Abstract
Caspase-8 is frequently deleted or silenced in neuroblastoma and other solid tumor such as medulloblastoma and small cell lung carcinoma. Caspase-8 expression can be re-established in neuroblastoma cell lines by treatment with demethylating agents or with IFN-γ Here we show that four different retinoic acid (RA) derivatives also increase caspase-8 protein expression in neuroblastoma, medulloblastoma and small cell lung carcinoma cell lines. This increase in protein expression is mirrored by an increase in RNA expression in NB cells. However, the promoter region of the caspase-8 gene was not responsible for the induction of caspase-8 expression. Rather, we identified another intronic region containing a CREB binding site that was required for maximal induction of caspase-8 via RA. DNA-protein interaction assays revealed increased phospho-CREB binding to this response element in RA-treated NB cells. Furthermore, both mutation of the CREB binding site completely blocked caspase-8 induction in the luciferase reporter system assay and transfection of dominant-negative form of CREB repressed the up-regulation of caspase-8 by RA. Importantly, RA-released cells maintained caspase-8 expression for at least 2–5 days and were more sensitive to doxorubicin and TNFα. Thus, RA treatment in conjunction with TNFα and/or subsets of cytotoxic agents may have therapeutic benefits.
Keywords: caspase-8; neuroblastoma; medulloblastoma, small cell lung carcinoma, retinoid, retinoic acid; CREB; apoptosis, transcription
1. Introduction
Neuroblastoma (NB) is the most common solid tumor in childhood, accounting for approximately 10% of pediatric cancers [1–3]. Several genetic abnormalities have been identified in these tumors, including N-myc amplification, chromosomal deletion and rearrangement of 1p36, 2q33, 11q23, and 14q23, and unbalanced gain of 17q23 [2–4]. Caspase-8 is also deleted or silenced in the majority of NB cell lines and patients [5–7]. Studies using animal model systems and NB cell lines have shown that loss of caspases-8 potentiates metastasis [8–10].
Caspase-8 has an important role in the death receptor mediated apoptotic pathway. The death receptor (or extrinsic) pathway is triggered by members of the death receptor superfamily (FasR, TNFRI, DR5, etc). Binding of the ligand (e.g., FasL) to its receptor (FasR) induces aggregation of the receptors, leading to the recruitment of an adaptor molecule known as FADD (Fas receptor associated death domain containing protein) and procaspase-8. Procaspase-8 is then cleaved and activated, resulting in the activation of downstream effector caspases and leading to apoptosis. Active caspase-8 can also cleave Bid, which is a Bcl2 family member. Cleaved Bid, termed tBid, translocates to the mitochondria and promotes cytochrome c release, thereby activating the mitochondrial (or intrinsic) pathway [11–13]. Caspase-8 expression is also lost in other solid tumors including medulloblastoma [14], small cell lung carcinoma [15] and colorectal carcinomas [16]. Loss of caspase-8 expression in medulloblastoma patients has been correlated with a poor prognosis [17].
All-trans retinoic acid (ATRA or RA), which is a derivative of vitamin A, is effective in inducing differentiation and inhibiting proliferation of NB cell lines [18–20]. The differentiation of NB cells induced by RA is accompanied by changes in gene expression, including N-myc down-regulation [21,22]. RA is well tolerated in clinical trials, improves survival in NB patients and even induces occasional long-term remission [21,23,24]. RA generally regulates gene transcription through its receptors, retinoic acid receptors (RARs), which form heterodimers with retinoid X receptors (RXRs). RAR-RXR heterodimers bind to RA response elements (RAREs) to regulate gene transcription. The RAREs are generally composed of two direct repeats (DR) with the consensus sequence AG(G/T)TCA spaced by 2 or 5 nucleotides (DR2 or DR5) [25,26]. Although the majority of RAREs are located in the promoter region of RA-responsive genes, they are also found in introns or exons or downstream of the RA-responsive genes [26,27]. However, recent studies showed that RA could stimulate other cellular signaling pathways, which are independent on the binding of receptors to RAREs [28–30]. For examples, RA can regulate PKCα activity by competing with phosphatidylserine to bind to the C2-domain of PKCα [29,30]. RA can also up-regulate the c-fos gene although it does not contain RAREs in its promoter [28]. The present study analyzed the effects of RA treatment on caspase-8 expression and cellular proliferation in NB cells. The results demonstrate that RA induces caspase-8 mRNA and protein expression in NB cell lines via induction of phosphorylated CREB and the binding of this transcription factor to sequences within the caspase-8 gene, thereby sensitizing the cells to TNFα- or drug- induced apoptosis.
2. Materials and Methods
2.1. Cell lines and culture methods
The human NB cell lines used in these studies have been described in detail previously [5,31]. All the human neuroblastoma, medulloblastoma (BT-3, BT-14) and small cell lung carcinoma (NCI-H82, NCI-H889) cell lines were cultured in RPMI 1640 medium supplemented with 10% FBS, 2 mM L-glutamine, and 0.1% gentamicin (Invitrogen, CA, USA) in a humidified atmosphere of 5% CO2 at 37°C.
2.2. Reagents and Antibodies
All-trans RA (ATRA or RA), 13-cis RA, 9-cis-RA, fenretinide (4HPR), doxorubicin, cycloheximide and MTT were purchased from Sigma (MO, USA). zVAD-fmk and z-IETD-fmk were purchased from Calbiochem (Germany). TNFα was purchased from Promega (WI, USA).
Anti-caspase-8 (C15) was a gift from Dr. M. Peter [32]. Anti-CREB, anti-phospho-CREB (Ser-133) and anti-PARP were obtained from Upstate Biotechnology (MA, USA). Actin, Fas and FADD antibodies were obtained from Santa Cruz Biotechnology (CA, USA). Caspase-3, DR4 and DR5 antibodies were purchased from BD Biosciences (CA, USA). Anti-TNFR1 was purchased from Cell Signaling Technology (MA, USA) and anti-GFP was obtained from Invitrogen (CA, USA). Horseradish peroxidase (HRP)-conjugated secondary antibodies were obtained from KPL (MD, USA).
2.3. Western Blot
Cells were lysed with the loading buffer (50 mM Tris, pH 6.8, 100 mM DTT, 2% SDS, 10% glycerol and 0.1% bromophenol blue). 30 µg protein from the cell lysates was subjected to SDS-PAGE analysis. Immunoblot analysis was carried out with unlabeled primary antibodies and HRP-conjugated secondary antibodies. The proteins were detected by enhanced chemiluminescence (ECL, GE Healthcare, NJ, USA).
2.4. Electrophoretic mobility shift assays
Electrophoretic mobility shift assays (EMSA) were carried out as described previously [33] with some modifications. Briefly, nuclear extracts (5 µg) prepared from RA-treated or untreated NB cells were incubated with 1 µl 32P-end-labeled double-stranded oligonucleotides (10000 cpm/µl) and 2 µg poly(dI-dC) in 20 µl binding buffer (10 mM HEPES, pH 8.0, 50 mM NaCl, 5 mM MgCl2, 2 mM DTT, 10% Glycerol) for 20 min at room temperature. 2 µg of anti-phospho-CREB (Ser133) antibody was added to the nuclear extract and incubated for 20 min for the supershift analysis. The DNA-protein complexes were resolved in a 4% native polyacrylamide gel. Following electrophoresis, the gels were dried and autoradiographed at −80°C for 3–16 hours. The oligonucleotides used in this study are listed in the figure legends.
2.5. Methylation-specific PCR and Real-time PCR
Genomic DNA isolated from NB cells was bisulfite-modified with an EZ DNA Methylation kit (Zymo research, CA, USA) according to the manufacturer's instructions. The modified genomic DNA was then used for methylation specific PCR (MSP) studies using the caspase-8 methylation primer sets previously described [5].
Total RNA was extracted from NB cells with TRIzol reagent (Invitrogen, CA, USA) and residual DNA was removed by the RNeasy Mini kit (Qiagen, CA, USA). Purified RNAs (2 µg) were then used to synthesize cDNA using Superscript II (Invitrogen, CA, USA) and random primers (Promega, WI, USA). The primers for the analysis for caspase-8 mRNA expression were 5’-TGTTGGAGGAAAGCAATCTG-3’ and 5’-CCTGGTGTCTGAAGTTCCCT-3’, which were designed by the GenScript Real Time Primer Design Program. GAPDH expression was used to as normalization control and the primers were 5’-GCCAAAAGGGTCATCATCTCT-3’ and 5’- GGTCATGAGTCCTTCCACGA-3’ [34]. Gene expression was normalized to GADPH using the comparative Ct method (2−ΔΔCT) [35].
2.6. Chromatin Immunoprecipitation Assay
The chromatin immunoprecipitation (ChIP) assay was performed as described previously [36] with some modifications. Briefly, cells were treated with 1% formaldehyde for 15 min. Then cells were lysed in SDS buffer (1 % SDS, 10 mM EDTA, 50 mM Tris, pH 8.1) containing protease inhibitor cocktail (Roche Applied Science, IN, USA) and the lysate was sonicated to shear the DNA to an average size of 300–500 base pairs. The cell lysate was then diluted in 9- fold dilution buffer (0.01 % SDS, 1.1% Triton X-100, 1.2 mM EDTA, 16.7 mM Tris-HCl, pH 8.1, 167 mM NaCl) and immunoprecipitated with antibodies against CREB, phospho-CREB-Ser133, and control rabbit IgG at 4 °C overnight. No antibody reactions served as a negative control. The immunoprecipitates were eluted in buffer (1 % SDS, 0.1 M NaHCO3) and then heated at 65°C to reverse the crosslinking. After RNase A and proteinase K digestion, the DNA was purified by standard phenol/chloroform extraction methods. The samples were analyzed by PCR with primer sets specific for the RA-responsive region in the caspase-8 gene containing putative CREB binding sites (5’-GACAGAGAAAGGGGAAATGG-3’; 5’- CCAGAAGCTTCTAGGGTATGATATG-3’) or HLA-G promoter region (5’- TGCTCAAGTGCCTGACATTC-3’; 5’-GCTCCTTTTCCTCACCTCCT-3’) as a positive control for the ChIP assay [37].
2.7. MTT Assay
Cells grown in 24-well plates were incubated with 200 µl phenol red-free RPMI 1640 containing 2 % FBS and 0.5 mg/ml MTT for 3 hours at 37 °C. Isopropanol (200 µl) was then added to dissolve the MTT formazan crystals. Absorbance was measured at 570 nm with background subtraction at 690 nm and the cell viability was represented as a percentage of the control cells.
2.8. Reporter Constructs and Expression Vectors
Different caspase-8 gene fragments were obtained by PCR amplification using genomic DNA from NB16 cells as template and oligonucleotide primers flanked by XhoI and HindIII restriction sites. The resulting fragments were then cloned into the XhoI and HindIII sites of 9 pGL3-basic vector (Promega, WI, USA). The sequences of the primers are available upon request. Mutant versions of the luciferase-reporter plasmids were prepared by the QuikChange site-directed mutagenesis kit (Stratagene, CA, USA) according to the manufacturer's instructions.
For luciferase assay, NB cells were co-transfected via electroporation with 25 µg of the luciferase reporter plasmids and 2.5 µg of pRL-TK plasmid (Promega, WI, USA) as an internal control for transfection efficacy. The luciferase activity was determined by dual LUC reporter system (Promega, WI, USA). Transfection efficiency was normalized by measuring Renilla luciferase activity.
cDNAs encoding wild-type CREB (CREB-wt) and dominant negative CREB (CREB-m1) containing a mutation at the phosphorylation site (S133A), kindly provided by Dr. D. Ginty [38], were subcloned into IRES2-EGFP vector (Clontech, CA, USA).
The pSUPER.retro.neo+GFP retroviral vector (OligoEngine, WA, USA) was used to generate a caspase-8 shRNA construct containing the 19-nucleotide targeting sequence (5’- GGACTTCAGCAGAAATCTT-3’) for RNA interference studies. The construct was then packaged and the resultant viruses were used to infect the NB cells as described previously [5].
3. Results
3.1. RA inhibits cell proliferation of NB cells
To confirm that our NB cell lines exhibited growth inhibition similar to that reported by others using different NB cell lines, we monitored the effect of RA on two of our NB cell lines, NB8 and NB10. As expected, the number of BrdU positive cells decreased significantly during RA treatment (Supplementary Fig. 1A). We also analyzed cell cycle alterations in the RA treated cells by measuring the DNA content and by examining expression of cell cycle regulatory proteins. The DNA content studies showed increases in the percentage of G0/G1 cells and in sub-G1 apoptotic cells. The percentage of these apoptotic cells varied between the different cell lines (Supplementary Fig. 1B). In agreement with the DNA content data, the levels of cyclin A, an essential protein for S-phase progression, and of phosphorylated Rb were decreased by RA treatment (Supplementary Fig. 1C). When cells were released from RA after a 2-day treatment, cyclin A was re-expressed and Rb was re-phosphorylated, indicating that cells were able to recover from RA-treatment and re-enter the cell cycle (Supplementary Fig. 1C). These data support the conclusion that RA inhibits proliferation of our NB cell lines and are consistent with previously reported results [22,39].
3.2. RA increases the expression of caspase-8 in NB cells
Due to the important role of caspase-8 in NB cells, we examined the effect of RA on caspase-8 expression. Immunoblot analysis revealed that RA treatment increased caspase-8 expression in a time and dose dependent manner (Fig. 1A). To determine whether similar effects could be observed in other cell lines, we treated 10 additional NB cell lines with high concentration of RA (100 µM). RA treatment increased the expression of caspase-8 in 11/12 NB cell lines (Table 1). Caspase-8 expression was even increased somewhat in NB16 cells (Fig. 1B), which have high initial levels of caspase-8 [5]. Consistent with the western blot results, quantitative PCR analysis demonstrated an 8-fold increase in caspase-8 mRNA abundance in NB8 and NB10 cells, while the increase was only about 2-fold in NB16 cells (Fig. 1C). These data suggest that RA regulated caspase-8 transcription or mRNA stability.
Fig. 1.
RA increased caspase-8 expression. (A) Induction of caspase-8 by RA is time and dose dependent. NB8 and NB10 cells were treated continuously with RA (10 µM or 25 µM) for 10 days or treated with 100 µM RA for 3 days. Aliquots were collected at the indicated times and subjected to immunoblot analysis with antibodies against caspase-8 and actin. (B) Time course analysis of caspase-8 levels in NB cells treated with 100 µM RA. NB8, NB10 and NB16 cells were treated continuously with RA for 1 to 4 days or treated with RA for 2 days and released into fresh medium. The cells were collected at the indicated times, lysed and subjected to immunoblot analysis. (C) Induction of caspase-8 mRNA measured by real-time PCR. NB8, NB10 and NB16 cells were treated with RA for 48 hours and caspase-8 mRNA content was analyzed by real-time PCR. The caspase-8 mRNA expression was normalized to GADPH and the value for the relative amount of caspase-8 in the untreated control samples was set as 1 for each cell lines. The values represent the means ± SD from at least 3 independent experiments. (D) Induction of caspase-8 by retinoids in NB cells. NB8, NB10 and NB16 cells were treated with either RA (ATRA), 9-cis RA, 13-cis RA (100 µM) or 4HPR (10 µM) for 3 days, lysed and subjected to immunoblot analysis. (E) Induction of caspase-8 by retinoids in medulloblastoma and small cell lung carcinoma cell lines. Medulloblastoma (BT-3, BT-4) and small cell lung carcinoma (NCI-H82, NCI-H889) cell lines were treated with the indicated retinoids for 3 days, prior to lysis and immunoblot analysis.
Table 1.
Analysis of CASP8 during and after RA treatment in human NB cell lines
| Control | RA treatment | RA release | |||
|---|---|---|---|---|---|
| Cell line | Casp8 expressiona | CASP8 Methylationb | Casp8 proteinup-regulationc | Methylation status changed | Maintenance of Casp8 up-regulation(d) |
| NB4 | − | Y | 1 | ND | 10 |
| NB6 | − | Y | 3 | ND | 5 |
| NB8 | − | Y | 3 | N | 15 |
| NB10 | − | Y | 3 | Y | >120 |
| NB12 | − | Y | 1 | N | 2 |
| NB13 | − | Y | 1 | ND | 2 |
| NB14 | − | Y | 2 | ND | 5 |
| NB15 | + | N | 1 | N | 2 |
| NB16 | + | N | 1 | N | 2 |
| NB17 | − | Y | 0 | ND | N |
| NB19 | − | Y | 1 | N | 2 |
| SKNDZ | − | ND | 1 | ND | 2 |
from Teitz et al. [5] and references therein; ND, not determined.
The relative level of caspase-8 expression during RA treatment on a scale of 0 (minimal increase) to 3 (highest).
The change of methylation status as determined by MS-PCR; N, no change; Y, changed (from methylation to demethylation); ND, not determined.
We also examined the effects of other forms of retinoids on caspase-8 expression. All the four forms of retinoids up-regulated caspase-8 expression in NB cells (Fig. 1D). RA and 13-cis RA were more effective than 9-cis RA in inducing caspsase-8, while 4HPR was the most effective (Fig 1D). Importantly, retinoids treatment increased caspase-8 protein levels in several medulloblastoma (BT-3, BT-4) and small cell lung carcinoma (NCI-H82, NCI-H889) cell lines which lack caspase-8 expression, indicating that the ability of these compounds to induce caspase-8 was not limited to neuroblastoma (Fig. 1E).
To investigate the duration of RA-mediated effects on caspase-8 expression, twelve NB cell lines were treated with RA for 2 days. RA was then removed and the cells were monitored to determine how long casapse-8 levels remained elevated. Although the maintenance time of caspase-8 up-regulation was cell line dependent, in all cases caspase-8 remained elevated for at least two days (Table 1). To our surprise, caspase-8 expression was retained at the same level for 38 days in NB10 cells. Furthermore, the level of caspase-8 mRNA and protein increase further on ~day 42. This increased level of caspase-8 was maintained for >120 days in NB10 cells (Fig. 2). Further studies to address the processes involved in this long term change in caspase-8 expression are ongoing.
Fig. 2.
(A) Increased expression of caspase-8 was maintained in NB10 cells. NB10 cells were released into fresh medium after 2-day RA treatment, collected at indicated times, lysed and subjected to immunoblot analysis. (B) Induction of caspase-8 mRNA in RA-released NB10 cells. NB10 cells were collected 4 days or 60 days after removal of RA and subjected to real-time PCR analysis. The caspase-8 mRNA expression was normalized to GADPH and the value for the relative amount of caspase-8 in the untreated control sample was set as 1. The values represent the means ± SD from at least 3 independent experiments.
Since previous studies established a correlation between reduced caspase-8 expression and methylation of an intragenic region of the caspase-8 gene [5,40,41], we used methylation-specific PCR to determine the methylation status of caspase-8 after RA treatment. Following RA treatment the caspase-8 gene remained methylated in NB8, NB12 and NB19 cells and unmethylated in NB16 cells (Fig. 3A). Surprisingly however, in NB10 cells RA treatment led to demethylation of the caspase-8 gene (Fig. 3B).
Fig. 3.
Long-term expression of caspase-8 is accompanied by decreases in intragenic methylation. (A) RA did not change the methylation status of the caspase-8 gene in most NB cells. NB8, NB12, NB19 and NB16 cell lines were treated with RA for 4 days and subjected to methylation specific PCR of the caspase-8 gene. The methylated primer set (upper, marked as “M”) produced a 321-bp product, and the un-methylated primer set (lower, marked as “U”) produced a 322-bp product. Water was used as a negative control of MS-PCR. (B) The caspase-8 gene is demethylated in RA treated NB10 cells. NB10 cells were treated continuously with RA for 1–4 days or treated with RA for 2 days and released into fresh medium. The cells were then collected at indicated time and subjected to MS-PCR.
3.3. Identification of a RA-responsive region in the caspase-8 gene
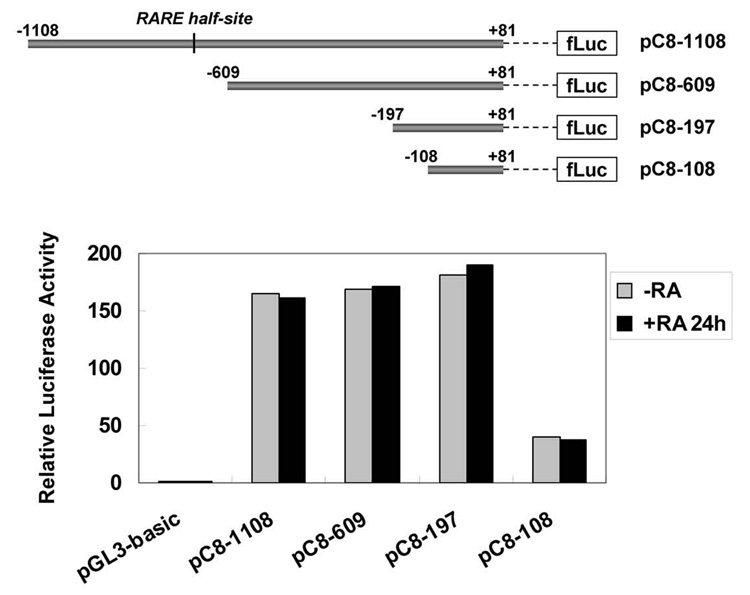
To determine the mechanism of caspase-8 up-regulation, we measured caspase-8 promoter activity after RA treatment by transfecting luciferase reporter plasmids containing either the entire functional promoter region [42–44] or smaller promoter fragments into NB8 cells (Fig. 4, upper panel). The transcriptional activity of these constructs was measured by comparison of the firefly luciferase activity to the control Renilla luciferase activity in the presence and absence of RA. The full length caspase-8 promoter (pC8–1108, −1108/+81) and the two longer truncation constructs (pC8–609: −609/+81; pC8–197: −197/+81) had similar transcriptional activity, while the smallest fragment (pC8–108: −108/+81) exhibited lower levels of expression, implying this fragment contains the caspase-8 basal promoter. Somewhat surprisingly, no changes in transcriptional activity of any of these constructs were observed after RA treatment despite the presence of the RARE half-site (AGGTCA) located at −694 (Fig. 4). These results suggest that other regions in the caspase-8 gene mediate RA-responsiveness.
Fig. 4.
The promoter region of the caspase-8 gene was not RA-responsive. Schematic representations of full length of caspase-8 gene promoter region and three fragments used for generating the deletion constructs are shown in the upper panel. The numbers indicate the location of these fragments in the caspase-8 gene relative to the transcriptional start site. The black lines represent a putative RARE half-site. NB8 cells were transiently transfected with these constructs and then treated with RA for 24 hours. The transcriptional activity of these constructs was determined by comparison of firefly luciferase activity with the Renilla luciferase activity (lower panel). The relative luciferase activity in vector-transfected control cells was set as 1.
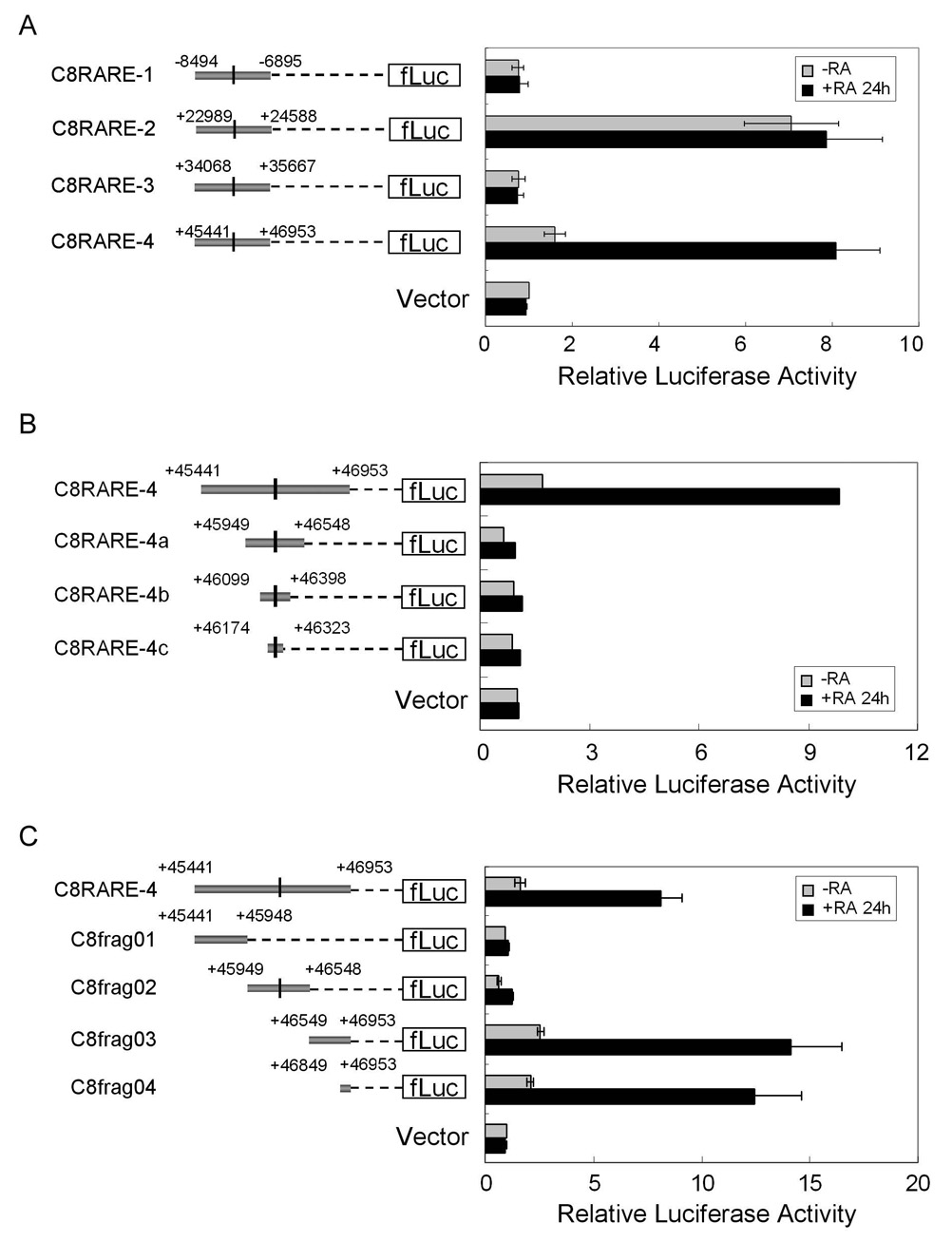
Computer analysis indicated that there are four potential RAREs located within the caspase-8 gene (−7733, +23778, +34847 and +46249), all of which have the same DR2 sequence (AGGTCAGGAGTTCA). To determine whether any of these sites were RA-responsive, caspase-8 genomic regions containing the four RAREs were cloned into the pGL3-basic vector (Fig. 5A, left panel). The fragments containing sites 1 and 3, C8RARE-1 (−8494/−6895) and C8RARE-3 (+34068/+35667), had no transcriptional activity even in the presence of RA. The fragment containing the second RARE (C8RARE-2, +22989/+24588) activated transcription in the absence of RA, although no increase in the transcriptional activity was observed upon the addition of RA (Fig. 5A, right panel). In contrast, RA induced the transcriptional activity of the construct containing the C8RARE-4 fragment (+45441/+46953) approximately 6-fold, indicating that this fragment was RA-responsive (Fig. 5A).
Fig. 5.
Identification of a RA-responsive region in the caspase-8 gene. (A) One of the four putative RAREs located within CASP8 was RA-responsive. Schematic representations of the four fragments containing the putative RAREs used for generating the different constructs are shown in the left panel. The black lines represent the putative RAREs. NB8 cells were transiently transfected with these constructs and pRL-TK plasmid as an internal control for transfection efficiency. The transcriptional activity was measured 24 hours after RA treatment (right panel). (B) RARE sequences are not responsible for RA-mediated induction of caspase-8. Schematic representations of the constructs containing the full length RA-responsive fragment (C8RARE-4) and the C8RARE-4 deletion fragments are shown in the left panel. The relative luciferase activity of these constructs is shown in the right panel. (C) Location of the RA-responsive region in CASP8. Schematic representations of the C8RARE-4 deletion fragments are shown in the left panel. The relative luciferase activity of these constructs is shown in the right panel. The values represent the means ± SD from at least 3 independent experiments.
To further localize the sequences that were required for RA-responsiveness, we performed a detailed analysis of this region by generating three deleted constructs containing progressively smaller sequence regions flanking the RARE-4 sequence (Fig. 5B, left panel). Surprisingly, none of the smaller constructs were induced by RA despite the presence of the RARE sequence in all three fragments, suggesting that this sequence was not responsible for RA responsiveness (Fig. 5B). Therefore, we generated additional constructs containing other portions of the C8RARE-4 fragment. Using these constructs we identified a 105 bp fragment (+46849/+46953, termed a C8frag04) that was induced by RA (Fig. 5C). This fragment contained two potential transcription factor binding sites: a CREB site and a c-Myb site.
To determine whether either of these transcription factor binding sites contributed to the RA induction, we generated constructs with point mutations in these sites. Mutation of the CREB binding site completely blocked RA induction, while mutation of the c-Myb binding site had no effect on RA responsive transcriptional activity. The double mutant construct blocked RA induction to the same extent as the CREB single mutant (Fig. 6).
Fig. 6.
Mutation of the CREB binding site blocked RA-mediated induction of caspase-8 transcription. The sequence of the 105 bp RA-responsive region (C8frag04) is shown in the upper right panel. The putative transcription factor binding sites for CREB and c-Myb are underlined. Constructs containing single mutations in CREB or c-Myb binding sites and a double mutant were generated and used for transient transfection (right panel, lower). The relative luciferase activity was determined (left panel). The values represent the means ± SD from at least 3 independent experiments.
3.4. RA induces CREB phosphorylation and increases DNA-binding activity of CREB
CREB (cAMP response element-binding protein) is a nuclear transcription factor which is activated by phosphorylation at serine 133 [45]. To investigate the role of CREB in caspase-8 induction by RA, we examined the effects of RA on CREB mRNA and protein expression. Real-time PCR results showed no significant change in CREB mRNA levels after RA treatment in NB8, NB10 or NB16 cell lines (data not shown). Similarly, RA treatment had no effect on expression of the CREB protein (Fig. 7A). However, RA increased the levels of Ser133-phosphorylated CREB, which is the active form of CREB in the NB cell lines (Fig. 7A).
Fig. 7.
RA induced CREB phosphorylation and increased CREB DNA-binding activity. (A) RA increased the phosphorylation of CREB at Ser133. Cells which had been treated continuously with RA for 1 to 4 days or treated with RA for 2 days and released into fresh medium were collected at the indicated times and subjected to immunoblot analysis with antibodies against CREB or phospho-CREB (Ser-133). (B) CREB binding activity was enhanced by RA in vitro. EMSA analysis was performed with nuclear extracts (NEs) from untreated NB10 (lanes 2–7) or RA-treated cells (lanes 8–13) and a 32P–labeled oligonucletotide encompassing the putative CREB binding site in CASP8 (5’-AAGAACGAAATGACGCAATCTCCAGAT-3’). The reactions were then subjected to gel electrophoresis and autoradiography. Free probe was also loaded as a negative control (lane 1). Competition assays were performed using a 100-fold excess of unlabeled oligonucleotide (lanes 3 & 9) or an unlabeled mutant oligonucleotide probe (5’-AAGAACGAAATGTGGCAATCTCCAGAT-3’) (lanes 4 & 10). Supershift assays were performed by addition of BSA (lanes 5 &11), serum (lanes 6 & 12), or an anti-phospho-CREB antibody (lanes 7 &13). The arrow indicates retarded bands and the asterisk indicates supershifted bands. (C) RA enhanced in vivo CREB binding. NB10 cells were treated with RA for 24 hours and subjected to ChIP analysis. The cells were fixed in 1% formaldehyde and sheared chromatin was immunoprecipitated with rabbit IgG, CREB, pCREB (Ser133) antibodies along with a no antibody control. PCR amplification was also performed using input DNA from the lysed cells prior to immunoprecipiation as a positive control. “−”: without RA treatment; “+”: with RA treatment. (D) RA treatment causes phosphorylation of the majority of cellular CREB. NB10 cells were treated with RA for 24 hours and subjected to ChIP analysis as described above. ChIP analysis was performed on NB10 cell lysates depleted with control, pCREB, CREB or both (“pCreb/Creb”) antibodies. “−”: without RA treatment; “+”: with RA treatment.
To determine whether RA-activated CREB binds to the RA-responsive CREB site we identified within caspase-8, we carried out gel shift (EMSA) analysis using a 32P-labeled oligonucleotide containing the putative CREB binding site and nuclear extracts isolated from control NB10 cells and NB10 cells which were treated with RA for 24-hours. Specific gel shift bands indicative of interaction between proteins and the probe were observed with both control and RA treated nuclear extracts, however, the intensity of these bands was dramatically increased when nuclear extracts from RA treated cells were used for the assay (Fig. 7B, lanes 2 and 8). This binding was specific because the band shift was eliminated by competition with a 100-fold excess of unlabeled oligonucleotide (Fig. 7B, lanes 3 and 9), but not by the addition of unlabeled mutant probe, BSA, or serum (Fig. 7B, lanes 4–6 and 10–12). The specificity of the interactions was further verified by the observation that the bands were shifted to a higher molecular mass upon addition of pCREB (Ser133) antibodies (Fig. 7B, lanes 7 and 13), confirming that the bands were due to the binding of CREB to the oligonucleotides.
To further investigate the effects of RA on DNA binding activity of CREB in vivo, we performed chromatin immunoprecipitation analysis (ChIP). Sheared cross-linked chromatin fragments were immunoprecipitated with control rabbit IgG, anti-CREB antibodies, and an antibody recognizing the Ser133-phosphorylated form of CREB antibodies along with a no antibody control. We also amplified the promoter region of HLA-G, which has been shown to be bound by CREB using the ChIP assay, as a positive control [37]. ChIP analysis demonstrated in vivo binding of CREB to the RA-responsive region in the caspase-8 gene (Fig. 7C). Consistent with the EMSA data, more PCR products were obtained in ChIP assays from RA-treated cells (Fig. 7C), suggesting RA induced increased DNA binding of CREB to the caspase-8 gene in vivo. Furthermore, no PCR product was obtained from CREB antibody immunoprecipitates after depleting the extract of phospho-Ser133-CREB, indicating that most of the CREB protein that binds to caspase-8 in RA-treated cells is phosphorylated (Fig. 7D).
3.5. Overexpression of a dominant-negative form of CREB represses caspase-8 induction by RA
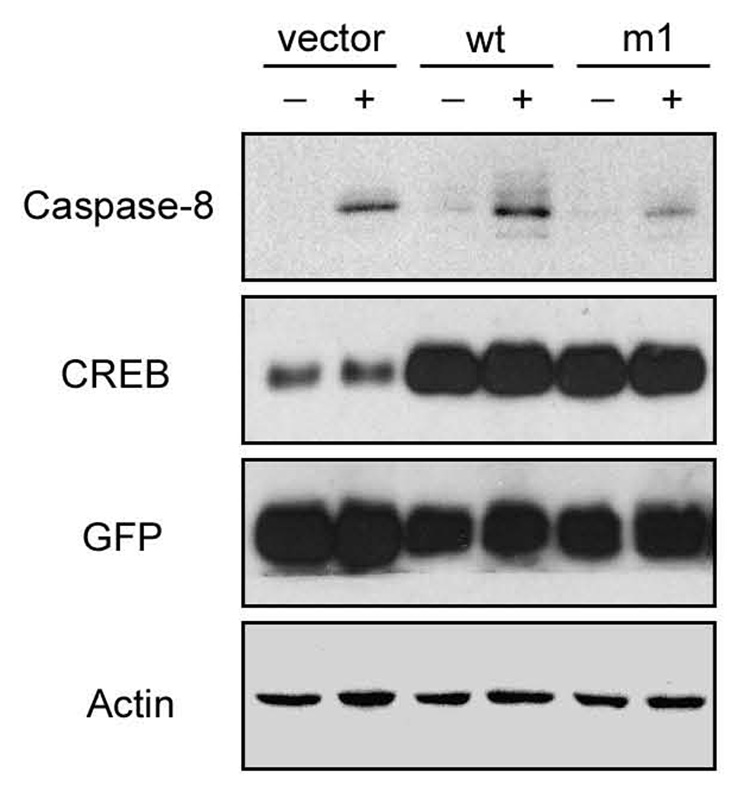
To determine whether CREB activation is necessary for caspase-8 up-regulation, we over-expressed wild-type (CREB-wt) and a dominant negative mutant CREB (CREB-m1, S133A) which prevents phosphorylation and the binding of CREB to co-activator proteins [38]. For these studies, NB8 cells were transfected with the CREB constructs for 2 days, and the sorted GFP-positive cells were exposed to RA for another 3 days. Immunoblot analysis revealed that caspase-8 expression was much lower in RA-treated cells transfected with CREB-m1 compared to cells transfected with the vector or a CREB-wt construct (Fig. 8), suggesting that activity of CREB is necessary for the induction of caspase-8 by RA.
Fig. 8.
Overexpression of a dominant-negative form of CREB repressed RA induced caspase-8 up-regulation. NB8 cells were transfected with control vector (IRES2-EGFP), CREB-wt (wild-type), or CREB-m1 (dominant-negative) constructs. Two days later, the GFP-positive cells were isolated and exposed to RA for 3 days prior to immunoblot analysis with the indicated antibodies. “−”: without RA treatment; “+”: with RA treatment.
3.6. RA-mediated increases in caspase-8 sensitize NB cells to TNFα- or drug- induced apoptosis
To determine whether the up-regulation of caspase-8 has functional significance, we examined the effect of RA-treatment and caspase-8 induction on cell death. For these studies, NB10 cells were treated for 2 days with RA and then released into fresh media. Ten days after release, the cells were treated with TNFα plus cycloheximide in the presence or absence of zVAD-fmk. RA-released cells were more sensitive to TNFα, while the addition of the caspase inhibitor zVAD repressed cell death (Fig. 9A). Similar results were obtained with more specific caspase-8 inhibitor z-IETD-fmk (data not shown), supporting the conclusion that caspase-8 was involved in this process. We next checked the cell response to another apoptosis-inducing reagent, doxorubicin (Dox). Dox has been reported to trigger apoptosis primarily through the death-receptor pathway in leukemic T cells and neuroblastoma cells [31,46,47], although this drug induces cell death via the mitochondrial pathway in other cell types [48,49]. RA treatment increased Dox sensitivity in NB10. This increase in apoptosis was inhibited by zVAD (Fig. 9B). Similar responses were observed in RA-released NB8 cells (data not shown). Importantly, expression of a dominant negative CREB mutant protein caused the transfected NB8 cells to become resistant to TNFα and Dox following RA treatment and release (Fig. 9C).
Fig. 9.
RA sensitizes NB cells to TNFα- or drug- induced apoptosis. (A) RA sensitizes NB10 cells to TNFα. NB10 cells were treated with RA for 2 days and released into fresh medium. Ten days later, the cells were treated with TNFα (100 ng/ml) plus cycloheximide (CHX, 1 µg/ml) with and without the caspase inhibitor zVAD-fmk (20 µM). The cells were also treated with CHX or zVAD alone as controls. Cell viability was measured using an MTT assay after a 24-h treatment. (B) RA sensitized NB10 cells to doxorubicin (Dox). The RA-released NB10 cells prepared as described above were treated with Dox (1 µg/ml) for 24 hours and used for a MTT assay. The student t-test indicates that responses of the control and RA treated cells are statistically significant different (p-values <0.05). (C) Overexpression of a dominant-negative form of CREB repressed RA-mediated sensitization to TNFα or Dox. NB8 cells were transfected with vector (IRES2-EGFP), or CREB-m1 (dominant-negative) constructs, sorted and treated with RA for 3 days. NB8 cells were released from RA and treated with TNFá and Dox for 24-h as described above. Cell viability was measured using an MTT assay. (D) RNA interference of caspase-8 inhibited RA-mediated sensitization. RA-released NB10 cells were infected with retrovirus supernatants containing vector (pSuper.retro.neo+GFP) or caspase-8 shRNA (C8-shRNA) for 2 days. Microscope analysis demonstrated that >80% of cells expressed GFP at this time. The cells were then treated with TNFα or Dox for 24 hours and used for a MTT assay. The values in (A–D) are means ± SD from at least three independent experiments. (E) Caspase-8 was activated during TNFα- or Dox-induced apoptosis. RA-released NB10 cells prepared as described above were treated with TNFα (T) or Dox (D) for 24 hours and subjected to immunoblot analysis using the indicated antibodies.
To further investigate the contribution of caspase-8 to RA-mediated sensitization, we used RNA interference to decrease caspase-8 expression. As shown in Fig. 9D, the RA-released NB10 cells which were expressing caspase-8 shRNA (C8-shRNA) were resistant to TNFα and Dox. We also explored the activation of caspase-8 by monitoring caspase-8 cleavage and activation of downstream caspases in TNFα and Dox treated cells. Caspase-8 was cleaved to an active form after TNFα and Dox treatment in vector infected cells, but not in C8-shRNA expressing cells (Fig. 9E). In addition, the caspase-8 substrate caspase-3 was cleaved only in vector infected cells, as was the caspase-3 substrate PARP (Fig. 9E). Furthermore, we found that expression of the upstream proteins Fas and FADD were increased in both vector and C8-ShRNA infected cells. TNF receptor 1 (TNFR1) was up-regulated only in TNFα treated cells and TRAIL receptor 2 (DR5) was induced only in Dox treated cells. These data support the conclusion that caspase-8 was activated during TNFα- and Dox- induced apoptosis after RA removal.
4. Discussion
Caspase-8 is deleted or silenced in most NB cell lines and many patient tumor samples [5–7]. Loss of caspase-8 expression has also been observed in other tumors such as medulloblastoma, small cell lung carcinoma and colorectal carcinomas [14–17]. In medulloblastoma, loss of caspase-8 correlates with unfavorable survival outcome [17]. Additionally, animal models and cell culture studies have demonstrated that the loss of caspase-8 enhances the metastatic potential of NB cells [8–10]. Caspase-8-deficient NB cells are resistant to death receptor signals and some chemotherapeutic drugs [5,31,41,50]. These defects can be corrected by re-expression of caspase-8 via demethylating agents, caspase-8 expressing retroviral vectors and IFN-γ treatment [5,31,41,50–53]. Re-expression of caspase-8 by IFN-γ or demethylating agents sensitizes cells to radio- and chemotherapy in medulloblastoma cells [53,54]. In a search of other agents that are more easily tolerated than those described above, we investigated the effects of all-trans retinoic acid (RA) treatment on caspase-8 expression and cellular proliferation in NB cells. Here we show that activation of caspase-8 by retinoic acid sensitizes neuroblastoma cells to TNFα- or drug- induced apoptosis.
Our results indicate that RA increases the expression of caspase-8 in most NB cell lines (11/12) to varying extents regardless of whether caspase-8 is silenced or not (Fig. 1 and Table 1). Further, we show that RA up-regulates the caspase-8 gene at the RNA level (Fig. 1C). Moreover, all the forms of retinoids (RA, 13-cis RA, 9-cis RA and 4HPR) increase caspase-8 expression not only in NB cell lines, but also in medulloblastoma and small cell lung carcinoma cell lines (Fig. 1D and 1E). Previous work has identified a region within the caspase-8 gene which is methylated. Methylation of this region has been correlated with decreased caspase-8 protein in some studies [5,40,41], but not in others [42,55]. Several studies have shown that demethylation of this region is not required for re-expression of caspase-8 in response to IFN-γ [31,50,56]. Here we show by methylation specific PCR that RA treatment does not alter the methylation status of this region in the majority of NB cell lines. Strikingly however, a single 2-day treatment of one cell line, NB10, with RA resulted in continued expression of caspase-8 for more than 120 days (Fig. 2 and Table 1). Furthermore, the sustained expression of caspase-8 correlated with the appearance of unmethylated caspase-8 intergenic DNA sequences (Fig. 3). While this correlation is noteworthy, many more studies are needed to determine whether this change in methylation is responsible for the sustained increase in casapse-8 expression we observed in this cell line.
The effects of RA on gene transcription are mainly mediated through the binding of retinoic acid receptors (RARs) and retinoid X receptors (RXRs) to RA response elements (RAREs) in the target gene [25,26]. Here we show that the transcriptional activity of the caspase-8 promoter (−1108/+81) was not induced by RA, despite the presence of a RARE half-site (Fig. 4). These data are in contrast to another study which showed that the transcriptional activity of the caspase-8 promoter (−1161/+16) was induced by 4HPR, an RA analog [43]. Although the reasons for this discrepancy are not clear, it should be noted that RA and 4HPR have been reported to act by different pathways [20,57,58].
Since we found that the caspase-8 promoter is not RA responsive, we also examined the four putative RAREs within the caspase-8 gene. None of these sequences were RA-inducible, suggesting that RA up-regulates caspase-8 independent of RAREs (Fig. 5). Several recent studies have shown that RA can also stimulate other cellular signaling pathways, which are independent of receptor binding to RAREs [28–30]. For example, RA can regulate PKCα activity by competing with phosphatidylserine for a binding site in the C2-domain of PKCα [29,30]. In addition, other studies have demonstrated that RA-mediated activation of CREB can increase expression of c-fos, which does not contain RAREs in its promoter [28]. Here we identify a RA-responsive region in intron 8 of the caspase-8 gene and localize the responsive element to a region containing a CREB binding site (Fig. 6). While we were somewhat surprised by this finding, intronic sequences have been found to be involved in transcriptional regulation of other genes. Intronic enhancement of gene expression was first identified in human alpha-1 collagen gene [59], and was further found in other genes including the CD21, ALAS2, delta1-crystallin, CRP1 and Opalin genes [60–64]. Intronic sequences can also function as gene silencer in some genes such as CD4 and OTR [65,66]. Intronic control of gene transcription usually involves the binding of transcription factors to the DNA sequence [61–64]. Importantly, previous studies have demonstrated that active CREB binding to intronic region of the CRP1 and Opalin genes results in increased expression of these genes [63,64]. Our gel shift and chromatin IP assays reveal an increase in CREB binding to an intronic region of the caspase-8 gene in RA-treated NB cells (Fig. 7B and C). This data in conjunction with data from studies using a dominant-negative CREB mutant support the conclusion that CREB binding to this responsive region is required for RA induced caspase-8 expression. This result is also consistent with reports indicating that DNA binding activity of CREB is increased by RA or other stimuli [67,68].
CREB is a nuclear transcription factor which is very important in controlling cell growth, survival, proliferation and cell fate decisions [45]. CREB forms dimers with the co-activator CREB-binding protein (CBP) which bind to the cAMP-response element (CRE, TGACGTCA) or the half-site of CRE (TGACG/CGTCA) [45,69]. In addition to CRE-binding, activation of CREB requires phosphorylation at serine 133. Numerous kinases including pp90rsk, PKA, PKC, AKT, MSK-1, MAPKAP-2, and calcium–calmodulin kinases II/IV phosporylate CREB in response to various stimuli [45,69,70]. Importantly, phosphorylation of CREB is required for the interaction of CREB with the co-activator protein CBP.
Our date demonstrates that RA increases the amount of CREB bound to the CREB binding site in intron 8 of CASP8 in NB cells (Fig. 7B and C). It is interesting that most of the CREB bound oligonucleotide in the retarded band is supershifted by the anti-phospho-CREB antibody (Fig. 7B). This data and similar data from another study using RA in human tracheobronchial epithelial cells [68] support the conclusion that most cellular CREB protein is phosphorylated following RA treatment. The chromatin IP data demonstrating that cellular lysates immunodepleted of phospho-CREB no longer contain any chromatin-bound CREB further support this conclusion (Fig. 7D). Finally, over-expression of a dominant-negative form of CREB repressed caspase-8 expression during RA treatment, further confirming that CREB function is necessary for induction of caspase-8 by RA (Fig. 8).
Although the mechanism of activation of CREB by RA is not well understood, several recent studies demonstrated that RA activates CREB in an ATM-dependent or a PKCα-dependent manner [70,71]. Further, induction of CREB phosphorylation is very rapid and seems to be independent of the binding of RA receptors to RAREs [28]. Two recently published papers strongly support this conclusion, since depletion of RARs and RXRα via siRNA did not block the activation of CREB by RA [68,70]. It remains to be determined whether CREB phosphorylation is induced by similar mechanisms in neuroblastoma cells. Importantly, these data also suggest multiple stimuli could induce and/or regulate caspase-8 expression via altering CREB phosphorylation. Further studies are clearly needed to address this possibility.
In addition to inducing CREB phosphorylation, RA inhibits cellular proliferation in NB cells as judged by analysis of DNA content, BrdU incorporation, decreased expression of cyclin A and dephosphorylation of Rb (Supplementary Fig. 1). RA treatment also results in neurite extension with increased cell length (data not shown), indicating that RA induces at least partial differentiation. However, when RA was removed the cells were capable of rapidly re-entering the cell cycle based on cyclin A re-expression and Rb phosphorylation (Supplementary Fig. 1C). These data indicate both that RA is cytostatic and that NB cells are likely to be resistant to many chemotherapeutic agents during RA treatment since cellular proliferation is required for the action of most chemotherapeutic agents. This suggests that agents such as TRAIL would be effective during RA treatment while most chemotherapeutics would not. However, since caspase-8 expression remains elevated in most NB cells for at least 2 days after the removal of RA, and in the case of NB8 and NB10 for 15 and >120 days, respectively (Fig. 2 and Table 1), and cellular proliferation resumes during this time period, RA released cells should be more sensitive to chemotherapeutic agents. Indeed, our data indicate that RA-released cells displayed increased apoptosis in response to doxorubicin and TNFα following RA release (Fig. 9A and B). This increased responsiveness is at least partially dependent on caspase-8 expression and activation, because not only is caspase-8 activated by cleavage, but also the caspase inhibitors zVAD and z-IETD repressed cell death (Fig. 9 and data not shown). Furthermore, specific inhibition of caspase-8 using RNA interference repressed cell death induced by TNFα and Dox (Fig. 9D). Thus, RA is a potential therapeutic agent for increasing the response of NB cells to both receptor-mediated death signals, such as TRAIL, and to standard cytotoxic reagents such as doxorubicin. Importantly, the data also indicate that further preclinical studies combining RA with other agents are clearly warranted.
Supplementary Material
Acknowledgements
We thank Drs. Tal Teitz, Akira Inoue, Janeen Trembley and members of the Lahti laboratory for their stimulating discussion and technical assistance; Dr. Marcus Peter (University of Chicago) for the C15 caspase-8 monoclonal antibody; and Dr. David Ginty (Johns Hopkins University Medical School) for the CREB-wt and CREB-mutant cDNAs. This work was supported by N.I.H grant (R01CA067938), the American Lebanese Syrian Associated Charities (ALSAC) and Cancer Center Support Grant (P30CA021765). M. Jiang is a recipient of a St. Jude Children’s Research Hospital Academic Programs fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schor NF. Neuroblastoma as a neurobiological disease. J. Neurooncol. 1999;41:159–166. doi: 10.1023/a:1006171406740. [DOI] [PubMed] [Google Scholar]
- 2.Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat. Rev. Cancer. 2003;3:203–216. doi: 10.1038/nrc1014. [DOI] [PubMed] [Google Scholar]
- 3.Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet. 2007;369:2106–2120. doi: 10.1016/S0140-6736(07)60983-0. [DOI] [PubMed] [Google Scholar]
- 4.Maris JM, Matthay KK. Molecular biology of neuroblastoma. J. Clin. Oncol. 1999;17:2264–2279. doi: 10.1200/JCO.1999.17.7.2264. [DOI] [PubMed] [Google Scholar]
- 5.Teitz T, Wei T, Valentine MB, Vanin EF, Grenet J, Valentine VA, Behm FG, Look AT, Lahti JM, Kidd VJ. Caspase 8 is deleted or silenced preferentially in childhood neuroblastomas with amplification of MYCN. Nat. Med. 2000;6:529–535. doi: 10.1038/75007. [DOI] [PubMed] [Google Scholar]
- 6.Fulda S, Poremba C, Berwanger B, Hacker S, Eilers M, Christiansen H, Hero B, Debatin KM. Loss of caspase-8 expression does not correlate with MYCN amplification, aggressive disease, or prognosis in neuroblastoma. Cancer Res. 2006;66:10016–10023. doi: 10.1158/0008-5472.CAN-05-4079. [DOI] [PubMed] [Google Scholar]
- 7.Yang Q, Kiernan CM, Tian Y, Salwen HR, Chlenski A, Brumback BA, London WB, Cohn SL. Methylation of CASP8, DCR2, and HIN-1 in neuroblastoma is associated with poor outcome. Clin. Cancer Res. 2007;13:3191–3197. doi: 10.1158/1078-0432.CCR-06-2846. [DOI] [PubMed] [Google Scholar]
- 8.Stupack DG, Teitz T, Potter MD, Mikolon D, Houghton PJ, Kidd VJ, Lahti JM, Cheresh DA. Potentiation of neuroblastoma metastasis by loss of caspase-8. Nature. 2006;439:95–99. doi: 10.1038/nature04323. [DOI] [PubMed] [Google Scholar]
- 9.Lahti JM, Teitz T, Stupack DG. Does integrin-mediated cell death confer tissue tropism in metastasis? Cancer Res. 2006;66:5981–5984. doi: 10.1158/0008-5472.CAN-06-0131. [DOI] [PubMed] [Google Scholar]
- 10.Teitz T, Stupack DG, Lahti JM. Halting neuroblastoma metastasis by controlling integrin-mediated death. Cell Cycle. 2006;5:681–685. doi: 10.4161/cc.5.7.2615. [DOI] [PubMed] [Google Scholar]
- 11.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 12.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 13.Krammer PH, Arnold R, Lavrik IN. Life and death in peripheral T cells. Nat. Rev. Immunol. 2007;7:532–542. doi: 10.1038/nri2115. [DOI] [PubMed] [Google Scholar]
- 14.Zuzak TJ, Steinhoff DF, Sutton LN, Phillips PC, Eggert A, Grotzer MA. Loss of caspase-8 mRNA expression is common in childhood primitive neuroectodermal brain tumour/medulloblastoma. Eur. J. Cancer. 2002;38:83–91. doi: 10.1016/s0959-8049(01)00355-0. [DOI] [PubMed] [Google Scholar]
- 15.Shivapurkar N, Toyooka S, Eby MT, Huang CX, Sathyanarayana UG, Cunningham HT, Reddy JL, Brambilla E, Takahashi T, Minna JD, Chaudhary PM, Gazdar AF. Differential inactivation of caspase-8 in lung cancers. Cancer Biol. Ther. 2002;1:65–69. doi: 10.4161/cbt.1.1.45. [DOI] [PubMed] [Google Scholar]
- 16.Kim HS, Lee JW, Soung YH, Park WS, Kim SY, Lee JH, Park JY, Cho YG, Kim CJ, Jeong SW, Nam SW, Kim SH, Lee JY, Yoo NJ, Lee SH. Inactivating mutations of caspase-8 gene in colorectal carcinomas. Gastroenterology. 2003;125:708–715. doi: 10.1016/s0016-5085(03)01059-x. [DOI] [PubMed] [Google Scholar]
- 17.Pingoud-Meier C, Lang D, Janss AJ, Rorke LB, Phillips PC, Shalaby T, Grotzer MA. Loss of caspase-8 protein expression correlates with unfavorable survival outcome in childhood medulloblastoma. Clin. Cancer Res. 2003;9:6401–6409. [PubMed] [Google Scholar]
- 18.Thiele CJ, Reynolds CP, Israel MA. Decreased expression of N-myc precedes retinoic acid-induced morphological differentiation of human neuroblastoma. Nature. 1985;313:404–406. doi: 10.1038/313404a0. [DOI] [PubMed] [Google Scholar]
- 19.Reynolds CP, Kane DJ, Einhorn PA, Matthay KK, Crouse VL, Wilbur JR, Shurin SB, Seeger RC. Response of neuroblastoma to retinoic acid in vitro and in vivo. Prog. Clin. Biol. Res. 1991;366:203–211. [PubMed] [Google Scholar]
- 20.Reynolds CP, Matthay KK, Villablanca JG, Maurer BJ. Retinoid therapy of high-risk neuroblastoma. Cancer Lett. 2003;197:185–192. doi: 10.1016/s0304-3835(03)00108-3. [DOI] [PubMed] [Google Scholar]
- 21.Redfern CP, Lovat PE, Malcolm AJ, Pearson AD. Gene expression and neuroblastoma cell differentiation in response to retinoic acid: differential effects of 9-cis and all-trans retinoic acid. Eur. J. Cancer. 1995;31A:486–494. doi: 10.1016/0959-8049(95)00066-r. [DOI] [PubMed] [Google Scholar]
- 22.Matsuo T, Thiele CJ. p27Kip1: a key mediator of retinoic acid induced growth arrest in the SMS-KCNR human neuroblastoma cell line. Oncogene. 1998;16:3337–3343. doi: 10.1038/sj.onc.1201830. [DOI] [PubMed] [Google Scholar]
- 23.Adamson PC, Reaman G, Finklestein JZ, Feusner J, Berg SL, Blaney SM, O'brien M, Murphy RF, Balis FM. Phase I trial and pharmacokinetic study of all-trans-retinoic acid administered on an intermittent schedule in combination with interferon-alpha2a in pediatric patients with refractory cancer. J. Clin. Oncol. 1997;15:3330–3337. doi: 10.1200/JCO.1997.15.11.3330. [DOI] [PubMed] [Google Scholar]
- 24.Adamson PC, Matthay KK, O'brien M, Reaman GH, Sato JK, Balis FM. A phase 2 trial of all-trans-retinoic acid in combination with interferon-alpha2a in children with recurrent neuroblastoma or Wilms tumor: A Pediatric Oncology Branch, NCI and Children's Oncology Group Study. Pediatr. Blood Cancer. 2007;49:661–665. doi: 10.1002/pbc.21011. [DOI] [PubMed] [Google Scholar]
- 25.Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell. 1995;83:841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 26.Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996;10:940–954. [PubMed] [Google Scholar]
- 27.Jiang SY, Wu MS, Chen LM, Hung MW, Lin HE, Chang GG, Chang TC. Identification and characterization of the retinoic acid response elements in the human RIG1 gene promoter. Biochem. Biophys. Res. Commun. 2005;331:630–639. doi: 10.1016/j.bbrc.2005.03.214. [DOI] [PubMed] [Google Scholar]
- 28.Canon E, Cosgaya JM, Scsucova S, Aranda A. Rapid effects of retinoic acid on CREB and ERK phosphorylation in neuronal cells. Mol. Biol. Cell. 2004;15:5583–5592. doi: 10.1091/mbc.E04-05-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ochoa WF, Torrecillas A, Fita I, Verdaguer N, Corbalan-Garcia S, Gomez-Fernandez JC. Retinoic acid binds to the C2-domain of protein kinase C(alpha) Biochemistry. 2003;42:8774–8779. doi: 10.1021/bi034713g. [DOI] [PubMed] [Google Scholar]
- 30.Radominska-Pandya A, Chen G, Czernik PJ, Little JM, Samokyszyn VM, Carter CA, Nowak G. Direct interaction of all-trans-retinoic acid with protein kinase C (PKC). Implications for PKC signaling and cancer therapy. J. Biol. Chem. 2000;275:22324–22330. doi: 10.1074/jbc.M907722199. [DOI] [PubMed] [Google Scholar]
- 31.Tekautz TM, Zhu K, Grenet J, Kaushal D, Kidd VJ, Lahti JM. Evaluation of IFN-gamma effects on apoptosis and gene expression in neuroblastoma--preclinical studies. Biochim. Biophys. Acta. 2006;1763:1000–1010. doi: 10.1016/j.bbamcr.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 32.Scaffidi C, Medema JP, Krammer PH, Peter ME. FLICE is predominantly expressed as two functionally active isoforms, caspase-8/a and caspase-8/b. J. Biol.Chem. 1997;272:26953–26958. doi: 10.1074/jbc.272.43.26953. [DOI] [PubMed] [Google Scholar]
- 33.Ma Y, Brewer JW, Diehl JA, Hendershot LM. Two distinct stress signaling pathways converge upon the CHOP promoter during the mammalian unfolded protein response. J. Mol. Biol. 2002;318:1351–1365. doi: 10.1016/s0022-2836(02)00234-6. [DOI] [PubMed] [Google Scholar]
- 34.Wu R, Lin L, Beer DG, Ellenson LH, Lamb BJ, Rouillard JM, Kuick R, Hanash S, Schwartz DR, Fearon ER, Cho KR. Amplification and overexpression of the L-MYC proto-oncogene in ovarian carcinomas. Am. J. Pathol. 2003;162:1603–1610. doi: 10.1016/S0002-9440(10)64294-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 36.Javed A, Zaidi SK, Gutierrez SE, Lengner CJ, Harrington KS, Hovhannisyan H, Cho BC, Pratap J, Pockwinse SM, Montecino M, van Wijnen AJ, Lian JB, Stein JL, Stein GS. Chromatin immunoprecipitation. Methods Mol. Biol. 2004;285:41–44. doi: 10.1385/1-59259-822-6:041. [DOI] [PubMed] [Google Scholar]
- 37.Gobin SJ, Biesta P, de Steenwinkel JE, Datema G, van den Elsen PJ. HLA-G transactivation by cAMP-response element-binding protein (CREB). An alternative transactivation pathway to the conserved major histocompatibility complex (MHC) class I regulatory routes. J. Biol. Chem. 2002;277:39525–39531. doi: 10.1074/jbc.M112273200. [DOI] [PubMed] [Google Scholar]
- 38.Riccio A, Alvania RS, Lonze BE, Ramanan N, Kim T, Huang Y, Dawson TM, Snyder SH, Ginty DD. A nitric oxide signaling pathway controls CREB-mediated gene expression in neurons. Mol. Cell. 2006;21:283–294. doi: 10.1016/j.molcel.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 39.Gaetano C, Matsumoto K, Thiele CJ. In vitro activation of distinct molecular and cellular phenotypes after induction of differentiation in a human neuroblastoma cell line. Cancer Res. 1992;52:4402–4407. [PubMed] [Google Scholar]
- 40.Hopkins-Donaldson S, Bodmer JL, Bourloud KB, Brognara CB, Tschopp J, Gross N. Loss of caspase-8 expression in neuroblastoma is related to malignancy and resistance to TRAIL-induced apoptosis. Med. Pediatr. Oncol. 2000;35:608–611. doi: 10.1002/1096-911x(20001201)35:6<608::aid-mpo25>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 41.Fulda S, Kufer MU, Meyer E, van Valen F, Dockhorn-Dworniczak B, Debatin KM. Sensitization for death receptor- or drug-induced apoptosis by re-expression of caspase-8 through demethylation or gene transfer. Oncogene. 2001;20:5865–5877. doi: 10.1038/sj.onc.1204750. [DOI] [PubMed] [Google Scholar]
- 42.Banelli B, Casciano I, Croce M, di Vinci A, Gelvi I, Pagnan G, Brignole C, Allemanni G, Ferrini S, Ponzoni M, Romani M. Expression and methylation of CASP8 in neuroblastoma: identification of a promoter region. Nat. Med. 2002;8:1333–1335. doi: 10.1038/nm1202-1333. [DOI] [PubMed] [Google Scholar]
- 43.Casciano I, Banelli B, Croce M, De Ambrosis A, di Vinci A, Gelvi I, Pagnan G, Brignole C, Allemanni G, Ferrini S, Ponzoni M, Romani M. Caspase-8 gene expression in neuroblastoma. Ann. N. Y. Acad. Sci. 2004;1028:157–167. doi: 10.1196/annals.1322.017. [DOI] [PubMed] [Google Scholar]
- 44.Ruiz-Ruiz C, Ruiz dA, Rodriguez A, Ortiz-Ferron G, Redondo JM, Lopez-Rivas A. The up-regulation of human caspase-8 by interferon-gamma in breast tumor cells requires the induction and action of the transcription factor interferon regulatory factor-1. J. Biol. Chem. 2004;279:19712–19720. doi: 10.1074/jbc.M313023200. [DOI] [PubMed] [Google Scholar]
- 45.Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat. Rev. Mol. Cell Biol. 2001;2:599–609. doi: 10.1038/35085068. [DOI] [PubMed] [Google Scholar]
- 46.Friesen C, Herr I, Krammer PH, Debatin KM. Involvement of the CD95 (APO-1/FAS) receptor/ligand system in drug-induced apoptosis in leukemia cells. Nat. Med. 1996;2:574–577. doi: 10.1038/nm0596-574. [DOI] [PubMed] [Google Scholar]
- 47.Fulda S, Sieverts H, Friesen C, Herr I, Debatin KM. The CD95 (APO-1/Fas) system mediates drug-induced apoptosis in neuroblastoma cells. Cancer Res. 1997;57:3823–3829. [PubMed] [Google Scholar]
- 48.Cuvillier O, Nava VE, Murthy SK, Edsall LC, Levade T, Milstien S, Spiegel S. Sphingosine generation, cytochrome c release, and activation of caspase-7 in doxorubicin-induced apoptosis of MCF7 breast adenocarcinoma cells. Cell Death. Differ. 2001;8:162–171. doi: 10.1038/sj.cdd.4400793. [DOI] [PubMed] [Google Scholar]
- 49.Childs AC, Phaneuf SL, Dirks AJ, Phillips T, Leeuwenburgh C. Doxorubicin treatment in vivo causes cytochrome C release and cardiomyocyte apoptosis, as well as increased mitochondrial efficiency, superoxide dismutase activity, and Bcl-2:Bax ratio. Cancer Res. 2002;62:4592–4598. [PubMed] [Google Scholar]
- 50.Fulda S, Debatin KM. IFNgamma sensitizes for apoptosis by upregulating caspase-8 expression through the Stat1 pathway. Oncogene. 2002;21:2295–2308. doi: 10.1038/sj.onc.1205255. [DOI] [PubMed] [Google Scholar]
- 51.Yang X, Merchant MS, Romero ME, Tsokos M, Wexler LH, Kontny U, Mackall CL, Thiele CJ. Induction of caspase 8 by interferon gamma renders some neuroblastoma (NB) cells sensitive to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) but reveals that a lack of membrane TR1/TR2 also contributes to TRAIL resistance in NB. Cancer Res. 2003;63:1122–1129. [PubMed] [Google Scholar]
- 52.Johnsen JI, Pettersen I, Ponthan F, Sveinbjornsson B, Flaegstad T, Kogner P. Synergistic induction of apoptosis in neuroblastoma cells using a combination of cytostatic drugs with interferon-gamma and TRAIL. Int. J. Oncol. 2004;25:1849–1857. [PubMed] [Google Scholar]
- 53.Fulda S, Debatin KM. 5-Aza-2′-deoxycytidine and IFN-gamma cooperate to sensitize for TRAIL-induced apoptosis by upregulating caspase-8. Oncogene. 2006;25:5125–5133. doi: 10.1038/sj.onc.1209518. [DOI] [PubMed] [Google Scholar]
- 54.Meister N, Shalaby T, von Bueren AO, Rivera P, Patti R, Oehler C, Pruschy M, Grotzer MA. Interferon-gamma mediated up-regulation of caspase-8 sensitizes medulloblastoma cells to radio- and chemotherapy. Eur. J. Cancer. 2007;43:1833–1841. doi: 10.1016/j.ejca.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 55.van Noesel MM, van Bezouw S, Salomons GS, Voute PA, Pieters R, Baylin SB, Herman JG, Versteeg R. Tumor-specific down-regulation of the tumor necrosis factor-related apoptosis-inducing ligand decoy receptors DcR1 and DcR2 is associated with dense promoter hypermethylation. Cancer Res. 2002;62:2157–2161. [PubMed] [Google Scholar]
- 56.Kim S, Kang J, Evers BM, Chung DH. Interferon-gamma induces caspase-8 in euroblastomas without affecting methylation of caspase-8 promoter. J. Pediatr. Surg. 2004;39:509–515. doi: 10.1016/j.jpedsurg.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 57.Maurer BJ, Metelitsa LS, Seeger RC, Cabot MC, Reynolds CP. Increase of ceramide and induction of mixed apoptosis/necrosis by N-(4-hydroxyphenyl)- retinamide in neuroblastoma cell lines. J. Natl. Cancer Inst. 1999;91:1138–1146. doi: 10.1093/jnci/91.13.1138. [DOI] [PubMed] [Google Scholar]
- 58.Sani BP, Shealy YF, Hill DL. N-(4-hydroxyphenyl)retinamide: interactions with retinoid-binding proteins/receptors. Carcinogenesis. 1995;16:2531–2534. doi: 10.1093/carcin/16.10.2531. [DOI] [PubMed] [Google Scholar]
- 59.Bornstein P, McKay J, Morishima JK, Devarayalu S, Gelinas RE. Regulatory elements in the first intron contribute to transcriptional control of the human alpha 1(I) collagen gene. Proc. Natl. Acad. Sci. U. S. A. 1987;84:8869–8873. doi: 10.1073/pnas.84.24.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zabel MD, Weis JH. Cell-specific regulation of the CD21 gene. Int.Immunopharmacol. 2001;1:483–493. doi: 10.1016/s1567-5769(00)00046-1. [DOI] [PubMed] [Google Scholar]
- 61.Surinya KH, Cox TC, May BK. Identification and characterization of a conserved erythroid-specific enhancer located in intron 8 of the human 5-minolevulinate synthase 2 gene. J Biol. Chem. 1998;273:16798–16809. doi: 10.1074/jbc.273.27.16798. [DOI] [PubMed] [Google Scholar]
- 62.Kondoh H, Uchikawa M, Kamachi Y. Interplay of Pax6 and SOX2 in lens development as a paradigm of genetic switch mechanisms for cell differentiation. Int. J Dev. Biol. 2004;48:819–827. doi: 10.1387/ijdb.041868hk. [DOI] [PubMed] [Google Scholar]
- 63.Najwer I, Lilly B. Ca2+/calmodulin-dependent protein kinase IV activates cysteine-rich protein 1 through adjacent CRE and CArG elements. Am. J Physiol Cell Physiol. 2005;289:C785–C793. doi: 10.1152/ajpcell.00098.2005. [DOI] [PubMed] [Google Scholar]
- 64.Aruga J, Yoshikawa F, Nozaki Y, Sakaki Y, Toyoda A, Furuichi T. An oligodendrocyte enhancer in a phylogenetically conserved intron region of the mammalian myelin gene Opalin. J Neurochem. 2007;102:1533–1547. doi: 10.1111/j.1471-4159.2007.04583.x. [DOI] [PubMed] [Google Scholar]
- 65.Sawada S, Scarborough JD, Killeen N, Littman DR. A lineage-specific transcriptional silencer regulates CD4 gene expression during T lymphocyte development. Cell. 1994;77:917–929. doi: 10.1016/0092-8674(94)90140-6. [DOI] [PubMed] [Google Scholar]
- 66.Mizumoto Y, Kimura T, Ivell R. A genomic element within the third intron of the human oxytocin receptor gene may be involved in transcriptional suppression. Mol. Cell Endocrinol. 1997;135:129–138. doi: 10.1016/s0303-7207(97)00195-0. [DOI] [PubMed] [Google Scholar]
- 67.Zhang B, Liu S, Perpetua MD, Walker WH, Harbrecht BG. Cytokines increase CRE binding but decrease CRE-mediated reporter activity in rat hepatocytes by increasing c-Jun. Hepatology. 2004;39:1343–1352. doi: 10.1002/hep.20200. [DOI] [PubMed] [Google Scholar]
- 68.Aggarwal S, Kim SW, Cheon K, Tabassam FH, Yoon JH, Koo JS. Nonclassical action of retinoic acid on the activation of the cAMP response element-binding protein in normal human bronchial epithelial cells. Mol. Biol. Cell. 2006;17:566–575. doi: 10.1091/mbc.E05-06-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carlezon WA, Jr, Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28:436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 70.Kim SW, Hong JS, Ryu SH, Chung WC, Yoon JH, Koo JS. Regulation of Mucin Gene Expression by CREB via a Nonclassical Retinoic Acid Signaling Pathway. Mol. Cell Biol. 2007;27:6933–6947. doi: 10.1128/MCB.02385-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fernandes ND, Sun Y, Price BD. Activation of the kinase activity of ATM by retinoic acid is required for CREB-dependent differentiation of neuroblastoma cells. J. Biol. Chem. 2007;282:16577–16584. doi: 10.1074/jbc.M609628200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.