Abstract
Leptospirosis, caused by the spirochete Leptospira, is a geographically widespread disease that affects a broad range of mammals, including marine mammals. Among pinniped populations, periodic epizootics of leptospirosis are responsible for significant die-offs. Along the west coast of North America, the most recent leptospirosis epizootic occurred in 2004, during which samples were collected from cases ranging from California to British Columbia. The primary objective of this study was to use this well-defined sample set to determine the feasibility of using PCR techniques to diagnose Leptospira infection among pinniped populations in comparison with diagnostic methodologies commonly used for marine mammals. Successful amplification was achieved from a variety of samples, including freshly collected urine, urine stored at −80°C for less than 6 months, and kidney (freshly collected, frozen, and decomposed), as well as feces- and urine-contaminated sand collected in the vicinity of a live-stranded animal. Pathological examination of tissue collected from Leptospira-infected animals revealed the presence of leptospiral antigen in the kidneys. The use of species-specific primer pairs revealed a pattern of host specificity for Leptospira interrogans in sea lions and Leptospira kirschneri in elephant seals. These studies indicate PCR is a sensitive and specific diagnostic tool for the detection of Leptospira infection in pinnipeds and reveal a potential source for epizootic, enzootic, and zoonotic spread of leptospirosis in a marine environment.
Leptospirosis is a ubiquitous disease with a global distribution that affects humans and a wide variety of domestic and wild animal species, including marine mammals such as California sea lions (Zalophus californianus) (7), northern fur seals (Callorhinus ursinus) (10), northern elephant seals (Mirounga angustirostris) (2), and harbor seals (Phoca vitulina) (11). Leptospirosis typically presents in these animals as interstitial nephritis with clinical signs of impaired renal function, including dehydration, polydipsia, vomiting, and depression (7). Disease outbreaks have occurred repeatedly in California sea lions off central and northern California, with hundreds of animals stranding and subsequently dying in each outbreak (1, 7). These large-scale epizootics are cyclic and have been recognized since the early 1970s, with a distinct 3- to 4-year periodicity that is separated by enzootic maintenance of the disease (3, 4, 7, 14). During the most recent outbreak in 2004, over 300 sea lions died along the central California coast and further mortalities were tracked off the coasts of Oregon, Washington, and British Columbia.
Current methods for diagnosing leptospirosis among live marine mammals rely upon a combination of the microscopic agglutination test (MAT) performed on sera, clinical observations, and detection of serum biochemistry changes typical of renal failure (1). If animals are dead, additional diagnostic tools include histopathology and immunohistochemistry, both of which may indicate the presence of Leptospira and, in conjunction with clinical chemistry, clinical signs, or necropsy findings, determine the extent of disease. Culture of organisms is possible from urine and harvested kidney of live and dead animals; however, as these organisms are fastidious and require selective media, bacterial isolation is not used as a routine diagnostic tool. Furthermore, without paired serum samples, MAT cannot distinguish an acute active infection from a previous recent infection. The use of molecular analytical techniques such as PCR has been established in terrestrial animals (5, 15, 16) and is ideally suited for the detection of Leptospira infection, in that PCR technology is sensitive, specific, widely available, and can be reliably performed on a range of templates, including urine and renal tissue. The studies reported herein evaluate the use of PCR technology for assessing Leptospira infection among marine mammal populations compared to conventional diagnostic methodologies.
MATERIALS AND METHODS
Animals and samples.
Live animals that stranded along the California coast were admitted to The Marine Mammal Center and examined clinically and, if they died, at postmortem as described by Greig et al. (6). Fresh urine samples were collected from stranded, captive, and wild animals by established methods (free flow capture, cystocentesis, or catheterization), separated into 1- to 3-ml aliquots, and stored at 4°C prior to analysis. Serum samples were collected from the caudal gluteal vein and used for serum biochemistry and MAT testing as described by Colagrass-Schouten et al. (1). The cutoff for a positive agglutination reaction was defined as a titer of ≥1:400 in a single sample. If animals died or were euthanized due to poor prognosis, gross necropsies were performed within 12 h of death. Gross necropsy findings consistent with leptospirosis included swollen kidneys, loss of renicular differentiation, pale tan cortices, or subcapsular hemorrhages and serum biochemistry results indicative of renal failure (blood urea nitrogen of >100 mg/dl, creatinine of >2 mg/dl, sodium of >155 meq/liter, and phosphorus > calcium) (1, 7). At necropsy, kidney tissue and urine samples were collected aseptically and frozen immediately at −80°C; samples 53 to 61 (Table 1) remained at −80°C for greater than 6 months. Tissue samples from multiple organs were fixed in 10% neutral buffered formalin, processed routinely for paraffin embedding, sectioned at 5 μm, and stained with hematoxylin and eosin. Representative slides were also prepared with Warthin-Starry and Gram stain (9). Immunohistochemical staining was performed on kidney sections using an established streptavidin-biotin staining protocol (12). Sections were incubated at room temperature with an anti-Leptospira polyclonal antibody (National Veterinary Services Laboratory, Ames, IA) at a 1:40,000 dilution. The antibody was directed against L. interrogans serovars Bratislava, Canicola, Hardjo, Icterohemorrhagiae, and Pomona and L. kirschneri serovar Grippotyphosa. Appropriate positive and negative controls were included in all cases.
TABLE 1.
Comparison of diagnostic methodologies for identification of Leptospira infection
| Casea | Accession no. | Species | Sample origin | PCR result/template | Clinical observation(s) consistent with leptospirosis | Serology | Histopathology | Immunohistochemistry | Status/primary cause of death or working diagnosis |
|---|---|---|---|---|---|---|---|---|---|
| Test samples | |||||||||
| 1 | ES2339 | Elephant seal | CA | + (L. kirschneri)/urine | Yes | NAb | Tubulointerstitial nephritis | Positive | Dead/leptospirosis |
| 2 | CSL6119 | CA sea lion | CA | + (L. interrogans)/urine | Yes | Positive | Tubulointerstitial nephritis | Positive | Dead/leptospirosis |
| 3 | CSL6146 | CA sea lion | CA | + (L. interrogans)/urine | Yes | Positive | Tubulointerstitial nephritis | Positive | Dead/leptospirosis |
| 4 | CSL6144 | CA sea lion | CA | + (L. interrogans)/urine/kidney | Yes | Positive | Tubulointerstitial nephritis | Positive | Dead/leptospirosis |
| 5 | CSL6139 | CA sea lion | CA | + (L. interrogans)/urine | Yes | NA | Tubulointerstitial nephritis | Positive | Dead/leptospirosis |
| 6 | CSL6079 | CA sea lion | CA | + (L. interrogans)/urine | No | Negative | Minimal nephritis | Negative | Dead/encephalomalcia |
| 7 | CSL6257 | CA sea lion | CA | + (L. interrogans)/urine | Yes | Positive | Tubulointerstitial nephritis | Positive (marked) | Dead/leptospirosis |
| 8 | CSL6138 | CA sea lion | CA | + (L. interrogans)/urine | No | Negative | Minimal pyelitis | Negative | Dead/trauma |
| 9 | CSL6175 | CA sea lion | CA | + (L. interrogans)/urine | Yes | Positive | Tubulointerstitial nephritis | Positive | Dead/leptospirosis |
| 10 | CSL6214 | CA sea lion | CA | + (L. interrogans)/urine | Yes | Positive | Tubulointerstitial nephritis | Positive | Dead/leptospirosis |
| 11 | CSL6263 | CA sea lion | CA | + (L. interrogans)/urine | No | Negative | Minimal nephritis | Negative | Dead/trauma |
| 12 | CSL6218 | CA sea lion | CA | + (L. interrogans)/urine | Yes | Positive | Tubulointerstitial nephritis | Positive | Dead/leptospirosis |
| 13 | CSL6250 | CA sea lion | CA | + (L. interrogans)/urine | Yes | Positive | Tubulointerstitial nephritis | Positive | Dead/leptospirosis |
| 14 | CSL6210 | CA sea lion | CA | + (L. interrogans)/urine | Yes | Positive | Tubulointerstitial nephritis | Positive | Dead/leptospirosis |
| 15 | CSL6187 | CA sea lion | CA | + (L. interrogans)/urine | Yes | NA | Tubulointerstitial nephritis | Positive | Dead/leptospirosis |
| 16 | CSL6176 | CA sea lion | CA | + (L. interrogans)/urine | Yes | Positive | Tubulointerstitial nephritis | Positive | Dead/leptospirosis |
| 17 | CSL6205 | CA sea lion | CA | + (L. interrogans)/urine | Yes | Positive | Tubulointerstitial nephritis | Positive | Dead/leptospirosis |
| 18 | CSL6308 | CA sea lion | CA | + (L. interrogans)/urine | Yes | Positive | NA | NA | Dead/leptospirosis |
| 19 | CSL6338 | CA sea lion | CA | + (L. interrogans)/urine | Yes | Positive | NA | NA | Released/leptospirosis |
| 20 | CSL6344 | CA sea lion | CA | + (L. interrogans)/urine | Yes | Positive | NA | NA | Released/leptospirosis |
| 21 | CSL6378 | CA sea lion | CA | + (L. interrogans)/urine | Yes | Positive | NA | NA | Released/leptospirosis |
| 22 | NA | CA sea lion | British Columbia | + (L. interrogans)/kidney | Yes | Positive | Tubulointerstitial nephritis | Positive | Dead/leptospirosis |
| 23 | ZL-NPP-04-01 | CA sea lion | WA | + (L. interrogans)/kidney | Yes | NA | NA | NA | Dead/leptospirosis |
| 24 | WDFW 0904-04 | CA sea lion | WA | + (L. interrogans)/urine | Yes | Positive | Interstitial nephritis | NA | Dead/leptospirosis |
| 25 | WDFW 0804-10 | Steller/CA sea lion cross | WA | + (L. interrogans)/urine | Yes | Positive | Interstitial nephritis | NA | Dead/leptospirosis |
| 26 | WDFW 0904-09 | CA sea lion | WA | + (L. interrogans)/urine | Yes | Positive | Interstitial nephritis | NA | Dead/leptospirosis |
| 27 | WDFW 1004-01 | CA sea lion | WA | + (L. interrogans)/urine | Yes | Positive | Interstitial nephritis | NA | Dead/leptospirosis |
| 28 | WDFW 1004-02 | Steller sea lion | WA | + (L. interrogans)/feces/urine/sand | Yes | NA | NA | NA | Unknown/leptospirosis |
| 29 | WDFW 1004-14 | CA sea lion | WA | + (L. interrogans)/urine | Yes | Positive | Interstitial nephritis | NA | Dead/leptospirosis |
| 30 | WDFW 1004-10 | CA sea lion | WA | + (L. interrogans)/urine | Yes | NA | NA | NA | Dead/leptospirosis |
| 31 | WDFW 1004-15 | CA sea lion | WA | + (L. interrogans)/kidney | NA | NA | Interstitial nephritis | NA | Dead/leptospirosis |
| 32 | CWB-04-ZC-02 | CA sea lion | WA | + (L. interrogans)/kidney | NA | NA | NA | NA | Dead/trauma (moderate decomposition of carcass) |
| 33 | WDFW 1004-16 | CA sea lion | WA | + (L. interrogans)/kidney | NA | NA | NA | NA | Dead/unknown (moderate decomposition of carcass) |
| 34 | WDFW 1004-11 | CA sea lion | WA | + (L. interrogans)/kidney | NA | NA | NA | NA | Dead/uknown (moderate decomposition of carcass) |
| 35 | WDFW 1004-06 | CA sea lion | WA | -/urine | Yes | Negative | Tubulointerstitial nephritis | NA | Dead/unknown |
| Samples from healthy animals | |||||||||
| 36 | CSL LML male | CA sea lion | CA | -/urine | No | NA | NA | NA | Alive, captive born |
| 37 | CSL LML female | CA sea lion | CA | -/urine | No | NA | NA | NA | Alive, captive born |
| 38 | WCSL ZC939 | CA sea lion | CA | -/urine | No | NA | NA | NA | Alive, wild sea lion |
| 39 | WCSL ZC1043 | CA sea lion | CA | -/urine | No | NA | NA | NA | Alive, wild sea lion |
| 40 | WCSL ZC1072 | CA sea lion | CA | -/urine | No | NA | NA | NA | Alive, wild sea lion |
| 41 | WCSL ZC1074 | CA sea lion | CA | -/urine | No | NA | NA | NA | Alive, wild sea lion |
| 42 | WCSL ZC1077 | CA sea lion | CA | -/urine | No | NA | NA | NA | Alive, wild sea lion |
| 43 | WCSL ZC1079 | CA sea lion | CA | -/urine | No | NA | NA | NA | Alive, wild sea lion |
| 44 | WCSL ZC845 | CA sea lion | CA | -/urine | No | NA | NA | NA | Alive, wild sea lion |
| 45 | WCSL ZC870 | CA sea lion | CA | -/urine | No | NA | NA | NA | Alive, wild sea lion |
| 46 | WCSL ZC952 | CA sea lion | CA | -/urine | No | NA | NA | NA | Alive, wild sea lion |
| 47 | WCSL ZC954 | CA sea lion | CA | -/urine | No | NA | NA | NA | Alive, wild sea lion |
| 48 | WCSL ZC956 | CA sea lion | CA | -/urine | No | NA | NA | NA | Alive, wild sea lion |
| 49 | WCSL ZC958 | CA sea lion | CA | -/urine | No | NA | NA | NA | Alive, wild sea lion |
| 50 | WCSL ZC960 | CA sea lion | CA | -/urine | No | NA | NA | NA | Alive, wild sea lion |
| 51 | WCSL ZC962 | CA sea lion | CA | -/urine | No | NA | NA | NA | Alive, wild sea lion |
| 52 | WCSL ZC963 | CA sea lion | CA | -/urine | No | NA | NA | NA | Alive, wild sea lion |
| Stored urine samples | |||||||||
| 53 | CSL6707 | CA sea lion | CA | -/urine | Yes | Positive | NA | NA | Released/leptospirosis |
| 54 | CSL6797 | CA sea lion | CA | +(L. interrogans)/urine | Yes | Positive | NA | NA | Dead/leptospirosis |
| 55 | CSL6809 | CA sea lion | CA | +(L. interrogans)/urine | Yes | Positive | NA | NA | Dead/leptospirosis |
| 56 | CSL6807 | CA sea lion | CA | -/urine | Yes | Positive | NA | NA | Released/leptospirosis |
| 57 | CSL6820 | CA sea lion | CA | -/urine | Yes | Positive | NA | NA | Released/leptospirosis |
| 58 | CSL6645 | CA sea lion | CA | -/urine | Yes | Positive | NA | NA | Released/leptospirosis |
| 59 | CSL6818 | CA sea lion | CA | -/urine | Yes | Positive | NA | NA | Dead/leptospirosis |
| 60 | CSL6824 | CA sea lion | CA | -/urine | Yes | Positive | NA | NA | Dead/leptospirosis |
| 61 | CSL6828 | CA sea lion | CA | -/urine | Yes | Positive | NA | NA | Dead/leptospirosis |
Cases 6, 8, and 11 represent potential carrier animals. Cases 36 to 52 represent samples taken from healthy captive (cases 36 and 37) and wild (cases 38 to 52) animals. Cases 53 to 61 represent samples stored at −80°C for >6 months.
NA, not available.
Animals that live-stranded along the Washington coast were observed for clinical signs of leptospirosis (dehydration, polydipsia, emaciation, and depression), and, if possible, agonal or postmortem urine and serum samples were collected. In one live-stranded sea lion that returned to the ocean prior to sample collection, feces- and urine-contaminated sand in the vicinity of the stranded animal was collected and analyzed. For animals along the British Columbia and Washington coasts that were found dead or were euthanized due to poor prognosis, tissue, serum, and urine samples were collected. In three instances, dead animals found along the Washington coast had moderate levels of decomposition. Histopathology and serology analyses and interpretations were performed as described above.
DNA isolation.
Total DNA was prepared from samples collected from the captive, wild, live-stranded, dead, and euthanized animals. Isolation of total DNA from renal tissue and feces- and urine-contaminated sand was accomplished using the Qiagen DNeasy kit (Qiagen, Valencia, CA). Isolation of total DNA from urine was performed by the method described by Zuerner et al. (15). Briefly, 1 ml of urine was concentrated by centrifugation (16,100 × g, 20 min). The resulting pellet was resuspended in an equal volume of 1 mM EDTA, concentrated, washed with an equal volume of distilled water, and subjected to a final concentration step prior to resuspension in 50 μl of distilled water.
PCR analyses. (i) Species-specific Leptospira PCR amplification.
To specifically amplify the species Leptospira interrogans, primers unique to the IS1500 insertion sequence (16) were used (P1, 5′-TTCGATTCAAAGCATGGCTAACG-3′; M16, 5′-AAAGAAGGACTCAGCGACTGCG-3′) with a two-step amplification protocol: 7 cycles of 94°C for 30 s and 72°C for 2 min and 35 cycles of 94°C for 30 s and 67°C for 2 min. To specifically amplify the species Leptospira kirschneri, the flagellum-specific primers B64-I (5′-ACTAACTGAGAAACTTCTAC-3′) and B64-II (5′-TCCTTAAGTCGAACCTATGA-3′) were used (5). The conditions for this amplification protocol were as follows: 1 cycle of 94°C for 2 min and 72°C for 3 min and 35 cycles of 94°C for 1.5 min, 55°C for 1 min, and 72°C for 2 min. Control amplification templates included water as a negative control and L. interrogans and L. kirschneri genomic DNA as positive controls. Amplified products were separated on 1% agarose gels, stained with ethidium bromide, and viewed using a UV light source.
RESULTS
Table 1 summarizes the data collection and sample analyses performed on the animals in our study. Cases 1 to 35 represent test samples. Cases 36 to 52 include samples collected from healthy captive animals (cases 36 and 37) and healthy wild animals (cases 38 to 52) to determine the rate of false-negative PCR amplifications, and test cases 53 to 61 comprise urine samples stored at −80°C for greater than 6 months to test for sample stability during long-term storage.
Clinical observations, serology, pathology, and immunohistochemistry.
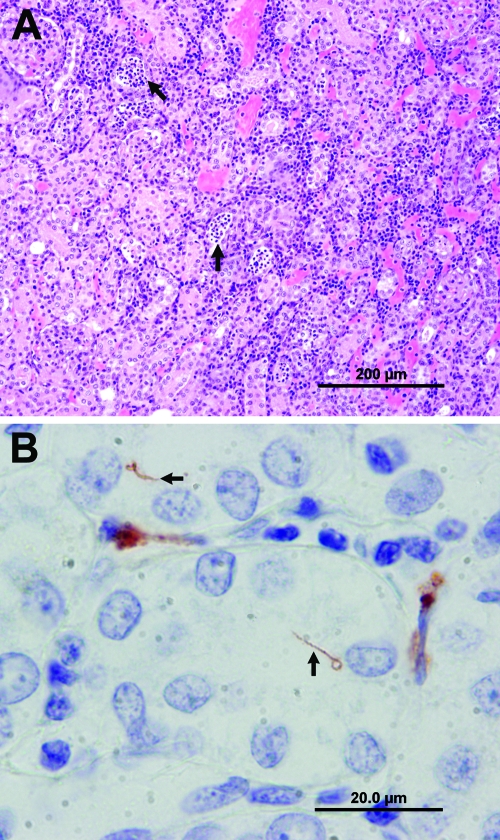
Clinical observations recorded for cases 1 to 5, 7, 9, 10, 12 to 30, 35, and 53 to 61 were consistent with leptospirosis, and serology performed on cases 2 to 4, 6 to 14, 16 to 22, 24 to 27, 29, and 35 were positive, with the exception of cases 6, 8, 11, and 35. The cause of death in sea lions 6, 8, 11, and 32 was determined to be secondary to trauma (cases 8, 11, and 32) and encephalomalacia (case 6). The cause of death in three animals was unknown (cases 33 to 35), with the latter case demonstrating clinical observations and pathological findings consistent with renal failure. Gross necropsy findings in cases 1 to 5, 7, 9, 10, 12 to 17, 27, 54, 55, and 59 to 61 included markedly swollen kidneys with pale tan cortices. There was loss of renicular and corticomedullary differentiation with occasional infarcts, consistent with nephritis. Histopathologic examination of samples collected from cases 1 to 5, 7, 9, 10, 12 to 17, 22, 24 to 27, 29, 31, and 35 suggested that leptospirosis was the cause of death as lesions were similar to those reported in past leptospirosis outbreaks in pinnipeds (1, 2, 11) (lymphoplasmacytic tubulointerstitial nephritis of various severities, with tubular degeneration, necrosis, and regeneration) (Fig. 1A). In sea lions 6, 8, and 11, renal lesions were limited to a few scattered aggregates of lymphocytes in the cortical interstitium and renal pelvis. Immunohistochemistry results for cases 1 to 17 and 22 were positive, with the exception of cases 6, 8, and 11, where no antigen staining was observed. Positive samples showed antigen within the lumen renal tubules, within the cytoplasm of renal tubular epithelia, and interspersed within associated peritubular inflammatory cells and directly correlated with sea lions that exhibited kidney tubulointerstitial nephritis. In some cases, whole spirochetes could be visualized within the lumina of renal tubules or the microvasculature (Fig. 1B).
FIG. 1.
Images taken from a California sea lion with leptospirosis. (A) Photomicrograph of a hematoxylin and eosin-stained section of kidney illustrating numerous lymphocytes and plasma cells infiltrating the renal cortical interstitium. Tubules are occasionally ecstatic, lined by flattened epithelial cells and contain eosinophilic fluid, necrotic epithelial cells, and neutrophils (arrows). (B) Photomicrograph of immunohistochemistry for Leptospira sp. demonstrating positive staining in renal interstitial inflammatory cells and of spirochetes within renal tubules (arrows). Images A and B were taken from case 16 (CSL6176).
Molecular analyses.
For the molecular analyses, Leptospira-specific PCR analyses were performed on DNA isolated from either urine (collected from live-stranded, dead, captive, and wild animals) and renal tissue samples (collected from dead animals) or, in one case, urine- and feces-contaminated sand samples (collected from a transiently stranded pinniped). Leptospiral DNA was successfully amplified from all sample sources, and the majority of samples that demonstrated positive amplification were obtained from animals dying from leptospirosis (cases 1 to 5, 7, 9, 10, and 12 to 31). Amplification was also observed in samples collected from three animals that were negative by clinical observations, immunohistochemistry, and serology but displayed mild interstitial nephritis upon histopathologic analysis (cases 6, 8, and 11). However, since immunohistochemistry and serological analyses detected neither leptospiral antigen in the renal tubules nor an antibody response in these potential carrier animals, respectively, an alternative, yet unlikely, possibility is that the positive PCR amplicons could have resulted from cross-contamination of the necropsy facility. Three animal carcasses in a moderate state of decomposition (cases 32 to 34), including two animals which died from unknown causes (cases 33 and 34), were positive by PCR. An additional sample (case 35) from an animal exhibiting clinical symptoms and histopathology consistent with renal failure was negative by both PCR analysis and serology; this animal likely died from a condition unrelated to leptospirosis. It should be noted that samples from all healthy animals investigated in this study (cases 36 to 52), including both captive and wild sea lions that were serologically negative by MAT, were also negative for Leptospira DNA by PCR.
Urine samples stored at −80°C for extended periods (6 months or longer) demonstrated a reduced amplification potential, as exemplified by cases 53 to 61, which were positive by clinical observations and serological investigations but demonstrated only a 22% amplification accuracy by PCR. No such reduction in amplification potential was observed in similarly stored renal tissue samples or in freshly isolated urine samples, as shown by the reliable amplification observed in cases 1 to 34.
Interestingly, samples collected from California sea lions and Steller sea lions exhibited positive amplification using the L. interrogans-specific primer pair, while the elephant seal sample (case 1) demonstrated positive amplification using the L. kirschneri-specific primer pair.
Table 2 shows a comparative summary of the methods used to detect the presence of Leptospira in our study. The use of PCR identified the highest number of Leptospira infections, with 34 samples showing positive PCR amplification. This number exceeds the number of cases deemed positive for leptospirosis via conventional methodology, which included clinical observations (27 cases) and serological (21 cases), histopathological (21 cases), and immunohistochemical (15 cases) investigations. An overall pattern of specificity was observed for the PCR analyses, with 0/17 healthy animals, 28/28 freshly isolated urine samples, and 7/7 kidney samples displaying amplification.
TABLE 2.
Comparative summary of methods to detect the presence of Leptospira in pinnipeds using fresh samples
| Animals (n) | No. positive/no. of samples by:
|
||||||
|---|---|---|---|---|---|---|---|
| PCR
|
Clinical observations | MAT | Histopathology | IHCa | |||
| Urine | Kidney | Total | |||||
| Captive healthy (2) | 0/2 | 0/0 | 0/2 | 0/2 | 0/2 | 0/0 | 0/0 |
| Wild healthy (15) | 0/15 | 0/0 | 0/15 | 0/15 | 0/15 | 0/0 | 0/0 |
| Leptospirosis cases (28) | 25/25b | 4/4b | 28/28 | 27/27 | 21/21 | 21/21 | 15/15 |
| Other clinical cases (4) | 3/3 | 1/1 | 4/4 | 0/3 | 0/3 | 0/3 | 0/3 |
| Unknown (3) | 0/1 | 2/2 | 2/3 | 1/1 | 0/1 | 1/1 | 0/0 |
| Total positive/total cases examined | 28/28b | 7/7b | 34/35 | 28/31 | 21/25 | 22/25 | 15/18 |
IHC, immunohistochemistry.
Includes case 4 (CSL6144), for which both urine and kidney were tested.
DISCUSSION
The widespread 2004 leptospirosis outbreak, in which sea lion mortalities were observed from California to British Columbia, afforded an opportunity to evaluate and compare PCR-based methods to existing methods for diagnosis of Leptospira infection among marine mammal populations. These data clearly demonstrate that Leptospira DNA can be successfully and accurately amplified from samples collected from stranded marine mammals, using DNA templates prepared from a wide variety of sources, including urine, renal tissue, and feces- and urine-contaminated sand samples collected from the vicinity of the stranded animal. The positive amplifications observed in the latter two sources of sample material are particularly noteworthy in that this result allows biologists an opportunity to collect samples from live pinnipeds that have clinical signs consistent with leptospirosis but return to the ocean prior to urine collection. Furthermore, these results suggest leptospirosis could be transmitted via contamination of the coastal environment if the excreted bacteria remain viable for a significant period of time. The suitability of PCR for detecting Leptospira infections in pinniped populations is further shown by the successful identification of infected animals that were unable to be analyzed by conventional diagnostic methodologies, including decomposed animal carcasses. Additionally, the sensitivity and specificity of PCR are useful in distinguishing renal failure due to leptospirosis from other causes of renal failure. A key finding revealed through these studies was the labile nature of isolated urine samples; templates prepared from fresh urine stocks provided reproducible amplifications, while templates prepared from urine samples stored at −80°C for 6 months or longer resulted in sporadic amplification and unreliable results. Collectively, these investigations highlight the versatility of PCR over conventional diagnostic methodologies, in that analyses can be tailored to the available sample type collected from stranded animals, but attention must be given to proper sample storage and processing to ensure valid results are achieved using this technique.
The three animals that did not exhibit clinical symptoms of disease and displayed only mild nephritis upon necropsy, but displayed positive PCR amplicons, suggest that PCR may be useful in the detection of carrier animals. Carrier animals could shed Leptospira sp. in their urine and in this way function as reservoirs for Leptospira transmission. Detection of carrier animals is vital to the understanding of enzootic and epizootic leptospirosis in marine mammals, since pathogenic Leptospira serovars have been shown to survive for only short periods of time in seawater (8, 13) and therefore the mode of transmission of this organism in a marine species is not understood. Further investigation of the carrier status of California sea lions using PCR is warranted.
Use of two primer sets that discriminate between L. interrogans and L. kirschneri suggests these two species many have different host preferences. Samples from California and Steller sea lions were positive for L. interrogans but negative for L. kirschneri. In contrast, the one sample obtained from an elephant seal was positive for L. kirschneri but negative for L. interrogans. Expansion of these experiments to include additional marine and terrestrial mammal species, combined with the use of supplementary Leptospira species-specific primer pairs, will provide invaluable information on transmission routes, both within the marine environment and between terrestrial and marine environments, and may identify additional incidental and maintenance hosts for this disease. Overall, this study has shown that PCR represents a powerful diagnostic technique that has many advantages over classic methods of leptospirosis diagnosis.
The majority of animals included in this study stranded along the California coast, a region that is routinely frequented by recreational sporting enthusiasts and densely populated with humans, pinnipeds, rodents, and domestic animals, thus establishing an optimal milieu for zoonotic disease transmission and long-term disease maintenance. Many of the animals stranded near freshwater estuaries, increasing the potential for disease transmission to humans and domestic animals due to enhanced leptospiral survival in freshwater. Furthermore, detection of Leptospira in sand contaminated by fecal material and urine in this study suggests a potential environmental source of pathogen exposure. Collectively, these observations reveal the significant zoonotic potential of leptospirosis within a marine environment. This investigation increases our understanding of potential routes of Leptospira transmission and will provide scientists and animal health experts with methodology to rapidly and accurately diagnose future outbreaks of leptospirosis.
Acknowledgments
We thank Janelle Kuroiwa, Liz Wheeler, and Denise Greig for technical assistance; Paul Cullen for support and helpful discussions; Teresa Francescutti for critical reading of the manuscript; and the NWRMMSN participants from Cascadia Research, Central Puget Sound Marine Mammal Stranding Network, WDFW, and Washington State Parks, especially Jessie Huggins, Sue Murphy, Bruce Kauffman, and Ed and Sheila Mitchell, for their assistance in monitoring and collecting live and dead sea lions from Washington State.
This work was supported by a Discovery Grant from the Natural Sciences and Engineering Research Council of Canada (327186; C.E.C.), the Canada Foundation for Innovation (C.E.C.), and the British Columbia Knowledge Development Fund (C.E.C.). C.E.C. is a CIHR Canada Research Chair in Molecular Pathogenesis.
Footnotes
Published ahead of print on 26 March 2008.
REFERENCES
- 1.Colagross-Schouten, A. M., J. A. Mazet, F. M. Gulland, M. A. Miller, and S. Hietala. 2002. Diagnosis and seroprevalence of leptospirosis in California sea lions from coastal California. J. Wildl. Dis. 387-17. [DOI] [PubMed] [Google Scholar]
- 2.Colegrove, K. M., L. J. Lowenstine, and F. M. Gulland. 2005. Leptospirosis in northern elephant seals (Mirounga angustirostris) stranded along the California coast. J. Wildl. Dis. 41426-430. [DOI] [PubMed] [Google Scholar]
- 3.Dierauf, L. A., D. J. Vandenbroek, J. Roletto, M. Koski, L. Amaya, and L. J. Gage. 1985. An epizootic of leptospirosis in California sea lions. J. Am. Vet. Med. Assoc. 1871145-1148. [PubMed] [Google Scholar]
- 4.Gerber, J. A., J. Roletto, L. E. Morgan, D. M. Smith, and L. J. Gage. 1993. Findings in pinnipeds stranded along the central and northern California coast, 1984-1990. J. Wildl. Dis. 29423-433. [DOI] [PubMed] [Google Scholar]
- 5.Gravekamp, C., K. H. Van de Kemp, M. Franzen, D. Carrington, G. J. Schoone, G. J. Van Eys, C. O. Everard, R. A. Hartskeerl, and W. J. Terpstra. 1993. Detection of seven species of pathogenic leptospires by PCR using two sets of primers. J. Gen. Microbiol. 1391691-1700. [DOI] [PubMed] [Google Scholar]
- 6.Greig, D. J., F. M. Gulland, and C. Kreuder. 2005. A decade of live California sea lion (Zalophus californianus) strandings along the central California coast: causes and trends, 1991-2000. Aquat. Mammals 3111-22. [Google Scholar]
- 7.Gulland, F. M., M. Koski, L. J. Lowenstine, A. Colagross, L. Morgan, and T. Spraker. 1996. Leptospirosis in California sea lions (Zalophus californianus) stranded along the central California coast, 1981-1994. J. Wildl. Dis. 32572-580. [DOI] [PubMed] [Google Scholar]
- 8.Khairani-Bejo, S., A. R. Bahaman, M. Zamri-Saad, and A. R. Mutalib. 2004. The survival of Leptospira interrogans serovar Hardjo in the Malaysian environment. J. Anim. Vet. Adv. 3123-129. [Google Scholar]
- 9.Luna, L. G. 1968. Manual of histologic staining methods of the Armed Forces Institute of Pathology, p. 258. McGraw-Hill Company, New York, NY.
- 10.Smith, A. W., R. J. Brown, D. E. Skilling, H. L. Bray, and M. C. Keyes. 1977. Naturally-occurring leptospirosis in northern fur seals (Callorhinus ursinus). J. Wildl. Dis. 13144-148. [DOI] [PubMed] [Google Scholar]
- 11.Stamper, M. A., F. M. Gulland, and T. Spraker. 1998. Leptospirosis in rehabilitated Pacific harbor seals from California. J. Wildl. Dis. 34407-410. [DOI] [PubMed] [Google Scholar]
- 12.Tizard, I. 1987. Veterinary immunology: an introduction, p. 401. W. B. Saunders Company, Philadelphia, PA.
- 13.Trueba, G., S. Zapata, K. Madrid, P. Cullen, and D. Haake. 2004. Cell aggregation: a mechanism of pathogenic Leptospira to survive in fresh water. Int. Microbiol. 735-40. [PubMed] [Google Scholar]
- 14.Vedros, N. A., A. W. Smith, J. Schonewald, G. Migaki, and R. C. Hubbard. 1971. Leptospirosis epizootic among California sea lions. Science 1721250-1251. [DOI] [PubMed] [Google Scholar]
- 15.Zuerner, R. L., D. Alt, and C. A. Bolin. 1995. IS1533-based PCR assay for identification of Leptospira interrogans sensu lato serovars. J. Clin. Microbiol. 333284-3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zuerner, R. L., and C. A. Bolin. 1997. Differentiation of Leptospira interrogans isolates by IS1500 hybridization and PCR assays. J. Clin. Microbiol. 352612-2617. [DOI] [PMC free article] [PubMed] [Google Scholar]