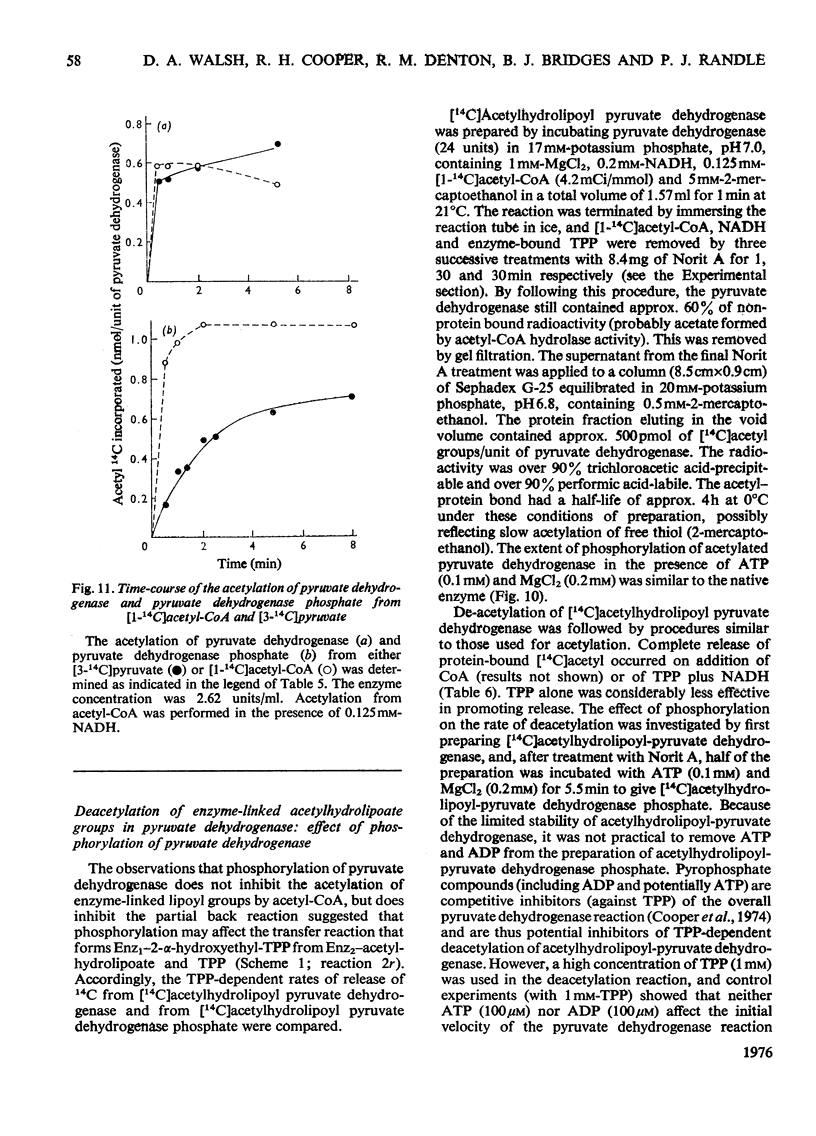
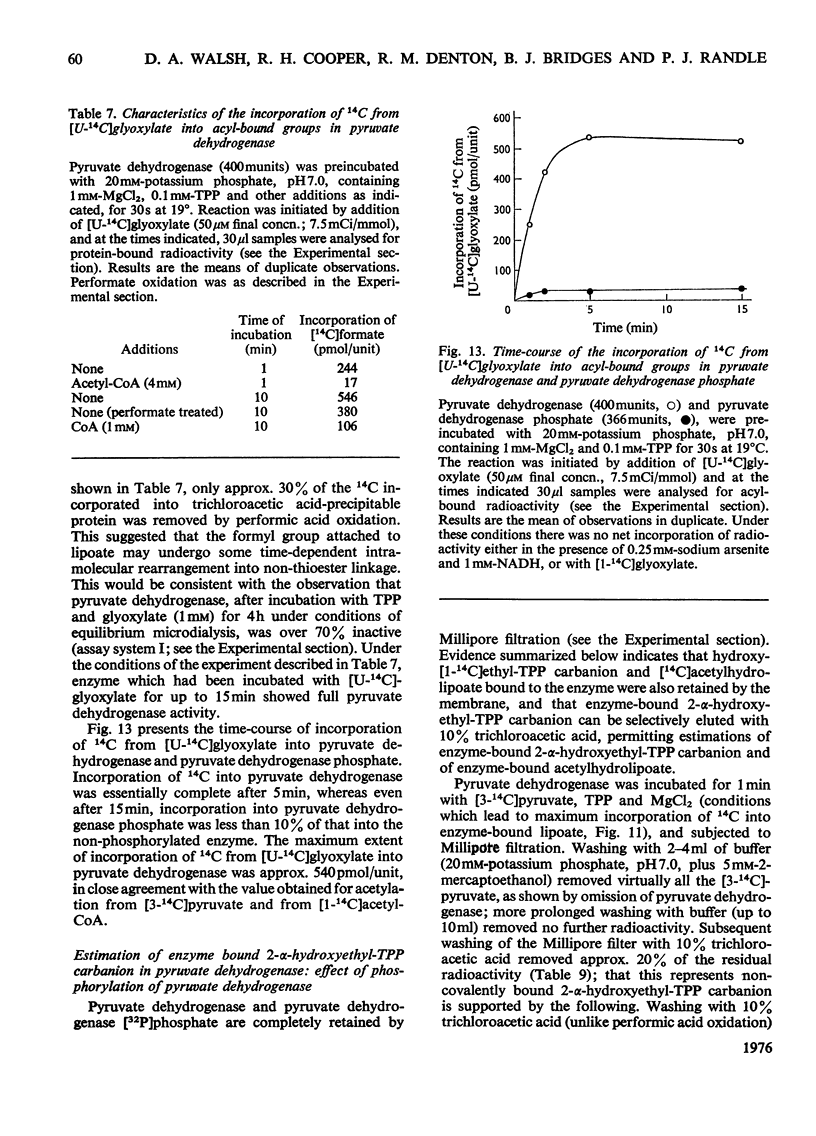
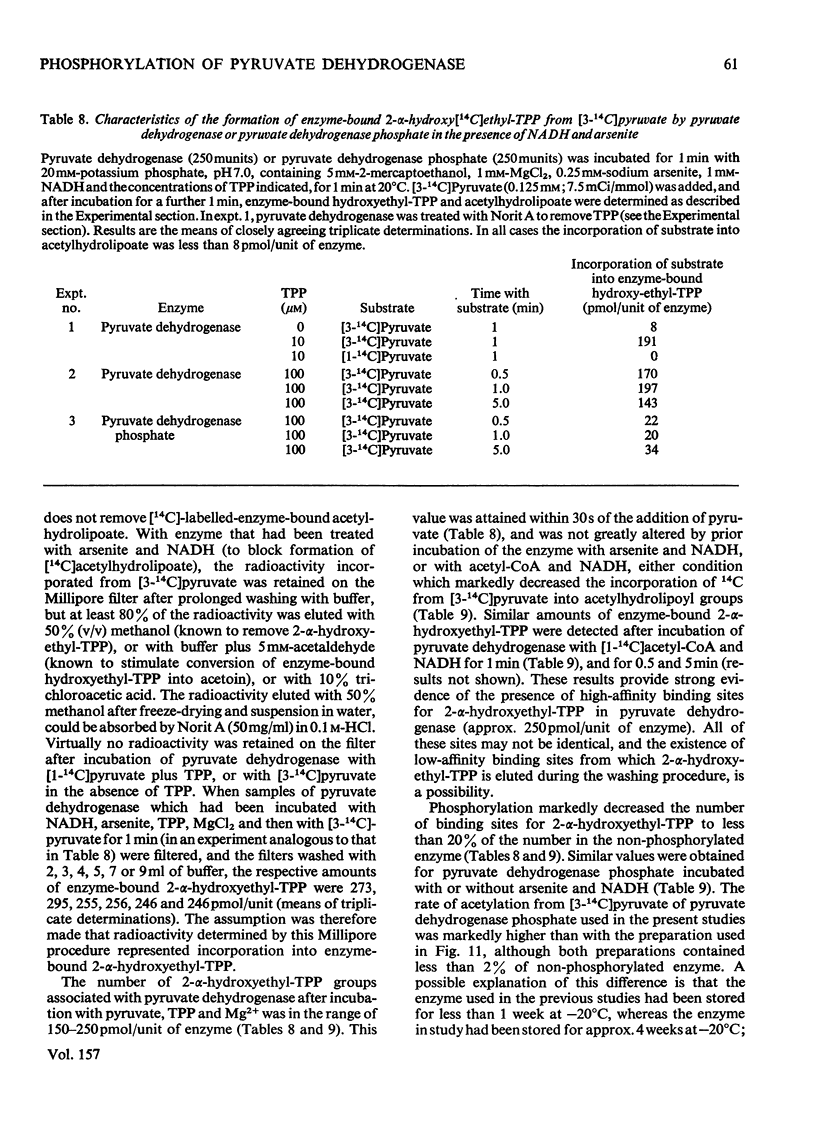
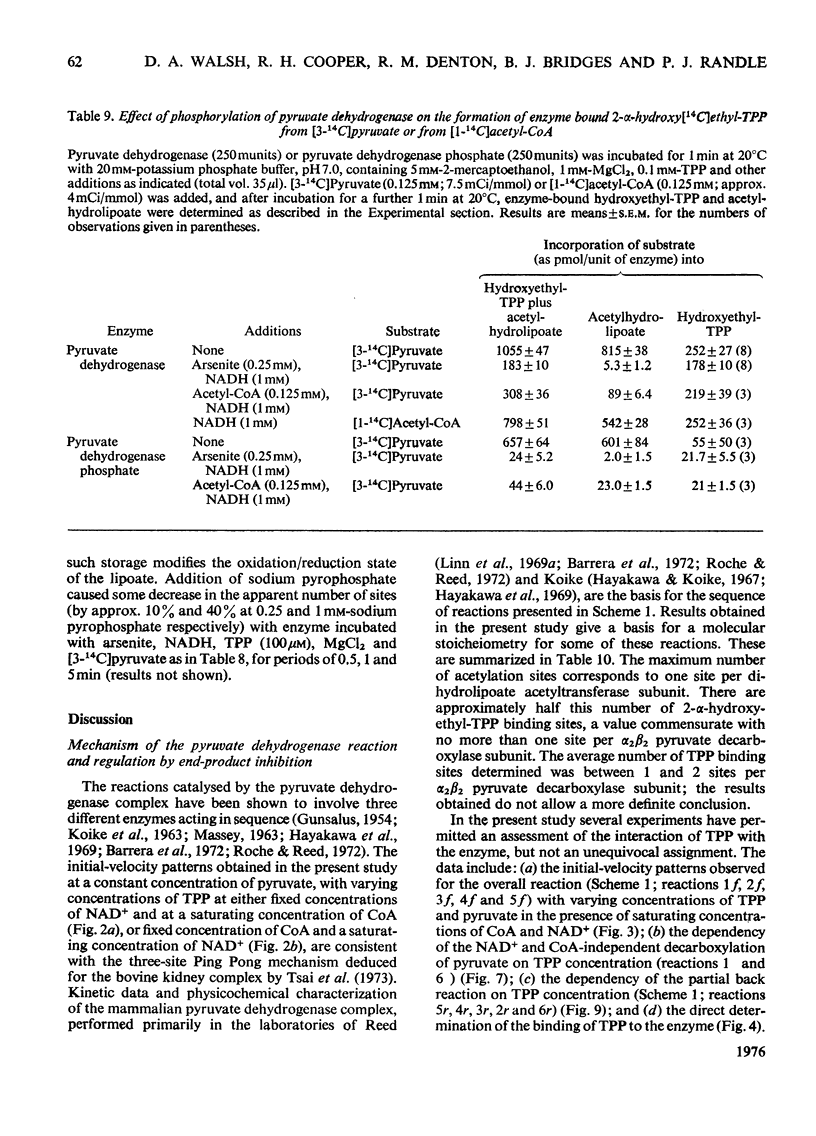
Abstract
1. A method was devised for preparing pig heart pyruvate dehydrogenase free of thiamin pyrophosphate (TPP), permitting studies of the binding of [35S]TPP to pyruvate dehydrogenase and pyruvate dehydrogenase phosphate. The Kd of TPP for pyruvate dehydrogenase was in the range 6.2-8.2 muM, whereas that for pyruvate dehydrogenase phosphate was approximately 15 muM; both forms of the complex contained about the same total number of binding sites (500 pmol/unit of enzyme). EDTA completely inhibited binding of TPP; sodium pyrophosphate, adenylyl imidodiphosphate and GTP, which are inhibitors (competitive with TPP) of the overall pyruvate dehydrogenase reaction, did not appreciably affect TPP binding. 2. Initial-velocity patterns of the overall pyruvate dehydrogenase reaction obtained with varying TPP, CoA and NAD+ concentrations at a fixed pyruvate concentration were consistent with a sequential three-site Ping Pong mechanism; in the presence of oxaloacetate and citrate synthase to remove acetyl-CoA (an inhibitor of the overall reaction) the values of Km for NAD+ and CoA were 53+/- 5 muM and 1.9+/-0.2 muM respectively. Initial-velocity patterns observed with varying TPP concentrations at various fixed concentrations of pyruvate were indicative of either a compulsory order of addition of substrates to form a ternary complex (pyruvate-Enz-TPP) or a random-sequence mechanism in which interconversion of ternary intermediates is rate-limiting; values of Km for pyruvate and TPP were 25+/-4 muM and 50+/-10 nM respectively. The Kia-TPP (the dissociation constant for Enz-TPP complex calculated from kinetic plots) was close to the value of Kd-TPP (determined by direct binding studies). 3. Inhibition of the overall pyruvate dehydrogenase reaction by pyrophosphate was mixed non-competitive versus pyruvate and competitive versus TPP; however, pyrophosphate did not alter the calculated value for Kia-TPP, consistent with the lack of effect of pyrophosphate on the Kd for TPP. 4. Pyruvate dehydrogenase catalysed a TPP-dependent production of 14CO2 from [1-14C]pyruvate in the absence of NAD+ and CoA at approximately 0.35% of the overall reaction rate; this was substantially inhibited by phosphorylation of the enzyme both in the presence and absence of acetaldehyde (which stimulates the rate of 14CO2 production two- or three-fold). 5. Pyruvate dehydrogenase catalysed a partial back-reaction in the presence of TPP, acetyl-CoA and NADH. The Km for TPP was 4.1+/-0.5 muM. The partial back-reaction was stimulated by acetaldehyde, inhibited by pyrophosphate and abolished by phosphorylation. 6. Formation of enzyme-bound [14C]acetylhydrolipoate from [3-14C]pyruvate but not from [1-14C]acetyl-CoA was inhibited by phosphorylation. Phosphorylation also substantially inhibited the transfer of [14C]acetyl groups from enzyme-bound [14C]acetylhydrolipoate to TPP in the presence of NADH. 7...
Full text
PDF






















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrera C. R., Namihira G., Hamilton L., Munk P., Eley M. H., Linn T. C., Reed L. J. -Keto acid dehydrogenase complexes. XVI. Studies on the subunit structure of the pyruvate dehydrogenase complexes from bovine kidney and heart. Arch Biochem Biophys. 1972 Feb;148(2):343–358. doi: 10.1016/0003-9861(72)90152-x. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. II. Inhibition: nomenclature and theory. Biochim Biophys Acta. 1963 Feb 12;67:173–187. doi: 10.1016/0006-3002(63)91815-8. [DOI] [PubMed] [Google Scholar]
- Cleland W. W. Derivation of rate equations for multisite ping-pong mechanisms with ping-pong reactions at one or more sites. J Biol Chem. 1973 Dec 25;248(24):8353–8355. [PubMed] [Google Scholar]
- Cleland W. W. The statistical analysis of enzyme kinetic data. Adv Enzymol Relat Areas Mol Biol. 1967;29:1–32. doi: 10.1002/9780470122747.ch1. [DOI] [PubMed] [Google Scholar]
- Cooper R. H., Randle P. J., Denton R. M. Regulation of heart muscle pyruvate dehydrogenase kinase. Biochem J. 1974 Dec;143(3):625–641. doi: 10.1042/bj1430625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R. H., Randle P. J., Denton R. M. Stimulation of phosphorylation and inactivation of pyruvate dehydrogenase by physiological inhibitors of the pyruvate dehydrogenase reaction. Nature. 1975 Oct 30;257(5529):808–809. doi: 10.1038/257808a0. [DOI] [PubMed] [Google Scholar]
- Denton R. M., Randle P. J., Martin B. R. Stimulation by calcium ions of pyruvate dehydrogenase phosphate phosphatase. Biochem J. 1972 Jun;128(1):161–163. doi: 10.1042/bj1280161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deus B., Blum H. E., Holzer H. Radiometric assay procedure for thiamine pyrophosphokinase activity. Anal Biochem. 1969 Mar;27(3):492–501. doi: 10.1016/0003-2697(69)90063-3. [DOI] [PubMed] [Google Scholar]
- Englund P. T., Huberman J. A., Jovin T. M., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. XXX. Binding of triphosphates to deoxyribonucleic acid polymerase. J Biol Chem. 1969 Jun 10;244(11):3038–3044. [PubMed] [Google Scholar]
- HELMREICH E., CORI C. F. THE ROLE OF ADENYLIC ACID IN THE ACTIVATION OF PHOSPHORYLASE. Proc Natl Acad Sci U S A. 1964 Jan;51:131–138. doi: 10.1073/pnas.51.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa T., Kanzaki T., Kitamura T., Fukuyoshi Y., Sakurai Y., Koike K., Suematsu T., Koike M. Mammalian alpha-keto acid dehydrogenase complexes. V. Resolution and reconstitution studies of the pig heart pyruvate dehydrogenase complex. J Biol Chem. 1969 Jul 10;244(13):3660–3670. [PubMed] [Google Scholar]
- Hayakawa T., Koike M. Mammalian alpha-keto acid dehydrogenase complexes. 3. Resolution and reconstitution of the pig heart pyruvate dehydrogenase complex. J Biol Chem. 1967 Mar 25;242(6):1356–1358. [PubMed] [Google Scholar]
- Hucho F., Randall D. D., Roche T. E., Burgett M. W., Pelley J. W., Reed L. J. -Keto acid dehydrogenase complexes. XVII. Kinetic and regulatory properties of pyruvate dehydrogenase kinase and pyruvate dehydrogenase phosphatase from bovine kidney and heart. Arch Biochem Biophys. 1972 Jul;151(1):328–340. doi: 10.1016/0003-9861(72)90504-8. [DOI] [PubMed] [Google Scholar]
- JAGANNATHAN V., SCHWEET R. S. Pyruvic oxidase of pigeon breast muscle. I. Purification and properties of the enzyme. J Biol Chem. 1952 May;196(2):551–562. [PubMed] [Google Scholar]
- JUNI E., HEYM G. A. Acyloin condensation reactions of pyruvic oxidase. J Biol Chem. 1956 Jan;218(1):365–378. [PubMed] [Google Scholar]
- KOHLHAW G., DEUS B., HOLZER H. ENZYMATIC PREPARATION, STRUCTURE, AND PROPERTIES OF THIAMINE PYROPHOSPHATE-ACTIVATED FORMALDEHYDE. J Biol Chem. 1965 May;240:2135–2141. [PubMed] [Google Scholar]
- KOIKE M., REED L. J., CARROLL W. R. alpha-Keto acid dehydrogenation complexes. IV. Resolution and reconstitution of the Escherichia coli pyruvate dehydrogenation complex. J Biol Chem. 1963 Jan;238:30–39. [PubMed] [Google Scholar]
- KREBS E. G., LOVE D. S., BRATVOLD G. E., TRAYSER K. A., MEYER W. L., FISCHER E. H. PURIFICATION AND PROPERTIES OF RABBIT SKELETAL MUSCLE PHOSPHORYLASE B KINASE. Biochemistry. 1964 Aug;3:1022–1033. doi: 10.1021/bi00896a003. [DOI] [PubMed] [Google Scholar]
- Kerbey A. L., Randle P. J., Cooper R. H., Whitehouse S., Pask H. T., Denton R. M. Regulation of pyruvate dehydrogenase in rat heart. Mechanism of regulation of proportions of dephosphorylated and phosphorylated enzyme by oxidation of fatty acids and ketone bodies and of effects of diabetes: role of coenzyme A, acetyl-coenzyme A and reduced and oxidized nicotinamide-adenine dinucleotide. Biochem J. 1976 Feb 15;154(2):327–348. doi: 10.1042/bj1540327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn T. C., Pelley J. W., Pettit F. H., Hucho F., Randall D. D., Reed L. J. -Keto acid dehydrogenase complexes. XV. Purification and properties of the component enzymes of the pyruvate dehydrogenase complexes from bovine kidney and heart. Arch Biochem Biophys. 1972 Feb;148(2):327–342. doi: 10.1016/0003-9861(72)90151-8. [DOI] [PubMed] [Google Scholar]
- Linn T. C., Pettit F. H., Hucho F., Reed L. J. Alpha-keto acid dehydrogenase complexes. XI. Comparative studies of regulatory properties of the pyruvate dehydrogenase complexes from kidney, heart, and liver mitochondria. Proc Natl Acad Sci U S A. 1969 Sep;64(1):227–234. doi: 10.1073/pnas.64.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn T. C., Pettit F. H., Reed L. J. Alpha-keto acid dehydrogenase complexes. X. Regulation of the activity of the pyruvate dehydrogenase complex from beef kidney mitochondria by phosphorylation and dephosphorylation. Proc Natl Acad Sci U S A. 1969 Jan;62(1):234–241. doi: 10.1073/pnas.62.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- REISS O. K. Pyruvate metabolism. II. Restoration of pyruvate utilization in heart sarcosomes by alpha-(+)-lipoic acid. J Biol Chem. 1958 Oct;233(4):789–793. [PubMed] [Google Scholar]
- ROSELL-PEREZ M., LARNER J. STUDIES ON UDPG-ALPHA-GLUCAN TRANSGLUCOSYLASE. V. TWO FORMS OF THE ENZYME IN DOG SKELETAL MUSCLE AND THEIR INTERCONVERSION. Biochemistry. 1964 Jan;3:81–88. doi: 10.1021/bi00889a014. [DOI] [PubMed] [Google Scholar]
- ROSSI-FANELLI A., SILIPRANDI N., FASELLA P. On the presence of the triphosphothiamine (TPT) in the liver. Science. 1952 Dec 26;116(3026):711–713. doi: 10.1126/science.116.3026.711. [DOI] [PubMed] [Google Scholar]
- Reed L. J., Oliver R. M. The multienzyme alpha-keto acid dehydrogenase complexes. Brookhaven Symp Biol. 1968 Jun;21(2):397–412. [PubMed] [Google Scholar]
- Roche T. E., Reed L. J. Function of the nonidentical subunits of mammalian pyruvate dehydrogenase. Biochem Biophys Res Commun. 1972 Aug 21;48(4):840–846. doi: 10.1016/0006-291x(72)90684-5. [DOI] [PubMed] [Google Scholar]
- Roche T. E., Reed L. J. Monovalent cation requirement for ADP inhibition of pyruvate dehydrogenase kinase. Biochem Biophys Res Commun. 1974 Aug 19;59(4):1341–1348. doi: 10.1016/0006-291x(74)90461-6. [DOI] [PubMed] [Google Scholar]
- SCHREIBER G., KOHLHAW G., GOEDDE H. W., HOLZER H. DIE BIOSYNTHESE VON ACETOIN IN SCHWEINEHERZMUSKEL. Biochem Z. 1963 Oct 14;339:83–93. [PubMed] [Google Scholar]
- Severson D. L., Denton R. M., Pask H. T., Randle P. J. Calcium and magnesium ions as effectors of adipose-tissue pyruvate dehydrogenase phosphate phosphatase. Biochem J. 1974 May;140(2):225–237. doi: 10.1042/bj1400225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. S., Burgett M. W., Reed L. J. Alpha-keto acid dehydrogenase complexes. XX. A kinetic study of the pyruvate dehydrogenase complex from bovine kidney. J Biol Chem. 1973 Dec 25;248(24):8348–8352. [PubMed] [Google Scholar]
- Ullrich J., Mannschreck A. Studies on the properties of (--)-2-alpha-hydroxyethyl-thiamine pyrophosphate ("active acetaldehyde"). Eur J Biochem. 1967 Mar;1(1):110–116. doi: 10.1111/j.1432-1033.1967.tb00051.x. [DOI] [PubMed] [Google Scholar]
- Wieland O. H., Siess E. A., Weiss L., Löffler G., Patzelt C., Portenhauser R., Hartmann U., Schirmann A. Regulation of the mammalian pyruvate dehydrogenase complex by covalent modification. Symp Soc Exp Biol. 1973;27:371–400. [PubMed] [Google Scholar]
- Wieland O., Von Jagow-Westermann B., Stukowski B. Kinetic and regulatory properties of heart muscle pyruvate dehydrogenase. Hoppe Seylers Z Physiol Chem. 1969 Mar;350(3):329–334. doi: 10.1515/bchm2.1969.350.1.329. [DOI] [PubMed] [Google Scholar]


