Abstract
Context: Ghrelin, an acylated peptide hormone secreted from the gut, regulates appetite and metabolism. Elucidating its pattern of secretion in the fed and fasted states is important in the face of the obesity epidemic.
Objective: Our objective was to examine changes in circulating ghrelin and des-acyl ghrelin in response to meals and fasting using newly developed two-site sandwich assays and sample preservation protocols to allow specific detection of full-length forms.
Design: Ten-minute sampling was done for 26.5 h during a fed admission with standardized meals and on a separate admission during the final 24 h of a 61.5-h fast and continuing for 2.5 h after terminating the fast.
Setting: The study was conducted at the University Hospital General Clinical Research Center.
Participants: Eight male volunteers participated, mean ± sd age 24.5 ± 3.7 yr and body mass index 24 ± 2.1 kg/m2.
Main Outcome Measures: Ten-minute sampling profiles were assessed for ghrelin and des-acyl ghrelin, fed and fasting.
Results: In the fed state, ghrelin and des-acyl ghrelin showed similar dynamics; both were sharply inhibited by meals and increased at night. During fasting, ghrelin decreased to nadir levels seen postprandially, and des-acyl ghrelin remained near peak levels seen preprandially. Total full-length ghrelin (acyl plus des-acyl) levels remained unchanged.
Conclusions: Meals inhibited secretion of both ghrelin and des-acyl ghrelin, yet long-term fasting inhibited acylation but not total secretion. Acylation may be regulated independently of secretion by nutrient availability in the gut or by esterases that cleave the acyl group. These studies highlight the importance of stringent conditions for sample collection and evaluation of full-length ghrelin and des-acyl ghrelin using specific two-site assays.
In healthy young men, meals suppress both ghrelin and des-acyl ghrelin levels, whereas long-term fasting inhibits acylation but not total secretion. These findings suggest that acylation may be regulated independently of secretion by nutrient availability in the gut or by esterases which cleave the acyl group. Specific assays to measure these two types of ghrelin are warranted.
Ghrelin, discovered as a natural ligand for the GH secretagogue receptor (now also called the ghrelin receptor), is a 28-amino-acid peptide produced predominantly by the enteroendocrine X/A-like cells in the oxyntic glands of the stomach (1,2). In addition to potently stimulating secretion of GH from the pituitary (1,3), ghrelin acts as an orexigenic peptide promoting appetite, food intake, body weight gain, and adipogenesis in rodents and humans (4,5,6,7,8).
During its synthesis, ghrelin is acylated on serine (3) with an ester-linked fatty acid group (9,10). This unusual acylation is essential for ghrelin's activity at the GH secretagogue receptor (1,11) but is readily cleaved by endogenous esterase activity (12,13,14). Both acyl- and des-acyl ghrelin are found in the circulation (1), with des-acyl being the more abundant form (15). Des-acyl ghrelin was first thought to be inactive (1), however, recent studies suggest that des-acyl ghrelin has multiple biological activities (16,17,18,19,20,21,22). Some of these actions of des-acyl ghrelin oppose those of acyl-ghrelin (18,23), suggesting that the ratio of acyl- to des-acyl ghrelin may determine the overall physiological response (18).
Most published studies have used single-antibody ghrelin assays that recognize an epitope unique to ghrelin (active ghrelin) or common to both ghrelin and des-acyl ghrelin (total ghrelin). Such assays also detect ghrelin fragments. Two-site sandwich assays, as used in this study, have greater specificity and can avoid cross-reactivity with peptide fragments (24).
Likely because of ghrelin's strong basic charge and hydrophobic acylation, it can stick to surfaces and be difficult to quantitate. In the circulation, ghrelin, but not des-acyl ghrelin, may be predominantly bound to carrier proteins (25), with ghrelin having specific lipoprotein interactions not seen with des-acyl ghrelin (26), and ghrelin antisera differ in their ability to detect these bound forms (25). Together these factors can affect ghrelin measurement as reported by Groschl et al. (27), who found that when two popular commercial RIA kits were compared on the same sample set, a 10-fold difference in the measured total ghrelin levels was seen.
There are also concerns about the specificity of available acyl-ghrelin assays. In one study examining patients with anorexia nervosa (28) the acyl-ghrelin levels measured in the same sample set were either increased, decreased, or unchanged in subjects with anorexia relative to normal controls, depending on which of three different active ghrelin assays was used.
Human plasma ghrelin levels increase preprandially and drop rapidly after meals, suggesting that ghrelin plays a role in short-term regulation of food intake (29,30). However, changes in plasma ghrelin levels accompanying a fast in excess of the typical inter-meal interval are not well understood. Previous studies have not examined whether ghrelin and des-acyl ghrelin respond differently to meals or long-term fasting.
Here we report the development of sensitive and specific two-site assays to measure both ghrelin and des-acyl ghrelin and validated protocols for sample preservation. Using these assays, we examined ghrelin and des-acyl ghrelin profiles in normal young men sampled every 10 min for 26.5 h in fed and fasting protocols.
Subjects and Methods
Peptide synthesis
The C-terminal region of human ghrelin [cys-ghrelin (21,22,23,24,25,26,27)] was synthesized using an Fmoc strategy on Rink amide resin. The product was then HPLC purified and verified by mass spectrometry.
Sources of other peptides
Peptides were obtained as follows (all human sequence unless noted): ghrelin (human and rat), des-acyl ghrelin (human and rat), ghrelin(17–28), and acyl-ghrelin(3–28) (rat) from Phoenix Pharmaceuticals (Burlingame, CA); ghrelin 1–5, 1–10, 1–14, and 1–18, from Peptides International (Louisville, KY); and GH-releasing peptide 6, motilin, galanin, somatostatin, cortistatin-14, cortistatin-17, GHRH, pituitary adenylate cyclase-activating peptide(1–38), secretin, gastric inhibitory polypeptide, PTH, and calcitonin, from Bachem (King of Prussia, PA).
Ghrelin antisera
Three different ghrelin antisera were used in these ghrelin assays: 1) C-terminal-specific antiserum, a rabbit polyclonal antiserum generated using full-length human acyl-ghrelin as antigen (gift from Bristol-Myers Squibb) and then affinity purified against ghrelin(21–27); 2) acyl-specific antiserum, a protein A purified polyclonal rabbit IgG generated using the N-terminal fragment of acyl-ghrelin(1–11) as antigen (gift from Merck Research Laboratories); and 3) des-acyl-specific antiserum, a mouse monoclonal antibody generated using full-length human ghrelin as antigen. This antibody recognizes the Gly1 through Pro7 region of the N terminus of des-acyl ghrelin (gift from Eli Lilly & Co.).
Affinity purification and biotinylation of antisera
A monospecific polyclonal antiserum for the ghrelin C terminus was obtained by affinity purification against cys-ghrelin(21–27) linked to iodoacetyl activated resin (Sulfo-Link; Pierce, Rockford, IL) following the manufacturer's protocol. Yield was 300–500 μg purified IgG/ml crude serum.
For biotinylation, purified antisera were bound to a nickel-chelated column (Pierce no. 21440) and reacted with N-hydrosuccinimide-biotin containing a polyethylene oxide spacer arm (Pierce no. 21329). Note that in our hands, it was crucial that the antiserum be free of Tris (introduced during affinity purification) to obtain satisfactory binding to the nickel column (not documented by Pierce).
Dot blotting
Ghrelin and its fragments were dotted on nitrocellulose membrane (Hybond-ECL; GE Healthcare, Piscataway, NJ), blocked, and then detected by standard Western blot techniques (West Pico; Pierce) and x-ray film (X-Omat RP; Kodak, Rochester, NY).
Collection of human plasma samples for ghrelin assay validation
Volunteers gave written informed consent, and all procedures followed a protocol approved by the Institutional Review Boards of the University of Virginia and the General Clinical Research Center (GCRC). Overnight fasting blood samples (collected before breakfast) were drawn into a cold syringe and added to chilled EDTA Vacutainer tubes preloaded with 4-[2-aminoethyl benzene] sulfonyl fluoride (AEBSF; Alexis Biochemicals, San Diego, CA) (4 mm final concentration) on ice. The blood was promptly centrifuged and the plasma separated and acidified with 200 μl 1 n HCl/ml. Aliquots were stored frozen at −20 C.
Stripping of ghrelin from plasma by C-18 extraction
Human plasma from the University of Virginia Hospital Blood Bank was dosed with AEBSF and HCl as above, and the plasma was then sequentially extracted three times on a C-18 column (3M Bioanalytical Technologies, St. Paul, MN). Previous experiments with [3H]ghrelin (Phoenix Pharmaceuticals) had established that under these acid conditions, both acyl- and des-acyl ghrelin are dissociated from carrier proteins and can be quantitatively retained on this resin (31).
Ghrelin sandwich assay
Plates (384-well Maxisorb; Nunc, Roskilde, Denmark) were coated with acyl-specific antiserum at 1 μg/ml overnight. The plate was then blocked, washed, and loaded with 25 μl/well wetting/neutralization buffer (0.5 m phosphate buffer with 1% BSA, pH 7.4) and 25 μl/well ghrelin standards or unknown samples and incubated overnight at 4C. The washed plate was then incubated 1 h with the biotinylated C-terminal ghrelin antiserum in blocking buffer and then for 30 min with streptavidin-poly-HRP80 (RDI Fitzgerald, Concord, MA). Finally, the plate was detected with the fluorescent substrate Amplex Red (Molecular Probes, Eugene, OR). Fluorescence was read using excitation/emission wavelengths of 535/590 nm (Tecan Genios plate reader; Phenix Research, Hayward, CA). All unknowns were run in duplicate, and all samples for each admission of each subject were run on the same plate. Standards were made up in acid/AEBSF-treated stripped plasma.
Des-acyl ghrelin sandwich assay
The protocol for des-acyl ghrelin assay follows that used for the ghrelin sandwich assay with the substitution of affinity-purified C-terminal ghrelin antiserum for the capture step and biotinylated N-terminal des-acyl ghrelin-specific monoclonal antiserum as the reporter. All other steps are unchanged.
Butyrylcholinesterase (BuChE) assay
BuChE was assayed in 96-well plates following established methods (32). Hydrolysis of butyrylthiocholine iodide substrate was detected with DTNB and absorbance read at 415 nm in a plate reader at 1-min intervals for 10 min (Tecan Genios plate reader; Phenix Research). The assay showed an intraassay coefficient of variation (CV) of 3.6% and an interassay CV of 7.2%. For the 10-min sampling studies, BuChE was assayed in serum samples without preservative. All unknowns were run in duplicate. Assay results are reported as percent inhibition relative to controls or as enzyme units relative to the activity units of purified bovine BuChE (no. C1057; Sigma-Aldrich, St. Louis, MO) run as a control on each plate.
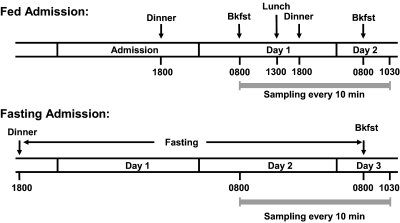
Subjects and study design for 10-min sampling protocols (Fig. 1)
Figure 1.
Protocol for 10-min sampling on fed and fasting admissions. Strenuous daily exercise was restricted to less than 1 h/d. Screening included a medical history questionnaire, physical examination, and a fasting blood profile. Exclusion criteria included smoking, acute illness, or medications known to affect GH release. On the fed admission, the 26.5-h sampling period included four standardized meals. On the fasting admission, the matched sampling period included the final 24 h of a 61.5-h fast and then the response to breaking the fast. Samples were collected for measurement of ghrelin, des-acyl ghrelin, and BuChE. The subjects were allowed to sleep after 2100 h. Bkfst, Breakfast.
The study protocol was approved by the Institutional Review Boards noted above. Eight healthy men were recruited by advertisement and gave written informed consent before participating in the study. Mean age ± sd was 24.5 ± 3.7 yr (range 18–28) and body mass index (BMI) ± sd was 24 ± 2.1 kg/m2 (range 20.6–26.2). Each subject was admitted for both a fed and a fasting admission in random order.
Fed admission
During the fed admission, subjects were admitted to the GCRC, served a standardized dinner at 1800 h and then fasted overnight except for water ad libitum. The following morning, a venous cannula was inserted into a forearm vein of each arm. Blood sampling started at 0800 h and continued every 10 min for 26.5 h. During the sampling period, four standardized meals were served. Subjects were asked to consume each meal in its entirety within 30 min. The total calories given each subject were calculated using the Harris-Benedict equation and supplied as 20% protein, 30% fat, and 50% carbohydrate. Each sample was assayed for ghrelin, des-acyl ghrelin, and BuChE.
Fasting admission
During the fasting admission, subjects were admitted to the GCRC in the early evening. Upon completion of dinner at 1830 h, no food was allowed for 61.5 h except for free access to water. Sampling at 10-min intervals was started at 0800 h, 37.5 h into the fast. Breakfast was served at 0800 h the following day ending the fast, but sampling continued for another 2.5 h (total of 26.5 h of sampling). Ketones were measured three times per day to verify compliance with fasting.
Statistical analysis
All results are expressed as mean ± sem unless otherwise noted. Statistical comparisons were performed using two-tailed paired t tests. P < 0.05 was considered statistically significant. In all cases, nonparametric Wilcoxon paired rank tests gave the same conclusions as t tests. Statistical, regression, curve fitting, and IC50 calculations were made using GraphPad Prism version 4.03 for Windows (GraphPad Software, San Diego, CA).
Results
Dot-blot characterization of antisera specificity
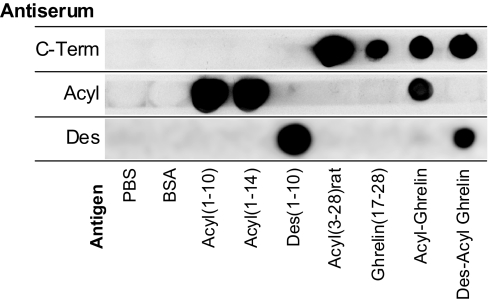
Figure 2 demonstrates that each antisera recognized the peptides and fragments containing its expected epitope.
Figure 2.
Dot-blot characterization of ghrelin antisera. Ghrelin and ghrelin fragments were spotted on nitrocellulose to test specificities of antisera used in sandwich assays as described in Subjects and Methods. Membrane strips dotted with antigens were probed with the three different antisera: C-Term, rabbit antiserum affinity purified to the ghrelin C terminus; Acyl, rabbit antiserum specific to an acylated ghrelin epitope; and Des, mouse monoclonal antiserum specific to a des-acylated ghrelin epitope. Spots were dotted with ghrelin fragments and controls as indicated in the figure. The acyl-specific antiserum did not recognize rat acyl(3–28), which lacks residues 1 and 2. Note that des(1–10) was included only in the test of the des-acyl-specific antiserum. A minor artifact of this experiment was that all peptides were dotted with an equal number of nanograms per spot; thus, the smaller fragments have more moles of the antigenic sites and generally appear darker.
Ghrelin assay characterization
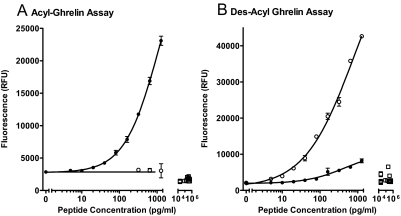
The dose-response curve for the ghrelin sandwich assay and lack of cross-reactivity with des-acyl ghrelin, ghrelin fragments, or other peptides is shown in Fig. 3A. The assay sensitivity was 6.7 pg/ml with an intraassay CV of 9.1% at 30 pg/ml, 12.6% at 100 pg/ml, and 16.8% at 300 pg/ml. The interassay CV was 17.8% at 50 pg/ml. Figure 3B shows the dose response of the des-acyl sandwich assay. The assay sensitivity was 4.6 pg/ml with an intraassay CV of 12.5% at 50 pg/ml, 10.7% at 150 pg/ml, and 18.0% at 500 pg/ml. The interassay CV was 20.8% at 30 pg/ml. There was no significant cross-reactivity with ghrelin fragments or nonspecific peptides, but the assay does show a cross-reactivity of less than 3% with acyl-ghrelin. Because acyl-ghrelin was generally less abundant in clinical samples, this is not a significant error in this study and was not corrected for.
Figure 3.
Dose-response curves for ghrelin sandwich assays. A, Acyl-ghrelin assay; B, des-acyl ghrelin assay. Acyl-ghrelin (•) and des-acyl ghrelin (○) were tested in both assays at varying doses shown on a log scale. Controls for nonspecific cross-reactivity were tested in both assays at one dose (all shown as open squares). Ghrelin fragments tested at 20 ng/ml were acyl-ghrelin(1–5), acyl-ghrelin(1–10), acyl-ghrelin(1–14), acyl-ghrelin(1–18), ghrelin(17–28), and ghrelin(3–28) (rat). Other peptides tested at 100 nm (87–506 ng/ml depending on molecular weight) were GH-releasing peptide 6, motilin, galanin, somatostatin, cortistatin-14, cortistatin-17, GHRH, pituitary adenylate cyclase-activating peptide (1–38), secretin, gastric inhibitory polypeptide, PTH, and calcitonin. Although the acyl-ghrelin assay showed no response to des-acyl ghrelin or other tested peptides, the des-acyl ghrelin assay had 3% cross-reactivity to acyl-ghrelin and is an inherent property of the monoclonal antibody's specificity (note faint cross-reactivity seen in Fig. 2) and no significant response to other peptides. RFU, Relative fluorescence units.
Stability of ghrelin acylation in human blood samples
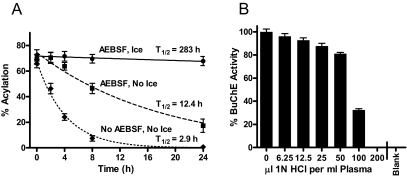
Studies were performed to evaluate methods to prevent degradation of ghrelin after blood samples are drawn. Figure 4A shows average percent acylation over time (time was defined as the delay in hours between blood draw and plasma acidification). The initial overnight fasting ghrelin levels in six subjects (three men and three women, ages 26–60 yr) varied from 26–234 pg/ml, but in the samples without AEBSF, all were degraded to below the level of detection within 24 h. In the samples incubated at room temperature with and without AEBSF, levels of acyl-ghrelin decreased over time, whereas des-acyl increased. Those incubated with AEBSF on ice showed only 6.2% loss of acylation in 24 h (not statistically different from zero loss). Samples on ice without AEBSF were tested in other runs (data not shown) and gave a deacylation half-life of about 6.4 h. All samples for additional studies were collected with AEBSF on ice and acidified within 1 h of collection.
Figure 4.
A, Stability of ghrelin acylation in human blood samples. The percentage of ghrelin that was acylated in human blood at increasing incubation times was fit as a single-phase exponential decay. Average of six subjects with three incubation conditions each: AEBSF plus chilling on ice (—•—), AEBSF without ice (– –▪– –), and no AEBSF and no ice (···♦···). B, Inhibition of BuChE activity by HCl. Plasma was incubated 1 h with the indicated dose of HCl (microliters per milliliter plasma) and then diluted into a neutralizing buffer for assay. Blank indicates no enzyme negative control. The y-axis is percentage of activity without acid.
As an additional test of our sample preservation protocols and also of assay reproducibility, a set of samples from one subject was assayed, frozen at −20 C for 9 months, and then thawed and reassayed for ghrelin. There was a Pearson's r correlation of 0.935 between the two runs of 160 samples and a linear regression slope of 1.024 (95% confidence interval 0.996–1.05), demonstrating that there was no detectable loss of ghrelin acylation after 9 months storage and an additional freeze-thaw cycle.
Recovery of acyl-ghrelin added to plasma samples
Spiking experiments were used to confirm the specificity of the assays and the efficacy of the protective agents. Two doses of ghrelin were spiked into plasma samples from four volunteers and into stripped blood bank plasma with and without AEBSF and acid. Fasting ghrelin levels (overnight, before breakfast) varied from 43–366 pg/ml in four volunteers. The increase due to the added exogenous ghrelin could be assayed quantitatively in all subjects and in the stripped plasma with recoveries of 102.9 ± 4.2% (n = 5 at 50 pg/ml) and 108.2 ± 8.9% (n = 5 at 200 pg/ml). Without AEBSF and acid, 100% of the 50 pg/ml dose and 53% of the 200 pg/ml dose of spiked acyl-ghrelin was lost during assay.
Examining BuChE inhibition in serum samples
BuChE is one of the activities found in human serum that must be inhibited to prevent ghrelin degradation (12). We found that AEBSF potently inhibited BuChE (see supplemental Fig. 1, published as supplemental data on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). At room temperature, the 4 mm concentration used in our ghrelin samples inhibited 86–96% of the BuChE activity, consistent with the partial loss of ghrelin acylation seen in Fig. 4A (middle curve).
Different ghrelin assay protocols specify 50, 100, or 200 μl of 1 n HCl/ml plasma to preserve samples. Figure 4B shows the result of adding varying amounts of HCl to plasma (without AEBSF), incubating 1 h, and then diluting the plasma into neutralizing buffer and assaying BuChE activity. This is relevant because samples must be thawed and neutralized before being incubated with antibodies for immunoassay. Figure 4B shows that 200 μl 1 n HCl/ml plasma (pH ∼3) inactivates this esterase within 1 h leaving no measurable activity. Using 100 μl of 1 n HCl/ml plasma, 32.5% of the BuChE remained after 1 h (Fig. 4B), but when incubated for 6 h, only 3% of the BuChE activity remained (not shown). With 50 μl acid, 81.6% of the enzyme activity was still present even after 6 h incubation (not shown). Thus, the 50 μl HCl/ml recommended in some protocols seems insufficient to prevent BuChE activity. Acidification is crucial to protect ghrelin against loss of acylation on storage and freeze/thaw (33), but the ester linkage on ghrelin can become unstable if the pH is allowed to drop to less than 2 (33).
Ghrelin profiles during fed and fasting admissions
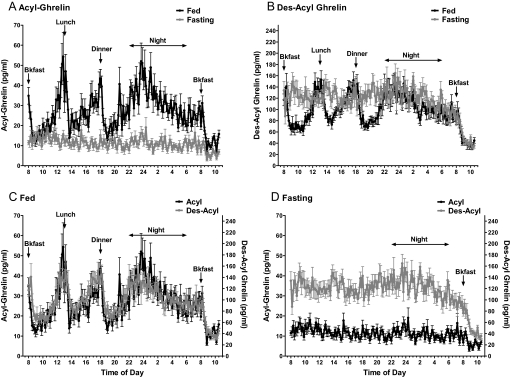
Figure 5 shows the fed and fasting profiles for acyl- and des-acyl ghrelin over the 26.5 h of the sampling protocol (Fig. 1).
Figure 5.
Profiles of plasma ghrelin and des-acyl ghrelin over 26.5 h on fed and fasting admissions, with 10-min sampling, mean ± sem of eight subjects, and each sample assayed in duplicate for both forms of ghrelin. A, Acyl-ghrelin of fed admission (black symbols and black line) and fasting admission (gray symbols and gray line). B, Des-acyl ghrelin of fed admission (black symbols and black line) and fasting admission (gray symbols and gray line). C, Fed acyl-ghrelin (black symbols and black line) and des-acyl ghrelin (gray symbols and gray line). Note that the scales are different, with des-acyl ghrelin present at higher levels. D, Fasting acyl-ghrelin (black symbols and black line) and des-acyl ghrelin (gray symbols and gray line). Bkfast, Breakfast.
Figure 5A shows the pulsatile pattern of acyl-ghrelin levels. During the fed admission, preprandial increases and postprandial suppression of acyl-ghrelin were observed. Marked suppression of acyl-ghrelin was seen in the same subjects on the fasting admission. Note that on the fasting admission, ghrelin did not rise before customary mealtimes, and ghrelin levels remained near the nadir seen on the fed admission. Despite this suppression, there was a further suppression of ghrelin after breaking the 61.5-h fast (P = 0.039, paired t tested for 1-h intervals before and after breakfast).
Figure 5B compares the profiles for des-acyl ghrelin on the fed and fasting admissions. During the fed day, the pattern was similar to that observed for acyl-ghrelin, but the levels (picograms per milliliter) were higher. With fasting, des-acyl ghrelin levels remained at preprandial (peak) levels without the postprandial suppression.
Figure 5C compares acyl- and des-acyl ghrelin on the fed admission. However, the patterns, including response to meals and rise at night, are nearly identical (acyl-ghrelin tends to show somewhat larger percent changes with a suggestion of more extreme peaks and valleys).
Figure 5D compares the acyl- and des-acyl ghrelin profiles on the fasting admission and demonstrates the change in the relative abundance of the two forms on fasting.
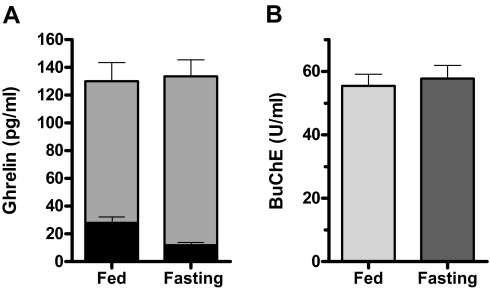
To quantitate the effects of fasting, we compared the 24-h sampling period while fasting (before breaking the fast at 0800 h) with the same time period on the fed admission. A value for each subject was computed as the average of the 144 time points over these 24 h. Figure 6A shows the average of these values for the eight subjects for both ghrelin and des-acyl ghrelin. On the fasting admission, ghrelin was decreased by 58% (27.9 ± 3.9 vs. 11.8 ± 1.7 pg/ml; P = 0.0025), whereas des-acyl ghrelin was increased by 19% (102.2 ± 13.4 vs. 121.9 ± 11.8 pg/ml; P = 0.040). The sum of acyl- plus des-acyl ghrelin was not different (130.1 ± 13.7 vs. 133.7 ± 12.5 pg/ml; P = 0.66).
Figure 6.
A, Average levels of ghrelin (black bars) and des-acyl ghrelin (gray bars) on fed and fasting admissions. B, Average levels of serum BuChE activity on fed and fasting admissions. Activity is in units per milliliter relative to the activity of purified bovine BuChE. In both A and B, each bar represents the average of 144 time points per subject over a matched 24-h period, mean ± sem for eight subjects.
Preliminary analysis suggests that ghrelin secretion is pulsatile and that the pulse mass but not pulse frequency is changed on fasting. But studies using more frequent sampling will be needed to clearly define pulse parameters (see supplemental Fig. 2).
BuChE on fed and fasting admissions
BuChE activity was examined to test the hypothesis that increased circulating activity of this esterase was the cause of the observed decrease in ghrelin acylation seen on fasting. Figure 6B shows that although BuChE activity was present, there was no significant change in activity with fasting (55.5 ± 3.7 fed vs. 57.8 ± 4.1 U/ml fasting; P = 0.13; n = 8; paired t test, 144 samples for each subject over the same time course as in Fig. 6A).
Discussion
Our studies describe the validation of new sandwich assays for measurement of full-length ghrelin and des-acyl ghrelin and examine specific requirements for sample preparation. Details of the assay development are found in the supplemental data. In the fed state, circulating levels of ghrelin and des-acyl ghrelin show similar changes in response to meals and at night. With prolonged fasting, the average daily levels of total ghrelin (full-length acyl-ghrelin plus des-acyl ghrelin) are not different from the fed admission, but the degree of acylation is sharply reduced despite no apparent change in BuChE. This suggests that the ghrelin acylation and secretion processes are controlled independently.
Assays for ghrelin and des-acyl ghrelin
Consistent with improved specificity for the two-site assays, ghrelin profiles show larger relative changes in response to meals than previously seen by single-site assays (29,30). The extensive literature on ghrelin measurements has generally assumed that single-antibody total ghrelin measurements reflect ghrelin activity; the results presented here from fasting subjects demonstrate that this assumption is not always correct and strongly argues for the specific assay of both forms. This is especially important in the face of a growing literature on the unique activities of des-acyl ghrelin.
Akamizu et al. (34), using their own sandwich assays for acyl- and des-acyl ghrelin, concluded that 40–60% of the signal in a standard total ghrelin RIA in plasma was actually from inactive C-terminal ghrelin fragments. Results with our sandwich assays confirm this, showing that the levels of full-length ghrelin are lower than suggested by single-site assay and that the fraction of acyl-ghrelin is higher. Reported values for ghrelin acylation in normal men were 18.0% in Akamizu's study and 22.0% in our study vs. 8.3–9.4% in similar acid-preserved samples assayed by RIA (35,36) and 1.8% acylation in plasma assayed by RIA in older studies without appropriate sample preservation (15).
Our results document the importance of measures to protect ghrelin acylation. The half-life of endogenous acyl-ghrelin in blood without AEBSF found here (2.9 h at room temperature) is comparable to that reported by De Vriese et al. (12) (∼4 h at 37 C), who added synthetic ghrelin(1–23) or ghrelin(1–28) to human serum and analyzed the acylation levels by HPLC.
Changes in ghrelin and des-acyl ghrelin in the fed state and during prolonged fasting
On the fed admission, ghrelin levels varied markedly with rises preprandially and at night. Under these conditions, ghrelin and des-acyl ghrelin levels changed in parallel with sharp declines on feeding, but the percentage of the circulating hormone that was acylated remained fairly constant (22.0 ± 0.3%, mean ± sem; sd = 3.8%). During the fasting admission, the levels of des-acyl ghrelin were near the peak levels observed during the fed admission, whereas acyl-ghrelin levels were near the nadirs observed on the fed day, and the percent acylation dropped (9.9 ± 0.2%; sd = 2.7%). The sum of acyl- plus des-acyl ghrelin was no different on the fasting than on the fed admission. This is consistent with other studies that found that in contrast to shorter fasts, on long-term fasting, total ghrelin levels were not increased (37,38,39,40). The dissociation between changes in ghrelin and des-acyl ghrelin seen in this study demonstrates the importance of measuring both forms simultaneously.
During fasting, the balance of ghrelin to des-acyl ghrelin was altered. This ratio has been proposed to determine ghrelin's overall adipogenic action (18) and may have other consequences related to the specific actions of des-acyl ghrelin. Our results suggest separate regulation of ghrelin secretion and ghrelin acylation. Consistent with independent mechanisms, the breakfast that was served after 61.5 h of fasting further suppressed ghrelin levels below the maximal suppression caused by fasting and also sharply suppressed des-acyl ghrelin (Fig. 5).
The change in the relative amounts of ghrelin and des-acyl ghrelin could be caused by either an inhibition of the acylation of ghrelin as it is synthesized (a process not well understood, see Note Added in Proof) (10) or an increase in the activity of ghrelin esterases. We have shown that serum BuChE, a significant ghrelin esterase in human serum (12), was not increased during fasting, but this does not rule out the possible contribution of other esterases, especially in tissues such as liver.
Interestingly, the data of Kim et al. (41) suggest that the decrease in ghrelin during the fasting admission might be counterbalanced by an increase in central ghrelin receptor expression, showing that in rodents, 48-h fasting resulted in an 8-fold increase in hypothalamic ghrelin receptor expression. This suggests that the changes in circulating ghrelin levels do not necessarily reflect the actual changes in its biological activity.
During fasting, we did not see changes in ghrelin levels around customary mealtimes. Such changes were seen in a study of a 33-h fast (39) but may not have been apparent here because we did not initiate sampling until 37.5 h of fasting.
Regulation of acylation
These new sandwich assays show that with normal feeding, ghrelin and des-acyl ghrelin rise and fall together in response to meals. But on long-term fasting, the proportion of ghrelin that is acylated falls, with no change in the total levels of circulating full-length ghrelin. Thus, under these conditions, the balance between ghrelin and des-acyl ghrelin may be changed by the regulation of ghrelin acylation. Toshinai et al. (42) report that in the stomach of rats fasted for 48 h, “the ratio of des-octanoylated ghrelin to n-octanoylated ghrelin markedly increased after fasting.” This result is consistent with our findings, and together these data suggest that long-term fasting regulates ghrelin activity by a mechanism that inhibits the addition of the acyl group as ghrelin is synthesized. It can be speculated that X/A cells require an intraluminal nutrient source to allow acylation. This is consistent with observations that fatty acids from the diet can be directly used for ghrelin acylation (43).
Conclusions
Our results suggest that acyl-ghrelin levels decline with long-term fasting, whereas des-acyl ghrelin levels are increased. It is interesting that increased appetite occurs during short-term fasting but wanes over time; this may relate to the lowering of acyl-ghrelin levels seen here. New assays that specifically measure the full-length forms of ghrelin and des-acyl ghrelin will be crucial to defining ghrelin's physiological role and delineating its regulation.
Note Added in Proof
Following acceptance of this manuscript. Yang et al. published the identification of the acyltransferase that octanoylates ghrelin [Cell 132:387–396, February 8, 2008 (44)]. Regulation of this enzyme could be a mechanism controlling the changes in ghrelin acylation levels seen in the present study.
Supplementary Material
Acknowledgments
We thank the University of Virginia GCRC nursing staff for their excellent support when conducting this study as well as the GCRC Core Laboratory staff and our volunteers who made this work possible. We also thank Dr. Mark L. Heiman for his generous help obtaining antisera.
Footnotes
This study was supported in part by National Institutes of Health Grants MO1 RR 00847 (to the GCRC at the University of Virginia), RO1 DK32632 (to M.O.T.), K23 RR018770 (to R.N.), an unrestricted grant from Bristol-Myers Squibb, and a gift to the laboratory by Mr. Salvatore Ranieri.
Disclosure Information: The authors have declared that no conflicts of interest exist. D.A.G reports a financial interest in Bristol-Myers Squibb. M.O.T. reports he is a paid consultant for Novo Nordisk and Tercica. Part of this work was supported by an unrestricted grant from Bristol-Myers Squibb.
First Published Online March 18, 2008
See article p. 1979
Abbreviations: AEBSF, 4-[2-Aminoethyl benzene] sulfonyl fluoride; BMI, body mass index; BuChE, butyrylcholinesterase; CV, coefficient of variation; GCRC, General Clinical Research Center.
References
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K 1999 Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402:656–660 [DOI] [PubMed] [Google Scholar]
- Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, Matsukura S, Kangawa K, Nakazato M 2000 Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology 141:4255–4261 [DOI] [PubMed] [Google Scholar]
- Takaya K, Ariyasu H, Kanamoto N, Iwakura H, Yoshimoto A, Harada M, Mori K, Komatsu Y, Usui T, Shimatsu A, Ogawa Y, Hosoda K, Akamizu T, Kojima M, Kangawa K, Nakao K 2000 Ghrelin strongly stimulates growth hormone release in humans. J Clin Endocrinol Metab 85:4908–4911 [DOI] [PubMed] [Google Scholar]
- Tschöp M, Smiley DL, Heiman ML 2000 Ghrelin induces adiposity in rodents. Nature 407:908–913 [DOI] [PubMed] [Google Scholar]
- Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S 2001 A role for ghrelin in the central regulation of feeding. Nature 409:194–198 [DOI] [PubMed] [Google Scholar]
- Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S, Kennedy AR, Roberts GH, Morgan DG, Ghatei MA, Bloom SR 2000 The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology 141:4325–4328 [DOI] [PubMed] [Google Scholar]
- Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, Dhillo WS, Ghatei MA, Bloom SR 2001 Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab 86:5992–5995 [DOI] [PubMed] [Google Scholar]
- Choi K, Roh SG, Hong YH, Shrestha YB, Hishikawa D, Chen C, Kojima M, Kangawa K, Sasaki S 2003 The role of ghrelin and growth hormone secretagogues receptor on rat adipogenesis. Endocrinology 144:754–759 [DOI] [PubMed] [Google Scholar]
- Hosoda H, Kojima M, Mizushima T, Shimizu S, Kangawa K 2003 Structural divergence of human ghrelin. Identification of multiple ghrelin-derived molecules produced by post-translational processing. J Biol Chem 278:64–70 [DOI] [PubMed] [Google Scholar]
- Zhu X, Cao Y, Voodg K, Steiner DF 2006 On the processing of proghrelin to ghrelin. J Biol Chem 281:38867–38870 [DOI] [PubMed] [Google Scholar]
- Bednarek MA, Feighner SD, Pong SS, McKee KK, Hreniuk DL, Silva MV, Warren VA, Howard AD, Van Der Ploeg LH, Heck JV 2000 Structure-function studies on the new growth hormone-releasing peptide, ghrelin: minimal sequence of ghrelin necessary for activation of growth hormone secretagogue receptor 1a. J Med Chem 43:4370–4376 [DOI] [PubMed] [Google Scholar]
- De Vriese C, Gregoire F, Lema-Kisoka R, Waelbroeck M, Robberecht P, Delporte C 2004 Ghrelin degradation by serum and tissue homogenates: identification of the cleavage sites. Endocrinology 145:4997–5005 [DOI] [PubMed] [Google Scholar]
- Beaumont NJ, Skinner VO, Tan TM, Ramesh BS, Byrne DJ, MacColl GS, Keen JN, Bouloux PM, Mikhailidis DP, Bruckdorfer KR, Vanderpump MP, Srai KS 2003 Ghrelin can bind to a species of high density lipoprotein associated with paraoxonase. J Biol Chem 278:8877–8880 [DOI] [PubMed] [Google Scholar]
- Shanado Y, Kometani M, Uchiyama H, Koizumi S, Teno N 2004 Lysophospholipase I identified as a ghrelin deacylation enzyme in rat stomach. Biochem Biophys Res Commun 325:1487–1494 [DOI] [PubMed] [Google Scholar]
- Hosoda H, Kojima M, Matsuo H, Kangawa K 2000 Ghrelin and des-acyl ghrelin: two major forms of rat ghrelin peptide in gastrointestinal tissue. Biochem Biophys Res Commun 279:909–913 [DOI] [PubMed] [Google Scholar]
- Cassoni P, Papotti M, Ghe C, Catapano F, Sapino A, Graziani A, Deghenghi R, Reissmann T, Ghigo E, Muccioli G 2001 Identification, characterization, and biological activity of specific receptors for natural (ghrelin) and synthetic growth hormone secretagogues and analogs in human breast carcinomas and cell lines. J Clin Endocrinol Metab 86:1738–1745 [DOI] [PubMed] [Google Scholar]
- Bedendi I, Alloatti G, Marcantoni A, Malan D, Catapano F, Ghe C, Deghenghi R, Ghigo E, Muccioli G 2003 Cardiac effects of ghrelin and its endogenous derivatives des-octanoyl ghrelin and des-Gln14-ghrelin. Eur J Pharmacol 476:87–95 [DOI] [PubMed] [Google Scholar]
- Thompson NM, Gill DA, Davies R, Loveridge N, Houston PA, Robinson IC, Wells T 2004 Ghrelin and des-octanoyl ghrelin promote adipogenesis directly in vivo by a mechanism independent of the type 1a growth hormone secretagogue receptor. Endocrinology 145:234–242 [DOI] [PubMed] [Google Scholar]
- Kleinz MJ, Maguire JJ, Skepper JN, Davenport AP 2006 Functional and immunocytochemical evidence for a role of ghrelin and des-octanoyl ghrelin in the regulation of vascular tone in man. Cardiovasc Res 69:227–235 [DOI] [PubMed] [Google Scholar]
- Dixit VD, Schaffer EM, Pyle RS, Collins GD, Sakthivel SK, Palaniappan R, Lillard Jr JW, Taub DD 2004 Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J Clin Invest 114:57–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toshinai K, Yamaguchi H, Sun YX, Smith RG, Yamanaka A, Sakurai T, Date Y, Mondal MS, Shimbara T, Kawagoe T, Murakami N, Miyazato M, Kangawa K, Nakazato M 2006 Des-acyl ghrelin induces food intake by a mechanism independent of the growth hormone secretagogue receptor. Endocrinology 147:2306–2314 [DOI] [PubMed] [Google Scholar]
- Gauna C, Delhanty PJ, van Aken MO, Janssen JA, Themmen AP, Hofland LJ, Culler M, Broglio F, Ghigo E, van der Lely AJ 2006 Unacylated ghrelin is active on the INS-1E rat insulinoma cell line independently of the growth hormone secretagogue receptor type 1a and the corticotropin releasing factor 2 receptor. Mol Cell Endocrinol 251:103–111 [DOI] [PubMed] [Google Scholar]
- Gauna C, Meyler FM, Janssen JA, Delhanty PJD, Abribat T, van Koetsveld P, Hofland LJ, Broglio F, Ghigo E, van der Lely AJ 2004 Administration of acylated ghrelin reduces insulin sensitivity, whereas the combination of acylated plus unacylated ghrelin strongly improves insulin sensitivity. J Clin Endocrinol Metab 89:5035–5042 [DOI] [PubMed] [Google Scholar]
- Nussbaum SR, Zahradnik RJ, Lavigne JR, Brennan GL, Nozawa-Ung K, Kim LY, Keutmann HT, Wang CA, Potts Jr JT, Segre GV 1987 Highly sensitive two-site immunoradiometric assay of parathyrin, and its clinical utility in evaluating patients with hypercalcemia. Clin Chem 33:1364–1367 [PubMed] [Google Scholar]
- Patterson M, Murphy KG, le Roux CW, Ghatei MA, Bloom SR 2005 Characterization of ghrelin-like immunoreactivity in human plasma. J Clin Endocrinol Metab 90:2205–2211 [DOI] [PubMed] [Google Scholar]
- De Vriese C, Hacquebard M, Gregoire F, Carpentier Y, Delporte C 2007 Ghrelin interacts with human plasma lipoproteins. Endocrinology 148:2355–2362 [DOI] [PubMed] [Google Scholar]
- Groschl M, Uhr M, Kraus T 2004 Evaluation of the comparability of commercial ghrelin assays. Clin Chem 50:457–458 [DOI] [PubMed] [Google Scholar]
- Hotta M, Ohwada R, Katakami H, Shibasaki T, Hizuka N, Takano K 2004 Plasma levels of intact and degraded ghrelin and their responses to glucose infusion in anorexia nervosa. J Clin Endocrinol Metab 89:5707–5712 [DOI] [PubMed] [Google Scholar]
- Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS 2001 A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 50:1714–1719 [DOI] [PubMed] [Google Scholar]
- Tschöp M, Wawarta R, Riepl RL, Friedrich S, Bidlingmaier M, Landgraf R, Folwaczny C 2001 Post-prandial decrease of circulating human ghrelin levels. J Endocrinol Invest 24:RC19–RC21 [DOI] [PubMed] [Google Scholar]
- Liu J, Gaylinn BD, Calore JD, Weiner RS, Gordon DA, Thorner MO 2004 A new enzyme-linked immunoassay for acylated and des-acylated ghrelin in plasma. Growth Horm IGF Res 14:131 (Abstract) [Google Scholar]
- Ellman GL, Courtney KD, Andres V, Featherstone RM 1961 A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–90 [DOI] [PubMed] [Google Scholar]
- Hosoda H, Doi K, Nagaya N, Okumura H, Nakagawa E, Enomoto M, Ono F, Kangawa K 2004 Optimum collection and storage conditions for ghrelin measurements: octanoyl modification of ghrelin is rapidly hydrolyzed to desacyl ghrelin in blood samples. Clin Chem 50:1077–1080 [DOI] [PubMed] [Google Scholar]
- Akamizu T, Shinomiya T, Irako T, Fukunaga M, Nakai Y, Kangawa K 2005 Separate measurement of plasma levels of acylated and desacyl ghrelin in healthy subjects using a new direct ELISA assay. J Clin Endocrinol Metab 90:6–9 [DOI] [PubMed] [Google Scholar]
- Yoshimoto A, Mori K, Sugawara A, Mukoyama M, Yahata K, Suganami T, Takaya K, Hosoda H, Kojima M, Kangawa K, Nakao K 2002 Plasma ghrelin and desacyl ghrelin concentrations in renal failure. J Am Soc Nephrol 13:2748–2752 [DOI] [PubMed] [Google Scholar]
- Nakai Y, Hosoda H, Nin K, Ooya C, Hayashi H, Akamizu T, Kangawa K 2004 Short-term secretory regulation of the active form of ghrelin and total ghrelin during an oral glucose tolerance test in patients with anorexia nervosa. Eur J Endocrinol 150:913–914 [DOI] [PubMed] [Google Scholar]
- Chan JL, Bullen J, Lee JH, Yiannakouris N, Mantzoros CS 2004 Ghrelin levels are not regulated by recombinant leptin administration and/or three days of fasting in healthy subjects. J Clin Endocrinol Metab 89:335–343 [DOI] [PubMed] [Google Scholar]
- Liu J, Gaylinn BD, Nass RM, Pezzoli SS, Clancy MA, Thorner MO, Effects of prolonged fasting on circulating ghrelin levels in healthy young men. Program of the 86th Annual Meeting of The Endocrine Society, New Orleans, LA, 2004, p 166 (Abstract P1-49) [Google Scholar]
- Natalucci G, Riedl S, Gleiss A, Zidek T, Frisch H 2005 Spontaneous 24-h ghrelin secretion pattern in fasting subjects: maintenance of a meal-related pattern. Eur J Endocrinol 152:845–850 [DOI] [PubMed] [Google Scholar]
- Espelund U, Hansen TK, Hojlund K, Beck-Nielsen H, Clausen JT, Hansen BS, Orskov H, Jorgensen JO, Frystyk J 2005 Fasting unmasks a strong inverse association between ghrelin and cortisol in serum: studies in obese and normal-weight subjects. J Clin Endocrinol Metab 90:741–746 [DOI] [PubMed] [Google Scholar]
- Kim MS, Yoon CY, Park KH, Shin CS, Park KS, Kim SY, Cho BY, Lee HK 2003 Changes in ghrelin and ghrelin receptor expression according to feeding status. Neuroreport 14:1317–1320 [DOI] [PubMed] [Google Scholar]
- Toshinai K, Mondal MS, Nakazato M, Date Y, Murakami N, Kojima M, Kangawa K, Matsukura S 2001 Up-regulation of ghrelin expression in the stomach upon fasting, insulin-induced hypoglycemia, and leptin administration. Biochem Biophys Res Commun 281:1220–1225 [DOI] [PubMed] [Google Scholar]
- Nishi Y, Hiejima H, Hosoda H, Kaiya H, Mori K, Fukue Y, Yanase T, Nawata H, Kangawa K, Kojima M 2005 Ingested medium-chain fatty acids are directly utilized for the acyl modification of ghrelin. Endocrinology 146:2255–2264 [DOI] [PubMed] [Google Scholar]
- Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL 2008 Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell 132:387–396 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.