Abstract
Background
The cyclin-dependent kinase (cdk) inhibitor p21 inhibits cellular proliferation of many cell types, including T cells. However, autoimmune models have yielded conflicting results regarding the role of cdk inhibitors and T cell function. The role of p21 in T cell function following transplantation has not been directly investigated. We hypothesized that p21 plays an important role in alloantigen-driven responses in vitro in mixed lymphocyte cultures (MLC) and in vivo using the heterotopic murine cardiac allograft model.
Methods
Wild type (WT) and p21-deficient (p21−/−) mice were employed as recipients, and the effects of p21 over expression were assessed by transplanting p21 adenoviral-transfected cardiac allografts. Enzyme linked immunospot (ELISPOT) and 3H-thymidine incorporation were used to evaluate for T cell priming and proliferation in vitro while graft histology was evaluated for rejection.
Results
When stimulated with alloantigens in vitro, splenocytes from p21−/− mice mounted enhanced proliferative responses and reduced Th2 responses relative to their WT counterparts. No differences in Th1 responses were noted when p21−/− cells were stimulated with alloantigens in vitro. However, following cardiac transplantation, Th1 responses were enhanced in p21−/− recipients relative to WT mice. This enhanced in vivo Th1 response was associated with exacerbated graft rejection in p21−/− recipients. Interestingly, p21 transfection of WT allografts inhibited graft rejection and Th1 priming.
Conclusion
p21 controls the intensity of the immune response post-transplantation, with over expression inhibiting allograft rejection. Our data demonstrate that p21 controls T cell priming, and also suggests p21 and other cdk inhibitors may serve as potential targets for therapeutic manipulation of alloimmune responses.
Introduction
Cell cycle control has been shown to play a critical role in T cell proliferation, apoptosis, and priming (1–3). Entry into the S phase of the cell cycle is governed by the G1 cyclins, which assemble with cyclin dependent kinases (cdk) to form functional holoenzymes that phosphorylate key substrates required for the G1/S phase transition (reviewed in (4–6). In mammalian cells, cyclin D-cdk4 or -cdk6, cyclin E-cdk2, and cyclin A-cdk2 complexes act sequentially during G1/S transition and are required for cell cycle progression. p21 and p27 are considered important regulators of cell proliferation because of their inhibitory interactions with cyclin/cdk complexes (reviewed in (5, 6). p21 and p27 belong to the Cip/Kip family of cdk inhibitors, which have the capacity to bind and inactivate many different cyclin/cdk complexes. Hence, the Cip/Kip family of cdk inhibitors is believed to regulate G1 and S phase cdks in vivo. Indeed, p21 and p27 have been shown to be important for cell cycle control of a variety of cell types, including T cells (7–10). Zhang et al. (9) reported that p27 controlled cytokine-induced T cell proliferation, while p21 played a lesser role in this process. One possible explanation for these findings is that p27 may control initial entry of T cells into S phase from G1, whereas p21 may regulate T cells that are already actively cycling (3). Upregulation of p21 has been observed in memory CD4+ T cells and in T cells after several rounds of proliferation and chronic activation (1). Further, p21, along with p27, is upregulated in anergic T cells (10). Thus, cdk inhibitors are considered potent suppressors of immune responses and may therefore be therapeutically important in transplantation and autoimmunity. Indeed, the commonly used immunosuppressant cyclosporine A (CsA) induces expression of p21 in T cells in a TGFβ-dependent manner (11). Hence, p21 may contribute to the anti-proliferative activities of CsA.
Studies in autoimmune models have reported conflicting activities for p21. For example, Balomenos et al. (1) reported that p21−/− mice develop female-prone lupus accompanied by anti-DNA antibodies, lymphadenopathy, and glomerulonephritis while Lawson et al. (12) found no evidence for autoimmunity in an independently derived stock of p21−/− mice. Santiago-Raber et al. (2) reported that p21−/− mice develop mild autoimmunity at a low incidence that is unaffected by gender. Hence, a spectrum of autoimmune disease states has been reported for p21−/− mice. The direct effects of p21 on in vivo immune responses have not been completely defined, and the impact of p21 deficiency vs. over-expression in the context of organ transplantation has not been rigorously investigated.
The current study explored the role of p21 in alloimmune responses in an in vitro mixed leukocyte culture (MLC) and, most importantily, in vivo following cardiac allograft transplantation. As anticipated, p21−/− cells mounted enhanced proliferative responses relative to WT cells in both settings, indicating a role for p21 in regulating clonal expansion of graft-reactive T cells. However, p21 appeared to differentially regulate Th1 and Th2 responses in the in vitro versus in vivo settings. In vitro, p21 deficiency led to reduced Th2 responses but did not alter the magnitude of the Th1 response. Following transplantation, p21 deficiency did not impact Th2 responses, even when Th2 were preferentially induced by in vivo depletion of CD8+ T cells as previously described (13). In contrast, p21−/− allograft recipients mounted enhanced Th1 responses relative to their WT counterparts, which was associated with exacerbated graft rejection. Over expression of p21 within the graft led to prolonged allograft survival and reduced Th1 responses. These data demonstrate a differential impact of p21 on Th1 versus Th2 responses, with p21 expression level correlating with graft outcome. This study suggests that p21 may provide a target for therapeutic strategies aimed at manipulating Th1 responses following solid organ transplantation.
Materials and Methods
Mice
Female WT and p21−/− C57BL/6/129 (H-2b) mice and BALB/c (H-2d) mice were purchased from The Jackson Laboratories (Bar Harbor, ME) and housed under specific pathogen free conditions in the Unit for Laboratory Animal Medicine at the University of Michigan. Mice were used between 6–12 weeks of age.
Media
The culture medium used in these studies was DMEM supplemented with 0.27 mM L-asparagine, 1.4 mM L-arginine HCl, 14 mM folic acid, 5 × 10−5 M 2-ME (all obtained from Sigma Chemical, St. Louis, MO), 1.6 mM L-glutamine, 10 mM HEPES buffer, 1.0 mM sodium pyruvate, 100 units/ml penicillin/streptomycin, 2% FCS (all obtained from Life Technologies, Grand Island, NY).
Heterotopic cardiac transplantation
WT and p21−/− C57BL/6/129 mice were transplanted with intact BALB/c cardiac allografts, as described (14). In this model, the donor heart is anastomosed to the great vessels of the abdomen, perfused with recipient mouse’s blood, and resumes contraction. Transplant function was monitored by daily abdominal palpation, and graft rejection was indicated by cessation of contractions. Histologic evidence of rejection (i.e. leukocytic infiltration, loss of myocyte nuclei and cross-striation) was verified by H & E staining of formalin fixed allograft fragments.
p21 transfection of cardiac allografts
Cardiac allografts were transfected by perfusion with E1/E3 deleted adenoviral vectors encoding human p21 (Adp21) or beta-galactosidase (Adβ-gal) as described (15, 16). Adp21 was kindly provided by Dr. Elizabeth Nabel (NIH) and its in vivo use has been previously described (8). Stocks of adenoviral vectors were produced for in vivo use in the Vector Core at the University of Michigan Medical Center. Prior to in situ perfusion of the donor heart, the left superior vena cava and the distal aorta were ligated to retain the perfusate. The aorta was then held in position by suture to facilitate perfusion using a syringe with a 30 gauge needle. The donor heart was flushed with 400 µl of heparin (250 U/ml), then perfused with 200 µl of lactated Ringer's solution containing adenoviral vectors (5 × 108 pfu). Following perfusion, donor grafts were harvested and placed in iced Ringer's for approximately 1 hour prior to transplantation.
Verifying p21 transgene expression
Expression of the p21 transgene was verified by RT-PCR as described (8). Adp21 contains an untranslated region of bovine growth hormone proximal to the polyadenylation site. The anti-sense PCR primer is located in this region and therefore is specific for the recombinant p21 and does not amplify endogenous p21 (8). Tissues were homogenized in 1 ml TRIzol® (Invitrogen Life Technologies, Carlsbad, CA) and RNA was isolated as per manufacturer’s protocol. Two µg of total RNA were reverse transcribed using a cDNA Cycle® Kit (Invitrogen Life Technologies) using oligo dT primers and AMV reverse transcriptase to generate cDNA. Primer sequences:
recombinant p21 sense 5’ GAG ACA CCA CTG GAG GGT GAC TTC G 3’,
anti-sense 5’ GGG CAA ACA ACA GAT GGC TGG CAA C 3’;
γ actin sense 5’ CCA CAC AGA GTA CTT GCG CTC AGG 3’,
anti-sense 5’ CAC CCT GTG CTG CTC ACC GAG GCC 3’.
Samples were amplified using AmpliTaq DNA polymerase (Perkin Elmer, Norwalk, CT) in a GeneAmp® PCR System 9700 (Applied Biosystems Inc, Foster City, CA).
In vivo depletion of CD8+ T cells
To deplete allograft recipients of CD8+ cells, mice were injected i.p. with 1 mg of anti-CD8 mAb (hybridoma 2.43, obtained from the American Type Culture Collection Rockville, MD), on days −2, 0, and 5 relative to transplantation. Preparations of anti-CD8 mAb were purified and re-suspended in PBS by Ligocyte Pharmaceuticals, Inc. (Bozeman, MT). Flow cytometric analysis of splenocytes confirmed CD8+ T cell depletion, with <1% of cells staining positive for CD8. Staining with FITC-conjugated rabbit anti-rat IgG (Biosource International, Camarillo, CA) was used to verify that mAb treatment resulted in depletion of CD8+ cells, rather than epitope coating with the 2.43 mAb.
MLC
To detect alloantigen-induced proliferation, WT or p21−/− splenocytes (1 × 106/ml) were stimulated with γ-irradiated (5000R) BALB/c splenocytes (1 × 106/ml) in microtiter wells for 5 days, including a terminal 8 hour pulse with 0.5 µCi 3H-thymidine. Microcultures were harvested by aspiration onto a filter mat (Tomtec Cell Harvester System, Hamden, CT) and 3H-thymidine incorporation determined using a BetaPlate liquid scintillation counter (Wallac, Gaithersburg, MD).
Enzyme-linked immunospot (ELISPOT) assay for cytokine production
The ELISPOT assay used to quantify alloantigen-primed cytokine producing cells has been described previously (17). Capture and detection mAb were obtained from BD-Pharmingen (San Diego, CA). Polyvinylidine fluoride-bottom plates (Cellular Technologies, Cleveland, OH) were coated overnight with rat anti-mouse IFNγ (R4-6A2, 4 µg/ml) or IL-4 (11B11, 2 µg/ml) capture mAb, blocked for 90 minutes with 1% BSA in PBS at room temperature, and washed three times with PBS. Irradiated (5000R) BALB/c splenocytes (4 × 105) were added to each well followed by 2 × 105 WT or p21−/− responder splenocytes. Plates were incubated for 24 hours at 37° C, and then washed 3 times with PBS followed by 4 times with PBS-Tween 20 (0.05%). Biotinylated rat anti-mouse IFNγ (XMG1.2, 4 µg/ml) or IL-4 (BVD6-24G2, 2µg/ml) detection mAb were added to each well and incubated overnight at 4° C. Plates were washed 3 times with PBS-Tween 20, then alkaline phosphatase-conjugated anti-biotin antibodies (1/1000 dilution; Vector Laboratories, Burlingame, CA) were added to the IFNγ wells and HRP-conjugated streptavidin (1:2000 dilution; Dako, Carpinteria, CA) was added to the IL-4 wells for 90 minutes at room temperature. Plates were washed 4 times with PBS, developed with NBT/BCIP or 3-amino-9-ethylcarbozole (AEC), washed with H2O, and air dried. Spots were enumerated using an ImmunoSpot Series 1 ELISPOT Analyzer (Cellular Technologies, Cleveland, OH).
Statistical analyses
Data are presented as mean +/− standard error of the mean (SEM). Statistical analyses were performed using StatView 5.0.1 software (SAS Institute, Inc., Cary, NC). Analyses of variance or Student’s t-tests were performed where appropriate between groups. Fisher’s exact tests were performed to determine least significant difference among groups. p values of less than 0.05 were considered significant. Significance of allograft survival was assessed by logrank analysis.
Results
Impact of p21 on alloantigen-driven T cell proliferation and Th1/Th2 priming in vitro
To begin exploring the role of p21 in alloantigen responses, we first compared the ability of p21−/− and WT splenocytes to proliferate in response to alloantigens in vitro. p21−/− cells mounted an approximate 110% increased proliferation response to alloantigens relative to their WT counterparts (p21−/− 210 +/− 31% vs. WT 100 +/− 14%, n = 6 experiments, p < 0.05). This enhanced proliferative response was not apparent when the polyclonal mitogen Con A was used as a stimulus (data not shown), suggesting that an overwhelming stimulus could over-ride the impact of p21 inhibition of T cell proliferation and p21 levels primarily affected alloantigen-dependent proliferation.
Since the impact of p21 on T cell priming for cytokine production in response to alloantigens has not been determined, we compared Th1 (IFNγ) and Th2 (IL-4) responses of p21−/− and WT splenocytes following in vitro stimulation. Splenocytes were stimulated in a 3 day MLC and plated in ELISPOT cultures that detect primed, but not quiescent precursor cytokine producing cells (17). Following in vitro stimulation with alloantigens, IFNγ responses by p21−/− and WT cells were similar (p21−/− 193 +/− 9 IFNγ spots per million cells vs. WT 212 +/− 15 IFNγ spots, one of three representative experiments, p value non-significant), suggesting that p21 plays a minor role in regulating Th1 responsiveness in MLC. In comparison, p21−/− cells mounted significantly reduced Th2 responses relative to WT cells as measured by IL-4 production (p21 −/− 29 +/− 3 IL-4 spots per million cells vs WT 111 +/− 9 IL-4 spots, one of three representative experiments, p < 0.05). These observations suggest that p21 may play a role in influencing Th1/Th2 balance in this in vitro culture system.
Flow cytometry revealed that the CD4:CD8 ratio following in vitro stimulation with alloantigen was also impacted by p21. While the percentage of CD8+ cells was comparable following culture of WT and p21−/− cells, CD4+ cells were preferentially expanded in the p21−/− MLC with mean a increase over WT of 56% for 4 independent experiments. Percentages of CD4+ and CD8+ T cells in naïve animals prior to stimulation in MLC were similar between WT and p21−/− mice (data not shown). This is in keeping with the findings of Santiago-Raber et al. (2) who reported that proliferative responses of CD4+ cells are more inhibited by p21 than are those of CD8+ cells in an autoimmune model.
Impact of p21 on cardiac allograft rejection in vivo
To assess the influence of p21 on alloimmune responses in vivo, WT and p21−/− mice were transplanted with vascularized cardiac allografts. p21−/− recipients uniformly rejected BALB/c cardiac allografts by day 7 (Table I), which was slightly accelerated relative to WT recipients (mean survival = 8.5 days, p<0.001). However, differences in the progression of rejection were revealed by histologic evaluation of functioning grafts (Figure 1). On day 5, allografts placed in WT recipients revealed only minor signs of rejection, including a mild mononuclear cell infiltrate with little or no myocyte necrosis (Figure 1A). In contrast, allografts placed in p21−/− recipients revealed a more intense mononuclear cell infiltrate, early signs of myocyte degeneration, vascular involvement (white arrow) and hemorrhage (black arrows) on day 5 (Figure 1B). As rejection progressed on day 7, histologic differences remained apparent. While grafts in WT recipients revealed a progressive cellular infiltrate and some myocyte necrosis on day 7 (Figure 1C), the signs of rejection were intensified in p21−/− recipients, with near total myocyte necrosis (as noted by loss of myocyte nuclei) and massive hemorrhage (Figure 1D). These data demonstrate that the rejection process was accelerated in p21−/− recipients.
Table I.
Impact of p21 on allograft rejection
| Allograft Survival (Days) | Mean | |
|---|---|---|
| WT | 8,8,8,9,9,9 | 8.5 |
| p21 −/− | 7,7,7,7,7,7 | 7.0* |
WT and p21−/− C57BL/6/129 mice were transplanted with vascularized BALB/c cardiac allografts. Graft function was monitored by daily palpation and rejection was recorded as the day on which the graft ceased to function.
p < 0.001 vs. WT.
Figure 1. Increased pathology of rejection in p21−/− cardiac allograft recipients.
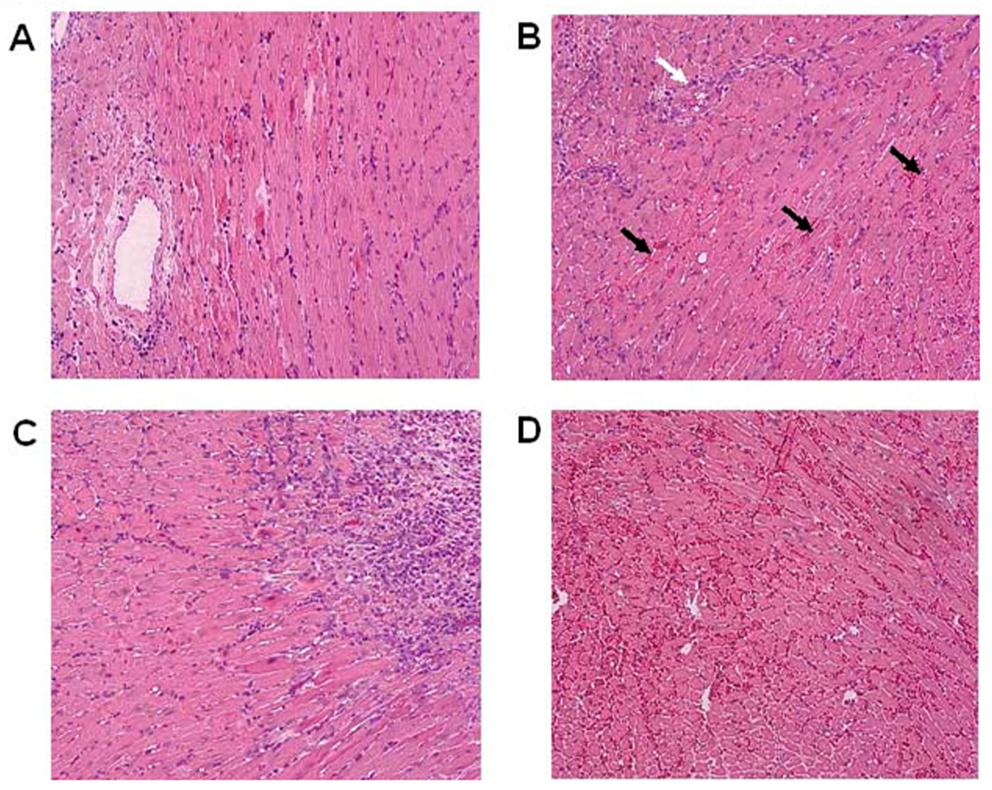
Representative H & E stained sections are depicted of rejecting BALB/c cardiac allografts in WT recipients on days 5 (Panel A) and 7 (Panel C) and in p21−/− recipients on day 5 (Panel B) and 7 (Panel D) post transplantation. Note early vascular involvement (white arrow) and hemorrhage (black arrows) on day 5 in p21−/− recipients (Panel B). Magnification = 200X.
Impact of p21 on in vivo T cell responses following transplantation
It is generally accepted that immune responses initiated in vitro may not accurately reflect the complexity of responses initiated in vivo where compartmentalization, microenvironmental parameters, and tissue specific expression of antigens may impact outcome (18–25). Therefore we assessed the ability of WT and p21−/− mice to mount proliferative and cytokine responses following cardiac transplantation. Donor alloantigen-driven proliferative responses following graft rejection were similar to those initiated in vitro, with splenocytes obtained from p21−/− recipients mounting significantly stronger proliferative responses than that of WT recipients (p21 −/− 182 +/− 25% vs. WT 100 +/− 12%, n = 9–14 mice per group, p < 0.05). However, in vitro and in vivo cytokine responses in the absence of p21 were disparate. Unlike the in vitro initiated response, IFNγ secreting Th1 were significantly increased in p21−/− allograft recipients (4830 +/− 777 IFNγ spots per million cells) when compared to WT recipients (1187 +/− 478 IFNγ spots, p < 0.05, n=3 mice per group for one representative experiment) . Further, IL-4 producing Th2 were relatively rare in both groups (p21−/− 49 +/− 13 IL-4 spots per million cells vs. WT 15 +/− 1 IL-4 spots).
The fact that IL-4 producing Th2 were rare comes as no surprise since we have previously reported that rejection in this model is characterized by a CD8+ Th1 dominated response (13, 26–28). Depletion of CD8+ cells reduces IFNγ production and allows Th2 responses to emerge (13, 27, 28). We next depleted cardiac allograft recipients of CD8+ cells to assess the impact of p21 on Th2 responses to alloantigens in vivo. In addition, depleting CD8+ cells would allow us to assess the impact of p21 on CD4-mediated rejection, since CD4+ cells have been reported to be influenced to a greater degree than CD8+ cells by p21 (2). Splenocytes were harvested at the time of rejection from WT and p21−/− allograft recipients depleted of CD8+ cells to assess the degree of Th1 and Th2 priming by ELISPOT. IL-4 producing Th2 were increased in both groups when compared to Th2 responses observed when CD8+ cells were not depleted. However, there was no difference in the level of Th2 priming in WT and p21−/− recipients (p21−/− 122 +/− IL-4 spots per million cells vs. WT 134 +/− 56 IL-4 spots, n = 5–9 mice per group, p value non-significant), indicating that p21 played little if any role in regulating Th2 responses in vivo. As expected (13), Th1 responses were reduced in recipients that were depleted of CD8+ cells. However, primed Th1 were increased in number in p21−/− recipients (648 +/− 86 IFNγ spots per million cells) relative to their WT counterparts (321 +/− 46 IFNγ spots per million cells), n = 5–9 mice per group, p < 0.05, further indicating a role for p21 in regulating Th1 responses in vivo.
Over-expression of p21 in cardiac allografts prolongs graft survival and inhibits Th1 priming in vivo
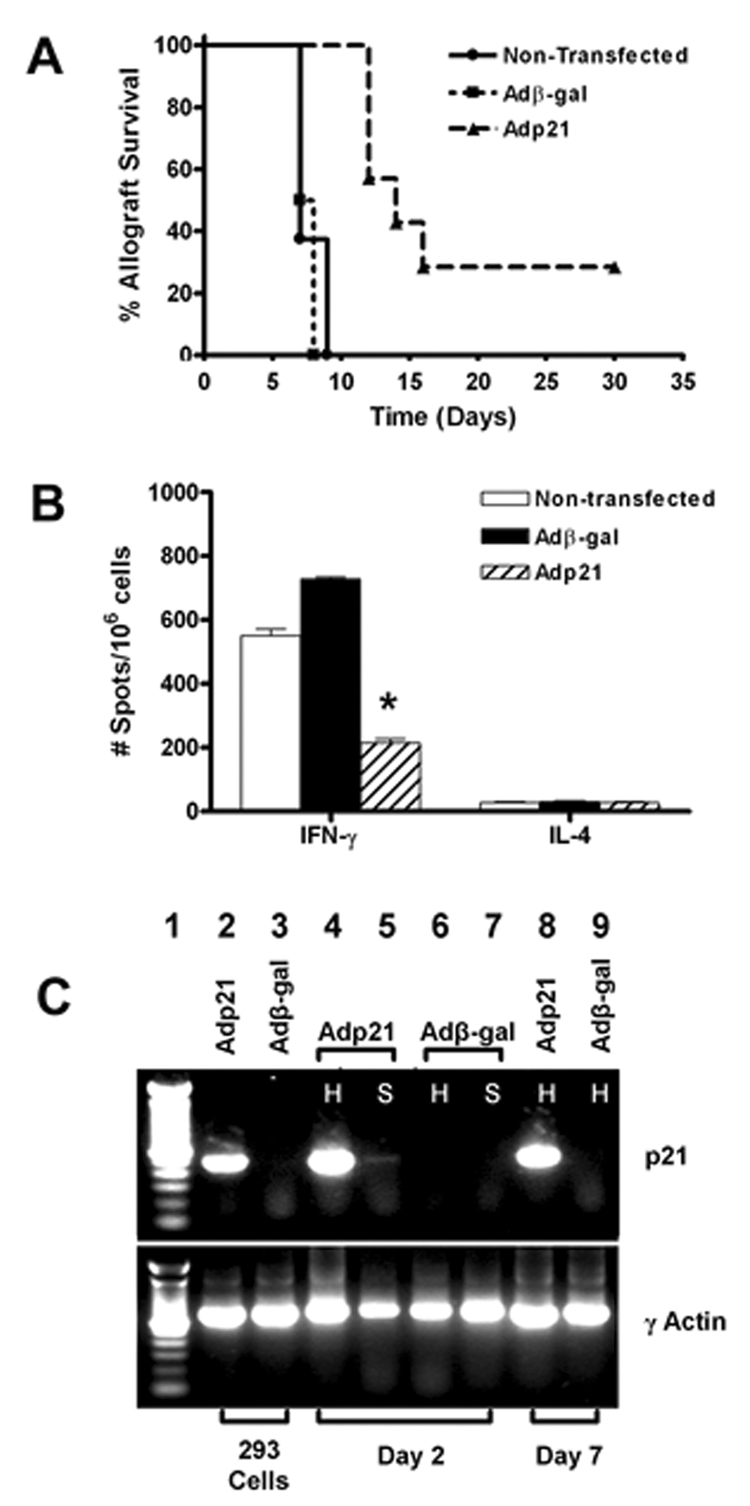
To further explore the role of p21 in alloimmune responses and transplant rejection, we transfected WT BALB/c cardiac allografts with adenoviral vectors encoding either p21 (Adp21) or β-galactosidase (Adβ-gal) prior to transplantation into WT C57BL/6 recipients as described (15, 16). Adenoviral-mediated transfection with p21 prolonged allograft survival (Figure 2A) with an increase in mean survival from 8 days to 18 days (p < 0.01). Indeed, two of the eight p21 transfected allografts continued to function normally until the termination of the experiment (30 days). Histologic evaluation of these two p21 transfected allografts that continued to function at 30 days post transplant revealed only minimal cellular infiltrate and no myocyte necrosis (data not shown).
Figure 2. Impact of p21 over-expression on allograft survival and donor-reactive Th1 responses.
BALB/c cardiac allografts were transfected with 5 × 108 pfu of Adβ-gal or Adp21 or left non-transfected prior to transplantation into WT C57BL/6 mice as described in the Materials and Methods section. Panel A: Graft function was monitored by daily palpation and rejection was recorded as the day on which the graft ceased to function (n = 6–8 transplants per group). p < 0.01 for the mean survival of Adp21 transfected allografts (18.6 days) compared to non-transfected (7.7 days) or Adβ-gal transfected allografts (7.5 days). Panel B: Splenocytes were harvested from recipients on the day of rejection and assessed for in vivo primed Th1 and Th2 responses by ELISPOT. Data represent the mean number of spots per 1 × 106 cells +/− SEM. *p < 0.05 vs. Adβ-gal transfected and non-transfected groups. Panel C: RT-PCR was performed using primers specific for recombinant p21 or γ actin (see Materials and Methods) to verify appropriate expression of p21 following transfection with Ad21, but not Adβ-gal. Lane 1 – MW ladder. Lanes 2 and 3 – 293 cells were transfected with Adp21 or Adβ-gal, respectively, at an M.O.I. of 25 and RNA was isolated 24 hours post-transfection. Lanes 4-7 – Allografts were perfused with Adp21 or Adβ-gal and RNA was isolated from the transplanted hearts (H) and recipient spleens (S) on day 2 post-transplantation. Lanes 8 and 9 – Allografts were transfected with Adp21 or Adβ-gal, respectively, and RNA was harvested on day 7 post transplant. Results are representative of 3 transplanted mice per group.
T cell priming was evaluated at the time of rejection or at the termination of the experiment in recipients of allografts transfected with Adp21 or Adβ-gal (Figure 2B). At the time of rejection, recipients of p21 transfected allografts mounted significantly reduced Th1 responses relative to recipients of β-gal transfected grafts. Further, the two mice bearing functioning p21 transfected allografts on day 30 mounted even smaller Th1 responses (140 ± 14 spots/106 splenocytes), further indicating that p21 inhibits allograft-reactive Th1 in vivo.
To verify that these observations were associated with expression of the p21 transgene, p21 or β-gal transfected allografts were analyzed on day 2 or day 7 post-transplantation. Spleens were also analyzed on day 2, since we have reported that transgene expression may be detected in the spleen at early time points post-transplantation of adenovirally transfected cardiac grafts (15). Figure 2C depicts RT-PCR for p21 transgene expression in Adp21 (Lane 2) or β-gal (Lane 3) transfected 293 cells, illustrating specificity of the PCR primers for recombinant p21 (8).
When allografts were perfused with Adp21 and tissues were harvested on day 2 post-transplant, expression of the p21 transgene was readily detectable in the transplanted heart (Lane 4) and to a lesser degree in the spleen (Lane 5). As expected, recombinant p21 was not expressed in the heart (Lane 6) or spleen (Lane 7) when allografts were perfused with Adβ-gal. The p21 transgene was expressed within grafts that were perfused with Adp21 (Lane 8) but not Adβ-gal (Lane 9) for at least 7 days post-transplantation. p21 transgene expression was also readily detectable in the two functioning Adp21 transfected grafts that were harvested on day 30 post transplant (data not shown).
Discussion
p21 and p27 are members of the Cip/Kip family of cdk inhibitors and have been shown to inhibit a wide variety of cdk, thus controlling cell cycle progression in a number of cell types (Reviewed in (4–6). Levels of p27 are high in resting T cells but decline following T cell activation (3, 29, 30). Costimulation through CD28 as well as responsiveness to IL-2 results in ubiquitinization and degradation of p27, thereby allowing the T cell to enter S-phase of the cell cycle (3, 31). Wolfram and Letterio (32) have reported that the requirement for CD28 costimulation is markedly reduced in CD8+, but not CD4+ T cells obtained from p27−/− mice. Further, Rowell et al. (33) have recently reported that p27−/− mice are less susceptible of co-stimulation blockade-induced allograft acceptance. However, while p27 regulates the expansion and homeostasis of T cells, Shen and Kaplan (34) reported that p27 does not influence Th1/Th2 differentiation. Taken together, these observations indicate that p27 regulates events that occur early in the T cell activation response. The current study reveals a role for p21 in regulating Th1 responses in vivo. Unlike p27, p21 levels are low in resting T cells and increase upon activation, where p21 is believed to play a role in controlling actively cycling T cells and IL-2 dependent proliferation (3, 31, 35). CD4+, rather than CD8+ T cells appear to be more inhibited by p21 (1, 2). Finally, high levels of both p27 and p21 are present in anergic T cells (10, 36), reflecting induction of p21 as well as a failure to down-regulate p27.
Diverse and controversial activities have been reported for p21 in activated T cells. For example, p21 has been reported to promote T cell apoptosis mediated by Fas/FasL interactions (37) but has also been reported to protect activated/memory T cells from apoptosis (38). In addition to its role as a cdk inhibitor, p21 has been shown to serve as an adaptor protein to promote the association of cdk4 with the D-type cyclins (39). p21 has also been shown to limit T cell responses to chronic stimulation (1). The in vivo impact of p21 on immune responses has been studied in models of autoimmunity, where a spectrum of disease states has been reported (1, 2, 12). Hence, the in vitro and in vivo effects of p21 on immune responses have not been clearly defined.
Given the diverse activities that have been ascribed to p21 in regulating T cell expansion and function, the current study assessed the impact of p21 on T cell function in response to allogeneic stimulation both in vitro and in vivo. To this end, we compared proliferative responses and Th1 vs. Th2 cytokine production by splenocytes obtained from p21−/− and WT mice. We also assessed the impact of over-expression of p21 on allograft survival and Th1/Th2 priming by transfecting grafts with Adp21 prior to transplantation.
When stimulated with alloantigens in vitro, p21−/− cells mounted enhanced proliferative responses when compared to WT cells. While Th1 responses were similar between the 2 groups, Th2 priming was reduced in p21−/− cells when compared to their WT counterparts. This in vitro observation suggested that p21 may positively influence Th2 differentiation, unlike what has been reported for p27 (34). However, an effect on in vivo Th2 priming was not observed when p21−/− mice were transplanted with cardiac allografts. Rather, p21−/− recipients mounted an exaggerated Th1 response relative to WT mice which was associated with accelerated graft rejection (Table I) and exacerbated graft pathology (Figure 1). These differences in the in vitro and in vivo scenarios are likely reflected by the fact that there are clear microenvironmental differences present in vivo following transplantation with differential expression of antigens and compartmentalization of responses that may occur (18–25). However, the in vivo correlate clearly contains the most relevance as this is most important clinically and reflects the net effect of all of the above variables. It should be noted that unmodified rejection in this transplant model is characterized by a dominant Th1 response and the emergence of Th2 requires manipulations which favor Th2 development (13, 40, 41). We have previously reported that CD8+ cells represent a major source of IFNγ in this model and depleting CD8+ cells reduces IFNγ production and induces Th2 responses (13, 27). Thus, depleting p21−/− vs. WT allograft recipients of CD8+ cells allowed us to investigate the impact of p21 on in vivo Th2 priming and CD4+ T cell mediated rejection (28). While depleting recipients of CD8+ cells did induce measurable Th2 responses in both groups, there was no difference in the level of Th2 priming in p21−/− vs. WT recipients. However, Th1 priming was significantly increased in p21−/− recipients even in a setting where Th2 were induced. These data indicate that p21 plays a role in controlling alloantigen-reactive responses following transplantation primarily via a Th1 mechanism. Whether Th1 responses in vivo are a result of altered cell cycle progression (39), altered apoptosis (37), or effects on T cell regulation (1) are unclear at the present time. However, many of these studies focused on in vitro situations, whereas the current study’s focus was to address the net effect of these postulated mechanisms in an in vivo transplantation model.
To further explore the role of p21 in modulating alloreactive T cell function in vivo, WT cardiac allografts were transfected with Adp21 and transplanted into WT recipients (Figure 2). Over-expression of p21 prolonged graft survival and reduced Th1 priming within the recipients’ spleens. This poses the question of how p21 transfection of the vascularized cardiac allograft could influence recipient T cell responses. We have previously reported that transfection of cardiac grafts with adenoviral vectors results in transient transgene expression within the marginal zones of the recipients’ spleens (15). Indeed, weak, albeit detectable expression of the p21 transgene was observed in the spleens of recipients of p21 transfected allografts on day 2 post-transplantation (Figure 2C). In this cardiac transplant model, dendritic cells (DC), which are readily transfected by adenoviral vectors (42, 43), are known to migrate out of the transplanted heart and localize into the recipient’s spleen (44). Therefore transfected DC may serve as a source of transgene product in the spleen. Alternatively, adenoviral vectors may be flushed out of the perfused heart upon revascularization and localize in the recipient’s spleen. Since priming of graft-reactive T cells occurs in the secondary lymphoid tissues (18, 19), it is possible that p21 expression within the spleen influences Th1 priming. However, T cells are known to be difficult to transfect with adenoviral vectors (45, 46). It has also been reported that exogenous p21 protein is capable of entering T cells, localizing in the nucleus, and inhibiting proliferation and proinflammatory cytokine production in response to mitogenic stimulation (47). Thus another possible scenario is that exogenous p21 transgene product secreted by DC or other transfected cells of the spleen could influence T cell behavior. Additionally, other cell types within the allograft that are capable of proliferation and interfacing with the immune system, such as endothelial cells or smooth muscle cells, may also explain some of the observations. Further studies are planned to investigate these potential cellular mechanisms of p21 action.
In summary, we provide evidence that p21 controls the intensity of the immune response using a cardiac transplant in vivo model, with over expression of p21 inhibiting allograft rejection. These data have important clinical applications. Immunosuppressive therapies currently used in transplantation are associated with numerous toxicities and side effects (Reviewed in (48, 49)). Hence, new approaches to immunosuppression are being explored. Manipulation of p21 may constitute such an approach. Indeed, the finding that recombinant p21 protein may inhibit T cell responses (47) points to the potential of this approach as a therapeutic modality and certainly warrants further investigation.
Acknowledgments
This work was supported by a Folkert Belzer Research Fellowship (THW) and R01 AI61469 (DKB) and R01 HL70613 (DKB) from the National Institutes of Health.
Abbreviations used in this paper
- cdk
cyclin dependent kinase
- CsA
cyclosporine A
- DC
dendritic cells
- ELISPOT
enzyme-linked immunospot
- H&E
hematoxylin and eosin
- MLC
mixed leukocyte culture
- p21−/−
p21 deficient
- WT
wild type
Footnotes
This work was supported by a Folkert Belzer Research Fellowship (THW) and R01 AI31946 (DKB) and R01 HL70613 (DKB) from the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Balomenos D, Martin-Caballero J, Garcia MI, Prieto I, Flores JM, Serrano M, et al. The cell cycle inhibitor p21 controls T-cell proliferation and sex-linked lupus development. Nat Med. 2000;6(2):171–176. doi: 10.1038/72272. [DOI] [PubMed] [Google Scholar]
- 2.Santiago-Raber ML, Lawson BR, Dummer W, Barnhouse M, Koundouris S, Wilson CB, et al. Role of cyclin kinase inhibitor p21 in systemic autoimmunity. J Immunol. 2001;167(7):4067–4074. doi: 10.4049/jimmunol.167.7.4067. [DOI] [PubMed] [Google Scholar]
- 3.Nourse J, Firpo E, Flanagan WM, Coats S, Polyak K, Lee MH, et al. Interleukin-2-mediated elimination of the p27Kip1 cyclin-dependent kinase inhibitor prevented by rapamycin. Nature. 1994;372(6506):570–573. doi: 10.1038/372570a0. [DOI] [PubMed] [Google Scholar]
- 4.Sherr CJ. G1 phase progression: cycling on cue. Cell. 1994;79(4):551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- 5.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1 - phase progression. Genes Dev. 1999;13(12):1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 6.Sherr CJ, Roberts JM. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9(10):1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 7.Perlman H, Bradley K, Liu H, Cole S, Shamiyeh E, Smith RC, et al. IL-6 and matrix metalloproteinase-1 are regulated by the cyclin-dependent kinase inhibitor p21 in synovial fibroblasts. J Immunol. 2003;170(2):838–845. doi: 10.4049/jimmunol.170.2.838. [DOI] [PubMed] [Google Scholar]
- 8.Yang ZY, Simari RD, Perkins ND, San H, Gordon D, Nabel GJ, et al. Role of the p21 cyclin-dependent kinase inhibitor in limiting intimal cell proliferation in response to arterial injury. Proc Natl Acad Sci U S A. 1996;93(15):7905–7910. doi: 10.1073/pnas.93.15.7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang S, Lawless VA, Kaplan MH. Cytokine-stimulated T lymphocyte proliferation is regulated by p27Kip1. J Immunol. 2000;165(11):6270–6277. doi: 10.4049/jimmunol.165.11.6270. [DOI] [PubMed] [Google Scholar]
- 10.Jackson SK, DeLoose A, Gilbert KM. Induction of anergy in Th1 cells associated with increased levels of cyclin-dependent kinase inhibitors p21Cip1 and p27Kip1. J Immunol. 2001;166(2):952–958. doi: 10.4049/jimmunol.166.2.952. [DOI] [PubMed] [Google Scholar]
- 11.Khanna AK, Hosenpud JD. Cyclosporine induces the expression of the cyclin inhibitor p21. Transplantation. 1999;67(9):1262–1268. doi: 10.1097/00007890-199905150-00011. [DOI] [PubMed] [Google Scholar]
- 12.Lawson BR, Kono DH. Theofilopoulos AN. Deletion of p21 (WAF-1/Cip1) does not induce systemic autoimmunity in female BXSB mice. J Immunol. 2002;168(11):5928–5932. doi: 10.4049/jimmunol.168.11.5928. [DOI] [PubMed] [Google Scholar]
- 13.Chan SY, DeBruyne LA, Goodman RE, Eichwald EJ, Bishop DK. In vivo depletion of CD8+ T cells results in Th2 cytokine production and alternate mechanisms of allograft rejection. Transplantation. 1995;59(8):1155–1161. [PubMed] [Google Scholar]
- 14.Corry RJ, Winn HJ, Russell PS. Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation. 1973;16(4):343–350. doi: 10.1097/00007890-197310000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Chan SY, Li K, Piccotti JR, Louie MC, Judge TA, Turka LA, et al. Tissue-specific consequences of the anti-adenoviral immune response: implications for cardiac transplants. Nat Med. 1999;5(10):1143–1149. doi: 10.1038/13467. [DOI] [PubMed] [Google Scholar]
- 16.Chan SY, Goodman RE, Szmuszkovicz JR, Roessler B, Eichwald EJ, Bishop DK. DNA-liposome versus adenoviral mediated gene transfer of transforming growth factor beta1 in vascularized cardiac allografts: differential sensitivity of CD4+ and CD8+ T cells to transforming growth factor beta1. Transplantation. 2000;70(9):1292–1301. doi: 10.1097/00007890-200011150-00006. [DOI] [PubMed] [Google Scholar]
- 17.Matesic D, Lehmann PV, Heeger PS. High-resolution characterization of cytokine-producing alloreactivity in naive and allograft-primed mice. Transplantation. 1998;65(7):906–914. doi: 10.1097/00007890-199804150-00008. [DOI] [PubMed] [Google Scholar]
- 18.Lakkis FG. Where is the alloimmune response initiated? Am J Transplant. 2003;3(3):241–242. doi: 10.1034/j.1600-6143.2003.00054.x. [DOI] [PubMed] [Google Scholar]
- 19.Lakkis FG, Arakelov A, Konieczny BT, Inoue Y. Immunologic 'ignorance' of vascularized organ transplants in the absence of secondary lymphoid tissue. Nat Med. 2000;6(6):686–688. doi: 10.1038/76267. [DOI] [PubMed] [Google Scholar]
- 20.Zhou P, Hwang KW, Palucki D, Kim O, Newell KA, Fu YX, et al. Secondary lymphoid organs are important but not absolutely required for allograft responses. Am J Transplant. 2003;3(3):259–266. doi: 10.1034/j.1600-6143.2003.00067.x. [DOI] [PubMed] [Google Scholar]
- 21.Kreisel D, Krupnick AS, Gelman AE, Engels FH, Popma SH, Krasinskas AM, et al. Non-hematopoietic allograft cells directly activate CD8+ T cells and trigger acute rejection: an alternative mechanism of allorecognition. Nat Med. 2002;8(3):233–239. doi: 10.1038/nm0302-233. [DOI] [PubMed] [Google Scholar]
- 22.Vriens PW, Nisco SJ, Hoyt EG, Lyu SC, Pierre P, Reitz BA, et al. Tissue-specific differences in the establishment of tolerance. Tolerogenic effects of lung allografts in rats. Transplantation. 1994;57(12):1795–1798. [PubMed] [Google Scholar]
- 23.Fuchimoto Y, Gleit ZL, Huang CA, Kitamura H, Schwarze ML, Menard MT, et al. Skin-specific alloantigens in miniature swine. Transplantation. 2001;72(1):122–126. doi: 10.1097/00007890-200107150-00024. [DOI] [PubMed] [Google Scholar]
- 24.Fedoseyeva EV, Zhang F, Orr PL, Levin D, Buncke HJ, Benichou G. De novo autoimmunity to cardiac myosin after heart transplantation and its contribution to the rejection process. J Immunol. 1999;162(11):6836–6842. [PubMed] [Google Scholar]
- 25.Mares DC, Heidler KM, Smith GN, Cummings OW, Harris ER, Foresman B, et al. Type V collagen modulates alloantigen-induced pathology and immunology in the lung. Am J Respir Cell Mol Biol. 2000;23(1):62–70. doi: 10.1165/ajrcmb.23.1.3924. [DOI] [PubMed] [Google Scholar]
- 26.Bishop DK, Shelby J, Eichwald EJ. Mobilization of T lymphocytes following cardiac transplantation: evidence that CD4-positive cells are required for cytotoxic T lymphocyte activation, inflammatory endothelial development, graft infiltration, and acute allograft rejection. Transplantation. 1992;53:849–857. [PubMed] [Google Scholar]
- 27.Piccotti JR, Li K, Chan SY, Ferrante J, Magram J, Eichwald EJ, et al. Alloantigen-reactive Th1 development in IL-12-deficient mice. J Immunol. 1998;160(3):1132–1138. [PubMed] [Google Scholar]
- 28.Bishop DK, Chan S, Li W, Ensley RD, Xu S, Eichwald EJ. CD4-positive helper T lymphocytes mediate mouse cardiac allograft rejection independent of donor alloantigen specific cytotoxic T lymphocytes. Transplantation. 1993;56(4):892–897. doi: 10.1097/00007890-199310000-00023. [DOI] [PubMed] [Google Scholar]
- 29.Kwon TK, Buchholz MA, Ponsalle P, Chrest FJ, Nordin AA. The regulation of p27Kip1 expression following the polyclonal activation of murine G0 T cells. J Immunol. 1997;158(12):5642–5648. [PubMed] [Google Scholar]
- 30.Mohapatra S, Agrawal D, Pledger WJ. p27Kip1 regulates T cell proliferation. J Biol Chem. 2001;276(24):21976–21983. doi: 10.1074/jbc.M009788200. [DOI] [PubMed] [Google Scholar]
- 31.Appleman LJ, Berezovskaya A, Grass I, Boussiotis VA. CD28 costimulation mediates T cell expansion via IL-2-independent and IL-2-dependent regulation of cell cycle progression. J Immunol. 2000;164(1):144–151. doi: 10.4049/jimmunol.164.1.144. [DOI] [PubMed] [Google Scholar]
- 32.Wolfraim LA, Letterio JJ. Cutting edge: p27Kip1 deficiency reduces the requirement for CD28-mediated costimulation in naive CD8+ but not CD4+ T lymphocytes. J Immunol. 2005;174(5):2481–2484. doi: 10.4049/jimmunol.174.5.2481. [DOI] [PubMed] [Google Scholar]
- 33.Rowell EA, Wang L, Hancock WW, Wells AD. The cyclin-dependent kinase inhibitor p27kip1 is required for transplantation tolerance induced by costimulatory blockade. J Immunol. 2006;177(8):5169–5176. doi: 10.4049/jimmunol.177.8.5169. [DOI] [PubMed] [Google Scholar]
- 34.Shen R, Kaplan MH. The homeostasis but not the differentiation of T cells is regulated by p27(Kip1) J Immunol. 2002;169(2):714–721. doi: 10.4049/jimmunol.169.2.714. [DOI] [PubMed] [Google Scholar]
- 35.Modiano JF, Mayor J, Ball C, Fuentes MK, Linthicum DS. CDK4 expression and activity are required for cytokine responsiveness in T cells. J Immunol. 2000;165(12):6693–6702. doi: 10.4049/jimmunol.165.12.6693. [DOI] [PubMed] [Google Scholar]
- 36.Boussiotis VA, Freeman GJ, Taylor PA, Berezovskaya A, Grass I, Blazar BR, et al. p27kip1 functions as an anergy factor inhibiting interleukin 2 transcription and clonal expansion of alloreactive human and mouse helper T lymphocytes. Nat Med. 2000;6(3):290–297. doi: 10.1038/73144. [DOI] [PubMed] [Google Scholar]
- 37.Hingorani R, Bi B, Dao T, Bae Y, Matsuzawa A, Crispe IN. CD95/Fas signaling in T lymphocytes induces the cell cycle control protein p21cip-1/WAF-1, which promotes apoptosis. J Immunol. 2000;164(8):4032–4036. doi: 10.4049/jimmunol.164.8.4032. [DOI] [PubMed] [Google Scholar]
- 38.Lawson BR, Baccala R, Song J, Croft M, Kono DH, Theofilopoulos AN. Deficiency of the cyclin kinase inhibitor p21(WAF-1/CIP-1) promotes apoptosis of activated/memory T cells and inhibits spontaneous systemic autoimmunity. J Exp Med. 2004;199(4):547–557. doi: 10.1084/jem.20031685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.LaBaer J, Garrett MD, Stevenson LF, Slingerland JM, Sandhu C, Chou HS, et al. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 1997;11(7):847–862. doi: 10.1101/gad.11.7.847. [DOI] [PubMed] [Google Scholar]
- 40.Piccotti JR, Chan SY, Goodman RE, Magram J, Eichwald EJ, Bishop DK. IL-12 antagonism induces T helper 2 responses, yet exacerbates cardiac allograft rejection. Evidence against a dominant protective role for T helper 2 cytokines in alloimmunity. J Immunol. 1996;157(5):1951–1957. [PubMed] [Google Scholar]
- 41.Bishop DK, Chan Wood S, Eichwald EJ, Orosz CG. Immunobiology of allograft rejection in the absence of IFN-gamma: CD8+ effector cells develop independently of CD4+ cells and CD40-CD40 ligand interactions. J Immunol. 2001;166(5):3248–3255. doi: 10.4049/jimmunol.166.5.3248. [DOI] [PubMed] [Google Scholar]
- 42.Giannoukakis N, Bonham CA, Qian S, Chen Z, Peng L, Harnaha J, et al. Prolongation of cardiac allograft survival using dendritic cells treated with NF-kB decoy oligodeoxyribonucleotides. Mol Ther. 2000;1(5 Pt 1):430–437. doi: 10.1006/mthe.2000.0060. [DOI] [PubMed] [Google Scholar]
- 43.Bonham CA, Peng L, Liang X, Chen Z, Wang L, Ma L, et al. Marked prolongation of cardiac allograft survival by dendritic cells genetically engineered with NF-kappa B oligodeoxyribonucleotide decoys and adenoviral vectors encoding CTLA4-Ig. J Immunol. 2002;169(6):3382–3391. doi: 10.4049/jimmunol.169.6.3382. [DOI] [PubMed] [Google Scholar]
- 44.Larsen CP, Morris PJ, Austyn JM. Migration of dendritic leukocytes from cardiac allografts into host spleens. A novel pathway for initiation of rejection. J Exp Med. 1990;171(1):307–314. doi: 10.1084/jem.171.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang S, Endo RI, Nemerow GR. Upregulation of integrins alpha v beta 3 and alpha v beta 5 on human monocytes and T lymphocytes facilitates adenovirus-mediated gene delivery. J Virol. 1995;69(4):2257–2263. doi: 10.1128/jvi.69.4.2257-2263.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horvath J, Weber JM. Nonpermissivity of human peripheral blood lymphocytes to adenovirus type 2 infection. J Virol. 1988;62(1):341–345. doi: 10.1128/jvi.62.1.341-345.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khanna AK, Plummer M, Nilakantan V, Pieper GM. Recombinant p21 protein inhibits lymphocyte proliferation and transcription factors. J Immunol. 2005;174(12):7610–7617. doi: 10.4049/jimmunol.174.12.7610. [DOI] [PubMed] [Google Scholar]
- 48.MacDonald A. Improving tolerability of immunosuppressive regimens. Transplantation. 2001;72(12 Suppl):S105–S112. [PubMed] [Google Scholar]
- 49.Kahan BD, Camardo JS. Rapamycin: clinical results and future opportunities. Transplantation. 2001;72(7):1181–1193. doi: 10.1097/00007890-200110150-00001. [DOI] [PubMed] [Google Scholar]