Abstract
Neuropathic pain occurs as a result of peripheral or central nervous system injury. Its pathophysiology involves mainly a central sensitization mechanism that may be correlated to many molecules acting in regions involved in pain processing, such as the spinal cord. It has been demonstrated that reactive oxygen species (ROS) and signaling molecules, such as the serine/threonine protein kinase Akt, are involved in neuropathic pain mechanisms. Thus, the aim of this study was to provide evidence of this relationship. Sciatic nerve transection (SNT) was used to induce neuropathic pain in rats. Western blot analysis of Akt and 4-hydroxy-2-nonenal (HNE)-Michael adducts, and measurement of hydrogen peroxide (H2O2) in the lumbosacral spinal cord were performed. The main findings were found seven days after SNT, when there was an increase in HNE-Michael adducts formation, total and p-Akt expression, and H2O2 concentration. However, one and 15 days after SNT, H2O2 concentration was raised in both sham (animals that were submitted to surgery without nerve injury) and SNT groups, showing the high sensibility of this ROS to nociceptive afferent stimuli, not only to neuropathic pain. p-Akt also increased in sham and SNT groups one day post injury, but at 3 and 7 days the increase occurred exclusively in SNT animals. Thus, there is crosstalk between intracellular signaling pathways and ROS, and these molecules can act as protective agents in acute pain situations or play a role in the development of chronic pain states.
Keywords: Neuropathic pain, Spinal cord, Sciatic nerve transection, 4-hydroxy-2-nonenal, Akt, Hydrogen peroxide
Introduction
Neuropathic pain can arise as a result of many diseases and involves different etiologies, such as diabetic neuropathy, postherpetic neuralgia, trigeminal neuralgia, and many other conditions, that cause functional abnormalities or direct injury in the peripheral or central nervous system (Finnerup et al. 2007). The underlying neuropathic pain mechanisms are related to peripheral and central sensitization. They originate from the release of inflammatory mediators around peripheral damaged tissue and ectopic discharges from the injured nerve leading to a hyperexcitable state in spinal dorsal horn neurons (Millan 1999). Central sensitization is the main contributor to the development of neuropathic pain. The related symptoms are the aberrant responses encountered in animal models and by patients, as the pain threshold decreased, perceived by pain evoked when there is mechanical or thermal light stimuli (Shim et al. 2005; Chizh et al. 2007).
It has been reported that several mediators and neurotransmitters participate in central sensitization (Woolf and Salter 2000). Reactive oxygen species (ROS) seems to be involved in this concern, since it was shown that neuropathic pain reduces antioxidant activity in the spinal cord (Guedes et al. 2006). In addition, systemic or intrathecal injections of antioxidants relieve allodynia (Kim et al. 2004, 2006). Thus, increasing evidence suggests a role for oxidative stress in neuropathic pain and in many neurological diseases, but the precise mechanisms that underlie pain and oxidative stress are still unclear. Increased production of ROS leads to marked changes in cell structure and function, as lipid peroxidation, protein oxidation and DNA damage. Some markers are useful to detect increased ROS, such as lipid peroxidation products. 4-hydroxy-2-nonenal (HNE) is a highly cytotoxic aldehyde generated from oxidation of polyunsaturated fatty acids. Thus, HNE analysis could provide an indication of lipid peroxidation, and also for protein oxidation since it reacts with the amino acids cysteine, histidine, and lysine forming Michael adducts (Uchida 2003). Several functions have been ascribed to HNE in the nervous system including neurotoxic and neurotrophic effects (Farooqui and Horrocks 2006). Recently it was demonstrated that HNE may enhance nociceptor excitability and consequent pain hypersensitivity in response to tissue injury (Trevisani et al. 2007). Besides HNE, hydrogen peroxide (H2O2) is a stable ROS that can be easily measured providing evidence of oxidative stress occurrence. The cell activity constantly produces superoxide anion (O•−2) from molecular oxygen. This anion can be toxic if it reacts with other molecules such as nitric oxide (NO), but antioxidant enzyme superoxide dismutase (SOD) converts the superoxide anion to H2O2. The damaging potential of H2O2 consists in its ability to yield hydroxyl radical (•OH) by the iron catalyzed Fenton and Haber–Weiss reactions. Thus, the increased H2O2 concentration may be harmful to cells. Moreover, H2O2 is a diffusible ROS contributing to the development of pathological pain states not only by generating dangerous ROS but also by modulating synaptic plasticity. This ROS play a role in releasing intracellular stored calcium and interfering in synaptic activity at dorsal horn interneurons (Takahashi et al. 2007).
Relationships among molecules related to neural processing are very intricate, and neurotransmission is also modulated by several signal transduction events that crosstalk with ROS, especially H2O2. An important signaling pathway in the nervous system involves Akt. Akt is a serine/threonine protein kinase often refered to as a survival and growth cell promoter (Manning and Cantley 2007). Its activation occurs as a result of phosphatidylinositol 3-kinase (PI3K) stimulation by neurotrophin, cytokines, and other cellular stimuli. It has been well established that the nerve growth factor (NGF) enhances the sensation of pain (Lewin et al. 1994; Bonnington and McNaughton, 2003). Thus, NGF might activate Akt in response to nociceptive stimulus. In cortical neurons it was observed that increased ROS can down-regulate the PI3K/Akt pathway enhancing neuron susceptibility to cell death (Taylor et al. 2005). Antioxidant treatment prevents neuronal injury induced by H2O2 by modulating cell signaling pathways, and these effects are more important in PI3K/Akt than in MAPK (mitogen-activated protein kinase) transduction cascades (Vauzour et al. 2007).
In despite of studies that correlate ROS generation and signaling pathway activation in the nervous system, no results on this issue are found in pain conditions. Accordingly, this study proposes to do this correlation by Western blot analysis of Akt and HNE-Michael adducts, and measurement of H2O2 in spinal cord of rats submitted to sciatic nerve transection, a useful neuropathic pain experimental model.
Materials and Methods
Animals
Experiments were conducted in adult male Wistar rats weighing 200–250 g. All animal procedures were approved by the Ethics Committee at the Federal University of Rio Grande do Sul. Under anesthesia (ketamine 80 mg/kg and xylazine 2 mg/kg) and sterile conditions, the right sciatic nerve was exposed and transected at mid-thigh level. In sham-operated animals, the right sciatic nerve was exposed but not transected. For further comparisons a naïve group was included in which the animals do not undergo surgical manipulation. Groups of five animals were sacrificed 1, 3, 7, and 15 days after surgery.
Preparation of Tissue Samples
Rats were killed by decapitation and their lumbosacral spinal cords were quickly dissected out. The tissues were immediately cooled in liquid nitrogen and processed to determine hydrogen peroxide. The Western blot samples were homogenized in lysis buffer containing protease inhibitors and detergents (20 mM Tris–HCl, 150 mM NaCl, 1 mM EDTA, 10% glycerol, 1% Nonidet P40, 200 μM phenylmethylsulfonil fluoride (PMSF), leupeptin (25 μg/ml), pH 7,4). The homogenates were centrifuged at 800×g for 20 min to discard nuclei and cell debris, and the supernatant fraction obtained was frozen at −70°C for further measurements.
Determination of Hydrogen Peroxide
The assay was based on horseradish peroxidase (HRPO)-mediated oxidation of phenol red by hydrogen peroxide, leading to the formation of a compound that absorbs at 610 nm. Slices of fresh tissue from lumbosacral spinal cord were incubated for 30 min. at 37°C in phosphate buffer 10 mmol/l (NaCl 140 mmol/l and dextrose 5 mmol/l). The supernatants were transferred to tubes with phenol red 0.28 mmol/l and 8.5 U/ml HRPO. After 5 min incubation, NaOH 1 mol/l was added, and it was read at 610 nm. The results were expressed in nmoles H2O2/g tissue (Pick and Keisari 1980).
Western Blot
Sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis (12%) was carried out using a miniprotein system (Bio-Rad). The molecular weights of the bands were determined by reference to a standard molecular weight marker (rainbow full range Amersham). Protein (50 μg) was loaded in each lane with loading buffer containing 0.375mol/l Tris (pH 6.8), 50% glycerol, 10% SDS, 0.5 mol/l mercaptoethanol, and 0.002% bromophenol blue. Samples were heated at 100°C for 2 min prior to gel loading. After electrophoresis, proteins were transferred to nitrocellulose membranes (Amersham) using an electrophoretic transfer system at 110V for 1 h. The membranes were then washed with TTBS (20 mmol/l Tris–HCl, pH 7.5; 150 mmol/l NaCl; 0.05% Tween-20, pH 7.4) and 8% nonfat dry milk for 1 h. The membranes were incubated overnight at 4°C with the primary antibodies diluted in TTBS plus 2.5% BSA. Rabbit anti-HNE-Michael adducts (1:1000, Calbiochem), rabbit anti-total-Akt polyclonal antibody and rabbit anti-phospho-Akt ser657 (Santa Cruz Biotechnology) were used as primary antibodies. After washing with TTBS, the membranes were incubated for 2 h at room temperature with secondary antibody (1:5000, anti-rabbit IgG peroxidase conjugated; Santa Cruz), washed with TBS (20 mmol/l Tris–HCl; 150 mM NaCl, pH 7.5) and revealed by chemiluminescence followed by apposition of the membranes to autoradiographic films. These films were analyzed with an image densitometer (Imagemaster VDS CI, Amersham). The results were expressed as % of pixels mean. The amount of protein per lane transfer was corrected by the Ponceau method (Klein et al. 1995).
Protein Measurement
Protein was measured by the Bradford method (1976), using bovine serum albumin as standard.
Statistical Analysis
The results were compared by one way ANOVA followed by Student Newman Keuls post hoc multiple comparison test. Differences were considered statistically significant when the P value <0.05. All statistical analyses were carried out with SPSS 13.0 software.
Results
Hydrogen Peroxide
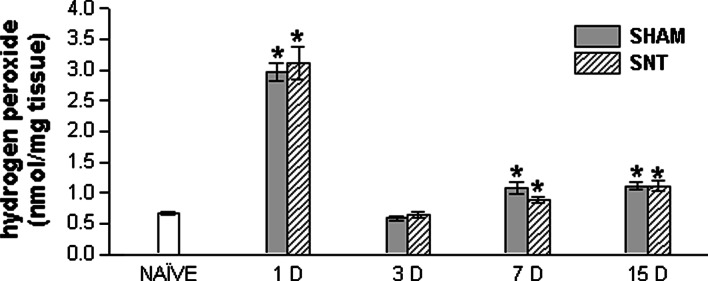
There was a significant increase in H2O2 concentration in lumbosacral spinal cord one day after surgery (P < 0.05). This rise was found in both groups: those that had undergone sciatic transection and sham surgery, indicating that H2O2 concentration is affected not only by neuropathic pain, but also by surgical manipulation (Fig. 1). Three days after surgery H2O2 concentration was similar to that of the naïve group. Seven and fifteen days after surgery, the H2O2 concentration values were lower than 1 day groups, but higher than the naïve one (P < 0.05). At these time points, results also do not differ between sham and SNT groups. These results demonstrate a dramatic increase in H2O2 concentration in lumbosacral spinal cord during an acute period of injury and a large sensibility in H2O2 formation, since sham animals also exhibited increased H2O2 levels.
Fig. 1.
Hydrogen peroxide (H2O2) concentration in lumbosacral spinal cord of naïve, sham, and SNT groups 1, 3, 7, and 15 D. * P < 0.05 (one way ANOVA followed by Student-Newman-Keuls post hoc test) compared to the naïve group. H2O2 values are expressed as nmol/mg of tissue. Data represent the means ± SEM (n = 5 for each group). SNT, sciatic nerve transection; 1, 3, 7, and 15 D, day(s) after surgery
4-hydroxy-2-nonenal (HNE)
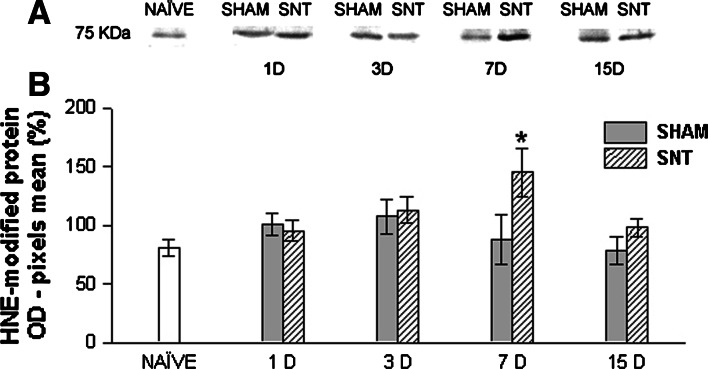
A few bands reacted with anti-HNE indicating HNE-protein adduct formation (Fig. 2). We chose the 75-KDa band to quantify the optical density, since it showed reaction in all samples and may provide a better parameter to compare the changes in the formation of Michael adducts due to lipid peroxidation among all samples analyzed. The results demonstrate a significant (P < 0.05) increase in lumbosacral spinal cord HNE-protein adduct formation 7 days after SNT as compared to naïve and sham animals. This result indicates an elevation in lipid peroxidation products, such as HNE, that may bind to cellular proteins, in response to neuropathic pain induction.
Fig. 2.
Western blot analysis for HNE-modified protein. (a) Representative Western blots showing HNE-protein adduct formation in lumbosacral spinal cord homogenates of naïve, sham, and SNT groups 1, 3, 7, and 15 D detected with anti-HNE antibody. (b) Mean ± SEM value percentages of pixel optical density of 75 KDa protein positive for anti-HNE antibody (expressed in arbitrary units) in spinal cord homogenates of naïve, sham, and SNT groups 1, 3, 7, and 15 days after surgery. * P < 0.05 (one way ANOVA followed by Student-Newman-Keuls post hoc test) in relation to naïve and sham (n = 5 for each group). OD, optical density; SNT, sciatic nerve transection; 1, 3, 7, and 15 D, day(s) after surgery
p-Akt and total-Akt
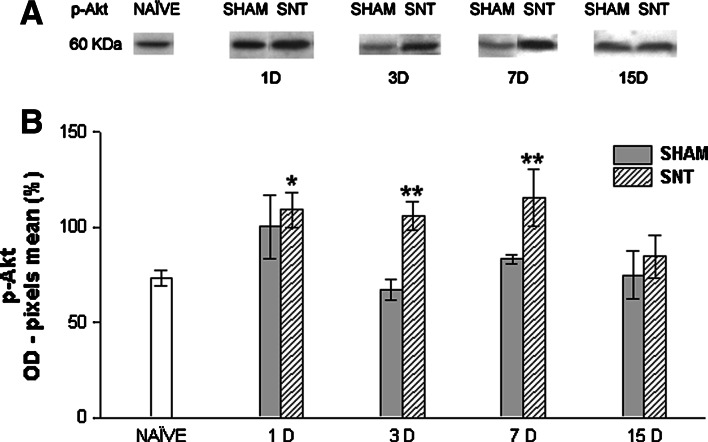
Sciatic nerve transection causes a significant (P < 0.05) rise in p-Akt expression (Fig. 3). The employed antibody recognizes phosphorylation on serine 657 residue. This increase was significant 1, 3, and 7 days after surgery, demonstrating that injury may affect and activate intracellular transduction pathways in earlier stages of neuropathic pain. One day post surgery sham animals also exhibit an increase in p-Akt expression in relation to the naïve group, as illustrated by Fig. 4b. However, it was not significant statistically (P > 0.05). Moreover, sham does not differ from SNT at this point in time. This result may be explained by activation of pain pathways by skin and muscle lesion a few hours after surgery due to surgical manipulation, and it leads to Akt pathway activation. At 3 and 7 days post injury, sham animals returned to naïve p-Akt expression levels. At 15 days post surgery, both sham and SNT groups decrease p-Akt expression, becoming similar to the naïve group.
Fig. 3.
Western blot analysis for phospho-Akt (p-Akt). (a) Representative Western blots showing expression of p-Akt (ser657) in lumbosacral spinal cord homogenates of naïve, sham, and SNT groups 1, 3, 7, and 15 D. (b) Mean ± SEM value percentages of pixel optical density of p-Akt (expressed in arbitrary units) in spinal cord homogenates of naïve, sham, and SNT groups 1, 3, 7, and 15 days after surgery. * indicates P < 0.05 between 1 D SNT and naïve. ** indicates P < 0.05 among 3 D SNT compared to 3 D sham and naïve, and 7 D SNT compared to 7 D sham and naïve. The statistical test employed was one way ANOVA followed by Student-Newman-Keuls post hoc test (n = 5 for each group). OD, optical density; SNT, sciatic nerve transection; 1, 3, 7, and 15 D, day(s) after surgery
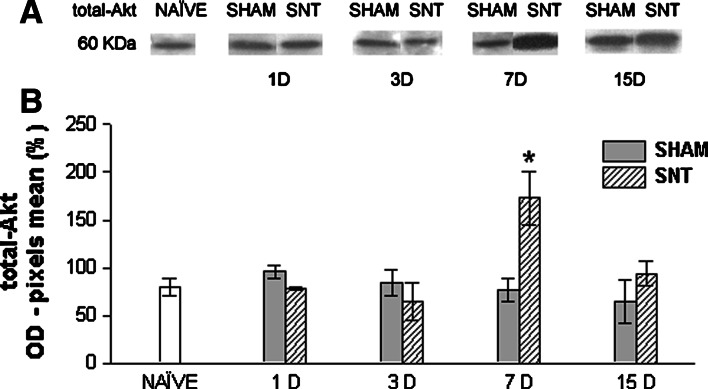
Fig. 4.
Western blot analysis for total-Akt. (a) Representative western blots showing expression of total-Akt in lumbosacral spinal cord homogenates of naïve, sham, and SNT groups 1, 3, 7, and 15 days after surgery. (b) Mean ± SEM value percentages of pixel optical density of total-Akt (expressed in arbitrary units) in spinal cord homogenates of naïve, sham, and SNT groups 1, 3, 7, and 15 D after surgery. * indicates P < 0.05 in relation to naïve and sham (one way ANOVA followed by Student-Newman-Keuls post hoc test) (n = 5 for each group). OD, optical density; SNT, sciatic nerve transection; 1, 3, 7, and 15 D, day(s) after surgery
Western blot analysis revealed that neuropathic pain leads to a striking rise in total-Akt (Fig. 4). Thus, not only does Akt phosphorylation increase, but there is also a rise in expression levels of Akt protein in response to nerve injury. This may help to provide a higher source to phosphorylation by transduction cascade activation following peripheral nerve damage, amplifying the nociceptive signal. The increase was found 7 days post sciatic nerve transection (P < 0.05).
Discussion
The present study demonstrated that painful stimulation to peripheral afferents via sciatic nerve axotomy causes activation of Akt, a serine/threonine protein kinase, and also increases production of ROS evidenced by H2O2. Moreover, cell damage is occurs, as supported by HNE, a marker of lipid peroxidation, in the lumbosacral spinal cord, the region where nociceptive information arrives in the central nervous system.
The reduction of O2 to H2O generates dangerously reactive intermediates, such as H2O2. Elevation in production of these intermediates and decrease in antioxidant defenses, are responsible for the toxicity of O2, producing oxidative damage (Fridovich 1998). ROS-mediated neuronal injury is a final common pathway for a number of brain disease processes (Sas et al. 2007). In pain studies, injection of formalin into the upper lip of rats causes an increase in H2O2 level in the spinal trigeminal nucleus. Treatment with 2-methoxyoestradiol (2-ME), a SOD inhibitor, and N-acetylcysteine (NAC), a ROS scavenger, reduces nociceptive behavioral response (Viggiano et al. 2005). Decline in SOD by 2-ME leads to a decrease in the production of H2O2, which may be related to decrease in nociceptive signals demonstrated by behavioral tests. Accordingly, H2O2 seems to be a major molecule in pain transmission. Furthermore, H2O2 increases the activity of substantia gelatinosa neurons in the dorsal horn via mobilization of calcium stores. These effects cause an increase in inhibitory current frequency of these neurons, hence, H2O2 acts in synaptic plasticity of sensory neurons in spinal cord interfering in nociceptive processing. However, the effects of H2O2 on the GABAergic inhibitory synapses are not well understood and the inhibition may occur on the inhibitory interneurons generating enhanced overall excitation (Takahashi et al. 2007).
In the present study the increase in H2O2 concentration in the spinal cord was more pronounced one day after surgery in both sham and SNT groups. This result can be explained since in sham animals lesions occur in the skin and slightly in adjacent connective tissue to expose the sciatic nerve, stimulating cutaneous afferents. However, three days after surgery there was a decrease in H2O2 concentration, but we cannot state that cells are being protected in this period because there is reduction in catalase activity as demonstrated in our previous work (Guedes et al. 2006). Catalase converts H2O2 to H2O, thus, with decreased catalase, H2O2 produced may have been converted to more toxic molecules, as hydroxyl radical, since there was a reduction in enzymatic antioxidant defense. Fifteen days after surgery, the H2O2 concentration was increased in relation to naïve animals, but antioxidant enzyme activities were near to basal levels, and this could at least in part protect the cells from oxidative damage. Therefore, changes encountered 7 days after SNT seem to be more dangerous to spinal cord cells. At this point in time, there was a remarkable decrease in catalase activity, and this may lead to greater toxicity. In addition, increased H2O2 production occurs in spite of decreased activity of SOD (Guedes et al. 2006), an enzyme that produces H2O2. This response was specific to the SNT group, in other words, generated exclusively by neuropathic pain. Sham animals also demonstrate increased H2O2 seven days after surgery, but in this time, SOD activity is near to basal levels showing that there is greater stimulation of H2O2 production post-axotomy.
Increased activity of sensory afferents generates greater oxygen expenditure, and if antioxidant defenses are low or absent, oxidative stress may occur. In experimental models of neuropathic pain mitochondrial ROS production has been demonstrated in the spinal cord (Park et al. 2006), and injection of ROS scavengers effectively reduces allodynia (Kim et al. 2004). Measurement of thiobarbituric acid reactive substances (TBARS) did not detect changes in lipid peroxidation post SNT (Guedes et al. 2006), but this result may be due to the limitation of this technique. On the other hand, HNE is a much more reliable index of lipid peroxidation than malondialdehyde (Moore and Roberts 1998). In the present study, the greater toxicity produced one week after SNT can be evidenced by increase in HNE-Michael adducts formation. Exposure of neurons to HNE causes cytotoxic effects leading to loss of cell viability and even to apoptosis (Malecki et al. 2000). Toxic effects of HNE can be attributed to its ability to disturb calcium homeostasis, since increases in calcium mitochondrial and cytoplasmic levels elicited by HNE were seen in cultured neural cells leading to apoptosis and necrosis (Kruman and Mattson 1999). The reactivity of HNE also can be observed by its protein adducts, that consequently changes protein conformation and function. The importance of HNE in modifying proteins and impairing the central nervous function may be illustrated by its role in the pathogenesis of neurodegenerative disorders (Yoritaka et al. 1996; Sayre et al. 1997). The apoptotic process in Alzheimer’s disease is linked to oxidative stress-dependent activation of signaling pathways, and HNE and H2O2 are directly related to this (Tamagno et al. 2003). On the other hand, HNE, under nontoxic levels, curiously also activates survival mechanisms against cytotoxic effects (Uchida 2003).
Akt is a downstream target of PI3K, and activation of this intracellular signaling cascade is an important stimulus to avoid neuron apoptosis. This effect is due to phosphorylation and inactivation of Bad, a pro-apoptotic member of the Bcl-2 family, that in turn, prevents caspase activation and cell death (Alessi and Cohen 1998). ROS such as H2O2 are stimulants for Akt phosphorylation and this provides a signal to protect cells against oxidative stress (Wang et al. 2000). In this study, SNT animals showed an elevation in phospho-Akt expression in the lumbosacral spinal cord, one, three, and seven days after axotomy, but 15 days after SNT the phospho-Akt expression returned to naïve levels. These changes in phospho-Akt expression are possibly related to increased cell susceptibility to oxidative damage as demonstrated in our work by increase in H2O2 concentration and HNE-protein adducts formation. In other studies, the increase in Akt phosphorylation occurs in early stages of pain transmission (Bonnington and McNaughton 2003) in both peripheral nerve injury (Xu et al. 2007) and pain elicited by capsaicin injection (Pezet et al. 2005). In these studies, inhibition of the PI3K/Akt pathway reduces hyperalgesia, so this intracellular signaling cascade is involved in enhancing the sensation of pain, and perhaps contributing to the development of neuropathic pain.
Moreover, we found an increase in total-Akt seven days after SNT. This finding emphasizes that one week post axotomy is a critical period in which the spinal cord is more exposed to oxidative stress, since in this period there was an increase in H2O2 and HNE-Michael adducts formation. Thus, elevation of total-Akt expression may mean a rise in protein synthesis of Akt aiming to protect the cell. The signals that activate Akt in pain transmission are unknown. Growth factors are putative molecules with this action. It is well established that neurotrophins bind to the same tyrosine kinase receptor that activates several signaling pathways, including PI3K/Akt (Malik-Hall et al. 2005; Chao et al. 2006), and growth factors are upregulated in pain conditions (Pezet and McMahon 2006). However it is likely that there is involvement of ROS as H2O2 or even HNE to activate signaling intracellular pathways in nociceptive processing. In brain endothelial cells, superoxide anion and H2O2 activated PI3K/Akt pathway and antioxidants inhibited Akt phosphorylation showing that ROS contributed to Akt activation (Schreibelt et al. 2007). In addition, it was demonstrated that protein oxidation is involved in Akt phosphorylation and neuroprotection in neuron culture (Delgado-Esteban et al. 2007). Accordingly, in pain transmission the role of ROS as second messengers that activate the Akt pathway may also be reasonable, since we show protein adducts formation by HNE and increase in H2O2 in our pain model.
Thus, Akt is related to pain behaviors, but their role in cell survival cannot be ruled out. Besides post-translational regulation of pro-nociceptive receptors and other pain-related molecules in the dorsal root ganglia and spinal cord dorsal horn, kinase proteins may interfere in gene expression (Woolf and Costigan 1999). It has been suggested that the mitogen-activated protein kinase (MAPK) cascade interferes in gene expression in neuropathic pain by phosphorylating the transcription factor cAMP response element binding protein (CREB). This may be related to increased expression of receptors or neurotransmitters/neuromodulators (Song et al. 2005). Nevertheless, there are no studies that demonstrate linkage between Akt activation and gene expression in pain conditions. However, in spite of the well established role of Akt in cell survival (Manning and Cantley 2007) and the crosstalk with MAPK cascade in pain conditions (Malik-Hall et al. 2005) we can suggest the hypothesis that this kinase protein may be acting in the spinal cord by post-translational and transcriptional changes activating or increasing gene expression of molecules that intensify pain transmission. Moreover, this signaling may be favorable because of the protective role of pain, i.e., after injury the increase in nociceptive threshold may prevent further injury.
In conclusion, this study provides evidence concerning the involvement of oxidative stress and Akt in neuropathic pain. The activation of Akt in response to neuropathic pain may be due to a signal originated by second messengers like H2O2 and oxidized proteins by HNE and this is an attempt by sensory neurons to stimulate the expression of nociceptive-related genes. Further studies are necessary to establish the precise role of oxidative stress and intracellular signaling pathways in pain transmission and can help to explain whether these molecules are protective in acute pain situations or may play a role in the development of long-lasting pathological pain states after nerve injury.
Acknowledgments
This work was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS).
References
- Alessi DR, Cohen P (1998) Mechanism of activation and function of protein kinase B. Curr Opin Genet Dev 8(1):55–62 [DOI] [PubMed] [Google Scholar]
- Bonnington JK, McNaughton PA (2003) Signalling pathways involved in the sensitisation of mouse nociceptive neurones by nerve growth factor. J Physiol 551(Pt 2):433–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254 [DOI] [PubMed] [Google Scholar]
- Chao MV, Rajagopal R, Lee FS (2006) Neurotrophin signalling in health and disease. Clin Sci (Lond) 110(2):167–173 [DOI] [PubMed] [Google Scholar]
- Chizh BA, Gohring M, Troster A, Quartey GK, Schmelz M, and Koppert W (2007) Effects of oral pregabalin and aprepitant on pain and central sensitization in the electrical hyperalgesia model in human volunteers. Br J Anaesth 98(2):246–254 [DOI] [PubMed] [Google Scholar]
- Delgado-Esteban M, Martin-Zanca D, Andres-Martin L, Almeida A, Bolaños JP (2007) Inhibition of PTEN by peroxynitrite activates the phosphoinositide-3-kinase/Akt neuroprotective signaling pathway. J Neurochem 102(1):194–205 [DOI] [PubMed] [Google Scholar]
- Farooqui AA, Horrocks LA (2006) Phospholipase A2-generated lipid mediators in the brain: the good, the bad, and the ugly. Neuroscientist 12(3):245–260 [DOI] [PubMed] [Google Scholar]
- Finnerup NB, Sindrup SH, Jensen TS (2007) Chronic neuropathic pain: mechanisms, drug targets and measurement. Fundam Clin Pharmacol 21(2):129–136 [DOI] [PubMed] [Google Scholar]
- Fridovich I (1998) Oxygen toxicity: a radical explanation. J Exp Biol 201(8):1203–1209 [DOI] [PubMed] [Google Scholar]
- Guedes RP, Bosco LD, Teixeira CM, Araujo AS, Llesuy S, Bello-Klein A, Ribeiro MF, Partata WA (2006) Neuropathic pain modifies antioxidant activity in rat spinal cord. Neurochem Res 31(5):603–639 [DOI] [PubMed] [Google Scholar]
- Kim HK, Park SK, Zhou JL, Taglialatela G, Chung K, Coggeshall RE, Chung JM (2004) Reactive oxygen species (ROS) play an important role in a rat model of neuropathic pain. Pain 111(1–2):116–124 [DOI] [PubMed] [Google Scholar]
- Kim HK, Kim JH, Gao X, Zhou JL, Lee I, Chung K, Chung JM (2006) Analgesic effect of vitamin E is mediated by reducing central sensitization in neuropathic pain. Pain 122(1–2):53–62 [DOI] [PubMed] [Google Scholar]
- Klein D, Kern RM, Sokol RZ (1995) A method for quantification and correction of proteins after transfer to immobilization membranes. Biochem Mol Biol Int 36(1):59–66 [PubMed] [Google Scholar]
- Kruman II, Mattson MP (1999) Pivotal role of mitochondrial calcium uptake in neural cell apoptosis and necrosis. J Neurochem 72(2):529–540 [DOI] [PubMed] [Google Scholar]
- Lewin GR, Rueff A, Mendell LM (1994) Peripheral and central mechanisms of NGF-induced hyperalgesia. Eur J Neurosci 6(12):1903–1912 [DOI] [PubMed] [Google Scholar]
- Malecki A, Garrido R, Mattson MP, Hennig B, Toborek M (2000) 4-Hydroxynonenal induces oxidative stress and death of cultured spinal cord neurons. J Neurochem 74(6):2278–2287 [DOI] [PubMed] [Google Scholar]
- Malik-Hall M, Dina OA, Levine JD (2005) Primary afferent nociceptor mechanisms mediating NGF-induced mechanical hyperalgesia. Eur J Neurosci 21(12):3387–3394 [DOI] [PubMed] [Google Scholar]
- Manning BD, Cantley LC (2007) AKT/PKB signaling: navigating downstream. Cell 129(7):1261–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ (1999) The induction of pain: an integrative review. Prog Neurobiol 57(1):1–164 [DOI] [PubMed] [Google Scholar]
- Moore K, Roberts LJ 2nd (1998) Measurement of lipid peroxidation. Free Radic Res 28(6):659–671 [DOI] [PubMed] [Google Scholar]
- Park ES, Gao X, Chung JM, Chung K (2006) Levels of mitochondrial reactive oxygen species increase in rat neuropathic spinal dorsal horn neurons. Neurosci Lett 391(3):108–111 [DOI] [PubMed] [Google Scholar]
- Pezet S, McMahon SB (2006) Neurotrophins: mediators and modulators of pain. Annu Rev Neurosci 29:507–538 [DOI] [PubMed] [Google Scholar]
- Pezet S, Spyropoulos A, Williams RJ, McMahon SB (2005) Activity-dependent phosphorylation of Akt/PKB in adult DRG neurons. Eur J Neurosci 21(7):1785–1797 [DOI] [PubMed] [Google Scholar]
- Pick E, Keisari Y (1980) A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J Immunol Methods 38(1–2):161–170 [DOI] [PubMed] [Google Scholar]
- Sas K, Robotka H, Toldi J, Vecsei L (2007) Mitochondria, metabolic disturbances, oxidative stress and the kynurenine system, with focus on neurodegenerative disorders. J Neurol Sci 257(1–2):221–239 [DOI] [PubMed] [Google Scholar]
- Sayre LM, Zelasko DA, Harris PL, Perry G, Salomon RG, Smith MA (1997) 4-Hydroxynonenal-derived advanced lipid peroxidation end products are increased in Alzheimer’s disease. J Neurochem 68(5):2092–2097 [DOI] [PubMed] [Google Scholar]
- Schreibelt G, Kooij G, Reijerkerk A, van Doorn R, Gringhuis SI, van der Pol S, Weksler BB, Romero IA, Couraud PO, Piontek J, Blasig IE, Dijkstra CD, Ronken E, de Vries HE (2007) Reactive oxygen species alter brain endothelial tight junction dynamics via RhoA, PI3 kinase, and PKB signaling. FASEB J 21(13):3666–3676 [DOI] [PubMed] [Google Scholar]
- Shim B, Kim DW, Kim BH, Nam TS, Leem JW, Chung JM (2005) Mechanical and heat sensitization of cutaneous nociceptors in rats with experimental peripheral neuropathy. Neuroscience 132(1):193–201 [DOI] [PubMed] [Google Scholar]
- Song XS, Cao JL, Xu YB, He JH, Zhang LC, Zeng YM (2005) Activation of ERK/CREB pathway in spinal cord contributes to chronic constrictive injury-induced neuropathic pain in rats. Acta Pharmacol Sin 26(7):789–798 [DOI] [PubMed] [Google Scholar]
- Takahashi A, Mikami M, Yang J (2007) Hydrogen peroxide increases GABAergic mIPSC through presynaptic release of calcium from IP3 receptor-sensitive stores in spinal cord substantia gelatinosa neurons. Eur J Neurosci 25(3):705–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamagno E, Robino G, Obbili A, Bardini P, Aragno M, Parola M, Danni O (2003) H2O2 and 4-hydroxynonenal mediate amyloid beta-induced neuronal apoptosis by activating JNKs and p38MAPK. Exp Neurol 180(2):144–155 [DOI] [PubMed] [Google Scholar]
- Taylor JM, Ali U, Iannello RC, Hertzog P, Crack PJ (2005) Diminished Akt phosphorylation in neurons lacking glutathione peroxidase-1 (Gpx1) leads to increased susceptibility to oxidative stress-induced cell death. J Neurochem 92(2):283–293 [DOI] [PubMed] [Google Scholar]
- Trevisani M, Siemens J, Materazzi S, Bautista DM, Nassini R, Campi B, Imamachi N, Andre E, Patacchini R, Cottrell GS, Gatti R, Basbaum AI, Bunnett NW, Julius D, Geppetti P (2007) 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc Natl Acad Sci USA 104(33):13519–13524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida K (2003) 4-Hydroxy-2-nonenal: a product and mediator of oxidative stress. Prog Lipid Res 42(4):318–343 [DOI] [PubMed] [Google Scholar]
- Vauzour D, Vafeiadou K, Rice-Evans C, Williams RJ, Spencer JP (2007) Activation of pro-survival Akt and ERK1/2 signalling pathways underlie the anti-apoptotic effects of flavanones in cortical neurons. J Neurochem 103(4):1355–1367 [DOI] [PubMed] [Google Scholar]
- Viggiano A, Monda M, Viggiano A, Viggiano D, Viggiano E, Chiefari M, Aurilio C, De Luca B (2005) Trigeminal pain transmission requires reactive oxygen species production. Brain Res 1050(1–2):72–78 [DOI] [PubMed] [Google Scholar]
- Wang X, McCullough KD, Franke TF, Holbrook NJ (2000) Epidermal growth factor receptor-dependent Akt activation by oxidative stress enhances cell survival. J Biol Chem 275(19):14624–14631 [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Costigan M (1999) Transcriptional and posttranslational plasticity and the generation of inflammatory pain. Proc Natl Acad Sci USA 96(14):7723–7730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ, Salter MW (2000) Neuronal plasticity: increasing the gain in pain. Science 288(5472):1765–1769 [DOI] [PubMed] [Google Scholar]
- Xu JT, Tu HY, Xin WJ, Liu XG, Zhang GH, Zhai CH (2007) Activation of phosphatidylinositol 3-kinase and protein kinase B/Akt in dorsal root ganglia and spinal cord contributes to the neuropathic pain induced by spinal nerve ligation in rats. Exp Neurol 206(2):269–279 [DOI] [PubMed] [Google Scholar]
- Yoritaka A, Hattori N, Uchida K, Tanaka M, Stadtman ER, Mizuno Y (1996) Immunohistochemical detection of 4-hydroxynonenal protein adducts in Parkinson disease. Proc Natl Acad Sci USA 93(7):2696–701 [DOI] [PMC free article] [PubMed] [Google Scholar]