Abstract
Patients suffering from invasive mycoses often receive concomitant antifungal therapy and antibacterial agents. Assessment of pharmacodynamic interactions between antifungal and antibacterial agents is complicated by the absence of a common antifungal end point for both agents. Ciprofloxacin has no intrinsic antifungal activity but may interact with antifungal agents, since it inhibits DNA gyrase (topoisomerase II), which is abundant in fungi. We therefore employed isobolographic analysis adapted to incorporate a nonactive agent in order to analyze the potential in vitro interaction between the fluoroquinolone ciprofloxacin and several representative antifungal agents against Candida albicans and Aspergillus fumigatus strains by using a microdilution checkerboard technique. In agreement with earlier in vitro studies, conventional fractional inhibitory concentration index analysis was unable to detect interactions between ciprofloxacin and antifungal agents. However, isobolographic analysis revealed significant pharmacodynamic interactions between antifungal agents and ciprofloxacin against C. albicans and A. fumigatus strains. Amphotericin B demonstrated concentration-dependent interactions for both species, with synergy (interaction indices, 0.14 to 0.81) observed at ciprofloxacin concentrations of <10.64 μg/ml. Synergy (interaction indices, 0.10 to 0.86) was also found for voriconazole and caspofungin against A. fumigatus. Isobolographic analysis may help to elucidate the pharmacodynamic interactions between antifungal and non-antifungal agents and to develop better management strategies against invasive candidiasis and aspergillosis.
Candida and Aspergillus spp. are the most common pathogens causing life-threatening invasive fungal infections in immunocompromised patients (22, 25, 41). Patients at risk for these invasive fungal infections are also at risk of developing other opportunistic infections, for which a wide range of non-antifungal therapeutic agents is used for prophylactic and therapeutic purposes concomitantly with antifungal agents (2). A potential interaction between these agents and antifungal agents may affect antifungal efficacy, with potentially important implications for clinical outcome.
Fluoroquinolones are broad-spectrum antibacterial agents that act on DNA gyrase (topoisomerase II) and topoisomerase IV, resulting in inhibition of DNA replication, recombination, and transcription, and ultimately bacterial death (40). Although fluoroquinolones have no intrinsic antifungal activity, high levels of topoisomerase I and II have been reported in pathogenic fungi (6, 34, 35), offering a potential mechanism of interaction between fluoroquinolones and antifungal agents. Shen and colleagues demonstrated that an isothiazoloquinolone inhibited Candida albicans topoisomerase II (34). Sugar et al., Sasaki et al., and Nakajima et al. demonstrated in vivo enhancement of antifungal activity by combined fluoroquinolone therapy (19, 29, 36). However, most in vitro studies using conventional fractional inhibitory concentration (FIC) indices did not find this synergistic interaction between quinolones and antifungal agents, raising uncertainties about the applicability of this analytical approach.
Assessment of in vitro pharmacodynamic interactions between antifungal and non-antifungal agents, such as fluoroquinolones, is complicated by the absence of a common antifungal end point. The FIC index is often used to analyze such potential antifungal interactions but has practical limitations because MIC end points are required for both agents. Therefore, new analytical tools are required in order to analyze interactions between antifungal agents and compounds without intrinsic antifungal activity.
Isobolographic analysis has previously been applied for the pharmacodynamic study of several classes of non-antimicrobial agents, including antineoplastic (8), cardiovascular (26), antiepileptic (9), analgesic (30), and anti-inflammatory (17) compounds. Isobolographic analysis has also been applied recently to the study of the pharmacodynamic interactions between azoles and polyenes (12, 14). However, to our knowledge, isobolographic analysis has not been reported in the study of the complex pharmacodynamic interactions between antifungal agents and agents without antifungal activity. Isobolographic analysis may be a more sensitive method by which to determine in vitro pharmacodynamic interactions between antifungal and non-antifungal agents (37). Therefore, we explored the utility of isobolographic analysis for analysis of pharmacodynamic interactions between systemic antifungal agents (amphotericin B, caspofungin, voriconazole, and fluconazole) and a fluoroquinolone (ciprofloxacin) against Aspergillus fumigatus and Candida albicans.
MATERIALS AND METHODS
Strains and medium.
Three clinical strains of C. albicans (CA 362, CA 8621, and CA 5685) and three clinical strains of A. fumigatus (AF 2025, AF 4215 [ATCC MYA-3626], and AF 2350) were used in this study. The strains were stored on potato dextrose agar slants at −70°C. Candida blastoconidia and Aspergillus conidia were collected with a wet swab from 1- to 2- and 5- to 7-day-old cultures in Sabouraud dextrose agar, respectively. Blastoconidial and conidial suspensions were adjusted spectrophotometrically at 530 nm to 75 to 77% and 80 to 82% transmittance, respectively. Conidial suspensions were diluted in order to obtain twice the final inoculum, which ranged from 5 × 102 to 2.5 × 103 CFU/ml for Candida strains and from 0.4 × 104 to 5 × 104 CFU/ml for Aspergillus strains, in a medium consisting of RPMI 1640 medium buffered at pH 7 with 0.165 M morpholinepropanesulfonic acid (MOPS) (BioWhittaker, Walkersville, MD). Candida parapsilosis (ATCC 22019), Candida krusei (ATCC 6258), and Escherichia coli (ATCC 259222) were used as quality controls.
Antimicrobial compounds and combination microtitration plates.
Ciprofloxacin (Bayer AG, Leverkusen, Germany), amphotericin B (Ben Venue Laboratories, Inc., Bedford, OH), caspofungin (Merck and Company, Rahway, NJ), fluconazole (Pfizer Pharmaceuticals, New York, NY), and voriconazole (Pfizer Pharmaceuticals, New York, NY) were provided as clinical formulations and prepared according to the manufacturer's guidelines in order to obtain working solutions of 200 μg/ml, 8 μg/ml, 2,040 μg/ml, 8 μg/ml, and 10 μg/ml, respectively, in assay medium. The drugs were serially diluted twofold in the medium in order to obtain four times the final concentrations; these concentrations were chosen so as to include the MIC of each compound. The ranges of concentrations of compounds studied against C. albicans blastoconidia were 0.05 to 50 μg of ciprofloxacin/ml, 0.03 to 2 μg of amphotericin B/ml, 0.015 to 1 μg of caspofungin/ml, and 0.03 to 2 μg of fluconazole/ml. The same ranges of concentrations of these drugs were tested against A. fumigatus conidia, except for caspofungin, for which the range was 8 to 512 μg/ml. Fifty microliters of each antifungal drug concentration and its drug-free control was combined with 50 μl of each concentration of ciprofloxacin and its drug-free control in order to obtain a 12-by-8 checkerboard in 96-well flat-bottom microtitration plates (Corning Inc., Corning, NY). The plates were stored at −70°C and thawed on the day of the experiment.
Susceptibility testing.
Microtitration plates were thawed, and 100 μl of conidial suspensions was inoculated into each well. Plates were incubated at 37°C for 24 h, and fungal growth in each well was assessed visually with the aid of a magnifying mirror. For amphotericin B, caspofungin, and voriconazole, the MIC was defined as the lowest drug concentration that showed no visible growth (20, 21). The MIC of fluconazole was defined as the lowest drug concentration showing slight growth (20% of the growth of the drug-free control) (7). Fungal growth was also assessed spectrophotometrically at 405 nm with a spectrophotometer (ELX808; Biotek Instruments, Winooski, VT), and the percentage of growth in each well was calculated based on the equation (A405 of a well − background A405)/(A405 of the drug-free well − background A405 of the drug-free well) × 100%, where the background A405 was measured for a plate inoculated with a conidium-free inoculum and handled in the same way as the inoculated plates with the conidium-containing inocula. All studies for each strain were conducted in three replicates. The MIC of each drug was defined visually with the aid of a concave mirror as the lowest drug concentration corresponding to the absence of growth, or 0% of the growth of the drug-free control (MIC-0); slight growth, or 25% of the growth of the control (MIC-1); prominent reduction of growth, or 50% of control growth (MIC-2); and slight reduction of growth, or 75% of control growth (MIC-3) (20, 21).
FIC index analysis.
For all wells of the microtitration plates that corresponded to a MIC, the sum of the FICs (ΣFIC) was calculated for each well as FICA + FICB, where A and B are the two drugs in the well. The FIC of each drug was calculated as (MIC of the drug in combination)/(MIC of the drug alone). ΣFIC was calculated for each MIC end point, i.e., MIC-0, MIC-1, MIC-2, and MIC-3. A ΣFIC of ≤0.5 was considered to indicate synergy, whereas if ΣFIC was >4, antagonism was inferred. In any other case (0.5 < ΣFIC ≤ 4), the interaction was considered indifferent (1, 7).
These cutoffs have been proposed for combinations between two active drugs. Based on these cutoffs, at least a fourfold reduction in the MICs of both drugs is required for defining synergy, whereas at least a fourfold increase in the MIC of at least one drug is required for defining antagonism (7). This implies that for double combinations with only one active drug, a fourfold reduction or increase in the MIC of the active drug would indicate synergy or antagonism, respectively. Therefore, synergy or antagonism between an antifungal agent and ciprofloxacin was concluded when the MIC of the antifungal agent was increased or reduced by 2 dilutions, respectively.
Isobolographic analysis. (i) Regression analysis.
For isobolographic analysis of the interaction of an antifungal drug with ciprofloxacin, the checkerboard data were analyzed by nonweighted, nonlinear regression analysis using Prism 4.0 software (GraphPad Inc., San Diego, CA). The Emax model was fitted to the concentration-effect curves of each drug alone (last column and last row of the microtiter plate for the antifungal drug and ciprofloxacin, respectively) and their combinations at various fixed ratios of the antifungal drug to ciprofloxacin based on weight. For example, for the 1:1 fixed ratio, combinations with equal amounts of the two drugs were chosen, while for the 1:25 fixed ratio, combinations of 0.5 μg of amphotericin B/ml plus 25 μg of ciprofloxacin/ml, 0.25 μg of amphotericin B/ml plus 12.5 μg of ciprofloxacin/ml, 0.125 μg of amphotericin B/ml plus 6.25 μg of ciprofloxacin/ml, etc., were chosen. The Emax model of each of the fixed ratios is described by
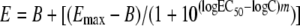 |
(1) |
where E is the percentage of growth (dependent variable) at drug concentration D (independent variable), Emax is the maximum percentage of growth observed for the drug-free control, B is the minimum percentage of growth at infinite drug concentration, EC50 is the drug concentration producing 50% of the Emax, and m is the slope of the concentration-effect curve (Hill coefficient). Equation 1 is a modification of the classic equation of the Emax model in order to account for the normal distribution of the log drug concentrations rather than the drug concentrations themselves (13, 18).
The minimum percentage of growth of the Emax model, B, was kept at 0% for all combination data sets except the combination of ciprofloxacin with fluconazole, where it was shared among all fixed ratios and fitted globally, since two out of three strains of C. albicans presented a trailing phenomenon. The maximum percentage of growth in the Emax model, Emax, was shared among all fixed ratios of each replicate experiment and fitted globally by the statistical software program. Regression analysis with global fitting may minimize the systematic pattern of growth observed previously, in which wells inside 96-well microplates showed different growth from wells on the periphery (5). The goodness of fit of the model was interpreted using the runs test, residuals, visual inspection, and R2 values, and poor fits (e.g., R2, <0.8; 95% confidence interval, <1 log2 unit; statistically significant deviation of residuals from a normal distribution with a mean of zero; and statistically significant deviation based on the runs test) were excluded from the analysis. Preliminary regression analysis showed that a weighting scheme was not necessary (the scatter in the percentage of growth was similar across different drug concentrations), and results using different weighting schemes were similar to those obtained by nonweighted regression analysis. Therefore, nonweighted regression analysis was used throughout.
(ii) Drug interaction analysis.
The isobolographic drug interaction analysis is based on the Loewe additivity (no-interaction) theory (3), which is described by the equation
 |
(2) |
where cAA and cCIP are the concentrations of the antifungal drug and ciprofloxacin, respectively, in the combination that elicit a certain effect, and ECAA and ECCIP are the isoeffective concentrations of the antifungal drug and ciprofloxacin, respectively, acting alone. In isobolographic analysis, the concentration-effect curve of the drugs in combination at a fixed ratio is compared with the theoretical additive concentration-effect curve calculated from equation 1, and the interaction is assessed at any effect level. Thus, for a particular growth level (e.g., 15%, 50%, or 85% of the growth of the drug-free control), the total concentration of both drugs (ECMIX) for a fixed ratio of each combination is compared with the isoeffective theoretical additive total concentration (ECTHE) (38, 39).
ECMIX is provided by the Emax model for each fixed-ratio combination as
 |
(3) |
where cAA and cCIP are the concentrations of the antifungal agent and ciprofloxacin, respectively.
The theoretical additive concentration, ECTHE, for the same growth level is calculated from equation 2 by substituting PAA·ECTHE for cAA and PCIP·ECTHE for cCIP, where PAA and PCIP are the proportions of the antifungal agent and ciprofloxacin, respectively, in the total concentration ECMIX (e.g., for a 1:2 fixed ratio of the antifungal agent to ciprofloxacin, the corresponding PAA and PCIP are 1/3 and 2/3, respectively), and ECAA and ECCIP are the isoeffective concentrations of the antifungal agent and ciprofloxacin alone, respectively, obtained from the Emax model of the concentration-effect curves of the drugs alone. After rearrangement, the following equation is obtained:
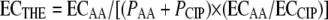 |
(4) |
where ECAA and ECCIP are the isoeffective concentrations of amphotericin B and ciprofloxacin alone, respectively, obtained from the Emax model of the concentration-effect curves of the drugs alone, and PAA and PCIP are the proportions of amphotericin B and ciprofloxacin, respectively, in the total concentration (e.g., for a 1:2 fixed ratio of amphotericin B to ciprofloxacin, the corresponding PAA is 1/3), based on which ECTHE can be calculated (38, 39). However, since ciprofloxacin has no direct antifungal activity, equation 4 becomes
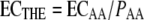 |
(5) |
based on reference 4.
The 95% confidence intervals of ECTHE were calculated based on its standard error (SE), which can be derived by
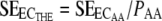 |
(6) |
where  is obtained by the nonlinear regression analysis software (39).
is obtained by the nonlinear regression analysis software (39).
An interaction index (I) for each fixed ratio at a specified growth level was then calculated as the ECMIX/ECTHE ratio for each replicate. (The interaction index can also be calculated as cAA/ECAA based on equation 2 or can be derived from equations 3 and 4.) The average of the interaction indices for the three replicates of each strain was calculated. In order to test whether the I's of three replicates were significantly lower or higher than 1 (P < 0.05), the I's were log-transformed to approximate a normal distribution, and the 95% confidence intervals were calculated. If the 95% confidence intervals of I's of a fixed-ratio combination were significantly lower or higher than 1, synergy or antagonism, respectively, was concluded for that particular fixed ratio. In any other case, additivity (indifference in the case of one active drug) was concluded. Three growth levels, i.e., 15%, 50%, and 85% of the growth of the drug-free control, were chosen in order to assess pharmacodynamic interaction at high, intermediate, and low drug concentrations, respectively, since high drug concentrations result in more inhibition of fungal growth. We defined the interaction between an antifungal agent and ciprofloxacin as synergistic or antagonistic for a strain when two or more sequential fixed ratios of each growth level had lower or higher interaction indices than 1, respectively.
The results of isobolographic analysis can be easily visualized using isobolograms. An isobologram is a two-dimensional plot in which the coordinates are the concentrations of the two drugs on an arithmetic scale. An isobol is a curve that starts from a concentration of drug A on the x axis and ends at an isoeffective concentration of drug B on the y axis, connecting the concentrations of all combinations showing the same effect. An additive isobol, the graphical representation of equation 1, is a straight line from the x axis to the y axis, which connects the isoeffective concentrations of drugs A and B alone. However, when only one drug is active, the additive isobol (called an indifferent isobol in this case) is parallel to the axis along which the concentrations of the inactive drug are plotted, starting from the concentration of the active drug that produces an effect. An isobol that deviates to the left or right from the indifferent isobol indicates synergy or antagonism, respectively.
Finally, the concentrations of drugs at which synergy or antagonism occurred were determined for each drug combination and strain, and the median (range) concentration of each drug was reported.
RESULTS
MICs and EC50s of single antifungal agents.
The MICs of amphotericin B, fluconazole, and caspofungin for C. albicans strains were 0.125 to 0.5 μg/ml, 0.125 to 0.25 μg/ml, and 1.0 μg/ml, respectively. The median (range) EC50s of amphotericin B, fluconazole, and caspofungin for C. albicans strains, as determined by the Emax model fitted to the concentration-effect data of each drug individually, were 0.25 (0.08 to 0.29) μg/ml, 0.09 (0.07 to 0.10) μg/ml, and 0.59 (0.33 to 0.65) μg/ml, respectively.
The MICs of amphotericin B, voriconazole, and caspofungin for A. fumigatus strains were 0.5 to 1.0 μg/ml, 0.5 μg/ml, and 128 μg/ml, respectively. The median (range) EC50s of amphotericin B, voriconazole, and caspofungin for A. fumigatus strains were 0.50 (0.25 to 0.54) μg/ml, 0.18 (0.15 to 0.24) μg/ml, and 28.79 (12.11 to 59.85) μg/ml, respectively.
FIC index analysis for interactions.
No more than a 1-dilution increase or decrease in the MICs of the antifungal agents was observed when they were combined with ciprofloxacin against C. albicans or A. fumigatus, indicating indifference for all strains based on the FIC index criteria.
Isobolographic analysis of interaction against C. albicans.
The results of the isobolographic analysis for C. albicans are summarized in Tables 1 and 2, where the median interaction indices for all strains for each fixed-ratio combination and the drug concentrations are presented at three different levels of fungal growth (15%, 50%, and 85% of the growth of the drug-free control). No more than a 1-dilution increase or decrease in the MICs of the antifungal agents was observed when they were combined with ciprofloxacin against C. albicans, indicating indifference based on the FIC index criteria for all strains. Based on isobolographic analysis, the combination of ciprofloxacin with amphotericin B was synergistic for all three growth levels (P < 0.05) and all strains of C. albicans at fixed ratios ranging from 1:0.08 to 1:50, with interaction indices ranging from 0.52 to 0.81 (Table 1). Antagonism was observed only at the fixed ratio of 1:100, with interaction indices ranging from 1.23 to 1.70. The concentrations at which this synergy was observed ranged from 0.12 to 0.19 μg/ml of amphotericin B and from 0.41 to 0.53 μg/ml of ciprofloxacin (Table 2). Antagonism was detected at 0.17 to 0.33 μg/ml of amphotericin B and 44.01 to 63.58 μg/ml of ciprofloxacin (Table 2). For the ciprofloxacin-plus-fluconazole combination against C. albicans, a strain dependency was observed that ranged from antagonism against strain 362 to synergy against strain 8621. Other combinations with ciprofloxacin (amphotericin B or caspofungin) did not display this degree of strain-dependent interaction. Significant synergistic or antagonistic interactions were seldom observed for the combination of ciprofloxacin with caspofungin.
TABLE 1.
Results of isobolographic analysis for combinations of antifungal drugs with ciprofloxacin against Candida albicans strains
| Drug combinationa | Growth (% of control) | Strain | Interaction indexb at the following fixed ratio of antifungal drug to CIP (PAA):
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1:100 (0.01) | 1:50 (0.02) | 1:25 (0.04) | 1:12.5 (0.07) | 1:6.25 (0.14) | 1:3.125 (0.24) | 1:1.6 (0.39) | 1:0.8 (0.56) | 1:0.4 (0.72) | |||
| AMB + CIP | 15 | 362 | 1.70 | 0.86 | 0.84 | 0.78 | 0.76 | 0.76 | 0.77 | 0.78 | |
| 8621 | 1.23 | 0.99 | 0.85 | 0.80 | 0.79 | 0.67 | 0.80 | 0.79 | |||
| 5685 | 1.31 | 0.82 | 0.69 | 0.61 | 0.62 | 0.58 | 0.61 | 0.60 | |||
| 50 | 362 | 1.58 | 0.82 | 0.81 | 0.73 | 0.72 | 0.71 | 0.71 | 0.69 | ||
| 8621 | 1.28 | 0.91 | 0.78 | 0.67 | 0.68 | 0.62 | 0.71 | 0.73 | |||
| 5685 | 1.14 | 0.73 | 0.62 | 0.56 | 0.55 | 0.52 | 0.54 | 0.57 | |||
| 85 | 362 | 1.46 | 0.79 | 0.79 | 0.67 | 0.68 | 0.67 | 0.66 | 0.61 | ||
| 8621 | 1.32 | 0.83 | 0.72 | 0.57 | 0.59 | 0.58 | 0.63 | 0.67 | |||
| 5685 | 0.99 | 0.66 | 0.56 | 0.52 | 0.48 | 0.47 | 0.49 | 0.55 | |||
| FLC + CIP | 15 | 362 | 1.03 | 1.06 | 1.08 | 1.07 | 1.04 | 1.05 | ND | ND | |
| 8621 | 0.91 | 0.89 | 0.88 | 0.88 | 0.92 | 0.94 | 0.91 | 0.96 | |||
| 5685 | 1.08 | 1.04 | 1.06 | 1.01 | 1.06 | 1.09 | 1.03 | 1.04 | |||
| 50 | 362 | 1.23 | 1.37 | 1.39 | 1.38 | 1.29 | 1.23 | 4.18 | 4.13 | ||
| 8621 | 0.91 | 0.90 | 0.91 | 0.89 | 0.90 | 0.92 | 0.91 | 0.93 | |||
| 5685 | 1.00 | 0.98 | 0.99 | 0.96 | 0.98 | 0.98 | 0.96 | 0.97 | |||
| 85 | 362 | 1.47 | 1.77 | 1.79 | 1.79 | 1.59 | 1.44 | ND | ND | ||
| 8621 | 0.92 | 0.92 | 0.95 | 0.90 | 0.88 | 0.91 | 0.92 | 0.91 | |||
| 5685 | 0.89 | 0.90 | 0.82 | 0.91 | 0.93 | 0.80 | 0.97 | 0.99 | |||
| CAS + CIP | 15 | 362 | 1.09 | 1.02 | 1.03 | 1.05 | 1.20 | 1.09 | 1.20 | 1.16 | |
| 8621 | 1.17 | 1.01 | 0.99 | 1.05 | 1.02 | 1.00 | 1.03 | ND | |||
| 5685 | 0.71 | 0.80 | 0.93 | 0.90 | 1.03 | 1.03 | 0.98 | 0.96 | |||
| 50 | 362 | 1.29 | 1.24 | 1.29 | 1.30 | 1.33 | 1.31 | 1.34 | 1.28 | ||
| 8621 | 0.87 | 0.99 | 0.98 | 0.99 | 0.99 | 0.98 | 0.99 | ND | |||
| 5685 | 0.73 | 0.79 | 0.88 | 0.87 | 0.96 | 1.00 | 0.94 | 0.96 | |||
| 85 | 362 | 1.53 | 1.52 | 1.62 | 1.61 | 1.47 | 1.58 | 1.48 | 1.40 | ||
| 8621 | 0.64 | 0.96 | 0.97 | 0.93 | 0.95 | 0.96 | 0.94 | ND | |||
| 5685 | 0.75 | 0.78 | 0.83 | 0.85 | 0.91 | 0.97 | 0.89 | 0.97 | |||
AMB, amphotericin B; CIP, ciprofloxacin; FLC, fluconazole; CAS, caspofungin.
Underlined interaction indices are significantly higher than 1, indicating antagonism; boldfaced interaction indices are significantly lower than 1, indicating synergy (P < 0.05). ND, not determined (because Emax model could not fit).
TABLE 2.
Drug concentrations at different levels of growth in combinations that showed statistically significant interactions for Candida albicansa
| Drug combinationb | Growth (% of control) | Median (range) drug concn (μg/ml)c for:
|
|||
|---|---|---|---|---|---|
| Synergistic interactions
|
Antagonistic interactions
|
||||
| Antifungal | Ciprofloxacin | Antifungal | Ciprofloxacin | ||
| AMB + CIP | 15 | 0.19 (0.11-0.3) | 0.41 (0.13-1.94) | 0.33 (0.25-0.42) | 63.58 (39.37-84.12) |
| 50 | 0.14 (0.1-1) | 0.44 (0.06-6.79) | 0.21 (0.16-0.5) | 56.36 (42.43-159.08) | |
| 85 | 0.12 (0.09-0.18) | 0.53 (0.05-4.57) | 0.17 (0.12-0.45) | 44.01 (31.29-142.05) | |
| FLC + CIP | 15 | 0.13 (0.12-0.14) | 1.13 (0.10-6.15) | NA | NA |
| 50 | NA | NA | 0.11 (0.08-0.11) | 2.04 (0.28-11.07) | |
| 85 | NA | NA | 0.09 (0.07-0.1) | 1.92 (0.23-10.39) | |
| CAS + CIP | 15 | 0.73 (0.69-0.96) | 11.62 (4.32-18.97) | NA | NA |
| 50 | 0.58 (0.58-0.59) | 1.36 (0.9-1.85) | NA | NA | |
| 85 | 0.32 (0.2-0.5) | 10.15 (0.39-38.2) | NA | NA | |
Synergistic and antagonistic interactions were observed for one of the strains tested.
AMB, amphotericin B; CIP, ciprofloxacin; FLC, fluconazole; CAS, caspofungin.
NA, not applicable, because no statistically significant interactions were observed.
Isobolographic analysis of interaction against A. fumigatus.
The results of the isobolographic analysis for A. fumigatus are summarized in Tables 3 and 4, where the median interaction indices for all strains for each fixed-ratio combination and the drug concentrations are presented at three different levels of fungal growth (15%, 50%, and 85% of the growth of the drug-free control). The overall pattern of interaction for each of the antifungal combinations with ciprofloxacin was primarily synergistic for all strains of A. fumigatus studied. Isobolographic analysis of ciprofloxacin plus amphotericin B revealed a shift of the amphotericin B concentration-effect curves in the presence of ciprofloxacin depending on the proportion of amphotericin B in the mixture (Fig. 1). This resulted in interaction indices that deviated significantly (P < 0.05) from 1 (indifference), as shown in Fig. 2.
TABLE 3.
Results of isobolographic analysis for combinations of antifungal drugs with ciprofloxacin against Aspergillus fumigatus strains
| Drug combinationa | Growth (% of control) | Strain | Interaction indexb at the following fixed ratio of antifungal drug to CIP (PAA):
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1:100 (0.01) | 1:50 (0.02) | 1:25 (0.04) | 1:12.5 (0.07) | 1:6.25 (0.14) | 1:3.125 (0.24) | 1:1.6 (0.39) | 1:0.8 (0.56) | |||
| AMB + CIP | 15 | 2025 | 1.25 | 4.44 | 1.18 | 0.91 | 0.83 | 0.75 | 0.74 | 1.29 |
| 4215 | 3.68 | 2.16 | 1.02 | 0.67 | 0.61 | 0.55 | 0.68 | 0.67 | ||
| 2350 | 1.49 | 0.92 | 0.76 | 0.65 | 0.69 | 0.88 | 0.69 | 0.69 | ||
| 50 | 2025 | 1.21 | 0.79 | 0.68 | 0.71 | 0.64 | 0.62 | 0.60 | 0.56 | |
| 4215 | 1.45 | 1.47 | 0.70 | 0.60 | 0.60 | 0.56 | 0.63 | 0.61 | ||
| 2350 | 1.58 | 0.64 | 0.61 | 0.60 | 0.48 | 0.39 | 0.51 | 0.50 | ||
| 85 | 2025 | 1.17 | 0.14 | 0.40 | 0.55 | 0.49 | 0.51 | 0.48 | 0.25 | |
| 4215 | 1.57 | 1.00 | 0.48 | 0.54 | 0.60 | 0.58 | 0.59 | 0.55 | ||
| 2350 | 1.67 | 0.45 | 0.49 | 0.55 | 0.33 | 0.18 | 0.37 | 0.36 | ||
| 1:200 (0.005) | 1:100 (0.01) | 1:50 (0.02) | 1:25 (0.04) | 1:12.5 (0.07) | 1:6.25 (0.14) | 1:3.125 (0.24) | 1:1.6 (0.39) | |||
| VOR + CIP | 15 | 2025 | 1.25 | 1.23 | 0.59 | 0.98 | 0.98 | 0.62 | 0.87 | 1.00 |
| 4215 | 1.44 | 1.00 | 0.80 | 0.80 | 1.08 | 0.94 | 0.74 | 0.98 | ||
| 2350 | 1.14 | 1.52 | 1.20 | 1.12 | 1.12 | 1.14 | 1.19 | 1.19 | ||
| 50 | 2025 | 0.70 | 0.62 | 0.54 | 0.78 | 0.51 | 0.50 | 0.68 | 0.71 | |
| 4215 | 0.50 | 0.50 | 0.41 | 0.44 | 0.34 | 0.36 | 0.34 | 0.46 | ||
| 2350 | 0.70 | 0.61 | 0.73 | 0.66 | 0.62 | 0.65 | 0.57 | 0.60 | ||
| 85 | 2025 | 0.39 | 0.32 | 0.49 | 0.62 | 0.27 | 0.41 | 0.54 | 0.51 | |
| 4215 | 0.18 | 0.25 | 0.21 | 0.24 | 0.10 | 0.14 | 0.16 | 0.21 | ||
| 2350 | 0.43 | 0.25 | 0.45 | 0.39 | 0.35 | 0.37 | 0.28 | 0.30 | ||
| 1:0.78 (0.56) | 1:0.39 (0.72) | 1:0.195 (0.84) | 1:0.1 (0.91) | 1:0.05 (0.95) | 1:025 (0.98) | 1:0125 (0.99) | 1:006 (0.99) | |||
| CAS + CIP | 15 | 2025 | 1.55 | 0.94 | 0.81 | 0.65 | 0.94 | 1.05 | 0.95 | 0.81 |
| 4215 | 0.87 | 0.99 | 0.86 | 0.60 | 1.85 | 0.71 | 0.96 | 0.81 | ||
| 2350 | 2.43 | 1.06 | 0.78 | 0.67 | 0.78 | 0.72 | 0.71 | 0.74 | ||
| 50 | 2025 | 0.62 | 0.58 | 0.56 | 0.49 | 0.56 | 0.60 | 0.62 | 0.60 | |
| 4215 | 0.71 | 0.96 | 0.72 | 0.66 | 1.14 | 0.67 | 0.75 | 0.75 | ||
| 2350 | 0.78 | 0.55 | 0.45 | 0.44 | 0.47 | 0.43 | 0.41 | 0.45 | ||
| 85 | 2025 | 0.25 | 0.36 | 0.39 | 0.37 | 0.34 | 0.35 | 0.41 | 0.45 | |
| 4215 | 0.58 | 0.93 | 0.60 | 0.73 | 0.71 | 0.64 | 0.59 | 0.69 | ||
| 2350 | 0.22 | 0.29 | 0.26 | 0.29 | 0.28 | 0.26 | 0.23 | 0.27 | ||
AMB, amphotericin B; CIP, ciprofloxacin; VOR, voriconazole; CAS, caspofungin.
Underlined interaction indices are significantly higher than 1, indicating antagonism; boldfaced interaction indices are significantly lower than 1, indicating synergy (P < 0.05).
TABLE 4.
Drug concentrations at different levels of growth in combinations that showed statistically significant interactions for Aspergillus fumigatus
| Drug combinationa | Growth (% of control) | Median (range) drug concn (μg/ml)b for:
|
|||
|---|---|---|---|---|---|
| Synergistic interactions
|
Antagonistic interactions
|
||||
| Antifungal | Ciprofloxacin | Antifungal | Ciprofloxacin | ||
| AMB + CIP | 15 | 0.39 (0.3-0.46) | 1.85 (0.23-8.66) | 1.12 (0.71-1.38) | 74.24 (69.15-101.82) |
| 50 | 0.29 (0.22-1) | 0.82 (0.11-10.64) | 0.62 (0.58-0.76) | 62.09 (58.25-75.72) | |
| 85 | 0.24 (0.03-0.31) | 0.78 (0.04-7.4) | 0.53 (0.35-0.61) | 52.75 (35.08-61) | |
| VOR + CIP | 15 | NA | NA | NA | NA |
| 50 | 0.11 (0.07-0.17) | 0.95 (0.08-29.92) | NA | NA | |
| 85 | 0.04 (0.02-0.09) | 0.53 (0.03-12.18) | NA | NA | |
| CAS + CIP | 15 | 70.85 (47.87-86.85) | 2.81 (0.66-7.23) | NA | NA |
| 50 | 19.95 (1-38.28) | 0.81 (0.05-12.55) | NA | NA | |
| 85 | 1.94 (0.47-3.12) | 0.27 (0.01-1.52) | NA | NA | |
AMB, amphotericin B; CIP, ciprofloxacin; VOR, voriconazole; CAS, caspofungin.
NA, not applicable, because no statistically significant interactions were observed.
FIG. 1.
Isobolographic analysis of pharmacodynamic interactions between amphotericin B and ciprofloxacin against Aspergillus fumigatus 2025. The concentration-effect relationships of amphotericin B alone (AMB regression curve) and in combination with ciprofloxacin in mixtures with an amphotericin B proportion of 0.24 (0.24 PAMB regression curve) or 0.02 (0.02 PAMB regression curve) are presented for one of three replicates. In order to assess the interactions at 15%, 50%, and 85% growth (dashed horizontal lines), the concentration-effect curves of the mixtures were compared with theoretical indifferent concentration-effect curves of the mixtures with 0.24 PAMB (0.24 PAMB additive curve) and 0.02 PAMB (0.02 PAMB additive curve). The concentration-effect curve of the mixture with 0.24 PAMB is on the left of the corresponding indifferent curve (i.e., a smaller drug concentration is required in combination in order to produce the same effect as the drug alone), indicating synergistic (S) interactions for all growth levels. The concentration-effect curve of the mixture with 0.02 PAMB is on the left of the corresponding indifferent curve at 85% of growth and on the right at 15% of growth (i.e., a higher drug concentration is required in combination in order to produce the same effect as the drug alone), indicating synergistic and antagonistic (A) interactions, respectively.
FIG. 2.
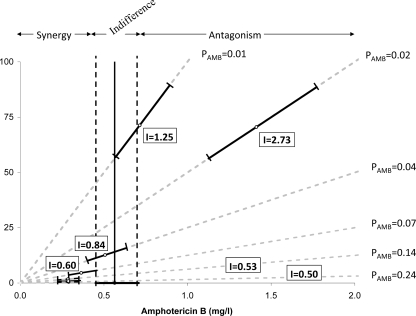
Two-dimensional isobolograms showing the interaction at a 15% growth level between amphotericin B and ciprofloxacin against A. fumigatus 2025 for one replicate. All points (shown as small circles) on the 15% isobologram represent drug concentrations, alone and in combination, that resulted in 15% growth. The isobol of indifference is the solid line, which starts from the EC15 of amphotericin B on the x axis (solid circle) and runs parallel to the y axis, because ciprofloxacin has no antifungal activity. The dashed lines around the indifferent isobol line represent the 95% confidence limits. Open circles represent the experimentally derived EC15,MIXs obtained from the regression analysis, and the error bars represent the 95% confidence limits. The experimental EC15,MIX of the mixtures with amphotericin B proportions (PAMB) of 0.01 and 0.02 were statistically significantly higher than the theoretical isobol of indifference, indicating antagonistic interactions, with interaction indices of 1.25 and 2.73, respectively. On the other hand, for mixtures with a PAMB of >0.14, statistically significant synergy was found, with interaction indices of 0.6 to 0.45. The shaded dashed lines starting from the origin of the axes represent mixtures with different PAMB. These interactions were considered significant if the interaction indices obtained from all three replicates were statistically significantly different from 1. For the exact magnitude of synergy and antagonism for mixtures with different PAMB, see Table 3.
The interaction indices ranged from 0.18 to 4.44 for all three growth levels, indicating the predominance of synergy and the presence of some antagonism (Table 3). Synergy was detected at low concentrations of ciprofloxacin (0.78 to 1.85 μg/ml) and amphotericin B (0.24 to 0.39 μg/ml), and antagonism was detected at higher concentrations of both ciprofloxacin (52.75 to 74.25 μg/ml) and amphotericin B (0.53 to 1.12 μg/ml) (Table 4). The concentrations of voriconazole and ciprofloxacin, where synergy was found in their combination, were 0.04 to 0.11 μg/ml and 0.53 to 0.95 μg/ml, respectively. For the combination of caspofungin and ciprofloxacin, synergy was found at 1.94 to 70.85 μg/ml of caspofungin and 0.27 to 2.81 μg/ml of ciprofloxacin (Table 4).
DISCUSSION
Significant in vitro pharmacodynamic interactions were found between antifungal agents and ciprofloxacin against C. albicans and A. fumigatus by using isobolographic analysis. Amphotericin B was found to interact with ciprofloxacin in a concentration-dependent manner against C. albicans and A. fumigatus, with synergy observed at low drug concentrations and antagonism at higher drug concentrations. However, we should note that the antagonistic interactions were observed at ciprofloxacin concentrations higher than those safely achieved in plasma (16). Synergy was also found between ciprofloxacin and caspofungin or low concentrations of voriconazole against A. fumigatus.
In a previous study by Faessel et al., a systematic pattern of differential cell growth was observed in 96-well plates (5). It was reported that cell growth was higher in the periphery of the plate and lower in the middle of the plate. This phenomenon does not affect our results, since the isobolographic analysis takes into consideration the growth in more than one well. Thus, wells with potential overgrowth will be taken into account. The results of these isobolographic analyses are in agreement with previous in vivo studies, which, however, had no in vitro correlates. For example, Sugar et al. (36) showed that the combination of ciprofloxacin (4 mg/kg of body weight/day) with amphotericin B (0.1 mg/kg) provided significantly more protection to mice infected by C. albicans than amphotericin B or ciprofloxacin alone. The dosages of ciprofloxacin and amphotericin B used in that study correspond to concentrations in plasma that fluctuate around the in vitro concentrations found to produce synergy in the current isobolographic study (23, 43). In the same study, mice that received ciprofloxacin (4 mg/kg/day) plus the lower dose of fluconazole (40 mg/kg/day) exhibited survival equivalent to that observed with the higher dose of fluconazole alone (80 mg/kg/day), indicating a synergistic interaction between these two agents, as also found in the present study. However, in our study, the in vitro pharmacodynamic interaction between fluconazole and ciprofloxacin was found to occur against only one of three strains of C. albicans, suggesting that other, nonpharmacodynamic interactions such as immunomodulatory effects may also contribute to the better outcome of combination therapy (32, 33). Furthermore, combination therapy with other quinolones and amphotericin B or fluconazole has resulted in better outcomes than monotherapy regimens against experimental models of candidiasis and aspergillosis (19). These findings indicate a potential synergistic interaction between quinolones and antifungal agents, raising questions about the pharmacodynamic nature of this interaction (19, 36).
In agreement with the results of our FIC index analysis, none of the previous in vitro studies has found a synergistic interaction between ciprofloxacin and amphotericin B using the same analysis (24, 27). Overbeek et al. found no significant interaction between amphotericin B and ciprofloxacin against C. albicans (24), whereas Petrou and Rogers found indifference at lower concentrations of quinolones and antagonism at higher quinolone concentrations (27). The antagonistic interaction of these combinations was also found in the present study at similar ciprofloxacin concentrations. Of note, Nakijama et al. (19) also found antagonism at high concentrations of quinolone DU-6859a (>25 μg/ml) against A. fumigatus, whereas synergy was found at lower concentrations against A. fumigatus and C. albicans by using yeast nitrogen base (YNB), but not RPMI, as the medium. Combinations of other quinolones with amphotericin B demonstrated indifferent interactions by the FIC index analysis, as with the previous studies (27, 28).
Petrou and Rogers found that the combination between different quinolones, including ciprofloxacin, and other azoles (ketoconazole, miconazole, and itraconazole) was synergistic at a quinolone concentration of 1 μg/ml and antagonistic at 10 μg/ml (27). A concentration-dependent interaction was also found in the present study for the ciprofloxacin-plus-fluconazole combination, with synergy and antagonism observed at different levels of growth (i.e., different drug concentrations) at the same range of ciprofloxacin concentrations. Nakajima et al. described synergistic interaction between DU-6859a and fluconazole against C. albicans by using only synthetic amino acid medium fungal (SAAMF) (19). The combinations of other quinolones with fluconazole were indifferent by FIC index analysis.
The mechanisms of these interactions are not easily understood and could be explained as either enhancement of a previously insignificant antifungal activity of ciprofloxacin or potentiation of the activity of antifungal agents by ciprofloxacin. Ciprofloxacin does not possess significant antifungal growth-inhibitory activity. However, it has been found that quinolones could bind to fungal topoisomerase II (6, 34, 35). Thus, ciprofloxacin may inhibit fungal DNA replication and thereby exhibit an antifungal effect. Since this effect is apparent only when ciprofloxacin is combined with antifungal agents, it is possible that antifungal agents may alter fungal cell membrane permeability and thereby increase intracellular ciprofloxacin levels. This hypothesis warrants further study.
Ciprofloxacin could also enhance the action of antifungal agents. Ciprofloxacin molecules are zwitterionic and are present as monomers at low concentrations, whereas at higher concentrations they are self-associated in a head-to-tail arrangement (11). Given that ciprofloxacin molecules interact with cholesterol-containing liposomes (11), ciprofloxacin at low concentrations may participate in pore formation on fungal cell membranes imposed by amphotericin B and thereby enhance the antifungal action of amphotericin B. However, the self-association of ciprofloxacin molecules at high concentrations could interfere with amphotericin B pore formation, thereby decreasing the antifungal activity of amphotericin B and thus producing an antagonistic effect, as found in the present study.
Ciprofloxacin also may enhance the action of azoles and caspofungin by overlapping substrate specificity of the ATP-binding cassette multidrug transporters, resulting in higher intracellular concentrations of antifungal agents. Ciprofloxacin was found to be transported by multiresistance-related proteins (15), homologs of the Candida drug resistance transporters involved in azole (42) and possibly caspofungin (31) efflux. In addition, ofloxacin, another quinolone, was found to increase intracellular levels of rhodamine 6G, which shares the same efflux pump with fluconazole in fluconazole-resistant C. albicans strains (29). Further studies are required to elucidate the mechanisms that could explain the different interactions between ciprofloxacin and antifungal agents.
Significant in vitro pharmacodynamic interactions between quinolones and antifungal agents against Candida and Aspergillus spp. may be lost when checkerboard results are analyzed with the FIC index. In order to account for the 1-dilution error using geometrically increased drug dilutions in checkerboard assays, the stringent FIC index cutoffs of 0.5 and 4 were used for defining additivity/indifference. In addition, a complete growth inhibition end point was used to analyze the in vitro combinations, which inevitably assess pharmacodynamic interactions only at high drug concentrations where a complete growth inhibition end point (MIC-0) is observed. When one is reporting an FIC index among the many FICs calculated for a data set, neither synergistic nor antagonistic interactions can be captured when present. Thus, information about concentration-dependent pharmacodynamic interactions within the 0.5-to-4 range and at lower drug concentrations than those corresponding to MIC-0 may be lost.
In the present study, in vitro pharmacodynamic interactions were assessed using the isobolographic analysis of Loewe additivity (no-interaction) theory. Although the latter theory is the same as the no-interaction theory on which the FIC index is based, isobolographic analysis enables one to detect small departures for additivity and at various growth inhibition end points. This analysis combines pharmacodynamic no-interaction theories and contemporary techniques of modeling in order to statistically describe complex pharmacodynamic interactions over the entire range of drug concentrations irrespective of the concentration-effect curves of the individual agents. Although this analysis has previously been used for combinations between two active antifungal agents (12), to our knowledge this is the first report where isobolographic analysis is used to analyze antifungal pharmacodynamic interactions of an active agent with an inactive agent.
Isobolographic analysis has been used previously to assess interaction between active and inactive drugs. In particular, it was used to assess the pharmacodynamic interactions between the novel antiepileptic drug gabapentin and a number of other antiepileptic drugs against electroshock-induced convulsions in mice. Gabapentin (<50 mg/kg) was ineffective on electroconvulsions produced by means of an alternative current. Isobolographic analysis revealed that combinations of gabapentin with other antiepileptic drugs, which were effective against electroconvulsions, resulted in synergistic interactions (4, 10). Notably, this particular analysis can provide a useful tool for the assessment of interactions between antifungal agents and non-antifungal compounds, which alone do not elicit any significant antifungal activity, and possibly of interactions against resistant isolates.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Published ahead of print on 25 February 2008.
REFERENCES
- 1.American Society for Microbiology. 2007. Instructions to authors. Antimicrob. Agents Chemother. 51:1-22. [Google Scholar]
- 2.Antoniadou, A., and H. Giamarellou. 2007. Fever of unknown origin in febrile leukopenia. Infect. Dis. Clin. N. Am. 21:1055-1090. [DOI] [PubMed] [Google Scholar]
- 3.Berenbaum, M. C. 1989. What is synergy? Pharmacol. Rev. 41:93-141. [PubMed] [Google Scholar]
- 4.Borowicz, K. K., M. Swiader, J. Luszczki, and S. J. Czuczwar. 2002. Effect of gabapentin on the anticonvulsant activity of antiepileptic drugs against electroconvulsions in mice: an isobolographic analysis. Epilepsia 43:956-963. [DOI] [PubMed] [Google Scholar]
- 5.Faessel, H. M., L. M. Levasseur, H. K. Slocum, and W. R. Greco. 1999. Parabolic growth patterns in 96-well plate cell growth experiments. In Vitro Cell. Dev. Biol. Anim. 35:270-278. [DOI] [PubMed] [Google Scholar]
- 6.Fostel, J. M., D. A. Montgomery, and L. L. Shen. 1992. Characterization of DNA topoisomerase I from Candida albicans as a target for drug discovery. Antimicrob. Agents Chemother. 36:2131-2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson, M. D., C. MacDougall, L. Ostrosky-Zeichner, J. R. Perfect, and J. H. Rex. 2004. Combination antifungal therapy. Antimicrob. Agents Chemother. 48:693-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levasseur, L. M., W. R. Greco, Y. M. Rustum, and H. K. Slocum. 1997. Combined action of paclitaxel and cisplatin against wildtype and resistant human ovarian carcinoma cells. Cancer Chemother. Pharmacol. 40:495-505. [DOI] [PubMed] [Google Scholar]
- 9.Luszczki, J. J., M. Czuczwar, J. Kis, J. Krysa, I. Pasztelan, M. Swiader, and S. J. Czuczwar. 2003. Interactions of lamotrigine with topiramate and first-generation antiepileptic drugs in the maximal electroshock test in mice: an isobolographic analysis. Epilepsia 44:1003-1013. [DOI] [PubMed] [Google Scholar]
- 10.Luszczki, J. J., and S. J. Czuczwar. 2006. Gabapentin synergistically interacts with topiramate in the mouse maximal electroshock seizure model: an isobolographic analysis. Pharmacol. Rep. 58:944-954. [PubMed] [Google Scholar]
- 11.Maurer, N., K. F. Wong, M. J. Hope, and P. R. Cullis. 1998. Anomalous solubility behavior of the antibiotic ciprofloxacin encapsulated in liposomes: a 1H-NMR study. Biochim. Biophys. Acta 1374:9-20. [DOI] [PubMed] [Google Scholar]
- 12.Meletiadis, J., V. Petraitis, R. Petraitiene, P. Lin, T. Stergiopoulou, A. M. Kelaher, T. Sein, R. L. Schaufele, J. Bacher, and T. J. Walsh. 2006. Triazole-polyene antagonism in experimental invasive pulmonary aspergillosis: in vitro and in vivo correlation. J. Infect. Dis. 194:1008-1018. [DOI] [PubMed] [Google Scholar]
- 13.Meletiadis, J., T. Stergiopoulou, E. M. O'Shaughnessy, J. Peter, and T. J. Walsh. 2007. Concentration-dependent synergy and antagonism within a triple antifungal drug combination against Aspergillus species: analysis by a new response surface model. Antimicrob. Agents Chemother. 51:2053-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meletiadis, J., D. T. Te Dorsthorst, and P. E. Verweij. 2006. The concentration-dependent nature of in vitro amphotericin B-itraconazole interaction against Aspergillus fumigatus: isobolographic and response surface analysis of complex pharmacodynamic interactions. Int. J. Antimicrob. Agents 28:439-449. [DOI] [PubMed] [Google Scholar]
- 15.Michot, J. M., F. Van Bambeke, M. P. Mingeot-Leclercq, and P. M. Tulkens. 2004. Active efflux of ciprofloxacin from J774 macrophages through an MRP-like transporter. Antimicrob. Agents Chemother. 48:2673-2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mimoz, O., V. Binter, A. Jacolot, A. Edouard, M. Tod, O. Petitjean, and K. Samii. 1998. Pharmacokinetics and absolute bioavailability of ciprofloxacin administered through a nasogastric tube with continuous enteral feeding to critically ill patients. Intensive Care Med. 24:1047-1051. [DOI] [PubMed] [Google Scholar]
- 17.Miranda, H. F., E. Silva, and G. Pinardi. 2004. Synergy between the antinociceptive effects of morphine and NSAIDs. Can. J. Physiol. Pharmacol. 82:331-338. [DOI] [PubMed] [Google Scholar]
- 18.Motulsky, H. C., and A. Christopoulos. 2003. Fitting models to biological data using linear and nonlinear regression. A practical guide to curve fitting. GraphPad Software, Inc., San Diego, CA.
- 19.Nakajima, R., A. Kitamura, K. Someya, M. Tanaka, and K. Sato. 1995. In vitro and in vivo antifungal activities of DU-6859a, a fluoroquinolone, in combination with amphotericin B and fluconazole against pathogenic fungi. Antimicrob. Agents Chemother. 39:1517-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.NCCLS. 2002. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard. M38-A, vol. 22, no 16. NCCLS, Wayne, PA.
- 21.NCCLS. 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard, 2nd ed. NCCLS document M27-A2. NCCLS, Wayne, PA.
- 22.Nosari, A., P. Oreste, R. Cairoli, M. Montillo, G. Carrafiello, A. Astolfi, G. Muti, L. Marbello, A. Tedeschi, E. Magliano, and E. Morra. 2001. Invasive aspergillosis in haematological malignancies: clinical findings and management for intensive chemotherapy completion. Am. J. Hematol. 68:231-236. [DOI] [PubMed] [Google Scholar]
- 23.Otsu, Y., K. Yanagihara, Y. Fukuda, Y. Miyazaki, K. Tsukamoto, Y. Hirakata, K. Tomono, J. Kadota, T. Tashiro, I. Murata, and S. Kohno. 2003. In vivo efficacy of a new quinolone, DQ-113, against Streptococcus pneumoniae in a mouse model. Antimicrob. Agents Chemother. 47:3699-3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Overbeek, B. P., M. Rozenberg-Arska, and J. Verhoef. 1985. Do quinolones really augment the antifungal effect of amphotericin B in vitro? Drugs Exp. Clin. Res. 11:745-746. [PubMed] [Google Scholar]
- 25.Perea, S., and T. F. Patterson. 2002. Invasive Aspergillus infections in hematologic malignancy patients. Semin. Respir. Infect. 17:99-105. [DOI] [PubMed] [Google Scholar]
- 26.Perez-Vizcaino, F., A. L. Cogolludo, F. Zaragoza-Arnaez, S. Fajardo, M. Ibarra, J. G. Lopez-Lopez, and J. Tamargo. 1999. Vasodilator effects of sodium nitroprusside, levcromakalim and their combination in isolated rat aorta. Br. J. Pharmacol. 128:1419-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petrou, M. A., and T. R. Rogers. 1988. In-vitro activity of antifungal agents in combination with four quinolones. Drugs Exp. Clin. Res. 14:9-18. [PubMed] [Google Scholar]
- 28.Polak, A. 1990. In vitro and in vivo activity of antifungal agents in combination with fleroxacin, a new quinolone. Mycoses 33:173-178. [PubMed] [Google Scholar]
- 29.Sasaki, E., S. Maesaki, Y. Miyazaki, K. Yanagihara, K. Tomono, T. Tashiro, and S. Kohno. 2000. Synergistic effect of ofloxacin and fluconazole against azole-resistant Candida albicans. J. Infect. Chemother. 6:151-154. [DOI] [PubMed] [Google Scholar]
- 30.Satyanarayana, P. S., N. K. Jain, A. Singh, and S. K. Kulkarni. 2004. Isobolographic analysis of interaction between cyclooxygenase inhibitors and tramadol in acetic acid-induced writhing in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 28:641-649. [DOI] [PubMed] [Google Scholar]
- 31.Schuetzer-Muehlbauer, M., B. Willinger, G. Krapf, S. Enzinger, E. Presterl, and K. Kuchler. 2003. The Candida albicans Cdr2p ATP-binding cassette (ABC) transporter confers resistance to caspofungin. Mol. Microbiol. 48:225-235. [DOI] [PubMed] [Google Scholar]
- 32.Shalit, I., D. Halperin, D. Haite, A. Levitov, J. Romano, N. Osherov, and I. Fabian. 2006. Anti-inflammatory effects of moxifloxacin on IL-8, IL-1β and TNF-α secretion and NF-κB and MAP-kinase activation in human monocytes stimulated with Aspergillus fumigatus. J. Antimicrob. Chemother. 57:230-235. [DOI] [PubMed] [Google Scholar]
- 33.Shalit, I., L. Horev-Azaria, I. Fabian, H. Blau, N. Kariv, I. Shechtman, H. Alteraz, and Y. Kletter. 2002. Immunomodulatory and protective effects of moxifloxacin against Candida albicans-induced bronchopneumonia in mice injected with cyclophosphamide. Antimicrob. Agents Chemother. 46:2442-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen, L. L., J. Baranowski, J. Fostel, D. A. Montgomery, and P. A. Lartey. 1992. DNA topoisomerases from pathogenic fungi: targets for the discovery of antifungal drugs. Antimicrob. Agents Chemother. 36:2778-2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen, L. L., and J. M. Fostel. 1994. DNA topoisomerase inhibitors as antifungal agents. Adv. Pharmacol. 29B:227-244. [DOI] [PubMed] [Google Scholar]
- 36.Sugar, A. M., X. P. Liu, and R. J. Chen. 1997. Effectiveness of quinolone antibiotics in modulating the effects of antifungal drugs. Antimicrob. Agents Chemother. 41:2518-2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tallarida, R. J. 2006. An overview of drug combination analysis with isobolograms. J. Pharmacol. Exp. Ther. 319:1-7. [DOI] [PubMed] [Google Scholar]
- 38.Tallarida, R. J. 1992. Statistical analysis of drug combinations for synergism. Pain 49:93-97. [DOI] [PubMed] [Google Scholar]
- 39.Tallarida, R. J., H. L. Kimmel, and S. G. Holtzman. 1997. Theory and statistics of detecting synergism between two active drugs: cocaine and buprenorphine. Psychopharmacology (Berlin) 133:378-382. [DOI] [PubMed] [Google Scholar]
- 40.Van Bambeke, F., J. M. Michot, J. Van Eldere, and P. M. Tulkens. 2005. Quinolones in 2005: an update. Clin. Microbiol. Infect. 11:256-280. (Erratum, 11:513.) [DOI] [PubMed] [Google Scholar]
- 41.Walsh, T. J., and A. H. Groll. 1999. Emerging fungal pathogens: evolving challenges to immunocompromised patients for the twenty-first century. Transpl. Infect. Dis. 1:247-261. [DOI] [PubMed] [Google Scholar]
- 42.White, T. C., K. A. Marr, and R. A. Bowden. 1998. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin. Microbiol. Rev. 11:382-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wiederhold, N. P., V. H. Tam, J. Chi, R. A. Prince, D. P. Kontoyiannis, and R. E. Lewis. 2006. Pharmacodynamic activity of amphotericin B deoxycholate is associated with peak plasma concentrations in a neutropenic murine model of invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 50:469-473. [DOI] [PMC free article] [PubMed] [Google Scholar]