Abstract
Recent studies indicate that reactive oxygen species (ROS) are critically involved in persistent pain primarily through spinal mechanisms, thus suggesting ROS involvement in central sensitization. To investigate ROS involvement in central sensitization, the effects of ROS scavengers and donors on pain behaviors were examined in mice. The capsaicin-induced hyperalgesia was used as a pain model since it has 2 distinctive pain components, primary and secondary hyperalgesia representing peripheral and central sensitization, respectively. Capsaicin (25 μg/5 μl) was injected intradermally into the left foot. Foot withdrawal frequencies in response to von Frey filament stimuli were measured and used as an indicator of mechanical hyperalgesia. The production of ROS was examined by using a ROS sensitive dye MitoSOX Red. Mice developed primary and secondary mechanical hyperalgesia after capsaicin injection. A systemic or intrathecal post-treatment with either phenyl-N-tert-butylnitrone (PBN) or 4-hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPOL), ROS scavengers, significantly reduced secondary hyperalgesia, but not primary hyperalgesia, in a dose dependent manner. Pretreatment with ROS scavengers also significantly reduced the magnitude and duration of capsaicin-induced secondary hyperalgesia. On the other hand, intrathecal injection of tert-butylhydroperoxide (t-BOOH, 5 μl), a ROS donor, produced a transient hyperalgesia in a dose dependent manner. The number of MitoSOX positive dorsal horn neurons was increased significantly after capsaicin treatment. This study suggests that ROS mediates the development and maintenance of capsaicin-induced hyperalgesia in mice, mainly through central sensitization and that the elevation of spinal ROS is most likely due to increased production of mitochondrial superoxides in dorsal horn neurons.
Keywords: free radicals, persistent pain, central sensitization, ROS
1. Introduction
Oxidative stress, cytotoxic effects of reactive oxygen species (ROS), is considered a prominent factor in many degenerative neurological conditions, such as Alzheimer disease, Parkinson disease, amyotrophic lateral sclerosis, and other brain dysfunctions ranging from brain injury to aging [10,14,16,25]. ROS are oxygen containing chemicals, free radicals and nonradicals, and are byproducts of normal cellular functions, such as oxidative phosphorylation and monoamine oxidase reaction [39]. Physiological concentrations of cellular ROS are tightly controlled by an endogenous antioxidant system which includes various enzymes and non-enzymatic molecules [6,16]. In some pathological conditions, however, cellular ROS levels may rise beyond the normal physiological ranges, and thus leads to oxidative stress. This can be resulted from either increased ROS production or decreased antioxidant capacity. The consequences of oxidative stress is variable from modification of cellular signaling pathways to irreversible structural and/or functional damages [6,16].
The mechanisms of persistent pain are not clearly understood and ROS have been proposed to contribute to persistent pain [19,21,41]. For instance, mechanical allodynia developed after a peripheral nerve injury in the rat was temporarily but almost completely relieved by a systemic injection of free radical scavengers, phenyl-N-tert-butyl-nitrone (PBN) and 5,5-dimetyl-pyrroline-N-oxide (DMPO) [21]. The hyperalgesia induced by an injection of formalin into the rat paw was significantly attenuated by a treatment with antioxidants, either systemically or intrathecally [12]. Furthermore, the number of neurons showing mitochondrial ROS production was significantly increased in the lumbar spinal dorsal horn in spinal nerve ligated neuropathic rats [28]. These studies thus suggest that increased levels of ROS in the spinal cord and/or in the peripheral tissue play an important role in persistent pain after peripheral nerve injury or tissue inflammation.
While different studies suggest that ROS are important for central or peripheral sensitization in pain, the exact site of ROS action in pain has not been clearly identified. Pain induced by intradermal capsaicin has well defined peripheral and central components [42]. Primary hyperalgesia, occurring at the capsaicin-injected site, is explained by peripheral nociceptor sensitization [2,22,36,37]. On the other hand, secondary hyperalgesia, which is observed in the surrounding neighboring region of the injection site, is known to be due to central sensitization in the spinal dorsal horn [42]. Thus the capsaicin induced pain model is useful to study the role of ROS in these two different components. Using this well established model, the present study investigates the differential role of ROS in primary and secondary hyperalgesia. The data indicate that spinal ROS are critical for secondary hyperalgesia and thus central sensitization. Parts of this study have been presented in abstract form [32,33].
2. Materials and methods
Experimental Animals
Young adult FVB/NJ male mice 4–5 week old, 20–25 g body weight, (The Jackson Laboratory, Bar Harbor, ME) were used for all studies. Animals were housed in groups of four to five in plastic cages with soft bedding and free access to food and water under a 12/12 hour light-dark cycle. All animals were acclimated for 1 week before any experimental procedures. All experimental protocols were approved by the Animal Care and Use Committee of the University of Texas Medical Branch and are in accordance with the National Institute of Health’s Guide for the Care and Use of Laboratory Animals.
2.2 Capsaicin Injection
For injection of capsaicin, each mouse was anesthetized with isoflurane (2.0% for induction and 1.5 % for maintenance) in a flow of O2 and placed in a prone position. Capsaicin (0.5% final concentration, Sigma, St. Louis, MO) was dissolved in vehicle containing 20% alcohol and 7% Tween 80 in saline and was injected intradermally (i.d.) using a 30 gauge needle attached to a Hamilton Syringe. For behavioral experiments 5 μl of 0.5% capsaicin was injected into the plantar region of the left foot. The needle was inserted into a site, marked X in Figure 1, near the heel of the left hind foot and advanced to the middle of the plantar surface (site I). A successful injection was noted by the formation of a “bleb” about 2 mm in diameter. The insertion site was pressed for 1 min to prevent leakage of the solution after removal of the needle. Anesthesia was discontinued and the mice were arisen within 5 min and then returned to their cages.
Figure 1.
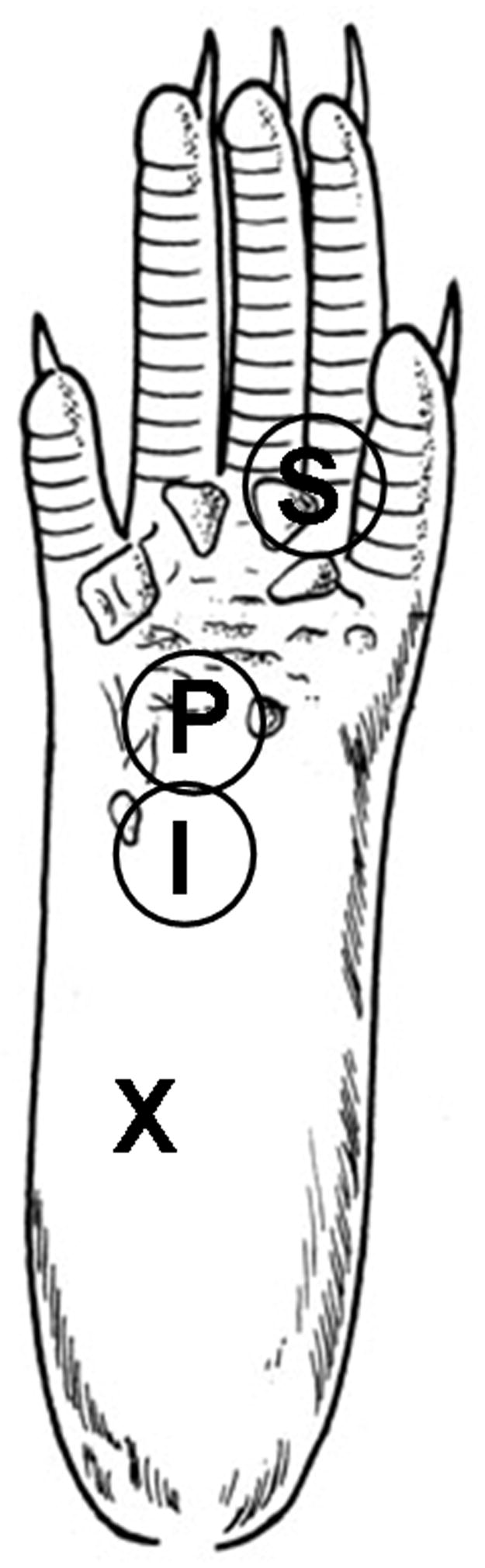
Sites of capsaicin injection and behavioral testing in the mouse hind foot. For capsaicin injection, a 30-gauge needle was inserted at the heel of the foot (X) and advanced to the injection site (I), and capsaicin (25 μg in 5 μl of vehicle) was injected intradermally (i.d.). Foot withdrawal frequencies in response to von Frey stimuli were measured at site P for primary hyperalgesia and at site S for secondary hyperalgesia.
For ROS imaging experiments, 25 μl of 0.5% capsaicin was injected on both the plantar (15 μl) and dorsal (10 μl) surfaces of the left hind foot. A larger volume of capsaicin was used than for the behavioral testing to increase receptive fields of capsaicin responsive afferents and thus to maximize the number of affected neurons in the spinal cord.
2.3 Behavioral Testing for assessment of foot withdrawal responses
Foot withdrawal frequencies in response to von Frey stimuli were measured and used as an indicator of mechanical hypersensitivity. Tests were conducted blindly and foot withdrawal responses were assessed before and 1, 2, 3, 5, 8, and 24 hr or (48 hr sometimes) after i.d. injection of capsaicin. For each test, the animal was placed in a plastic chamber on top of a mesh screen platform and was habituated for at least 10 min. Mechanical stimuli were applied from underneath to the plantar surface of the left hind foot. Mechanical sensitivity of the foot was determined by the amount of positive foot withdrawal responses in response to von Frey stimuli. Two von Frey monofilaments 2.48 and 3.0,0 (vF#2.48 and vF#3.00) which are equivalent to 0.03 and 0.1 g force, respectively, were used and the first stimulus was always with the 2.48 filament. For mechanical stimulation, a von Frey filament was applied perpendicular to the stimulation site with a sufficient force to bend the filament for 2–3 sec and then removed. An abrupt withdrawal of the foot with or without licking during stimulation or immediately after stimulus removal was considered a positive response. Each test was composed of 10 stimuli for each area. The number of positive responses was converted into a percentage with 10 positive responses corresponding to 100%. To assess primary hyperalgesia a von Frey monofilament was applied to site P in Figure 1, which is 3 mm distal from the injection site (site I). For secondary hyperalgesia, a von Frey monofilament was applied at the base and/or proximal part of the third and fourth toes (site S). This area is an adequate distance from the capsaicin injection site, and thus not directly affected by capsaicin, but becomes extremely sensitive and is thus identified as a site of secondary hyperalgesia. Mechanical sensitivity was also tested on the contralateral (non-injected) side in all experimental mice.
2.4 Systemic Injection of ROS Scavengers
To determine the effects of ROS scavengers on capsaicin-induced hyperalgesia, two different ROS scavengers were tested: phenyl N-tert-butylnitrone (PBN; Sigma, St Louis, MO) and 4-hydroxy-2,2,6,6-tetramethylpiperidine 1-oxyl (TEMPOL; Sigma, St Louis, MO). Both scavengers were dissolved in saline. For injection of ROS scavengers, each mouse was anesthetized with isoflurane (2.0% for induction and 1.5% for maintenance) in a flow of O2 and placed in a prone position. For PBN experiments, 20 mg of PBN was dissolved in 1 ml of saline, and 100 μl of this solution was injected intraperitoneally (i.p) per 20 g of body weight (100 mg or 0.56 mmol/kg of body weight). The same volume of saline was used as a control. To determine the role of ROS in the induction or maintenance of capsaicin-induced hyperalgesia, PBN treatment was administered either 0.5 hours before capsaicin treatment (pre-treatment) or 1.5 hours after (post-treatment).
For TEMPOL experiments, 60 mg of TEMPOL was dissolved in 1 ml of saline, and 100 μl of this solution was injected per 20 g of body weight intraperitoneally (300 mg or 1.7 mmol/kg of body weight). The same volume of saline was used as a control. To determine the role of ROS in the induction or maintenance of capsaicin-induced hyperalgesia, TEMPOL was administered either 0.5 hours before capsaicin treatment (pre-treatment) or 1.5 hours after (post-treatment).
To examine the dose response of PBN, one of three doses of PBN [20 mg (0.11 mmol), 50 mg (0.28 mmol), or 100 mg (0.56 mmol)/kg of body weight] or saline was injected i.p., 1.5 hours (post-treatment) after intradermal injection of capsaicin. For TEMPOL, either 200 mg/kg (1.2 mmol/kg), 300 mg/kg (1.7 mmol/kg), or saline was injected i.p., 1.5 hours (post-treatment) after intradermal injection of capsaicin.
2.5 Direct transcutaneous intrathecal injection of ROS Scavengers
For direct transcutaneous intrathecal injection, a modified method [23] of intrathecal injection developed by Hylden and Wilcox [15], was used. Either 1.13 μmol of PBN in 5 μl of saline or 2.90 μmol of TEMPOL in 5 μl of saline was injected intrathecally (i.t.) 1.5 hrs following capsaicin injection. Mice were anesthetized (2.0% for induction and 1.5 % for maintenance) with isoflurane in a flow of O2, placed in a prone position, and the hair on their back was clipped. The caudal paralumbar region, just cranial to the iliac crests, was securely held by the thumb and middle finger of the left hand and the index finger was placed on the tip of sixth lumbar (L6) spinous process, the highest point of the vertebral column. A 30 gauge needle connected to a Hamilton syringe was positioned underneath the index finger with the beveled side facing upward in a forward direction. The needle was inserted into the tissue at a 45° angle. The angle of the needle was maintained until the needle went through the L5/L6 intervertebral space and “slipped in” causing a sudden lateral movement of the tail. Solution was injected at a rate of 1 μl/sec. The needle was held in position for 10 seconds and removed slowly to avoid any outflow of the solution. Anesthesia was discontinued and the mice were aroused within 5 min and then returned to their cages.
To examine the dose response of PBN, one of three doses of PBN: 0.28 μmol, 0.56 μmol, 1.13 μmol, or saline was injected i.t., 1.5 hours (post-treatment) after intradermal injection of capsaicin. For TEMPOL, either 0.58 μmol, 1.74 μmol, 2.90 μmol, or saline was injected i.t., 1.5 hours (post-treatment) after intradermal injection of capsaicin.
2.6 Testing the effects of intrathecal injection of ROS donor, t-BOOH
A bolus of tert-butylhydroperoxide (t-BOOH; Sigma, St. Louis, MO) was injected intrathecally using a direct transcutaneous intrathecal injection method into the mouse. Three different doses of t-BOOH (0.55, 1.10, and 2.80 μmol in 5 μl saline) and the same volume of saline (control) were tested. Ten minutes later, the mechanical hypersensitivity of various spots on the plantar surface of the left hind paw were tested and the most sensitive area was used to measure mechanical hyperalgesia. Mechanical sensitivity of the paw was measured every 15 min for 2 hrs following i.t. injection of t-BOOH.
2.7 In vivo detection of mitochondrial superoxide production using MitoSOX-Red
MitoSox Red (Molecular Probes, Eugene, OR) was dissolved in a 1:1 mixture of dimethylsulfoxide (DMSO) and saline to a final concentration of 33 μM. Under isoflurane anesthesia, 10 μl of MitoSox was injected intrathecally by using a direct transcutaneous intrathecal injection method described previously. Approximately 23 hrs after MitoSox injection, mice received an i.d. injection of either capsaicin (0.5%, 25 μl) or the same volume of vehicle on both the plantar and dorsal surface of the left hind paw. A larger volume (25 μl) of capsaicin (as compared to behavioral testing) was injected to increase receptive fields, including the dorsal surface of the foot, in order to expose a large number of afferents to capsaicin, which would maximize the number of affected neurons in the spinal cord. Mice remained under 1.5 % isoflurane anesthesia for 30 min to suppress any side effects induced by capsaicin. Mice were perfused through the aorta with saline followed by fixative containing 4% paraformaldehyde 1 hr after capsaicin injection and the L4-L5 spinal cord segments were removed. The cord was postfixed 4–15 hr in the perfusion fixative, equilibrated in 30% sucrose, cryosectioned at 30 μm, and mounted on gelatin coated slides. The sections were examined under a fluorescence microscope with a rhodamine filter. Two different regions of the dorsal horn were photographed from 10 randomly selected sections from each animal: the lateral part of laminae I -II and laminae III-V. Photographs were taken with a Q-Imaging Retiga 2000R digital camera attached to an Olympus BX50 microscope using a 63x oil objective lens and saved as digital image files. The number of MitoSOX positive cellular profiles with distinctive nuclei (dark oval shaped space surrounded by red granules) was counted from these pictures.
Some spinal cord sections from each treatment group were also immunostained for neurons, astrocytes, and microglia. The primary antibodies used are NeuN (Chemicon, Temecula, CA; 1:1000 dilution), GFAP (Chemicon, Temecula, CA; 1:500) and OX-42 (AbD Serotec, Raleigh, NC 1:200) and Alexa fluor 488 conjugated goat-anti-mouse IgG (Molecular Probes, Eugene, OR; 1:200 dilution) as secondary antibodies. The sections were washed with distilled water, mounted on slides, air dried, and then coverslipped. The sections were examined under a fluorescent microscope with rhodamine and FITC filters. Photographs of the same regions were taken with proper filters and saved as digital image files. The number of single and double labeled, MitoSox only and MitoSox and NeuN, GFAP, or OX-42 cellular profiles were determined.
2.8 Statistical Analysis
Data are presented as mean ± standard error of the mean (SEM) and were analyzed using the statistical program SigmaStat (Version 3.1, Systat Software, CA). Statistical analyses were performed using two-way analysis of variance (ANOVA) with one repeated factor (time), followed by Duncan’s post hoc test.
3. Results
3.1 Capsaicin induces long lasting primary and secondary hyperalgesia in mice
Mechanical sensitivity of the foot was determined by the amount of positive foot withdrawals in response to von Frey stimuli applied 10 times. An abrupt withdrawal of the foot with or without licking during stimulation or immediately after stimulus removal was considered a positive response. However, animals did not exhibit any spontaneous licking or shaking of the paw after intradermal capsaicin injection in the absence of von Frey stimulation. To assess primary hyperalgesia, a von Frey monofilament was applied to site P in Figure 1, which is 3 mm distal from the injection site (site I). For secondary hyperalgesia, a von Frey monofilament was applied at the base and/or the proximal part of the third and fourth toes (site S).
In normal mice, the average % of foot withdrawal frequencies was 2.1 ± 1.8 % (mean ± SEM, n=6) and 3.2 ± 2.3 % (n=6) in response to mechanical stimuli with vF filament # 2.48 (0.03 g force) and # 3.00 (0.10 g force), respectively. One hour after i.d. capsaicin injection, foot withdrawal frequencies increased to 36.3 ± 2.3 % (vF #2.48, n=6) and 45.1 ± 2.3 % (vF #3.0, n=6) in the area where inflammation developed due to capsaicin injection (site P in Fig. 1) compared to 2.1 ± 1.3 % (vF #2.48, n=6) and 3.3 ± 2.7 % (vF #3.00, n=6) in the vehicle treated mice (Fig. 2). The data indicate that primary hyperalgesia is developed at the capsaicin injection site. Foot withdrawal responses were also significantly increased in the neighboring non-inflamed region (site S in Fig. 1) to 40.4 ± 4.6 % (vF # 2.48, n=6) and 50.2 ± 3.7 % (vF #3.00, n=6) with capsaicin treatment compared to 1.5 ± 1.1 % (vF #2.48, n=6) and 4.2 ± 1.7 % (vF #3.00, n=6) with vehicle treatment, indicating the development of secondary hyperalgesia. The peak responses, ranging from 64 to 82 %, were observed 2 hr after capsaicin injection for both primary and secondary hyperalgesia, and then started to decline gradually afterward. A significant hyperalgesia was observed up to 5–8 hrs (Fig. 2) after capsaicin injection. Data suggest that i.d. capsaicin induces both primary and secondary hyperalgesia that last approximately 5–8 hrs. The foot withdrawal responses to mechanical stimuli recovered to the pretreatment levels by 24 hrs after capsaicin injection for both primary and secondary hyperalgesia.
Figure 2.
Time course of primary and secondary hyperalgesia after intradermal (i.d.) capsaicin (25 μg in 5 μl saline) injection in the mouse hind foot. Asterisks (*), p ≤ 0.05 (n=6), indicate values significantly different from corresponding values in the vehicle treated group according to Duncan’s post hoc test after two-way repeated ANOVA. Arrowheads indicate time of capsaicin (or vehicle) injection
3.2 Post-treatment with ROS scavengers reduces capsaicin-induced secondary hyperalgesia, but not primary hyperalgesia, in a dose-dependent manner
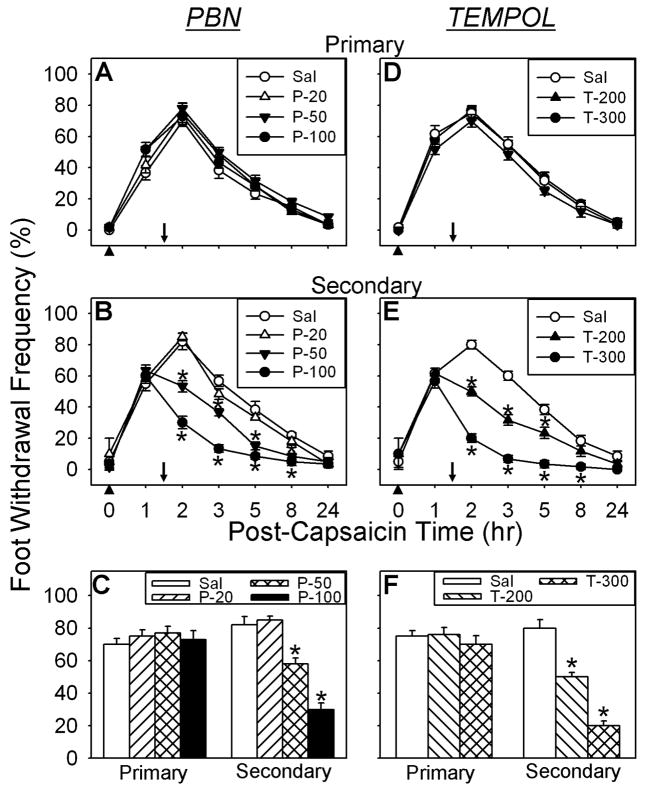
To test whether ROS are involved in the maintenance of capsaicin-induced hyperalgesia, the effects of ROS scavengers on pain were examined and the data are shown in Fig. 3. Systemic PBN treatment (100 mg/kg, i.p.), 1.5 hours after capsaicin injection, had no effect on the primary hyperalgesia. The same treatment, however, showed a significant reduction in secondary hyperalgesia, which lasted approximately 6 hours in a dose-dependent manner. Foot withdrawal frequencies in response to vF stimuli were 67.2 ± 4.4 % (vF #2.48, n=6) and 72.2 ± 5.2 % (vF #3.00, n=6) 0.5 hrs systemic saline treatment on the secondary hyperalgesia site. With PBN treatment, these values were 32.1 ± 3.4 % (vF #2.48, n=6) and 35.3 ± 4.1 % (vF #3.00, n=6) 0.5 hrs after PBN treatment (Figs. 3B & C). In the dose-response study, both 50 and 100 mg/kg of PBN (n=6 for each dose) showed graded antihyperalgesic responses with an ED50 of 57.7 mg/kg (CI95% 32.4–83.0 mg/kg), whereas, 20 mg/kg of PBN did not show a significant reduction in secondary hyperalgesia as compared to the saline treated group (Fig. 3B&C). A systemic TEMPOL treatment (200 and 300 mg/kg, i.p. n=6) also showed a similar antihyperalgesic effect only on secondary hyperalgesia with an ED50 of 214.6 mg/kg (CI95% 151.5–277.7 mg/kg) (Figs. 3E&F). The foot withdrawal frequencies on the secondary decreased to 35.6 ± 3.4 % (vF #2.48, n=6) and 40.5 ± 4.2 % (vF #3.00, n=6) compared to 55.2 ± 4.7 % (vF #2.48, n=6) and 60.3 ± 3.7 % (vF #3.00, n=6) with saline treatment (Figs. 3E&F). The results show that ROS scavengers are effective in producing antihyperalgesia only on secondary hyperalgesia, thus suggesting that ROS are mainly involved in central sensitization.
Figure 3.
Effects of systemic (i.p.) PBN or TEMPOL post-treatment on capsaicin-induced hyperalgesia. A,D Time course of PBN or TEMPOL effects on primary hyperalgesia. B,E Time course of PBN or TEMPOL effects on secondary hyperalgesia. C,F The dose response of PBN or TEMPOL on secondary hyperalgesia 2 hr after i.d. capsaicin injection and 30 min post ROS scavenger. Asterisks (*), p ≤ 0.05 (n=6), indicate values significantly different from corresponding values in the vehicle treated group according to Duncan’s post hoc test after two-way repeated ANOVA. Arrowheads indicate time of capsaicin injection. Downward arrows indicates time of tested compound (PBN, TEMPOL, or saline) injection.
3.3 The main action site of ROS is the spinal cord
To check whether ROS are acting mainly by the spinal mechanism, the effects of intrathecally injected PBN and TEMPOL on capsaicin-induced secondary hyperalgesia were examined. After confirming the development of the primary and secondary hyperalgesia 1 hr after i.d. capsaicin treatment, a bolus of either PBN (1.13 μmol) or TEMPOL (2.9 μmol) was injected intrathecally 1.5 hr after capsaicin treatment. The foot withdrawal frequencies were then measured on both primary and secondary hyperalgesia sites at various times after i.t. injection. The time course of behaviors and the averaged values of the foot withdrawal frequencies at 2 hr after capsaicin, representing 0.5 hr after PBN or TEMPOL treatment, are shown in Figure 4. As shown in Fig. 4, intrathecal injection of both PBN and TEMPOL produced significant analgesic effects on secondary hyperalgesia. Vehicle treatment did not affect secondary hyperalgesia. In the dose-response study, all three doses of PBN: 0.28, 0.56, and 1.13 μmol (n=6 for each dose) showed graded antihyperalgesic responses with an ED50 of 88.7 mg/kg (CI95% 69.7–107.7 mg/kg) However, 0.28 μmol of PBN did not show a significant reduction in secondary hyperalgesia as compared to the saline treated group (Fig. 4B&C). We also tested the effects of i.t. ROS scavengers on the contralateral paw but there was no change in foot withdrawal frequencies compared to the pre-drug values, which show no response.
Figure 4.
Effects of intrathecal (i.t.) PBN or TEMPOL treatment on capsaicin-induced hyperalgesia. A,D Time course of PBN or TEMPOL effects on primary hyperalgesia. B,E Time course of PBN or TEMPOL effects on secondary hyperalgesia. C,F The effect of PBN or TEMPOL on secondary hyperalgesia 2 hr after i.d. capsaicin injection and 30 min after either PBN, TEMPOL, or Saline treatment. Asterisks (*), p ≤ 0.05 (n=6), indicate values significantly different from corresponding values in the vehicle treated group according to Duncan’s post hoc test after two-way repeated ANOVA. Arrowheads indicate time of capsaicin injection. Downward arrows indicate time of tested compound (PBN, TEMPOL, or saline) injection.
In the dose-response study of TEMPOL, all three doses of intrathecal treatment, 0.58, 1.16, and 2.9 μmol (n=6), also showed graded antihyperalgesic response only on secondary hyperalgesia with an ED50 of 185.5 mg/kg (CI95% 130.5–240.5 mg/kg) (Figs. 4E&F). The foot withdrawal frequencies on the secondary hyperalgesia site, 0.5 hr after TEMPOL injection (2.9 μmol), was significantly decreased to 35.6 ± 3.4 % (vF #2.48, n=6) and 40.5 ± 4.2 % (vF #3.00, n=6) compared to 55.2 ± 4.7 % (vF #2.48, n=6) and 60.3 ± 3.7 % (vF #3.00, n=6) with saline treatment (Figs. 4E&F). The results show that ROS scavengers are effective in producing antihyperalgesia only on secondary hyperalgesia, thus suggesting that ROS are mainly involved in central sensitization.
These results are almost identical as the systemic effects of PBN and TEMPOL at their highest doses. This antihyperalgesic effect of intrathecal PBN or TEMPOL on secondary hyperalgesia lasted approximately 3.5 hours. The result that intrathecal PBN or TEMPOL is effective in producing analgesia only on secondary hyperalgesia suggests that ROS may be important for central sensitization in the spinal cord.
3.4 Intrathecal administration of a ROS donor, t-BOOH, produced transient pain behaviors in normal mice in a dose-dependent manner
To test whether an artificial increase of spinal ROS will induce pain behaviors, a ROS donor t-BOOH was injected intrathecally and mechanical sensitivity of the paw was measured. The most sensitive area of the paw to vF stimuli was identified at the base or proximal parts of the 3rd and 4th digits. Three different doses of t-BOOH, 0.55, 1.10, and 2.80 μmol in 5 μl saline, were tested by using a transcutaneous intrathecal injection method. As shown in Figure 5, the foot withdrawal frequencies started to increase approximately 15 minutes after t-BOOH in all three doses and reached the maximum responses 45 min after and then returned to near baseline levels 90 minutes later. The hyperalgesic effect after 2.80 μmol of t-BOOH showed the highest increase of 75.2 ± 4.7 % (vF #2.48, n=10) and 95.4 ± 3.3 % (vF #3.00, n=10) compared to the saline treated group of 1.7 ± 1.3 % (vF #2.48, n=10) and 2.9 ± 1.4 % (vF #3.00, n=10). All three tested doses of t-BOOH produced transient but statistically significant mechanical hyperalgesia as compared to the saline treated group in a dose-dependent manner. The vehicle (saline) treated mice did not produce mechanical hyperalgesia.
Figure 5.
Effects of i.t. t-BOOH on mechanical hyperalgesia. A: i.t. t-BOOH transiently induced mechanical hyperalgesia in normal mice in a dose-dependent manner. Saline did not show any changes in mechanical sensitivity. B: The bar graph shows the effect of t-BOOH on mechanical hyperalgesia 45 min after injection. *, p ≤ 0.05 (n=10). The open arrowhead indicates time of t-BOOH (or saline) injection.
3.5 Pretreatment with ROS scavengers significantly reduced the development of secondary hyperalgesia
To test whether ROS are involved in the development of pain, the effects of a ROS scavenger pretreatment on mechanical hyperalgesia were examined after i.d. capsaicin injection. A ROS scavenger, PBN (100 mg/kg) or TEMPOL (300 mg/kg), was injected intraperitoneally 0.5 hr prior to capsaicin treatment and then mechanical hyperalgesia were measured as described before. PBN or TEMPOL pretreatment had no affect on the development of capsaicin-induced primary hyperalgesia (Fig. 6A & D). On the other hand, it significantly reduced capsaicin-induced secondary hyperalgesia. The foot withdrawal frequencies at 1 hr after capsaicin injection were 30.5 ± 3.7 % (vF #2.48, n=6) and 38.2 ± 4.1 % (vF #3.00, n=6) with PBN pretreatment compared to 55.6 ± 4.7 % (vF #2.48, n=6) and 60.4 ± 3.7 % (vF #3.00, n=6) with saline pretreatment (Fig. 6B & C). TEMPOL (300 mg/kg, i.p.) pretreatment also decreased foot withdrawal frequencies significantly to 25.00 ± 3.67 (vF #2.48, n=6) and 30.00 ± 4.12 (vF #3.00, n=6) compared to 55.00 ± 4.69 (vF #2.48, n=6) and 60.00 ± 3.74 (vF #3.00, n=6) with saline pretreatment (Figure 6E & F) at 1 hr after capsaicin treatment. From this point on mechanical hyperalgesia started to decrease without any further increase. The results showed that with ROS scavenger pretreatment, capsaicin-induced hyperalgesia only lasted 2 hours rather than 5–8 hours with capsaicin only. Therefore, pretreatment of ROS scavengers reduces the magnitude and duration of capsaicin-induced secondary hyperalgesia in mice.
Figure 6.
Effects of systemic PBN or TEMPOL pre-treatment on capsaicin-induced hyperalgesia. A,D Time course of PBN or TEMPOL effects on primary hyperalgesia. B,E Time course of PBN or TEMPOL effects on secondary hyperalgesia. C,F The effect of PBN or TEMPOL on secondary hyperalgesia 2 hr after i.d. capsaicin injection and 2.5 hrs after either PBN, TEMPOL, or Saline treatment. Asterisks (*), p ≤ 0.05, indicate values significantly different from corresponding values in the vehicle treated group according to Duncan’s post hoc test after two-way repeated ANOVA. Arrowheads indicate time of capsaicin (or saline) injection. Downward arrows indicate time of tested compound (PBN, TEMPOL, or saline) injection.
3.6 Intradermal capsaicin induces increased ROS production in spinal dorsal horn neurons
To determine whether intradermal injection of capsaicin causes increased ROS production in the spinal cord, the number of cells showing oxidized MitoSox Red was counted in the spinal dorsal horn. The reduced form of MitoSOX Red, a cell-permeant mitochondrial superoxide indicator, has no color and sequesters in mitochondria when absorbed by cells. They fluoresce red when oxidized by ROS that are produced by mitochondria. MitoSOX Red was injected intrathecally and capsaicin was injected into the paw 23 hr later. One hour after capsaicin injection, the spinal cord was removed after perfusion fixation, cryosectioned and examined under a fluorescent microscope. MitoSOX positive cellular profiles, containing red fluorescent granules in the cytoplasm (Fig. 7A & B), were counted in two different regions of the spinal dorsal horn: superficial (laminae I-II) and deep (laminae III-V) layers. Only the cellular profiles that contained red fluorescent granules in the cytoplasm, which surrounds a round to oval shaped dark nucleus, were counted from each sampled picture. After measuring the sampled area of each section, the density of MitoSOX positive neurons (average # of cells per 10,000 μm2 of sample area ± SEM) was calculated and the data are presented in Fig. 8. In vehicle treated (n=7) mice, the average density of MitoSOX positive cells was 3.73 ± 0.55 and 5.13 ± 0.28 in the superficial and deep layers, respectively (Fig. 8A). The result indicates that there are a small number of cells that produce a sufficient amount of mitochondrial superoxide to oxidize sequestered MitoSOX in vehicle treated mice. In capsaicin-treated mice, the density of MitoSOX positive cell profiles increased to 4.55 ± 0.59 for laminae I-II and 10.95 ± 0.44 for laminae III-V. These increases are significant on both regions but much greater in the deep layers (Fig. 8A). The results show that mitochondrial superoxide generation is increased significantly in both the superficial and deeper layers of the dorsal horn in capsaicin-treated mice.
Figure 7.
MitoSOX positive cells in the deep layer of the L4-L5 spinal dorsal horn. A&B: MitoSOX labeled cells: vehicle (A) and capsaicin (B) treated mice: C&D: MitoSOX+NeuN label: NeuN label (C); MitoSOX label (D); combined (E). F&H: MitoSOX+GFAP label: GFAP label (F); MitoSOX label (G); combined (H).
Figure 8.
A: Density of MitoSOX positive cells (average # of cells per 10,000 μm2 of sample area ± SEM) in superficial (La I/II) and deep (La III/V) layers of the dorsal horn after vehicle (n=7) and capsaicin (n=7) treatment. *, p ≤ 0.05 (n=7). B: The average proportion of neurons and glia among MitoSOX positive cellular profiles in the deep layer (La III-IV) of the dorsal horn after i.d. capsaicin injection (n=7).
To identify the type of cells producing ROS, tissues were immunostained for NeuN, GFAP, and OX-42 in combination with MitoSOX labeling. Examples of MitoSOX positive and NeuN immunoreactive dorsal horn cells are shown in Figure 7C-7E and examples of GFAP immunoreactive astrocytes are shown in Figure 7F-H. In these double labeled sections, MitoSOX labeling was identified as red fluorescent granules in the cytoplasm while NeuN immunostaining was observed as green fluorescence in the nucleus. When these two images were merged, 523 out of 608 MitoSOX positive cells enclosed green nuclei, thus indicating that greater than 85% of ROS producing cells are neurons (Figure 8B). In astrocyte immunostained sections, GFAP immunostaining was observed as green fluorescence in cytoplasm. Colocalization of GFAP was observed only in 31 cells out of 516 MitoSOX positive cells, thus indicating that less than 5% of ROS producing cells are astrocytes. In OX-42 immunostained tissue, microglial staining was almost absent as was colocalization with MitoSOX. The data show that increased mitochondrial superoxide production occurs mainly in neurons in the dorsal horn of the spinal cord after capsaicin treatment. The big increase in the number of MitoSOX positive dorsal horn neurons in capsaicin-treated mice provides a strong evidence that increased mitochondrial superoxide production contributes to elevated spinal ROS levels and thus central sensitization in the persistent pain condition.
As a positive control, we examined OX-42 immunoreactivity and MitoSOX labeling in the spinal cord 3 days after L5 spinal nerve ligation, a peripheral neuropathy model, in mice. In these mice, we detected many OX-42 immunoreactive profiles that also contain MitoSOX labeling in the L5 spinal cord. Thus it is possible that mitochondrial ROS of glial origin can be detected by MitoSOX with the method we used in this study. Since we did not detect any cellular profiles that were positive for both MitoSOX and OX-42, we hypothesize that mitochondrial superoxide production in activated microglia may not be a significant contributor for ROS elevation in the capsaicin-induced hyperalgesia model at the time point we have chosen to study.
4. Discussion
The present study shows that both PBN and TEMPOL, ROS scavengers, produce a significant antihyperalgesic effect on capsaicin-induced secondary hyperalgesia, but not primary hyperalgesia, indicating that ROS seem to be important for secondary hyperalgesia. The significant antihyperalgesic effect of intrathecal ROS scavengers and the induction of hyperalgesia with an intrathecal ROS generator indicate that ROS may act through a spinal mechanism to induce pain. Furthermore, the significant increase in the number of MitoSOX positive neurons after i.d. capsaicin suggests that mitochondrial ROS production from the dorsal horn neurons may be a major contributor for spinal ROS increase.
A few studies report that various ROS scavengers and antioxidants reduce hyperalgesic behaviors in rat models of persistent pain [19,20,21,41]. In addition, increased production of mitochondrial superoxides in spinal dorsal horn neurons [28] and increased levels of extracellular hydrogen peroxides in the spinal trigeminal nucleus [40] are observed in animal models of neuropathic and inflammatory pain, respectively. Showing that ROS scavengers reduce secondary hyperalgesia and ROS levels are increased in the dorsal horn neurons in capsaicin treated mice, further support that the main action site of ROS is in the spinal cord. However, it has been shown that an intracerebroventricular injection of ROS scavengers also produces a small but significant antihyperalgesic effect in capsaicin-induced secondary hyperalgesia in rats [24]. Thus, the supraspinal involvement of ROS can not be completely ruled out because it is possible that intrathecally injected ROS scavengers might have spread and acted on supraspinal structures. On the other hand, several other studies have shown that peripheral ROS are important for persistent pain. In a rat model of chronic constriction injury, significantly increased free radical activities are detected in the injured sciatic nerve [18]. A neuropathic-like pain is produced in rats following prolonged hind paw ischemia and reperfusion, a condition known to induce free radical generation [5]. The present study, however, suggests that peripheral ROS production may not contribute significantly to capsaicin-induced primary hyperalgesia, since ROS scavengers do not affect primary hyperalgesia. In addition, a significant reduction in intensity and duration of secondary hyperalgesia after PBN or TEMPOL pretreatments suggests that ROS are most likely involved in both the maintenance and development of secondary hyperalgesia in capsaicin-induced hyperalgesia. Detailed mechanisms of ROS in the development and maintenance of secondary hyperalgesia need to be explored for better understanding of ROS roles in capsaicin-induced hyperalgesia.
It is well known that too much ROS produce tissue damage [6,16]. Thus it is possible that increased spinal ROS either after i.d. capsaicin or i.t. t-BOOH might have induced spinal cord damage. The common consequence of oxidative damage in the nervous system is lipid peroxidation due to the presence of large amounts of polyunsaturated fatty acids, thereby compromising cell integrity [1]. It is possible that the hyperalgesic effect of i.d. capsaicin or i.t. t-BOOH might have resulted from oxidative damage of the spinal cord. However, the hyperalgesia induced by i.t. t-BOOH only lasts 80 minutes, the paw sensitivity then returns to normal levels. Furthermore, the hyperalgesia induced i.d. capsaicin can be reversed by ROS scavengers within 30 minutes. The reversible nature of the antihyperalgesic effect of ROS scavengers and the hyperalgesic effect of a ROS donor, suggest that the ROS in pain transmission is unlikely due to permanent cellular damage. In support of this, one study showed that lipid peroxidation, a sign of tissue damage, in the spinal cord does not increase in the animal model of neuropathic pain with sciatic nerve transection [11]. Thus we propose that the neurons that are less severely stressed by moderately elevated levels of ROS survive but become dysfunctional, and that their function is restored by removal of ROS as shown in the present study.
The spinal cord is a region where nociceptive processing occurs and nerve injury can cause various neurochemical adaptations, up- or down-regulations of neurotransmitters, neuromodulators, and various signaling molecules [45]. Several recent studies suggest that ROS may modulate the functions of crucial proteins that are involved in persistent pain [4,27]. It has been shown that ROS affect the activities of cellular signaling molecules, such as activating protein kinase A, protein kinase C and CaMKII and inactivating protein phosphatases [34,43,44]. Studies also report the involvement of hydrogen peroxide enhancing protein tyrosine phosphorylation by inactivating protein tyrosine phosphatases and activating protein tyrosine kinases [29] and its involvement on neurotransmitter receptors by altering ligand-receptor interactions [30]. Based on these, we speculate that increased ROS in the spinal dorsal horn after an i.d. capsaicin may be involved for modification of cellular signal transduction pathways. We propose that elevated ROS modify redox sensitive signaling enzymes, such as protein kinases and protein phosphatases and thus gates cellular mechanisms toward central sensitization. The ROS induced signal transduction pathway changes in persistent pain need to be explored in future studies.
While data suggest that elevated spinal ROS seem to be involved in capsaicin-induced central sensitization, the sources and critical types of ROS are not clear. There are multiple sources of ROS in nervous tissue: superoxide generation from mitochondrial oxidative phosphorylation; NO production by activation of NO synthase [7]; activation of phospholipase A2 leading to an arachidonate cascade, and production of xanthine oxidase by activation of proteases. Among these, mitochondria have been recognized as the major source of intracellular ROS in neurons during excitotoxicity [31]. Since capsaicin-induced hyperalgesia is most likely a mild form of excitotoxicity, mitochondrial superoxide production in neurons is speculated as the major source of the enhanced spinal ROS. Since TEMPOL is known to remove superoxides preferentially [38] and had a strong analgesic effect on capsaicin-induced secondary hyperalgesia, data suggest that superoxides produced by mitochondria may be a major culprit. The significant increase of dorsal horn neurons that show staining for MitoSOX, specific mitochondrial superoxides, also supports the increased production of mitochondrial superoxides in the dorsal horn neurons in response to capsaicin. Although ROS production in glia has been well documented in cultured systems, this study shows that mitochondrial superoxide production in glia may not be the major source of spinal ROS in capsaicin induced hyperalgesia. This is based on our result that >85% of MitoSOX positive cells are neurons. Furthermore, less than 5% of MitoSOX positive cells were identified as astrocytes and no MitoSOX positive microglia was detected. Since we have observed that many OX-42 immunoreactive microglia were also labeled with MitoSOX in the spinal cord of neuropathic mice, we believe the absence of MitoSOX containing microglia in capsaicin treated mice is not due to technical problems. However, additional contribution of glia through other types of ROS production methods, such as NADPH oxidase, could not be ruled out.
While it becomes evident that ROS are involved in central sensitization, it is not known what drives the increased mitochondrial ROS production in capsaicin-induced pain. Previous studies have shown that application of glutamate induces an increase in intracellular ROS in cultured forebrain neurons [3,17], thus implicating glutamate mediated ROS production in neurons, A possible cause of enhanced neuronal ROS production is increased glutamate receptor activation. Intradermal capsaicin injection will induce intense activation of small myelinated and unmyelinated afferent fibers and thus release excitatory amino acids from their central terminals in the spinal dorsal horn [8, 9,35]. Glutamate will activate both ionotrophic and metabotrophic glutamate receptors, including NMDA receptors, which will lead to massive Ca2+ influx, thus increasing intracellular Ca2+ levels. High levels of intracellular Ca2+ will overload mitochondria, destabilize mitochondrial membrane potentials and accelerate the mitochondrial electron transport chain resulting in increased mitochondrial ROS generation [7,13]. This increased ROS can then affect redox sensitive signaling pathways towards sensitization of dorsal horn neurons [26]. Thus we propose that peripheral capsaicin treatment causes excessive release of glutamate in the spinal cord and excessive glutamate receptor activation leads to increased production of ROS in neurons. We propose that spinal ROS are a small class of molecules that are produced as a result of calcium influx and act as cellular messengers in central sensitization. Further studies are warranted to explore how ROS modify signaling molecules and what signaling molecules are modified in central sensitization after capsaicin treatment.
In conclusion, this study demonstrates that ROS may contribute to the development and maintenance of capsaicin-induced hyperalgesia in mice, through central sensitization. The elevation of spinal ROS is most likely due to increased production of mitochondrial superoxides.
Acknowledgments
This work was supported by NIH grants R01 NS31680 and P01 NS11255. We also would like to express our gratitude to Ms. Denise Broker for her excellent assistance in editing the manuscript.
There were no financial or other relationships that led to a conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Abe K, Saito H. Characterization of t-butyl hydroperoxide toxicity in cultured rat cortical neurones and astrocytes. Pharmacol Toxicol. 1998;83:40–46. doi: 10.1111/j.1600-0773.1998.tb01440.x. [DOI] [PubMed] [Google Scholar]
- 2.Baumann TK, Simone DA, Shain CN, LaMotte RH. Neurogenic hyperalgesia: The search for the primary cutaneous afferent fibers that contribute to capsaicin-induced pain and hyperalgesia. J Neurophysiol. 1991;66:212–227. doi: 10.1152/jn.1991.66.1.212. [DOI] [PubMed] [Google Scholar]
- 3.Bindokas VP, Jordán J, Lee CC, Miller RJ. Superoxide production in rat hippocampal neurons: selective imaging with hydroethidine. J Neurosci. 1996;16(4):1324–1336. doi: 10.1523/JNEUROSCI.16-04-01324.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan SF, Sucher NJ. An NMDA receptor signaling complex with protein phosphatase 2A. J Neurosci. 2001;21:7985–7992. doi: 10.1523/JNEUROSCI.21-20-07985.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coderre TJ, Xanthos DN, Francis L, Bennett GJ. Chronic post-ischemia pain (CPIP): a novel animal model of complex regional pain syndrome-Type I (CRPS-I; reflex sympathetic dystrophy) produced by prolonged hind paw ischemia and reperfusion in the rat. Pain. 2004;112:94–105. doi: 10.1016/j.pain.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Contestabile A. Antioxidant strategies for neurodegenerative diseases. Exp Opin Therap Patents. 2001;11:573–585. [Google Scholar]
- 7.Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262:689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- 8.Dougherty PM, Palecek J, Zorn S, Willis WD. Combined application of excitatory amino acids and substance P produces long-lasting changes in responses of primate spinothalamic tract neurons. Brain Res Rev. 1993;18:227–246. doi: 10.1016/0165-0173(93)90003-i. [DOI] [PubMed] [Google Scholar]
- 9.Galeazza MT, Garry MG, Yost HJ, Strait KA, Hargreaves KM, Seybold VS. Plasticity in the Synthesis and Storage of Substance-P and Calcitonin-Gene-Related Peptide in Primary Afferent Neurons During Peripheral Inflammation. Neurosci. 1995;66:443–458. doi: 10.1016/0306-4522(94)00545-g. [DOI] [PubMed] [Google Scholar]
- 10.Gerlach M, Ben Shachar D, Riederer P, Youdim MB. Altered brain metabolism of iron as a cause of neurodegenerative diseases? J Neurochem. 1994;63:793–807. doi: 10.1046/j.1471-4159.1994.63030793.x. [DOI] [PubMed] [Google Scholar]
- 11.Guedes RP, Bosco LD, Teixeira CM, Araujo AS, Llesuy S, Bello-Klein A, Ribeiro MF, Partata WA. Neuropathic pain modifies antioxidant activity in rat spinal cord. Neurochem Res. 2006;31:603–609. doi: 10.1007/s11064-006-9058-2. [DOI] [PubMed] [Google Scholar]
- 12.Hacimuftuoglu A, Handy CR, Goettl VM, Lin CG, Dane S, Stephens RL. Antioxidants attenuate multiple phases of formalin-induced nociceptive response in mice. Beh Brain Res. 2006;173:211–216. doi: 10.1016/j.bbr.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 13.Han DS, Cadenas E, Kobayashi MS, Packer L. Oxidative stress in glutamate neurotoxicity. In: Poli G, Cadenas E, Packer L, editors. Free radicals in brain pathophysiology. New York: Dekker; 2000. pp. 127–157. [Google Scholar]
- 14.Hensley K, Pye QN, Tabatabaie T, Stewart CA, Floyd RA. Reactive oxygen involvement in neurodegenerative pathways. In: Wood PL, editor. Neuroinflammation: Mechanisms and Management. Totowa, NJ: Humana Press; 1997. pp. 265–281. [Google Scholar]
- 15.Hylden JLK, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol. 1980;67:313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- 16.Jenner P. Oxidative damage in neurodegenerative disease. Lancet. 1994;344:796– 798. doi: 10.1016/s0140-6736(94)92347-7. [DOI] [PubMed] [Google Scholar]
- 17.Kahlert S, Zündorf G, Reiser G. Glutamate-mediated influx of extracellular Ca2+ is coupled with reactive oxygen species genertion in cultured hippocampal neurons but not in astrocytes. J Neurosci Res. 2005;79:262–271. doi: 10.1002/jnr.20322. [DOI] [PubMed] [Google Scholar]
- 18.Khalil Z, Khodr B. A role for free radicals and nitric oxide in delayed recovery in aged rats with chronic constriction nerve injury. Free Radic Biol Med. 2001;31:430–439. doi: 10.1016/s0891-5849(01)00597-4. [DOI] [PubMed] [Google Scholar]
- 19.Khalil Z, Liu T, Helme RD. Free radicals contribute to the reduction in peripheral vascular responses and the maintenance of thermal hyperalgesia in rats with chronic constriction injury. Pain. 1999;79:31–37. doi: 10.1016/S0304-3959(98)00143-2. [DOI] [PubMed] [Google Scholar]
- 20.Kim HK, Kim JH, Gao X, Zhou JL, Lee I, Chung K, Chung JM. Analgesic effect of vitamin E is mediated by reducing central sensitization in neuropathic pain. Pain. 2006;122:53–62. doi: 10.1016/j.pain.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 21.Kim HK, Park SK, Zhou J-L, Taglialatela G, Chung K, Coggeshall RE, Chung JM. Reactive oxygen species (ROS) play an important role in a rat model of neuropathic pain. Pain. 2004;111:116–124. doi: 10.1016/j.pain.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 22.Koul B, Vesterqvist O, Egberg N, Steen S. 24-Hour Heparin-Free Veno Right Ventricular Eco - An Experimental-Study. Ann Thor Surg. 1992;53:1046–1051. doi: 10.1016/0003-4975(92)90386-i. [DOI] [PubMed] [Google Scholar]
- 23.Lee I, Park ES, Kim HK, Wang JG, Chung K, Chung JM. A modified direct lumbar puncture method in rats. Neurosci Abst. 2006:832.20. [Google Scholar]
- 24.Lee I, Kim HK, Kim JH, Chung K, Chung JM. The role of reactive oxygen species in capsaicin-induced mechanical hyperalgesia and in the activities of dorsal horn neurons. Pain. 2007;133:9–17. doi: 10.1016/j.pain.2007.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewen A, Matz P, Chan PH. Free radical pathways in CNS injury. J Neurotrauma. 2000;17:871–890. doi: 10.1089/neu.2000.17.871. [DOI] [PubMed] [Google Scholar]
- 26.Maher P, Schubert D. Signaling by reactive oxygen species in the nervous system. Cell Mol Life Sci. 2000;57:1287–1305. doi: 10.1007/PL00000766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Odell TJ, Huang PL, Dawson TM, Dinerman JL, Snyder SH, Kandel ER, Fishman MC. Endothelial Nos and the Blockade of Ltp by Nos Inhibitors in Mice Lacking Neuronal Nos. Science. 1994;265:542–546. doi: 10.1126/science.7518615. [DOI] [PubMed] [Google Scholar]
- 28.Park E-S, Gao X, Chung JM, Chung K. Levels of mitochondrial reactive oxygen species increase in rat neuropathic spinal dorsal horn neurons. Neurosci Lett. 2006;391:108–111. doi: 10.1016/j.neulet.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 29.Rhee SG, Bae YS, Lee SR, Lee C, Kwon J, Yang KS. Hydrogen peroxide in peptide growth factor signaling. Faseb Journal. 2000;14:A1505. [Google Scholar]
- 30.Sah P, Faber ESL. Channels underlying neuronal calcium-activated potassium currents. Prog in Neurobio. 2002;66:345–353. doi: 10.1016/s0301-0082(02)00004-7. [DOI] [PubMed] [Google Scholar]
- 31.Schapira AH, Cooper JM. Mitochondrial function in neurodegeneration and aging. Mutant Res. 1992;275:1133–1143. doi: 10.1016/0921-8734(92)90018-k. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz E, Lee I, Chung K, Chung JM. Oxidative stress in the spinal cord plays an important role in capsaicin-induced hyperalgesia. Neurosci Abst. 2006:551.21. [Google Scholar]
- 33.Schwartz E, Lee I, Kim HY, Chung K, Chung JM. The role of free radicals in capsaicin-induced pain. American Pain Society Abst. 2007 [Google Scholar]
- 34.Servitja JM, Masgrau R, Pardo R, Sarri E, Picatoste F. Effects of oxidative stress on phospholipid signaling in rat cultured astrocytes and brain slices. J Neurochem. 2000;75:788–794. doi: 10.1046/j.1471-4159.2000.0750788.x. [DOI] [PubMed] [Google Scholar]
- 35.Seybold VS, Galeazza MT, Garry MG, Hargreaves KM. Plasticity of Calcitonin-Gene-Related Peptide Neurotransmission in the Spinal-Cord During Peripheral Inflammation. Can J Phys and Pharmacol. 1995;73:1007–1014. doi: 10.1139/y95-141. [DOI] [PubMed] [Google Scholar]
- 36.Simone DA, Baumann TK, LaMotte RH. Dose-dependent pain and mechanical hyperalgesia in humans after intradermal injection of capsaicin. Pain. 1989;38:99–107. doi: 10.1016/0304-3959(89)90079-1. [DOI] [PubMed] [Google Scholar]
- 37.Steen KH, Reeh PW, Anton F, Handwerker HO. Protons Selectively Induce Lasting Excitation and Sensitization to Mechanical Stimulation of Nociceptors in Rat Skin, Invitro. J Neurosci. 1992;12:86–95. doi: 10.1523/JNEUROSCI.12-01-00086.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thiemermann C. Membrane-permeable radical scavengers (tempol) for shock, ischemia-reperfusion injury, and inflammation. Crit Care Med. 2003;31:S76–S84. doi: 10.1097/00003246-200301001-00011. [DOI] [PubMed] [Google Scholar]
- 39.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 40.Viggiano A, Monda M, Viggiano A, Viggiano D, Viggiano E, Chiefari M, Aurilio C, De Luca B. Trigeminal pain transmission requires reactive oxygen species production. Brain Res. 2005;1050:72–78. doi: 10.1016/j.brainres.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 41.Wang Z-Q, Porreca F, Cuzzocrea S, Galen K, Lightfoot R, Masini E, Muscoli C, Mollace V, Ndengele M, Ischiropoulos H, Salvemini D. A newly identified role for superoxide in inflammatory pain. J Pharmacol Exp Ther. 2004;309:869–878. doi: 10.1124/jpet.103.064154. [DOI] [PubMed] [Google Scholar]
- 42.Willis WD. Role of neurotransmitters in sensitization of pain responses. Ann NY Acad Sci. 2001;933:142–156. doi: 10.1111/j.1749-6632.2001.tb05821.x. [DOI] [PubMed] [Google Scholar]
- 43.Zhang X, Wu J, Fan L, Willis WD. The effects of protein phosphatase inhibitions on nociceptive behavioral responses of rats following intradermal injection of capsaicin. Pain. 2003;106:443–451. doi: 10.1016/j.pain.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 44.Zhang X, Wu J, Lei Y, Fang L, Willis WD. Protein phosphatase modulates the phosphorylation of spinal cord NMDA receptors in rats following intradermal injection of capsaicin. Pain. 2005;138:264–272. doi: 10.1016/j.molbrainres.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 45.Zimmermann M. Pathobiology of neuropathic pain. Eur J Pharmacol. 2001;429:23–37. doi: 10.1016/s0014-2999(01)01303-6. [DOI] [PubMed] [Google Scholar]