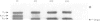Abstract
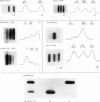
An investigation was made of the effect of NAD+ analogues on subunit interactions in yeast and rabbit muscle glyceraldehyde 3-phosphate dehydrogenases by using the subunit exchange (hybridization) method described previously [e.g. see Osborne & Hollaway (1975) Biochem. J. 151, 37-45]. The ligands ATP, ITP, ADP, AMP, cyclic AMP and ADP-ribose like NADH, all caused an apparent weakening of intramolecular subunit interactions, whereas NAD+ caused an apparent increase in the stability of the tetrameric enzyme molecules. A mixture of NMN and AMP, although it did not simulate completely the NAD+-induced 'tightening' of the enzyme structure, did result in a more than 20-fold decrease in the rate of subunit exchange compared with that in the presence of AMP alone. These results show that occupancy of the NMN subsite of the enzyme NAD+-binding site is insufficient in itself to give the marked tightening of the enzyme structure induced by NAD+. The 'tightening' effect is specific in that it seems to require a phosphodiester link between NMN and ADP-ribose. These effects are discussed in terms of the detailed X-ray structure of the lobster holoenzyme [Buehner et al. (1974) J. Mol. Biol. 90, 25-49].
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. J., McPherson A., Jr, Rossmann M. G., Schevitz R. W., Wonacott A. J. The structure of the nicotinamide-adenine dinucleotide coenzyme when bound to lactate dehydrogenase. J Mol Biol. 1970 Jul 14;51(1):31–38. doi: 10.1016/0022-2836(70)90267-6. [DOI] [PubMed] [Google Scholar]
- Bolotina I. A., Markovich D. S., Volkenstein M. V., Zavodsky P. Investigation of the conformation of D-glyceraldehyde-3-phosphate dehydrogenase. Biochim Biophys Acta. 1967 Mar 15;132(2):260–270. doi: 10.1016/0005-2744(67)90145-3. [DOI] [PubMed] [Google Scholar]
- Buehner M., Ford G. C., Olsen K. W., Moras D., Rossman M. G. Three-dimensional structure of D-glyceraldehyde-3-phosphate dehydrogenase. J Mol Biol. 1974 Nov 25;90(1):25–49. doi: 10.1016/0022-2836(74)90254-x. [DOI] [PubMed] [Google Scholar]
- Cantau B. N., Jaureguiberry G. J., Pudles J. Studies on the oxidative fraction of rabbit muscle glyceraldehyde-3-phosphate dehydrogenase. Influence of ligands on its obtention enzymic properties. Eur J Biochem. 1970 Oct;16(2):208–216. doi: 10.1111/j.1432-1033.1970.tb01073.x. [DOI] [PubMed] [Google Scholar]
- Constantinides S. M., Deal W. C., Jr Reversible dissociation of tetrameric rabbit muscle glyceraldehyde 3-phosphate dehydrogenase into dimers or monomers by adenosine triphosphate. J Biol Chem. 1969 Oct 25;244(20):5695–5702. [PubMed] [Google Scholar]
- Eby D., Kirtley M. E. Interaction of nicotinamide-adenine dinucleotide and its analogs with glyceraldehyde 3-phosphate dehydrogenase. Biochemistry. 1971 Jul 6;10(14):2677–2682. doi: 10.1021/bi00790a004. [DOI] [PubMed] [Google Scholar]
- Fenselau A. Nicotinamide adenine dinucleotide as an active site director in glyceraldehyde 3-phosphate dehydrogenase modification. J Biol Chem. 1970 Mar 25;245(6):1239–1246. [PubMed] [Google Scholar]
- Fensleau A. Structure-function studies on glyceraldehyde 3-phosphate dehydrogenase. IV. Subunit interactions of the rabbit muscle and yeast enzymes. J Biol Chem. 1972 Feb 25;247(4):1074–1079. [PubMed] [Google Scholar]
- Foucault G., Traore F., Levilliers J., Pudles J. Structure-function relationship in rabbit muscle glyceraldehyde-3-phosphate dehydrogenase. Trinitrophenylation of the lysine residues. Eur J Biochem. 1974 Jul 1;46(1):43–57. doi: 10.1111/j.1432-1033.1974.tb03595.x. [DOI] [PubMed] [Google Scholar]
- Francis S. H., Meriwether B. P., Park J. H. Interaction between adenine nucleotides and 3-phosphoglyceraldehyde dehydrogenase. I. Inhibition of the hydrolysis of S-acetyl-enzyme intermediate in the esterase activity. J Biol Chem. 1971 Sep 10;246(17):5427–5432. [PubMed] [Google Scholar]
- Francis S. H., Meriwether B. P., Park J. H. Interaction between adenine nucleotides and 3-phosphoglyceraldehyde dehydrogenase. II. A study of the mechanism of catalysis and metabolic control of the multi-functional enzyme. J Biol Chem. 1971 Sep 10;246(17):5433–5441. [PubMed] [Google Scholar]
- GUIDOTTI G., KONIGSBERG W., CRAIG L. C. ON THE DISSOCIATION OF NORMAL ADULT HUMAN HEMOGLOBIN. Proc Natl Acad Sci U S A. 1963 Oct;50:774–782. doi: 10.1073/pnas.50.4.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huehns E. R. The properties and reactions of haemoglobin F(1) and their bearing on the dissociation equilibrium of haemoglobin. Biochem J. 1966 Dec;101(3):852–860. doi: 10.1042/bj1010852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirtley M. E., Koshland D. E., Jr The properties of rabbit muscle glyceraldehyde 3-phosphate dehydrogenase labeled with a "reporter group". J Biol Chem. 1970 Jan 25;245(2):276–286. [PubMed] [Google Scholar]
- Listowsky I., Furfine C. S., Betheil J. J., Englard S. Coenzyme-induced changes in the optical rotatory dispersion properties of glyceraldehyde 3-phosphate dehydrogenase. J Biol Chem. 1965 Nov;240(11):4253–4258. [PubMed] [Google Scholar]
- Nagradova N. K., Vorona M. K., Asriiants R. A. Vliianie adeninovykh nukleotidov na aktivnost' glitseral'degid-3-fosfatdegidrogenaz razlichnogo proiskhozhdeniia. Biokhimiia. 1969 May-Jun;34(3):627–632. [PubMed] [Google Scholar]
- Oguchi M., Meriwether B. P., Park J. H. Interaction between adenosine triphosphate and glyceraldehyde 3-phosphate dehydrogenase. 3. Mechanism of action and metabolic control of the enzyme under simulated in vivo conditions. J Biol Chem. 1973 Aug 25;248(16):5562–5570. [PubMed] [Google Scholar]
- Osborne H. H., Hollaway M. R. The hybridization of glyceraldehyde 3-phosphate dehydrogenases from rabbit muscle and yeast. Kinetics and thermodynamics of the reaction and isolation of the hybrid. Biochem J. 1974 Dec;143(3):651–662. doi: 10.1042/bj1430651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne H. H., Hollaway M. R. The investigation of substrate-induced changes in subunit interactions in glyceraldehyde 3-phosphate dehydrogenases by measurement of the kinetics and thermodynamics of subunit exchange. Biochem J. 1975 Oct;151(1):37–45. doi: 10.1042/bj1510037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polgár L. The effect of coenzyme on the S--N acyl migration in glyceraldehyde-3-phosphate dehydrogenase. Biochim Biophys Acta. 1966 May 5;118(2):276–284. doi: 10.1016/s0926-6593(66)80036-x. [DOI] [PubMed] [Google Scholar]
- Sapag-Hagar M. Kinetics of rabbit muscle D-glyceraldehyde 3-phosphate dehydrogenase: inhibition by adenine nucleotides. Rev Esp Fisiol. 1969 Sep;25(3):201–206. [PubMed] [Google Scholar]
- Smith G. D., Schachman H. K. Effect of D2O and nicotinamide adenine dinucleotide on the sedimentation properties and structure of glyceraldehyde phosphate dehydrogenase. Biochemistry. 1973 Sep 25;12(20):3789–3801. doi: 10.1021/bi00744a001. [DOI] [PubMed] [Google Scholar]
- Stancel G. M., Deal W. C., Jr Reversible dissociation of yeast glyceraldehyde 3-phosphate dehydrogenase by adenosine triphosphate. Biochemistry. 1969 Oct;8(10):4005–4011. doi: 10.1021/bi00838a017. [DOI] [PubMed] [Google Scholar]
- TUCKER D., GRISOLIA S. Inactivation of muscle triosephosphate dehydrogenase by reduced diphosphopyridine nucleotide at physiological concentrations. J Biol Chem. 1962 Apr;237:1068–1073. [PubMed] [Google Scholar]
- Vas M., Boross L. Heat inactivation of D-glyceraldehyde-3-phosphate dehydrogenase apoenzyme. Acta Biochim Biophys Acad Sci Hung. 1972;7(2):105–114. [PubMed] [Google Scholar]
- Yang S. T., Deal W. C., Jr Metabolic control and structure of glycolytic enzymes. VI. Competitive inhibition of yeast glyceraldehyde 3-phosphate dehydrogenase by cyclic adenosine monophosphate, adenosine triphosphate, and other adenine-containing compounds. Biochemistry. 1969 Jul;8(7):2806–2813. doi: 10.1021/bi00835a017. [DOI] [PubMed] [Google Scholar]
- Yang S. T., Deal W. C., Jr Metabolic control and structure of glycolytic enzymes. VII. Destabilization and inactivation of yeast glyceraldehyde 3-phosphate dehydrogenase by adenosine phosphates and chymotrypsin. Biochemistry. 1969 Jul;8(7):2814–2820. doi: 10.1021/bi00835a018. [DOI] [PubMed] [Google Scholar]