Abstract
Background
Beta3-adrenergic receptor (beta3-AR) stimulation inhibits cardiac contractility.
Objective
To test the hypothesis that beta3-AR stimulation is antiarrhythmic.
Methods
We implanted a radiotransmitter for continuous ECG monitoring in 18 dogs with a tendency for high incidence of spontaneous ventricular tachycardia (VT). Ten of 18 had subcutaneous continuous BRL37344 (beta3-AR agonist) infusion (experimental group) for 1 month. The other dogs were controls. Western blotting studies were performed on tissues sampled from the noninfarcted left ventricular free wall of all dogs that survived the 60-day follow up period.
Results
Phase-2 VT appeared significantly later in the experimental group than in the control group (p<0.05). The number of VT episodes in the experimental group was significantly lower than control during both the first month (0.5 ± 0.95 episode/d vs. 2.6 ± 2.3 episode/d) and the second month (0.2 ± 0.2 episode/d vs. 1.2 ± 1.1 episode/d, p<0.05 for both). The experimental group had shorter QTc than control (p<0.002). The experimental group had decreased protein levels for sodium calcium exchanger and dihydropyridine receptor, increased beta3-AR expression, without changes in beta1-AR, beta2-AR. The average heart weight and the left ventricular free wall thickness in the experimental group (226 ± 17 g and 15.1 ± 1.2 mm, respectively) was significantly lower than control (265 ± 21 g and 17.4 ± 2.5 mm, respectively, p<0.05 for both). There was no difference in the incidences of sudden cardiac death (SCD) in these two groups of dogs.
Conclusion
Beta3-AR stimulation significantly reduces the occurrence of ventricular tachycardia.
Introduction
Beta3-adrenergic receptors (ARs) have generated considerable interest as a potential target for antiobesity and antidiabetic drugs.1 In addition to brown fat, beta3-ARs also are present in cardiomyocytes, and beta3-AR stimulation inhibits cardiac contractility.2, 3 Beta3-AR stimulation also has been shown to affect the electrical properties of the heart. For example, Kathofer et al4 found that beta3-AR was functionally coupled to KvLQT1/minK, and KvLQT1/minK current was increased after stimulation with isoproterenol. Gauthier et al2 reported that in human endomyocardial biopsies, selective beta3-AR stimulation decreased action potential amplitude and duration. Beta3-AR stimulation also was found to induce direct inhibition of intracellular calcium ([Ca2+]i) transient and ICa-L.3 However, little information exists regarding the effects of beta3-AR stimulation on cardiac arrhythmogenesis in-vivo. Recently, we observed that beta3-AR was upregulated in a canine model of sudden cardiac death (SCD).5 An upregulation of beta3-AR also was observed in human hearts with ischemic or dilated cardiomyopathy,6 in the failing canine myocardium,3 and in hearts of long-term diabetic rats.7 Whether or not the upregulation of beta3-AR is merely in response to excess catecholamines or confer additional risk or benefit for arrhythmia is unclear. Beta3-AR was suggested to function as a buffer against excessive beta1-AR and beta2-AR stimulation in disease conditions associated with hyperadrenergic activity, such as heart failure.8, 9 The purpose of the present study, therefore, was to test the hypothesis that chronic beta3-AR stimulation with selective beta3-AR agonist BRL37344 infusion suppresses the occurrence of ventricular tachycardia (VT) in a canine model with a high incidence of spontaneous VT.
Methods
The animal research protocol was approved by the Institutional Animal Care and Use Committee and conforms to the guidelines of the American Heart Association. We studied a total of 18 mongrel dogs (20 to 28 Kg).
Surgical procedure during first surgery
All dogs were prepared according to previously published procedures.10 These dogs with continuous infusion of nerve growth factor (NGF) to the left stellate ganglion (LSG) have a high incidence of spontaneous VT.10 Briefly, we first created complete atrioventricular block by radiofrequency catheter ablation of the atrioventricular junction and myocardial infarction by ligating the left anterior descending coronary artery below the first diagonal branch. We then implanted a pacemaker for back up pacing (40 bpm), and an osmotic pump (Model 2ML4, Alzet Osmotic Pumps, Cupertino, CA) for continuous infusion of NGF to LSG. For Long-term continuous ECG recordings,11 we implanted a D70-EEE Data Sciences International (DSI) transmitter in all dogs. ECG was continuously recorded in the implanted transmitter, transmitted to RMC-1 receivers, transferred to a computer via Matrix, and automatically stored by Dataquest ART acquisition software in a hard drive for both on-line and off-line analyses. This wireless recording system allowed an uninterrupted, 24 hrs a day and 7 days a week (24/7), ECG recording in ambulatory dogs.
To test the effects of beta3-AR stimulation, we implanted an osmotic pump subcutaneously in 10 dogs (experimental group) to continuously infuse a beta3-AR specific agonist BRL37344 (Sigma) at 1.5μg/kg/hr.12 BRL37344 infusion was predicted to last about one month based on the flow rate of the osmotic pump. DSI monitoring continued afterwards for an additional month to determine if there was a rebound arrhythmia. The remaining 8 dogs without BRL37344 infusion were also monitored for up to 2 months and were used as controls.
Western Blotting Studies
To explore the possible effects of chronic beta3-AR agonist stimulation on the expression of beta-ARs, Ca handling proteins, potassium channel proteins, and sympathetic nerve related proteins, we did Western blotting studies on hearts from all dogs that survived the 60-day follow up period. Tissues were sampled from the noninfarcted left ventricular free wall. For Ca handling proteins and potassium channel proteins, we used membrane protein prepared as previously described.5 For beta-ARs, NGF, tyrosine hydroxylase (TH) and growth associated protein 43 (GAP43), we used whole protein.13 Equal amounts of denatured proteins (If membrane protein was used, 5 μg was loaded in each well; If whole protein was used, 60 μg was loaded in each well) were fractionated on 4-20% Gradient gel (Bio-Rad) and transferred to PVDF membranes (Bio-Rad). The membranes were blocked with 5% nonfat dry milk in PBST (containing 0.05% Tween 20), followed by overnight incubation at 4°C with the primary antibody (hERG, 1:200, beta1-AR, 1:200, beta2-AR, 1:200, beta3-AR, 1:100, NGF, 1:200, Santa Cruz Biotechnology; GAP43, 1:1000 and TH, 1:500, Chemicon; sarcoplasmic reticulum Ca2+-ATPase (SERCA2a) 1:2500, type 2 ryanodine receptor (RyR2) 1:5000, sodium calcium exchanger (NaCaX), 1:1000, KvLQT1, 1:800, Aviva Systems Biology, MinK, 1:200, Alomone Labs, dihydropyridine receptor (DHPR) 1:500, Research Diagnostics Inc.), washed in PBST, incubated with horseradish peroxidase–conjugated secondary antibody, and revealed by Immun-Star HRP Substrate (Bio-Rad). For normalization of gel loading, the same Western blots were reprobed with anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH), 1:10,000, Research Diagnostics Inc.). The density of bands on Western blots was quantified by the use of ImagePro software. Densitometric values for each sample were expressed as a ratio of the mean value obtained for the control samples analyzed on the same gel.
Immunohistochemical Studies
Three specimens were taken from the noninfarcted myocardium of the left ventricle around the posterior and anterior papillary muscles in each dog that survived the 60- day postoperative period. A modified immunohistochemical ABC method was used for immunostaining for GAP43 and TH. The density of stained nerves was determined using ImagePro software and expressed as the nerve area divided by the total area examined (μm2/mm2).14 The nerve density of each slide was determined by the average of 3 fields with the highest nerve density. The average nerve density of three specimens of each dog was used to compare.
Statistical Analysis
We manually reviewed all recordings from the two groups and quantified the episodes of VT and SCD. All quantitative data were presented as means ± SD. The QT intervals of spontaneous (non-paced) beats14 was measured based on the ECG recorded by DSI transmitter. QTc is expressed as the QT interval in ms divided by the square root of the proceeding RR interval in seconds. Multivariate ANOVA was used to determine the statistical significance of time (factor 1) and treatment (factor 2) effect on QT interval. For other data sets, the Student t tests were used to compare the means of two groups. Analysis of Variance (ANOVA) with Newman-Keuls test was used for multiple comparisons. A p value ≤ 0.05 was considered significant.
Results
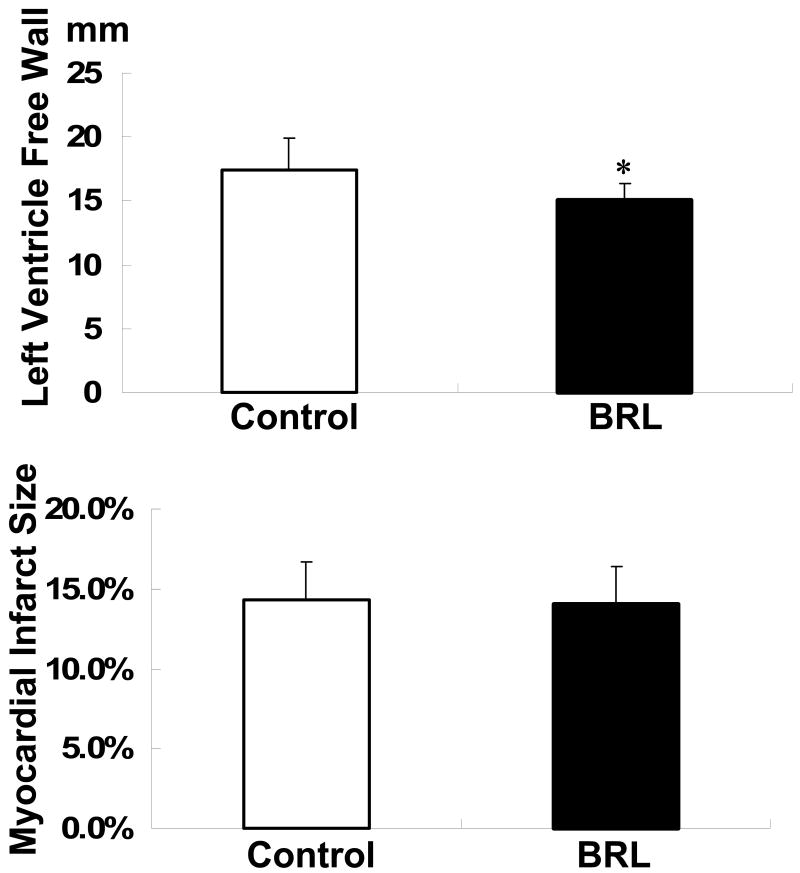
All dogs survived the first surgery without significant complications. However, the dogs in the experimental group were observed to have worse appetites but appeared more active compared to the dogs in the control group. At first surgery, there was no significant difference in average body weight between the experimental group (23.3 ± 1.8 kg) and the control group (24.4 ± 2.8 kg). In the experimental group, the average body weight at the end of follow up was significantly lower than that at first surgery (21± 2.1 kg, p<0.05). There were no significant changes of body weight in the control group. The average heart weight in the experimental group was also significantly lower than that in the control group (226 ± 17 g vs. 265 ± 21 g, p<0.05) at autopsy. However, there was no significant difference in heart weight/body weight ratio between the experimental group (10.8 ± 1.5 g/kg) and the control group (10.9 ± 1.4 g/kg). The left ventricle free wall was thinner in the experimental group (15.1 ± 1.2 mm) than the control group (17.4 ± 2.5 mm, p<0.05) (Figure 1). The size of infarct measured as described previously 10 in the experimental group was not significantly different from the control group (14.0 ± 2.4% vs. 14.3 ± 2.4%) (Figure 1).
Figure 1.
The thickness of left ventricle free wall and myocardial infarct size in two groups. *, p< 0.05; versus control group.
Effects of Beta3-AR Stimulation on VT and SCD
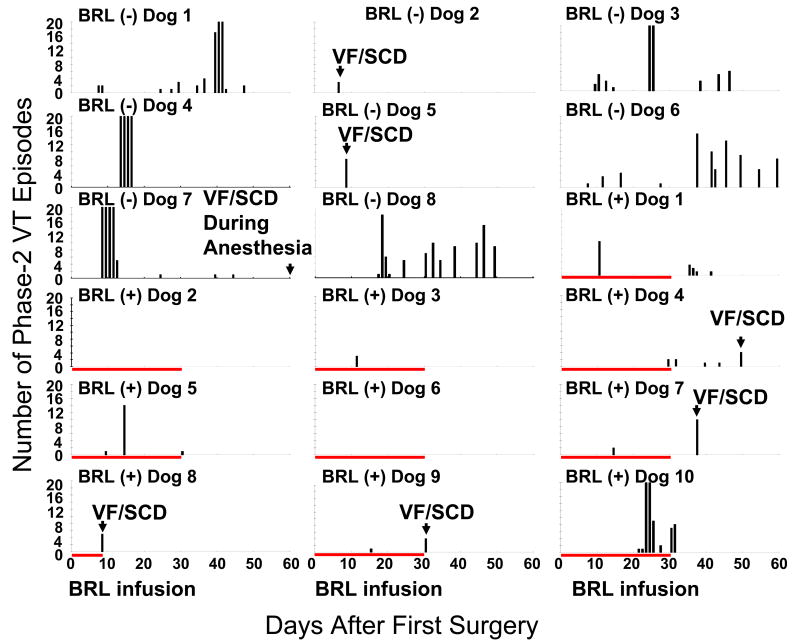
We defined phase 1 VT as VT induced by acute ischemia. The phase 1 VT usually continued for a few days, followed by an arrhythmia-free period. Phase 2 VT is the VT that occurred after this arrhythmia free period.10 All dogs had frequent phase-1 VT immediately after surgery. Phase-1 VT lasted 2-3 days. All 8 dogs in the control group developed paroxysmal episodes of phase-2 VT after an arrhythmia-free period of 6.5 ± 4.1 days. All of our dogs had complete atrioventricular block, so if the spontaneous ventricular rate exceeded 100 bpm for more than 4 beats, we defined it as one episode of VT. In the experimental group, 2 dogs did not have phase-2 VT at all and 8 dogs developed phase-2 VT after 13.8 ± 7.7 days of an arrhythmia-free period, significantly later compared with the control group (p<0.05). Figure 2 shows the number of phase-2 VT episodes per day and time of SCD in all dogs studied. Note that dogs 2 and 6 of the experimental group had no phase 2-VT during 2 months of follow up. The average VT episodes from postoperative day (POD) 7 to 30 in the experimental group was significantly lower compared to the control group (0.5 ± 0.95 episode/d (n=9) vs. 2.6 ± 2.3 episodes/d (n=6, p<0.05). From POD 31 to POD 60, the number of VT episodes in dogs that continued to survive in the experimental group (0.2 ± 0.2 episodes/d, n=6) were also lower than that of the control group (1.2 ±1.1 episodes/d, n=6, p<0.05). There was no significant difference in the duration of VT between the control and experimental groups (15.4 ± 8.1 s vs. 14.7 ± 9.6 s, p=NS).
Figure 2.
Number of spontaneous phase-2 VT episodes after surgery. VT episodes more than 20/day were clipped. BRL (-) indicates control group; BRL (+) indicates experimental group. Red bar indicates BRL37344 infusion interval. Note that dogs 2 and 6 of the experimental group had no VT for an entire 60-day period.
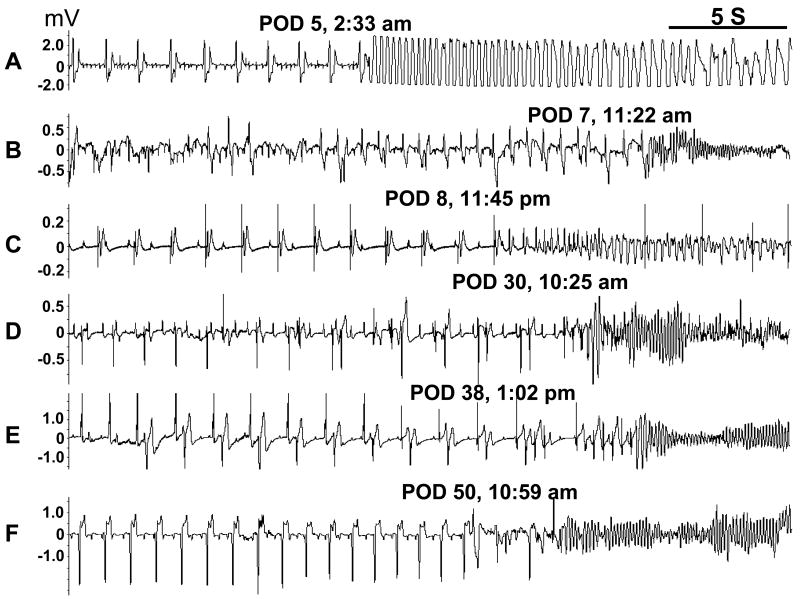
SCD occurred in 3/8 control dogs and in 4/10 experimental dogs (p=NS). The time of death is shown in Figure 2 and the rhythm recorded at the time of SCD was ventricular fibrillation in 6 dogs (Figure 3). A control dog died at day 60 during the induction of anesthesia and the rhythm was not recorded. All ventricular fibrillation episodes were initiated by a premature ventricular contraction that occurred on the T wave (R on T), including 3 episodes (Figures 3D, 3E and 3F) with short-long-short coupling preceding the onset of VF. Of note is that the majority of SCD episodes (3/4) in the experimental group occurred in the second 30 days of monitoring, when BRL37344 infusion had stopped.
Figure 3.
Spontaneous VF and SCD. These episodes were from both the control group (A-B) and the experimental group (C-F). All VF episodes were initiated by a premature ventricular contraction that occurred before the end of the T wave (R on T).
Effects of Beta3-AR Stimulation on QTc Intervals
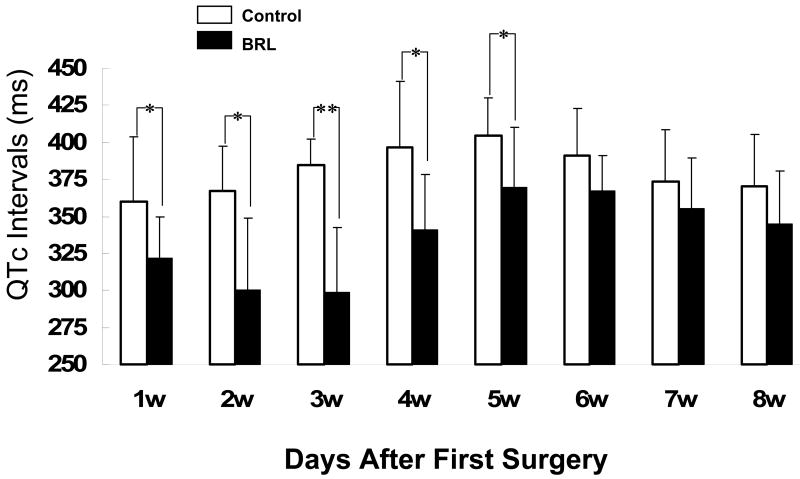
Figure 4 shows the time-course of QTc interval changes. Multivariate ANOVA showed that both time (p=0.0019) and BRL37344 treatment (p<0.0001) were significantly related to the QTc. Figure 3 also shows that the QTc intervals were shorter in experimental group than control group in all 8 weeks. However, the differences were particularly large during the 4 weeks of BRL infusion. Also note that the longest QTc in the control group occurred in the fifth week after surgery, consistent with our previous observation in the same model.14
Figure 4.
QTc intervals at different time points. The vertical bar indicates the standard deviation. *, p< 0.05; **, p<0.01 versus control group
Effects of Beta3-AR Stimulation on Beta-ARs Protein Expression
Beta-ARs protein expression was performed on tissues harvested at the completion of 60-day follow up, when BRL37344 infusion had been stopped for one month. We found that the signal ratio of beta3-AR to GAPDH in the experimental group (1.29 ± 0.23, n=6) was significantly higher than that in the control group (1.0 ±0.15, n=6), indicating upregulation of beta3-AR (Figure 5). The upregulation could be explained by the withdrawal of BRL37344 infusion. However, there were no significant differences in beta1-AR and beta2-AR protein expression between the experimental group and the control group (Figure 5).
Figure 5.
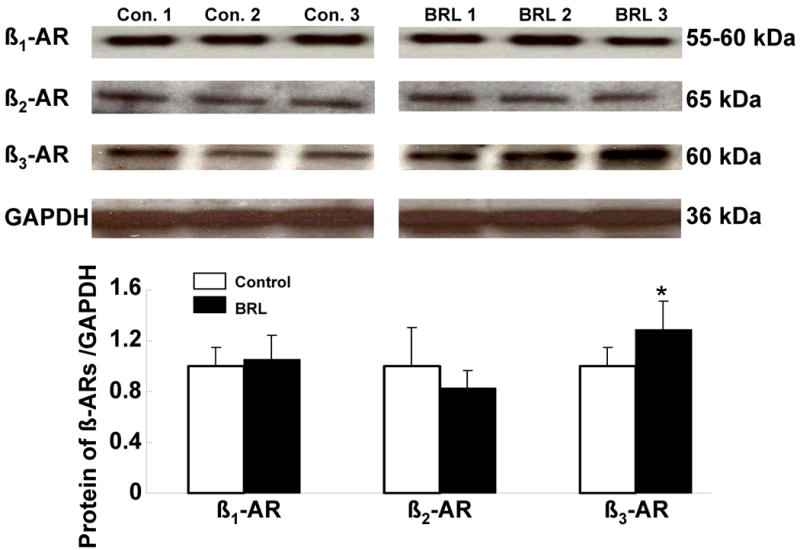
Protein expression of beta-ARs. The immunoblots are representative of the experiments summarized in the graph. Each bar represents the mean ± SD. Data are expressed as the ratio of control group. *, p< 0.05; versus control group.
Effects of Beta3-AR Agonist Stimulation on Ca Handling Protein and Potassium Channel Protein Expression
The abundance of key calcium regulatory proteins NaCaX, DHPR, RyR2 and SERCA2a in the two groups is presented in table 1. Immunoblotting (Figure 6) of NaCaX showed two prominent bands at 160 kDa and 120 kDa, corresponding to nonreduced exchanger protein and mature exchanger protein, respectively. The signals of two bands were summed to give the final NaCaX signals. We observed significant decreases in NaCaX and DHPR protein abundance in the experimental group compared with the control group (Figure 6). No significant differences were observed in SERCA2a and RyR2 protein abundance between the groups. When NaCaX and DHPR density bands were normalized to SERCA2a, they also showed NaCaX and DHPR were significantly decreased in the experimental group compared with the control group. In comparison, there were no significant differences in hERG, KvLQT1 and MinK expression between the control and experimental groups (1.0 ± 0.07 vs. 0.93 ± 0.11, 1.0 ± 0.14 vs. 1.0 ± 0.18, 1.0 ± 0.16 vs. 1.0 ± 0.07, respectively, p=NS for all) (Figure 6).
Table 1.
Summary of Ca Handling Protein Abundance
| Control (n=6) | Experimental (n= 6) | |
|---|---|---|
| NaCaX | 1 ± 0.34 | 0.5 ± 0.31* |
| DHPR | 1 ± 0.22 | 0.65 ± 0.25* |
| SERCA2a | 1 ± 0.11 | 0.96 ± 0.09 |
| RyR2 | 1 ± 0.31 | 0.91 ± 0.56 |
| DHPR/SERCA2a ratio | 1 ± 0.24 | 0.66 ± 0.3* |
| NaCaX/SERCA2a ratio | 1 ± 0.33 | 0.51 ± 0.27* |
Arbitrary unit was used. Values are mean ± SD.
p < 0.05
Figure 6.
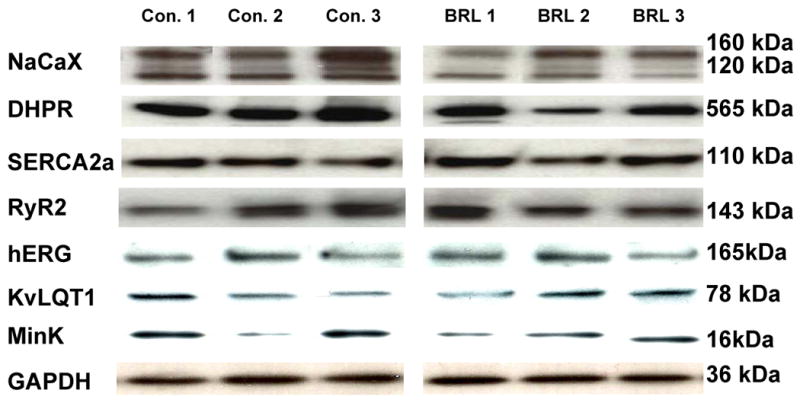
Examples of Western blots of sodium-calcium exchanger (NaCaX), dihydropyridine receptor (DHPR), sarcoplasmic reticulum Ca2+-ATPase (SERCA2a), type-2 ryanodine receptor (RyR2), hERG, KvLQT1, MinK and GAPDH in left ventricular free wall homogenates of 3 controls dogs and 3 experimental group dogs. The band signals of DHPR and NaCax were significantly decreased in the experimental group. There was no significant difference in SERCA2a, RyR2, hERG, KvLQT1 and MinK expression between the control group and the experimental group.
Effects of Beta3-AR Agonist Stimulation on Nerve Sprouting
To assess whether or not beta3-AR agonist stimulation had effects on neural remodeling, we quantified NGF, GAP43 and TH with immunoblotting (Figure 7). We found no significant differences in NGF, GAP43 and TH protein expression between the two groups. To confirm the Western blotting data, we performed immunohistochemical staining of TH and GAP43. Both groups showed that there was abundant of TH and GAP43 staining (Figure 8). Computerized morphometry showed that there were no significant differences (p=NS) in densities of GAP43 (13,655 ± 4,563 μm2/mm2 vs. 11, 177 ± 3,928 μm2/mm2) and TH (35,134 ± 16,045 μm2/mm2 vs. 30,052 ± 13,906 μm2/mm2) stained structures between the control group and the experimental group, respectively. The results suggest that beta3-AR agonist stimulation did not significantly affect nerve sprouting or sympathetic innervation.
Figure 7.
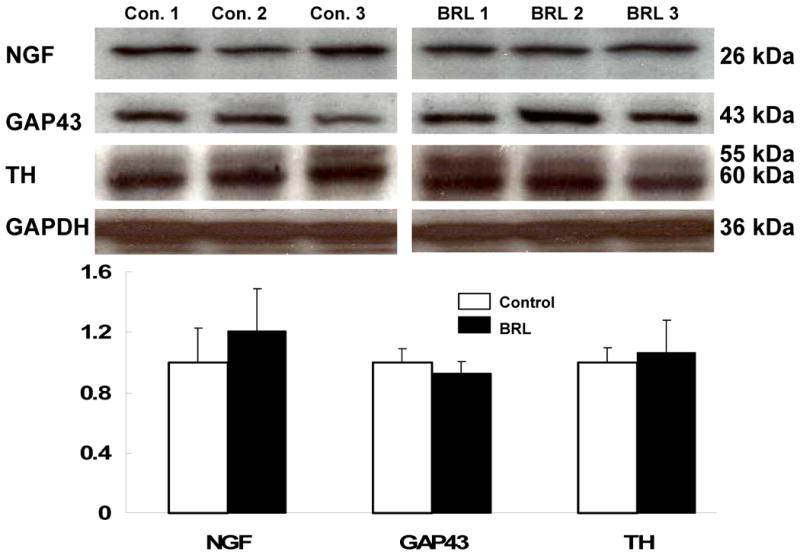
Protein expression of nerve group factor (NGF), growth associated protein 43 (GAP43), and tyrosine hydroxylase (TH) in left ventricle free wall. Representative Western blotting bands of NGF, GAP 43, TH, and GAPDH in three control dogs and three experimental group dogs are shown. Each bar represents the mean ± SD. Data are expressed as the ratio of control group. There was no significant difference in NGF, TH and GAP43 expression between the control and experimental groups.
Figure 8.
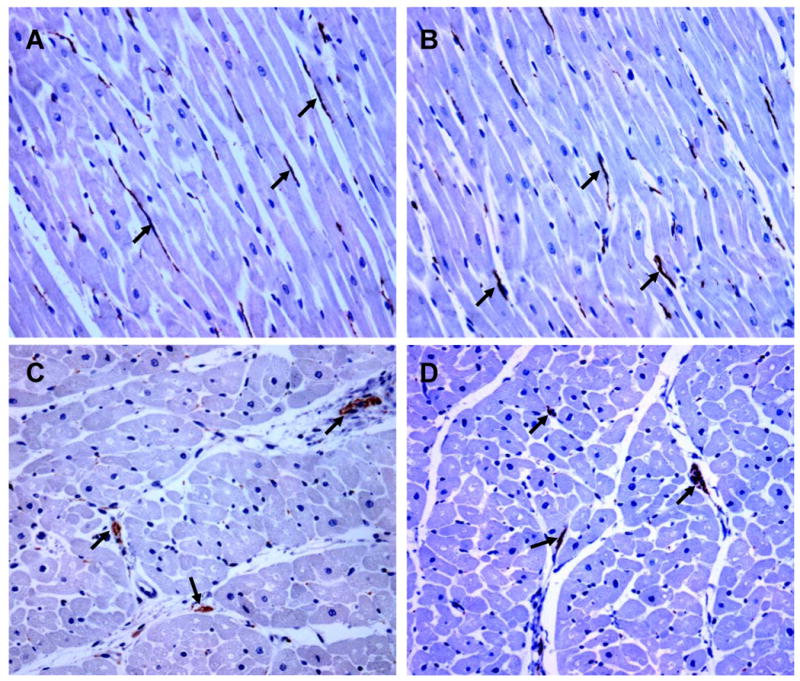
Examples of immunostaining of growth associated protein 43 (GAP43, A-B) and tyrosine hydroxylase (TH, C-D) in the control (A, C) and experimental group (B, D). A and B are longitude section, while C and D are transverse section. Both TH and GAP43 immunoreactivity (brown, arrow) was abundant in both control and experimental groups. However, there was no significant difference in either TH or GAP43 immunoreactivity between the two groups. Objective: 20×, Scale bar, 100 μm.
Discussion
The present study demonstrates that chronic beta3-AR stimulation reduces body weight, heart weight and the incidence of VT in dogs with complete heart block, myocardial infarction, and chronic NGF infusion. However, beta3-AR stimulation does not prevent SCD in this model.
Beta3-AR Stimulation and Ventricular Tachycardia
The osmotic pump is activated by water that enters its semipermeable surface. We primed the pump in 37°C sterile saline prior to implantation according to the manufacturer's instructions so that the pump was immediately activated. However, because we did not give a large bolus dose injection, it might have taken some time before the drug reached therapeutic level. Therefore, the presence of phase 1 VT in all dogs does not rule out the possible antiarrhythmic efficacy of beta-3 stimulation during acute phase of myocardial infarction. We have documented previously that the canine model used in this study has a high incidence of phase 2 VT, with an average incidence of 2.0±2.0 episodes/d (N=9) during chronic monitoring.10 The VT incidence of the control group of the present study is consistent with that report. Therefore, the absence of VT in two dogs and the overall low incidence of VT in the experimental group of the present study strongly support the conclusion that beta3-AR stimulation suppresses the incidence of VT. In addition, we found that BRL37344 infusion significantly reduced the QTc both during infusion (weeks 1 to 4) and afterwards (weeks 5-8). We observed no change of expression of beta1-AR and beta2-AR and no alteration of the magnitude of nerve sprouting. However, there were significant differences in NaCaX and DHPR between these two groups.
Intracellular Ca2+ Homeostasis and the Antiarrhythmic Effects of Beta3-AR Stimulation
We found no significant difference in potassium channel protein expression between the control and experimental groups. Therefore, the antiarrhythmic effects of BRL37344 might not be explained by potassium channel subunit modifications. Intracellular Ca2+ dynamics play a pivotal role in regulating electrical activity and cardiac arrhythmogenesis.15 Cheng et al3 demonstrated that BRL37344 inhibits L-type Ca2+ channels and attenuates intracellular Ca2+ transients in canine ventricular myocytes with an associated dose-dependent decrease in contractility. In this study, we found that the protein expression of DHP receptor, part of the L-type calcium channel complex, is significantly downregulated in the experimental group. The downregulation of DHPR may also contribute to reduced L-type Ca2+ channel activity and thus a decrease in Ca2+ influx and shortened QT intervals. The persistently reduced QTc in the experimental group at the end of the follow-up is consistent with the reduced L-type Ca2+ channel activity at that time, when the tissue was harvested for Western blotting. We also detected the reduced protein expression of NaCaX in the experimental group compared to the controls. Increased activity of the NaCaX has been implicated in arrhythmogenesis16 while NaCaX blockade may be an approach to arrhythmia control. It is possible that the reduced NaCaX protein expression contributes to the antiarrhythmic effects of BRL37344 infusion.
Effects of Beta3-AR Stimulation on Structural and Electrophysiological Remodeling
While the estimated duration of BRL37344 infusion was only 4 weeks, we observed persistent reduction of QTc interval and VT incidences in the following 4 weeks (weeks 5-8). These findings cannot be explained by beta3-AR inhibition alone. We propose that beta3-AR stimulation in the early phase of acute myocardial infarction and atrioventricular block might have reduced the structural and electrophysiological remodeling normally associated with acute myocardial damage. Therefore, even after the cessation of BRL37344 infusion, the QT interval and the arrhythmia incidence continue to be lower than the dogs without BRL37344 infusion.
Effects of Beta3-AR Stimulation on Neural Remodeling
It has been suggested that beta3-AR stimulation serves as a buffer against excessive stimulation of beta1-AR and beta2-AR.8, 9 Because beta1-AR and beta2-AR stimulation is proarrhythmic17 and their blockade reduces the incidence of SCD,18, 19 beta3-AR stimulation might be beneficial in arrhythmic control. In other words, beta3-AR is an inherent beta blocker that can be overexpressed to suppress the proarrhythmic effects of beta1-AR and beta2-AR after myocardial infarction. The magnitude of beta receptor activation depends on both the abundance of cardiac sympathetic innervation and the receptor density and function. Cao et al20 have previously demonstrated that there is an association between a history of spontaneous ventricular arrhythmia and an increased density of sympathetic nerves in human patients. These findings suggest that abnormally increased postinjury sympathetic nerve density may be in part responsible for the occurrence of ventricular arrhythmia and sudden cardiac death in these patients. Zhou et al13 subsequently demonstrated that postinjury myocardial nerve sprouting and sympathetic hyperinnervation can occur after canine myocardial infarction, and that the increased magnitude of sympathetic hyperinnervation correlates with the increased incidence of VT.10, 11 These latter studies imply that excessive beta1-AR and beta2-AR activation during sympathetic discharges play a role in causing VT in this model. By serving as a buffer to the beta1-AR and beta2-AR activation, beta3-AR stimulation might reduce the effects of sympathetic activation on the diseased myocardium and thereby reduced the incidence of VT in this model.
Beta3-AR Stimulation and Sudden Cardiac Death
In this study, we found no significant differences in the occurrence of SCD within 2 months monitoring between the experimental and control groups. However, we observed that most of SCD (3/4) in the experimental group occurred after 30 days, when the osmotic pump had emptied its content of BRL37344. These data suggest that rapid withdrawal of beta3-AR stimulation might induce a rebound increase of SCD. The mechanisms for the discrepancy between protection from VT but lack of protection from VF/SCD are unclear. Because the incidence of VT as well as QTc remained reduced in the experimental group in the second month of the monitoring, the increased incidence of SCD cannot be explained by the increased incidence of arrhythmia. It is possible that QTc shortening and downregulation of Ca handling proteins in the experimental group helped suppress triggered arrhythmias but not prevent reentrant arrhythmias. Therefore, there is dissociation between antiarrhythmic efficacy and the ability to prevent SCD. Previous clinical trials have indicated that the suppression of ventricular arrhythmias by pharmacological agents did not result in a reduction of incidence of SCD.21 The results of the present study are consistent with that clinical observation.
Beta3-AR Stimulation and Beta-ARs Expression
One unexpected finding of the present study was that administration of beta3-AR agonist for one month upregulated beta3-AR protein expression, but did not change beta1-AR and beta2-AR expression. The finding of paradoxical increase of beta3-AR protein expression is consistent with an in vitro study22 in which it was observed that beta-3 AR expression increased approximately 165% from basal expression during exposure up to 30 hrs to a high concentration of (-)-isoproterenol. Caprene et al 23 reported that a chronic (6-day) (-)-norepinephrine infusion in the hamster, which induces a downregulation of beta1- and beta2-ARs, does not affect the beta3-AR. These observations may partially explain the interesting ability of beta3-AR signaling to maintain or even increase its activity following chronic exposure to receptor stimulation.24, 25 Finally, we harvested the tissues roughly one month after the end of beta-3 stimulation. It is possible that there is a rebound increase of beta-3 AR expression due to the withdrawal of the beta-3 stimulation. It was impossible to test the latter hypothesis without harvesting the tissues in the same animals at different time points of BRL37344 infusion.
Clinical Implication
Our data suggest that beta3-AR agonist could decrease the incidence of VT, reduce the body weight and minimize the cardiac hypertrophy after MI and atrioventricular block. The predicted therapeutic indications for beta3-AR agonists are obesity and obesity-associated diabetes, so the advantages of beta3-AR agonist vs. other therapeutic measures would be to treat obesity, diabetes and ventricular arrhythmia at the same time. Our findings also suggest that beta-AR blocking agents with beta3-AR agonist properties might be useful for cardiac arrhythmia control after MI. We noted that 3 dogs in control groups had > 20 VT episodes a day (Figure 1). The same was not seen in dogs receiving beta3 agonist infusion except for dog 8, which developed VT after 22 days. These findings suggest that beta3-AR agonist infusion might be useful in treating VT storms. However, beta3-AR stimulation does not prevent SCD and should not be used for SCD prevention. In conclusion, the present study has shown that specific beta3-AR agonist stimulation exerts antiarrhythmic effects. Beta3-AR might provide a novel molecular target for antiarrhythmic therapy.
Acknowledgments
We thank Jian Tan, Hongmei Li, Avile McCullen, Lei Lin and Elaine Lebowitz for their assistance.
This study was supported by an American Heart Association Scientist Development Grant 0435135N; a Heart Rhythm Society Postdoctoral Fellowship Award; the NIH Grants P01 HL78931, R01s HL78932, 58533, 66389, 71140; AHA Established Investigator Award (#0540093N); a Price Endowment and a Medtronic-Zipes Endowment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Yen TT. Beta-agonists as antiobesity, antidiabetic and nutrient partitioning agents. Obes Res. 1995;3 Suppl 4:531S–6S. doi: 10.1002/j.1550-8528.1995.tb00223.x. [DOI] [PubMed] [Google Scholar]
- 2.Gauthier C, Tavernier G, Charpentier F, Langin D, Le Marec H. Functional beta3-adrenoceptor in the human heart. J Clin Invest. 1996;98:556–62. doi: 10.1172/JCI118823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng HJ, Zhang ZS, Onishi K, Ukai T, Sane DC, Cheng CP. Upregulation of functional beta(3)-adrenergic receptor in the failing canine myocardium. Circ Res. 2001;89(7):599–606. doi: 10.1161/hh1901.098042. [DOI] [PubMed] [Google Scholar]
- 4.Kathofer S, Zhang W, Karle C, Thomas D, Schoels W, Kiehn J. Functional coupling of human beta 3-adrenoreceptors to the KvLQT1/MinK potassium channel. J Biol Chem. 2000;275(35):26743–7. doi: 10.1074/jbc.M003331200. [DOI] [PubMed] [Google Scholar]
- 5.Zhou S, Paz O, Cao JM, Asotra K, Chai NN, Wang C, Chen LS, Fishbein MC, Sharifi B, Chen PS. Differential -adrenoceptor expression induced by nerve growth factor infusion into the canine right and left stellate ganglia. Heart Rhythm. 2005;2:1347–55. doi: 10.1016/j.hrthm.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 6.Moniotte S, Kobzik L, Feron O, Trochu JN, Gauthier C, Balligand JL. Upregulation of beta(3)-adrenoceptors and altered contractile response to inotropic amines in human failing myocardium. Circulation. 2001;103(12):1649–55. doi: 10.1161/01.cir.103.12.1649. [DOI] [PubMed] [Google Scholar]
- 7.Dincer UD, Bidasee KR, Guner S, Tay A, Ozcelikay AT, Altan VM. The effect of diabetes on expression of beta1-, beta2-, and beta3-adrenoreceptors in rat hearts. Diabetes. 2001;50(2):455–61. doi: 10.2337/diabetes.50.2.455. [DOI] [PubMed] [Google Scholar]
- 8.Gauthier C, Langin D, Balligand JL. Beta3-adrenoceptors in the cardiovascular system. Trends Pharmacol Sci. 2000;21(11):426–31. doi: 10.1016/s0165-6147(00)01562-5. [DOI] [PubMed] [Google Scholar]
- 9.Verrier RL. Beta3-adrenoceptor: friend or foe? Heart Rhythm. 2005;2(12):1356–8. doi: 10.1016/j.hrthm.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 10.Cao JM, Chen LS, KenKnight BH, Ohara T, Lee MH, Tsai J, Lai WW, Karagueuzian HS, Wolf PL, Fishbein MC, Chen PS. Nerve sprouting and sudden cardiac death. Circ Res. 2000;86:816–21. doi: 10.1161/01.res.86.7.816. [DOI] [PubMed] [Google Scholar]
- 11.Swissa M, Zhou S, Gonzalez-Gomez I, Chang CM, Lai AC, Cates A, Fishbein MC, Karagueuzian HS, Chen PS, Chen LS. Long-term subthreshold electrical stimulation of the left stellate ganglion and a canine model of sudden cardiac death. J Am Coll Cardiol. 2004;43:858–64. doi: 10.1016/j.jacc.2003.07.053. [DOI] [PubMed] [Google Scholar]
- 12.Shen YT, Cervoni P, Claus T, Vatner SF. Differences in beta 3-adrenergic receptor cardiovascular regulation in conscious primates, rats and dogs. J Pharmacol Exp Ther. 1996;278(3):1435–43. [PubMed] [Google Scholar]
- 13.Zhou S, Chen LS, Miyauchi Y, Miyauchi M, Kar S, Kangavari S, Fishbein MC, Sharifi B, Chen PS. Mechanisms of cardiac nerve sprouting after myocardial infarction in dogs. Circ Res. 2004;95:76–83. doi: 10.1161/01.RES.0000133678.22968.e3. [DOI] [PubMed] [Google Scholar]
- 14.Zhou S, Cao JM, Tebb ZD, Ohara T, Huang HL, Omichi C, Lee MH, KenKnight BH, Chen LS, Fishbein MC, Karagueuzian HS, Chen P-S. Modulation of QT interval by cardiac sympathetic nerve sprouting and the mechanisms of ventricular arrhythmia in a canine model of sudden cardiac death. J Cardiovasc Electrophysiol. 2001;12(9):1068–73. doi: 10.1046/j.1540-8167.2001.01068.x. [DOI] [PubMed] [Google Scholar]
- 15.Weiss JN, Karma A, Shiferaw Y, Chen PS, Garfinkel A, Qu Z. From pulsus to pulseless: the saga of cardiac alternans. Circ Res. 2006;98(10):1244–53. doi: 10.1161/01.RES.0000224540.97431.f0. [DOI] [PubMed] [Google Scholar]
- 16.Sipido KR, Varro A, Eisner D. Sodium calcium exchange as a target for antiarrhythmic therapy. Handb Exp Pharmacol. 2006;(171):159–99. doi: 10.1007/3-540-29715-4_6. [DOI] [PubMed] [Google Scholar]
- 17.Kaumann AJ, Molenaar P. Modulation of human cardiac function through 4 beta-adrenoceptor populations. Naunyn Schmiedebergs Arch Pharmacol. 1997;355(6):667–81. doi: 10.1007/pl00004999. [DOI] [PubMed] [Google Scholar]
- 18.Freemantle N, Cleland J, Young P, Mason J, Harrison J. beta Blockade after myocardial infarction: systematic review and meta regression analysis. BMJ. 1999;318:1730–7. doi: 10.1136/bmj.318.7200.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gottlieb SS, McCarter RJ, Vogel RA. Effect of beta-blockade on mortality among high-risk and low-risk patients after myocardial infarction. N Engl J Med. 1998;339(8):489–97. doi: 10.1056/NEJM199808203390801. [DOI] [PubMed] [Google Scholar]
- 20.Cao JM, Fishbein MC, Han JB, Lai WW, Lai AC, Wu TJ, Czer L, Wolf PL, Denton TA, Shintaku IP, Chen PS, Chen LS. Relationship between regional cardiac hyperinnervation and ventricular arrhythmia. Circulation. 2000;101:1960–9. doi: 10.1161/01.cir.101.16.1960. [DOI] [PubMed] [Google Scholar]
- 21.Echt DS, Liebson PR, Mitchell LB, Peters RW, Obias-Manno D, Barker AH, Arensberg D, Baker A, Friedman L, Greene HL, Huther ML, Richardson DW, The CAST Investigators Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991;324:781–8. doi: 10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]
- 22.Thomas RF, Holt BD, Schwinn DA, Liggett SB. Long-term agonist exposure induces upregulation of beta 3-adrenergic receptor expression via multiple cAMP response elements. Proc Natl Acad Sci U S A. 1992;89(10):4490–4. doi: 10.1073/pnas.89.10.4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carpene C, Galitzky J, Collon P, Esclapez F, Dauzats M, Lafontan M. Desensitization of beta-1 and beta-2, but not beta-3, adrenoceptor-mediated lipolytic responses of adipocytes after long-term norepinephrine infusion. J Pharmacol Exp Ther. 1993;265(1):237–47. [PubMed] [Google Scholar]
- 24.Rouget C, Breuiller-Fouche M, Mercier FJ, Leroy MJ, Loustalot C, Naline E, Frydman R, Croci T, Morcillo EJ, Advenier C, Bardou M. The human near-term myometrial beta 3-adrenoceptor but not the beta 2-adrenoceptor is resistant to desensitisation after sustained agonist stimulation. Br J Pharmacol. 2004;141(5):831–41. doi: 10.1038/sj.bjp.0705616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nantel F, Bonin H, Emorine LJ, Zilberfarb V, Strosberg AD, Bouvier M, Marullo S. The human beta 3-adrenergic receptor is resistant to short term agonist-promoted desensitization. Mol Pharmacol. 1993;43(4):548–55. [PubMed] [Google Scholar]