6 | This study |
| JB377-6C | MATα leu2 ura3-52 his3 ypi1::kanMX6 SDS22-mYFP:SpHIS5 POM34-mCherry:SpHIS5 pJB27 | This study |
| JB378-7D | MATaleu2 ura3-52 his3 ypi1::kanMX6 SDS22-mYFP:SpHIS5 POM34-mCherry:SpHIS5 pJB43 | This study |
| JB385-1A | MATα leu2 ura3-52 his3 trp1-1 YPI1-13Myc:kanMX6 | This study |
| JB402-10D | MATα leu2 ura3-52 his3 YPI1-GFP:kanMX6 SDS22-mYFP:SpHIS5 | This study |
| JB403-5C | MATaura3-52 his3 trp1-1 sds22::TRP1 leu2::sds22-6ts:LEU2 YPI1-13Myc:kanMX6 | This study |
| JB409-1C | MATaleu2 ura3-52 his3 YPI1-13Myc:kanMX6 glc7-129 | This study |
| JB412-1B | MATα leu2 ura3-52 his3 GLC7-13Myc:kanMX6 | This study |
Gene deletion, PGAL1-3HA, 13Myc, and fluorescent protein fusion cassettes were generated using PCR. Each amplified cassette was transformed into a KT1112xKT1113 diploid strain, drug-resistant or amino acid-prototrophic transformants were sporulated, and haploid meiotic segregants were isolated by tetrad analysis. Primers are listed in Table 2. To create the ypi1Δ strain, the deletion cassette was amplified with primers JB90-F and JB91-R, using pFA6a-kanMX6 (Longtine et al., 1998) as a template. To create the PGAL1-3HA-YPI1 allele, the cassette was amplified with primers LUG-1F and LUG-2R using pFA6a-kanMX6-PGAL1-3HA (Longtine et al., 1998) as a template. The 13Myc-integration cassettes were amplified with primers JB99-F and JB101-R for YPI1–13Myc, primers JB107-F and JB101-R for ypi1-Δ114-13Myc, primers JB108-F and JB101-R for ypi1-Δ147-13Myc, primers GSH1-F and GSH1-R for GLC7-13Myc and using pFA6a-13Myc-His3MX6 and pFA6a-13Myc-kanMX6 (Longtine et al., 1998) as templates. The YPI1-GFP cassette was amplified with primers JB99-F and JB101-R, using pLK3 (Kozubowski et al., 2003) as a template. To create YPI1-EmCitrine the cassette was amplified using primers JB119-F and JB101-R with pKT211 (Sheff and Thorn, 2004) as a template. The SDS22-EmCitrine cassette was amplified with primers JB109-F and JB110-R, using pKT211 as a template. To create POM34-mCherry, the cassette was amplified with primers JB111-F and JB112-R using pKT355 (pFA6a-L-mCherry-HIS3MX6; K. Thorne, personal communication) as a template. To create GLC7-EmCitrine, GLC7-mCherry, and GLC7-tdimer2, the cassettes were amplified with primers CAT138-F and GSH1-R using pKT211, pKT146 (Sheff and Thorn, 2004), and pKT355, respectively, as templates. For simplicity, the EmCitrine and mCherry fusions are referred to as mYFP and mRFP, respectively, throughout this article. All deletions, truncations, and integrations were confirmed by genomic PCR.
Table 2.
Primers
| Name | Sequence (5′–3′) |
|---|---|
| JB81-F | ACGCGTCGACCTCCGGTACCCGATTGAGGCA |
| JB82-R | GCTCTAGATTTGTTTATATCTATATATCA |
| JB90-F | CCAGGAGTTGCGAGCTAAGTCTTCAATTAAGTCTATAAGGCGGATCCCCGGGTTAATTAA |
| JB91-R | TTGCTGCTTCATCGAATATTTTGGCTTTCGTTGTACAAAGCCGAATTCGAGCTCGTTTAAAC |
| JB94-F | AGGCACAATGTAAGAGCTGAAGAAAATGTGATTG |
| JB95-R | CAATCACATTTTCTTCAGCTCTTACATTGTGCCT |
| JB99-F | TTATTCTGAATACAGGCGAAAACAGCAGGAAAAGAAGGACCGGATCCCCGGGTTAATTAA |
| JB101-R | GCTGCTTCATCGAATATTTTGGCTTTCGTTGTACAAAGCCGAATTCGAGCTCGTTTAAAC |
| JB102-F | CCTACAAGGCACAATGCAAGAGCTGAAGAAAATG |
| JB103-R | CATTTTCTTCAGCTCTTGCATTGTGCCTTGTAGG |
| JB105-F | GCTCTAGAGATGAGTGGAAATCAAATGGCT |
| JB106-R | ACGCGTCGACGCAGTCCTTCTTTTCCTGCT |
| JB107-F | CAGAATCTGAGAATGAGAAGGATCTTGACTTTAACGAACGCGGATCCCCGGGTTAATTAA |
| JB108-F | CCAATGCTTATGAAATCCAACCAGATTATTCTGAATACAGCGGATCCCCGGGTTAATTAA |
| JB109-F | TCCATCCCTACAGAAGATTGATGCGACATATATAAGAGGCGGTGACGGTGCTGGTTTA |
| JB110-R | GTTTGTGTGTGTATATAAAAAAAAAATCATTTCTTGATCAAGATCGATGAATTCGAGCTCG |
| JB111-F | AATATGCATATATGATGAACTCACAGTCCCCAAGGGGGAAAATAGGTGACGGTGCTGGTTTA |
| JB112-R | TATATAGCTATGGAAAGTATTAAATGTTTTTTTGCTGTTTTCGATGAATTCGAGCTCG |
| JB119-F | TTATTCTGAATACAGGCGAAAACAGCAGGAAAAGAAGGACGGTGACGGTGCTGGTTTA |
| LUG1-F | CTGCCAGGAGTTGCGAGCTAAGTCTTCAATTAAGTCTATAAGGGAATTCGAGCTCGTTTAAAC |
| LUG2-R | TCTGTTGTTGTTCTGATCCCATAGCCATTTGATTTCCACTGCACTGAGCAGCGTAATCTG |
| LD1-F | CCTACAAGGCACAATGCAAGATGGGAAGAAAAT |
| LD2-R | ATTTTCTTCCCATCTTGCATTGTGCCTTGTAGG |
| GSH1-F | AAAAAGTCTACCAAGGCAAGCTGGGGGTAGAAAGAAAAAACGGATCCCCGGGTTAATTAA |
| GSH1-R | TATTTTCCTTTTTAAACTTTGATTTAGGACGTGAATCTATGAATTCGAGCTCGTTTAAAC |
| CAT138F | AAAAAGTCTACCAAGGCAAGCTGGGGGTAGAAAGAAAAAAGGTGACGGTGCTGGTTTA |
Cells were processed for flow cytometry as described (Baker et al., 1997) with the following modifications. Cells were treated with RNase A overnight at 37°C, harvested, resuspended in phosphate-buffered saline at a cell density of 1 × 107 cells/ml, and stained with propidium iodide (Sigma, St. Louis, MO) at a final concentration of 50 μg/ml. Flow cytometry was performed at the LSUHSC Flow Cytometry Facility.
Plasmid Construction and Site-directed Mutagenesis
Plasmids used in this study are listed in Table 3. Standard techniques were used for DNA manipulation (Maniatis et al., 1989). Restriction and modification enzymes were used as recommended by the manufacturers (Promega, Madison, WI; New England Biolabs, Beverly, MA; Roche, Mannheim, Germany). The YPI1 gene (ORF plus 500 bp upstream and 100 bp downstream sequence) was cloned by PCR using primers JB81-F and JB82-R into pGEM-T (using the pGEM-T Easy Vector Systems kit, Promega) to generate pJB25. The entire sequence was confirmed at the Arizona State University DNA facility (Tempe, AZ). The gene was subcloned from pJB25 as a SalI-XbaI fragment into pRS315, pRS316, and pRS306 vectors to create pJB27, pJB33, and pJB45, respectively. The ability of the plasmid-borne YPI1 to complement the lethality of the ypi1Δ mutant was tested in a ypi1Δ heterozygous diploid strain (JB256). YPI1 was also subcloned as a SalI-SacII fragment into pRS425 to create pJB29. The YPI1 ORF was cloned using a similar strategy and primers JB105-F and JB106-R to create pJB25. The sequence was verified as mentioned above. Mutations in YPI1 were created with the QuikChange kit (Stratagene, La Jolla, CA), using pJB27 as a template (unless mentioned otherwise). Primers were designed according to manufacturer's instructions. Primers JB94-F and JB95-R were used to create ypi1W53A (pJB43). Primers JB102-F and JB103-R were used to generate ypi1V51A in pJB43 to create ypi1V51A/W53A (pJB49). Primers LD1-F and LD2-R were used to create ypi1V51A (pLD1). For integration, YPI1, ypi1V51A, and ypi1W53A were subcloned as SalI-XbaI fragments into pRS306 to create pJB45, pLD3, and, pJB47, respectively. These were digested within URA3 with StuI and transformed into the ypi1Δ heterozygous diploid (JB256). Transformants were sporulated and segregants analyzed by tetrad analysis. Alternatively, the LEU2 bearing plasmids were introduced into a ypi1Δ haploid containing pJB33 (pRS316-YPI1, URA3), and transformants were selected on SC-Ura-Leu. The Ura+ Leu+ transformants were then grown on 5-fluoro-orotic acid (5-FOA) media to select for viable cells that had lost the URA3 plasmid but contained the LEU2 plasmid.
Table 3.
Plasmids
| Name | Description | Source |
|---|---|---|
| pJB25 | YPI1 in pGEM-T | This study |
| pJB27 | pRS315 CEN LEU2 YPI1 | This study |
| pJB29 | pRS425 2 μ LEU2 YPI1 | This study |
| pJB33 | pRS316 CEN URA3 YPI1 | This study |
| pJB43 | pRS315 CEN LEU2 ypi1W53A | This study |
| pJB45 | pRS306 URA3 YPI1 | This study |
| pJB47 | pRS306 URA3 ypi1W53A | This study |
| pJB49 | pRS315 CEN LEU2 ypi1V51A/W53A | This study |
| pLD1 | pRS315 CEN LEU2 ypi1V51A | This study |
| pLD3 | pRS306 URA3 ypi1V51A | This study |
| pRS425 | LEU2 2 μ vector | Christianson et al. (1992) |
| pRS315 | LEU2 CEN vector | Sikorksi and Hieter (1989) |
| pRS316 | URA3 CEN vector | Sikorksi and Hieter (1989) |
| pRS306 | URA3 vector | Sikorksi and Hieter (1989) |
Immunoblot Analysis
To assess tagged protein levels, total protein was prepared from cultures by lysis in trichloroacetic acid (Davis et al., 1993; Stuart et al., 1994). Immunoblot analysis was performed as described (Stuart et al., 1994) using anti-Myc 9E10 ascites antibody or BD Living Colors A.v. monoclonal anti-green fluorescent protein (GFP; JL-8) antibody with subsequent detection using the Enhanced Chemiluminescence System (ECL or ECL Plus, Amersham Biosciences, Piscataway, NJ). Protein levels were quantitated from immunoblots using Image Quant software (Molecular Dynamics, Sunnyvale, CA), with phosphoglycerate kinase (Pgk1) as a loading control.
Microscopy
For live cell imaging, cells were placed onto a pad of 2% agarose in SC or SCGal medium, as indicated, and imaged for GFP, yellow fluorescent protein (YFP), and red fluorescent protein (RFP) as previously described (Kozubowski et al., 2003). DIC and fluorescence images in a series of Z-axis planes (0.5 μm apart) were acquired using Slidebook software (Intelligent Imaging Innovations, Denver, CO). Indirect immunofluorescence was performed as described previously (Kozubowski et al., 2003). Cells were stained with anti-Myc 9E10 ascites mAb followed by goat anti-mouse TRITC-conjugated secondary antibody (Sigma). Quantitative analysis of the levels of GFP fluorescence was performed as described (Kozubowski et al., 2003). For quantitative analysis of spindle length in large-budded cells, the diameters of the mother and bud/daughter cells were measured along the mother-daughter axis using Slidebook software. Cells with bud/daughter cell at least 75% the size of the mother cell were considered large-budded cells for this analysis.
RESULTS
Ypi1 Depletion Induces a Delay in G2/M and Activation of the Spindle Assembly Checkpoint
To determine the essential role(s) of Ypi1 in vivo, we placed the endogenous YPI1 gene under the control of a galactose-regulated promoter using the pFA6a-kanMX6:PGAL1-3HA cassette (Longtine et al., 1998) as described in Materials and Methods. The strain grows normally on galactose (inducing) medium (YPGal) but fails to thrive on glucose (repressing) medium (YPD). To study the consequence(s) of Ypi1 depletion, we grew PGAL1-3HA-YPI1 and congenic YPI1 strains to log phase in YPGal medium and assayed cell number, viability, morphology, and DNA content after transfer to YPD medium. Immunoblot analysis revealed that 3HA-Ypi1 levels were largely depleted after 2 h in YPD (Figure 1A), but the cells continued to divide at a normal rate for at least 8 h, after which time they grew at progressively slower rates (Figure 1B). The delay in growth was accompanied by an increase in the population of budded cells with 2C DNA content (Figure 1C). By 16 h in YPD, the majority (60%) of the PGAL1-3HA-YPI1 cells were delayed in the G2 phase of the cell cycle with large buds and short spindles (88%), assayed using a GFP-Tub1 fusion to visualize spindle microtubules (Figure 1D, Supplementary Figure S1, and Table 4). These results are similar to those reported for depletion of Ypi1 by placing YPI1 under the control of the tetO7 promoter (Pedelini et al., 2007).
Figure 1.
Depletion of Ypi1 results in G2 cell cycle delay and activation of the spindle assembly checkpoint. (A) Immunoblot analysis with anti-HA antibody of 3HA-Ypi1 in extracts from WT (KT2422) and PGAL1-3HA-YPI1 (KT2432) strains, after shifting cells from YPGal to YPD medium as described in Materials and Methods. Pgk1 serves as a loading control. (B) Growth curves of the WT (JB289-1A), PGAL1-3HA-YPI1 (JB284-2C), mad1Δ (JB289-1C), and PGAL1-3HA-YPI1 mad1Δ (JB289-4B) strains after the shift to glucose. (C) Flow cytometry analysis of DNA content for the strains in B at 12, 16, and 20 h in YPD. (D) Tub1-GFP fluorescence in WT (JB289-1A) and PGAL1-3HA-YPI1 (JB284-2C) cells after 16 h in YPD medium. Bar, 5 μm. (E) GFP-lacI fluorescence in the WT (JB338-3A), PGAL1-3HA-YPI1 (KT2668), mad1Δ (JB338-14D), and PGAL1-3HA-YPI1 mad1Δ (JB338-13B) strains at 12 h in YPD. DIC and GFP images are shown. White arrow, a large-budded cell with no spot; white arrowhead, a large-budded cell with no spot in the mother and two spots in the daughter cell.
Table 4.
Quantitative analysis of spindle length in large-budded cells after Ypi1 depletion
| Strain | % Cells with large buds | % Large-budded cells with spindle length |
||
|---|---|---|---|---|
| 0.5–2 μm | >2 μm | Undetermineda | ||
| WTb | ||||
| 0 h | 32 | 33 | 62 | 4 |
| 8 h | 31 | 20 | 80 | 0 |
| 12 h | 34 | 18 | 82 | 0 |
| 16 h | 36 | 28 | 71 | 3 |
| 20 h | 42 | 35 | 60 | 5 |
| PGAL1-YPI1 | ||||
| 0 h | 30 | 17 | 79 | 4 |
| 8 h | 30 | 54 | 44 | 2 |
| 12 h | 44 | 87 | 10 | 3 |
| 16 h | 60 | 88 | 5 | 7 |
| 20 h | 34 | 77 | 13 | 11 |
| PGAL1-YPI1 mad1Δ | ||||
| 0 h | 28 | 30 | 63 | 7 |
| 8 h | 23 | 28 | 67 | 6 |
| 12 h | 32 | 56 | 42 | 2 |
| 16 h | 37 | 32 | 55 | 14 |
| 20 h | 26 | 26 | 71 | 11 |
| mad1Δ | ||||
| 0 h | 27 | 46 | 50 | 4 |
| 8 h | 38 | 15 | 84 | 1 |
| 12 h | 40 | 37 | 63 | 0 |
| 16 h | 42 | 25 | 71 | 3 |
| 20 h | 41 | 49 | 47 | 5 |
a Large-budded cells without a clearly defined spindle were included in this category.
b The following strains were used: WT, JB289-1A; pGAL-YPI1, JB284-2C; pGAL-YPI1 mad1Δ, JB289-4B; and mad1Δ, JB289-1C.
The G2 arrest of some GLC7 mutants is due to activation of the spindle assembly checkpoint (Bloecher and Tatchell, 1999; Sassoon et al., 1999). To determine if the cell cycle arrest that accompanies Ypi1 depletion is also dependent on this checkpoint, we assayed the cell cycle distribution in a PGAL1-3HA-YPI1 strain that also contained a deletion of the MAD1 gene (Mitotic Arrest Deficient), an essential component of the checkpoint (Hardwick and Murray, 1995). As shown in Figure 1B, mad1Δ had no effect on cell proliferation, but largely suppressed the cell cycle arrest induced by Ypi1 depletion (Figure 1C). The lack of G2 arrest was consistent with an observed increase in the frequency of long anaphase spindles in PGAL1-3HA-YPI1 mad1Δ cells after Ypi1 depletion (Table 4).
The release of cell cycle arrest in GLC7 mutants by inactivation of the spindle assembly checkpoint results in an increase in chromosome loss (Bloecher and Tatchell, 1999; Sassoon et al., 1999). We tested whether depletion of Ypi1 in the mad1Δ mutants also results in chromosome segregation defects by assaying the mitotic segregation of chromosome IV that had been tagged with a tandem array of Lac operators (lacO array). Expression of GFP-lacI in these cells allows direct visualization of the tagged chromosome (Straight et al., 1997). The lacO array is normally observed as a single nuclear spot until anaphase, at which point the spot divides into two, and the two spots move to opposite poles of the anaphase spindle. As shown in Figure 1E and Table 5, depletion of Ypi1 in an otherwise wild-type background results in the accumulation of budded cells with one spot (62% at 12 h in YPD), as expected for cells delayed in G2. In contrast, no such increase occurs after Ypi1 depletion in the mad1Δ strain (25% at 12 h in YPD). We noted a high percentage of cells with either too many or no spots (35% at 16 h in YPD; Figure 1E, arrow). Interestingly, the percentage of abnormal cells (cells with no spots and unbudded cells with two spots) was also higher (21%) in the PGAL1-3HA-YPI1 mad1Δ strain compared with the single mutants when grown on YPGal medium (Table 5), suggesting that the high level of 3HA-Ypi1 in these cells might also perturb chromosome stability. We also noted that relatively few PGAL1-YPI1 cells growing in YPGal medium have short spindles (Table 4), further suggesting that Ypi1 function is not normal in the PGAL1-YPI1 strains. This could be due to either altered levels of Ypi1 or the presence of the HA epitope tag.
Table 5.
Quantitative analysis of chromosome segregation after Ypi1 depletion
| No. of of loci | Normal |
Abnormal |
|||||
|---|---|---|---|---|---|---|---|
 |
 |
 |
 |
 |
 |
 |
|
| 1 | 1 | 2 | 2 | 0 | 2 | >2 | |
| YPI1a | |||||||
| 0 h | 53b | 28 | 1 | 17 | 0 | 0 | 0 |
| 8 h | 35 | 37 | 3 | 25 | 0 | 0 | 0 |
| 12 h | 40 | 37 | 2 | 19 | <1 | 2 | 0 |
| 16 h | 41 | 37 | 3 | 18 | 1 | 0 | 0 |
| pGAL-YPI1 | |||||||
| 0 h | 54 | 28 | <1 | 18 | <1 | 0 | 0 |
| 8 h | 41 | 41 | 1 | 16 | 0 | 0 | 0 |
| 12 h | 23 | 62 | 3 | 5 | <1 | 5 | 0 |
| 16 h | 19 | 55 | 11 | 2 | 14 | 8 | <1 |
| pGAL-YPI1 mad1Δ | |||||||
| 0 h | 39 | 24 | 1 | 14 | 15 | 6 | 0 |
| 8 h | 40 | 26 | 2 | 17 | 12 | 3 | 0 |
| 12 h | 40 | 25 | 7 | 12 | 14 | <1 | 1 |
| 16 h | 28 | 20 | 3 | 3 | 34 | 9 | 1 |
| mad1Δ | |||||||
| 0 h | 78 | 10 | 0 | 9 | 0 | 3 | 0 |
| 8 h | 38 | 27 | 2 | 27 | 3 | 2 | 1 |
| 12 h | 41 | 28 | 5 | 21 | 2 | 1 | 1 |
| 16 h | 40 | 31 | 3 | 17 | 3 | 2 | 3 |
a The following strains were used: YPI1, JB338-3A; pGAL-YPI1, KT2668, pGAL-YPI1 mad1Δ, JB338-13B; and mad1Δ, JB338-14D.
b Numbers represent the percentage of cells in each category for a particular strain at the denoted time in YPD medium. n = 100–200 cells for each strain at each time point.
We monitored cell viability in Ypi1-depleted cultures by plating serial dilutions of the cultures grown in YPD onto YPGal medium. Ypi1 depletion results in a ∼10–20-fold decrease in cell viability 24 h after the shift to YPD medium but surprisingly, viability is not reduced further in the mad1Δ mutant (data not shown), as is the case for glc7-129 mad1Δ strains (Bloecher and Tatchell, 1999). These results suggest that the cell death induced by Ypi1 depletion is caused by a YPI1-dependent process(es) separate from or in addition to spindle microtubule attachment.
Ypi1 Is Required for the Nuclear Localization of Glc7 and Sds22
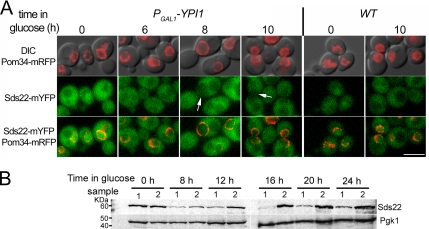
Ypi1 forms a complex with Glc7 in vivo and in vitro (Hazbun et al., 2003; Pedelini et al., 2007). We therefore assayed the cellular distribution of Glc7 and Sds22 in the PGAL1-3HA-YPI1 strain to determine if Ypi1 depletion alters the subcellular location of either protein. In both cases, we used fusions to the monomeric form of the codon-optimized YFP variant, EmCitrine (hereafter referred to as mYFP) (Griesbeck et al., 2001). In a separate study (Tatchell, unpublished data), genomic GLC7-fluorescent protein (FP) fusions were constructed using a variety of different FP variants, and growth rates of the resulting strains were assayed at different temperatures. Although all fusions suppressed the lethality of a GLC7 deletion, many variants conferred slow growth. In contrast, the GLC7-mYFP strain exhibited a growth rate similar to that of the wild-type strain at temperatures ranging from 11 to 37°C. Therefore, we used this FP fusion for our studies of Glc7 and Sds22. As observed previously for the GFP-Glc7 fusion (Bloecher and Tatchell, 2000), Glc7-mYFP accumulates in the cytoplasm, nucleus, and at the bud neck (Figure 2B). In cells from late log and early stationary phase cultures, Glc7-mYFP accumulates to highest levels in the nucleolus, as reported previously (Bloecher and Tatchell, 2000). Sds22-3Myc was found by indirect immunofluorescence to localize largely to the nucleus (Peggie et al., 2002). However, direct visualization of Sds22-mYFP fluorescence in live cells revealed a more equal distribution between the nucleus and cytoplasm. Although nuclear fluorescence levels were higher than those in the cytoplasm, in some cells, the nuclear and cytoplasmic levels were nearly equal, making it difficult to unambiguously identify the nucleus. Expresssion of a RFP fusion to Pom34, a nuclear pore protein (Pom34-mCherry) allowed us to delineate the nuclear periphery in these cells. Total fluorescence was much lower in an Sds22-mYFP strain than in the Glc7-mYFP strain. This apparent difference in expression was confirmed by immunoblot analysis (Figure 2A), from which we estimate that Sds22-mYFP is expressed at 10–20-fold lower levels than is Glc7, a greater difference than the approximately fourfold previously estimated from detection of TAP-tagged fusion proteins (Ghaemmaghami et al., 2003).
Figure 2.
Ypi1 is required for the nuclear localization of Glc7. (A) Immunoblot analysis of extracts from WT (KT1112), GLC7-mYFP (KT2422), and SDS22-mYFP (JB320-1B) strains with anti-GFP antibody. Pgk1 serves as a loading control. (B) Subcellular distribution of Glc7-mYFP in WT (KT2771) and PGAL1-3HA-YPI1 (KT2767) strains at the indicated time after shifting to YPD. Cells were examined by fluorescence microscopy with a GFP filter set for Glc7-mYFP and a rhodamine filter set for Pom34-mRFP. Bar, 5 μm. (C) Quantitative analysis of the ratio of nuclear to cytoplasmic levels of Glc7-mYFP in the WT (KT2422) (□ and ○) and PGAL1-3HA-YPI1 (KT2432; ■ and •) cells shown in B. Squares and circles indicate data from two separate experiments. To accurately compare the changes in fluorescence levels between experiments we determined the nuclear/cytoplasmic fluorescence ratio rather than the absolute signal, which can fluctuate with changes in Hg-Arc intensity. More than 50 cells were examined for each strain at each time point. Bright fluorescent punctae were excluded from the analysis. (D) Immunoblot analysis with anti-GFP antibody of Glc7-mYFP in extracts from the strains in B. Left and right panels, lane 1, GLC7-mYFP strain (KT2422); lane 2, GLC7-mYFP PGAL1-3HA-YPI1 strain (KT2432); and lane 3, WT strain (KT1112). Pgk1 serves as a loading control.
Ypi1 protein levels were depleted in the GLC7-mYFP and SDS22-mYFP strains containing the PGAL1-3HA-YPI1 promoter fusion. As was previously reported for depletion of Ypi1 protein using a tetO7:YPI1 strain (Pedelini et al., 2007), we observed that nuclear Glc7 levels were reduced after transfer from YPGal to YPD. As shown in Figure 2B, nuclear Glc7-mYFP fluorescence was greatly reduced after 8 h in YPD, and after 12 h Glc7-mYFP fluorescence was equally distributed between the nucleus and cytoplasm. We quantitated the levels of Glc7-mYFP fluorescence in the nucleus and in the cytoplasm after Ypi1 depletion and plotted the ratio of nuclear to cytoplasmic fluorescence in Figure 2C. The nuclear/cytoplasmic ratio was the same for WT and PGAL1-3HA-YPI1 cells on YPGal and through 4 h on YPD, after which time the nuclear/cytoplasmic ratio dropped in the PGAL1-3HA-YPI1 cells (Figure 2C, filled symbols). We also observed bright fluorescent foci (Figure 2B, arrows) in the Ypi1-depleted cells, as noted previously (Pedelini et al., 2007). The spots were occasionally found in PGAL1-3HA-YPI1 cells growing in YPGal medium but were prominent after 4 h in YPD. The number, size, and brightness of these punctae varied widely from cell to cell, suggesting they do not represent a well-defined organelle or structure. The spots are similar to the bright punctae of GFP-Glc7 that accumulate in sds22-6 mutant cells at the nonpermissive temperature (Peggie et al., 2002) and are reminiscent of aggresomes that result from the accumulation of unfolded proteins in mammalian cells (Johnston et al., 1998). Immunoblot analysis for Glc7-mYFP in total cell extracts revealed that Ypi1 depletion had little influence on the total amount of Glc7 (Figure 2D). Thus, Ypi1 is important for maintaining the normal cellular distribution of Glc7 in the cell but it does not influence Glc7 levels.
Ypi1 depletion also results in loss of Sds22-mYFP from the nucleus (Figure 3A) but in contrast to Glc7-mYFP, which becomes equally distributed between the nucleus and the cytoplasm, Sds22-mYFP becomes largely excluded from the nucleus. After 6 h in YPD, nuclear Sds22-mYFP fluorescence was generally lower than in the cytoplasm. By 8–10 h, Sds22 was largely excluded from the nucleus in a majority of cells (Figure 3A, arrows). However, fluorescent punctae were not observed in these cells, even after long periods in YPD medium. As for Glc7, Ypi1 depletion did not result in a reduction of Sds22-mYFP protein levels. In fact, by immunoblot, total endogenous Sds22 levels increased after Ypi1 depletion (Figure 3B).
Figure 3.
Ypi1 is required for the nuclear localization of Sds22. (A) Subcellular distribution of Sds22-mYFP in WT (JB328-12C) and PGAL1-3HA-YPI1 (JB331-3A) strains at the indicated times in YPD. Pom34-mRFP serves as a nuclear periphery marker. White arrows indicate loss of Sds22 from the nucleus. Cells were examined by fluorescence microscopy with a YFP filter set for Sds22-mYFP and a rhodamine filter set for Pom34-mRFP. Bar, 5 μm. (B) Immunoblot analysis of Sds22-mYFP in extracts from the strains in A. Left and right panels, lane 1, SDS22-mYFP strain (JB328-12C); lane 2, SDS22-mYFP PGAL1-3HA-YPI1 strain (JB331-3A). The Sds22 protein level at the 16-h time point is inconsistently low.
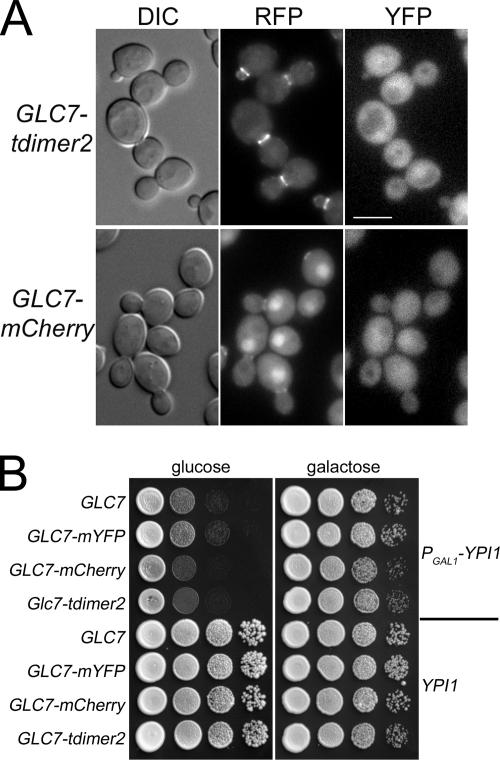
The reduction in the levels of nuclear Glc7 and Sds22 induced by Ypi1 depletion suggests that Ypi1 could act to facilitate nuclear import of Glc7. Consistent with the possibility that high nuclear Glc7 levels are necessary for normal cell cycling, Pinsky et al. (2006a) observed that overexpression of the cytoplasmic Glc7 targeting subunits Gip3 and Gip4 results in nuclear depletion of Glc7 and concomitant G2/M arrest. However, it is possible that the reduction in nuclear Glc7 in the YPI1 mutants is only indirectly responsible for the cell cycle arrest. This possibility is supported by the observation that two Glc7 FP fusions that contain tandem copies of RFP variants, tdimer2 and tdTomato, fail to accumulate to high levels in the nucleus. As shown in Figure 4A, Glc7-tdimer2 is targeted to the bud neck but does not accumulate in the nucleus to levels above those in the cytoplasm. The subcellular distribution of Sds22-mYFP in this strain is similar to that observed in wild-type cells. A similar pattern of fluorescence is observed for Glc7-tdTomato (data not shown). In contrast, Glc7-mCherry, a fusion to a monomeric RFP variant, has a subcellular distribution similar to that of other Glc7-FP fusions (Figure 4A). Nevertheless, GLC7-tdimer2 and GLC7-mCherry strains grow at similar rates in a wild-type genetic background or with PGAL1-YPI1 (Figure 4B). We also note that GLC7-mCherry and GLC7-tdimer2 strains containing PGAL1-3HA-YPI1 arrest more rapidly on glucose medium than the congenic GLC7-mYFP or GLC7 strains (Figure 4B). A complete understanding of this genetic interaction will require a more thorough characterization but the results indicate that the cell cycle arrest induced by Ypi1 depletion is not simply due to the depletion of Glc7 from the nucleus.
Figure 4.
Variation in nuclear Glc7 levels between Glc7-RFP variants. (A) Yeast strains KT2729 (GLC7-tdimer2 SDS22-mYFP) and KT2732 (GLC7-mCherry SDS22-mYFP) were grown to exponential phase in YPD medium, and cells were examined by DIC and fluorescence microscopy. Bar, 5 μm. (B) Cultures of strain KT2779 (GLC7 PGAL1-YPI1), KT2432 (GLC7-mYFP PGAL1-YPI1), KT2780 (GLC7-mCherry PGAL1-YPI1), KT2739 (GLC7-tdimer2 PGAL1-YPI1), KT1112 (GLC7 YPI1), KT2422 (GLC7-mYFP), KT2487 (GLC7-mCherry), and KT2453 (GLC7-tdimer2) were serially diluted onto YPD (glucose) and YPGal (galactose) plates and incubated at 30°C for 48 h before imaging.
Ypi1 also interacts with the Ppz1 phosphatase in yeast two-hybrid (Venturi et al., 2000) and GST-pulldown assays (Garcia-Gimeno et al., 2003) and can inhibit Ppz1 phosphatase activity in vitro (Garcia-Gimeno et al., 2003). These results suggest that Ypi1 could also act on the Ppz1 phosphatase in vivo. We therefore examined the subcellular location of a GFP-Ppz1 fusion protein after Ypi1 depletion. GFP was inserted near the N-terminus of the protein, after the N-myristoylation site, and the fusion gene was integrated ectopically at the URA3 locus. GFP-Ppz1 fluorescence appeared to be on internal membranes (Supplementary Figure S2), consistent with myristoylation of the protein (Clotet et al., 1996). After 8 h in YPD medium, the GFP-PPZ1 PGAL1-3HA-YPI1 cells displayed the same cell cycle delay previously observed for other PGAL1-3HA-YPI1 strains. However, the fluorescence pattern did not change even after 20 h after Ypi1 depletion (Supplementary Figure S2). Although these results do not rule out the possibility that Ypi1 influences Ppz1 activity, they do indicate that Ypi1 is not necessary for the normal subcellular distribution of Ppz1.
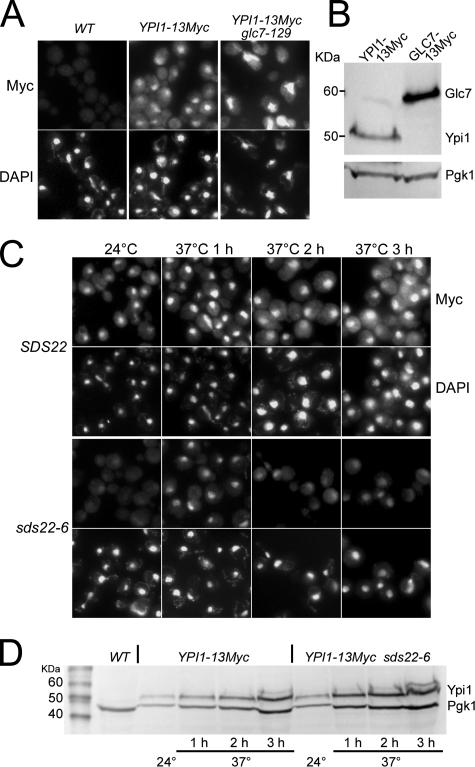
Ypi1 Is a Nuclear Protein
The mammalian PP1 inhibitor-3 (Inh3) localizes to nucleoli and centrosomes within nuclei (Huang et al., 2005). Pedelini et al. (2007) observed that a Ypi1-GFP fusion protein was also largely nuclear. However, we were unable to observe GFP fluorescence in our yeast strains containing a chromosomally integrated Ypi1-GFP fusion (our unpublished data), as previously reported (Huh et al., 2003). This YPI1-GFP allele is partially defective for Ypi1 activity, as indicated by the strong genetic interactions between YPI1- GFP and different alleles of GLC7 and SDS22 (see below). The YPI1-mYFP allele shows less severe genetic interactions with GLC7 and SDS22 alleles than did the YPI1-GFP fusion, but it still displays some defects. Little or no fluorescence was detected for the mYFP fusion, and very little protein was detected by immunoblot analysis (our unpublished data), suggesting that these fusions are not stable. In contrast, a YPI1–13Myc allele is functional (see Table 6). Our studies of Ypi1 localization were therefore conducted with this fusion protein. Indirect immunofluorescence with anti-Myc antibody revealed that Ypi1 is located predominantly in the nucleus (Figure 5A), consistent with the previous report (Pedelini et al., 2007). Ypi1-13Myc is present in the nucleus of all cells regardless of bud size, indicating that there is no cell cycle dependence for its nuclear localization. Immunoblot analysis revealed that the levels of Ypi1–13Myc in total cell extracts are lower than a comparable Glc7-13Myc fusion (Figure 5B).
Table 6.
Genetic interactions between GLC7, SDS22, and YPI1 alleles
| YPI1-GFP | YPI1-mYFP | YPI1-13Myc | sds22-6 | SDS22-mYFP | |
|---|---|---|---|---|---|
| glc7-129 | Lethala | ± at 24°− at 30°C, 37°C | + | Lethal | Lethal |
| glc7-F256A | Lethal | ± at 30°C− at 37°C | + at 30°C++/− at 37°C | ± at 30° | + at 30°C− at 37°C |
| glc7-127 | ± | + | + | ± | + |
| glc7-132 | + at 30°C | + | + | + | + |
| ± at 37°C | |||||
| glc7-109 | + at 30°Cb | + | + | + | + |
| ± at 37°C | |||||
| sds22-6 | ± at 30°C | + at 30°C | + | ||
| − at 37° | ++/− at 37°C | ||||
| SDS22-mYFP | ± | + | + |
a The following descriptors are used for the growth of double mutants: lethal, double mutants did not grown into colonies at 24 or 30°C; +, normal growth; ±, slow growth, at all temperatures if not stipulated; −, no growth at a specific temperature; ++/−, slight but perceptible slower growth of the double mutant.
b Low glycogen accumulation at all temperatures, as assayed by staining colonies with iodine vapor.
Figure 5.
Ypi1 is a nuclear protein. (A) Indirect immunofluorescence with anti-Myc antibody of YPI1–13Myc (JB385-1A) and YPI1-13Myc glc7-129 (JB409-1C) strains. An untagged WT strain (KT1112) serves as the negative control. (B) Immunoblot analysis with anti-Myc antibody of extracts from the YPI1-13Myc (JB385-1A) and GLC7-13Myc (JB412-1B) strains grown to midlog phase in YPD at 30°C. (C) Indirect immunofluorescence of Ypi1-13Myc in WT (JB385-1A) and sds22-6 (JB403-5C) strains at the permissive (24°C) and nonpermissive (37°C) temperatures. (D) Immunoblot analysis of Ypi1-13Myc with anti-Myc antibody of extracts from the strains described in C.
The dependence of nuclear Glc7 and Sds22 on Ypi1 led us to determine if the nuclear localization of Ypi1 is dependent on normal Glc7 and/or Sds22 activity. Glc7 dependence was assayed by localizing Ypi1 in a glc7-129 mutant, which delays at G2/M in the cell cycle due to activation of the mitotic checkpoint (Bloecher and Tatchell, 1999) and exhibits genetic interactions with YPI1 mutants (see below). Ypi1-13Myc levels appear to be elevated in the nuclei of glc7-129 mutant cells (Figure 5A), but total levels of Ypi1-13Myc appear to be unaltered, as judged by immunoblot analysis of whole cell extracts (Supplementary Figure S3). We also tested other GLC7 mutants with reported cell cycle defects (glc7-127, glc7-F256A) and in all cases nuclear Ypi1 levels are the same or elevated relative to the wild-type strain (data not shown). These results indicate that defects in Glc7 activity do not result in depletion of Ypi1 from the nucleus.
The dependence of Ypi1 localization on Sds22 was assayed in the temperature-sensitive sds22-6 mutant (Peggie et al., 2002). Nuclear levels of Ypi1-13Myc appear reduced in the sds22-6 mutant at the permissive temperature (24°C) relative to the wild type (Figure 5C), but transiently increase after shifting cells to the nonpermissive temperature (37°C) for 1 h. Two and 3 h after the temperature shift, the nuclear levels appear similar to those observed at the permissive temperature. However, nuclear Ypi1 levels appear to increase in the wild-type strain after 1 h at 37°C, suggesting that the increase in nuclear localization of Ypi1 upon temperature upshift is not related to the SDS22 mutation. Immunoblot analysis revealed that total levels of Ypi1-13Myc protein are similar in the WT and sds22-6 strains (Figure 5D). Together, these results indicate that the ability of Ypi1 to localize to the nucleus may be influenced by Sds22 activity.
The Glc7-binding Motif in Ypi1 Is Necessary for Activity In Vivo
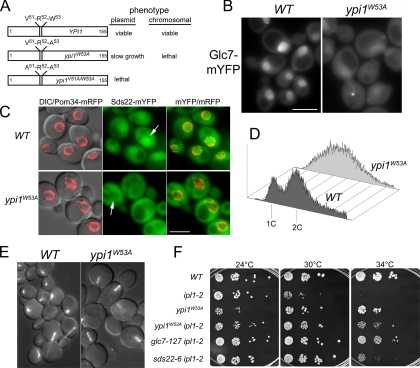
A mutation in the consensus VXF Glc7 binding motif (VRW in Ypi1), ypi1W53A, reduces Glc7 binding and blocks the ability of Ypi1 to inhibit Glc7 activity in vitro (Garcia-Gimeno et al., 2003), but the biological activity of this mutant has not been reported. To address this, we assayed the ability of ypi1W53A, ypi1V51A, and ypi1V51A/W53A alleles to complement the lethality of a YPI1 deletion. The mutants were expressed from the native promoter either on a CEN vector (low-copy) or by ectopic integration at the URA3 locus as outlined in Materials and Methods. The ypi1W53A allele expressed from a low copy CEN plasmid is able to complement the lethality of ypi1Δ. However, the doubling time of the resulting strain is 180 min, twice that of the wild-type strain. ypi1W53A is lethal when integrated into the chromosome (Figure 6A), suggesting that the increased dosage of the gene on the CEN plasmid provides sufficient activity for viability. The ypi1V51A/W53A mutant expressed from the CEN vector is unable to complement the lethality due to ypi1Δ (Figure 6A). These results indicate that a direct interaction between Ypi1 and Glc7 is likely to be essential for the activity of Ypi1.
Figure 6.
Interaction with Ypi1 via the VXW motif is necessary for nuclear targeting and activity of Glc7 in vivo. (A) Diagram of Ypi1 variants and their phenotypes, expressed either from a CEN plasmid or by ectopic integration into the genome. (B) Subcellular distribution of Glc7-mYFP in a ypi1Δ strain carrying either pYPI1 (JB300-3A) or pypi1W53A (JB278-16B). Bar, 5 μm. (C) Subcellular distribution of Sds22-mYFP in a ypi1Δ strain carrying either pYPI1 (JB377-6C) or pypi1W53A (JB378-7D). White arrows indicate nuclei. Bar, 5 μm. (D) Flow cytometry analysis of ypi1Δ mutant cultures containing either pYPI1 (JB300-3A) or pypi1W53A (JB278-16B). Transformants were grown in synthetic medium to midlog phase and processed as described in Materials and Methods. (E) GFP-Tub1 fluorescence in WT (JB309-8B) and ypi1W53A (JB308-7A) cells. Merged DIC and fluorescence images are shown. (F) Serial dilutions of strains were imaged after incubation for 2 d on YPD at the indicated temperatures. The strains used are WT (JB275-1A), ipl1-2 (JB280-5B), ypi1W53A (JB276-8B), ipl1-2 ypi1W53A (JB279-2C), and glc7-127 ipl1-2 (KT1968).
The viability of ypi1W53A strains allowed us to test genetically whether the essential function of Ypi1 is to regulate Glc7. If the phenotype of ypi1W53A strains resembles that caused by Ypi1 depletion, then it is likely that the major cellular role of Ypi1 is to regulate Glc7 or some other protein that binds to the VXF motif. Our results support this hypothesis. Nuclear Glc7-mYFP levels are reduced and bright fluorescent spots are apparent in ypi1W53A mutant cells (Figure 6B). Sds22-mYFP is largely excluded from the nucleus (Figure 6C, arrows) in ypi1W53A mutants. Quantitative analysis revealed that in 85% of WT cells, Sds22-mYFP fluorescence is greater in the nucleus than in the cytoplasm (n = 116 cells). In contrast, for the ypi1W53A mutant, only 8% of the cells show higher levels of fluorescence in the nucleus (n = 131 cells).
As observed for Ypi1-depleted cultures, ypi1W53A cultures contain many large-budded cells with short and misaligned spindles (Figure 6E). In some cells, the spindle is located fully in the bud. Flow cytometry analysis of these cells showed a distribution with broad DNA content centered at 2C (Figure 6D). We were unable to recover mad1Δ ypi1W53A double mutants, suggesting that the spindle assembly checkpoint is essential for the viability of the ypi1W53A mutant. Using the GFP-LacI lacO array system, we observed extensive evidence for chromosome instability. ypi1W53A mutant cells contain multiple chromosome IV spots (data not shown). In conclusion, the similar phenotypes of ypi1W53A and PGAL1-3HA-YPI1 argue that the essential function of Ypi1 is to associate with and regulate Glc7.
YPI1 Mutations Suppress the Temperature Sensitivity of the ipl1-2 Mutant
If Ypi1 regulates the nuclear activity of Glc7, we predict that YPI1 mutations should suppress the temperature-sensitive growth of mutants in IPL1, as has been shown for GLC7 and SDS22 mutants (Hsu et al., 2000; Peggie et al., 2002; Pinsky et al., 2006a). Indeed, ypi1W53A restores growth of an ipl1-2ts mutant at 34°C, similar to the glc7-127 control (Figure 6F).
As discussed above, the inability to detect Ypi1-GFP by fluorescence microscopy or by immunoblot (data not shown) suggests that YPI1-GFP may not be fully functional. Consistent with this idea, nuclear levels of Glc7-mYFP and Sds22-mYFP are reduced in YPI1-GFP cells (Supplementary Figure S4). Interestingly, Glc7-mYFP is retained at the neck of small and medium budded cells and quantitative analysis of fluorescence revealed a significant increase in Glc7-mYFP at the bud neck (Supplementary Figure S4). Although YPI1-GFP strains do not show an obvious growth defect, this allele partially suppresses the temperature-sensitive growth of the ipl1-2 mutant (Supplementary Figure S4) and shows genetic interactions with GLC7 and SDS22 mutations (see below). These results further support the hypothesis that Ypi1 is an activator of nuclear Glc7.
Genetic Interactions between GLC7, SDS22, and YPI1
The dependence on Ypi1 activity for the nuclear accumulation of Glc7 and Sds22 is consistent with biochemical evidence that the three proteins form a ternary complex (Hazbun et al., 2003; Pedelini et al., 2007). If this complex is essential for Glc7 function in vivo, then we would predict strong genetic interactions between mutants in the three genes. Thus, the phenotype of a partial loss of function mutation in Ypi1 should be exacerbated by a mutation in one of its binding partners. The nature of the genetic interactions may also inform us concerning the precise roles of Ypi1 and Sds22. For example, the ability of Ypi1 and Sds22 to inhibit Glc7 activity in vitro (Garcia-Gimeno et al., 2003; Pedelini et al., 2007) could be interpreted to indicate that the complex has an inhibitory role in vivo. To explore these interactions, we assayed the phenotypes of a collection of GLC7 mutants that also contained mutations in either YPI1 or SDS22. We tested alleles of GLC7 that confer cell cycle–related defects (glc7-129, glc7-127, and glc7-F256A; Hsu et al., 2000), and alleles that confer no cell cycle defect (glc7-132 and glc7-109). glc7-132 mutants grow slowly and fail to accumulate glycogen but show no obvious cell cycle defect (Baker et al., 1997), whereas glc7-109 mutants are hypersensitive to many cations and accumulate high levels of glycogen (Williams-Hart et al., 2002). We crossed these mutants to ypi1-GFP and sds22-6 mutant strains, and the double mutants were recovered as haploid meiotic spore clones.
An example of our genetic analysis is provided in Figure 7 and the work is summarized in Table 6. One of the most striking observations is the incompatibility between GLC7 mutations that confer cell cycle defects with YPI1-GFP and sds22-6. In some cases the double mutants are inviable. This was true for glc7-129 with YPI1-GFP or sds22-6. Similar genetic interactions were observed for glc7-127 and glc7-F256A. Because these three GLC7 alleles have recessive defects, the lethality or detrimental effects in combination with recessive YPI1 and SDS22 mutations is consistent with a positive effect of Ypi1 and Sds22 on the cell cycle function(s) of Glc7. Interestingly, the dominant hyperglycogen trait of glc7-109 is suppressed by YPI1-GFP (Figure 7B) and sds22-6 (data not shown), again pointing to a positive or activating role for Ypi1 and Sds22. The known roles for Glc7 in glycogen metabolism are the dephosphorylation of glycogen synthase and phosphorylase (François et al., 1992; Hardy et al., 1994; Wilson et al., 2005). Because glycogen is synthesized in the cytoplasm, the influence of YPI1 on glycogen levels suggests that Ypi1 activity may not be restricted to the nucleus. We never observed a growth condition in which either YPI1-GFP or sds22-6 actually suppress a recessive trait conferred by a GLC7 mutant. These results together with those described above indicate that Ypi1 and Sds22 have positive effect(s) on Glc7 function in vivo.
Figure 7.
Genetic interactions between GLC7 and YPI1. (A and B) Images of serial dilutions of the strains after incubation for 2 d on YPD at designated temperatures. The strains used are YPI1-GFP (JB282-1B), glc7-127 (JB290-1B), YPI1-GFP glc7-127 (JB290-1D), glc7-132 (JB298-5B), YPI1-GFP glc7-132 (JB298-7A), WT (KT1112), glc7-109 (JB297-2A), YPI1-GFP glc7-109 (JB297-1A). The last image in B is that of strains grown on synthetic complete medium that were stained with iodine to assay glycogen levels. Dark color indicates high glycogen levels.
DISCUSSION
Ypi1, Sds22, and Glc7 exist as a ternary complex in cell extracts (Hazbun et al., 2003; Pedelini et al., 2007) and two-hybrid studies indicate that Ypi1 may have separable binding sites for Glc7 and Sds22 within its N- and C-terminus, respectively (Pedelini et al., 2007). YPI1 and SDS22 are evolutionarily conserved and essential for cell viability, suggesting that this ternary complex could play a key role in the regulation of PP1/Glc7. What is the nature of this regulation? Ypi1 was originally identified as an in vitro inhibitor of Glc7 phosphatase activity toward p-nitrophenylphosphate and protein substrates of Glc7 (Garcia-Gimeno et al., 2003; He and Moore, 2005). More recently, Sds22, in a complex with Ypi1, was shown to be a more effective Glc7 inhibitor (Pedelini et al., 2007). Consistent with the possibility that Sds22 and Ypi1 inhibit Glc7 in vivo, overexpression of Ypi1 reduces glycogen levels and overexpression of Sds22 and Ypi1 suppress the temperature-sensitive growth defect of the ipl1-321 mutant (Garcia-Gimeno et al., 2003; Pedelini et al., 2007). The most closely related mammalian protein, Inhibitor-3, is also an effective inhibitor of PP1 activity in vitro (Zhang et al., 1998). These data support the hypothesis that the Ypi1/Sds22/Glc7 ternary complex has little phosphatase activity in vitro, but they do not directly address its physiological role. To address this, we investigated the physiological consequences of reducing Ypi1 activity by either depleting cells of Ypi1 or in cells expressing YPI1 mutants. Our results strongly support the hypothesis that Ypi1 has a positive regulatory or modulatory role on Glc7 in cells.
The first line of evidence to suggest that Ypi1 acts as a positive Glc7 regulator is the cell cycle arrest at G2/M brought about by depletion of Ypi1 using the GAL1 promoter as described here or with the tetO promoter as previously reported (Pedelini et al., 2007). We show here that the arrest is dependent on the spindle assembly checkpoint because mutational inactivation of the checkpoint with a MAD1 mutation reverses the arrest. This phenotype is similar to that of several GLC7 mutants that activate the mitotic checkpoint (Bloecher and Tatchell, 1999; Sassoon et al., 1999). Furthermore, ypi1-W53A mutant cells also accumulate in G2/M with short spindles and are inviable in the absence of the spindle checkpoint. The ypi1-W53A protein is partially defective in Glc7 binding (Garcia-Gimeno et al., 2003), supporting the hypothesis that the major role of Ypi1 is to regulate Glc7 activity. Furthermore, ypi1W53A and YPI1-GFP alleles both suppress partially the temperature sensitivity of ipl1-2 mutants, as do recessive mutations in SDS22 and GLC7 (Francisco and Chan, 1994; Francisco et al., 1994; Sassoon et al., 1999; Hsu et al., 2000; Peggie et al., 2002; Zhang et al., 2005). The results of our genetic analysis further support the conclusion that Ypi1 is a positive regulator of Glc7. GLC7 mutants with cell cycle defects (glc7-129, glc7-127, and glc7-F256A) are inviable in combination with ypi1-W53A and YPI1-GFP, whereas GLC7 mutants with major defects unrelated to the cell cycle are only marginally influenced by ypi1W53A and YP1-GFP. It is important to stress that in no case did we see any alleviation or partial suppression of GLC7-dependent defects by YPI1 mutations, as might be expected if Ypi1 has an inhibitory role in vivo. Together, the genetics and cell biological data point to a positive role of Ypi1 in regulating Glc7 activity.
What is the biochemical role of the Glc7-Sds22-Ypi1 complex? Ypi1 could act as a targeting subunit for Glc7 toward nuclear substrates. The physiologically relevant PP1 holoenzyme toward many substrates consists of the conserved PP1/Glc7 catalytic subunit and a VXF-containing targeting subunit (reviewed in Cohen, 2002; Ceulemans and Bollen, 2004). The targeting subunit acts as a specificity factor by either direct association with the substrate and/or by increasing the catalytic activity toward the specific substrate and decreasing it toward others. Ypi1 is a candidate for a nuclear targeting subunit because it binds to PP1 via a VXF motif and because loss of Ypi1 activity results in a phenotype similar to that of GLC7 mutants. However, at this time there is no evidence that Ypi1 binds to potential nuclear substrates of Glc7, such as Dam1 or Ndc80.
Rather than targeting Glc7 to relevant nuclear substrates, Ypi1 and Sds22 could perform another essential modulatory role. Ypi1 could help target Glc7 and Sds22 to the nucleus. Although mammalian PP1 isoforms and Glc7 are abundant in the nucleus, the mechanism of their nuclear targeting is poorly understood. Many PP1/Glc7 isoforms contain poly-basic sequences at their COOH termini, suggesting that they could be imported by the classic karyopherin-dependent system, but mutational analysis of these sequences indicates that they are not essential for nuclear targeting (Bloecher and Tatchell, 2000; Lesage et al., 2004). However, variants of Glc7 and PP1 that contain mutations in the RVXF-binding channel are less abundant in the nucleus (Wu and Tatchell, 2001; Lesage et al., 2004), suggesting that an RVXF-containing protein is responsible for cotransporting PP1/Glc7 into the nucleus. Overexpression of the nuclear PP1 targeting subunits NIPP1 and PNUTS facilitates nuclear accumulation of PP1γ1-F257A, one variant in the RVXF-binding channel (Lesage et al., 2004), and conversely, overexpression of cytoplasmic Glc7-binding proteins results in decreased levels of nuclear Glc7 (Pinsky et al., 2006a). However, these studies involving protein overexpression do not necessarily inform us on the mechanism of nuclear transport. Ypi1 has a potential NLS near its C-terminus (120ERRHRK125) and a precise deletion of this sequence results in a slow growth phenotype similar to the ypi1W53A mutant (our unpublished observations), but further experiments must be done before we can definitively assign the sequence as an NLS. Nevertheless, it is possible that Glc7 and Sds22 could be imported into the nucleus by piggybacking on Ypi1.
Consistent with a role for Ypi1 in the nuclear targeting of Glc7 and Sds22, nuclear Glc7 levels fall and Sds22 becomes largely excluded from the nucleus after Ypi1 depletion. Our immunoblot analyses of total Ypi1, Sds22, and Glc7 proteins indicate that Glc7 is more abundant than Sds22 and Ypi1, suggesting that a ternary Glc7-Sds22-Ypi1 complex may contain most of the cellular Ypi1 and Sds22 but only a small portion of the total Glc7. This possibility would explain why Ypi1 depletion results in the exclusion of Sds22 from the nucleus but only a reduction in nuclear Glc7 levels.
Ypi1 and Sds22 could also have a chaperone-like role on Glc7 that only indirectly influences phosphatase activity toward nuclear substrates. Precedent for this possibility comes from studies on mammalian inhibitor 2 (I-2) and the related yeast protein, Glc8. I-2 was originally identified as a PP1 inhibitor (Brandt et al., 1975; Huang and Glinsmann, 1976) but was subsequently found to have positive or chaperone-like activity toward PP1 (Alessi et al., 1993; Park et al., 1994). Glc8 also inhibits Glc7 activity in vitro (Tung et al., 1995), but the phenotype of glc8 mutants is consistent with a positive role (Cannon et al., 1994; Tung et al., 1995; Nigavekar et al., 2002; Tan et al., 2003). As observed for YPI1, overexpression of GLC8 and a glc8 null mutation can partially suppress the temperature sensitivity of an ipl1-2 mutation (Tung et al., 1995). However, there are clear differences between Glc8 and Ypi1. YPI1 is an essential gene, whereas glc8 null mutants have little or no growth defect. This could be interpreted to suggest that Glc8 modulates predominantly cytoplasmic Glc7, whereas Ypi1 is specialized toward nuclear Glc7 activity. However, the activities of Glc8 and Ypi1 may not be completely separate, because both influence glycogen metabolism, and mutations in both genes partially suppress ipl1-2. This possibility is also consistent with our recent observation that glc8Δ YPI1-GFP mutants are very slow growing (unpublished observations). We also find it interesting that the levels of Glc7 at the bud neck actually increase in the YPI1-GFP mutant (Figure 6B). This suggests that the Ypi1 activity that is required for nuclear Glc7 activity antagonizes other forms of Glc7 or Glc7 holoenzymes, such as that which binds to the bud neck.
In summary, we have presented evidence that Ypi1 forms an essential positive regulatory complex with Sds22 that largely governs Glc7 activity in the nucleus. Although the precise role of this complex is unknown, the fact that YPI1, SDS22, and GLC7 are all essential and evolutionarily conserved suggests that understanding its role in S. cerevisiae could be relevant to studies of PP1 in metazoans.
Supplementary Material
ACKNOWLEDGMENTS
We thank Kurt Thorn (University of California, San Francisco) for reagents and Lucy Robinson (Louisiana State University Health Sciences Center, Shreveport) for reading the manuscript and thoughtful discussion. This work was supported by the National Institutes of Health Grant GM-47789.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-05-0499) on January 2, 2008.
REFERENCES
- Alessi D. R., Street A. J., Cohen P., Cohen P. T. Inhibitor-2 functions like a chaperone to fold three expressed isoforms of mammalian protein phosphatase-1 into a conformation with the specificity and regulatory properties of the native enzyme. Eur. J. Biochem. 1993;213:1055–1066. doi: 10.1111/j.1432-1033.1993.tb17853.x. [DOI] [PubMed] [Google Scholar]
- Andrews P. D., Stark M. J. Type 1 protein phosphatase is required for maintenance of cell wall integrity, morphogenesis and cell cycle progression in Saccharomyces cerevisiae. J. Cell Sci. 2000;113:507–520. doi: 10.1242/jcs.113.3.507. [DOI] [PubMed] [Google Scholar]
- Axton J. M., Dombradi V., Cohen P.T.W., Glover D. M. One of the protein phosphatase 1 isoenzymes in Drosophila is essential for mitosis. Cell. 1990;63:33–46. doi: 10.1016/0092-8674(90)90286-n. [DOI] [PubMed] [Google Scholar]
- Baker S. H., Frederick D. L., Bloecher A., Tatchell K. Alanine scanning mutagenesis of protein phosphatase type 1 in the yeast Saccharomyces cerevisiae. Genetics. 1997;145:615–626. doi: 10.1093/genetics/145.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black S., Andrews P. D., Sneddon A. A., Stark M. J. A regulated MET3-GLC7 gene fusion provides evidence of a mitotic role for Saccharomyces cerevisiae protein phosphatase 1. Yeast. 1995;11:747–759. doi: 10.1002/yea.320110806. [DOI] [PubMed] [Google Scholar]
- Bloecher A., Tatchell K. Defects in Saccharomyces cerevisiae protein phosphatase type I activate the spindle/kinetochore checkpoint. Genes Dev. 1999;13:517–522. doi: 10.1101/gad.13.5.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloecher A., Tatchell K. Dynamic localization of protein phosphatase type 1 in the mitotic cell cycle of Saccharomyces cerevisiae. J. Cell Biol. 2000;149:125–140. doi: 10.1083/jcb.149.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt H., Lee E. Y., Killilea S. D. A protein inhibitor of rabbit liver phosphorylase phosphatase. Biochem. Biophys. Res. Commun. 1975;63:950–956. doi: 10.1016/0006-291x(75)90661-0. [DOI] [PubMed] [Google Scholar]
- Cannon J. F., Pringle J. R., Fiechter A., Khalil M. Characterization of glycogen-deficient glc mutants of Saccharomyces cerevisiae. Genetics. 1994;136:485–503. doi: 10.1093/genetics/136.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon J. F., Tatchell K. Characterization of Saccharomyces cerevisiae genes encoding subunits of cyclic AMP-dependent protein kinase. Mol. Cell. Biol. 1987;7:2653–2663. doi: 10.1128/mcb.7.8.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceulemans H., Bollen M. Functional diversity of protein phosphatase-1, a cellular economizer and reset button. Physiol. Rev. 2004;84:1–39. doi: 10.1152/physrev.00013.2003. [DOI] [PubMed] [Google Scholar]
- Cheeseman I. M., Anderson S., Jwa M., Green E. M., Kang J., Yates J. R., 3rd, Chan C. S., Drubin D. G., Barnes G. Phospho-regulation of kinetochore-microtubule attachments by the Aurora kinase Ipl1p. Cell. 2002;111:163–172. doi: 10.1016/s0092-8674(02)00973-x. [DOI] [PubMed] [Google Scholar]
- Cheeseman I. M., Chappie J. S., Wilson-Kubalek E. M., Desai A. The conserved KMN network constitutes the core microtubule-binding site of the kinetochore. Cell. 2006;127:983–997. doi: 10.1016/j.cell.2006.09.039. [DOI] [PubMed] [Google Scholar]
- Christianson T. W., Sikorski R. S., Dante M., Shero J. H., Hieter P. Multifunctional yeast high-copy-number shuttle vectors. Gene. 1992;110:119–122. doi: 10.1016/0378-1119(92)90454-w. [DOI] [PubMed] [Google Scholar]
- Cimini D., Wan X., Hirel C. B., Salmon E. D. Aurora kinase promotes turnover of kinetochore microtubules to reduce chromosome segregation errors. Curr. Biol. 2006;16:1711–1718. doi: 10.1016/j.cub.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Clotet J., Posas F., de Nadal E., Ariño J. The NH2-terminal extension of protein phosphatase PPZ1 has an essential functional role. J. Biol. Chem. 1996;271:26349–26355. doi: 10.1074/jbc.271.42.26349. [DOI] [PubMed] [Google Scholar]
- Cohen P. T. Protein phosphatase 1—targeted in many directions. J. Cell Sci. 2002;115:241–256. doi: 10.1242/jcs.115.2.241. [DOI] [PubMed] [Google Scholar]
- Davis N. G., Horecka J. L., Sprague G. F., Jr Cis- and trans-acting functions required for endocytosis of the yeast pheromone receptors. J. Cell Biol. 1993;122:53–65. doi: 10.1083/jcb.122.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca J. G., Gall W. E., Ciferri C., Cimini D., Musacchio A., Salmon E. D. Kinetochore microtubule dynamics and attachment stability are regulated by Hec1. Cell. 2006;127:969–982. doi: 10.1016/j.cell.2006.09.047. [DOI] [PubMed] [Google Scholar]
- Doonan J. H., Morris N. R. The bimG gene of Aspergillus nidulans, required for completion of anaphase, encodes a homolog of mammalian phosphoprotein phosphatase 1. Cell. 1989;57:987–996. doi: 10.1016/0092-8674(89)90337-1. [DOI] [PubMed] [Google Scholar]
- Fernandez A., Brautigan D. L., Lamb N.J.C. Protein phosphatase type 1 in mammalian cell mitosis: chromosome localization and involvement in mitotic exit. J. Cell Biol. 1992;116:1421–1430. doi: 10.1083/jcb.116.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francisco L., Chan C. S. Regulation of yeast chromosome segregation by Ipl1 protein kinase and type 1 protein phosphatase. Cell. Mol. Biol. Res. 1994;40:207–213. [PubMed] [Google Scholar]
- Francisco L., Wang W., Chan C.S.M. Type 1 protein phosphatase acts in opposition to Ipl1 protein kinase in regulating yeast chromosome segregation. Mol. Cell. Biol. 1994;14:4731–4740. doi: 10.1128/mcb.14.7.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- François J. M., Thompson-Jaeger S., Skroch J., Zellenka U., Spevak W., Tatchell K. GAC1 may encode a regulatory subunit for protein phosphatase type 1 in Saccharomyces cerevisiae. EMBO J. 1992;11:87–96. doi: 10.1002/j.1460-2075.1992.tb05031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadde S., Heald R. Mechanisms and molecules of the mitotic spindle. Curr. Biol. 2004;14:R797–R805. doi: 10.1016/j.cub.2004.09.021. [DOI] [PubMed] [Google Scholar]
- Garcia-Gimeno M. A., Munoz I., Arino J., Sanz P. Molecular characterization of Ypi1, a novel Saccharomyces cerevisiae type 1 protein phosphatase inhibitor. J. Biol. Chem. 2003;278:47744–47752. doi: 10.1074/jbc.M306157200. [DOI] [PubMed] [Google Scholar]
- Ghaemmaghami S., Huh W. K., Bower K., Howson R. W., Belle A., Dephoure N., O'Shea E. K., Weissman J. S. Global analysis of protein expression in yeast. Nature. 2003;425:737–741. doi: 10.1038/nature02046. [DOI] [PubMed] [Google Scholar]
- Gietz D., St. Jean A., Woods R. A., Schiestl R. H. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 1992;20:1425. doi: 10.1093/nar/20.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesbeck O., Baird G. S., Campbell R. E., Zacharias D. A., Tsien R. Y. Reducing the environmental sensitivity of yellow fluorescent protein. Mechanism and applications. J. Biol. Chem. 2001;276:29188–29194. doi: 10.1074/jbc.M102815200. [DOI] [PubMed] [Google Scholar]
- Hardwick K. G., Murray A. W. Mad1p, a phosphoprotein component of the spindle assembly checkpoint in budding yeast. J. Cell Biol. 1995;131:709–720. doi: 10.1083/jcb.131.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy T. A., Huang D., Roach P. J. Interactions between cAMP-dependent and SNF1 protein kinases in the control of glycogen accumulation in Saccharomyces cerevisiae. J. Biol. Chem. 1994;269:27907–27913. [PubMed] [Google Scholar]
- Hauf S., Cole R. W., LaTerra S., Zimmer C., Schnapp G., Walter R., Heckel A., van Meel J., Rieder C. L., Peters J. M. The small molecule Hesperadin reveals a role for Aurora B in correcting kinetochore-microtubule attachment and in maintaining the spindle assembly checkpoint. J. Cell Biol. 2003;161:281–294. doi: 10.1083/jcb.200208092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauf S., Watanabe Y. Kinetochore orientation in mitosis and meiosis. Cell. 2004;119:317–327. doi: 10.1016/j.cell.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Hazbun T. R., et al. Assigning function to yeast proteins by integration of technologies. Mol. Cell. 2003;12:1353–1365. doi: 10.1016/s1097-2765(03)00476-3. [DOI] [PubMed] [Google Scholar]
- He X., Moore C. Regulation of yeast mRNA 3′ end processing by phosphorylation. Mol. Cell. 2005;19:619–629. doi: 10.1016/j.molcel.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Hisamoto N., Frederick D. L., Sugimoto K., Tatchell K., Matsumoto K. The EGP1 gene may be a positive regulator of protein phosphatase type 1 in the growth control of Saccharomyces cerevisiae. Mol. Cell. Biol. 1995;15:3767–3776. doi: 10.1128/mcb.15.7.3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisamoto N., Sugimoto K., Matsumoto K. The Glc7 type 1 protein phosphatase of Saccharomyces cerevisiae is required for cell cycle progression in G2/M. Mol. Cell. Biol. 1994;14:3158–3165. doi: 10.1128/mcb.14.5.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho Y., et al. Systematic identification of protein complexes in Saccharomyces cerevisiae by mass spectrometry. Nature. 2002;415:180–183. doi: 10.1038/415180a. [DOI] [PubMed] [Google Scholar]
- Hsu J.-Y., et al. Mitotic phosphorylation of histone H3 is governed by Ipl1/aurora kinase and Glc7p/PP1 phosphatase in budding yeast and nematodes. Cell. 2000;102:279–291. doi: 10.1016/s0092-8674(00)00034-9. [DOI] [PubMed] [Google Scholar]
- Huang F. L., Glinsmann W. H. Separation and characterization of two phosphorylase phosphatase inhibitors from rabbit skeletal muscle. Eur. J. Biochem. 1976;70:419–426. doi: 10.1111/j.1432-1033.1976.tb11032.x. [DOI] [PubMed] [Google Scholar]
- Huang H. S., Pozarowski P., Gao Y., Darzynkiewicz Z., Lee E. Y. Protein phosphatase-1 inhibitor-3 is co-localized to the nucleoli and centrosomes with PP1gamma1 and PP1alpha, respectively. Arch. Biochem. Biophys. 2005;443:33–44. doi: 10.1016/j.abb.2005.08.021. [DOI] [PubMed] [Google Scholar]
- Huh W. K., Falvo J. V., Gerke L. C., Carroll A. S., Howson R. W., Weissman J. S., O'Shea E. K. Global analysis of protein localization in budding yeast. Nature. 2003;425:686–691. doi: 10.1038/nature02026. [DOI] [PubMed] [Google Scholar]
- Janke C., Ortiz J., Lechner J., Shevchenko A., Magiera M. M., Schramm C., Schiebel E. The budding yeast proteins Spc24p and Spc25p interact with Ndc80p and Nuf2p at the kinetochore and are important for kinetochore clustering and checkpoint control. EMBO J. 2001;20:777–791. doi: 10.1093/emboj/20.4.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston J. A., Ward C. L., Kopito R. R. Aggresomes: a cellular response to misfolded proteins. J. Cell Biol. 1998;143:1883–1898. doi: 10.1083/jcb.143.7.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J., Cheeseman I. M., Kallstrom G., Velmurugan S., Barnes G., Chan C. S. Functional cooperation of Dam1, Ipl1, and the inner centromere protein (INCENP)-related protein Sli15 during chromosome segregation. J. Cell Biol. 2001;155:763–774. doi: 10.1083/jcb.200105029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozubowski L., Panek H., Rosenthal A., Bloecher A., DeMarini D. J., Tatchell K. A Bni4-Glc7 phosphatase complex that recruits chitin synthase to the site of bud emergence. Mol. Biol. Cell. 2003;14:26–39. doi: 10.1091/mbc.E02-06-0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogan N. J., et al. Global landscape of protein complexes in the yeast Saccharomyces cerevisiae. Nature. 2006;440:637–643. doi: 10.1038/nature04670. [DOI] [PubMed] [Google Scholar]
- Lesage B., Beullens M., Nuytten M., Van Eynde A., Keppens S., Himpens B., Bollen M. Interactor-mediated nuclear translocation and retention of protein phosphatase-1. J. Biol. Chem. 2004;279:55978–55984. doi: 10.1074/jbc.M411911200. [DOI] [PubMed] [Google Scholar]
- Lew D. J., Burke D. J. The spindle assembly and spindle position checkpoints. Annu. Rev. Genet. 2003;37:251–282. doi: 10.1146/annurev.genet.37.042203.120656. [DOI] [PubMed] [Google Scholar]
- Li Y., Bachant J., Alcasabas A. A., Wang Y., Qin J., Elledge S. J. The mitotic spindle is required for loading of the DASH complex onto the kinetochore. Genes Dev. 2002;16:183–197. doi: 10.1101/gad.959402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine M. S., McKenzie A., 3rd, Demarini D. J., Shah N. G., Wach A., Brachat A., Philippsen P., Pringle J. R. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998;14:953–961. doi: 10.1002/(SICI)1097-0061(199807)14:10<953::AID-YEA293>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- MacKelvie S. H., Andrews P. D., Stark M.J.R. The Saccharomyces cerevisiae gene SDS22 encodes a potential regulator of the mitotic function of yeast type 1 protein phosphatase. Mol. Cell. Biol. 1995;15:3777–3785. doi: 10.1128/mcb.15.7.3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis, Sambrook T. J., Fritsch E. F. Molecular Cloning a Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Nigavekar S. S., Tan Y. S., Cannon J. F. Glc8 is a glucose-repressible activator of Glc7 protein phosphatase-1. Arch. Biochem. Biophys. 2002;404:71–79. doi: 10.1016/s0003-9861(02)00231-x. [DOI] [PubMed] [Google Scholar]
- Ohkura H., Kinoshita N., Miyatani S., Toda T., Yanagida M. The fission yeast dis2+ gene required for chromosome disjoining encodes one of two putative type 1 protein phosphatases. Cell. 1989;57:997–1007. doi: 10.1016/0092-8674(89)90338-3. [DOI] [PubMed] [Google Scholar]
- Ohkura H., Yanagida M. S. pombe gene sds22+ essential for a midmitotic transition encodes a leucine-rich repeat protein that positively modulates protein phosphatase-1. Cell. 1991;64:149–157. doi: 10.1016/0092-8674(91)90216-l. [DOI] [PubMed] [Google Scholar]
- Park I. K., Roach P., Bondor J., Fox S. P., DePaoli-Roach A. A. Molecular mechanism of the synergistic phosphorylation of phosphatase inhibitor-2. Cloning, expression, and site-directed mutagenesis of inhibitor-2. J. Biol. Chem. 1994;269:944–954. [PubMed] [Google Scholar]
- Pedelini L., Marquina M., Arino J., Casamayor A., Sanz L., Bollen M., Sanz P., Garcia-Gimeno M. A. YPI1 and SDS22 proteins regulate the nuclear localization and function of yeast type 1 phosphatase Glc7. J. Biol. Chem. 2007;282:3282–3292. doi: 10.1074/jbc.M607171200. [DOI] [PubMed] [Google Scholar]
- Peggie M. W., MacKelvie S. H., Bloecher A., Knatko E. V., Tatchell K., Stark M. J. Essential functions of Sds22p in chromosome stability and nuclear localization of PP1. J. Cell Sci. 2002;115:195–206. doi: 10.1242/jcs.115.1.195. [DOI] [PubMed] [Google Scholar]
- Pinsky B. A., Biggins S. The spindle checkpoint: tension versus attachment. Trends Cell Biol. 2005;15:486–493. doi: 10.1016/j.tcb.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Pinsky B. A., Kotwaliwale C. V., Tatsutani S. Y., Breed C. A., Biggins S. Glc7/protein phosphatase 1 regulatory subunits can oppose the Ipl1/aurora protein kinase by redistributing Glc7. Mol. Cell. Biol. 2006a;26:2648–2660. doi: 10.1128/MCB.26.7.2648-2660.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinsky B. A., Kung C., Shokat K. M., Biggins S. The Ipl1-Aurora protein kinase activates the spindle checkpoint by creating unattached kinetochores. Nat. Cell Biol. 2006b;8:78–83. doi: 10.1038/ncb1341. [DOI] [PubMed] [Google Scholar]
- Sassoon I., Severin F. F., Andrews P. D., Taba M.-R., Kaplan K. B., Ashford A. J., Stark M.J.R., Sorger P. K., Hyman A. A. Regulation of Saccharomyces cerevisiae kinetochores by the type 1 phosphatase Glc7p. Genes Dev. 1999;13:545–555. doi: 10.1101/gad.13.5.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang C., Hazbun T. R., Cheeseman I. M., Aranda J., Fields S., Drubin D. G., Barnes G. Kinetochore protein interactions and their regulation by the Aurora kinase Ipl1p. Mol. Biol. Cell. 2003;14:3342–3355. doi: 10.1091/mbc.E02-11-0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheff M. A., Thorn K. S. Optimized cassettes for fluorescent protein tagging in Saccharomyces cerevisiae. Yeast. 2004;21:661–670. doi: 10.1002/yea.1130. [DOI] [PubMed] [Google Scholar]
- Sherman F., Fink G. R., Hicks J. B. Methods in Yeast Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1986. [Google Scholar]
- Sikorksi R. S., Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straight A. F., Marchall W. F., Sedat J. W., Murray A. W. Mitosis in living budding yeast: anaphase A but no metaphase plate. Science. 1997;277:574–578. doi: 10.1126/science.277.5325.574. [DOI] [PubMed] [Google Scholar]
- Stuart J. S., Frederick D. L., Varner C. M., Tatchell K. The mutant type 1 protein phosphatase encoded by glc7-1 from Saccharomyces cerevisiae fails to interact productively with the GAC1-encoded regulatory subunit. Mol. Cell. Biol. 1994;14:896–905. doi: 10.1128/mcb.14.2.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y. S., Morcos P. A., Cannon J. F. Pho85 phosphorylates the Glc7 protein phosphatase regulator Glc8 in vivo. J. Biol. Chem. 2003;278:147–153. doi: 10.1074/jbc.M208058200. [DOI] [PubMed] [Google Scholar]
- Tanaka T. U., Rachidi N., Janke C., Pereira G., Galova M., Schiebel E., Stark M. J., Nasmyth K. Evidence that the Ipl1-Sli15 (Aurora kinase-INCENP) complex promotes chromosome bi-orientation by altering kinetochore-spindle pole connections. Cell. 2002;108:317–329. doi: 10.1016/s0092-8674(02)00633-5. [DOI] [PubMed] [Google Scholar]
- Tanaka T. U., Stark M. J., Tanaka K. Kinetochore capture and bi-orientation on the mitotic spindle. Nat. Rev. Mol. Cell Biol. 2005;6:929–942. doi: 10.1038/nrm1764. [DOI] [PubMed] [Google Scholar]
- Trinkle-Mulcahy L., Andersen J., Lam Y. W., Moorhead G., Mann M., Lamond A. I. Repo-Man recruits PP1 gamma to chromatin and is essential for cell viability. J. Cell Biol. 2006;172:679–692. doi: 10.1083/jcb.200508154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinkle-Mulcahy L., Andrews P. D., Wickramasinghe S., Sleeman J., Prescott A., Lam Y. W., Lyon C., Swedlow J. R., Lamond A. I. Time-lapse imaging reveals dynamic relocalization of PP1gamma throughout the mammalian cell cycle. Mol. Biol. Cell. 2003;14:107–117. doi: 10.1091/mbc.E02-07-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung H. Y., Wang W., Chan C. S. Regulation of chromosome segregation by Glc8p, a structural homolog of mammalian inhibitor 2 that functions as both an activator and an inhibitor of yeast protein phosphatase 1. Mol. Cell. Biol. 1995;15:6064–6074. doi: 10.1128/mcb.15.11.6064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venturi G. M., Bloecher A., Williams-Hart T., Tatchell K. Genetic interactions between GLC7, PPZ1 and PPZ2 in Saccharomyces cerevisiae. Genetics. 2000;155:69–83. doi: 10.1093/genetics/155.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Burke D. J. Checkpoint genes required to delay cell division in response to nocodazole respond to impaired kinetochore function in the yeast Saccharomyces cerevisiae. Mol. Cell. Biol. 1995;15:6838–6844. doi: 10.1128/mcb.15.12.6838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermann S., Cheeseman I. M., Anderson S., Yates J. R., 3rd, Drubin D. G., Barnes G. Architecture of the budding yeast kinetochore reveals a conserved molecular core. J. Cell Biol. 2003;163:215–222. doi: 10.1083/jcb.200305100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams-Hart T., Wu X., Tatchell K. Protein phosphatase type 1 regulates ion homeostasis in Saccharomyces cerevisiae. Genetics. 2002;160:1423–1437. doi: 10.1093/genetics/160.4.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson W. A., Wang Z., Roach P. J. Regulation of yeast glycogen phosphorylase by the cyclin-dependent protein kinase Pho85p. Biochem. Biophys. Res. Commun. 2005;329:161–167. doi: 10.1016/j.bbrc.2005.01.106. [DOI] [PubMed] [Google Scholar]
- Wu X., Tatchell K. Mutations in yeast protein phosphatase type 1 that affect targeting subunit binding. Biochemistry. 2001;40:7410–7420. doi: 10.1021/bi002796k. [DOI] [PubMed] [Google Scholar]
- Zhang J., Zhang L., Zhao S., Lee E. Y. Identification and characterization of the human HCG V gene product as a novel inhibitor of protein phosphatase-1. Biochemistry. 1998;37:16728–16734. doi: 10.1021/bi981169g. [DOI] [PubMed] [Google Scholar]
- Zhang K., Lin W., Latham J. A., Riefler G. M., Schumacher J. M., Chan C., Tatchell K., Hawke D. H., Kobayashi R., Dent S. Y. The set 1 methyltransferase opposes ipl 1 aurora kinase functions in chromosome segregation. Cell. 2005;122:723–734. doi: 10.1016/j.cell.2005.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.